Abstract
We evaluated the sensitivity and specificity of a new confirmatory test for treponemal antibodies, INNO-LIA Syphilis (Innogenetics NV, Ghent, Belgium), on a large number of sera from a clinical laboratory. This multiparameter line immunoassay (LIA) uses recombinant and synthetic polypeptide antigens derived from Treponema pallidum proteins. In a single-blinded cross-sectional retrospective study, 289 seronegative sera, 219 seropositive sera, and 23 sera with an indeterminate serological status for syphilis were analyzed. All sera were tested by the T. pallidum hemagglutination assay (TPHA), the immunoglobulin (IgG)-fluorescent T. pallidum absorption assay (IgG-FTA-ABS), and the Venereal Disease Research Laboratory (VDRL) test. In addition, some seropositive samples were analyzed by the 19S-IgM-FTA-ABS test, an enzyme immunoassay (IgM-EIA), and the MarDx immunoblotting assay. Based on a consensus diagnosis derived from conventional serology, all of the sera were classified as positive, negative, or indeterminate, and the results were compared with the findings of the INNO-LIA Syphilis assay. The sensitivity and specificity of the LIA were 100% (219 of 219) and 99.3% (286 of 288), respectively. Compared to TPHA and IgG-FTA-ABS, the new test gave a significantly higher number (P = 0.021 and P < 0.0001, respectively) of correct results than either of the other two tests. The multiparameter INNO-LIA Syphilis assay is a useful confirmatory test for syphilis because it increases the reliability of syphilis diagnosis with respect to current conventional techniques.
As detection of Treponema pallidum subsp. pallidum by dark-field microscopy or direct immunofluorescence is possible only in the early stages of the disease, serology has become the method of choice for syphilis diagnosis. In many countries, nontreponemal tests such as the Venereal Disease Research Laboratory (VDRL) or the rapid plasma reagin tests are routinely used (8, 9, 14). However, the large number of false-positive results and the low sensitivity of these tests have led to the introduction of T. pallidum-specific tests such as the Treponema pallidum hemagglutination assay (TPHA) or enzyme immunoassays (EIA) for syphilis screening (1, 6, 12). Irrespective of the screening method employed, there is general agreement that confirmation of a reactive screening test is essential (8, 9, 12-14), especially as false-positive results are associated with conditions such as pregnancy, autoimmune diseases, borreliosis, human immunodeficiency virus infection, malignancy, and chronic liver disease (8, 9, 12, 14).
The classic T. pallidum immobilization (Nelson-Mayer) test is no longer routinely used because of its complexity and consequent high cost, and it has largely been replaced by the fluorescent T. pallidum absorption (FTA-ABS) test. However, this confirmatory test can also lead to false-positive or false-negative results if the fluorescence intensity is misinterpreted or if the testing equipment is suboptimal (7). False-positive results have been reported for patients with other spirochetal infections or immunological disorders (e.g., systemic lupus erythematosis, rheumatoid factors, antinuclear and anti-DNA antibodies or cross-reacting antibodies such as those from Borrelia burgdorferi) (4, 7-9, 11, 12, 14). The T. pallidum Western immunoblot assay is also used as an alternative confirmatory test to improve sensitivity and specificity, but indeterminate and false-positive reactivity patterns have been reported (2, 8, 10).
A newly validated line immunoassay (LIA) based on recombinant antigens and synthetic peptides derived from T. pallidum proteins, the INNO-LIA Syphilis assay (Innogenetics NV, Ghent, Belgium), was previously shown to provide highly reliable confirmatory diagnostic information (3). We therefore evaluated the new assay using a large number of sera from a clinical laboratory in order to determine the sensitivity and specificity of the test and compare the results with those of other confirmatory assays.
MATERIALS AND METHODS
Study design and sera.
