Abstract
Sixty-seven human strains of enterohemorrhagic Escherichia coli (EHEC) (from patients with more or less severe symptoms) were serogrouped and arranged according to pulsed-field gel electrophoresis (PFGE) patterns. We used PCR to investigate the strains according to known or putative virulence factors, and associations with disease were studied. All EHEC strains with the same PFGE pattern belonged to the same serogroup. On the contrary, two serogroups (O157 and O8) included strains with different PFGE patterns. We found several different combinations of chromosomal and plasmid-borne determinants, encoding the putative virulence factors, among the strains. As judged from clinical symptoms, there was no marked difference in pathogenicity among the strains and their combinations of virulence traits. All strains of O157 had the genes coding for verocytotoxin (VT) 2, intimin (eaeA), E. coli hemolysin (E-hly), and secreted serine protease (espP). Among EHEC non-O157 strains, the genes coding for VT1 and VT2 were equally dispersed. EaeA positivity was just as common among VT1- as VT2-positive strains. Among the plasmid-borne determinants, E-hly and espP were the most common and E-hly might be a pathogenicity marker among EHEC non-O157 strains. The conclusion is that PFGE is a very useful tool in epidemiological studies. The EHEC plasmids are heterogeneous in their gene composition, with the four plasmid-borne determinants found in many combinations. There was no reliable correlation between chromosomal and plasmid-borne virulence factors and human disease.
Enterohemorrhagic Escherichia coli (EHEC) was first associated with the diseases hemolytic-uremic syndrome (HUS) and hemorrhagic colitis (HC) in the early 1980s (15). HC and HUS are severe diseases which frequently require hospitalization. HUS appears especially among children and may be fatal in up to 5% of cases. EHEC has also been associated with uncomplicated diarrhea and has even been isolated from stools of healthy individuals (14).
The most thoroughly studied potential virulence factors among EHEC strains are the verocytotoxins (VTs) (15). VT1 is identical or almost identical (15) to Shiga toxin of Shigella dysenteriae type 1 in the nucleotide sequence of its gene and its amino acid sequence. The gene coding for VT2 is distinct from VT1 and Shiga toxin, with an overall nucleotide sequence homology of approximately 55 to 60% (13, 15).
Another well-studied potential virulence factor is the eaeA gene (for E. coli attaching and effacing), which encodes intimin. This is an adhesin that enables some EHEC strains to tightly attach to epithelial cells of the intestine. The eaeA gene is clustered in the locus for enterocyte effacement (1, 6, 8, 9, 12).
Recently, the genetic analysis of a new plasmid-encoded hemolysin of EHEC called enterohemorrhagic E. coli hemolysin (E-hly) was reported by Schmidt and coworkers. This hemolysin seemed to be associated with severe clinical disease in humans (17, 20).
The plasmid harboring the E-hly gene, called pO157, is large, approximately 90 kb. It has been shown to harbor other genes for putative virulence factors: the bifunctional catalase peroxidase katP (5), the etp gene cluster, which presumably encodes a type II secretion pathway system (19), and the secreted serine protease (espP), which can cleave human coagulation factor V (4).
Sorbitol-nonfermenting E. coli O157 has been said to be the predominant pathogenic EHEC in the world (21). The reason for this may be that sorbitol-nonfermenting E. coli O157 isolates are easier to detect in cultures than are pathogenic non-O157 EHEC strains, and these may have been overlooked.
In the present investigation, we characterized isolated EHEC strains, of both serogroup O157 and non-O157, from Swedish patients by using PCR to analyze possible genetic variability among the above-mentioned possible virulence factors. The information is useful for development of techniques to detect pathogenic EHEC among patients. It also may be important in considering the role of specific virulence factors.
Clinical microbiologists, besides being asked about the pathogenicity of a bacterium, are also often asked to determine the relatedness of a group of bacterial isolates. Pulsed-field gel electrophoresis (PFGE) is a molecular method with the potential to type bacterial strains of almost any kind. With this rather well-characterized collection of strains we wanted to establish that PFGE can be a powerful tool for epidemiological studies of EHEC.
MATERIALS AND METHODS
Bacterial strains.
The strains were isolated from patients' stool specimens during 1997 to 1999; altogether, 67 strains from 5,617 specimens were isolated. All routine specimens sent to the Bacteriology Laboratory, Sahlgrenska University Hospital in Göteborg, Sweden, from patients under 11 years of age with diarrhea or from older patients with sorbitol-negative colonies on sorbitol-MacConkey agar and/or a reported diagnosis of severe or bloody diarrhea were included. Some individuals were family members of patients with diagnosed EHEC and they were also included, even if they had no symptoms.
