Abstract
The purpose of the study was to evaluate the effect of origin and age of ducks on physical and morphological composition of eggs, as well as determine the level and activity of lysozyme. Eggs taken from three (3) breeding flocks of Pekin-type ducks were studied: P-9 (Pekin of French origin), K-2 Mini-duck, and LsA (Pekin of English origin – synthetic line). Eggs were collected from birds that were in the 13th week of reproduction of the first and second year of laying. In a given research period, 90 eggs were randomly selected for evaluation from each analysed duck flock, always up to 24 h after laying. A total of 540 duck eggs (first and second laying seasons) were analysed. The results showed significant differences (p < 0.05 and p < 0.01) in the evaluated egg parameters between the three experimental groups of ducks. The effect of the genetic origin of the ducks was significant (p < 0.001) on all studied egg traits, except for the percentage of albumen. Older ducks laid eggs with more (p < 0.05) elongated shape and favourable albumen quality parameters (height and Haugh units). The effect significant (p < 0.001) of duck age on albumen height, Haugh units, yolk pH, shell colour and porosity was observed, as well as the effect of origin × age interaction on egg weight, surface area and shape index, albumen and yolk pH, and shell parameters (colour, weight, porosity, elastic deformation). Eggs from ducks in the second period of laying were characterized by the significantly highest (P ≤ 0.01) content and activity of lysozyme. There was an effect (p < 0.001) of origin, season of use and interaction between these factors on the content and activity of lysozyme.
Keywords: Egg, Duck, Age, Origin, Physicochemical parameters, Lysozyme
Subject terms: Zoology, Animal breeding, Genotype
Introduction
Pekin-type ducks are mostly bred to create hybrids for meat production , and their eggs are mainly used for reproduction. These birds are characterized by seasonality of egg production - it averages only six months (from February to August). During this period, females lay an average of 150 eggs. The number of eggs obtained and the length of lay depend largely on genetic, age and nutrition factors, but especially on the light programme1,2. Although the lifespan of ducks is 8 to 12 years, they are used commercially for two seasons at most. This is related to the economics of rearing, for in the second season of duck use the laying rate declines at a significantly faster rate than during the first period of egg production, and after about 25–30 weeks it is usually no longer profitable to maintain the flock, since the number of eggs obtained is 20–25% lower3.
The morphological composition and biological quality of farmed poultry eggs that shape their hatchability depend on, among other things, the origin (species, breed, pedigree, variety), age and nutrition of the birds, as well as environmental factors4–6.
Flock age has been shown to be a major determinant of albumen and yolk content in eggs. A greater increase in yolk relative to albumen occurs as the laying flock ages7. During incubation, the embryo covers all of its nutrient requirements from albumen and yolk. Therefore, the egg content primarily affects the weight and body dimensions of the chick and overall hatchability rate8.
Since the period for obtaining duck eggs is relatively short, it is important that the eggs have good biological quality to ensure the desired results during hatching. The physical properties of the egg play an important role in the processes of embryo development and hatching. Among the most important are: eggs weight, shell thickness and porosity, shape index, and content morphology. The average values of physical characteristics mostly meet the requirements of embryonic development9. For eggs whose parameters do not fall within the average range (shape and egg size), incubation is more successful if the shell is thicker than average, the eggs are more pointed than round, and the contents are firm10,11. The results of studies of hatching eggs whose weight is not within the average range are contradictory. Both thick shells and proper colloidal balance of egg content, considered to be higher than average, lead to an increase in egg weight, likely resulting in more efficient hatching of embryos from heavier eggs12,13.
The albumen of avian eggs contains many biologically valuable substances in its composition, including lysozyme – a valuable hydrolytic enzyme with strong antimicrobial activity. Belonging to biologically active proteins, it provides natural, primary protection of the embryo until the first antibodies are formed14. Its content and activity have been shown to depend on a number of factors, as the origin and age of laying hens, nutrition, rearing system or health status of the flock15–17. Previous studies of the effect of female age on the amount and lytic intensity of this enzyme, have mainly concerned changes in successive weeks of laying within a single season of use18–20. In contrast, there are few studies on the formation of lysozyme in poultry eggs from two laying seasons20.
It was assumed that the age and origin of ducks would affect the physical parameters of the eggs and thus the content and activity of lysozyme, therefore the aim of the study was to evaluate the effect of age and origin of Pekin-type ducks of three breeding flocks on physical and morphological parameters of eggs, as well as the content and activity of lysozyme.
Materials and methods
Research material
The experimental material consisted of duck eggs from three breeding flocks (180 in each strain): P-9 (Pekin of French origin), K-2 Mini-duck and LsA (Pekin of English origin – synthetic line), which are maintained at the Waterfowl Genetic Resources Station in Dworzyska of the National Research Institute of Animal Production in Kołuda Wielka in Poland. The numerical ratio of males to females was 1:4.
