Abstract
A total of 180 one-day-old white-feathered broiler chicks were selected and randomly divided into 4 treatments, namely the control group (CON), Escherichia coli groups (E. coli), 2 g/kg polyherbal mixture group (PHM2), and the 4 g/kg polyherbal mixture group (PHM4). The CON and E. coli groups were fed a basal diet, while the PHM2 and PHM4 groups were fed the basal diet supplemented with 2 g/kg and 4 g/kg PHM, respectively. Each group had 3 replicates, with 15 broilers per replicate. On day 17 of the experiment, broilers in the E. coli, PHM2, and PHM4 groups were intraperitoneally injected with 0.8 mL of 1 × 108 CFU/mL of E. coli O78. Broilers in the control group received an equivalent volume of saline. Chicks were euthanized 48 h postinjection for collecting serum, liver, spleen, jejunum, ileum, ileal mucosa, and cecal contents. Our results showed that PHM significantly reversed the weight loss and decreased the diarrhea rate and the mortality of chicks caused by E. coli infection (P < 0.05). In the serum of chicks infected with E. coli, PHM significantly enhanced the antioxidant capacity (P < 0.05), increased the levels of immunoglobulins and anti-inflammatory cytokines (P < 0.05), and decreased the concentrations of proinflammatory cytokines (P < 0.05). Meanwhile, PHM also promoted the mRNA expression of antioxidant-related genes and decreased the expression of proinflammatory cytokines and apoptosis-related genes in the liver, spleen, jejunum, and ileum (P < 0.05). In addition, PHM repaired the intestinal barrier and injury to further reduce the serum concentrations of d-lactate (DAO) and lipopolysaccharide (LPS) (P < 0.05). More importantly, PHM significantly regulated the composition of cecal microbiota, especially by up-regulating the relative abundance of beneficial bacteria, including Faecalibacterium, Bacteroides, Butyricicoccus, and Lactobacillus, and down-regulating the relative abundance of pathogenic bacteria, including Enterococcus, Escherichia, and Shigella (P < 0.05). These beneficial bacteria were significantly positively correlated with antioxidant capacity and intestinal barrier function, while pathogenic bacteria were significantly positively correlated with proinflammatory cytokines (P < 0.05). In conclusion, PHM may be a potential preventive strategy for E. coli-infected poultry, which is closely related to its modulation of gut microbiota.
Keywords: Polyherbal mixture, Chick, Escherichia coli O78, Immune response, intestinal health
Introduction
Immune stress leads to damage to the intestinal barrier and digestive disorders in poultry, triggering various inflammatory diseases, reducing their production performance, and even leading to death, resulting in huge economic losses (Zhang et al., 2020). Avian pathogenic Escherichia coli (APEC) is the main Gram-negative bacteria responsible for immunosuppressive diseases or environmental stress and is one of the most common pathogens in poultry production, causing sepsis, hemorrhagic sepsis, and enteritis (Kathayat et al., 2021; Ren et al., 2024). Gram-negative bacteria release a large amount of endotoxin (such as LPS) during the propagation and growth of infected hosts, which is an important component of pathogenic microorganisms to damage the body (Park and Lee, 2013). Although E. coli is an extraintestinal pathogen, its colonization, and growth mainly occurs in the intestinal lumen, causing severe diarrhea, reduced growth performance, and feed intake, especially in chicks with insufficient immune function growth and development (La Ragione and Woodward, 2002; Mellata, 2013; Hashem et al., 2022).
The diseases associated with APEC are primarily caused by environmental factors and host susceptibility, resulting in significant economic losses to the poultry industry (Xu et al., 2024). In recent years, herbal products have been used in poultry diets due to their growth-promoting and natural immuno-stimulating effects (Kuralkar and Kuralkar, 2021). Liu et al. showed that adding Chinese herbal medicine to the feed of laying hens improved their production performance, egg quality, antioxidant capacity, immunity, and intestinal health (Liu et al., 2023). Chinese herbal is a strong stimulant of poultry digestion and immunity because of its rich active ingredients, including organic acids, polyphenols, flavonoids, polysaccharides, etc., and has a positive effect on poultry (Liu et al., 2022; Abo Ghanima et al., 2023; Rafeeq et al., 2023). Many studies have shown that Chinese herbal medicine reduced oxidative stress through Nrf2 signaling and inflammation through NF-κB, repaired intestinal barriers through tight junction proteins, and enhanced immunity by reducing apoptosis, which is disrupted in poultry infected with E. coli (Wang et al., 2016; Wan et al., 2021, 2023).
The symbiotic relationship between the microbiota and the host is mutually beneficial, with the gut microbiota assisting the host to absorb nutrients and the host providing nutrients and suitable habitat for the gut microbes (Guo et al., 2022). Gut microbiota forms a multi-layer microbial barrier in the gut, which plays a crucial role in intestinal defense and immune function regulation (Murga-Garrido et al., 2021). Once the body is stimulated, gut microbiota will suffer drastic changes. When poultry is attacked by bacteria, viruses, etc., the integrity of the intestine will be damaged, and intestinal development, digestion, and absorption of nutrients will be affected, accompanied by changes in intestinal microbial composition, which also occurs in E. coli-infected poultry (Gonzalez-Quilen et al., 2020; Li et al., 2024, 2024). More importantly, many traditional Chinese medicines (TCM) have been reported to improve bacterial, viral, and other diseases by regulating gut microbiota (Wu et al., 2018; Li et al., 2022).
In our previous research, a polyherbal mixture (PHM) of five TCMs, including Portulaca oleracea L., Radix Sophorae Flavescentis, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin, significantly improved growth performance, regulated immune function, reduced oxidative stress, and repaired intestinal damage (Liu et al., 2023). Therefore, we wanted to further explore whether PHM alleviated E. coli damage to poultry. In the current study, we investigated the effects of PHM on antioxidant capability, immune response, intestinal health, and gut microbiota composition of chicks infected with E. coli O78. We therefore aimed to further explore the role of the interaction between gut microbiota and antioxidant, anti-inflammatory, and anti-apoptotic in the process of PHM attenuating E. coli injury.
Materials and methods
Preparation and composition of polyherbal mixture
The PHM was prepared according to a previously described method (Liu et al., 2023). PHM is composed of 5 TCMs, including Portulaca oleracea L., Radix Sophorae Flavescentis, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin, in a 1:1:1:1:1 ratio. Then dry, crush, and sieve through an 80-mesh sieve to obtain the final PHM powder. The effective active ingredients and nutritive composition of PHM powder were listed in our previous research (Liu et al., 2023).
Animals and treatments
All experimental protocols were approved by the Animal Care and Use Committee of the South China Agricultural University (approval number: SYXK 2022–0136, Guangzhou, China). One hundred and eighty 1-day-old chicks (Guangming No.2 white-feathered broiler, bred jointly by the Beijing Institute of Animal Husbandry and Veterinary Sciences of the Chinese Academy of Agricultural Sciences and Foshan Xanguang Farming Co., LTD.) were randomly divided into the CON group (basal diet), E. coli group (basal diet), PHM2 group (basal diet supplemented with 2 g/kg PHM), and PHM4 group (basal diet supplemented with 4 g/kg PHM), with 45 chicks per group in three cages, and were given free access to food and water. The E. coli O78 (repository number: CVCC1569; China Veterinary Microorganism Strains Preservation Management Center, Beijing, China) was obtained from cultivating it for 24 h at 37°C in Luria-Bertani (LB) broth (Difco, Sparks, USA). At the age of 17 days, except for the CON group, all the chicks were intraperitoneally injected with E. coli O78 (0.8 mL × 108 CFU/mL), and the experiment was terminated after 48 h.