Two different outbreaks (PFGE patterns A and S) were included in the study (Table 1and Fig. 1). The strains with pattern A were from stool specimens from 19 patients. Eighteen of these were collected in 2 months during the late summer of 1997, and one was collected 2 years later. The origin of this outbreak is not known, but a linking factor for some of the patients was a lake used for bathing. None of the patients harboring this strain had been abroad the month before giving the stool specimen. The strains with pattern S had a food-borne origin. The EHEC-positive stool specimens from this outbreak all belonged to patients who had visited the same dinner party in August 1999 (C. Welinder-Olsson, Å. Brandberg, K. Stenquist, B. Trollfors, M. Badenfors, K. Florén, E. Kjellin, and B. Kaijser, unpublished data).
TABLE 1.
Characterization of isolated EHEC strains together with date of specimen collection, patient's age, and symptoms
| Patient no. | Date of specimen collection (mo-yr) | Age (yr) | Disease associationa | PFGE patternb | Serogroupc | Sorbitol/lactose | Chromosomal genes
|
Plasmid genes
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VT1 | VT2 | eaeA | E-hly | etpD | katP | espP | |||||||
| 1 | 07-97 | 29 | D | H | O157 | −/+ | + | + | + | + | + | + | + |
| 2 | 06-98 | 47 | BD | M | O157 | −/+ | + | + | + | + | + | − | + |
| 3 | 08-99 | 3 | BD | X | O157 | −/+ | + | + | + | + | + | + | − |
| 4 | 09-97 | 1 | D | E | O157 | −/+ | + | + | + | + | + | + | − |
| 5 | 09-97 | 1 | D | E | O157 | −/+ | + | + | + | + | + | + | − |
| 6 | 09-97 | 25 | SF | E | O157 | −/+ | + | + | + | + | + | + | − |
| 7 | 08-99 | 24 | D | E2 | O157 | −/+ | − | + | + | + | + | + | + |
| 8 | 09-97 | 2 | BD | F | O157 | −/+ | − | + | + | + | + | + | + |
| 9 | 09-97 | 28 | SF | F | O157 | −/+ | − | + | + | + | + | + | + |
| 10 | 08-97 | 5 | BD | C | O157 | −/+ | − | + | + | + | + | + | + |
| 11 | 05-97 | 12 | D | G | O157 | −/+ | − | + | + | + | + | + | + |
| 12 | 08-97 | 2 | D | I | O157 | −/+ | − | + | + | + | + | + | + |
| 13 | 11-98 | 63 | D | 12 | O157 | −/+ | − | + | + | + | + | + | + |
| 14 | 08-97 | 2 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 15 | 08-97 | 1 | HUS | A | O157 | −/+ | − | + | + | + | + | + | + |
| 16 | 09-99 | 1 | D | A | O157 | −/+ | − | + | + | + | + | + | + |
| 17 | 08-97 | 1 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 18 | 08-97 | 2 | D | A | O157 | −/+ | − | + | + | + | + | + | + |
| 19 | 08-97 | 3 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 20 | 08-97 | 7 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 21 | 08-97 | 1 | HUS | A | O157 | −/+ | − | + | + | + | + | + | + |
| 22 | 08-97 | 3 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 23 | 08-97 | 7 | SF | A | O157 | −/+ | − | + | + | + | + | + | + |
| 24 | 08-97 | 9 | HUS | A | O157 | −/+ | − | + | + | + | + | + | + |
| 25 | 08-97 | 6 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 26 | 08-97 | 32 | SF | A | O157 | −/+ | − | + | + | + | + | + | + |