Environment
The birds were kept under identical environmental and nutritional conditions - during the rearing period up to the 3rd week, the ducks were kept in an enclosed room with a controlled microclimate on straw without windows. From the 3rd to the 4th week, the birds were housed in a hall with outdoor access and from the 4th week onwards, they were kept in the open air and in a straw, partially covered enclosure. In autumn season, in the 27th–28th week of reproduction, flocks of ducks were put together and moved again to the hall for the winter period (artificial lighting 3–5 W/m2 13–14 h; pastel light (matte bulbs). Eggs intended for tests were collected in the morning and placed in the egg carton. Then, a detailed selection was subjected, which mainly concerned the assessment of mass, the regularity of the shape and its shell. Crushed and mechanically damaged eggs were eliminated. Then the surface of the shell was cleaned (washed with running water at a temperature of about 20 °C), and then disinfected with a 0.5% Virkon solution. Technological conditions (environmental and zoohygienic conditions, feeding, prevention) during the birds’ rearing were in accordance with general assumptions for this type of production. Ducks were fed ad libitum with identical feed mixes with a content of 19.7% total protein, 4.1% fat and 4.3% fiber. Due to the fact that no animal experiments were conducted and only eggs were collected for analysis, APPROVAL NUMBER our IACUC was not required. Duck eggs were used in the experiment with the knowledge and consent of the National Research Institute of Animal Production in Kraków (Poland).
Morphometric analysis of eggs
Eggs were taken from birds that were in the 13th week of laying of first and second year of reproduction. A total of 90 eggs for evaluation from each duck flock analysed, always up to 24 h after laying were randomly selected. Eggs were collected daily – they were first washed with running water and then disinfected with 1.0% Virkon S (Antec International Limited, UK).
Eggs were subjected to quality assessment using EQM system (Egg Quality Micro-Technical (EQM) Services and Supplies Limited). This instrument was used to determine the weight (g) of different egg fractions, albumen height (mm), Haugh units, colour of the shell (% reflectance), and density (mg/cm2). The pH values of albumen (on whole egg white) and yolk were determined using a portable pH-meter (Seven2Go, Mettler-Toledo) after they were transferred into sterile containers. Also, the percentage of albumen, yolk and shell in the egg weight was calculated. Egg shape index (%) was calculated by dividing egg length by egg width. Measurements were made to the nearest 0.5% with a Shape-Meter (B.V. Apparatenfabriek Van Doorn, De Bilt, The Netherlands), scaled 65–85%. The formula of Paganelli et al.23 was used to calculate eggshell area (cm2.
Eggshell analysis
Shell thickness without inner shell membranes was measured with a Mitutoyo micrometer (Japan) to the nearest 1 μm, at three locations on the egg – at the equator and at both poles. The average of three measurements was taken as the final result. The pores (number of pores/cm2) were determined and counted by the method of Tyler23. Tests of the elastic deformation of the shell and its strength were carried out using a TA.XT PLUS texture analyser (Stable Micro Systems) and a set of appropriate attachments for testing the quality of the shell. The elastic deformation of shell was measured to the nearest 1 μm, at three measuring points of the egg (at the periphery along the short axis and at both poles – at the sharp and blunt ends of the egg along the long axis) under three different loads, i.e. 0.50 kg, 1.00 kg and 1.50 kg (the average of three measurements was taken as the final result). This test made it possible to determine the degree of elastic deformation of the shell under the influence of the applied force. Analysing the mechanical strength of the eggshell, pressure (N) was applied and gradually increased until the shell broke. This measurement made it possible to determine the pressure force at which the analysed shell will crack, crush or puncture.
Lysozyme analysis
To make a detailed analysis of the active ingredient (lysozyme) in egg albumen fractions (hydrolytic activity, percentage), the previously collected and weighed eggs from different experimental groups of the birds were broken and separated into albumen and yolk. Next, the albumen was separated into thick and thin fractions and placed in disposable sterile containers. The so prepared albumen samples were used to make preparations to determine the concentration (%) and hydrolytic activity of lysozyme (U/ml) in the albumen fractions. The content and activity of lysozyme in the fresh albumen fractions was determined with spectrophotometry, consisting in the use of the lysis of cell walls of Micrococcus lysodeikticus bacteria24. Hydrolytic activity of lysozyme was expressed in lysozyme activity units (U/ml). One such unit is defined as the amount of lysozyme that in one minute decreases the absorbance of a Micrococcus lysodeikticus bacterial suspension by 0.001, measured at a wavelength of 450 nm and a temperature of 25 °C. The reaction occurred in a mixture of a volume of 2.6 ml (2.5 ml of bacterial suspension + 0.1 ml lysozyme solution) and pH 6.24, in a cuvette with a length of optical path being 1 cm. After calculating the value of absorbance decrease (ΔA) for the working solution of lysozyme, a curve of correlations between ΔA and lysozyme concentration was graphed. Next, based on the standard curve, hydrolytic activity of lysozyme was determined in the studied preparations. The value of the decrease in the solution absorbance (ΔA) was calculated from the following equation:
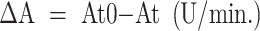 |
where:
At0 – absorbance value for bacterial suspension at time t0.
At – absorbance value for bacterial suspension after time t.
Data were analysed using Statistica 13.1 PL software. Mean values for all analysed parameters were calculated. A two-way model of ANOVA was used to analyse the effects (variable 1: genotype; variable 2: laying period). The significance of differences was verified using Duncan’s test. Interactions between experimental variables were assessed. The significance of differences was set at P ≤ 0.05 and P ≤ 0.01. Pearson’s coefficients of correlation were calculated between all the analysed parameters.