Sample collection
48 h after infection with E. coli, a 5 mL blood sample was collected from the vein, and the chick was euthanized by cervical dislocation. Six sacrificed experimental chicks from each group were dissected, and the thymus, liver, spleen, and bursa of Fabricius were removed and weighed. Blood samples were centrifuged at 3000 × g for 20 min at 4°C, and serum was collected. A rapid separation of jejunum and ileum was performed, and a 0.5 cm sample of mid jejunum and ileum was placed in 4 % paraformaldehyde for intestinal morphometry. The ileal mucosa was scraped with a sterile scraper, and samples were collected into 2 mL cryotubes and flash-frozen in liquid nitrogen. Serum and frozen mucosa were stored at −80°C for further analysis.
Production performance
Body weight (BW), body weight gain (BWG), and average daily gain (ADG) were analyzed at the end of the trial, along with the incidence of diarrhea and mortality after intraperitoneal injection of E. coli. The fatality rate of the chicks ((number of dead chicks/total number of chicks in each group) × 100 %) was calculated on the nineteenth day.
Serum biochemical parameters
Serum biochemical indexes, including total protein (TP), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and UREA, were determined via the biochemical analytical instrument PUZS-600B (Medical Equipment Co., Ltd., Beijing, China) using respective commercial assay kits (Shenzhen Mindray Biomedical Electronics Co., Ltd., Shenzhen, China).
Determination of antioxidant enzyme activity and immunologic indices
The inflammation-related factors such as interleukin-6 (IL-6), interferon-γ (IFN-γ), interleukin-10 (IL-10), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and caspase 8 were measured in chick serum using the corresponding kit according to the instruction manual of the kit manufacturer (Shanghai Coibo Bio Technology Co., Ltd., Shanghai, China). Serum levels of three factors related to intestinal barrier function: diamine oxidase (DAO), lipopolysaccharide (LPS), and D-lactate (D-LA) were also measured. Jejunum and ileum were homogenized in ice-cold phosphate-buffered saline at a ratio of 1:10 (g/mL) and centrifuged at 3000 × g, 4°C for 10 min.
Histopathological analysis of jejunum and ileum
According to the previous method (Liu et al., 2023), the jejunal and ileal tissues were collected, washed with PBS, fixed with 4 % paraformaldehyde solution, dehydrated with ethanol, embedded in paraffin, sliced, and finally stained with hematoxylin and eosin (H&E).
Tissue RNA extraction and analysis of relative expression levels of genes
Total RNA was extracted from cecal mucosa (50∼100 mg) using a Trizol kit (Invitrogen, Carlsbad), and NanoDrop Lite (ThermoFisher Scientific, Waltham) was employed to measure the RNA concentration. Thereafter, 1 μg of the RNA sample was used to synthesize cDNA using a reverse transcription kit (Vazyme, Nanjing, China). Real-time fluorescence quantitative PCR (qRT-PCR) analysis was conducted on a CFX96 contact real-time PCR detection system (Bio-Rad, Hercules) using a qRT-PCR kit (Vazyme, Nanjing, China), with GAPDH as the internal reference gene. The qRT-PCR premix contained quantitative primers and the cDNA template. The experiment was conducted in triplicate, and the expression level of the target gene was calculated using the delta-delta (2−ΔΔCt) method. Primers for genes related to antioxidants (CAT, SOD1, GSH-Px, Keap-1, Nrf2, and HO-1), immunity (IL-1β, IL-10, IL-6, iNOS, COX-2, TNF-α, TLR4, NF-κB, and MyD88), apoptosis (Bax, Bcl-2, Caspase 3, and Caspase 8), and intestinal barrier function (Claudin-1, Occludin, ZO-1, and Mucin-2) were designed using Primer Premier 6.0 software (Premier Biosoft International, United States) and synthesized by Tsingke Biotechnology Co., Ltd. (Beijing, China) (Table S1).
16S rRNA sequencing and gut microbiota analysis
The fresh cecal digest samples of E. coli-infected chicks in the 4 treatment groups (CON, E. coli, PHM2, and PHM4) were used to evaluate the cecal microflora community (n = 5). The cecal digesta microbiota genomic DNA 16S rRNA v3-v4 region was sequenced using a high-throughput sequencing method (Illumina NovaSeq 6000, 250 PE). Briefly, microbial genomic DNA was extracted from the samples using the E.Z.N.A. DNA Kit (Omega Bio-tek, Norcross, GA), according to the manufacturer's protocol. After the 16S rRNA gene v3-v4 region amplification, the resulting PCR products were extracted and purified. The purified amplicons were then pooled in equimolar amounts and subjected to paired-end sequencing on an Illumina MiSeq platform (Illumina, San Diego). The sequencing process and instruments were provided by Personalbio Technology CO., Ltd. (Shanghai, China). Microbiological data analysis, PICRUSt, and correlation analysis were performed using the software in the cloud platform of Shanghai Maggie Biomedical Technology Co., Ltd. (Shanghai, China). The repository names and accession numbers are listed below: https://www.ncbi.nlm.nih.gov/sra/PRJNA1203957.
Statistical analysis
All data was normalized using SPSS software (version 19.0) and subjected to statistical analysis using one-way analysis of variance (ANOVA). Duncan's multiple comparison test was used to compare differences among the different groups. Data is expressed as the mean ± standard error of the mean (SE). Differences were considered significant at P < 0.05.
Results
Effects of PHM on body weight and mortality rates of chicks
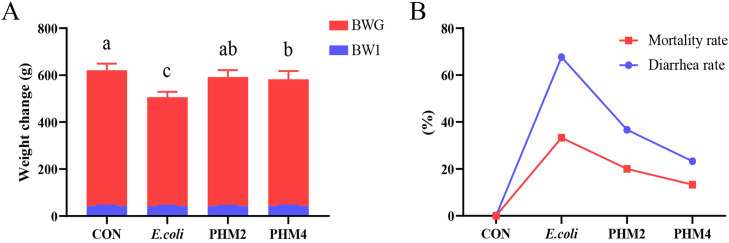
The effect of PHM on clinical symptoms of E. coli infection in chicks, including body weight, mortality rate, diarrhea rate, etc., was monitored (Table 1, Fig. 1). At 19 days of age, which is the endpoint of the experiment, Escherichia coli caused a significant decrease in the weight of the chicks (P < 0.05), and the weight was increased by different doses of PHM in chicks induced by E. coli (P < 0.05). Meanwhile, PHM markedly reversed the ADG in E. coli induced chicks (P < 0.05) (Fig. 1A). BWG also obviously increased in E. coli-infected chick treatment with PHM (P < 0.05). In addition, the mortality and diarrhea rates of chicks infected with E. coli were reduced by PHM (Fig. 1B).
Table 1.
The growth performance of chicks infected with E. coli and fed on PHM at 19 days.
| Items | CON | E. coli | PHM2 | PHM4 | SEM | P-value |
|---|---|---|---|---|---|---|
| BW 1 (g) | 42.87 | 42.57 | 42.75 | 42.83 | 0.383 | 0.994 |
| BW 19 (g) | 621.25a | 537.92c | 592.42ab | 582.50b | 8.542 | 0.001 |
| ADG (g) | 27.54a | 23.59c | 26.17ab | 25.70b | 0.404 | 0.001 |
a-c Means within a row with no common superscript differ significantly (P < 0.05). Values are means (n = 30). Abbreviations: BW 1 and BW 19, body weight in 1-day-old and 19-days-old. ADG, average daily gain.
Fig. 1.
(A) Body-weight changes of chicks fed luteolin for 19 days. (B) The mortality rates (%) and diarrhea rates (%) of chicks in the control group, E. coli group, PHM2 (2 g/kg) group, and PHM4 (4 g/kg) group after E. coli injection. The data are expressed as mean ± SEM. A statistical difference (P < 0.05) was indicated using different letters. BMG, body weight gain. BW 1, 1-day-old weight.