| 27 | 08-97 | 2 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 28 | 08-97 | 2 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 29 | 09-97 | 4 | BD | A | O157 | −/+ | − | + | + | + | + | + | + |
| 30 | 09-97 | 4 | D | A | O157 | −/+ | − | + | + | + | + | + | + |
| 31 | 08-97 | 3 | BD | A2 | O157 | −/+ | − | + | + | + | + | + | + |
| 32 | 08-97 | 5 | BD | A2 | O157 | −/+ | − | + | + | + | + | + | + |
| 33 | 07-99 | 8 | BD | R | O157 | −/+ | − | + | + | + | + | + | + |
| 34 | 08-99 | 3 | D | AE | O157 | −/+ | − | + | + | + | + | − | − |
| 35 | 09-99 | 53 | D | S | O157 | −/+ | − | + | + | + | + | + | + |
| 36 | 09-99 | 62 | BD | S | O157 | −/+ | − | + | + | + | + | + | + |
| 37 | 09-99 | 59 | D | S | O157 | −/+ | − | + | + | + | + | + | + |
| 38 | 09-99 | 43 | D | S | O157 | −/+ | − | + | + | + | + | + | + |
| 39 | 09-99 | 57 | D | S | O157 | −/+ | − | + | + | + | + | + | + |
| 40 | 09-99 | 51 | D | S | O157 | −/+ | − | + | + | + | + | + | + |
| 41 | 09-99 | 50 | D | S | O157 | −/+ | − | + | + | + | + | + | + |
| 42 | 09-99 | 16 | BD | T | O157 | −/+ | − | + | + | + | + | + | + |
| 43 | 09-99 | 2 | BD | T2 | O157 | −/+ | − | + | + | + | + | + | + |
| 44 | 07-99 | 12 | HUS | T | O157 | −/+ | − | + | + | + | + | + | + |
| 45 | 07-99 | 34 | D | AC | O91 | −/+ | + | + | − | − | − | + | − |
| 46 | 07-98 | 1 | HUS | O | OR | +/+ | + | − | + | + | − | − | + |
| 47 | 06-99 | 4 | D | AD | ON | +/+ | + | − | + | + | − | − | + |
| 48 | 10-98 | 52 | Not known | AF | ON | +/+ | + | − | + | + | + | − | + |
| 49 | 02-99 | 32 | BD | V | O118 | +/+ | + | − | + | + | − | + | + |
| 50 | 06-99 | 1.5 | D anemia | NT | E43478/86d | +/+ | + | − | + | − | − | − | − |
| 51 | 05-98 | 8 mo | BD | NT | ON | +/+ | + | − | − | + | − | − | − |
| 52 | 08-99 | 20 | BD | Y | OR | −/− | + | − | + | + | + | − | − |
| 53 | 01-98 | 8 | BD | NT | O76 | +/+ | + | − | − | + | − | − | − |
| 54 | 09-98 | 32 | D | AB | ON | −/+ | + | − | + | − | − | − | − |
| 55 | 10-98 | 2 | SF | AG | O117 | −/− | + | − | − | − | − | − | − |
| 56 | 10-98 | 2 | SF | AG2 | O117 | −/− | + | − | − | − | − | − | − |
| 57 | 08-99 | 26 | D | Z | O46 | +/+ | − | + | + | − | − | − | − |
| 58 | 11-98 | 1.5 | D | AH | O22 | +/+ | − | + | − | − | − | − | − |
| 59 | 04-97 | 28 | Not known | K | O8 | −/+ | − | + | − | − | − | − | + |
| 60 | 04-98 | 16 | BD | N | O8 | +/+ | − | + | − | + | − | − | − |
| 61 | 10-97 | 4 mo | BD | J | ON | +/+ | − | + | − | − | − | − | − |
| 62 | 05-97 | 1.5 | BD | B | O121 | +/+ | − | + | + | + | − | − | + |
| 63 | 06-97 | 2 | BD | B | O121 | +/+ | − | + | + | + | − | − | + |
| 64 | 09-98 | 2 | D | B2 | O121 | +/− | − | + | + | + | − | − | + |
| 65 | 08-98 | 3 | HUS | B | O121 | +/− | − | + | + | + | − | − | + |
| 66 | 09-97 | 16 | BD | D | O145 | +/+ | − | + | + | + | − | − | + |
| 67 | 09-99 | 2 | SF | AK | OR | −/− | − | + | + | + | − | + | + |
D, diarrhea; BD, bloody diarrhea; SF, symptom-free.
NT, not typeable in PFGE pattern. Boldface indicates an epidemic outbreak.
OR, rough; ON, not typeable in serogroup.
Provisional serotype.
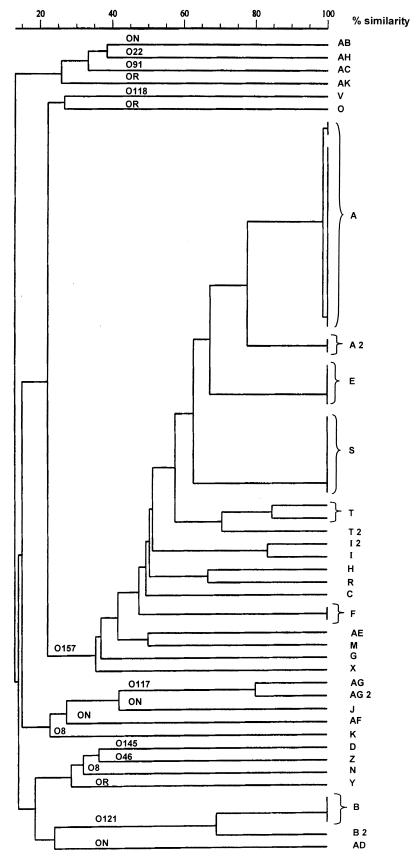
FIG. 1.
