Abstract
Between 24 July and 31 August 1998, thousands of domestic pigs died of hemorrhagic shock in three adjunct counties along the YangZi River in Jiangshu Province, China. From 28 July to 6 September 1998, 40 local farmers (36 males and 4 females, ages 23 to 78 years) were hospitalized with severe illness characterized by high fever, erythematous rash or petechiae, and profound lethargy after contact with sick pigs. Twelve (30%) of these patients died of respiratory failure and shock. Eleven bacterial isolates recovered from 11 blood and cerebrospinal fluid specimens collected from seven patients and two pigs were identified as Enterococcus faecium based on biochemical reactions and 16S rRNA gene sequence analysis. Both pig and human E. faecium isolates displayed indistinguishable antibiotic susceptibility and pulsed-field gel electrophoresis patterns. These data strongly suggest the spread of an outbreak of E. faecium-related sepsis from pigs to humans.
Enterococci normally inhabit the gastrointestinal tracts of humans and animals. They are less commonly found at other biologic sites, such as the vagina and mouth, and the clinical features of enterococcal infections are variable (21). Such infections may involve almost any anatomic site and may be life-threatening during bacteremia and endocarditis. Despite their inherent low virulence, enterococci have emerged as major nosocomial pathogens. Enterococcus faecalis and Enterococcus faecium are the two most commonly encountered enterococcal species, accounting for approximately 85 and 10% of clinical isolates, respectively.
Epidemiological investigations of E. faecium outbreaks were initially hampered by the lack of a highly discriminatory typing method, but newer DNA-based methods have solved this problem (17). It has been suggested that E. faecium may enter the community via the foodchain (10). van den Bogaard et al. (31) found indistinguishable pulsed-field gel electrophoresis (PFGE) patterns of vancomycin-resistant enterococci (VRE) strains isolated from a Dutch farmer and one of his turkeys, indicating that humans and animals in close contact may harbor identical strains. This study also showed that vancomycin-resistant E. faecium isolates from pigs, poultry, and humans could be divided according to base-pair variation in the vanX gene. All poultry isolates belonged to one type, whereas all but one of the porcine isolates belonged to another (31), indicating that horizontal exchange of vancomycin-resistant E. faecium or Tn1546-like elements between poultry and pigs is uncommon (15). On the other hand, both types were found in humans, indicating that human acquisition may arise from both animal sources. This also suggests directional transmission from livestock to humans (31). Jensen et al. (16) reported highly similar PFGE patterns in vancomycin-resistant E. faecium strains containing similar Tn1546-like elements isolated from pigs and a hospitalized patient in Denmark, further suggesting foodborne transmission of VRE from animals to humans.
An outbreak of E. faecium-related sepsis in both humans and pigs occurred from July to September 1998 along the YangZi River in Jiangshu Province, China. The outbreak began in domestic pigs in the same area; the deaths of thousands of pigs began in one village and spread to adjoining areas. A total of 40 humans were infected, 12 of whom died. The present study applied molecular techniques to investigate the epidemiology of the outbreak in pigs and humans.
(This work was presented in part at the 39th Annual Meeting of the Infectious Diseases Society of America, San Francisco, Calif., 25 to 28 October 2001.)
MATERIALS AND METHODS
Clinical data collection.
Human cases were reported to the local Diseases Control and Prevention Station. Clinical specimens were collected, medical records were reviewed, and a questionnaire was completed that included clinical diagnosis, underlying illnesses, prior contact with sick pigs, and social activities within the previous 2 weeks.
Bacterial strain isolation and identification.
A total of 11 whole-blood and/or cerebrospinal fluid (CSF) specimens were collected from two sick pigs and seven patients during acute illness and immediately placed onto 5% sheep blood agar plates. Plates were incubated for 24 to 48 h at 35°C with 5% carbon dioxide. Isolates were identified presumptively by colony appearance, hemolysis pattern, and Gram stain. Other biochemical tests performed at local hospitals included tests for optochin sensitivity, spore-forming ability, oxidase, and catalase; a panel of biochemical reactions; and bile solubility tests (12). The isolates were further identified phenotypically by the API 20 Strep kit (bioMérieux, Inc., Hazelwood, Mo.) at the Huashan Hospital in Shanghai (13). Isolates were stored at −70°C in Trypticase soy broth for further analysis.