This single-blinded, cross-sectional, retrospective study included 289 sera that were seronegative for syphilis, 219 sera seropositive for syphilis, and 23 sera with an indeterminate status. Testing with INNO-LIA Syphilis, including that of proficiency panel, was carried out at the investigator's site and performed blinded from other test results and related clinical information. In case of discrepancies between LIA and the consensus as defined by the investigator (based on combined conventional testing and clinical information), the sample was retested by an enzyme immunoassay (Enzygnost Syphilis; Behringwerke AG, Marburg, Germany). All 531 serum or plasma samples were stored at −20 or −80°C until used. The status of treponemal antibodies in the samples was classified according to currently applicable guidelines in Germany (6, 12). Briefly, a sample is considered positive if there is unequivocal reactivity in screening (e.g., by TPHA) and confirmatory assays (e.g., FTA-ABS).
Sample groups.
Four groups of samples were tested: (i) 43 samples from Russian blood donors for whom no clinical information was available; (ii) 195 sera reacting to TPHA with a minimum low titer of 1:80; (iii) 203 sera from pregnant women which were negative by TPHA; and (iv) 90 sera from patients with the following characteristics: antinuclear antibody positive (n = 10), rheumatoid factor positive (n = 10), antimitochondrial antibody positive (n = 1), double-stranded DNA positive (n = 1), acute Epstein-Barr virus infection (n = 10), cytomegalovirus infection (n = 5), Lyme borreliosis (n = 10), parvovirus B19 infection (n = 3), rubella virus infection (n = 5), human immunodeficiency virus infection (n = 10), hepatitis B virus infection (n = 10), anticardiolipin antibodies (n = 5), and hepatitis C virus infection (n = 10). Subsequently, syphilis determination was performed to complete the VDRL, FTA-ABS, and TPHA reactivity patterns for all the studied groups.
Screening and confirmatory assays used in the study. (i) TPHA.
The TPHA was performed with the Serokit LD test (Innogenetics GmbH, Heiden, Germany) according to the manufacturer's instructions. Typically, a qualitative (yes/no answer) TPHA is performed at a unique serum dilution of 1:80. All reactive samples were subsequently tested to determine the end dilution titers.
(ii) FTA-ABS.
The FTA-ABS confirmatory test for syphilis was performed with some modifications to the standard procedure (5, 8, 12), using the T. pallidum antigen and the Treponema phagedenis ultrasonicate (Ultrasorb; BAG, Lich, Germany) as well as an immunoglobulin G (IgG)-specific anti-human fluorescein isothiocyanate-conjugated antibody (Gull Laboratories, Bad Hamburg, Germany). To confirm the reliability of screening results, each sample was tested at dilutions of 1:5 and 1:20 and scored as follows: 1+ (indeterminate), serum reacting weakly only in the 1:5 dilution; 2+ (weak positive), serum with a more intense fluorescence at 1:5 but negative at the 1:20 dilution; 3+ (positive), serum reactive at the 1:5 dilution and weak fluorescence at 1:20; 4+ (strong positive), serum with intense fluorescence at 1:20.
(iii) 19S-IgM-FTA-ABS test.
For the determination of specific IgM antibodies, a modification of the 19S-FTA-ABS procedure (5, 12) was used. To eliminate the IgG fraction, 20 μl of sera was first incubated overnight with 150 μl of an anti-IgG serum (GullSORB; Gull Laboratories) and then diluted with 30 μl of T. phagedenis ultrasonicate (Ultrasorb; BAG). The final serum dilution was 1:10. The test was performed using slides coated with treponemal organisms (FTA-ABS slides; Innogenetics GmbH). Antibody detection was carried out with an IgM-specific anti-human fluorescein isothiocyanate-conjugated globulin (Gull Laboratories). Results were read with a standard fluorescence microscope (Zeiss, Oberkochem, Germany) at a magnification of ×400 and classified qualitatively as negative, 1+ (indeterminate), 2+ (weak positive), 3+ (positive), or 4+ (strong positive).
(iv) IgM EIA.
The test kit from Gull Laboratories (Captia M-Syphilis EIA) was used according to the manufacturer's instructions. Results were calculated as an index as follows: negative, <0.9; indeterminate, 0.9 to 1.1; positive, >1.1.
(v) VDRL.
