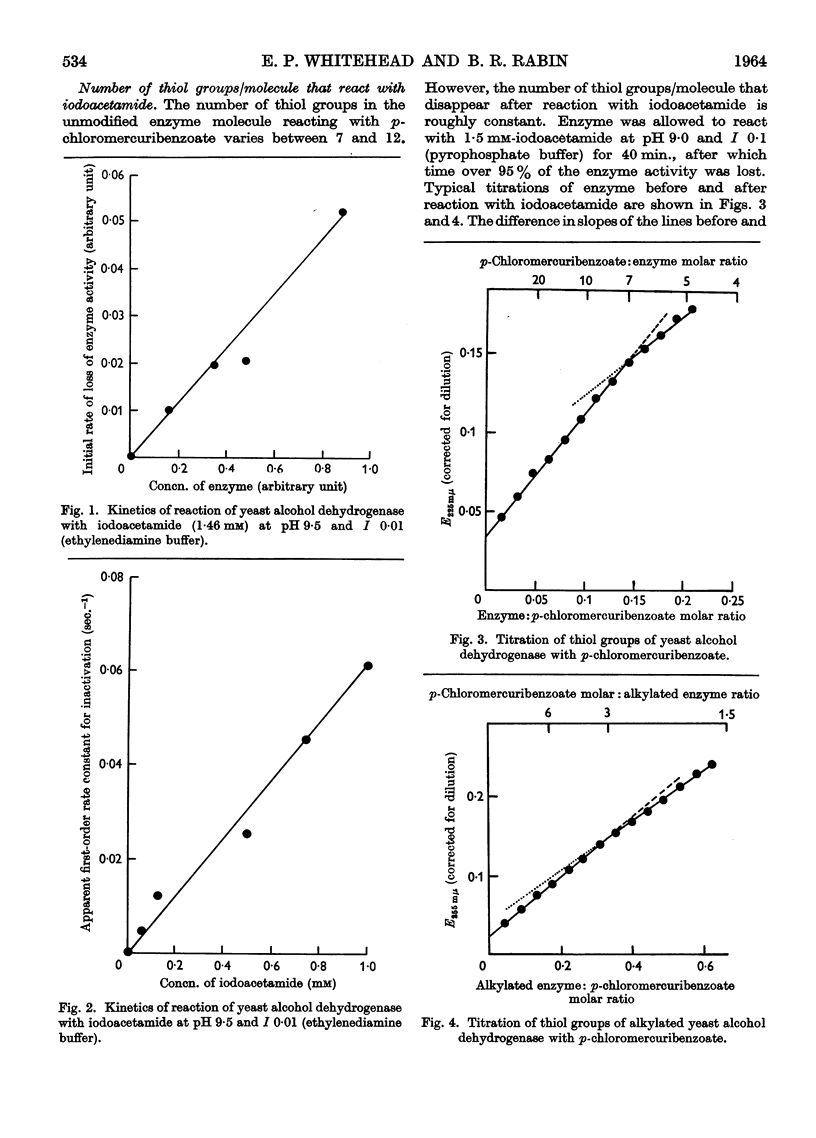
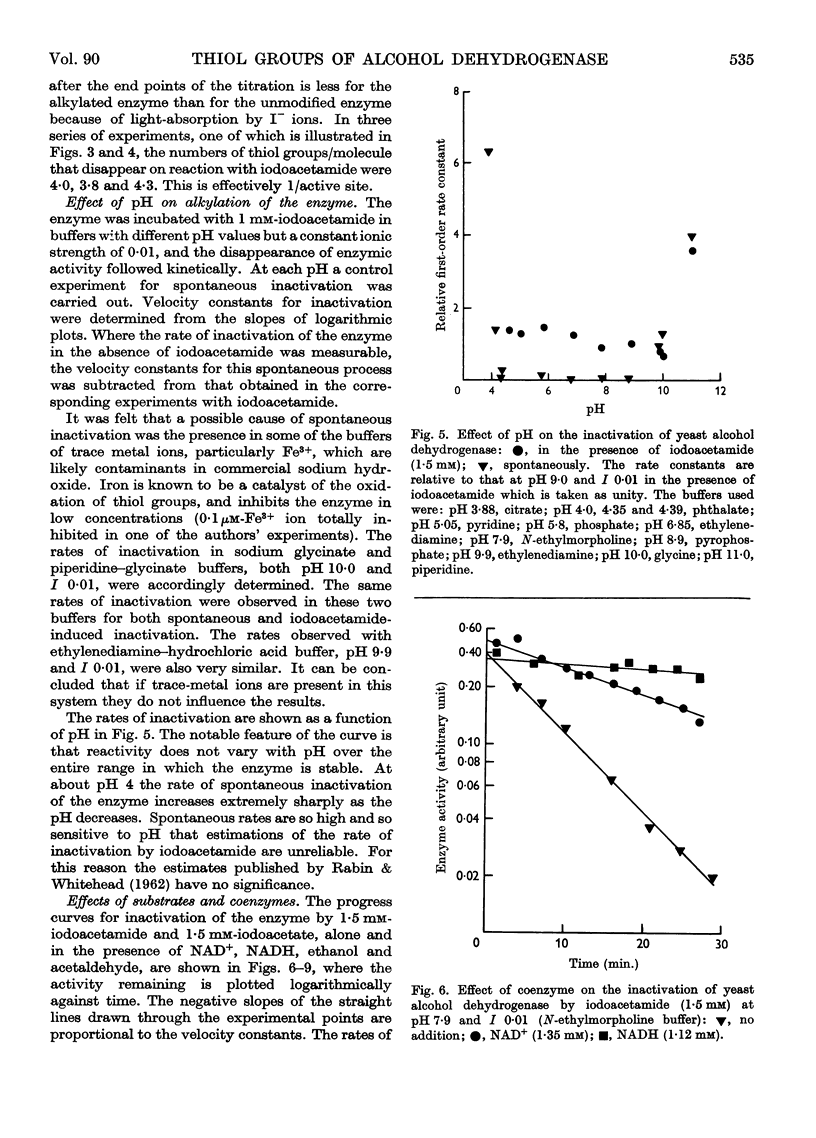
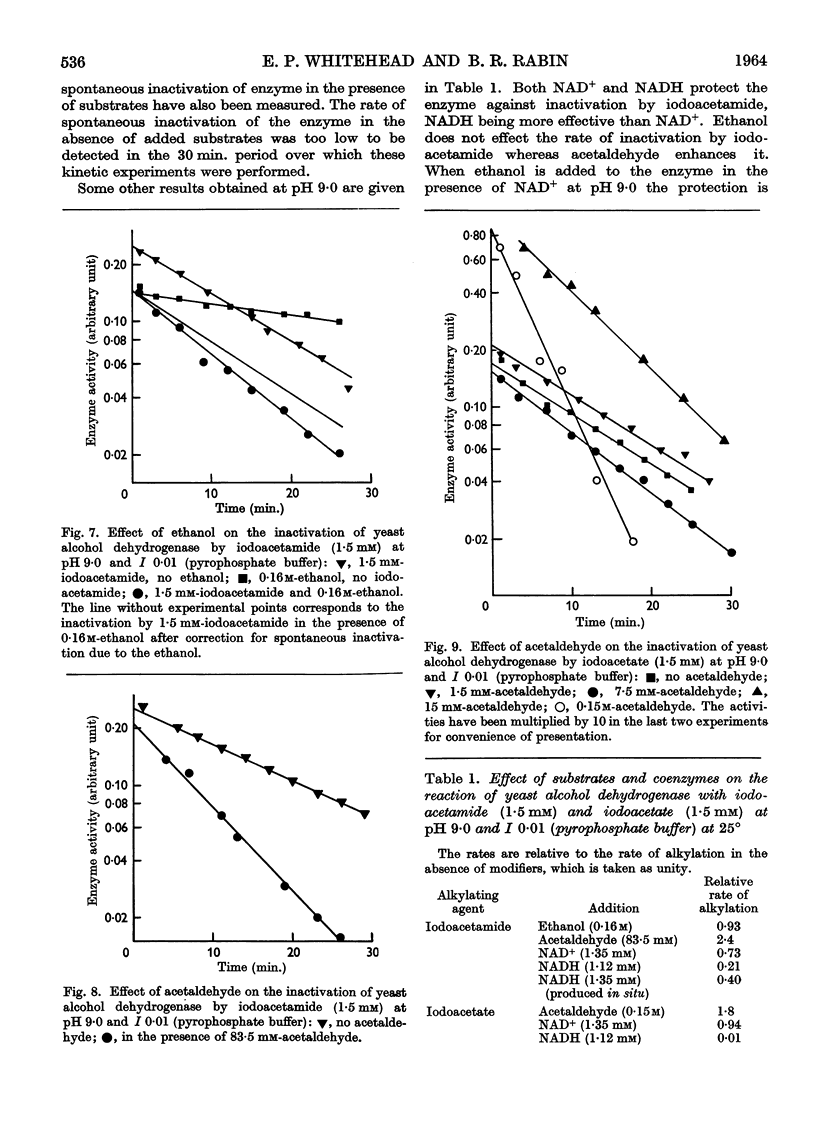
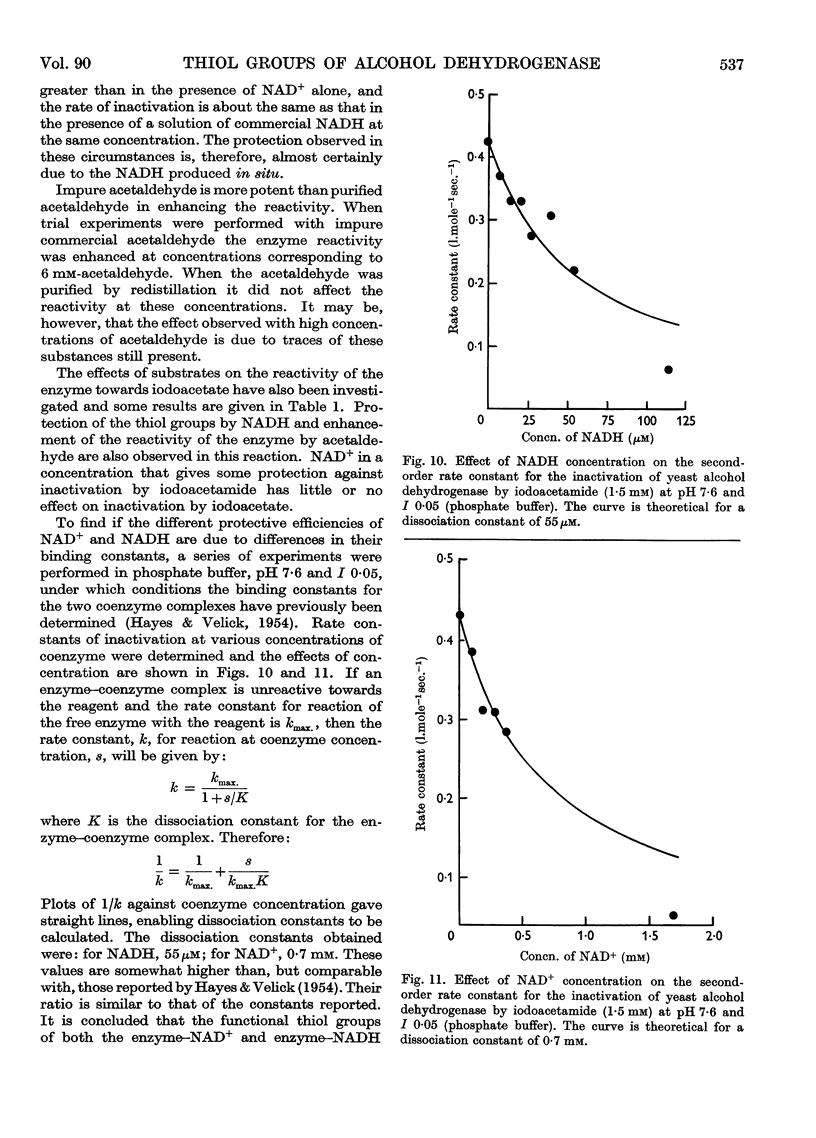
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRON E. S. G., LEVINE S. Oxidation of alcohols by yeast alcohol dehydrogenase and by the living cell; the thiol groups of the enzyme. Arch Biochem Biophys. 1952 Nov;41(1):175–187. doi: 10.1016/0003-9861(52)90518-3. [DOI] [PubMed] [Google Scholar]
- Dickens F. Interaction of halogenacetates and SH compounds: The reaction of halogenacetic acids with glutathione and cysteine. The mechanism of iodoacetate poisoning of glyoxalase. Biochem J. 1933;27(4):1141–1151. doi: 10.1042/bj0271141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES J. E., Jr, VELICK S. F. Yeast alcohol dehydrogenase: molecular weight, coenzyme binding, and reaction equilibria. J Biol Chem. 1954 Mar;207(1):225–244. [PubMed] [Google Scholar]