Results
Younger ducks of the K-2 and LsA families laid eggs with greater (p < 0.05) weight (by an average of 2.37 and 0.68 g, respectively) and egg surface area (by an average of 1.78 and 0.48 cm2, respectively). The opposite situation was observed in the case of eggs of P-9 ducks, where eggs with greater (p < 0.05) weight (88.70 g) and egg surface area (94.11 cm2 were obtained from ducks in the second year of laying. Older ducks laid eggs with a more elongated shape (p < 0.05). The egg shape index ranged from 71.72 (LsA) to 73.42% (K-2) for older and from 72.14 (LsA) to 74.65% (K-2) for younger ducks. The above parameters were influenced (p < 0.001) by origin and the interaction origin × season of ducks. On the other hand, the egg shape index was also influenced (p < 0.001) by season (Table 1).
Table 1.
Selected quality parameters of egg yolk from selected ducks breeds in two seasons of use.
| Item | Season | K-2 | P-9 | LsA | P value | ||
|---|---|---|---|---|---|---|---|
| Effect of origin | Effect of season | Effect of interaction |
|||||
| Egg weight (g) | 1 | 75.38C | 85.75B | 88.55A | < 0.001 | 0.975 | < 0.001 |
| 2 | 73.01B* | 88.70A* | 87.87A | ||||
| Egg surface area (cm2) | 1 | 84.50C | 92.04B | 94.00A | < 0.001 | 0.956 | < 0.001 |
| 2 | 82.72B* | 94.11A* | 93.52A | ||||
| Egg shape index (%) | 1 | 74.65A | 73.57B | 72.14C | < 0.001 | < 0.001 | < 0.001 |
| 2 | 73.42A* | 72.38B* | 71.72B* | ||||
A, B values in rows with different letters differ significantly (P ≤ 0.01); a, b- P ≤ 0.05.
*values in the same column with different superscripts differ significantly P < 0.05.
O – origin.
S - season.
In older ducks in all experimental groups the percentage of egg albumen and eggshell decreased (by an average of 0.46 and 0.13% points /p.p., respectively), and the percentage of yolk increased (by an average of 0.53 p.p.). The yolk and shell percentages in the egg were influenced (p < 0.001) by the origin of birds (Table 2).
Table 2.
Percentage of main egg fractions of ducks of the selected breeds in the two seasons of use.
| Item | Season | K-2 | P-9 | LsA | P value | ||
|---|---|---|---|---|---|---|---|
| Effect of origin | Effect of season | Effect of interaction |
|||||
| % of albumen | 1 | 52.58 | 53.20 | 52.95 | 0.014 | 0.241 | 0.071 |
| 2 | 51.87B | 52.68a | 52.80Aa | ||||
| % of yolk | 1 | 36.28A | 35.23B | 35.74 | < 0.001 | 0.013 | 0.187 |
| 2 | 37.23A* | 35.87B | 35.75B | ||||
| % of shell | 1 | 11.04B | 11.57A | 11.45A | < 0.001 | 0.012 | 0.970 |
| 2 | 10.90B | 11.45A | 11.30A | ||||
A, B - values in rows with different letters differ significantly (P ≤ 0.01); a, b…- P ≤ 0.05.
* - values in the same column with different superscripts differ significantly P < 0.05.
O – origin.
S - season.
It was shown that in the first year of laying, regardless of duck origin, albumen weight was higher (p < 0.05), on average by about 1.8 g (Table 3) compared to second year of laying. Regardless of ducks age, the most favourable (p < 0.01) albumen parameters, i.e. the highest albumen height (on average 7.54 mm) and Haugh units (on average 79.26) were characteristic of the eggs of LsA ducks. The albumen pH value in experimental groups of ducks was at a similar level. In the first year of use, it ranged from 8.94 (P-9) to 8.99 (K-2). However, with the age of the birds, the magnitude of this parameter increased (p < 0.05) by 0.03 (K-2), 0.07 (P-9) and 0.06 (LsA). The origin of the ducks affected (p < 0.001) egg white quality parameters, while the year of laying only affected the height of albumen and Haugh units.
Table 3.
Selected egg albumen quality parameters of ducks of selected breeds in two seasons of use.
| Item | Season | K-2 | P-9 | LsA | P value | ||
|---|---|---|---|---|---|---|---|
| Effect of origin | Effect of season |
Effect of interaction |
|||||
| Albumen weight (g) | 1 | 39.68B | 46.78A | 46.83A | < 0.001 | 0.378 | 0.003 |
| 2 | 37.88B* | 45.64A* | 46.55A | ||||
| Albumen height (mm) | 1 | 6.47B | 6.51B | 7.51A | < 0.001 | < 0.001 | 0.019 |
| 2 | 6.80Bb* | 7.09Ab* | 7.57A | ||||
| Haugh units | 1 | 75.06Aa | 72.43Bb | 78.93A | < 0.001 | < 0.001 | 0.128 |
| 2 | 78.45A* | 75.65B* | 79.59A | ||||
| Albumen pH | 1 | 8.99A | 8.94B | 8.95B | < 0.001 | 0.004 | < 0.001 |
| 2 | 9.02* | 9.01* | 9.01* | ||||
A, B… - values in rows with different letters differ significantly (P ≤ 0.01); a, b…- P ≤ 0.05.
* - values in the same column with different superscripts differ significantly P < 0.05.
O – origin.
S - season.
Yolk weight of eggs laid in the first year of laying ranged (p < 0.01) from 27.32 g for K-2 to 31.58 g for LsA (Table 4). Similarly, in the case of yolk pH, there were differences (p < 0.05) in its value between the groups K-2 and P-9 (6.07 and 6.02, respectively) and K-2 and LsA ( 6.07 and 6.20, respectively). Both studied yolk parameters were significantly (p < 0.001) influenced by the origin of the birds. pH value also was affected by the season of use and the interaction between the two analysed factors.