The quantitative VDRL test was performed according to standard procedures using the VDRL-Cardiolipin-Antigen (Behringwerke AG).
(vi) Enzygnost Syphilis EIA.
As an additional test for discrepant sera, the Enzygnost Syphilis EIA (Behringwerke AG) was used according to the manufacturer's instructions and interpretation criteria. The test simultaneously detects specific IgG and IgM antibodies to T. pallidum and is routinely used in Germany as a syphilis screening test.
(vii) T. pallidum immunoblotting.
The IgG and IgM immunoblot assay based on native antigens of T. pallidum (MarDx, Carlsbad, Calif.) was used as an additional confirmatory test. According to the manufacturer's interpretation criteria, a specific immune response can be assumed if reactivity for two of the three antigens (with molecular masses of 47, 17, and 15.5 kDa) is demonstrated.
(viii) INNO-LIA Syphilis.
INNO-LIA Syphilis, a LIA for the simultaneous detection of antitreponemal antibodies, makes use of recombinant antigens and synthetic peptide antigens derived from T. pallidum (Nichols strain) membrane proteins. The antigens consist of three immunodominant proteins (TpN47, TpN17, and TpN15) expressed as full-size proteins in Escherichia coli and one synthetic peptide (TmpA) derived from transmembrane protein A (for further details, see reference 3). In addition to the syphilis antigens on the test strip, control lines are used for semiquantitative evaluation of the results and for the verification of sample addition. Potential occurrence of antibodies that might react with nontreponemal components present on each strip can be visualized on the negative-control line.
The assay procedure can briefly be described as follows. Serum or plasma samples were diluted 1:100 and incubated at room temperature (20°C) overnight, followed by three washing steps with washing buffer before addition of a goat anti-human IgG (heavy and light chains) conjugated to alkaline phosphatase. After incubation, three washing steps were again performed, followed by the addition of a chromogen. Color development was then stopped with an appropriate stop solution. In a visual reading protocol, after color development, each line was compared to the control lines, and the intensities were scored as follows: 0, no line or a line less intense than the cutoff line; 1, a line with an intensity between that of the cutoff line and that of the 1+ control line; 2, a line with an intensity between that of the 1+ control line and that of the 3+ control line; 3, a line equal to that of the 3+ control line; 4, a line with an intensity greater than that of the 3+ control line.
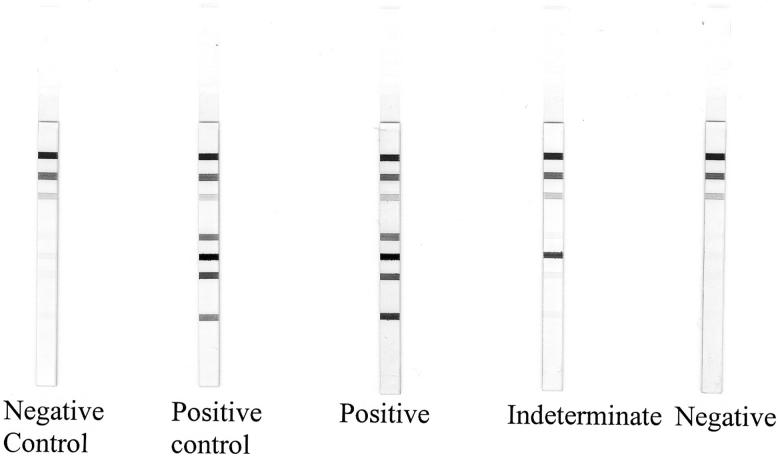
The interpretation algorithm of the INNO-LIA Syphilis kit was initially optimized by the manufacturer for visual reading with an independent set of negative and positive samples. A sample is considered T. pallidum antibody negative if no band or a single band with a maximum intensity of 0.5 is present. If multiple bands with a minimum intensity of 0.5 are visible, the sample is considered T. pallidum antibody positive. Finally, a sample is considered indeterminate if a single band is visible with a minimum intensity of 1. Examples of INNO-LIA Syphilis patterns are shown in Fig. 1.
FIG. 1.
Representative patterns of reactivity with the INNO-LIA Syphilis.
