Abstract
Background
Chronic limb- threatening ischemia (CLTI) is a serious condition that can lead to amputation, and in some cases, it can be associated with mortality. Current clinical evaluation methods have several limitations. Therefore, new methods to assess CLTI are needed to better understand and measure underlying causes and functionality, and hence potentially improve the treatment. In this study, we use dynamic 18F-FAZA PET-imaging as a method of measuring hypoxia as a marker associated with CLTI, on twelve patients identified with CLTI who underwent 18F-FAZA PET-MR imaging.
Results
The kinetic modelling goodness-of-fit metrics using AIF from independent limb with the irreversible-2TC3K model distinguished between index and contralateral limbs better than the reversable-2TC4K model. The Spearman correlation coefficients between the standardized uptake value (SUV) SUV-to-SUVmed ratio and the perfusion parameter, 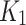 , was rs = -0.07 for index and rs = 0.22 for contralateral limbs. For the SUV-to-SUVmed ratio correlation with diffusion parameter,
, was rs = -0.07 for index and rs = 0.22 for contralateral limbs. For the SUV-to-SUVmed ratio correlation with diffusion parameter, 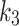 , it is found to be negative for both index (rs = -0.16) and contralateral (rs = -0.11).
, it is found to be negative for both index (rs = -0.16) and contralateral (rs = -0.11).
Conclusions
The kinetic modelling of 18F-FAZA dynamic PET-MR was able to differentiate between index and contralateral limbs in CLTI patients, and the diffusion metric from the kinetic modelling can potentially be used as a metric to measure hypoxia in CLTI.
Trial registration
ClinicalTrials.gov, NCT04054609. Registered 20,190,611, https//clinicaltrials.gov/study/NCT04054609.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13550-025-01243-5.
Keywords: Hypoxia, Kinetic modelling, PET/MRI, CLTI, 18F-FAZA.
Introduction
Chronic limb-threatening ischemia (CLTI) is a serious condition that can lead to amputation, and in some cases, it can be associated with mortality. CLTI is normally diagnosed by hemodynamic metrics, such as the ankle-brachial index (ABI) of ≤ 0.4, the ankle systolic pressure (ASP) of ≤ 50 mmHg, the toe brachial index (TBI), and the toe systolic pressure (TSP) of ≤ 30 mmHg [1]. However, these metrics have limitations, being that they are partly operator-dependent. Additionally, although the ABI is the current gold standard for monitoring CLTI, high ABI (> 1.3) can be associated with kidney diseases or diabetes due to the presence of calcification in the arteries [2, 3]. Such calcification can cause a high and incorrect ABI value, hence, other diagnostic tests or methods in these populations are required. Furthermore, CLTI is routinely thought of as reduction of blood flow to tissue, which limits the methods by which CLTI can be assessed and understood [4, 5]. Hence, there is a need for quantitative assessment, complementing diagnostic metrics for CLTI using new methods and approaches.
Ischemia is defined by the lack of oxygenation in the tissues (hypoxia), and measurement of hypoxia can be conducted invasively or non-invasively [6, 7]. However, invasive methods are partly not feasible in clinical routine and may increase patient discomfort. A potential non-invasive method for measuring hypoxia is the use of imaging modalities, such as magnetic resonance imaging (MRI) and/or positron emission tomography (PET) [8, 9].
For example, 18F-Fluoroazomycin arabinoside (18F-FAZA) PET imaging has become a successful non-invasive method for studying tumor hypoxia with the aid of graphical analysis and mathematical models [10–15]. 18F-FAZA accumulates in low oxygenated tissues via a hypoxia-specific uptake mechanism, and passively diffuses through the cell membrane and becomes trapped. This mechanism is reversed if the cell is well oxygenated, and hence the tracer accumulates less / does not accumulate in those tissues. Therefore, 18F-FAZA tracer can differentiate hypoxic from none hypoxic tissues [16, 17]. The characterization of hypoxia formerly relied on information from static PET images, which did not provide temporal resolution of the uptake. As a result, it was not possible to measure important parameters, such as the tracer uptake rate in the tissues (uptake as a function of time). Only with the introduction of dynamic PET imaging, were the rates of transfer between the blood pool (artery) and the target tissue and vice-versa, as well as the rate of transfer within two adjacent tissues or compartments, possible using mathematical kinetic modelling. Using the dynamic PET imaging, the concentration of the radioactive tracer carried by the arterial blood can be extracted directly from the images and used as the arterial input function (AIF) to estimate the kinetic model. Likewise, for the target tissue region, the standardized uptake value (SUV) of the radiotracer can be measured over time and presented as the time activity curve (TAC), which is necessary for the modeling of the tracer kinetic. However, the accuracy of the kinetic modelling depends on a few factors, the most important being that the quality of both the AIF and the TAC provided for the model are free from contamination and influence from one another.
