Abstract
Background
This report examines how European geriatricians understand the concept of ‘cognitive frailty’, which was first formally defined by the International Academy on Nutrition and Aging (IANA) and the International Association of Gerontology and Geriatrics (IAGG) in 2013.
Methods
An online survey about delirium, dementia and frailty relationships and pathways was distributed across Europe through appropriate professional groups. Eligible participants were geriatricians or trainees in their final two years of specialist geriatric training, in a European country. Snowball sampling was used. In total, 440 people replied to the survey, of which 324 responded to the section on cognitive frailty. Respondents were predominantly female and there was a marked under-representation of Eastern European participants.
Results
From a list of possible definitions, only one in four of the 324 respondents identified cognitive frailty as defined by the IANA and the IAGG, i.e., a combination of physical frailty and mild cognitive impairment. Almost two thirds of those who stated that they currently use the term in their work did not choose the IANA-IAGG definition. After the definition was shared with respondents, only 44% strongly agreed with it as an apt description of cognitive frailty, with some considering it too narrow (by omitting delirium and dementia) while others considered it too broad (by including physical frailty).
Conclusions
There is no clear consensus opinion among geriatricians in Europe on the definition of ‘cognitive frailty’. While there is some core support for the IANA-IAGG definition, it is not intuitive to those not already familiar with the term. The variance in the current understanding of cognitive frailty among geriatricians suggests the time is right for a meaningful debate on this issue. While there is ongoing, growing research on a shared pathophysiology between physical frailty and cognitive impairment, further studies are required to evaluate the added benefit of this particular conceptual theorization in older persons care rather than its single components, and if beneficial, how awareness, understanding and correct usage of the concept can be improved.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-025-05930-9.
Keywords: Cognitive frailty, Frailty, Cognitive impairment, Delirium, Dementia
Background
The term ‘cognitive frailty’ first appeared in a 2001 US study exploring clock-drawing for measuring cognitive function [1]. A few years later, Panza et al. (2005) used the term when assessing mild cognitive impairment (MCI) and vascular risk factors [2]. The first formal definition of cognitive frailty, from the International Academy on Nutrition and Aging (IANA) and the International Association of Gerontology and Geriatrics (IAGG) in 2013, had three diagnostic criteria: i) physical frailty, defined by Fried’s phenotype [3], ii) cognitive impairment (CI) with a clinical dementia rating (CDR) of 0.5, i.e. MCI [4], and iii) no Alzheimer’s or other dementias [5]. In 2018, alterations were proposed to address the challenge of using CDR in epidemiologic studies and clinical settings: cognitive test scores more than 1.5 standard deviations below the mean for age-, gender- and education- adjusted norms, and independence for instrumental activities of daily living [6].
Since this formal definition, publications on cognitive frailty have increased [7], accompanied by some proposals to expand the definition to include social, nutritional, psychological and biopsychosocial domains [8–11]. Two cognitive frailty subtypes have been proposed: potentially reversible cognitive frailty (physical frailty and MCI) and reversible cognitive frailty (physical frailty and “pre-MCI subjective cognitive impairment”) [12].
The IANA-IAGG definition evolved from evidence that CI and physical frailty often coexist [13]. A four-year study of 6,030 community-dwelling older adults found CI in 22% of frail people [14]. Physical frailty is associated with faster cognitive decline and higher risk of incident cognitive disorders [15–17]. Slower gait speed predicts CI; lower grip strength is associated with greater risk of cognitive decline and dementia; and CI is a significant predictor of reduced muscular strength [18, 19]. The link between cognitive and physical functions is thus bidirectional; each can influence and accelerate the other [20, 21]. There is increasing research on mutual pathophysiology and shared biological markers between physical frailty and CI [21–23].
The aim of this study was to investigate European geriatricians’ understanding of the term ‘cognitive frailty’ and their agreement with the IANA-IAGG definition of this. Standardised terminology in clinical settings helps healthcare providers communicate efficiently and accurately, thereby contributing to improved patient care [24].
Methods
Data was collected using an online Qualtrics survey form as part of a broader, anonymous survey of European geriatricians’ views on frailty, delirium and dementia. The survey was piloted in English and refined, and then translated into 11 European languages. The survey (ref. supplementary material) was available via an embedded link to a project website, hosted by University College Cork, Ireland. Respondents were requested to provide answers in English only and participation was voluntary. Eligible participants were geriatricians, or trainees in their final two years of specialist geriatric training, in a European country. Physicians with a special interest in geriatric medicine were eligible in countries without this discipline. The survey was distributed to all members of the European Geriatric Medicine Society (EuGMS), with EuGMS members asked to respond and share the link with colleagues and trainees. The survey was also promoted by EuGMS members through their national geriatric professional bodies.