Statistical analysis.
Statistical comparison for significant differences between groups of samples was carried out using McNemar's test. All P values were calculated using binomial distribution, and a P value of <0.05 was considered to be significant.
RESULTS
Comparison of INNO-LIA Syphilis with consensus results.
Sensitivity and specificity were calculated in relation to the consensus determined by the investigator (Table 1). For the clear-cut positive (n = 219) or negative (n = 289) samples, the sensitivity of the INNO-LIA Syphilis was 100% (219 of 219) and the specificity was 99.3% (286 of 288), respectively. One sample was invalid due to cross-reactive antibodies (Table 1). The two other discrepant samples (no. 283 and 322) and the 23 indeterminate samples were retested by the Enzygnost Syphilis EIA. The two samples that scored indeterminate on LIA due to TmpA reactivity (no. 283 and 322) (Table 2) and which were classified as negative by the investigator also scored negative on the Enzygnost Syphilis EIA. For five samples (no. 216, 217, 219, 228, and 237), an additional test result of T. pallidum immunoblotting (MarDx) was available. The results without Western blotting are presented in Table 2, and those including Western blotting are shown in Table 3.
TABLE 1.
Comparison of INNO-LIA Syphilis results with consensus of multiple testing for syphilis serology
| LIA result | No. of samples with indicated consensus result
|
Total | ||
|---|---|---|---|---|
| Positive | Negative | Indeterminate | ||
| Positive | 219 | 12 | 231 | |
| Negative | 286 | 11 | 296 | |
| Indeterminate | 2 | 3a | ||
| Total | 219 | 288 | 23 | 531 |
One sample was invalid on the INNO-LIA Syphilis (reactivity on the negative-control band) and was therefore excluded from the analysis.
TABLE 2.
Detailed reactivities of discrepant samples on multiple testing in comparison to INNO-LIA Syphilisa
| Sample no. | TPHA titer | IgG-FTA score or result | 19S-IgM-FTA result | IgM-EIA result | VDRL titer | Consensus result | Enzygnost EIA result | INNO-LIA Syphilis score
|
INNO-LIA result | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TpN47 | TpN 17 | TpN15 | TmpA | |||||||||
| 216a | 320 | 2+ | Ind | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg | |
| 217 | <80 | 1+ | Neg | Neg | 2 | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 218 | 80 | 1+ | Neg | Neg | Neg | Ind | Pos | 1 | 3 | 0.5 | 2 | Pos |
| 219b | 160 | 1+ | Pos | Pos | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 220 | 160 | 1+ | Neg | Pos | Neg | Ind | Pos | 0.5 | 2 | 2 | 1 | Pos |
| 221 | 160 | 1+ | Ind | Ind | Neg | Ind | Pos | 2 | 0 | 1 | 0 | Pos |
| 222 | 160 | 1+ | Neg | Neg | Neg | Ind | Pos | 0.5 | 2 | 2 | 0 | Pos |
| 223 | 160 | 1+ | Neg | 1 | Ind | Ind | 0 | 2 | 1 | 0 | Pos | |
| 224 | 160 | 1+ | W Pos | Neg | Ind | Pos | 1 | 2 | 1 | 0 | Pos | |
| 225 | 80 | 1+ | Neg | Neg | Ind | Pos | 1 | 4 | 1 | 0 | Pos | |
| 226 | 80 | 1+ | Neg | Neg | 4 | Ind | Pos | 0.5 | 2 | 2 | 0 | Pos |
| 227 | 160 | 1+ | W Pos | Neg | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 228 | 80 | 1+ | Neg | Neg | Ind | Neg | 1 | 2 | 0 | 0.5 | Pos | |
| 229 | 320 | 1+ | Neg | Neg | Ind | Pos | 1 | 2 | 4 | 0 | Pos | |
| 230 | 80 | 1+ | Neg | Neg | 2 | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 231 | 80 | 1+ | Neg | Neg | 1 | Ind | Pos | 1 | 4 | 1 | 1 | Pos |
| 232 | 80 | 1+ | Neg | Neg | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 233 | 80 | 1+ | Ind | Neg | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 234 | 80 | 1+ | Ind | Neg | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 235 | 160 | 1+ | Neg | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg | |
| 236 | 160 | 1+ | Neg | Neg | Neg | Ind | Neg | 0 | 0 | 0 | 0 | Neg |
| 237 | 80 | 1+ | Ind | Neg | Ind | Neg | 1 | 0.5 | 1 | 1 | Pos | |
| 238 | 160 | 1+ | Neg | 4 | Ind | Neg | 0 | 0 | 0 | 0 | Neg | |
| 283 | <80 | Neg | Neg | Neg | Neg | 0 | 0 | 0 | 1 | Ind | ||
| 322 | <80 | Neg | 1 | Neg | Neg | 0 | 0 | 0 | 2 | Ind | ||
Ind, indeterminate; Neg, negative; Pos, positive; W pos, weakly positive.
