Abstract
G3 rotaviruses have been reported rarely in cattle, and none have been characterized. We report the first genomic characterization of a bovine G3 rotavirus, CP-1, which had been biologically characterized in vivo and shown to cause age-independent diarrhea. CP-1 was a G3 rotavirus as its VP7 had 92 to 96% deduced amino acid identity to those of G3 rotaviruses. However, initially, CP-1 was identified as a G10 rotavirus by RT-PCR even though the CP-1 VP7 had only 81 to 85% deduced amino acid identity to those of G10 rotaviruses. Rotavirus CP-1 was of P[5] specificity, a type common in cattle, and had a bovine NSP1 and NSP4. These results added another animal species to those in which G3 rotaviruses have been found, characterized a bovine rotavirus which caused age-independent diarrhea in calves, and raised the possibility that bovine G3 rotaviruses may be misdiagnosed as G10 rotaviruses.
Group A rotaviruses are a significant cause of neonatal diarrhea in humans and several animal species, including calves (31). They have been differentiated by neutralizing antigens on their VP4 and VP7 proteins, and this has led to classification by G type, based on VP7, and P type, based on VP4 (19). Initially, these types were based on serology, but increasingly, G and P typing has become genomically based. The G and P types circulating in different animal populations have been sought in order to design effective vaccines and to assess rotavirus cross-species infectivity. Specific G and P types have been associated with specific animal species. For example, human rotaviruses most commonly belong to G types 1 to 4, whereas bovine rotaviruses most commonly belong to G types 6, 8, and 10 and have P types [1], [5], or [11]. As more rotaviruses have been characterized from diverse locations throughout the world, the host species specificity of P and G types has become less distinct. G types 6, 8, and 10, once thought of as specific for cattle, have been found in humans (9, 13, 25, 29). Substantial sequence heterogeneity has also been identified in two rotavirus nonstructural proteins, NSP1 and NSP4, and rotaviruses from the same animal species have often, although not always, grouped together (8, 18, 27, 32). With bovine rotaviruses, their NSP1s clustered together as did their NSP4s into the proposed NSP4 genotype A. In the present paper we report the characterization of the VP4 (P type), VP7 (G type), NSP1, and NSP4 of the bovine rotavirus CP-1, which is well-established in our laboratory as a pathogen in experimental calves, producing age-independent diarrhea with small intestinal villous atrophy and malabsorption to at least 116 days of age (6, 17).
MATERIALS AND METHODS
Bovine rotavirus CP-1 was obtained from an outbreak of calf diarrhea in the United Kingdom in 1973, serially passaged in gnotobiotic calves, and cloned in cell culture, and its pathogenicity for gnotobiotic calves was reestablished (4, 6, 7, 17). The bovine-porcine G3P[7] reassortant rotavirus PP-1 was obtained from the same outbreak of calf diarrhea but by passage in experimental pigs (4, 11). The G3P[8] human rotavirus 200/97 was obtained from a sporadic case of diarrhea in a 10-month-old child in Newcastle, United Kingdom, in 1997 during a surveillance study of rotaviruses in the United Kingdom (24). Rotavirus genome profiles were determined on vertical 10% polyacrylamide gels as described previously (5). For G typing reverse transcription (RT)-PCR and nucleotide sequencing, viral RNA was extracted from cell culture fluids by phenol-chloroform or by the method of Boom et al. (3). The G-typing RT-PCR was conducted as described by Gouvea et al. (16) with the G5, G6, G8, and G10 primers but using the Expand High Fidelity PCR System (Roche). Rotaviruses OSU, UK, 678, and B223 were used as reference G5, G6, G8, and G10 rotaviruses, respectively. A G5-G6-G8-G10 primer pool or individual G-type-specific primers were used. Amplicons were electrophoresed on 2% agarose gels and visualized by short wave UV light after staining with ethidium bromide.