Table 4.
Selected quality parameters of egg yolk from selected ducks breeds in two seasons of use.
| Item | Season | K-2 | P-9 | LsA | P value | ||
|---|---|---|---|---|---|---|---|
| Effect of origin | Effect of season |
Effect of interaction |
|||||
| Yolk weight (g) | 1 | 27.32C | 30.20B | 31.58A | < 0.001 | 0.075 | 0.003 |
| 2 | 27.28B | 31.77A* | 31.41A | ||||
| Yolk pH | 1 | 6.07Ba | 6.02Bb | 6.20A | < 0.001 | < 0.001 | < 0.001 |
| 2 | 6.08A* | 6.06A* | 6.02B* | ||||
A, B… - values in rows with different letters differ significantly (P ≤ 0.01); a, b…- P ≤ 0.05.
* - values in the same column with different superscripts differ significantly P < 0.05.
O – origin.
S - season.
A significant effect (p < 0.001) of origin was shown in the shell characteristics of duck eggs (Table 5). The birds, regardless of their origin, showed a tendency to decrease shell weight (by 0.27 g on average), thickness (by 1.6 μm on average) and density (by 1.4 mg/cm2) with the age of the ducks. Shell porosity and colour were significantly (p < 0.001) affected on the season of duck use and its interaction with the origin of the birds. In the first year of laying, eggshell strength and elasticity differed significantly (p < 0.01) among the three flocks studied. The maximum differences were observed between P-9 and K-2 flocks, being 15.13 N and 6.80 μm, respectively, while in the following year no significant differences were recorded between the P-9 and LsA ducks. The influence of the season of ducks on the formation of the discussed two mechanical characteristics of the shell was not shown.
Table 5.
Selected egg shell quality parameters of ducks of selected breeds in two seasons of use.
| Item | Season | K-2 | P-9 | LsA | P value | ||
|---|---|---|---|---|---|---|---|
| Effect of origin | Effect of season |
Effect of interaction |
|||||
| Shell colour (%) | 1 | 62.45B | 78.18A | 63.76B | < 0.001 | < 0.001 | < 0.001 |
| 2 | 58.54C* | 65.18B* | 73.89A* | ||||
| Shell weight (g) | 1 | 8.31B | 10.15A | 10.14A | < 0.001 | 0.010 | 0.001 |
| 2 | 7.95B* | 9.91A* | 9.93A | ||||
| Shell thickness (µm) | 1 | 365.9C | 412.5A | 400.4B | < 0.001 | 0.439 | 0.896 |
| 2 | 365.4C | 411.1A | 397.5B | ||||
| Shell density (mg/cm2) | 1 | 98.36B | 107.8A | 107.7A | < 0.001 | 0.011 | 0.128 |
| 2 | 96.04B* | 107.6A | 106.0A* | ||||
|
Shell porosity (no. of pores/cm2) |
1 | 15.20A | 15.30A | 12.37B | < 0.001 | < 0.001 | < 0.001 |
| 2 | 18.78A* | 9.75C* | 12.13B | ||||
| Shell strenght (N) | 1 | 37.27C | 52.40A | 46.38B | < 0.001 | 0.498 | 0.273 |
| 2 | 37.51B | 49.39A* | 47.07A* | ||||
| Elastic deformation (µm) | 1 | 70.70A | 63.90C | 67.00B | < 0.001 | 0.114 | < 0.001 |
| 2 | 72.00A* | 66.60B* | 65.30B* | ||||
A, B… - values in rows with different letters differ significantly (P ≤ 0.01); a, b…- P ≤ 0.05.
* - values in the same column with different superscripts differ significantly P < 0.05.
O – origin.
S - season.
The content and hydrolytic activity of lysozyme was significantly (p < 0.001) affected by both the origin, age of the ducks and the interaction between the factors evaluated. Such an effect was shown for thick and thin albumen (Table 6). Regardless of the genotype of birds, albumen fractions showed significantly (p < 0.05) higher content and hydrolytic activity in albumen of eggs from the second year of laying. For the thick albumen fraction, the content of lysozyme and hydrolytic activity were higher in eggs from older birds, with an average of 0.02 p.p. and 2770 U/ml, respectively. The content of lysozyme in thick albumen of eggs from birds from the first year of laying did not differ significantly, ranging from 0.11 for LsA to 0.12% for K-2 and P-9. In contrast, its activity, which ranged from 22,021 (LsA) to 25,352 U/ml (K-2), differed significantly (p < 0.01) between three groups studied. On the other hand, the thin albumen fraction was characterized by higher lysozyme content, which ranged from 0.16 for P-9 and LsA in the first year to 0.19% for K-2 in the second year, and its hydrolytic activity ranged from 33,959 (LsA in the first year) to 40,987 U/ml (K-2 in the second year) compared to thick albumen fraction.
Table 6.