There are a few models that can be used to calculate kinetic parameters from which hypoxia can be measured. For example, the two-tissue compartmental (2TC) model has been reported to be sufficient for modelling of the 18F-FAZA radiotracer kinetics [12, 13, 17–21]. Although 18F-FAZA kinetic modelling has shown to be robust in characterizing tumor hypoxia, there is no evaluation available for non-cancerous hypoxic tissues, such as in CLTI.
Most recently, and at the time of writing this manuscript, measurement of perfusion in lower extremity skeletal muscles (LESM) and CLTI patients were reported using [15O] H2O PET/CT [22], and 18F-NaF PET/CT [23], respectively. Both tracers are not dedicated to study hypoxia in tissues. While 18F-NaF is primarily used for bone imaging, it has limited ability to accurately assess tissue perfusion due to its rapid clearance from the bloodstream. As for the most recent LESM study utilizing [15O] H2O, the authors measured rest perfusion and employed a one-tissue compartment (1TC) modeling in only healthy subjects, but not in patients with CLTI conditions. Nevertheless, to our knowledge, the measurement of hypoxia in CLTI using 18F-FAZA kinetic modelling has not been established before, therefore, quality control and discrimination of different approaches in producing the AIF and TAC must be performed. Furthermore, a validation of the 2TC suitability and stability for CLTI cases should be provided.
In this study, we performed different methods to extract the AIF and TAC, which we then used with 2TC 18F-FAZA kinetic modelling to characterize hypoxia in CLTI patients. The goals of this study were 1- to investigate the effect of using the AIF from the ischemic limb only vs. the AIF from both limbs for the kinetic modelling; 2- to use the kinetic parameters for differentiation between the critically ischemic (index) vs. non-ischemic (contralateral) leg in patients with CLTI; and 3- to quantify the hypoxia levels in patients with chronic limb ischemia. We hypothesize that the correlation between static SUV and perfusion is positive for non-ischemic, and negative to no correlation for critically ischemic tissue.
Methods
Patient recruitment
This was a prospective study that, so far, utilized a total of 12 patients, who were diagnosed with CLTI in one limb and underwent 18F-FAZA PET-MR before angioplasty, as presented in Table-1. All patients had rest pain and a non-healing ulcer of the index limb for extended time. All patients were evaluated clinically by a vascular surgeon/interventionalist for appropriateness of intervention. The study followed a Research Ethics Board-approved research setting (REB number: 18-6114.3). All patients gave informed consent before being included in this study.
Patient PET-MR acquisition
All PET image acquisitions were performed on a 3.0T PET-MRI scanner (Biograph mMR Software Version VE11P, Siemens Healthineers, Erlangen, Germany). The Patient’s calf was isocentred, so that the region just below the knee and up to the ankle is covered as seen in Fig. 1. Patients were injected peripherally (i.v.antecubital) with 5 MBq/kg of 18F-FAZA on the scanner table. The first scan began simultaneously with the injection, and dynamic PET-imaging was acquired for the initial 20 min, considering that 18F-FAZA has a faster diffusion and clearance time than other hypoxia-sensitive radioactive tracers [17]. The dynamic data were reconstructed for multiple sets of time points, as follows: 18 sets @ 5s, 3 sets @ 10s, 8 sets @ 30s, and 7 sets @ 120s (36 data sets overall). Two hours after the injection, a static PET data set (3D-PET) was acquired for 20 min for the same limb region as the dynamic scan. PET image reconstruction was performed using conventional ordinary Poisson-ordered subset maximum expectation maximization (OP-OSEM) with 3 iterations, 21 subsets, FWHM = 4.5 filtering, and resolution = 2.08 × 2.08 × 2.03mm3. As part of the PET-MR protocol, MR Dixon-based T1-weighted imaging (T1-WI) was acquired simultaneously with the PET. ABI measurements were performed on the limbs for all patients.