For VP4 gene sequencing, a VP4 cDNA fragment of 811 nucleotides encoding VP8*, the connecting peptide, and the terminus of VP5* was produced by RT-PCR using forward (EBH1, 5′-GGTACCCGGGATCCGCGGCTATAAAATGGCTTCGC-3′) and reverse (RVA4-1, 5′-AGATCTCGAGCGCTGCAGTATATTGCATTTCTTTCCA-3′) primers which were based on nucleotides 1 to 19 and nucleotides 793 to 811 (underlined) of the bovine rotavirus UK VP4 gene plus added cloning sites. The cDNA was cloned into pGEM-3Z (Promega Corp.) after digestion with PstI and BamHI restriction enzymes, and the plasmid was transfected into competent XL1-Blue Escherichia coli cells (Stratagene). Two or three gene clones from two PCR products were sequenced in both forward and reverse directions. The CP-1 VP7and NSP4 genes were reverse transcribed using hexamer primers. The VP7 gene was amplified using the forward and reverse VP7 gene-specific primers Beg9, End9, End9 UK, and CRW-8 (15). The NSP4 gene was amplified using the reverse primer 5′-CAT(A/C/G)G(A/C)(C/T)GCAGT(C/T)ACTTC-3′ and a pool of two forward primers: 5′-ATGGAAAAGTTTCCGACCTC-3′ and 5′-GGCTTTTAAAAGTTCTGTTT-3′ (modified from reference 25). VP7 and NSP4 amplicons were cloned into pCR2.1 vector and using a TOPO TA cloning kit (Invitrogen) as described by the manufacturer, and two clones were sequenced in both directions using standard M13 forward and reverse primers (5′-GTAAAACGACGGCCAG-3′ and 5′-CAGGAAACAGCTATGAC-3′, respectively). The CP-1 NSP1 gene was reverse transcribed using a gene-specific primer representing the 3′ end of NSP1 (5′-GGTCACATTTTATGCTGCCTA-3′) and amplified using the same primer and a forward primer representing the 5′ end (5′-GGCCGGCTTTTTTTATGA-3′) (modified from reference 30). NSP1 amplicons were sequenced directly from PCR products after gel purification using a Qiagen Quick kit. Sequencing was conducted with an ALF automated sequencer (Pharmacia) or an ABI 3700 sequencing system (MWG-Biotech). Consensus sequences were compared using the DNAstar or the NCBI BLAST sequence analysis programs. Multiple sequence alignments were performed using either the NCBI or Clustal X or EBI Clustal W programs. Phylogenetic analyses were conducted using the Clustal W neighbor-joining method. The VP7 monoclonal antibodies B223-N7 and B223/3 raised against the G10P[11] bovine rotavirus B223 were supplied by D. Snodgrass and G. N. Woode. MA104 cells were infected overnight, fixed, and stained with monoclonal antibodies at a 1:500 dilution, followed by a 1:200 dilution of peroxidase-conjugated rabbit anti-mouse immunoglobulin (DAKO). Color was developed with 3,3-diaminobenzidene tetrahydrochloride tablets (Kem-en-Tec, Copenhagen, Denmark).
The VP4 gene sequences used were as follows (accession numbers are given in parentheses): C486 (Y00127), UK (M22306), B223 (M92986), 999/83 (D16352), B641 (P25173), KK3 (P36308), A44 (P36307), 61A (P36306), A5 (P36305), and NCDV (P17465). The VP7 gene sequences used were as follows (accession numbers are given in parentheses): T449 (M92651), CRW-8 (edited from reference 20), AU-1 (D86271), SA11 (X66158), UK (X00896), 993/83 (X98869), 678 (L20883), B223 (X57852), P343 (edited from reference 28), B60 (M64680), A44 (D01055), B11 (M64679), 61A (X53403), 2292B (U14996) KK3 (D01056), EW (U08430), 97-S48 (AF260957), 98-B31 (AF260958), MP126 (AF386915), 02/92 (D86264),and 107e1b (U04350). The NSP1 gene sequences used were as follows (accession numbers are given in parentheses): UKtc (Z12108), RF (M22308), A44 (U23726), OSU (U08432), Gottfried (U08431), YM (D38154), Wa (L18943), DS1 (L18945), and H2 (D38157). The NSP4 gene sequences used were (accession numbers are given in parentheses): UK (K03384), NCDV (X06806), B223 (AF144805), BRV033 (AF144804), OSU (D88831), A253(AF144797), YM (X69485), A131 (AF144798), A411 (AF144799), Wa (AF093199), AU32 (D88830), KUN (D88829), ST3 (U59110), M37 (U59109), RV4 (U59108), 1076 (U59105), AU-1 (D89873), VA70 (U83798), RV5 (U59103), FRV64 (D88833), CU-1 (AF144806), EW (U96335), EHP (U96336), and EC (U96337). Accession numbers for the following rotavirus CP-1 genes are as indicated: VP4, AF448851; VP7, AF448852; NSP1, AF448853; and NSP4, AF448854.
