Abstract
We have evaluated a real-time PCR procedure based on the LightCycler technology for rapid detection of herpes simplex virus (HSV) in genital lesions. Two sets of primers, corresponding to the thymidine kinase and DNA polymerase regions, were used for the amplification reactions in separate capillaries containing the SYBR Green I dye as detection signal. In 28 of 118 samples (24%), HSV was isolated by conventional cell culture. All cell culture-positive samples were also positive by real-time PCR. Six additional cell culture-negative samples were positive by PCR with both sets of primers. Total processing time was less than 3 h. Real-time PCR using SYBR Green I as detection signal is a sensitive procedure for the rapid diagnosis of HSV in genital lesions.
Herpes simplex virus (HSV) is frequently detected in mucocutaneous lesions in the clinical virology laboratory (2, 13). Conventional cell culture still remains the diagnostic method of choice, although enzyme-linked immunosorbent assay and immunofluorescence techniques are available (3). Nucleic acid technology based on PCR has shown an interesting role in detection of HSV DNA (8, 10), and several protocols, adapted to clinical laboratories, have been reported (6, 14). However, conventional PCR techniques have been so far relatively cumbersome, difficult to interpret, and prone to contamination. Very recently, real-time PCR has started to demonstrate its potential utility in the field of clinical virology and, specifically, in the detection of herpesvirus DNA (1, 2, 9, 11, 12). The main features making this new technology so attractively suitable for these applications, in comparison with conventional PCR, are rapidness, possibility of accurate quantification and, very important, reduction of likelihood of contamination, since no postamplification analysis of the tubes is required. Nevertheless, the value of this technology for specific applications and the development of protocols to be included in clinical laboratory routines remain to be established through well-controlled clinical studies.
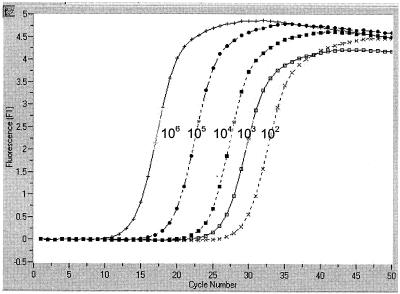
We have used a real-time PCR strategy, based on the Light Cycler technology, to detect HSV DNA in genital lesions from patients attending the sexually transmitted disease clinics of our institution, and the results were compared to those obtained by conventional cell culture. Swabs, taken from the surfaces of genital lesions and sent to the virology laboratory in 2 ml of viral transport medium (ViralPack; Biomedics S.L., Madrid, Spain), were inoculated to monolayers of A-549 and MRC-5 cells in tubes. These tubes were inoculated with 0.2 ml, incubated at 37°C in stationary phase, and scored daily for cytopathic effect (CPE) for 7 days or until CPE developed. When a characteristic HSV CPE was observed, a passage was done to two homologous monolayers in shell vials. These shell vials were incubated 24 h at 37°C and stained with specific fluorescent reagents to HSV type 1 (HSV-1) and HSV-2 (MicroTrak HSV 1-2 culture identification/typing test; Dade-Behring, Marburg, Germany). For the real-time PCR technique, 0.5 ml of the remaining transport medium was centrifuged at 12,000 × g for 10 min and the supernatant was vacuum aspirated so that the cellular pellet and 200 μl of transport media were left in the tube. DNA from this product was purified by a commercial procedure (High Pure Viral Nucleic Acid kit; Roche, Mannheim, Germany) and resuspended in water to a final volume of 50 μl. Two microliters of this purified DNA was used for real-time amplification in a final 10-μl reaction volume, using 1× Fast Start SYBR Green I Master Mix (Roche), MgCl2 (3 mM), and 0.5 μM concentrations of each primer. The capillary tubes were taken into the LightCycler instrument (Roche) and amplified as follows: 95°C for 7 min followed by 50 cycles of 95°C for 30 s, 62°C for 10 s, and 72°C for 12 s, followed by denaturation of amplification samples by slow increase of temperature (0.2°C/s) up to 99°C. Total processing time was less than 3 h. Samples were processed in duplicate with two different sets of primers, amplifying regions from thymidine kinase (Tk; 335 bp) and DNA polymerase (Pol; 215 bp), respectively (Tk-forward, GAC MAG CGC CCA GAT AAC AA; Tk-reverse, MCA GCA TRG CCA GGT CAA GC [GenBank accession no. M16321]; Pol-forward, GCT CGA GTG CGA AAA AAC GTT C; Pol-reverse, CGG GGC GCT CGG CTA AC [GenBank accession no. X03764 and X01712]; M being A or C and R being A or G) (2, 14). In order to use a quantitative control in the assay, these regions were amplified by conventional PCR from a control HSV-1 strain, and the fragments were cloned into pUC19. DH5α Escherichia coli was transformed with both constructions and, after expansion in culture, the plasmid was purified by double-banding CsCl ultracentrifugation and quantified. A panel of copies of the pUC19-Tk and Pol constructions, ranging from 1 to 106 copy numbers, was used for quantification (Fig. 1). Based on the fact that the 102-copy control of this panel was consistently detected by real-time PCR in repeated experiments, we decided to consider positive those samples showing specific signals for both sets of primers (melting temperature between 93 and 95°C) (Fig. 2) and a quantification higher than 10 DNA copies. Also for control purposes, two known samples and two cell cultures positive for varicella-zoster virus were processed, with negative results.