Classified as indeterminate because the immunoblot with native antigens showed negative results (Table 3).
TABLE 3.
Complementary analysis by immunoblotting for some discrepant samples
| Sample no. | Consensus result | Result obtained with indicated testa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzygnost EIA | INNO-LIA Syphilis | IgG (MarDx) immunoblotting
|
IgM (MarDx) immunoblotting
|
||||||||
| 47 | 17 | 15 | Overall | 47 | 17 | 15 | Overall | ||||
| 216 | Ind | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 217 | Ind | Neg | Neg | W pos | Neg | Pos | Pos | Pos | Neg | Neg | Ind |
| 219 | Ind | Neg | Neg | Ind | Neg | Neg | Neg | Ind | Neg | Neg | Neg |
| 228 | Ind | Neg | Pos | Ind | Ind | Ind | Pos | Ind | Neg | Neg | Neg |
| 237 | Ind | Neg | Pos | W pos | Neg | W pos | Pos | Neg | Neg | Neg | Neg |
Ind, indeterminate; Neg, negative; Pos, positive; W pos, weakly positive; 47, 17, and 15, T. pallidum native antigens of 47, 17, and 15.5 kDa, respectively.
In the remaining 23 cases which were scored by the consensus as indeterminate, there were only three samples (no. 223, 228, and 237) (Table 2) for which the LIA results did not match the Enzygnost Syphilis EIA results. In two of the three cases (no. 228 and 237) (Table 3), an additional test with the MarDx immunoblot was performed. Sample no. 228, which had a borderline reactivity in TPHA (titer of 80), Enzygnost EIA (signal/noise, 1.01), and Western blot-IgG (three bands very weakly reactive), gave a clear-cut reactivity by INNO-LIA Syphilis. This sample was additionally shown to test positive on a newly commercialized antibody-capturing assay (data not shown). The sample for which the MarDx immunoblotting could not be performed (no. 223) (Table 2) was from an 80-year-old woman who had probably been treated for a syphilis infection.
For serum no. 219 (Tables 3, 4, and 5), a primary syphilis was clinically suspected and treated very early. A number of follow-up samples showed a decrease of the TPHA titer, negative results in the IgM-EIA, and indeterminate reactions in 19S-IgM-FTA-ABS and IgG-FTA-ABS tests (Table 4). The original and the first follow-up sample were also tested by MarDx immunoblotting, giving repeatedly inconclusive results.
TABLE 4.
Longitudinal testing of patient 219 sample
| Test date (day/mo/yr) | TPHA titer | IgG FTA-ABS score | Resulta for indicated test
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19S-IgM-FTA-ABS | IgM-EIA | VDRL | IgG (MarDx) immunoblotting
|
IgM (MarDx) immunoblotting
|
|||||||||
| 47 | 17 | 15 | Overall | 47 | 17 | 15 | Overall | ||||||
| 14/09/98 | 160 | 1+ | Pos | Pos | Neg | Ind | Neg | Neg | Neg | Ind | Neg | Neg | Neg |
| 9/10/98 | 80 | 1+ | Pos | Neg | Neg | Pos | Neg | Neg | Ind | Ind | Neg | Neg | Neg |
| 23/11/98 | <80 | 1+ | Ind | Neg | Neg | ||||||||
| 6/01/99 | <80 | 1+ | Ind | Neg | Neg | ||||||||
| 17/02/99 | <80 | 1+ | Ind | Neg | Neg | ||||||||
| 26/05/99 | <80 | 1+ | Ind | Neg | Neg | ||||||||
Pos, positive; Neg, negative; Ind, indeterminate; 47, 17, 15, T. pallidum native antigens of 47, 17, and 15.5 kDa, respectively.