RESULTS AND DISCUSSION
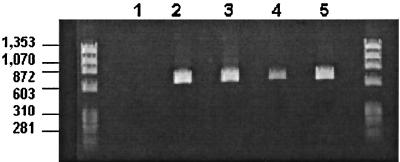
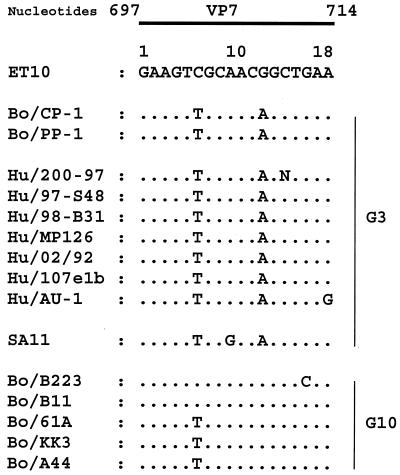
CP-1 had a typical group A rotavirus genome profile on 10% polyacrylamide gels (Fig. 1). Gene segments 1 to 3, 5, 6, 10, and 11 migrated indistinguishably from the equivalent segments of the G6P[5] rotavirus UK run in the parallel track. There were differences between the two rotaviruses in the 7-8-9 triplet region and in the 3-4 region, where CP-1 showed a slightly bigger gap between segments 3 and 4 than UK. By RT-PCR using the published ET10 primer for G10 rotaviruses (16), CP-1 was initially typed as a G10 rotavirus (Fig. 2). The expected 715-bp amplicon was produced with CP-1, the reference G10 bovine rotavirus B223, the recently characterized human G3P[8] rotavirus 200/97, and the recently characterized G3P[7] bovine-porcine reassortant PP-1 (11). However, immunoperoxidase tests with the G10-specific monoclonal antibodies B223/3 and B223-N7 failed to confirm G10 specificity for CP-1. Previously, sequence analysis of the ET10 amplicon of the bovine-porcine reassortant PP-1 showed G3, not G10, specificity: the amplicon had 93% deduced amino acid identity to G3 rotaviruses and 77% identity to G10 rotaviruses (11). The initial misdiagnosis of CP-1 and PP-1 as G10 rotaviruses by PCR was explained by the similarity of the CP-1 and PP-1 nucleotide sequences to the ET10 primer in the primer binding region, nucleotides 697 and 714 (Fig. 3). Both viruses differed from the ET10 G10 primer by only two nucleotides located in the middle of the G10 primer. Furthermore, the human G3P[8] rotavirus 200/97, which produced an amplicon with the ET10 primer of the size expected for G10 rotaviruses in our hands, had an identical or almost-identical (one nucleotide was undetermined) composition to CP-1 and PP-1 in the primer binding region. At least five additional human G3 rotaviruses (Hu/97-S48, Hu/98-B31, Hu/MP126, Hu/02/92, and Hu/107elb) also have nucleotide sequences identical to CP-1 and PP-1 in this region. Furthermore, the established G3 rotavirus SA11 has the same two differences as CP-1 plus one other.
FIG. 1.
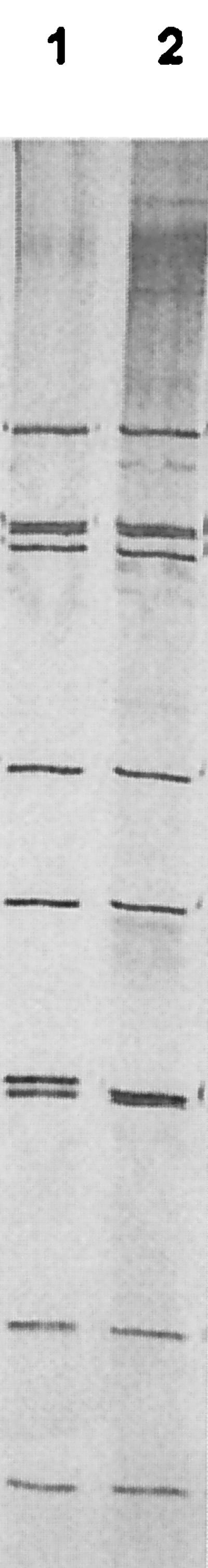
Genome profile analysis of the bovine rotaviruses CP-1 and UK on 10% polyacrylamide gels. Lane 1, the G6P[5] rotavirus UK; lane 2, the G3P[5] rotavirus CP-1.