Responses were gathered from September 2023 to June 2024. Quantitative data was analysed in Excel, with descriptive statistics displayed as the number of participants endorsing a particular response and/or percentages, and frequency distributions. One question required respondents to answer using a scale of 0–10, with 10 indicating ‘very strongly agree’ and 0 indicating ‘very strongly disagree’. Content analysis was used to analyse and interpret the responses from open-text boxes, categorising them into groups and then counting their associated frequency. Ethical approval was obtained from the Social Research Ethics Committee in University College Cork, Ireland. The survey did not collect personal data and IP addresses were not recorded.
Results
Understanding of the term ‘cognitive frailty’
Respondent demographics (n = 440) are outlined in the supplementary material and detailed elsewhere [25]. Participants spanned 30 countries (under-representing Eastern Europe) and with a 2:1 female/male ratio and a 5:1 consultant/trainee ratio. Participants who responded to this part of the survey (n = 324) first selected their familiarity with or use of the term ‘cognitive frailty’, from seven possible answers (Fig. 1). Some used it frequently (15%) or occasionally (14%) in work; 23% were ‘pretty sure they would recognise a definition’ and 21% believed they ‘might’ recognise it. In contrast, 17% had not heard the term before, while 8% could not recall the details. Two people were involved in defining the term.
Fig. 1.
Self-assessed familiarity with ‘cognitive frailty’ (purple bar) and respondents’ selection of IANA-IAGG definition (green bar)
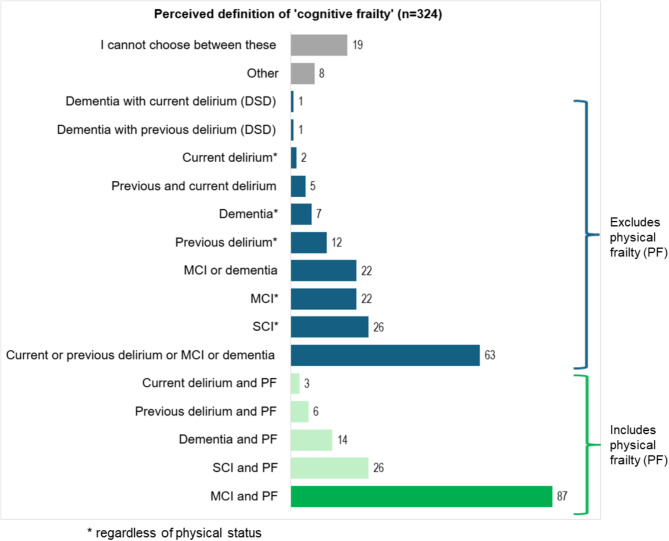
Participants were then asked for their opinion (without checking the literature) on which term best matches cognitive frailty as described in academic literature, regardless of whether they agreed with the definition. Figure 2 shows their selection within the 17 offered terms: subjective cognitive impairment (SCI) plus physical frailty; SCI (any physical status); MCI plus physical frailty; MCI (any physical status); dementia plus physical frailty; dementia (any physical status); MCI OR dementia; dementia with previous superimposed delirium (DSD); dementia with current DSD; previous delirium plus physical frailty; previous delirium (any physical status); current delirium plus physical frailty; current delirium (any physical status); previous delirium plus current delirium; current or previous delirium OR MCI OR dementia; other (please specify); or ‘cannot choose’.
Fig. 2.
Potential ‘cognitive frailty’ definitions selected by respondents; the dark green bar is the IANA-IAGG definition (n = 87)
While the most popular answer (26.8%) was the IANA-IAGG definition (MCI plus physical frailty), almost as many (19.6%) believed the term related to current or previous delirium or MCI or dementia, in other words broad cognitive vulnerability, without physical frailty necessarily. Other common answers were SCI plus physical frailty (8.1%); SCI (8.1%); MCI (6.9%); and MCI or dementia (6.2%). Overall, 46% of respondents selected one of the five options which included physical frailty, while 54% selected one of the ten options without physical frailty.
Of those who had heard the term before and were ‘pretty sure’ of or ‘might’ recognise a definition of it, only 33% chose the IANA-IAGG definition (See Fig. 1). Moreover, only 26.3% of respondents who stated that they frequently or occasionally use the term in work selected the IANA-IAGG definition. For those naïve to the term, or who could not recall it, only 15% selected the IANA-IAGG definition.