Antibiotic susceptibility testing.
Susceptibility to penicillin, amikacin, ampicillin, nitrofurantoin, ciprofloxacin, ceftazidime, vancomycin, imipenem, and ampicillin-sulbactam was tested. MICs were determined by E-test according to the manufacturer's instructions. NCCLS breakpoints were used (23).
16S rRNA gene sequencing.
Bacterial genomic DNA was extracted by using a QIAamp DNA Mini kit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions (24). A primer set spanning the region of the 16S rRNA gene corresponding to 5 to 1,540 nucleotide positions of Escherichia coli was used to amplify the DNA fragment by PCR (28, 29). The PCR-amplified products were sequenced by using six additional internal primers as previously described (28). Double orientation sequences of the whole 16S rRNA gene were determined by using the OpenGene sequencing system (Visible Genetics, Inc., Toronto, Ontario, Canada). Sequence sample files were compared with >1,100 validated 16S rRNA gene sequences in the MicroSeq database library (Applied Biosystems, Foster City, Calif.).
Genomic DNA analysis by PFGE.
Genomic DNA was extracted from log-phase bacterial cultures grown in brain heart infusion broth (22). The extracted DNA was prepared in low-melting-point agarose (pulsed-field certified agarose; Bio-Rad, Hercules, Calif.) plugs and was digested with the 20 U of SmaI enzyme (New England Biolabs, Beverly, Mass.) as described previously (9). The DNA size standards used were a bacteriophage lambda ladder consisting of concatemers of from 48.5 to approximately 1,000 kbp (Bio-Rad). Electrophoresis was performed with a CHEF DR III system (Bio-Rad). Run conditions were 240 V while switching from 10 to 50 s for 20 h at 14°C. Gels were stained with ethidium bromide, rinsed, and photographed under UV light (9). Interpretation of PFGE interrelationships was performed according to criteria recommended elsewhere (30).
Statistics.
Phylogenetic analysis by using the neighbor-joining method was performed as described elsewhere (1, 28).
RESULTS
Clinical epidemiology.
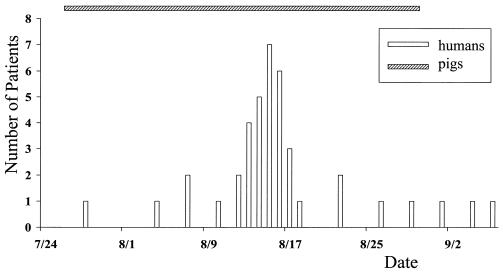
On 24 July 1998, the first sick domestic pig was noted in a local village in Nantong County, Jiangshu Province. Hundreds of pigs subsequently died in this and adjoining villages. By the end of August, thousands of pigs had died of the same disease in hundreds of villages in three counties. The onset was sudden. Pigs had sustained high fever associated with confluent purpuric-papular rash over their trunks and conjunctival hyperemia within 24 h. Sick pigs became flaccid, with mouth and snout bleeding, tachypnea, followed by death within 3 days. No other livestock, such as cattle, sheep, or goats, were affected. The first confirmed human case was reported on 28 July, when a slaughterman showed signs similar to toxic shock syndrome. Every suspected patient who presented to an emergency department had a history of exposure to infected pigs, had direct contact with livestock, or had handled sick or dead pigs within 7 days before the onset of disease. Concurrent with the pig epidemic, 40 patients were diagnosed clinically at three local central county hospitals. The number of cases peaked in mid-August, and the last human case was confirmed on 6 September 1998 (Fig. 1).
FIG. 1.
Temporal distribution of 40 cases. All patients had high fever, erythematous rash or petechiae, and profound lethargy. The hatched bar indicates the period during which sick pigs were reported.