FIG. 2.
G-typing PCR with the G10 primer ET10 described by Gouvea et al. (15). Lane 1, negative control; lane 2, G3P[8] human rotavirus 200/97; lane 3, G3P[5] bovine rotavirus CP-1; lane 4, bovine-porcine G3P[7] reassortant rotavirus PP-1; lane 5, G10P[11] bovine rotavirus B223. Molecular weight markers are contained in the two outer lanes and are indicated (in base pairs) at left.
FIG. 3.
Comparison of the VP7 nucleotide sequence of the ET10 G10 primer (reverse complement); the G3P[5] bovine rotavirus CP-1; the G3P[7] bovine-porcine reassortant rotavirus PP-1; the G3 human rotaviruses 200/97, 97-S48, 98-B31, MP126, 02/92, 107elb, and AU-1; the G3 simian rotavirus SA11; and the G10 bovine rotaviruses B223, B11, 61A, KK3, and A44. Accession numbers are given in the text.
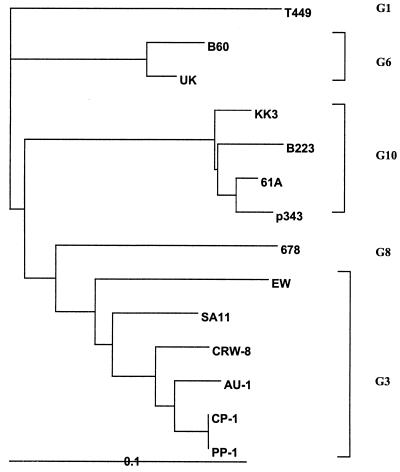
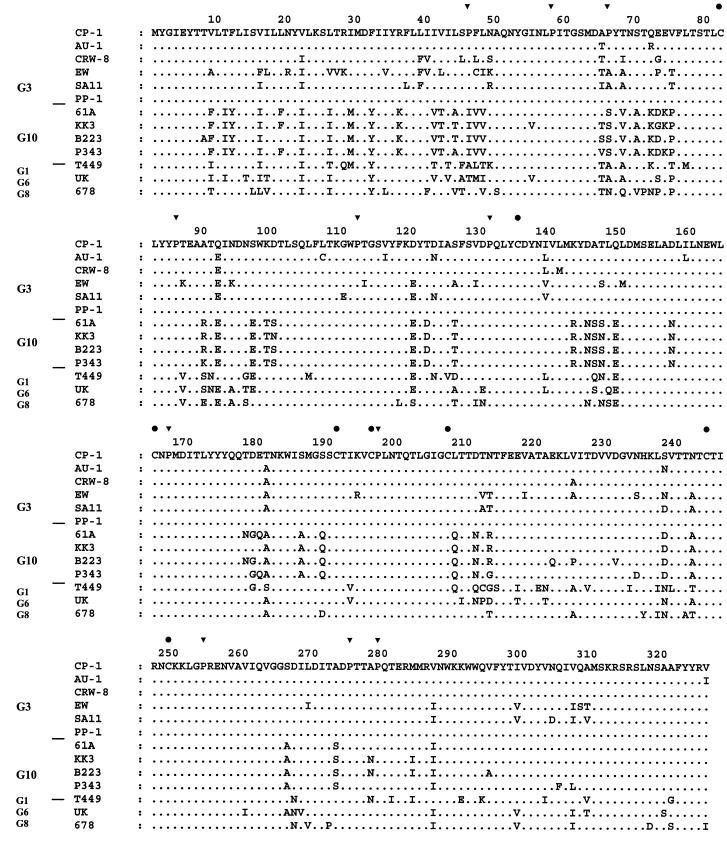
Sequence analysis of the full-length CP-1 VP7 showed it to be a G3, not G10, rotavirus with 92 to 96% deduced amino acid (81 to 90% nucleotide) identity to the porcine G3 rotavirus CRW-8, the human G3 rotavirus AU-1, and the simian G3 rotavirus SA11 and only 82 to 85% deduced amino acid (75 to 77% nucleotide) identity to the bovine G10 rotaviruses B223, 61A, KK3, A44, 2292B, and B11. The CP-1 VP7 had 57 to 83% amino acid (65 to 76% nucleotide) identity to the other G types found in cattle, the G1 rotavirus T449, the G6 rotaviruses UK and B60, the G7 rotavirus 993/83, and the G8 rotavirus 678. Phylogenetic analysis confirmed the grouping of CP-1 with G3 and not G10 rotaviruses (Fig. 4). Eight cysteine residues were identified in the CP-1 VP7 protein, in common with other rotaviruses (Fig. 5). Eleven proline residues were identified. Nine were common to, and at the same positions as, the G3 rotaviruses AU-1, CRW-8, EW, and SA11; the G10 rotaviruses 61A, KK3, B223, and P343; and the bovine G1, G6, and G8 rotaviruses T449, UK, and 678. The CP-1 VP7 also had proline residues at position 46, in common with the four G3 but not the G10 rotaviruses, and at position 66, in common with two of the four G3 rotaviruses. In antigenic regions B (amino acids 145 to 150) and C (amino acids 211 to 223) (26), the CP-1 VP7 protein was identical to those of