Determination of serum biochemical indicators, antioxidant capacity and caspase 8
The levels of serum biochemical indicators, antioxidants, and caspase 8 were measured in serum and shown in Table 2. The levels of AST, T-AOC, and UREA in serum were not significantly altered, but the levels of ALB, TP, SOD, and GSH-Px were markedly reduced (P < 0.05), while ALT, MDA, and caspase 8 were markedly increased (P < 0.05) in the serum of chicks infected with E. coli. PHM significantly reversed (P < 0.05) the level of ALT, TP, SOD, GSH-Px, MDA, and caspase 8 and decreased UREA (P < 0.05) in E. coli-infected chicks, and the effect of the PHM4 group was better than that of the PHM2 group.
Table 2.
Serum biochemical indices and antioxidant enzyme activity of E. coli-infected chicks fed diets supplemented with PHM.
| Items | CON | E. coli | PHM2 | PHM4 | SEM | P-value |
|---|---|---|---|---|---|---|
| AST (U/L) | 266.60 | 286.60 | 279.40 | 281.16 | 3.675 | 0.275 |
| ALT (U/L) | 4.20c | 6.60a | 5.5ab | 5.12bc | 0.282 | 0.010 |
| ALB (g/L) | 11.06a | 9.04b | 9.24b | 9.72ab | 0.277 | 0.027 |
| TP (g/L) | 29.52a | 25.20b | 28.00a | 28.04a | 0.488 | 0.005 |
| UREA (mmol/L) | 1.48ab | 1.78a | 1.42b | 1.02c | 0.080 | 0.002 |
| SOD (U/mL) | 154.84a | 121.49c | 123.49bc | 149.68ab | 5.297 | 0.029 |
| GSH-Px (U/mL) | 58.04a | 36.85c | 41.36bc | 50.03ab | 2.454 | 0.003 |
| T-AOC (U/mL) | 0.78 | 0.72 | 0.79 | 0.75 | 0.021 | 0.692 |
| MDA (nmol/mL) | 1.89b | 2.73a | 2.26ab | 1.96b | 0.122 | 0.027 |
| Caspase 8 (pmol/L) | 137.59b | 159.34a | 130.27b | 133.07b | 3.467 | 0.003 |
a-c Means within a row with no common superscript differ significantly (P < 0.05). Values are means (n = 6). Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture; AST, aspartate aminotransferase; ALT, alanine transaminase; ALB, albumin; TP, total protein; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; T-AOC, total antioxidant capacity; MDA, malondialdehyde.
Organ indices and determination of SIgA and serum immunoglobulins and cytokines
The effects of PHM on the immune function of chicks infected with E. coli, including organ index, immunoglobulin, and cytokines, were also tested (Table 3). The liver index (P < 0.05) and spleen index (P < 0.05) in E. coli-infected chicks were markedly higher than that in the CON group. PHM treatment markedly reduced liver index (P < 0.05) and spleen index (P < 0.05) and raised bursa index (P < 0.05) in E. coli-infected chicks. After infecting chicks with Escherichia coli, the levels of IgA, IgG, and IL-10 in the serum were significantly reduced (P < 0.05), and the levels of TNF-α and IFN-γ in the serum were significantly increased (P < 0.05). Meanwhile, after supplementing with PHM, the concentrations of IgA, IgG, IL-10, TNF-α, and IFN-γ in the serum were reversed (P < 0.05). In addition, the concentrations of sIgA (P < 0.05) in the jejunum and ileum were also reduced in E. coli-infected chicks and increased by PHM supplementation.
Table 3.
Immune-related indices of E. coli-infected chicks fed diets supplemented with PHM.
| Items | CON | E. coli | PHM2 | PHM4 | SEM | P-value |
|---|---|---|---|---|---|---|
| Liver index (%) | 2.70b | 4.56a | 2.80b | 2.63b | 0.215 | < 0.001 |
| Spleen index (%) | 0.08b | 0.18a | 0.11b | 0.10b | 0.014 | 0.037 |
| Bursa index (%) | 0.18ab | 0.15b | 0.22a | 0.23a | 0.011 | 0.010 |
| Thymus index (%) | 0.33 | 0.23 | 0.32 | 0.28 | 0.015 | 0.075 |
| IgA (μg/mL) | 212.38b | 170.36c | 213.88b | 256.56a | 9.213 | 0.003 |
| IgG (μg/mL) | 354.67b | 360.44b | 378.03ab | 427.93a | 10.431 | 0.037 |
| IgM (μg/mL) | 851.80 | 836.60 | 832.80 | 847.20 | 9.936 | 0.913 |
| IL-1β (pg/mL) | 274.89 | 307.89 | 282.44 | 299.67 | 6.266 | 0.226 |
| IL-10 (pg/mL) | 47.79b | 39.92c | 46.46b | 51.33a | 1.078 | < 0.001 |
| TNF-α (pg/mL) | 45.75b | 52.90a | 38.65c | 37.12c | 1.619 | < 0.001 |
| IFN-γ (pg/mL) | 47.97b | 56.86a | 40.92c | 45.03b | 1.471 | < 0.001 |
| Jejunal sIgA (mg/g protein) |
6.35a | 5.37b | 6.12a | 6.02a | 0.101 | < 0.001 |
| Ileal sIgA (mg/g protein) |
6.06a | 5.53b | 5.88ab | 6.18a | 0.081 | 0.012 |
a-c Means within a row with no common superscript differ significantly (P < 0.05). Values are means (n = 6). Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture; IgA, immunoglobulin A; IgG, immunoglobulin G; IgG, immunoglobulin M; IL-1β, interleukin-1β; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; sIgA, secreted immunoglobulin A.
Effect of PHM on the mRNA expression of antioxidant-related genes in E. coli-infected chicks
The relative mRNA expression of antioxidant-related genes was presented in Fig. 2. Compared with the CON group, the mRNA expression of CAT, GSH-Px, and Nrf2 was reduced (P < 0.05) and Keap-1, COX-2, and INOS were increased (P < 0.05) in the spleen of the E. coli-infected chicks. Unlike the results in the spleen, the expression of CAT and GSH-Px in the liver, CAT and INOS in the jejunum, and CAT, GSH-Px, Nrf2, and INOS in the ileum were not altered by E. coli, but the expression of HO-1 (P < 0.05) in the jejunum was obviously reduced by E. coli. The supplementation of PHM significantly restored the changes in antioxidant-related genes caused by E. coli in the liver, spleen, jejunum, and ileum of chicks (P < 0.05).
Fig. 2.
PHM effects on antioxidant-related gene expression in E. coli-infected chicks. (A-D) The expression levels of antioxidant-related genes (CAT, GSH-Px, SOD1, Keap-1, Nrf2, HO-1, COX-2, and INOS) in the liver, spleen, jejunum, and ileum, respectively. Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture; CAT, catalase; SOD1, copper and zinc superoxide dismutase; GSH-Px, Glutathione peroxidase; Keap-1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor E2-related factor 2; HO-1, heme oxygenase-1. Data are presented as means ± SEM (n = 6). Different letters indicate significant differences in the interaction effect (P < 0.05).
Effect of PHM on the mRNA expression of inflammation-related genes in E. coli-infected chicks
The mRNA expression of inflammation-related genes was also observed in chicks (Fig. 3). The expression of IL-1β and TNF-α in the liver, spleen, jejunum, and ileum (P < 0.05); IL-6 and TLR-4 in the liver, spleen, and jejunum (P < 0.05); TGF-β in the spleen and jejunum (P < 0.05); MyD88 in the jejunum and ileum (P < 0.05); IL-10 and NF-κB in the liver, jejunum, and ileum showed significant differences between the CON group and E. coli groups (P < 0.05). However, PHM treatment markedly reduced the expression of IL-1β, IL-6, TNF-α, TIR4, MyD88, and NF-κB and increased IL-10 and TGF-β in the liver, spleen, jejunum, and ileum of E. coli-infected chicks (P < 0.05).