Agreement with the IANA-IAGG definition
The IANA-IAGG definition of cognitive frailty was then shared with respondents, who were asked to rate, on a scale of 0–10, their agreement with it. 14 respondents selected the option unsure, one of whom had selected the IANA-IAGG definition. The results of the remaining 301 respondents who selected a scale are shown in Fig. 3. Clustering these into three approximately equal intervals, 44% agreed (7–10 scale), 29% were neutral (4–6 scale), and 27% disagreed (0–3 scale) with the IANA-IAGG definition. Figure 3 also shows that most of the respondents who selected the IANA-IAGG definition agreed with it, compared to those not selecting it. However, of respondents who selected the IANA-IAGG definition, 28% either disagreed or were neutral.
Fig. 3.
Respondents’ level of agreement with the IANA-IAGG definition of cognitive frailty (orange bars represent the whole group, n = 301; green bars demonstrate the spread of agreement for respondents who correctly selected the IANA-IAGG definition, n = 86)
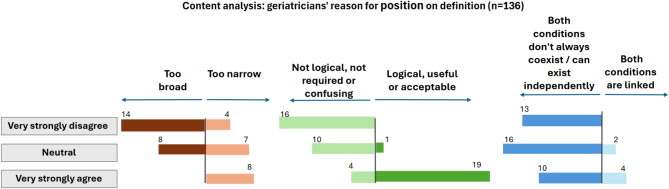
To explore the geriatricians’ reasons for their position on the IANA-IAGG definition of cognitive frailty, content analysis was performed on respondents’ explanations of their agreement or disagreement (Fig. 4). Responses (n = 152) to the open-ended question of why they selected a particular level of agreement in Fig. 3 were coded and grouped as follows in descending order: (i) Both conditions (physical frailty and MCI) don’t always coexist / can exist independently (26%); (ii) ‘Not logical, not required or confusing’ (20%); (iii) Too broad (14%); (iv) Too narrow (12.5%); (v) ‘Logical, useful or acceptable’ (12.5%); vi); ‘Other’ (10.5%); vii) Both conditions are linked (4%). Figure 4 shows how the responses (n = 136, excluding the ‘Other’ category) relate to the geriatricians’ agreement, neutrality or disagreement with cognitive frailty being ‘MCI and physical frailty’. The respondents considering the term too broad generally felt that it should only relate to CI, e.g. “Cognitive is cognitive, we usually separate these frailties”. Those viewing the term as too narrow wanted conditions such as dementia and delirium included, e.g. “Delirium and dementia… are part of cognitive frailty”.
Fig. 4.
Content analysis on geriatricians’ rationale for position on IANA-IAGG definition of cognitive frailty
Discussion
Among geriatricians surveyed across Europe there is a large variation in the recognition of, and agreement with, the IANA-IAGG definition of cognitive frailty. Only 26.8% selected the IANA-IAGG definition as their understanding of the term, although having multiple similar options may have been confusing. However, once presented with the definition, only 43% of respondents agreed with it.
Perhaps the variance is not surprising, as there is no agreed single operational definition for frailty [26, 27]. Some respondents believed that ’cognitive frailty’ should include dementia and delirium, mirroring previous support to include other domains and sub-types [8–12]. Others believed cognitive frailty should refer to cognitive decline only.
The IANA-IAGG definition may not be intuitive, as 85% of respondents naïve to the term, or who could not recall it, did not select it from the list of possible definitions. Equally, 74% of those using the term frequently or occasionally in their work did not select it. Some respondents found the IANA-IAGG definition not logical, not required or confusing. Grammatically, cognitive frailty suggests frailty that is cognitive, since cognitive is an adjective which modifies the noun frailty. This is similar to ‘cognitive reserve’ meaning a reserve of cognition [28], and ‘cognitive impairment’ meaning impairment in cognition. The linguistically logical definition of “frailty which is cognitive” also aligns with the earlier use of the phrase [1, 2].