the porcine CRW-8 and the human AU-1 G3 rotaviruses. In antigenic region A (amino acids 87 to 99), it differed from the G3 rotaviruses AU-1, CRW-8, and SA11 by one amino acid at position 92, with a mutation of glutamine (Q) to glutamic acid (E). It differed from the G10 rotaviruses B223, 61A, KK3, and P343 in each of the three regions by two to four amino acids.
FIG. 4.
Phylogenetic tree of VP7 deduced amino acid sequences of rotaviruses of G types 1, 3, 6, 8, and 10 and CP-1 produced by the Clustal W neighbor-joining method.
FIG. 5.
Multiple sequence alignment of the deduced amino acid sequence of the CP-1 VP7 with that of human AU-1, porcine CRW-8, murine EW, and simian SA11 G3 rotaviruses; bovine G1, G6, G8, and G10 rotaviruses; and the porcine G10 rotavirus P343. Accession numbers are given in the text. Symbols: ▾, CP-1 proline residues; •, CP-1 cysteine residues.
Sequence analysis of 267 deduced amino acids at the amino terminus of the CP-1 VP4 (representing VP8*, the interconnecting peptide and the 5′ terminus of VP5*) identified CP-1 as a P[5] rotavirus with 92 to 99% deduced amino acid (91 to 99.5% nucleotide) identity to the bovine P[5] rotaviruses B641, 61A, and UK but 67% or less deduced amino acid (60% nucleotide) identity to representatives of the other P types found to date in cattle, the P[1] rotaviruses C486, A5, and NCDV; the P[11] rotaviruses B223, A44, and KK3; and the P[17] rotavirus 993/83. The CP-1 amino acid sequence was most closely related to that of the G6P[5] bovine rotavirus UK isolated from the same fecal sample (7), including the sequence at the putative trypsin cleavage region, RSIKPRE (results not shown). Sequence analysis of 229 amino acids at the 5′ end of the CP-1 NSP1 showed that it had a bovine NSP1 with 97% deduced amino acid (99% nucleotide) identity to the bovine rotavirus UK and 92% amino acid (87% nucleotide) identity to the bovine rotaviruses RF and A44. It had less than 70% amino acid (72% nucleotide) identity to the porcine rotaviruses OSU, Gottfried, and YM; the human rotaviruses Wa and DS1; and the equine rotavirus H2 (data not shown)--thus endorsing previous findings that bovine rotavirus NSP1s segregate into a distinct group (27, 32).