Fig. 3.
PHM effects on inflammation-related gene expression in E. coli-infected chicks. (A-D) The expression levels of immune-related genes (IL-1β, IL-10, IL-6, INOS, COX-2, TNF-α, TLR4, NF-κB, and MyD88) in the liver, spleen, jejunum, and ileum, respectively. Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture; IL-1β, interleukin-1β; IL-10, interleukin-10; IL-6, interleukin-6; INOS, inducible nitric oxide sythase; COX-2, cyclooxygenase-2; TNF-α, tumor necrosis factor-α; TLR4, toll like receptor 4; NF-κB, nuclear factor kappa B; MyD88, myeloid differentiation factor 88. Data are presented as means ± SEM (n = 6). Different letters indicate significant differences in the interaction effect (P < 0.05).
Effect of PHM on the mRNA expression of apoptosis-related genes in E. coli-infected chicks
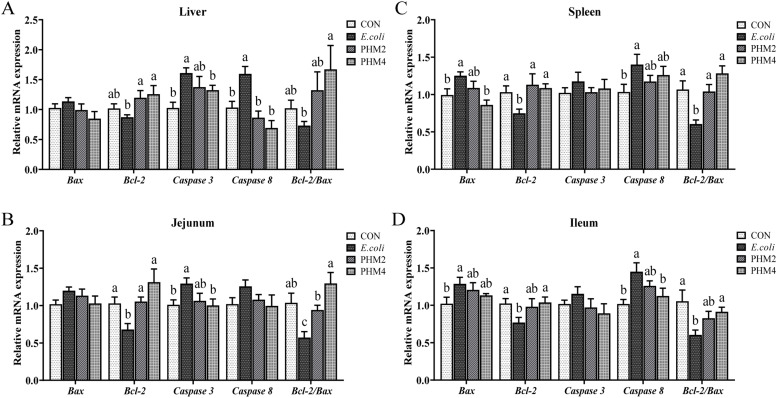
The results of apoptosis-related genes were shown in Fig. 4. Compared with the CON group, chick infection with E. coli increased the expression of Bax (P < 0.05) in the spleen and ileum, caspase 3 (P < 0.05) in the liver and jejunum, and caspase 8 (P < 0.05) in the liver, spleen, and ileum; decreased the expression of Bcl2 (P < 0.05) in the liver, spleen, jejunum, and ileum; and decreased the ratio of Bcl2/Bax (P < 0.05) in the spleen, jejunum, and ileum. PHM significantly restored the expression of Bax, Bcl2, Caspase 3, and Caspase 8 and the ratio of Bcl2/Bax in E. coli-infected chicks (P < 0.05).
Fig. 4.
PHM effects on anti-apoptotic ability in E. coli-infected chicks. (A-D) The expression levels of apoptosis-related genes (Bax, Bcl-2, Caspase 3, and Caspase 8) and the ratio of Bax/Bcl-2 in the liver, spleen, jejunum, and ileum, respectively. Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2. Data are presented as means ± SD (n = 6). Different letters indicate significant differences in the interaction effect (P < 0.05).
Effect of PHM on Intestinal permeability biomarkers and barrier function in E. coli-infected chicks
Intestinal permeability was assessed by measuring the levels of D-lactate, DAO, and LPS in plasma (Fig. 5). Compared with the CON group, the concentration of D-lactate, DAO, and LPS in the serum of the E. coli group was significantly increased. Supplementing PHM markedly decreased the levels of D-lactate, DAO, and LPS in the serum. The H&E staining (Fig. S1) showed a significant difference in intestinal morphology among the four groups. Compared with the CON group, the E. coli group showed significant pathological changes in the jejunal and ileal morphology, including epithelial cell loss and destruction of the villi structure. Compared with the E. coli group, the PHM group obviously decreased the villi structural damage in the jejunum and ileum of broilers infected with APEC. Meanwhile, the barrier function in the jejunum and ileum was also analyzed in Fig. 5. The expression of intestinal barrier-related genes, including claudin-1 (P < 0.05) and occludin (P < 0.05) in the jejunum and ileum and occludin (P < 0.05) in the ileum, was significantly lower in the E. coli group than that in the CON group, whereas it was significantly higher in the PHM group than that in the E. coli group. Meanwhile, PHM significantly upregulated the expression of ZO-1 (P < 0.05) and Mucin-2 (P < 0.05), which were not significantly reduced by E. coli.
Fig. 5.
Effects of dietary PHM supplementation on intestinal permeability biomarkers and barrier function of E. coli-infected chicks. (A) The concentration of DAO, d-lactate, and LPS in serum. (B-C) The mRNA expression of intestinal tight junction proteins (Claudin-1, Occludin, and ZO-1) and Mucin-2 in jejunum and ileum. Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture; DAO, diamine oxidase; LPS, lipopolysaccharide; ZO-1, zonula occludens-1. Data are presented as means ± SEM (n = 6). Different letters indicate significant differences in the interaction effect (P < 0.05).
Microbial diversity of the cecal digesta in E. coli-infected chicks
To explore the role of intestinal microbiota in E. coli-infected chicks with dietary supplementation of PHM, we profiled the composition of intestinal microbiota in all groups using 16S rRNA gene high-throughput sequencing. The effects of PHM supplementation on microbial diversity in the cecal contents of E. coli-infected chicks are shown in Fig. 6. The Venn diagram results showed that there were 5703 OTUs in the CON group, 5161 OTUs in the E. coli group, 5158 OTUs in the PHM2 group, and 6375 OTUs in the PHM4 group. Among them, there were a total of 762 OTUs in the CON group and E. coli group, 722 and 767 OTUs in E. coli group and PHM2 and PHM4 groups, and 902 OTUs in PHM2 and PHM4 groups, and 423 OTUs in these four groups (Fig. 6A). The plot of PCoA showed significant differences between the CON group and E. coli group, while the E. coli group and PHM2 group showed similar distribution trends, but the PCoA distribution in PHM4 showed significant changes (Fig. 6B). The alpha diversity index, including the Chao1 index, Observed-species, Shannon index (P < 0.05), and Simpson index (P < 0.05), was significantly reduced by E. coli, while the supplementation of PHM increased the levels of these indices, especially PHM4 (Fig. 6C).
Fig. 6.
Effects of dietary PHM supplementation on the microbial diversity in the cecal contents of E. coli-infected chicks. (A) Venn diagram of observed taxonomic unit (OTU) levels in cecal contents. (B) β diversity was shown as PCoA analysis. (C) Alpha diversity at the OTU level. Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture. Data are presented as means ± SEM (n = 5). Different letters indicate significant differences in the interaction effect (P < 0.05).