- KAPLAN N. O., CIOTTI M. M. Evolution and differentiation of dehvdrogenases. Ann N Y Acad Sci. 1961 Nov 2;94:701–722. doi: 10.1111/j.1749-6632.1961.tb35567.x. [DOI] [PubMed] [Google Scholar]
- LINDLEY H. A study of the kinetics of the reaction between thiol compounds and choloracetamide. Biochem J. 1960 Mar;74:577–584. doi: 10.1042/bj0740577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFLEIDERER G., JECKEL D., WIELAND T. Bedeutung von SH-Gruppen für die enzymatische Aktivität: Studien an Milchsäuredehydrogenase aus Schweineherz. Arch Biochem Biophys. 1959 Jul;83(1):275–282. doi: 10.1016/0003-9861(59)90033-5. [DOI] [PubMed] [Google Scholar]
- PIHL A., LANGE R. The interaction of oxidized glutathione, cystamine monosulfoxide, and tetrathionate with the-SH groups of rabbit muscle D-glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1962 Apr;237:1356–1362. [PubMed] [Google Scholar]
- RABIN B. R., WATTS D. C. A theory of the mechanism of action of creatine phosphokinase. Nature. 1960 Dec 31;188:1163–1165. doi: 10.1038/1881163a0. [DOI] [PubMed] [Google Scholar]
- RABIN B. R., WHITEHEAD E. P. Mechanism of action of yeast alcohol dehydrogenase. Nature. 1962 Nov 17;196:658–660. doi: 10.1038/196658a0. [DOI] [PubMed] [Google Scholar]
- RACKER E. Crystalline alcohol dehydrogenase from baker's yeast. J Biol Chem. 1950 May;184(1):313–319. [PubMed] [Google Scholar]
- Rabin B. R., Cruz J. R., Watts D. C., Whitehead E. P. The reaction of yeast alcohol dehydrogenase with iodoacetamide as determined with a silver-silver iodide electrode. Biochem J. 1964 Mar;90(3):539–542. doi: 10.1042/bj0900539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRITTMATTER P. The reactive sulfhydryl groups of microsomal cytochrome reductase. J Biol Chem. 1959 Oct;234:2661–2664. [PubMed] [Google Scholar]