TABLE 5.
Confirmation of TPHA results by INNO-LIA Syphilisa
| TPHA | No. of samples with indicated INNO-LIA Syphilis result
|
||||
|---|---|---|---|---|---|
| Positive | Negative | Indeterminate | Invalid | Total | |
| Positive (>1:80) | 206 | 206 | |||
| Negative (<1:80) | 286 | 2 | 1 | 289 | |
| Indeterminate (1:80) | 13 | 13 | |||
| Total | 219 | 286 | 2 | 1 | 508 |
Compared to the investigator's classification, INNO-LIA Syphilis gave a significantly higher number of correct answers than did TPHA (McNemar's test, P = 0.021).
Serum no. 216, from an 18-year-old Russian female with an unknown history, was classified as indeterminate by the investigator due to a negative result by MarDx immunoblotting (Tables 3 and 4), although TPHA and IgG-FTA-ABS produced a positive reaction. The negative Enzygnost Syphilis was congruous with the result of INNO-LIA Syphilis.
For sample no. 217, from a Russian blood donor, the MarDx IgG immunoblot was positive and the IgM immunoblot was indeterminate (Tables 3 and 4). A further immunoblot with recombinant antigens (under evaluation by the investigator) showed a negative IgG reaction and an indeterminate IgM reaction. The main reason for the classification as indeterminate was the overall heterogeneity of test results for this sample by different treponemal tests approved by the Paul-Ehrlich-Institute (Langen, Germany).
Comparison of INNO-LIA Syphilis versus TPHA and IgG-FTA-ABS tests, with consensus results as reference.
For the comparison of the INNO-LIA Syphilis kit with other tests, the consensus classification was used as a “gold standard.” The investigator, blinded for INNO-LIA Syphilis data, determined a consensus classification for each of the specimens based on the overall interpretation of assay results. In general, a sample was considered positive if reactive in a screening test (VDRL and/or TPHA) and in a confirmation test (FTA-ABS and/or Western blotting and/or EIA). For the purpose of this comparison, samples for which clear-cut results (positive or negative) were mostly available (508 out of 531 samples) were used. Twenty-three samples could not be classified by the existing data; they were therefore considered to be indeterminate.
(i) Comparison with TPHA as a screening test.
The comparison between TPHA and INNO-LIA Syphilis is shown in Table 5. Two samples had a negative TPHA result, while LIA classified them as indeterminate. There were 13 samples with a weakly reactive TPHA titer of 1:80, classified as indeterminate, although the samples were categorized as positive in the context of other results by the investigator, and this was congruous with the INNO-LIA Syphilis findings. When the two tests were compared for correct and incorrect answers as defined by the investigator, INNO-LIA Syphilis gave a significantly higher number of correct answers than did TPHA (McNemar's test, P value = 0.021).
(ii) Comparison with IgG-FTA-ABS as a confirmatory test.
There were six samples from pregnant women which were weakly positive (2+ reaction) by the IgG-FTA-ABS test but classified as negative by the investigator because the TPHA test was negative. The consensus classification agreed with the INNO-LIA Syphilis findings. The two samples (no. 283 and 322) (Table 2) with an indeterminate LIA result and a negative TPHA test also had a negative IgG-FTA-ABS result. Two samples with an indeterminate IgG-FTA-ABS result were classified as positive by the investigator. One sample was from a Russian blood donor (no. 36), and one was from a patient in the early seroconversion phase of a clinically suspected primary syphilis (no. 215). Both sera demonstrated strongly positive IgM antibody reactions with the IgM-EIA test and a weakly positive (2+) result in the 19S-IgM-FTA-ABS test. For 32 samples with a negative TPHA result and an indeterminate (1+) IgG-FTA-ABS test (25 samples from pregnant women and 7 samples from patients with various other conditions) (data not shown), the negative LIA findings also agreed with the consensus classification of the investigator. When analyzing the results of these two tests and comparing them to the investigator's classification, INNO-LIA Syphilis gave a significantly higher number of correct answers than did the IgG-FTA-ABS test (McNemar's test, P value < 0.0001) (Table 6).