Sequence analysis of the full-length CP-1 NSP4 also showed it to be almost identical to that of the bovine rotavirus UK, differing by two nucleotides but with an identical amino acid composition. It had 92 to 96% deduced amino acid (80 to 88% nucleotide) identity with the NSP4s of bovine rotaviruses NCDV, B223, and BRV033 but 83 to 86% deduced amino acid (77 to 80% nucleotide) identity to the porcine rotaviruses OSU, A253, YM, A131, and A411; 82 to 85% deduced amino acid (78 to 82% nucleotide) identity to the human rotaviruses Wa, AU32, M37, and AU-1; and 63 to 64%deduced amino acid (62 to 63% nucleotide) identity to the murine rotaviruses EW, EC, and EHP. The CP-1 NSP4 clustered with other bovine rotaviruses into genotype A by phylogenetic analysis (Fig. 6) (8). In the enterotoxin peptide region (amino acids 114 to 135) which was associated with age-dependent diarrhea in mice (2), CP-1, which caused age-independent diarrhea in cattle (6), differed by only one amino acid at position 135, with a mutation of threonine to methionine (data not shown). CP-1 was identical to the bovine rotavirus UK in the variable region spanning amino acids 130 to 141 but differed from the bovine rotaviruses NCDV, B223, and BRV033 by three to six residues.
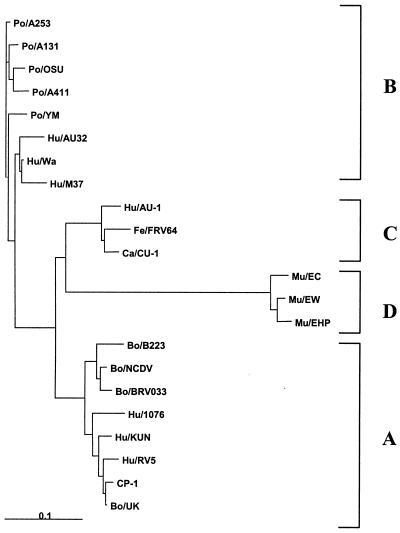
FIG. 6.
Phylogenetic tree of the deduced amino acid sequences of bovine, porcine, human, murine, feline, and canine rotavirus NSP4s and the CP-1 NSP4 produced by the Clustal W neighbor-joining method. Genotypes A to D are shown on the right.
Thus, rotavirus CP-1 was characterized as a G3P[5] rotavirus with a bovine NSP1 and NSP4. The high sequence identities of the CP-1 VP4, NSP1, and NSP4 to that of the G6P[5] bovine rotavirus UK were consistent with CP-1 and UK being isolated from the same fecal sample, albeit by different passage methods (4). These similarities were supported by the polyacrylamide gel electrophoresis genome analysis. The occasional G3 rotavirus has been found in cattle previously using PCR or PCR-generated cDNA probes (10, 22) but the present study is the first to report a bovine G3 VP7 sequence and the first to characterize the VP4, VP7, NSP1, and NSP4 of a bovine rotavirus which has been characterized experimentally in vivo (6, 17). The present study raises the possibility that G3 rotaviruses may be more common in cattle than currently thought, as CP-1 was initially misdiagnosed as a G10 rotavirus by RT-PCR. In surveys in which G10 bovine rotaviruses have been found by PCR, G10 specificity has rarely been confirmed by sequencing (10, 12, 14, 21, 23). Misdiagnosis by PCR has been recorded previously, for example between G3 and G8 rotaviruses (1). Clearly, more sequence information is required from G3, G8, and G10 bovine rotaviruses from diverse locations so that primer specificity can be improved.
The finding that CP-1 had P[5] specificity and a bovine NSP1 and NSP4 was unremarkable except for showing that these three proteins can form viable infectious virions with the G3 VP7 protein and that these virions are capable of causing age-independent diarrhea in experimental calves (6), producing villous atrophy and malabsorption (17). It is interesting that CP-1 differed by only one amino acid from the NSP4 enterotoxin peptide which caused age-dependent diarrhea in mice (2). Whether G3 specificity is a molecular marker for the ability to cause age-independent diarrhea in cattle needs further investigation. The prevalence of G3 rotaviruses and the disease associated with natural bovine G3 rotavirus infections also needs to be assessed, but this will require diagnostic methods which differentiate between G3 and G10 bovine rotaviruses. Genomic characterization of further bovine rotaviruses which have been characterized experimentally in vivo is in progress in our laboratory to correlate VP4, VP7, NSP1, and NSP4 type with the ability to infect and cause age-dependent or age-independent diarrhea in cattle.