Lysozyme - content and its enzymatic activity in Duck egg albumen of selected breeds in two seasons of use.
| Item | Season | K-2 | P-9 | LsA | P value | ||
|---|---|---|---|---|---|---|---|
| Effect of origin | Effect of season |
Effect of interaction |
|||||
| Thick albumen | |||||||
| Lysozyme content (%) | 1 | 0.12 | 0.11 | 0.11 | < 0.001 | 0.001 | 0.001 |
| 2 | 0.16A* | 0.12B* | 0.12B* | ||||
| Lysozyme activity (U/ml) | 1 | 25,352A | 24,426B | 22,021C | < 0.001 | < 0.001 | 0.001 |
| 2 | 32,944A* | 25,173B* | 22,049C* | ||||
| Thin albumen | |||||||
| Lysozyme content (%) | 1 | 0.18A | 0.16B | 0.16B | < 0.001 | 0.003 | < 0.001 |
| 2 | 0.19a* | 0.18b* | 0.17c* | ||||
| Lysozyme activity (U/ml) | 1 | 39,090A | 34,573B | 33,959B | < 0.001 | < 0.001 | < 0.001 |
| 2 | 40,987A* | 38,655A* | 35,485B* | ||||
A, B… - values in rows with different letters differ significantly (P ≤ 0.01); a, b…- P ≤ 0.05.
* - values in the same column with different superscripts differ significantly P < 0.05.
O – origin.
S - season.
Table 7 shows the results for correlations between individual physical characteristics of eggs, as well as lysozyme content and activity of the duck breeds. Negative correlation coefficients were observed between lysozyme parameters and most of the assessed determinants (parameters of albumen, yolk and eggshell except for Haugh units, albumen pH, shell porosity). They ranged from − 0.303* (yolk weight/lysozyme content) to -0.092 (yolk colour/lysozyme content). In turn, positive correlation coefficients were found between the lysozyme content and its activity and albumen pH (respectively 0.140 and 0.146) and shell porosity (respectively 0.207 and 0.197) and also Haugh unit and lysozyme activity – 0.085.
Table 7.
Selected correlations between individual physical characteristics of the eggs and the lysozyme content and activity of the evaluated Duck breeds.
| Item | Egg weight (g) |
Albumen weight (g) |
Yolk weight (g) |
Shell weight (g) | Albumen height (mm) |
Haugh units | Yolk colour (La Roche scale) |
Albumen pH |
Yolk pH |
Shell thickness (µm) | Shell porosity (pore number/cm2) |
Shell density (mg/cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysozyme content | −0.300* | −0.264* | −0.303* | −0.283* | −0.141 | 0.068 | −0.092 | 0.140 | −0.098 | −0.195 | 0.207 | −0.201 |
| Lysozyme activity | −0.295* | −0.269* | −0.300* | −0.293* | −0.143 | 0.085 | −0.098 | 0.146 | −0.097 | −0.194 | 0.197 | −0.216 |
*Dependencies statistically significant.
Discussion
Kaewmanee et al.25 showed that duck eggs from an indigenous strain of birds from Suratthani province (Thailand) were characterized by albumen, yolk and shell proportions of 54.73%, 33.94% and 10.87%, respectively. On the other hand, Al-Obaidi and Al-Shadeedi26, evaluating the basic morphological composition of Mallard duck and Pekin duck eggs, demonstrated that the percentage of albumen had the values of 55.59 and 55.35%, yolk 33.84 and 32.26%, and shell 10.57 and 12.39%, respectively. These results indicate that the morphological composition of the egg of Pekin ducks depends on the genetic origin of the birds. Our study showed that the genotype of the bird highly significantly influenced the weight of the eggs obtained, their surface area and shape index In the case of productive period, it only affected their shape. Wang et al.26, evaluating the eggs of two purebred ducks (Jinding duck and Shaoxing duck), noted that the eggs of Shaoxing ducks were characterized by a more rounded shape while having a smaller egg surface area (by an average of 1.85 p.p. and 2.82 cm2, respectively) compared to Jinding ducks. In contrast, Galić et al.27, who evaluated physical characteristics of eggs collected from two duck breeds (Pekin and Cherry Valley) found that Cherry Valley duck eggs were significantly heavier (94.23 vs. 71.91 g) than Pekin duck eggs (P < 0.01) and had higher shape index (73.80 vs. 70.16). In our study, in the first laying season, the heaviest eggs (88.55 g) with the most elongated shape (72.14%) were obtained from LsA ducks, and in all genetic groups females laid elongated eggs in the second laying season. A significant effect of age, but within a single reproduction season, on the aforementioned parameters was shown by Kokoszyński et al.29 and Baéza and Huang30. Also Biesiada-Drzazga et al.31, who evaluated the eggs of STAR 53 ducks at four different periods of egg production (6, 12, 18, 24 weeks of laying) in the first year of birds’ life, noted that eggs from the youngest ducks were characterized by the highest weight (95.56 g) and the most rounded shape (72.85%). Convergent results were obtained by Onbaşilar et al.32 and Chepiha et al.2.
Bondoc et al.33, evaluating eggs from 7 mallard breeds (Itik-Pinas, Itik-Pinas Khaki, Kayumanggi, Khaki Campbell, Pekin, Tsaiya, White Mallard), recorded significantly lower values of the tested albumen parameters, which ranged from 23.13 (Tsaiya) to 35.07 g (Pekin) for albumen weight; from 4.09 (White Mallard) to 4.90 mm (Pekin) for albumen height; and from 50.97 (White Mallard) to 55.49 (Pekin) for Haugh units. In contrast, Sumiati et al.34, who evaluated the albumen of eggs from Pajajaran laying ducks between 20 and 26 weeks of age, showed that these eggs were characterized by an albumen of 50.91 g and a high value of Haugh units amounting to 91.99. Lower values of the aforementioned albumen parameters were presented by Ismoyowati et al.35 and Cao et al.1 On the other hand, the study of Biesiada-Drzazga et al.31 involving eggs obtained from four laying periods in the first year of STAR 53 H.Y. ducks showed that the youngest birds laid eggs with the highest albumen weight (51.07 g) and the lowest acidity (8.10). This is consistent with our study, which obtained higher albumen weight with lower acidity from younger ducks from the first laying season. In K-2 and P-9 flocks, all studied albumen parameters in the first and second reproduction seasons were statistically different. However, for LsA birds, such a difference was recorded only for pH. Both our own research and data from the literature indicate that albumen traits are shaped by the genetic background of the birds. However, in the realized experiment, the effect of the season of use of ducks on albumen height and Haugh units was confirmed.