Dendrogram of EHEC strains, both O157 and non-O157, isolated from routine fecal specimens sent to the Bacteriological Laboratory of the Sahlgrenska University Hospital in Göteborg. The dendrogram was constructed with the use of the unweighted pair group method with arithmetic averages to a matrix by comparison of XbaI PFGE patterns. A, E, and S are examples of genetically related clusters of strains, while AB, AH, and AC are examples of strains not related.
The specimens were analyzed using PCR to detect bacteria harboring VT genes. PCR-positive stool specimens were recultured to identify the VT gene-harboring bacteria. All stool specimens were cultured for Salmonella, Campylobacter, Shigella, and Yersinia species, and each specimen was inoculated into a sorbitol-MacConkey agar plate to screen for E. coli O157. Isolated VT-positive strains were also analyzed for the presence of the eaeA gene by PCR.
PCR for detection of EHEC.
For DNA isolation, the method described previously was used (23). In short, bacterial growth from the primary agar plate culture of the stool specimens was suspended in 4 ml of double-distilled, sterile water to McFarland 4. One milliliter of the suspension was boiled for 15 min before centrifugation at 7,000 × g for 1 min. Five microliters of the supernatant was used for each PCR. During the first 2 years of the study (1997 and 1998), PCR was also performed as previously described (23). Briefly, the PCR was performed in a total volume of 50 μl containing 200 μM concentrations of each deoxynucleoside triphosphate, 5 μl of 10-fold-concentrated polymerase synthesis buffer, 6 mM MgCl2, and 1.25 U of Taq polymerase. Primers detecting the VT1 (VT1 l/VT1 r), VT2 (VT2 l/VT2 r) or eaeA (eaeA l/eaeA r) gene sequences (10 pmol each) were added separately. The PCRs were done with the Gene Amp PCR system 9600 (PE Biosystems, Stockholm, Sweden) using an initial denaturation step of 4 min at 96°C, followed by 20 s at 94°C, 45 s at 55°C, and 10 s at 72°C for 35 cycles. The amplified fragments were separated in a 2% agarose gel stained with ethidium bromide. Primers VT1 l and VT1 r, VT2 l and VT2 r, and eaeA l and eaeA r gave PCR fragments of 130, 298, and 376 bp in length, respectively.
To improve the method of analysis, a multiplex PCR was used starting from the year 1999. Primers detecting both VT1 and VT2 gene sequences were added to the same reaction mixture. This allowed detection of VT1 and VT2 positivity in the same PCR. The MgCl2 concentration was changed to 2.5 mM, and Gold Taq (PE Biosystems) was used instead of Ampli Taq DNA polymerase. The thermocycling was then started with 10 instead of 4 min of incubation at 94°C instead of at 96°C. The sensitivity with this new protocol was the same as before (data not shown). Even detection of the eaeA gene was by this time performed with Gold Taq with a prolonged incubation time (10 min instead of 4) at 94°C to initialize the thermocycling.
Preparation of plasmids.
Plasmids were prepared from the strains according to the manufacturers' (Qiagen and MERCK Eurolab, Göteborg, Sweden) instructions for large plasmids. One milliliter of overnight culture of EHEC resulted in a plasmid preparation dissolved in 50 μl of 10 mM Tris-HCl, pH 8.5.
PCR of the plasmid genes E-hly, etpD, katP, and espP.
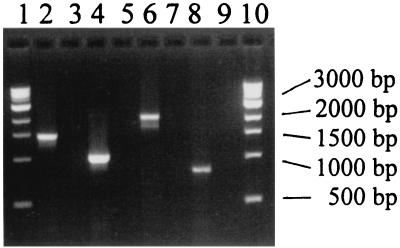
PCR of the plasmid genes was performed as described earlier by Schmidt et al. (18) for E-hly, etpD, and katP, but the gene coding for espP was detected by PCR instead of with hybridization (Fig. 2). Primers espP1 and espP3 were designed according to the nucleotide sequence reported by Brunder et. al (4) and found in the EMBL database library under accession number X97542. Amplification gave rise to an 800-bp fragment, with forward primer espP1 (5′AGG CAC TTG AAC GTT ACG GGG T 3′) and reverse primer espP3 (5′ ACC GTT GTA TTC ACC GCC AGA C 3′). The PCR was performed in a total volume of 10 μl containing 4 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, 1 μM concentrations of each primer, 1 μl of Taq DNA polymerase (5 U/μl; Applied Biosystems) diluted 1:12.5 in enzyme dilution buffer (10 mM Tris [pH 8.3], 2.5 mg of bovine serum albumin/ml [Labora, Göteborg, Sweden]). The amplification was performed in a thermal reactor (Rapid Cycler; Idaho Technology, Biotech-IgG A/S, Copenhagen, Denmark). Amplification reactions were performed for 40 cycles under the following conditions: DNA was denatured at 94°C for 1 s, annealing was at 59°C for 1 s, and extension was at 72°C for 32 s.