Acknowledgments
We acknowledge financial assistance from the Wellcome Trust to J.C.B. and W.D.
REFERENCES
- 1.Adah, M. I., A. Rohwedder, O. D. Olaleyle, and H. Werchau. 1997. Nigerian rotavirus serotype G8 could not be typed by PCR due to nucleotide mutation at the 3′ end of the primer binding site. Arch. Virol. 142:1881-1887. [DOI] [PubMed] [Google Scholar]
- 2.Ball, J. M., P. Tian, C. Q. Y. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. [DOI] [PubMed] [Google Scholar]
- 3.Boom, R., C. J. Sol, M. M. Salismans, C. L. Jansen, P. M. Wertheim-Van Dillen, and J. Van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridger, J. C., and J. F. Brown. 1984. Antigenic and pathogenic relationship of three bovine rotaviruses and a porcine rotavirus. J. Gen. Virol. 65:1151-1158. [DOI] [PubMed] [Google Scholar]
- 5.Bridger, J. C., W. Dhaliwal, M. J. V. Adamson, and C. R. Howard. 1998. Determinants of rotavirus host range restriction: a heterologous bovine NSP1 gene does not affect replication kinetics in the pig. Virology 245:47-52. [DOI] [PubMed] [Google Scholar]
- 6.Bridger, J. C., and D. H. Pocock. 1986. Variation in virulence of bovine rotaviruses. J. Hyg. Camb. 96:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridger, J. C., and G. N. Woode. 1975. Neonatal calf diarrhoea: identification of a reovirus-like (rotavirus) agent in faeces by immunofluorescence and immune electron microscopy. Br. Vet. J. 131:528-535. [PubMed] [Google Scholar]
- 8.Ciarlet, M., F. Liprandi, M. E. Conner, and M. K. Estes. 2000. Species specific and interspecies relatedness of NSP4 genetic groups by comparative NSP4 sequence analyses of animal rotaviruses. Arch. Virol. 145:371-383. [DOI] [PubMed] [Google Scholar]
- 9.Das, M., S. J. Dunn, G. N. Woode, H. B Greenberg, and D. Rao. 1993. Both surface proteins (VP4 and VP7) of an asymptomatic neonatal rotavirus strain (I321) have high levels of sequence identity with the homologous proteins of a serotype 10 bovine rotavirus. Virology 194:374-379. [DOI] [PubMed] [Google Scholar]
- 10.De Verdier Klingenberg, K., M. Nilsson, and L. Svensson. 1999. Rotavirus G-type restriction, persistence, and herd type specificity in Swedish cattle herds. Clin. Diagn. Lab. Immunol. 6:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Attar, L., W. Dhaliwal, C. R. Howard, and J. C. Bridger. 2001. Rotavirus cross-species pathogenicity; molecular characterisation of a bovine rotavirus pathogenic for pigs. Virology 291:172-182. [DOI] [PubMed]
- 12.Falcone, E., M. Tarantino, L. D. Trani, P. Cordioli, A. Lavazza, and M. Tollis, 1999. Determination of bovine rotavirus G and P serotypes in Italy by PCR. J. Clin. Microbiol. 37:3879-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerna, G., A. Sarasini, M. Parea, S. Arista, P. Miranda, H. Brussow, Y. Hoshino, and J. Flores. 1992. Isolation and characterization of two distinct human rotavirus strains with G6 specificity. J. Clin. Microbiol. 30:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati, B. R., O. Nakagomi, Y. Koshimura, T. Nakagomi, and R. Pandey. 1999. Relative frequencies of G and P types among rotaviruses from Indian diarrheic cow and buffalo calves. J. Clin. Microbiol. 37:2074-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouvea, V., C. Ramirez, B. Li, N. Santos, L. Saif, H. F. Clark, and Y. Hoshino. 1993. Restriction endonuclease analysis of the VP7 genes of human and animal rotaviruses. J. Clin. Microbiol. 31:917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouvea, V., N. Santos, and M. C. Timenetsky. 1994. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 32:1338-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall, G. A., J. C. Bridger, K. R. Parsons, and R. Cook. 1993. Variation in rotavirus virulence: a comparison of pathogenesis in calves between two rotaviruses of different virulence. Vet. Pathol. 30:223-233. [DOI] [PubMed] [Google Scholar]