Kokoszyński et al.35, who evaluated the egg yolks of ducks of two strains P11 and P22, showed their differentiation due to the genetic origin of the birds. Namely, eggs obtained from the P22 flock were characterized by higher yolk weight (33.2 g) and higher pH value (6.13). In our study, yolk pH was highest in eggs of LsA ducks in the first laying season (6.20). Varying yolk weight depending on the genetic origin of the birds was also recorded by Galić et al.27 at 25.26 g (Pekin duck) and 31.21 g (Cherry Valley). Also Biesiada-Drzazga et al.31, while evaluating the yolks of STAR 53 H.Y. ducks, showed their weight to increase in successive weeks of laying of the first year of use. The value of this trait ranged from 30.11 (12th week of laying) to 31.29 g (24th week of laying). The author and her team further noted that egg yolks from the youngest ducks (6th week of laying) were characterized by the highest pH value (5.75). Also in our study, we showed higher pH in the first laying season (LsA) and confirmed the effect of genetic origin and season of use on this yolk parameter.
The physical and mechanical characteristics of the eggshell are determined by the influence of many factors, including the age and origin of the birds, the housing system or nutrition[37,37. Other authors also showed the influence of the origin of the ducks on the formation of eggshell weight. The reported values of this parameter varied and ranged from 9.07 (Pekin) to 11.30 g (Cherry Valley)28; from 8.48 (Pekin) to 10.51 g (Khaki Campbell)33; from 8.2 (P22) to 8.4 g (P11)36. Our study confirmed the effect of genetic background on all evaluated shell parameters, as well as the effect of season of use on the formation of colour and porosity and weight and density. Analysing selected parameters of duck eggs36 showed that the shells of P11 strains were significantly (p ≤ 0.05) thicker, less flexible and lighter in colour (0.399 mm, 22.1 μm and 59.0%, respectively) compared to the evaluated shells of P22 duck eggs. In addition, the same author and his team29, when evaluating the shells of P44 ducks, found that the elastic deformation of the shells decreased with the age of the birds (23.2 μm at 44 weeks of age), and the shells were characterized by darker colour. In our study, eggshells with older birds were characterized by lower weight (by an average of 2.83%) and density (by an average of 1.36%). In a study by Galić et al.28 shell thickness ranged from 0.366 (Pekin) to 0.357 mm (Cherry Valley) and in that by Bondoc et al.33, from 0.330 (Pekin) to 0.410 mm (Khaki Campbell and Kayumanggi-IP ducks). The average thickness of the evaluated eggshells in our study was 0.393 mm in the first laying season and 0.391 mm in the following season. The value of this parameter was shaped solely by the origin of the birds. Mazanowski et al.5, when determining the shell parameters of ducks of five strains (A44, A55, P66, P77, K11) found that shells with more pores (25.0/0.25 cm2) and higher density (1.931 g/cm3) were obtained from older ducks. Both studied mechanical characteristics of the shell, i.e. its strength and elastic deformation, were dependent on the genetic origin of the ducks. In an earlier study37, evaluating the crushing strength of shells, showed that the shells of eggs of LsA and P-9 ducks, which were in the 22nd week of egg production in the first year of use, were characterized by a significantly higher value of this parameter (44.50 N and 44.00 N, respectively), compared to the eggshell strength of ducks of the K-2 flock (35.30 N). In turn, in the study of Galić et al.28 the average force required to rupture Cherry Valley duck eggs in all three axes was 50.32 N, which was 18.01% higher than the average force required to rupture Pekin duck eggs (42.64 N). The average breaking strength for Pekin duck eggs has been reported to range from 24.81 to 37.11 N6 and from 28.4 to 35.2 N39. In our study the least strong (37.27 and 37.51 N) but most flexible (70.70 and 72.00 μm) shells were found in K-2 duck eggs.
A review of the international literature shows that relatively few papers have been devoted to determining the level and hydrolytic activity of lysozyme in duck eggs. According to Araki and Torikata40, Wellman-Labadie et al.41 and Kawamura et al.14, the level of these parameters is determined by the origin of the bird, among other factors. Lewko and Gornowicz16 evaluated the level and hydrolytic activity of lysozyme in ducks from three flocks: P-9, K-2 and LsA that were in their final laying period (21–22 weeks) and showed that the most favourable lysozyme parameters (percentage content of 0.20% for thin albumen and 0.14% for thick albumen, hydrolytic activity of 41782 U/ml for thin albumen and 31291 U/ml for thick albumen) were characteristic of the egg whites of K-2 ducks. The same team20, when determining the level and hydrolytic activity of chicken egg white lysozyme, noted that with the age of the hens the value of these parameters increased, reaching a maximum at the end of the laying cycle (0.23% and 48086U/ml, respectively). Also in the discussed study of duck eggs from two years of egg production, there was an effect of origin and season of use and interaction between them on the content and hydrolytic activity of lysozyme, both in thick and in thin albumen. Eggs from older ducks were characterized by higher values of the mentioned parameters. These were the following differences: for enzyme content by 0.02 p.p. in thick albumen and by 0.01 p.p. in thin albumen, and for hydrolytic activity by 2,789 and 2,502 U/ml, respectively. An inverse relationship was presented by Adamski et al.20, who determined the content of the above parameters of lysozyme in the amniotic fluid of White Kołuda® goose eggs in four successive production seasons. The authors proved that the content and activity of lysozyme decreased with age, reaching a minimum in the 4th year of egg production at 36,742 U/ml and 0.173%, respectively.