Microbial composition of the cecal digesta in E. coli-infected chicks
To look for species with significant differences in abundance between taxa and species with significant differences between taxa at different levels, we compared the microbial communities of the four experimental groups by LEfSe analysis, and the results are shown in Fig. 7. At the phylum levels, Firmicutes, Bacteroidetes, and Proteobacteria were dominant, while at the genus level, Bacteroidetes, Faecalibacterium, and Ruminococcus dominated in abundance (P < 0.05) (Fig. 7A, C). Chicks infected with E. coli showed a reduction in the relative abundance of Bacteroidetes and Tenericutes and an increase in Proteobacteria, while chicks supplemented with PHM reversed the relative abundance of Bacteroidetes, Proteobacteria, and Tenericutes (P < 0.05) (Fig. 7B). At the genus level, the relative abundance of Bacteroides, Faecalibacterium, Butyricicoccus, Lactobacillus, and Lachnospiraceae-Clostridium was markedly decreased, and Enterococcus, Escherichia, and Shigella were significantly increased in cecal digesta by E. coli (P < 0.05) (Fig. 7D). However, dietary supplementation of PHM restored these changes (P < 0.05) (Fig. 7D). Finally, a linear discriminant analysis (LDA) effect size (LEfSe) analysis was conducted to further identify the enriched microbial community from phylum to genus among four groups with an LDA score >2.5 (Fig. 8A, B). The results of LEfSe analysis showed that some bacterial groups, including the genera Faecalibacterium in the CON group, had a higher score; the phylum Proteobacteria, the class Gammaproteobacteria, the family Enterobacteriaceae, the order Enterobacteriales, and the genera Escherichia in the E. coli group had a higher score, while some other bacterial groups, such as the genera Butyricicoccus in the PHM2 group and the family Lachnospiraceae and the genera Ruminococcus in the PHM4 group, had a higher score.
Fig. 7.
Effects of PHM2 group on the microbial composition in the cecal contents of E. coli-infected chicks. The relative abundance of gut bacteria at the (A) phylum and (C) genus level in different treatments. Analysis of the significant differences in microbiota composition on the (B) phylum and (D) genus level. Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture. Data are presented as means ± SEM (n = 5). Different letters indicate significant differences in the interaction effect (P < 0.05).
Fig. 8.
Changes and functional prediction of cecal microflora in broilers infected with APEC after addition of PHM. (A, B) LEfSe analysis and branching diagram of the evolution scatter plot. (C, D) Analysis of functional prediction between the three groups. Abbreviations: CON, control; E. coli, Escherichia coli; PHM, polyherbal mixture. * represents a significant difference (P < 0.05), ** represents a significant difference (P < 0.01).
Functional prediction of cecal microbiota
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was performed to analyze the possible levels of KEGG pathways (Fig. 8C, D). The prediction analysis identified 16 functional pathways between CON and E. coli groups and 7 functional pathways between E. coli and PHM4 groups. Among the 16 pathways identified, broilers infected with APEC notably increased the microbial gene abundance of membrane transport (bacterial secretion system), lipid metabolism (glycerolipid metabolism and biosynthesis of unsaturated fatty acids), and metabolism of cofactors and vitamins (riboflavin metabolism), while suppressing the microbial gene abundance of energy metabolism (carbon fixation pathways in prokaryotes), glycan biosynthesis and metabolism (N-glycan biosynthesis and other glycan degradation), lipid metabolism (sphingolipid metabolism and linoleic acid metabolism), and metabolism of cofactors and vitamins (thiamine metabolism). The dietary inclusion of PHM4 led to a significant increase in the microbial gene abundance related to the metabolism of cofactors and vitamins (thiamine metabolism) and lipid metabolism (linoleic acid metabolism) across all three levels of the KEGG pathways compared to the E. coli group. Conversely, the PHM4 group resulted in a decrease in the gene abundance associated with membrane transport (bacterial secretion system), lipid metabolism (glycerolipid metabolism), and metabolism of cofactors and vitamins (riboflavin metabolism).
Analysis of key bacterial genera altered by PHM in E. coli-infected chicks
To further explore the relationship between cecum microbiota and PHM treatment of E. coli in chicks, we conducted correlation analysis on changes between cecum microbiota and intestinal anti-inflammatory, antioxidant, and barrier-related functions treated with PHM. Correlation analysis showed stronger correlations between key gut bacterial genera and barrier, anti-inflammatory, and antioxidant functions of the jejunum, whereas Escherichia and Lachnospiraceae-clostridium were more strongly associated with the jejunum, and Butyricicoccus, Bilophila, and Lachnospiraceae-Clostridium were more strongly associated with the ileum (Fig. 9). And Butyricicoccus, Bilophila, Lachnospiraceae-clostridium, and Shigella were the shared key gut bacterial genera associated with the ileum and jejunum. More importantly, the regulation of PHM on the jejunum was positively correlated with the abundance of Lachnospiraceae-clostridium and Butyricicoccus and negatively correlated with Escherichia, Bilophila and Shigella (Fig. 9A), while PHM was positively correlated with Butyricicoccus and Lachnospiraceae-clostridium and negatively correlated with Bilophila and Shigella in the ileum (Fig. 9B). Taken together, these significantly correlated gut microbiota may be the key to improving the anti-inflammatory, antioxidant, and intestinal barrier effects of PHM in chicks infected with E. coli.
Fig. 9.
Correlation of intestinal microbiota with APEC-affected measurements of PHM. (A, B) The dominant differential microbials in the gut were related to the immune indexes, antioxidant-related genes, anti-apoptosis-related genes, and barrier function genes of the jejunum and ileum, respectively. * was judged as a trend with a difference at P < 0.05, ** represents a significant difference at P < 0.01 (n = 5).
Discussion
Pathogenic E. coli is one of the important challenges faced by the broiler farming industry, which seriously inhibits growth performance, reduces immune function and antioxidant capacity, exacerbates intestinal inflammation, and leads to cross-infection of various diseases, reducing economic benefits. Our previous study showed that PHM improved the growth performance, antioxidant capacity, and immune function of broilers (Liu et al., 2023). However, the effect of PHM on E. coli-infected broilers is still unclear. Therefore, in this study, we further investigated whether PHM could also improve the increase in inflammation, decrease in antioxidant capacity, and immunosuppression caused by E. coli in broilers.
In the present study, our results showed that PHM significantly inhibited the weight loss, mortality, and diarrhea rate caused by E. coli. Consistent with our findings, He et al. and Meng et al. showed that E. coli also caused weight loss and increased mortality, which were significantly reversed by the Chinese herbal compound (He et al., 2014; Meng et al., 2024). Consistent with previous studies, chickens infected with E. coli showed elevated spleen and liver organ indexes and abnormal blood biochemical markers related to liver function (Galal et al., 2018; Meng et al., 2024). The addition of PHM to the diet significantly improved the changes caused by E. coli. Immunosuppression is a common disaster in broiler breeding, which is induced by a variety of factors, and E. coli infection also induces and aggravates immunosuppression (Awad et al., 2019). In the present study, PHM significantly improved the concentration of immunoglobulin in serum and up-regulated the content of sIgA in jejunum and ileum, indicating that PHM significantly restored the immunosuppression caused by E. coli. Although it has not been reported that PHM enhances immunity, many studies have shown that Sophora flavescens, the Chinese herbal medicine in PHM, regulates immunity (Zhou et al., 2021).
Oxidative stress, TLR4/Myd88/NF-κB related inflammatory signaling, and apoptosis are important body responses in response to bacterial infection. Early supplementation of PHM in chickens infected with E. coli significantly inhibited inflammatory response, reduced oxidative stress, and reduced cell apoptosis, thereby alleviating the damage caused by E. coli. This is consistent with reports that Portolaca oleracea L. and Sophora flavescens, which are included in PHM, reduce inflammation levels and enhance antioxidant capacity (Wu et al., 2012; Lv et al., 2022; Zhang et al., 2024). Meanwhile, flavonoids and alkaloids from Portolaca oleracea L. and Sophora flavescens have also been shown to have anti-inflammatory and anti-oxidative effects (He et al., 2015; Yoon et al., 2019; Wang et al., 2024). The intestinal barrier, which is composed of intestinal epithelial cells and junctional complexes, is the first line of defense against pathogen invasion (Chen et al., 2021). The destruction of the intestinal barrier increases intestinal permeability, which increases the possibility of invasion by commensal bacteria and antigens, resulting in more inflammation and immunosuppression (Chelakkot et al., 2018; Zhang et al., 2022). Then, DAO, D-LA, and LPS are released into the blood after damage to the intestinal mucosa (Chen et al., 2017). Consistent with this, the present study showed that E. coli disrupted intestinal barrier integrity and increased serum DAO, D-LA, and LPS concentrations in chicks. However, PHM enhances intestinal barrier integrity and reduces intestinal permeability. These results suggested that PHM maintained intestinal barrier integrity disrupted by E. coli in chicks. Consistent with this, studies have reported that other formulations containing Sophora flavescens significantly repair the intestinal barrier (Wu et al., 2021).