FIG. 2.
Electrophoretic analysis of the PCR products obtained by amplification of the plasmid-borne DNA sequences coding for E-hly (lane 2), etpD (lane 4), katP (lane 6), and espP (lane 8). The target DNA was obtained from an EHEC strain with PFGE pattern A isolated from a patient. Lanes 3, 5, 7, and 9 are amplifications with primers without template DNA. Lanes 1 and 10 are a 1-kb DNA ladder (New England BioLabs, Inc.).
Serogrouping.
For O157 serogrouping, an E. coli O157 test kit (Oxoid LTD, Hampshire, England) was used. Serogrouping of groups other than O157 was performed as previously described by Lidin-Janson et al. (10) or as described by the Laboratory of Enteric Pathogens, Central Public Health Laboratory, Colindale, England (7).
PFGE.
PFGE was used to establish clonal relatedness and diversity among the strains. PFGE was performed as previously described (23). Briefly, DNA was digested with the enzyme XbaI following electrophoresis performed with the Gene Path system (Bio-Rad Laboratories, Sunbyberg, Sweden). The PFGE types were interpreted according to methods described by Tenover et. al. (22). Strains with zero or two to three fragment differences in PFGE patterns were considered related to or probably related to each other. The gels were also digitized for computer-aided analysis. The Molecular Analyst software package (Bio-Rad Laboratories) was used for analysis. Calculation of the similarity matrix was done with the Jacquard algorithm after defining each band between sizes 145 and 582 kb. The clustering was achieved with the unweighted pair group method.
RESULTS
Sorbitol and lactose.
Sixty-seven strains of EHEC were identified in this study. Forty-four of these were of serogroup O157 and 23 were non-O157. All EHEC O157 isolates in this study were sorbitol negative and lactose positive. Thirty percent (7 of 23) of the non-O157 strains were sorbitol negative, and 74% (17 of 23) were lactose positive (Table 1).
PFGE and serogroups.
The PFGE patterns obtained by XbaI digestion of the chromosomal DNA were compared in 64 of 67 strains. Three of the strains gave no bands. Among the 44 EHEC strains of serogroup O157, we found 13 different patterns with differences of more than three bands. Nineteen of the strains belonged to pattern A (Table 1 and Fig. 1), including pattern A2. Eighteen of the strains with pattern A, A2 belonged to the same outbreak (1997), but one strain, from patient 16, was isolated 2 years after the outbreak. Seven strains had the pattern S (the food-borne outbreak in 1999). Four strains had pattern E. Three of these strains, patients 4 to 6, were isolated in September 1997, but one from patient 7 was isolated in August 1999. Two strains were pattern F, two strains were pattern I, I2, and three were pattern T, T2. Seven strains had unique band patterns.
The 23 strains of non-O157, containing strains of serogroups O8, O22, O46, O76, O91, O117, O118, O121, O145, and E43478/86, and 8 strains not typeable or rough were divided into 16 different PFGE patterns (Table 1). Four strains showed pattern B; two of these were isolated about the same time, but two with pattern B, B2 (patients 64 and 65) were isolated 1 year later. The strains with pattern B, B2 all belonged to the same serogroup, O121. Two other strains, also possibly related, had patterns AG and AG2. Even though not quite identical, they were isolated during the same summer of 1998 but were from patients arriving from Kuwait and Turkey. These two strains also belonged to the same serogroup, O117. Two strains belonged to serogroup O8 but had quite different PFGE patterns. The remaining nine isolates had unique patterns and they also belonged to different serogroups or strains not typeable in serogroups or rough.
VT genes.
All strains of serogroup O157 were VT2 positive (Table 1). Six strains, three of PFGE pattern E and one each of PFGE patterns M, H, and X, were both VT1 and VT2 positive. This was demonstrated by PCR with primers complementary to the gene sequences. Among non-O157 strains, VT1 and VT2 positivities were about equally dispersed. Only one strain, with PFGE pattern AC (patient 45), was both VT1 and VT2 positive. Two of the HUS patients were infected by non-O157 strains: one strain was VT1 positive (patient 46) and one was VT2 positive (patient 65).
eaeA.