Conclusions
Research has shown that the age and origin of ducks instantly influenced on the physical and morphological parameters of eggs, as well as the content and activity of lysozyme. The older ducks laid eggs with more elongated shape, less weight, more favourable albumen and yolk quality parameters with worse eggshell quality parameters. Moreover with the age of the birds, there was an increase in the level and hydrolytic activity of lysozyme in the albumen of duck eggs.
Author contributions
L.L.—wrote the main manuscript text and prepared tables. E.G.—reviewed the manuscript and made comments.All the authors read and approved the final manuscript before submission.
Funding
This study was financially supported by the National Research Institute of Animal Production, Poland (subvention number: 01-12-11-11).
Data availability
Data availabilityThe datasets used and/or analyzed during the current study are available from the corresponding author onreasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao, Y., Xun, M., Ren, S. & Wang, J. Effects of dietary organic acids and probiotics on laying performance, egg quality, serum antioxidants and expressions of reproductive genes of laying ducks in the late phase of production. Poult. Sci.101(12), 102189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chepiha, A. M. et al. Monitoring of eggs productivity of the Shaoxing breed ducks of different age. Ser. Technol. Prod. Process. Anim. Husb. Prod.289, 77–85 (2018). Scientific Bulletin of the National University of Life and Environmental Science of Ukraine. [Google Scholar]
- 3.Cherry, P. & Morris, T. R. Domestic Duck Production: Science and Practice 1–251 (CABI, 2008).
- 4.Kuru, B., Kirmizibayrak, T., Cengiz, M. & Ișik, S. The effect of egg weight on egg external quality characteristics and hatching performance in Pekin ducks. Kafkas Univ. Vet. Fak Derg.29(4), 415–422 (2023). [Google Scholar]
- 5.Mazanowski, A., Bernacki, Z. & Kisiel, T. Comparing the structure and chemical composition of Duck eggs. Ann. Anim. Sci.5(1), 53–66 (2005). [Google Scholar]
- 6.Okruszek, A. et al. Effect of laying period and Duck origin on egg characteristics. Arch. Tierz Dummerstorf49, 400–410 (2006). [Google Scholar]
- 7.Nangsuay, A., Ruangpanit, Y., Meijerhof, R. & Attamangkune, S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult. Sci.90, 2648–2655 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Uni, Z., Yadgary, L. & Yair, R. Nutritional limitations during poultry embryonic development. J. Appl. Poult. Res.21, 175–184 (2012). [Google Scholar]
- 9.Narushin, V. G. & Romanov, M. N. Egg physical characteristics and hatchability. J. World’s Poult. Sci58, 297–303 (2002).
- 10.King’Ori, A. M. Review of the factors that influence egg fertility and hatchability in poultry. Int. J. Poult. Sci.10(6), 483–492 (2011).
- 11.Iqbal, J., Khan, S. H., Mukhtar, N., Ahmed, T. & Pasha, R. A. Effects of egg size (weight) and age on hatching performance and chick quality of broiler breeder. J. Appl. Anim. Res.44(1), 54–64 (2016). [Google Scholar]
- 12.Applegate, T. J., Harper, D. & Lilburn, M. S. Effect of Hen production age on egg composition and embryo development in commercial Pekin ducks. Poult. Sci.77, 1608–1612 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Gualhanone, A., Furlan, R. L., Fernandez-Alarcon, M. F. & Macari, M. Effect of breeder age on eggshell thickness, surface temperature, hatchability and chick weigh. Braz J. Poult. Sci.14, 09–14 (2012). [Google Scholar]
- 14.Kawamura, S., Toshima, G., Imoto, T., Araki, T. & Torikata, T. Amino acid residues in subsites e and F responsible for the characteristic enzymatic activity of Duck egg-white lysozyme. J. Biochem.131(5), 663–670 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Krawczyk, J., Lewko, L., Sokołowicz, Z., Koseniuk, A. & Kraus, A. Effect of Hen genotype and laying time on egg quality and albumen lysozyme content and activity. Animals13(10), 1611 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewko, L. & Gornowicz, E. Comparison of the egg white quality of K-2, P-9 and LsA Duck eggs in terms of lysozyme content and activity. Wiad Zoot. 4, 17–24 (2012a). (in Polish). [Google Scholar]
- 17.Wengerska, K., Spustek, D., Krakowiak, D., Drabik, K. & Batkowska, J. Rearing system, utility type and age of hens as factors modifying the hydrolytic activity of lysozyme. Rocz. Nauk. Pol. Tow Zootech17(3), 19–29 (2021). [Google Scholar]
- 18.Banaszewska, D., Biesiada-Drzazga, B., Ostrowski, D., Drabik, K. & Batkowska, J. The impact of breeder age on egg quality and lysozyme activity. Turk. J. Vet. Anim. Sci.43(5), 583–589 (2019). [Google Scholar]
- 19.Krawczyk, J., Lewko, L. & Calik, J. Effect of laying Hen genotype, age and some interior egg quality traits on lysozyme content. Ann. Anim. Sci.21(3), 1119–1132 (2021). [Google Scholar]
- 20.Lewko, L. & Gornowicz, E. The enzymatic activity of lysozyme depending on the age of hens. XXIV International Poultry Science Symposium PB WPSA, Science for poultry practice – poultry practice for science Kołobrzeg, 165 (2012).