Brain health is strongly connected to physical health, and there are physiological links between physical frailty and CI [21, 29, 30]. Physical frailty is associated with cognitive decline [14, 15] and CI improves the predictive validity of Fried’s phenotype for negative health outcomes [14]. There is a distinct frailty-CI bidirectional relationship [20, 21]. However, just because two conditions often coexist doesn’t mean they should be joined conceptually. Consider frailty and disability: they overlap but are distinct concepts [31, 32]. Similarly, not everyone with CI has physical frailty, and not every physically frail person has CI, as highlighted by several survey respondents. Furthermore, while decline in cognitive and physical functions can be concurrent, they may develop at different rates [20]. Some theorize the temporal order of frailty and CI development may represent different etiologies, whereby frailty before CI may have a vascular or inflammatory etiology, while CI development subsequent to frailty may have different origins [33]. However, Xue et al. (2019) affirm further longitudinal studies are required to understand frailty-CI associations and advise caution in integrating phenotypes that may not have common etiologies and pathways [27]. A final question is whether the IANA-IAGG definition adds anything to frailty as a term, or to models such as the Frailty Index, and the commonly used Clinical Frailty Scale. These cumulative deficit frailty instruments, and comprehensive geriatric assessment, avoid focusing attention on just physical frailty and MCI, but reflect a holistic approach to an older person that includes cognition, co-morbidities, medication burden, and psychosocial factors.
While strengths of this pan-European study include an easily accessible survey and access to professional networks to enable a relatively large sample size to be recruited, it has some limitations. As the number of geriatricians is not readily available for all countries, it is difficult to ascertain an exact survey response rate. However, where it is known, it suggests the country response rate varies from 0 to 35%, averaging less than 10% for participating countries. Although professional networks were used to disseminate the survey, participants’ inclusion criteria were not verified. Eastern Europe as a region was under-represented, while some northern and western European countries may be over-represented in the results. While the survey was available in twelve European languages, it had to be answered in English.
Conclusions
The infrequent selection of the decade-old IANA-IAGG definition as the meaning of cognitive frailty among European geriatric medicine specialists suggests this definition has not achieved widespread consensus and adoption across Europe. The frequent disagreement with this definition in our survey should prompt a meaningful debate on four possible future directions for “cognitive frailty”: broadening the definition to consider specific frailty domains; narrowing the definition to cognitive vulnerability only; abandoning the term in favour of phenotyping the variables in the frailty index; or broader adoption and championing of the current IANA-IAGG definition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the geriatricians and trainee geriatricians who volunteered their time to participate in the survey.
Abbreviations
- CDR
Clinical Dementia Rating
- CI
Cognitive Impairment
- DSD
Delirium Superimposed on Dementia
- IANA
International Academy on Nutrition and Aging
- IAGG
International Association of Gerontology and Geriatrics
- MCI
Mild Cognitive Impairment
- PF
Physical Frailty
- SCI
Subjective Cognitive Impairment
Author contributions
All authors contributed to the study conception and design. The survey was designed by all authors except MF. Data collection and analysis were performed by CC, MF and ST. The first draft of the manuscript was written by ST and MF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for completing this study.
Data availability
The complete survey dataset will be available in Open Science Framework from September 2025 onwards, once all papers are published (noting that the dataset contains other survey elements will be the subject of future publications by the author group).
Declarations
Ethics approval and consent to participate
This research was performed in compliance with the principles of the Declaration of Helsinki. Ethics approval was obtained from the Social Research Ethics Committee in University College Cork, Ireland. The survey did not collect personal data and IP addresses were not recorded. A detailed information front page was provided to all participants outlining the purpose of the survey and how their data would be used. Informed consent was via a tick box in advance of starting the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paganini-Hill A, Clark LJ, Henderson VW, Birge SJ. Clock drawing: analysis in a retirement community. J Am Geriatr Soc. 2001;49:941–7. [DOI] [PubMed] [Google Scholar]
- 2.Panza F, D’Introno A, Colacicco AM, Capurso C, Parigi AD, Capurso SA, et al. Cognitive frailty: predementia syndrome and vascular risk factors. Neurobiol Aging. 2006;27:933–40. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. Volume 56. Biological sciences and medical sciences: Journals of Gerontology Series A; 2001. [DOI] [PubMed] [Google Scholar]