All O157 strains were eaeA positive as tested by PCR. Only 14 of the 23 non-O157 strains were eaeA positive (Table 1). Two of the eaeA-positive strains belonged to patients who had HUS and five were found in patients with bloody diarrhea. Five of the eaeA-positive strains belonged to patients who had only diarrhea, and one was symptom-free. For one patient we had no information concerning symptoms. Among the nine non-O157 strains without the eaeA gene, four belonged to patients with bloody diarrhea, two had just diarrhea, and two were symptom-free. For one patient we had no information concerning symptoms. There was no correlation between eaeA positivity and the presence of the genes coding for VT1 or VT2.
Plasmid-encoded determinants.
All EHEC O157 strains tested possessed the EHEC hlyA gene as demonstrated by PCR with primer pair hlyA1 and hlyA4 (Table 1). The etpD gene cluster was also present in all EHEC O157 strains investigated. However, two strains of serogroup O157 (PFGE patterns M and AE) failed to produce an amplification product with the primers wkat-B and wkat-F, indicating the katP gene. Five strains of serogroup O157 (PFGE patterns E, X, and AE) did not produce an amplification product with primers espP1 and espP2, indicating the gene espP. The isolated E. coli strains of serogroup O157 were from both patients with mild symptoms or diarrhea only and patients with severe symptoms such as bloody diarrhea or HUS, even if they had strains possessing all four plasmid-encoded determinants together with eaeA positivity and one or both of the VT genes.
Among the 23 non-O157 strains, 14 were E-hly positive, 10 of which were isolated from patients who had HUS or bloody diarrhea. Three strains were isolated from patients who had milder symptoms or were symptom-free. One strain was from a patient for whom we have no information about symptoms. Only one patient with bloody diarrhea had a strain which was E-hly negative.
The etpD gene cluster was only present in two non-O157 strains. These were strains of pattern AF and Y. The strain with pattern Y was isolated from a patient with bloody diarrhea and the AF pattern belongs to the strain from one of the patients for whom we have no information about symptoms.
PCR with primers wkat-B and wkat-F demonstrated that only 3 of the 23 non-O157 EHEC strains harbored the katP gene. These strains had different PFGE patterns (V, AK, and AC) with different serogroups (O118, O91, and rough). One of the patients had bloody diarrhea, one had diarrhea only, and one was symptom-free.
EspP could be detected in 11 strains. Six belonged to patients who had bloody diarrhea or HUS. In 12 strains, espP could not be detected. Five of these strains were isolated from patients who had bloody diarrhea.
Seven strains were negative for all four plasmid-encoded determinants. Among these, only one belonged to a patient with symptoms as severe as bloody diarrhea.
Correlation between PFGE pattern and chromosomal and plasmid-borne determinants.
Strains with the same PFGE pattern often have the same chromosomal determinants (VT1, VT2, and eaeA). There was one exception. One strain with PFGE pattern E lost its VT1 positivity, in contrast to the other three strains with the same pattern. This strain was, however, isolated 2 years after the first three strains with pattern E. Even plasmid-encoded determinants were related to PFGE pattern. Even though most strains of serogroup O157 had a plasmid encoding all four determinants, strains with pattern E isolated at the same time were negative for espP. Strain M was negative for katP, strain X was negative for espP, and strain AE was negative for both katP and espP. Strains with pattern B, B2 (non-O157 EHEC) were all positive for E-hly and espP but negative for katP and etpD, and strains with pattern AG, AG2 were negative for all four determinants.
DISCUSSION
EHEC O157 has been considered to be the most common and important among the different EHEC strains. In our present studies we found, however, that non-O157 EHEC is just as common among the sporadic cases diagnosed in Sweden. It seems, however, that EHEC O157 is more often associated with outbreaks than is non-O157.
All EHEC O157 strains in this study were sorbitol negative and lactose positive. This was not the case concerning non-O157 strains. Thirty percent were sorbitol negative and 74% were lactose positive. This shows that there is no benefit in screening for sorbitol-negative, lactose-positive strains when looking for pathogenic non-O157 EHEC strains.