TABLE 6.
Confirmation of IgG FTA-ABS results by INNO-LIA Syphilisa
| IgG-FTA-ABS result | No. of samples with indicated INNO-LIA Syphilis result
|
||||
|---|---|---|---|---|---|
| Positive | Negative | Indeterminate | Invalid | Total | |
| Positive | 217 | 6 | 223 | ||
| Negative | 248 | 2 | 1 | 251 | |
| Indeterminate | 2 | 32 | 34 | ||
| Total | 219 | 286 | 2 | 1 | 508 |
Compared to the investigator's classification, INNO-LIA Syphilis gave a significantly higher number of correct answers than did IgG FTA-ABS (McNemar's test, P < 0.0001).
DISCUSSION
The data in this study emphasize the need for the confirmation of positive syphilis screening test results. For many years, the FTA-ABS test was the only confirmation test available for use in a routine laboratory, but in order to perform the FTA-ABS test correctly, a considerable degree of experience is required on the part of the investigator, combined with a reliable quality control system. In particular, the delineation of indeterminate (1+) and weakly positive (2+) reacting samples can be influenced by the person who reads the test results. This is the reason for the introduction of a modification to the FTA-ABS procedure, using two dilution steps for classification of the fluorescence intensity, in order to obtain more-objective results. Indeterminate (1+) IgG-FTA-ABS reactions are found mostly for sera with a low antibody titer of 1:80 or 1:160 in the TPHA. In our experience, nonspecific TPHA test results are found more often in pregnant women than in patients with other conditions and in sera from women more often than in those from men. The same problem occurs when the VDRL is used as a screening test in pregnancy (1, 9, 10, 12). In our study, the IgG-FTA-ABS test was undoubtedly negative for most of these cases. However, of the eight pregnant women with a low TPHA titer and an indeterminate (1+) IgG-FTA-ABS result (sera no. 230 to 237) (Table 2), INNO-LIA Syphilis demonstrated specific antibodies in two cases, indicating a possible former treponemal infection. From the serological point of view it is not possible to correctly classify sera with a weakly reacting TPHA screening test and an indeterminate (1+) reactivity of the IgG-FTA-ABS test as specific or nonspecific (9, 10). Samples like no. 228 (Tables 2 and 3) that contain low levels of residual antibodies are typically very difficult to diagnose. Although the clinical relevance of low-level antibodies can be questioned, it remains important to observe their presence for serosurveillance and epidemiological purposes.
The findings of this study also demonstrate the high number of nonspecific indeterminate IgG-FTA-ABS results for patients with no history of treponemal infection: 27 samples (13%) of the 203 TPHA-negative pregnant women showed 1+ reactivity, and two samples (1%) showed 2+ reactivity. The VDRL test (data not shown) reacted in 24 samples (12%) of the sera from the same panel. For all these sera, the INNO-LIA Syphilis test was clearly negative.