- 4.Stephan BCM, Hunter S, Harris D, Llewellyn DJ, Siervo M, Matthews FE, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17:1056–76. [DOI] [PubMed] [Google Scholar]
- 5.Kelaiditi E, Cesari M, Canevelli M, van Abellan G, Ousset P-J, Gillette-Guyonnet S, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17:726–34. [DOI] [PubMed] [Google Scholar]
- 6.Won CW, Lee Y, Kim S, Yoo J, Kim M, Ng T-P, et al. Modified criteria for diagnosing cognitive frailty. Psychiatry Investig. 2018;15:839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui Z, Wang X, Zhou Y, Li Y, Ren X, Wang M. Global research on cognitive frailty: A bibliometric and visual analysis of papers published during 2013–2021. Int J Environ Res Public Health. 2022;19:8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gobbens RJ, van Assen MA, Luijkx KG, Schols JM. Testing an integral conceptual model of frailty. J Adv Nurs. 2012;68:2047–60. [DOI] [PubMed] [Google Scholar]
- 9.Guo C-Y, Sun Z, Tan C-C, Tan L, Xu W. Multi-Concept frailty predicts the Late-Life occurrence of cognitive decline or dementia: an updated systematic review and Meta-Analysis of longitudinal studies. Front Aging Neurosci. 2022;14. [DOI] [PMC free article] [PubMed]
- 10.Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23:771–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng TP, Feng L, Nyunt MSZ, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. Am J Med. 2015;128:1225–e12361. [DOI] [PubMed] [Google Scholar]
- 12.Solfrizzi V, Scafato E, Lozupone M, Seripa D, Giannini M, Sardone R, et al. Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: the Italian longitudinal study on aging. Am J Geriatric Psychiatry. 2017;25:1236–48. [DOI] [PubMed] [Google Scholar]
- 13.Kiiti Borges M, Oiring de Castro Cezar N, Silva Santos Siqueira A, Yassuda M, Cesari M, Aprahamian I. The relationship between physical frailty and mild cognitive impairment in the elderly: A systematic review. J Frailty Aging. 2019;8:192–7. [DOI] [PubMed] [Google Scholar]
- 14.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57:453–61. [DOI] [PubMed] [Google Scholar]
- 15.Auyeung TW, Lee JSW, Kwok T, Woo J. Physical frailty predicts future cognitive decline — A four-year prospective study in 2737 cognitively normal older adults. J Nutr Health Aging. 2011;15:690–4. [DOI] [PubMed] [Google Scholar]
- 16.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borges MK, Canevelli M, Cesari M, Aprahamian I. Frailty as a predictor of cognitive disorders: A systematic review and Meta-Analysis. Front Med (Lausanne). 2019;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grande G, Triolo F, Nuara A, Welmer A-K, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol. 2019;124:110625. [DOI] [PubMed] [Google Scholar]
- 19.Cui M, Zhang S, Liu Y, Gang X, Wang G. Grip strength and the risk of cognitive decline and dementia: A systematic review and Meta-Analysis of longitudinal cohort studies. Front Aging Neurosci. 2021;13. [DOI] [PMC free article] [PubMed]
- 20.Basile G, Sardella A. From cognitive to motor impairment and from sarcopenia to cognitive impairment: a bidirectional pathway towards frailty and disability. Aging Clin Exp Res. 2021;33:469–78. [DOI] [PubMed] [Google Scholar]
- 21.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment–a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–51. [DOI] [PubMed] [Google Scholar]
- 22.Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013;80:2055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Facal D, Burgo C, Spuch C, Gaspar P, Campos-Magdaleno M. Cognitive frailty: an update. Front Psychol. 2021;12. [DOI] [PMC free article] [PubMed]
- 24.Fennelly O, Grogan L, Reed A, Hardiker NR. Use of standardized terminologies in clinical practice: A scoping review. Int J Med Informatics. 2021;149:104431. [DOI] [PubMed] [Google Scholar]
- 25.Faherty M, Curtin C, Bellelli G, Brunetti E, Bo M, Morandi A et al. The perceptions of European geriatricians on the co-occurrence and links between dementia, delirium and frailty. Eur Geriatr Med. 2025. [DOI] [PMC free article] [PubMed]
- 26.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol Biol Sci Med Sci. 2013;68:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue Q-L, Buta B, Ma L, Ge M, Carlson M. Integrating frailty and cognitive phenotypes: why, how, now what?? Curr Geriatr Rep. 2019;8:97–106. [PMC free article] [PubMed] [Google Scholar]
- 28.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. [PubMed] [Google Scholar]
- 29.Sargent L, Brown R. Assessing the current state of cognitive frailty: measurement properties. J Nutr Health Aging. 2017;21:152–60. [DOI] [PubMed] [Google Scholar]
- 30.Ma L, Chan P. Understanding the physiological links between physical frailty and cognitive decline. Aging Disease. 2020;11:405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical Paradigm—Issues and controversies. Journals Gerontology: Ser A. 2007;62:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried LP, Cohen AA, Xue Q-L, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu NM, Bandeen-Roche K, Tian J, Kasper JD, Gross AL, Carlson MC, et al. Hierarchical development of frailty and cognitive impairment: clues into etiological pathways. J Gerontol Biol Sci Med Sci. 2019;74:1761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete survey dataset will be available in Open Science Framework from September 2025 onwards, once all papers are published (noting that the dataset contains other survey elements will be the subject of future publications by the author group).