FIG. 1.
Performance of real-time PCR on a panel of control samples with quantified number of copies of HSV DNA. The graphic shows the evolution of the fluorescent signal related to cycle number on a panel of quantified copies of a plasmid containing the Tk fragment of an HSV-1 isolate. Similar results were obtained with the DNA Pol plasmid panel.
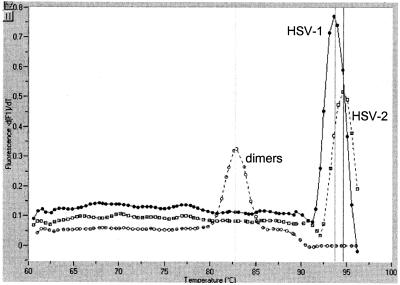
FIG. 2.
Melting curves of the amplified fragments generated by real-time PCR. These melting curves were generated on clinical samples with primers for Tk. Specific signals had melting temperatures ranging from 93 to 95°C. Similar results were obtained with the Pol set of primers. HSV-1 and HSV-2 amplification products were clearly differentiated from unspecific amplification (primer dimmers), exhibiting melting temperatures below 85°C.
HSV was isolated in 28 of 118 samples (24%) processed by conventional cell culture. Ten isolates (36%) were identified as HSV-1, and 18 (64%) were identified as HSV-2. All cell culture-positive samples were positive by real-time PCR. Quantification of HSV for these samples was in a range from 520 to 53 × 106 DNA copies (geometric mean, 171,879 copies) for Tk primers and from 57 to 35 × 106 DNA copies (geometric mean, 38,795 copies) for the Pol primer set. The global performance of the real-time PCR on clinical samples is as follows: of 28 samples testing positive with conventional cell culture, real-time PCR detected 28 samples as positive and none as negative; of 90 samples testing negative with conventional cell culture, real-time PCR detected 84 as negative and 6 as positive. Taking conventional culture as the “gold standard,” sensitivity was 100%, whereas specificity was 93%. However, if we consider that in six culture-negative samples the positivity of PCR was achieved with both sets of primers, in independent reactions, and at copy numbers ranging from 360 to 7.8 × 106 with Tk primers (geometric mean, 70,498 DNA copies) and from 15 to 3 × 106 with Pol primers (geometric mean, 16,328 DNA copies), we believe that these were false-negative culture results rather than false-positive PCRs. Moreover, these real-time PCR-positive cell culture-negative results were confirmed by gel electrophoresis, showing amplification bands of the expected molecular weights with both sets of primers.
Fluorescently labeled probes to detect the amplified products have been used in the majority of reports on real-time PCR for diagnostic purposes (5, 7). However, labeled probes are expensive and introduce an additional complexity to both the design and the parameters of the amplification reaction. Moreover, despite the use of specific probes, artifacts can occur, especially at amplification cycles beyond the 30th (1). No specific probes were used in our study, and the SYBR Green I dye provided the fluorescent signal (4). This approach is simpler, cheaper, and probably more sensitive, since many fluorescent labels, instead of just one molecule, are incorporated into the amplified fragment. On the other hand, the melting temperature of the amplified DNA (Fig. 2) allows a clear distinction of the specific products from artifacts, such as primer dimmer, that are also minimized by the hot-start step included in the procedure. To avoid unspecific signals and based in the quantification panel, we have established the positive cutoff at the 10-copy level, which represents a reasonable technical and clinical threshold. The only drawback of the system was the inability to differentiate accurately HSV-1 from HSV-2 in a single reaction, since the melting temperature of the fragments amplified in each type was measured within a too-narrow range. However, we think that HSV type identification is secondary to a rapid and consistent diagnosis and might well be performed, in a second step, on PCR-positive samples.