- SZABOLCSI G., ELODI P. Comparative studies on D-glyceraldehyde-3-phosphate dehydrogenases. III. The inhibitory effect of p-chlormercuribenzoate in the presence of different substrates. Acta Physiol Acad Sci Hung. 1958;13(3):207–211. [PubMed] [Google Scholar]
- VAN EYS J., KRETSZCHMAR R., TSENG N. S., CUNNINGHAM L. W., Jr A NAD analogue which can be covalently bound to dehydrogenases. Biochem Biophys Res Commun. 1962 Jun 19;8:243–247. doi: 10.1016/0006-291x(62)90271-1. [DOI] [PubMed] [Google Scholar]
- VERLICK S. F. Fluorescence spectra and polarization of glyceraldehyde-3-phosphate and lactic dehydrogenase coenzyme complexes. J Biol Chem. 1958 Dec;233(6):1455–1467. [PubMed] [Google Scholar]
- WALLENFELS K., ARENS A. [Amino acids of alcohol dehydrogenase from yeast]. Biochem Z. 1960;332:217–246. [PubMed] [Google Scholar]
- WATTS D. C., RABIN B. R. A study of the 'reactive' sulphydryl groups of adenosine 5'-triphosphate-creatine phosphotransferase. Biochem J. 1962 Dec;85:507–516. doi: 10.1042/bj0850507. [DOI] [PMC free article] [PubMed] [Google Scholar]