Local governmental authorities immediately initiated measures to control the outbreak through livestock screening and deep burial of the dead pigs. Veterinarians and epidemiologists were transported to the area to implement various measures to combat the outbreak. The outbreak was gradually controlled, and no sick pigs or human cases were reported after 31 August or 6 September 1998, respectively. No more sick pigs were identified in the same villages during the same season in 1999, but seven patients were reported with similar but mild clinical illnesses, and all recovered rapidly.
Clinical manifestations.
All 40 patients (36 males and 4 females, ages 23 to 78 years) presented with fever (i.e., a temperature of 39 to 40.5°C), erythematous rash or petechiae, and profound lethargy. Other manifestations in some patients included chills and arthralgias, diffuse sunburn-like erythroderma, intermittent confusion, and prodromal respiratory illness, or sore throat. Central nervous system findings, including headache, stiff neck, and vomiting, were present in 19 (47.5%) of the patients, 2 of whom had lumbar puncture revealing CSF polymorphonuclear pleocytosis, low glucose, and elevated protein. Fourteen (35%) of the patients fulfilled the major criteria for toxic shock syndrome (a temperature of >39°C, hypotension, erythematous rash, and desquamation) and a number of minor criteria (severe conjunctival hyperemia, myalgias without an increased serum creatine kinase, thrombocytopenia, hypoproteinemia, and hypocalcemia). The syndrome progressed within 24 h to shock. Seven (17.5%) of the patients showed only fever and lethargy, with no more than two major organ systems involved (hepatic, renal, gastrointestinal, respiratory, or hematologic).
Bacterial isolate identification and antibiotic susceptibility testing.
Eleven bacterial isolates were recovered from 11 whole-blood and/or CSF specimens that were collected from seven patients and two sick pigs during the outbreak. The recovery rate was 100%. Of nine human isolates, seven were recovered from blood and two were recovered from CSF. All 11 isolates exhibited the same colony and microscopic morphology and were initially identified as alpha-hemolytic streptococci in the laboratories of local hospitals. They were further identified as E. faecium based on phenotypic identification schemes in the Clinical Diagnostic Laboratory at Huashan Hospital. All 11 isolates recovered from both humans and pigs had indistinguishable susceptibility patterns. They were sensitive to vancomycin, intermediate to nitrofurantoin, and resistant to penicillin, amikacin, ampicillin, ciprofloxacin, ceftazidime, imipenem, and ampicillin-sulbactam.
16S rRNA gene sequence analysis.
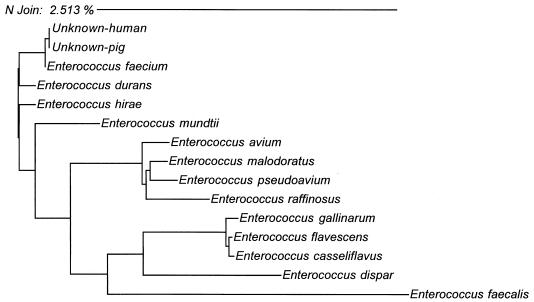
Genotypic identification was performed further on one human and one pig isolate. Genomic DNAs were extracted, and their small rRNA (16S rRNA) genes were amplified and sequenced. Sequences determined from both human and pig isolates were 100% identical and most closely related to E. faecium, diverging from the prototype sequence by one nucleotide (99.9% similarity) (Fig. 2).
FIG. 2.
Neighbor-joining analysis of DNA sequences from several specimens with homology to human and pig isolates. Phylogenetic analysis was based on whole 16S rRNA gene sequences. The scale indicates the relative phylogenetic distance.
Molecular typing.