As a general rule, laboratory test results should be reviewed by the clinician in the light of clinical symptoms and the history of infection and treatment. However, for patients with suspected or previous syphilis there are often no valid data available, and it is therefore very important for the laboratory diagnosis to be as reliable as possible. The INNO-LIA Syphilis as a new confirmatory test for treponemal infections seems to fulfill this need. It can nevertheless be argued that a comparison of the LIA with the consensus results derived from the TPHA and the IgG-FTA-ABS is open to possible criticism, since the investigator's interpretation is not independent of individual test results. However, the INNO-LIA Syphilis was found to correlate significantly better with the reference results than any of the individual tests alone. Moreover, when the consensus results based on the data derived from conventional serological testing are compared to the INNO-LIA Syphilis results, a close-to-perfect match of results is obtained (100% sensitivity and 99.3% specificity). Our data also confirm the previous findings of Ebel et al. (3), who determined the sensitivity and specificity of the INNO-LIA Syphilis to be 99.6 and 99.5%, respectively. The INNO-LIA Syphilis can therefore be considered to be a valid, if not superior, alternative to the FTA-ABS test for the confirmation of syphilis screening test findings (TPHA, VDRL, or EIA), and its use can improve the reliability of syphilis serology.
Acknowledgments
We thank Tessa James and Fred Shapiro for editorial assistance.
REFERENCES
- 1.Anonymous. 1979. Richtlininen 1979 für die Serodiagnose der Syphilis. Bundesgesundheitsblatt 22:398-400.
- 2.Byrne, R. E., S. Laska, M. Bell, D. Larson, J. Phillips, and J. Todel. 1992. Evaluation of a Treponema pallidum Western blot assay as a confirmatory test for syphilis. J. Clin. Microbiol. 30:115-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebel, A., L. Vanneste, M. Cardinaels, E. Sablon, L. Samson, K. De Bosschere, F. Hulstaert, and M. Zrein. 2000. Validation of the INNO-LIA Syphilis kit as a confirmatory assay for Treponema pallidum antibodies. J. Clin. Microbiol. 38:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enders, G., M. Bieber, R. Baier, H. Hlobil, and H.-J. Wellensieck. 1998. Luesverdacht in der Schwangerschaft bei Borrelieninfektion. Dtsch. Med. Wochenschr. 113:1511-1514. [DOI] [PubMed] [Google Scholar]
- 5.Hagedorn, H.-J. 1995. Serodiagnostik der Syphilis. Diagn. Labor 45:94-104. [Google Scholar]
- 6.Hagedorn, H.-J., H. Mauch, and R. Lüticken (ed.). 2001. Qualitätsstandards in der mikrobiologisch-infektiologischen diagnostik loseheftausgabe: syphilis, p. 1-40. Urban & Fischer, Munich, Germany.
- 7.Hunter, E. F., H. Russell, C. E. Farshy, J. S. Sampson, and S. A. Larson. 1986. Evaluation of sera from patients with Lyme disease in the fluorescent antibody absorption test for syphilis. Sex. Transm. Dis. 13:232-236. [DOI] [PubMed] [Google Scholar]
- 8.Larsen, S. A., S. J. Norris, and V. Pope. 1999. Treponema and other host-associated spirochetes, p. 759-776. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology. ASM Press, Washington, D.C.
- 9.Larsen, S. A., B. M. Steiner, and A. H. Rudolph. 1995. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 8:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen, S. A., E. F. Hunter, and E. T. Creighton. 1990. Syphilis, p. 927-934. In K. K. Holmes, P.-A. Mardh, and P. F. Sparling (ed.), Sexually transmitted diseases. McGraw-Hill, New York, N.Y.
- 11.Luther, B., and M. Moskophidis. 1990. Antigenic cross-reactivity between Borrelia burgdorferi, Borrelia recurrentis, Treponema pallidum and Treponema phagedenis. Zentbl. Bakteriol. 274:214-226. [DOI] [PubMed] [Google Scholar]
- 12.Müller, F., and H.-J. Hagedorn. 1998. Syphilis, p. 1203-1212. In L. Thomas (ed.), Clinical laboratory diagnosis. TH-Books, Frankfurt/Main, Germany.
- 13.Norris, S. J., and the Treponema pallidum Research Group. 1993. Polypeptides of Treponema pallidum: progress toward understanding their structural, functional and immunological roles. Microbiol. Rev. 57:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh, A. E., and B. Romanowksi. 1999. Syphilis: review with emphasis on clinical, epidemiological, and some biologic features. Clin. Microbiol. Rev. 12:187-209. [DOI] [PMC free article] [PubMed] [Google Scholar]