Chicken gut microbiota plays an important role in the occurrence and development of diseases. When the gut is destroyed, opportunistic pathogens will proliferate rapidly and further destroy the homeostasis of intestinal flora (Rangan and Hang, 2017). Therefore, to further investigate the in-depth reasons why PHM attenuates E. coli damage, the regulation of intestinal flora by PHM was observed. The abundance of Bacteroidetes, which degrades high molecular weight compounds in the intestine to help the host obtain nutrients from the diet and promote normal gastrointestinal development, was significantly restored by PHM (Tremaroli and Backhed, 2012; Chen et al., 2018). In the present study, the abundance of Proteobacteria increased significantly after E. coli infection, which is consistent with previous reports that Proteobacteria are a marker of gut microbiota imbalance and that PHM significantly reduced its abundance (Shin et al., 2015). At the genus level, the abundance of Faecalibacterium, a butyrate producer and promoter of spermidine production (Zhu et al., 2024), and Bacteroides, Butyricicoccus, and Lactobacillus, which produce butyrate to reduce inflammation, were significantly reduced by E. coli (Shi et al., 2019; Li et al., 2021; Fan et al., 2023). Enterococcus, the natural inhabitants of the gastrointestinal tract, are known as opportunistic pathogens causing severe infections, and Escherichia and Shigella, the main harmful bacteria in the gut, were enriched in the cecum of chicks infected with E. coli (Zhang et al., 2013; Dufossé et al., 2023; Ben et al., 2024). The results of Spearman correlation analysis showed that the key differential bacteria, including Butyricicoccus, Bilophila, Lachnospiraceae-clostridium, Shigella, etc., were closely related to the antioxidant, anti-inflammatory, and intestinal barrier function altered by PHM. The abundance of the beneficial bacteria Butyricicoccus and Lachnospiraceae-clostridium, which have been shown to produce SCFA, was significantly positively correlated with PHM improvement in E. coli-infected chicks (Sorbara et al., 2020). Bilophila has been shown to exert deleterious effects on the gut and exacerbate intestinal inflammation, while Bilophila wadsworthia, belonging to the genus Bilophila, is associated with low degrees of systemic inflammatory disease (Salvado et al., 2024; Gan et al., 2025). Collectively, the effects of PHM on reducing inflammation, enhancing antioxidant capacity and immune function, and restoring intestinal barrier were positively correlated with the abundance of beneficial bacteria and negatively correlated with the abundance of harmful bacteria. Therefore, PHM treats diseases by reducing harmful bacteria in the gut and increasing beneficial bacteria to protect the intestinal barrier and reduce inflammation. This is consistent with previous studies on adjusting the composition of gut microbiota to treat diseases (Ahmed and Al-Massri, 2022; Cai and Kang, 2023). Similarly, gut microbiota also stimulated the production of metabolites by TCM to exert more important biological functions. Oxyberberine, the intestinal secondary metabolite of Sophonae flavescens, alleviates inflammation and maintains intestinal barrier integrity by inhibiting the NF-κB pathway (Li et al., 2020). Therefore, gut microbiota and TCM also have a mutually beneficial relationship, synergistically regulating intestinal homeostasis. KEGG analysis showed that the improvement of E. coli-infected chicks by PHM were related to the reversal of metabolic pathways cofactors and vitamins (thiamine metabolism and riboflavin metabolism) and lipid metabolism (linoleic acid metabolism and glycerolipid metabolism), membrane transport (bacterial secretion system) through differential gut microbiota. Thiamine and riboflavin, whose metabolic pathways are significantly upregulated by PHM, are cofactors of hundreds of enzymes that are essential for cellular and mitochondrial energy metabolism (Takeda and Dai, 2024). The linoleic acid metabolism, as a key pathway for the production of omega-6 fatty acids, is a precursor of the known inflammatory mediator arachidonic acid (Hu et al., 2024). And glycerolipid metabolism was positively correlated with the peroxisomal pathway (Wang et al., 2022). In the present study, PHM significantly reduced the linoleic acid and glycerolipid metabolism, which may be the key to PHM reducing inflammation and enhancing antioxidant function. Bacterial pathogens use secretion systems to secrete effectors into the environment or directly into the cytoplasm of target cells to achieve pathogenicity or survival (Wang et al., 2024). Bacterial pathogens, mainly Gram-negative bacteria, use bacterial secretion system to secrete effectors into the environment or directly into the cytoplasm of target cells to achieve pathogenicity or survival. The reduction of bacterial secretion system by PHM also led to a decrease in the abundance of pathogenic bacteria in the gut, especially Gram-negative bacteria, which was consistent with the reduction of Bilophila and Shigella in the gut by PHM. Taken together, these results indicated that the alleviation of PHM in chicks infected with E. coli was closely related to the gut microbiota, which is consistent with many reports (Lv et al., 2017; Ye et al., 2022).
Conclusions
According to this study, our results showed that PHM significantly reversed the weight loss, diarrhea rate, and mortality in E. coli-infected chicks. PHM significantly enhanced the antioxidant capacity, repaired the immune response and intestinal barrier, and decreased proinflammatory cytokines and apoptosis-related genes in serum and different tissues, respectively. In addition, PHM also repaired intestinal injury to further reduce the serum concentrations of D-lactate, DAO, and LPS. PHM also significantly regulated the composition of cecal gut microbiota, especially the key phylotypes, which were significantly correlated with the improvement of PHM on E. coli-induced chicks. Among them, the antioxidant capacity, intestinal barrier function, and anti-inflammatory effects of PHM were significantly positively correlated with Butyricicoccus, Lachnospiraceae-clostridium, and significantly negatively correlated with Bilophila and Shigella. These findings provide evidence for PHM as a therapeutic strategy for E. coli diseases, but the underlying causes need to be further explored.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Key Research and Development Program (2022YFD1801103) and Key Research and Development Project of Hainan Province (ZDYF2023XDNY067).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2025.105156.