There was a large variety of PFGE patterns among both EHEC O157 and non-O157, which demonstrated a great clonal diversity. This is in agreement with the findings reported by Rios et. al. (16), who have studied the clonal diversity of Chilean isolates of EHEC from patients with different severities of symptoms. As mentioned above, PFGE types were interpreted according to the methods of Tenover et. al. (22). Strains with a band difference of more than three bands differ by about 70% or more in the dendrogram. This is consistent with the occurrence of two or more independent genetic events. Strains belonging to the same PFGE pattern, for natural reasons, often have the same chromosomal determinants, VT1, VT2, and eaeA. There is one exception among strains with pattern E. One strain isolated 2 years after the first three was VT2 positive only. Even if the whole gene of the approximate size 1,500 bp coding for VT1 is lost, this is not large enough to be seen as the missing of a band in the PFGE pattern, where each band size is in the range 20 to 1,500 kb.
The different PFGE patterns gave valuable epidemiological information and made it possible to assess the presence of an outbreak and to differentiate between outbreaks. PFGE typing has a high degree of discriminatory power that is superior to, for example, that of ribotyping or other molecular techniques (11).
In contrast, the determination of serogroup added very little information about clonality. Without PFGE we would not have been able to distinguish between the different EHEC strains of serogroup O157. All strains harboring the VT2 coding sequence would have appeared to belong to the same outbreak. Strains of serogroup O121 were all closely related in the dendrogram (pattern B, B2), as were strains of serogroup O117 (pattern AG, AG2). Two strains both of serogroup O8, however, had different PFGE patterns (K and N) and are placed relatively far from each other in the dendrogram. This is only the result of the higher discriminatory power of PFGE. From three strains in this study, however, we did not manage to extract intact genomic DNA. These bacteria seem to have a high amount of nuclease activity and destroy their own DNA in an early stage of preparation. Attempts to inactivate a possible nonspecific DNase were unsuccessful, even though we believe it should not be impossible to overcome this problem in the future.
In order to characterize the different EHEC strains, we have studied the presence of the different toxin genes and other presumptive virulence factors encoded by chromosomal as well as plasmid genes. Sixty-seven isolates of EHEC (44 O157 and 23 non-O157) were isolated from patient stool specimens. At least 58 of these patients had diarrhea, bloody diarrhea, or HUS, which shows that E. coli of serogroup O157 is not the only pathogenic EHEC. Serogroups other than O157 have, however, not been as thoroughly characterized for virulence traits as has E. coli O157.
It is well known that children with EHEC infection are suffering from more severe symptoms, such as bloody diarrhea or HUS, than are adults. This is probably the main reason for the high incidence of bloody diarrhea or HUS, seen in 14 of 19 of the patients with EHEC of PFGE pattern A and 3 of 4 with PFGE pattern B. The patients infected with EHEC of these two PFGE patterns are almost all children. In the group of seven patients infected with EHEC of PFGE pattern S, all were adults and six of seven patients had diarrhea without blood.
In our investigation all EHEC O157 harbored the gene coding for VT2, and a few simultaneously harbored the VT1 coding sequence. All strains harbored the DNA sequence eaeA, coding for intimin. This characterization also shows that EHEC strains of serogroup O157, with few exceptions, have a plasmid harboring all four determinants (E-hly, etpD, katP, and espP) that we screened for with PCR. The genes coding for VT1 and VT2 are more equally dispersed among the non-O157 EHEC strains. Sixty-one percent (14 of 23) of the strains harbored the eaeA DNA sequence. Seven belonged to VT1-positive strains and seven to VT2-positive strains. That means that we could not find any correlation between eaeA positivity and the presence of VT1 and/or VT2 gene sequences, as was suggested by Boerlin et. al (2). Surprisingly, non-O157 EHEC strains often failed to give an amplification product of the four plasmid-borne determinants, or at least all four concurrently. Sixty-one percent (14 of 23) were positive for the E-hly sequence, 48% (11 of 23) for the espP sequence, 13% for the katP sequence, and 8.7% for the etpD sequence. However, they seemed to be as pathogenic as EHEC O157. We found strains inducing HUS and bloody diarrhea among those with EHEC O157 as well as with non-O157 strains. This is not to say that the plasmid is not important to EHEC O157, but merely that it adds to the pathogenicity of these strains. There is, however, a possible relation between pathogenicity and non-O157 strains harboring the gene coding for E-hly. Ten of 11 patients with bloody diarrhea or HUS had strains with a plasmid encoding the E-hly gene sequence, but only 3 of 10 patients who were symptom-free or had only milder symptoms had strains harboring this plasmid. Another conceivable possibility is that the markers are not located on the plasmid but in the chromosome. Brunder et. al. (3) found three strains which seemed to have the genes coding for espP and/or E-hly located in the chromosome. We intend to investigate non-O157 EHEC strains concerning this trait.