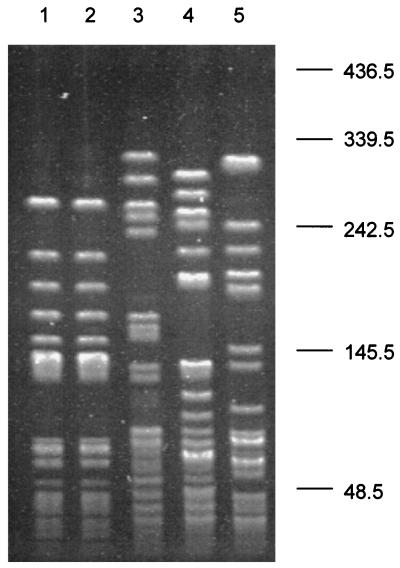
The epidemiologic relatedness of human and pig strains was examined by typing the same two representative isolates recovered from humans and pigs. The PFGE patterns of the isolates were indistinguishable and were completely different from unrelated E. faecium isolates (Fig. 3). These data demonstrate that the two isolates recovered from both patients and sick pigs were epidemiologically related, indicating that both human and pig sepsis cases were caused by the same clone of E. faecium.
FIG. 3.
PFGE patterns of SmaI-digested genomic DNA of human and pig E. faecium isolates. Lanes 1 and 2, E. faecium isolates recovered from a patient and a sick pig, respectively; lanes 3, 4, and 5, unrelated E. faecium isolates recovered from healthy individuals at the local hospital, the Huashan Hospital in Shanghai, and Vanderbilt University Medical Center, respectively. Molecular sizes are given in kilobases on the right.
DISCUSSION
In this study we investigated a sepsis outbreak involving thousands of pigs and 40 hospitalized patients over a period of 3 months. E. faecium was recovered from the blood of both patients and sick pigs, based on phenotypic and genotypic characteristics, including biochemical profiles and 16S rRNA gene sequences. Representative isolates from humans and pigs yielded indistinguishable PFGE patterns, suggesting that these isolates were clonal.
Enterococcus is the second most common cause of nosocomial infection in the United States (14, 19, 21), with E. faecalis causing most enterococcal infections. E. faecium is more commonly associated with resistance to beta-lactams, fluoroquinones, and glycopeptides and with greater morbidity and mortality (2, 6, 11). E. faecium isolates recovered in the present study were resistant to most antibiotics other than vancomycin. The rational administration of vancomycin is extremely important for such life-threatening E. faecium infections since vancomycin-resistant E. faecium has been reported to emerge in the community.
The spread of E. faecium-related disease from animals to humans has not been reported previously, although several molecular studies have indicated such horizontal transmission in nature (15, 31). The ability of enterococci to colonize the gastrointestinal tract, together with intrinsic and acquired resistance, allows these organisms to cause disease despite relatively low intrinsic virulence (25). Based on a study by van den Bogaard et al. (31), in which one strain from a farmer and one from his turkey flock appeared to be indistinguishable by PFGE and transposon typing, it was suggested that animal enterococci can colonize the human gut. All poultry isolates belonged to one type, while almost all porcine isolates belonged to another. On the other hand, both types were found among humans, indicating that humans may be infected from both sources (31). This observation suggests that the primary transmission is from animals to humans and not vice versa (31). It was also reported that vancomycin-resistant E. faecium strains with highly similar PFGE patterns contained similar Tn1546-like elements isolated from a hospitalized patient and pigs in Denmark, providing further evidence for the foodborne transmission of VRE from animals to humans (16). We report here that an E. faecium-related sepsis outbreak involved both humans and domestic pigs. E. faecium isolates recovered from blood and/or CSF specimens of both patients and sick pigs presented indistinguishable PFGE patterns, providing strong molecular evidence for the transmission of E. faecium from pigs to humans. This is, to our knowledge, the first indication of a horizontal transmission of E. faecium-related disease among humans and pigs.
Enterococcal bacteremia most often occurs in the absence of endocarditis. The source of bacteremia is often the urinary tract (19, 26). Intra-abdominal, biliary, pelvic, and wound sources are also common. The mortality associated with enterococcal bacteremia ranges from 34 to 44% and is often related to underlying complicating illnesses (20, 26). Enterococcal meningitis in adults has been reported in patients with coexistent chronic underlying illnesses and immunosuppressive therapy (27). Primary meningitis occurred in 25% of pediatric patients with enterococcal infections, especially in neonates (3). Patients involved in the present outbreak did not have underlying diseases during this study period.