Appendix. Supplementary materials
References
- Abo Ghanima M., Aljahdali N., Abuljadayel D., Shafi M., Qadhi A., Elgammal M., Mohamed L. Effects of dietary supplementation of Amla, Chicory and Leek extracts on growth performance, immunity and blood biochemical parameters of broilers. Ital. J. Anim. Sci. 2023;22(1):24–34. [Google Scholar]
- Ahmed L., Al-Massri K. Gut microbiota modulation for therapeutic management of various diseases: a new perspective using stem cell therapy. Curr. Mol. Pharmacol. 2022;15 doi: 10.2174/1874467215666220222105004. [DOI] [PubMed] [Google Scholar]
- Awad N., Abd E.M., Hashem Y.M., Erfan A.M., Abdelrahman B.A., Mahmoud H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: an in vivo perspective. J. Appl. Microbiol. 2019;127(2):396–405. doi: 10.1111/jam.14303. [DOI] [PubMed] [Google Scholar]
- Ben Y.H., Trabelsi I.., Arous F., Garcia-Vela S., Torres C., Ben S.K. Detection of linezolid and vancomycin resistant Enterococcus isolates collected from healthy chicken caecum. J. Appl. Microbiol. 2024;135(2) doi: 10.1093/jambio/lxae027. [DOI] [PubMed] [Google Scholar]
- Cai Y., Kang Y. Gut microbiota and metabolites in diabetic retinopathy: insights into pathogenesis for novel therapeutic strategies. Biomed. Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114994. [DOI] [PubMed] [Google Scholar]
- Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018;50(8):1–9. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.L., Zheng P.., Zhang C., Yu B., He J., Yu J., Luo J.Q., Mao X.B., Huang Z.Q., Chen D.W. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101(6):1137–1146. doi: 10.1111/jpn.12627. [DOI] [PubMed] [Google Scholar]
- Chen S., Zheng Y., Ran X., Du H., Feng H., Yang L., Wen Y., Lin C., Wang S., Huang M., Yan Z., Wu D., Wang H., Ge G., Zeng A., Zeng Y.A., Chen J. Integrin alphaEbeta7(+) T cells direct intestinal stem cell fate decisions via adhesion signaling. Cell Res. 2021;31(12):1291–1307. doi: 10.1038/s41422-021-00561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.M., Chang W.., Zheng A.J., Zhang S., Cai H., Liu G. Comparison of gut microbial diversity in Beijing oil and arbor acres chickens. Revista Brasileira de Ciência Avícola. 2018;20:37–44. [Google Scholar]
- Dufossé M., Lima J., Sampaio A., Rosa A., Silva J.B., Moraes C., Roos T. Molecular identification of pathogenic Escherichia coli virulence genes and histopathological analysis in chickens condemned for airsacculitis. Ciência Rural. 2023;53(11) [Google Scholar]
- Fan Y., Ju T., Bhardwaj T., Korver D.R., Willing B.P. Week-old chicks with high Bacteroides abundance have increased short-chain fatty acids and reduced markers of gut inflammation. Microbiol. Spectr. 2023;11(2) doi: 10.1128/spectrum.03616-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galal H.M., Tawfek A..M., Abdrabou M.I., Hessain A.M., Alhaaji J.H., Kabli S.A., Elbehiry A., Alwarhi W.K., Moussa I.M. Recent approaches for control of E. coli and respiratory complex in Middle East. Saudi. J. Biol. Sci. 2018;25(7):1302–1307. doi: 10.1016/j.sjbs.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan F., Luo S., Wei X., Zuo S. First case report of bacteremia caused by Bilophila wadsworthia in China, and literature review. BMC Infect. Dis. 2025;25(1):168. doi: 10.1186/s12879-025-10570-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Quilen C., Rodriguez-Gallego E., Beltran-Debon R., Pinent M., Ardevol A., Blay M.T., Terra X. Health-promoting properties of proanthocyanidins for intestinal dysfunction. Nutrients. 2020;12(1):130. doi: 10.3390/nu12010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Okpara E.S., Hu W., Yan C., Wang Y., Liang Q., Chiang J., Han S. Interactive relationships between intestinal flora and bile acids. Int. J. Mol. Sci. 2022;23(15):8343. doi: 10.3390/ijms23158343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem M.A., Hassan A.., Abou-Elnaga H., Abdo W., Dahran N., Alghamdi A.H., Elmahallawy E.K. Modulatory effect of dietary probiotic and prebiotic supplementation on growth, immuno-biochemical alterations, DNA damage, and pathological changes in E. coli-infected broiler chicks. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.964738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Fu B., Yi P., Wei X., Yin Z., Lv C., Zhang W., Han X., Cun X. Effectiveness of Xiang-Qi-Tang against avian pathogenic Escherichia coli. Pak. Vet. J. 2014;34:127–129. [Google Scholar]
- He X., Fang J., Huang L., Wang J., Huang X. Sophora flavescens ait.: traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015;172:10–29. doi: 10.1016/j.jep.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhou L., Yang J., Bai R., Marchioni E., Zhao M., Zhou L. Anti-inflammatory mechanism of Houttuynia cordata polysaccharides against ulcerative colitis based on multi-omics conjoint analysis. Int. J. Biol. Macromol. 2024;283(Pt 1) doi: 10.1016/j.ijbiomac.2024.137311. [DOI] [PubMed] [Google Scholar]
- Kathayat D., Lokesh D., Ranjit S., Rajashekara G. Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors. Zoonotic Poten., Cont. Strateg.. Pathog. 2021;10(4):467. doi: 10.3390/pathogens10040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuralkar P., Kuralkar S.V. Role of herbal products in animal production - an updated review. J. Ethnopharmacol. 2021;278 doi: 10.1016/j.jep.2021.114246. [DOI] [PubMed] [Google Scholar]
- La Ragione R.M., Woodward M.J. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res. Vet. Sci. 2002;73(1):27–35. doi: 10.1016/s0034-5288(02)00075-9. [DOI] [PubMed] [Google Scholar]
- Li A., Ding J., Shen T., Han Z., Zhang J., Abadeen Z.U., Kulyar M.F., Wang X., Li K. Environmental hexavalent chromium exposure induces gut microbial dysbiosis in chickens. Ecotoxicol. Environ. Saf. 2021;227 doi: 10.1016/j.ecoenv.2021.112871. [DOI] [PubMed] [Google Scholar]
- Li C., Ai G., Wang Y., Lu Q., Luo C., Tan L., Lin G., Liu Y., Li Y., Zeng H., Chen J., Lin Z., Xian Y., Huang X., Xie J., Su Z. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kappaB pathway. Pharmacol. Res. 2020;152 doi: 10.1016/j.phrs.2019.104603. [DOI] [PubMed] [Google Scholar]
- Li P., Zhao S., Teng Y., Han S., Yang Y., Wu M., Guo S., Ding B., Xiao L., Yi D. Dietary supplementary with ellagic acid improves the intestinal barrier function and flora structure of broiler chicken challenged with E. coli K88. Poult. Sci. 2024;103(12) doi: 10.1016/j.psj.2024.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Li W., Zhao Q., Wu P., Huang X., Jin W., Wang B., Li S., Liu W., Zhang G., Kang X. Combined analysis of the microbiome, metabolome and transcriptome of silkie chickens in response to avian pathogenic E. coli (APEC) Microb. Pathog. 2024;189 doi: 10.1016/j.micpath.2024.106586. [DOI] [PubMed] [Google Scholar]
- Li X., Wang M., Liu C., Xiao Y., Li M., Ban C., Huang Y., Cheng M., Song L., Liu G., Lu S., Wang C., Ren Z. Qingfeiyin decoction inhibits H1N1 virus infection via modulation of gut microbiota and inflammatory pathways in a Murine model. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.874068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Ma R., Yang Q., Yang Y., Fang Y., Sun Z., Song D. Effects of traditional Chinese herbal feed additive on production performance, egg quality, antioxidant capacity, immunity and intestinal health of laying hens. Animals (Basel) 2023;13(15):2510. doi: 10.3390/ani13152510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wang S., Xiang Z., Xu T., He M., Xue Q., Song H., Gao P., Cong Z. The chemistry and efficacy benefits of polysaccharides from atractylodes macrocephala koidz. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.952061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhou J., Li Y., Ding Y., Lian J., Dong Q., Qu Q., Lv W., Guo S. Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers. Poult. Sci. 2023;102(7) doi: 10.1016/j.psj.2023.102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W.J., Huang J..Y., Li S.P., Gong X.P., Sun J.B., Mao W., Guo S.N. Portulaca oleracea L. extracts alleviate 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.986943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W., Liu C., Ye C., Sun J., Tan X., Zhang C., Qu Q., Shi D., Guo S. Structural modulation of gut microbiota during alleviation of antibiotic-associated diarrhea with herbal formula. Int. J. Biol. Macromol. 2017;105(Pt 3):1622–1629. doi: 10.1016/j.ijbiomac.2017.02.060. [DOI] [PubMed] [Google Scholar]