In conclusion, real-time PCR based in the capillary format of the LightCycler instrument and using the SYBR Green I fluorescent dye as the detection signal is a simple, rapid, sensitive, and specific tool for detection of HSV DNA from genital lesions.
Acknowledgments
We acknowledge the technical assistance of C. Prieto, S. Maldonado, and M. J. Babiano. C.P.A. is supported by Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, SEIMC.
This work was partially supported by grants from Fondo de Investigación Sanitaria FIS 99/514 and 01/1430 and FIPSE 3026/99 to R.D.
REFERENCES
- 1.Espy, M. J., T. K. Ross, R. Teo, K. A. Svien, A. D. Wold, J. R. Uhl, and T. F. Smith. 2000. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J. Clin. Microbiol. 38:3116-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espy, M. J., J. R. Uhl, P. S. Mitchell, J. N. Thorvilson, K. A. Svien, A. D. Wold, and T. F. Smith. 2000. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J. Clin. Microbiol. 38:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston, S. L., and C. S. Siegel. 1990. Comparison of enzyme immunoassay, shell vial culture, and conventional cell culture for the rapid detection of herpes simplex virus. Diagn. Microbiol. Infect. Dis. 13:241-244. [DOI] [PubMed] [Google Scholar]
- 4.Karlsen, F., H. B. Steen, and J. M. Nesland. 1995. SYBR green I DNA staining increases the detection sensitivity of viruses by polymerase chain reaction. J. Virol. Methods 55:153-156. [DOI] [PubMed] [Google Scholar]
- 5.Lewin, S. R., M. Vesanen, L. Kostrikis, A. Hurley, M. Duran, L. Zhang, D. D. Ho, and M. Markowitz. 1999. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J. Virol. 73:6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell, P. S., M. J. Espy, T. F. Smith, D. R. Toal, P. N. Rys, E. F. Berbari, D. R. Osmon, and D. H. Persing. 1997. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J. Clin. Microbiol. 35:2873-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullah, B., K. Livak, A. Andrus, and P. Kenney. 1998. Efficient synthesis of double dye-labeled oligodeoxyribonucleotide probes and their application in a real time PCR assay. Nucleic Acids. Res. 26:1026-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell, K. F., N. E. Anderson, R. W. Frith, and M. C. Croxson. 1990. Non-invasive diagnosis of herpes simplex encephalitis. Lancet 335:357-358. [DOI] [PubMed] [Google Scholar]
- 9.Read, S. J., J. L. Mitchell, and C. G. Fink. 2001. LightCycler Multiplex PCR for the laboratory diagnosis of common viral infections of the central nervous system. J. Clin. Microbiol. 39:3056-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowley, A. H., R. J. Whitley, F. D. Lakeman, and S. M. Wolinsky. 1990. Rapid detection of herpes-simplex-virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis. Lancet 335:440-441. [DOI] [PubMed] [Google Scholar]
- 11.Schalasta, G., A. Arents, M. Schmid, R. W. Braun, and G. Enders. 2000. Fast and type-specific analysis of herpes simplex virus types 1 and 2 by rapid PCR and fluorescence melting-curve-analysis. Infection 28:85-91. [DOI] [PubMed] [Google Scholar]
- 12.Schalasta, G., M. Eggers, M. Schmid, and G. Enders. 2000. Analysis of human cytomegalovirus DNA in urines of newborns and infants by means of a new ultrarapid real-time PCR-system. J. Clin. Virol. 19:175-185. [DOI] [PubMed] [Google Scholar]
- 13.Smith, T. F., A. D. Wold, M. J. Espy, and W. F. Marshall. 1993. New developments in the diagnosis of viral diseases. Infect. Dis. Clin. N. Am. 7:183-201. [PubMed] [Google Scholar]
- 14.Tang, Y. W., P. S. Mitchell, M. J. Espy, T. F. Smith, and D. H. Persing. 1999. Molecular diagnosis of herpes simplex virus infections in the central nervous system. J. Clin. Microbiol. 37:2127-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]