One-third of patients met criteria for toxic shock-like syndrome. These findings suggest that a Streptococcus toxic shock-like syndrome producing gene might be carried by this E. faecium. Toxic shock-like syndrome is a severe, multisystem illness characterized by the rapid onset of fever and hypotension and is a subset of invasive streptococcal disease (8). Preliminary experiments in a pig model have demonstrated that 2- to 3-month-old pigs inoculated subcutaneously with the pig E. faecium isolate died within 10 days, whereas pigs inoculated with unrelated E. faecium recovered from normal pigs remain well. The streptococcal pyrogenic exotoxins (SPEs; also known as erythrogenic toxins or scarlet fever toxins) include the serologically distinct types A, B, C, D, F, G, and H, as well as streptococcal superantigen and streptococcal mutogenic exotoxin Z (5, 7, 18). SPEs are responsible for the fever, rash, and severe clinical manifestations of toxic shock-like syndrome. We have probed these E. faecium isolates with known SPE genes and have yet to identify homology (4). Efforts are focused on isolating and characterizing a possibly novel spe gene.
Acknowledgments
We thank our colleagues from local hospitals and antiepidemic and health stations for their hard work and strong support. We also thank Yu-Mei Wen, Zheng-Shi Yang, Karen Bloch, and David Haas for thoughtful discussions and review of the manuscript.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baele, M., P. Baele, M. Vaneechoutte, V. Storms, P. Butaye, L. A. Devriese, G. Verschraegen, M. Gillis, and F. Haesebrouck. 2000. Application of tRNA intergenic spacer PCR for identification of Enterococcus species. J. Clin. Microbiol. 38:4201-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker, D. P., P. W. Buss, N. Marlow, N. M. Brown, and M. R. Millar. 1994. Enterococcus faecium meningitis. Arch. Dis. Child. Fetal Neonatal Ed. 70:F78-F79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, C. M., D. F. Talkington, T. O. Messmer, R. R. Facklam, E. Hornes, and O. Olsvik. 1993. Detection of streptococcal pyrogenic exotoxin genes by a nested polymerase chain reaction. Mol. Cell. Probes 7:255-259. [DOI] [PubMed] [Google Scholar]
- 5.Bohach, G. A., D. J. Fast, R. D. Nelson, and P. M. Schlievert. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 17:251-272. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D. K., L. Pearce, A. McGeer, D. E. Low, and B. M. Willey. 2000. Evaluation of d-xylose and 1% methyl-α-d-glucopyranoside fermentation tests for distinguishing Enterococcus gallinarum from Enterococcus faecium. J. Clin. Microbiol. 38:3652-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleary, P. P., E. L. Kaplan, J. P. Handley, A. Wlazlo, M. H. Kim, A. R. Hauser, and P. M. Schlievert. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518-521. [DOI] [PubMed] [Google Scholar]
- 8.Cone, L. A., D. R. Woodard, P. M. Schlievert, and G. S. Tomory. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N. Engl. J. Med. 317:146-149. [DOI] [PubMed] [Google Scholar]
- 9.D'Agata, E. M., H. J. Li, C. Gouldin, and Y. W. Tang. 2001. Clinical and molecular characterization of vancomycin-resistant Enterococcus faecium during endemicity. Clin. Infect. Dis. 33:511-516. [DOI] [PubMed] [Google Scholar]
- 10.Descheemaeker, P. R., S. Chapelle, L. A. Devriese, P. Butaye, P. Vandamme, and H. Goossens. 1999. Comparison of glycopeptide-resistant Enterococcus faecium isolates and glycopeptide resistance genes of human and animal origins. Antimicrob. Agents Chemother. 43:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmond, M. B., J. F. Ober, J. D. Dawson, D. L. Weinbaum, and R. P. Wenzel. 1996. Vancomycin-resistant enterococcal bacteremia: natural history and attributable mortality. Clin. Infect. Dis. 23:1234-1239. [DOI] [PubMed] [Google Scholar]