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013;10(11):916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Wang W., Ding J., Gu B., Zhou F., Wu D., Fu X., Qiao M., Liu J. The synergy effect of matrine and berberine hydrochloride on treating colibacillosis caused by an avian highly pathogenic multidrug-resistant Escherichia coli. Poult. Sci. 2024;103(10) doi: 10.1016/j.psj.2024.104151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murga-Garrido S.M., Hong Q.., Cross T.L., Hutchison E.R., Han J., Thomas S.P., Vivas E.I., Denu J., Ceschin D.G., Tang Z.Z., Rey F.E. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome. 2021;9(1):117. doi: 10.1186/s40168-021-01061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.S., Lee J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013;45(12):e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafeeq M., Bilal R., Batool F., Yameen K., Farag M., Madkour M., Elnesr S., El-Shall N., Dhama K., Alagawany M. Application of herbs and their derivatives in broiler chickens: a review. World's. Poul. Sci. J. 2023;79:1–23. [Google Scholar]
- Rangan K.J., Hang H.C. Biochemical mechanisms of pathogen restriction by intestinal bacteria. Trends. Biochem. Sci. 2017;42(11):887–898. doi: 10.1016/j.tibs.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Cao Q., Ye H., Dong Z., Zhang C., Feng D., Zuo J., Wang W. Supplemental xylooligosaccharide attenuates growth retardation and intestinal damage in broiler chickens challenged by avian pathogenic Escherichia coli. Agriculture. 2024;14:1684. [Google Scholar]
- Salvado R., Santos-Minguez S., Lugones-Sanchez C., Gonzalez-Sanchez S., Tamayo-Morales O., Quesada J., Benito R., Rodríguez-Sánchez E., Gomez-Marcos M., Guimaraes P., Hernandez-Rivas J., Mira A., Garcia-Ortiz L. Gut microbiota and its relationship with early vascular ageing in a Spanish population (MIVAS study) Eur. J. Clin. Invest. 2024;54 doi: 10.1111/eci.14228. [DOI] [PubMed] [Google Scholar]
- Shi D., Bai L., Qu Q., Zhou S., Yang M., Guo S., Li Q., Liu C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019;98(6):2405–2413. doi: 10.3382/ps/pez026. [DOI] [PubMed] [Google Scholar]
- Shin N.R., Whon T..W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Sorbara M.T., Littmann E..R., Fontana E., Moody T.U., Kohout C.E., Gjonbalaj M., Eaton V., Seok R., Leiner I.M., Pamer E.G. Functional and genomic variation between Human-derived isolates of lachnospiraceae reveals inter- and intra-species diversity. Cell Host. Microbe. 2020;28(1):134–146. doi: 10.1016/j.chom.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Dai P. Functional roles of pantothenic acid, riboflavin, thiamine, and choline in adipocyte browning in chemically induced human brown adipocytes. Sci. Rep. 2024;14(1) doi: 10.1038/s41598-024-69364-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Wan J., Zhang J., Xu Q., Yin H., Chen D., Yu B., He J. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury. Carbohydr. Polym. 2021;270 doi: 10.1016/j.carbpol.2021.118316. [DOI] [PubMed] [Google Scholar]
- Wang C., Guo S., Zhao Y., Wang J., Yang Y., Ying X. Two novel alkaloids from Portulaca oleracea L. and their anti-inflammatory bioactivities. Nat. Prod. Res. 2024;0(0):1–8. doi: 10.1080/14786419.2024.2303597. [DOI] [PubMed] [Google Scholar]
- Wang L., Gou X., Ding Y., Liu J., Wang Y., Wang Y., Zhang J., Du L., Peng W., Fan G. The interplay between herbal medicines and gut microbiota in metabolic diseases. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1105405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Mu L., Yu C., He Y., Hu X., Jiao Y., Xu Z., You S., Liu S.L., Bao H. Microbial collaborations and conflicts: unraveling interactions in the gut ecosystem. Gut. Microbes. 2024;16(1) doi: 10.1080/19490976.2023.2296603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li Z., Han Q., Guo Y., Zhang B., D'Inca R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 2016;116(11):1878–1888. doi: 10.1017/S0007114516004116. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhang Z., Zhang K., Zhou Q., Chen S., Zheng H., Wang G., Cai S., Wang F., Li S. Multi-omics characterization of a glycerolipid metabolism-related gene enrichment score in colon cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.881953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Chen Q.Y., Wang W.Z., Chu S., Liu X.X., Liu Y.J., Tan C., Zhu F., Deng S.J., Dong Y.L., Yu T., Gao F., He H.X., Leng X.Y., Fan H. Compound sophorae decoction enhances intestinal barrier function of dextran sodium sulfate induced colitis via regulating notch signaling pathway in mice. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110937. [DOI] [PubMed] [Google Scholar]
- Wu T., Lin C., Chang C., Lin T., Martel J., Ko Y., Ojcius D., Lu C., Young J., Lai H. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2018;68:2017. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- Wu Y., Gong Q., Fang H., Liang W., Chen M., He R. Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish. Shellfish. Immunol. 2012;34(1):220–227. doi: 10.1016/j.fsi.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Xu P., Wang J., Chen P., Ding H., Wang X., Li S., Fan X., Zhou Z., Shi D., Li Z., Cao S., Xiao Y. Effects of pomegranate (Punica granatum L.) peel on the growth performance and intestinal microbiota of broilers challenged with Escherichia coli. Poult. Sci. 2024;103(2) doi: 10.1016/j.psj.2023.103304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C., Qu Q., Bai L., Chen J., Cai Z., Sun J., Liu C., Shi D. Effect of traditional Chinese medicine on the gut microbiota in heat-stressed laying hens. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.905382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Lim C., Cha D.S., Han Y. Synthesis and evaluation of the lifespan-extension properties of Oleracones D–F. Antioxidative Flavonoids Portulaca Oleracea L. Applied Sciences. 2019;9:4014. [Google Scholar]
- Zhang H., Wang R., Bao H. Phage inactivation of foodborne Shigella on ready-to-eat spiced chicken. Poult. Sci. 2013;92(1):211–217. doi: 10.3382/ps.2011-02037. [DOI] [PubMed] [Google Scholar]
- Zhang H., Chen Y., Chen Y., Li Y., Jia P., Ji S., Zhou Y., Wang T. Dietary pterostilbene supplementation attenuates intestinal damage and immunological stress of broiler chickens challenged with lipopolysaccharide. J. Anim. Sci. 2020;98(1):skz373. doi: 10.1093/jas/skz373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Yang J., Wang Q., Hu D., Zhao Q., Zhu S., Qiao Y., Zhao F., Wang Z., Wang J., Yu Y., Han H., Hao L., Dong H. Comparative efficacy of plant extracts and probiotics on growth and gut health in chickens with necrotic enteritis. Animals (Basel) 2024;14(22):3312. doi: 10.3390/ani14223312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gan Y., Wang J., Feng Z., Zhong Z., Bao H., Xiong Q., Wang R. Dysbiosis of gut microbiota and intestinal barrier dysfunction in pigs with pulmonary inflammation induced by Mycoplasma hyorhinis infection. mSystems. 2022;7(4) doi: 10.1128/msystems.00282-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.H., Yang J..Y., Ding H.Y., Chen Q.P., Tian E.J., Wang H.W. Anticoccidial effect of toltrazuril and Radix Sophorae Flavescentis combination: reduced inflammation and promoted mucosal immunity. Vet. Parasitol. 2021;296 doi: 10.1016/j.vetpar.2021.109477. [DOI] [PubMed] [Google Scholar]
- Zhu L.A., Li D.., Cheng Z., Wen Q., Hu D., Jin X., Liu D., Feng Y., Guo Y., Cheng G., Hu Y. Enzymatically prepared neoagarooligosaccharides improve gut health and function through promoting the production of spermidine by faecalibacterium in chickens. Sci. Total. Environ. 2024;912 doi: 10.1016/j.scitotenv.2023.169057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.