The conclusion concerning the plasmid-borne determinants is, however, that the four markers are found in many combinations, and we are in agreement with Brunder et. al. (3) in that the plasmids of EHEC are not uniform genetic elements but heterogeneous in their gene composition. The association of the plasmids and disease in humans is still obscure.
Boerlin et al. (2) have also studied associations between virulence factors of VT-producing E. coli and disease in humans, including strains isolated from bovine. They found significant associations of the genes for VT1 (Shiga toxin 1) and the espP protease. In the future, in an extended study including strains from animals and healthy children, we would like to investigate whether or not it is possible to use the typing procedures presented here to distinguish between virulent strains and nonvirulent or less virulent strains. In the present study, where all strains originated from humans and most of them were from patients with more or less severe symptoms of HUS or diarrhea, the typing was valuable for epidemiological investigations but gave little information concerning the virulent capacity of the strains.
Acknowledgments
The studies were supported by grants from the University of Göteborg and Sahlgrenska University Hospital, Göteborg, Sweden (I33912).
REFERENCES
- 1.Agin, T. S., and M. K. Wolf. 1997. Identification of a family of intimins common to Escherichia coli causing attaching-effacing lesions in rabbits, humans, and swine. Infect. Immun. 65:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunder, W., H. Schmidt, M. Frosch, and H. Karch. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005-1014. [DOI] [PubMed] [Google Scholar]
- 4.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 5.Brunder, W., H. Schmidt, and H. Karch. 1996. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:3305-3315. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg, M. S., J. Yu, and J. B. Kaper. 1993. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J. Bacteriol. 175:4670-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross, R., and B. Rowe. 1985. Serotyping of Escherichia coli, p. 235-240. In M. Sussman (ed.), The virulence of Escherichia coli. Review and methods. Academic Press, London, United Kingdom.
- 8.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenny, B., L. C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-323. [DOI] [PubMed] [Google Scholar]
- 10.Lidin-Janson, G., E. Falsen, U. Jodal, B. Kaijser, and K. Lincoln. 1977. Characteristics of antibiotic-resistant Escherichia coli in the rectum of healthy school-children. J. Med. Microbiol. 10:299-308. [DOI] [PubMed] [Google Scholar]
- 11.Martin, I. E., S. D. Tyler, K. D. Tyler, R. Khakhria, and W. M. Johnson. 1996. Evaluation of ribotyping as epidemiologic tool for typing Escherichia coli serogroup O157 isolates. J. Clin. Microbiol. 34:720-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newland, J. W., N. A. Strockbine, and R. J. Neill. 1987. Cloning of genes for production of Escherichia coli Shiga-like toxin type II. Infect. Immun. 55:2675-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai, C. H., N. Ahmed, H. Lior, W. M. Johnson, H. V. Sims, and D. E. Woods. 1988. Epidemiology of sporadic diarrhea due to verocytotoxin-producing Escherichia coli: a two-year prospective study. J. Infect. Dis. 157:1054-1057. [DOI] [PubMed] [Google Scholar]
- 15.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rios, M., V. Prado, M. Trucksis, C. Arellano, C. Borie, M. Alexandre, A. Fica, and M. M. Levine. 1999. Clonal diversity of Chilean isolates of enterohemorrhagic Escherichia coli from patients with hemolytic-uremic syndrome, asymptomatic subjects, animal reservoirs, and food products. J. Clin. Microbiol. 37:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt, H., C. Geitz, P. I. Tarr, M. Frosch, and H. Karch. 1999. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J. Infect. Dis. 179:115-123. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt, H., B. Henkel, and H. Karch. 1997. A gene cluster closely related to type II secretion pathway operons of gram-negative bacteria is located on the large plasmid of enterohemorrhagic Escherichia coli O157 strains. FEMS Microbiol. Lett. 148:265-272. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt, H., E. Maier, H. Karch, and R. Benz. 1996. Pore-forming properties of the plasmid-encoded hemolysin of enterohemorrhagic Escherichia coli O157:H7. Eur. J. Biochem. 241:594-601. [DOI] [PubMed] [Google Scholar]
- 21.Tarr, P. I. 1995. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 20:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welinder-Olsson, C., E. Kjellin, M. Badenfors, and B. Kaijser. 2000. Improved microbiological techniques using the polymerase chain reaction and pulsed-field gel electrophoresis for diagnosis and follow-up of enterohaemorrhagic Escherichia coli infection. Eur. J. Clin. Microbiol. Infect. Dis. 19:843-851. [DOI] [PubMed] [Google Scholar]