- 21.Adamski, M. et al. Effect of Goose age on morphological composition of eggs and on level and activity of lysozyme in Thick albumen and amniotic fluid. Eur. Poult. Sci.80, 148 (2016). [Google Scholar]
- 22.Paganelli, C. V., Olszowka, A. & Ar, A. The avian egg: Surface area, volume, and density. Condor76(3), 319–325 (1974). [Google Scholar]
- 23.Tyler, C. Studies on egg shells. II. A method for marking and counting pores. J. Sci. Food Agric.4(6), 266–272 (1953). [Google Scholar]
- 24.Leśnierowski, G., Yang, T. & Cegielska-Radziejewska, R. Unconventional effects of long-term storage of microwave-modified chicken egg white lysozyme preparations. Sci. Rep.11(1), 10707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaewmanee, T., Benjakul, S. & Visessanguan, W. Changes in chemical composition, physical properties and microstructure of Duck egg as influenced by salting. Food Chem.112(3), 560–569 (2009). [Google Scholar]
- 26.Al-Obaidi, F. A. & Al-Shadeedi, S. M. Comparison study of egg morpho Logy, component and chemical composition of Mallard Duck and domestic Peking Duck. J. Bioinform. 5(4), 555–562 (2016). [Google Scholar]
- 27.Wang, L. C. et al. Geometrical characteristics of eggs from 3 poultry species. Poult. Sci.100(3), 100965 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galić, A. et al. The comparison of quality characteristics of Pekin Duck and Cherry Valley Duck eggs from free-range Raising system. J. Cent. Europ Agric.20(4), 1099–1110 (2019). [Google Scholar]
- 29.Kokoszyński, D., Bernacki, Z. & Korytkowska, H. Eggshell and egg content traits in Peking Duck eggs from the P44 reserve flock Raised in Poland. J. Cent. Europ Agric.8(1), 9–16 (2007). [Google Scholar]
- 30.Baéza, E. & Huang, J. F. Nutritive value of Duck meat and eggs. In: Duck Production and Management Strategies (385–402). Singapore: Springer Nature Singapore (2022). [Google Scholar]
- 31.Biesiada-Drzazga, B., Charuta, A. & Banaszewska, D. Evaluation of particular traits of Pekin Duck breed star 53 of French origin eggs during egg laying. Vet. Ir. Zootech67(89) (2014).
- 32.Onbaşılar, E. E., Erdem, E., Hacan, Ö. & Yalçın, S. Effects of breeder age on mineral contents and weight of yolk sac, embryo development, and hatchability in Pekin ducks. Poult. Sci.2, 473–478 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Bondoc, O. L., Santiago, R. C., Bustos, A. R., Ebron, A. O. & Ramos, A. R. Evaluation of egg characteristics using the size classification and grading system for Mallard Duck eggs. Philipp J. Sci.47(2) (2021).
- 34.Sumiati, S., Darmawan, A. & Hermana, W. Performance and egg quality of laying ducks fed diets containing cassava (Manihot esculenta Crantz) leaf meal and golden snail (Pomacea canaliculata). Trop. Anim. Sci. J.43(3), 227–232 (2020). [Google Scholar]
- 35.Ismoyowati, I., Indrasanti, D., Ratriyanto, A., Egg & Production Egg quality, and fatty acid profile of Indonesian local ducks fed with turmeric, curcuma, and probiotic supplementation. Trop. Anim. Sci. J.45(3), 319–326 (2022). [Google Scholar]
- 36.Kokoszyński, D. Quality of Pekin Duck eggs from conservation flock P11 i P22. Zeszyty Naukowe. Zootechnika. Uniwersytet Technologiczno-Przyrodniczy W Bydgoszczy37 (2009). (in Polish).
- 37.Lewko, L. & Gornowicz, E. Development of strength and elastic deformation of the egg shell of ducks from selected conservation flocks. Wiad Zoot52(1) (2014). (in Polish).
- 38.Mohiti-Asli, M. et al. Effects of feeding regimen, fiber inclusion, and crude protein content of the diet on performance and egg quality and hatchability of eggs of broiler breeder hens. Poult. Sci.91, 3097–3106 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Okruszek, A. et al. Selected egg quality traits of Pekin type ducks from Conservative flocks. Arch. Geflugelkd. 72(6), 269–274 (2008). [Google Scholar]
- 40.Araki, T. & Torikata, T. The amino acid sequence of wood Duck lysozyme. Biosci. Biotechnol. Biochem.63(1), 220–2222 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Wellman-Labadie, O., Picman, J. & Hincke, M. T. Enhanced C-type lysozyme content of wood Duck (Aix sponsa) egg white: An adaptation to cavity nesting? Physiol. Biochem. Zool.81(2), 235–245 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availabilityThe datasets used and/or analyzed during the current study are available from the corresponding author onreasonable request.