- 18.Horie, Y., O. Masamune, and O. Nakagomi. 1997. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. J. Gen. Virol. 78:2341-2346. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino, Y., and A. Z. Kapikian. 1994. Rotavirus vaccine development for the prevention of severe diarrhea in infants and young children. Trends Microbiol. 2:242-248. [DOI] [PubMed] [Google Scholar]
- 20.Huang, J., S. Nagesha, M. L. Dyall-Smith, and I. H. Holmes. 1989. Comparative sequence analysis of VP7 genes from five Australian porcine rotaviruses. Arch. Virol. 109:173-183. [DOI] [PubMed] [Google Scholar]
- 21.Hussein, H. A., E. Frost, B. Talbot, M. Shalaby, E. Cornaglia, and Y. el-Azhary. 1996. Comparison of polymerase chain reaction and monoclonal antibodies for G-typing of group A bovine rotavirus directly from fecal material. Vet. Microbiol. 51:11-17. [DOI] [PubMed] [Google Scholar]
- 22.Hussein, H. A., A. V. Parwani, B. Rosen, A. Lucchelli, and L. J. Saif. 1993. Detection of rotavirus serotypes G1, G2, G3, and G11 in feces of diarrheic calves by using polymerase chain reaction-derived cDNA probes. J. Clin. Microbiol. 31:2491-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishizaki, H., T. Sakai, T. Shirahata, K. Taniguchi, T. Urasawa, S. Urasawa, and H. Goto. 1996. The distribution of G and P types within isolates of bovine rotavirus in Japan. Vet. Microbiol. 48:367-372. [DOI] [PubMed] [Google Scholar]
- 24.Iturriza-Gómara, M., J. Green, D. Brown, M. Ramsay, U. Desselberger, and J. J. Gray. 2000. Molecular epidemiology of human rotavirus infections in the United Kingdom between 1995 and 1998. J. Clin. Microbiol. 38:4094-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagannath, M. R., R. R. Vethanayagam, B. S. Y. Reddy, S. Raman, and C. D. Rao. 2000. Characterisation of human symptomatic rotavirus isolates MP409 and MP480 having long RNA electropherotype and subgroup I specificity, highly related to the P6[1], G8 type bovine rotavirus A5, from Mysore, India. Arch. Virol. 145:1339-1357. [DOI] [PubMed] [Google Scholar]
- 26.Kapikian, A. Z., and R. M. Chanock. 1996. Rotaviruses, p. 1657-1708. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 27.Kojima, K., K. Taniguchi, and N. Kobayashi. 1996. Species-specific and interspecies relatedness of NSP1 sequences in human, porcine, bovine, feline and equine rotavirus strains. Arch. Virol. 141:1-12. [DOI] [PubMed] [Google Scholar]
- 28.Pongsuwanna, Y., K. Tanuguchi, M. Chiwakul, T. Urasawa, F. Wakasugi, C. Jayavasu, and S. Urasawa. 1996. Serological and genomic characterization of porcine rotaviruses in Thailand: detection of a G10 porcine rotavirus. J. Clin. Microbiol. 34:1050-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos, N., R. C. C. Lima, C. F. A. Pereira, and V. Gouvea. 1998. Detection of rotavirus types G8 and G10 among Brazilian children with diarrhea. J. Clin. Microbiol. 36:2727-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian, Y., A. Ballard, U. Desselberger, and M. A. McCrae. 1993. Genomic concatermerization/deletion in rotaviruses: a new mechanism for generating rapid genetic change of potential epidemiological importance. J. Virol. 67:6625-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theil, K. W. 1990. Group A rotaviruses, p. 35-72. In L. J. Saif and K. W. Theil (ed.),Viral diarrheas of man and animals. CRC Press, Boca Raton, Fla.
- 32.Xu, L., O. Tian, D. Tarlow, D. Harbour, and M. A. McCrae. 1994. Molecular biology of rotaviruses. IX. Conservation and divergence in genome segment 5. J. Gen. Virol. 75:3413-3421. [DOI] [PubMed] [Google Scholar]