- 12.Facklam, R. R., and M. D. Collins. 1989. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J. Clin. Microbiol. 27:731-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fertally, S. S., and R. Facklam. 1987. Comparison of physiologic tests used to identify non-beta-hemolytic aerococci, enterococci, and streptococci. J. Clin. Microbiol. 25:1845-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gin, A. S., and G. G. Zhanel. 1996. Vancomycin-resistant enterococci. Ann. Pharmacother. 30:615-624. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, L. B. 1998. Differences in the occurrence of two base pair variants of Tn1546 from vancomycin-resistant enterococci from humans, pigs, and poultry. Antimicrob. Agents Chemother. 42:2463-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen, L. B., A. M. Hammerum, R. L. Poulsen, and H. Westh. 1999. Vancomycin-resistant Enterococcus faecium strains with highly similar pulsed-field gel electrophoresis patterns containing similar Tn1546-like elements isolated from a hospitalized patient and pigs in Denmark. Antimicrob. Agents Chemother. 43:724-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordens, J. Z., J. Bates, and D. T. Griffiths. 1994. Faecal carriage and nosocomial spread of vancomycin-resistant Enterococcus faecium. J. Antimicrob. Chemother. 34:515-528. [DOI] [PubMed] [Google Scholar]
- 18.Kamezawa, Y., T. Nakahara, S. Nakano, Y. Abe, J. Nozaki-Renard, and T. Isono. 1997. Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin produced by a T1 strain of Streptococcus pyogenes. Infect. Immun. 65:3828-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki, D. G., and W. A. Agger. 1988. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine 67:248-269. [PubMed] [Google Scholar]
- 20.Malone, D. A., R. A. Wagner, J. P. Myers, and C. Watanakunakorn. 1986. Enterococcal bacteremia in two large community teaching hospitals. Am. J. Med. 81:601-606. [DOI] [PubMed] [Google Scholar]
- 21.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray, B. E., K. V. Singh, J. D. Heath, B. R. Sharma, and G. M. Weinstock. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Patel, R., K. E. Piper, M. S. Rouse, J. M. Steckelberg, J. R. Uhl, P. Kohner, M. K. Hopkins, F. R. Cockerill III, and B. C. Kline. 1998. Determination of 16S rRNA sequences of enterococci and application to species identification of nonmotile Enterococcus gallinarum isolates. J. Clin. Microbiol. 36:3399-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poyart, C., G. Quesnes, and P. Trieu-Cuot. 2000. Sequencing the gene encoding manganese-dependent superoxide dismutase for rapid species identification of enterococci. J. Clin. Microbiol. 38:415-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlaes, D. M., J. Levy, and E. Wolinsky. 1981. Enterococcal bacteremia without endocarditis. Arch. Intern. Med. 141:578-581. [PubMed] [Google Scholar]
- 27.Stevenson, K. B., E. W. Murray, and F. A. Sarubbi. 1994. Enterococcal meningitis: report of four cases and review. Clin. Infect. Dis. 18:233-239. [DOI] [PubMed] [Google Scholar]
- 28.Tang, Y. W., N. M. Ellis, M. K. Hopkins, D. H. Smith, D. E. Dodge, and D. H. Persing. 1998. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J. Clin. Microbiol. 36:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang, Y. W., M. K. Hopkins, C. P. Kolbert, P. A. Hartley, P. J. Severance, and D. H. Persing. 1998. Bordetella holmesii-like organisms associated with septicemia, endocarditis, and respiratory failure. Clin. Infect. Dis. 26:389-392. [DOI] [PubMed] [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bogaard, A. E., L. B. Jensen, and E. E. Stobberingh. 1997. Vancomycin-resistant enterococci in turkeys and farmers. N. Engl. J. Med. 337:1558-1559. [DOI] [PubMed] [Google Scholar]