Fig. 1.

Axial images and sagittal, and coronal reconstructions of a patient’s limb after automatic threshold contouring for tissue dynamic assessment (A) and during early radiotracer injection for Arterial input function (AIF) determination (B). (A) The parasagittal and posterior coronal MR reconstructions transversing the area of major muscle thickness shows the contoured muscle tissue with exclusion of bones and subcutaneous fat. The axial image better depicts the exclusion of the deep posterior vessels in the neurovascular bundle at the level of the posterior tibial artery. (B) Fused PET/MR axial, sagittal, and coronal images at the 8th set of the dynamic PET where manual VOI placement within the popliteal artery for AIF determination is seen in the three planes. It is worth noting that while the sagittal plane seen in B clearly shows the popliteal and posterior tibial artery path, the sagittal image in A does not include those structures as the slice is lateral to that plane for the purpose of exemplifying how muscle was contoured
Dynamic image processing
All segmentation and modelling for this study was performed using PMOD software (PMOD Technologies Ltd., Zürich, Switzerland). The AIF- and muscle-VOI segmentation on dynamic PET was performed semi-automatically with careful observation by one experienced reader (T.S.), who has 8 years of experience interpreting PET-MR imaging, and is a board-certified in both nuclear medicine and radiology. For the AIF-VOI segmentation process, the popliteal artery was contoured on the slices that have visible signal while excluding noisy slices at all 36 data sets. The AIF signal was visible on only three slices for any given data set. Thereafter, the proximal one-third of slices were segmented in each limb, and in each VOI, the hottest 15 pixels were extracted as AIF-VOI (Fig. 1).
For contouring the muscle-VOI, the T1-WI was registered to the averaged 36 imaging sets of the dynamic PET. Thereafter, a selected limb region was extracted and one-pixel erosion was applied to exclude pixels with partial volume effect. The vessels were excluded with optimal thresholding on the arterial imaging set generated during the AIF-VOI segmentation step. Then, the cortical bone, whose signal is low on both fat-suppressed and in-phase T1-WI, was excluded. This was followed by the exclusion of fatty regions of bone and fat between muscles, whose signal intensity is low on fat-suppressed T1-WI, and high on in-phase T1-WI (Fig. 1).
Independent AIF vs. joint AIF
In order to examine the effect of AIF selection on the 18F-FAZA kinetic model outcome, the following two scenarios for image-based AIF were considered during contouring and modelling: 1- the AIF from the indexed-limb independently; and 2- the averaged AIF from both limbs jointly. Index limb was determined based on labelling provided by the interventionist.
Kinetic modelling
The TAC data set was generated from the muscle-VOIs, while the AIF-VOI produced the arterial input function. These two time-activity data sets were used as the input for modelling, where the AIF was 3-exponentialy fitted before estimating the transfer rate parameters according to Eq. 1.
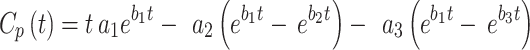 |
1 |
Where 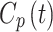 is the smoothed fitted AIF as a function of time, also known as the parent concentration of the tracer, and
is the smoothed fitted AIF as a function of time, also known as the parent concentration of the tracer, and 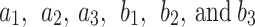 are the fitting parameters of the model amplitude and ranges. The image-derived time activity curves of the tissues and the AIF were decay-corrected, and then, used to perform the 2TC. In the 2TC model, the assumptions are: 1- the transfer rate constant of the blood from the artery to the first tissue compartment, representing the un-bind18F-FAZA concentration (
are the fitting parameters of the model amplitude and ranges. The image-derived time activity curves of the tissues and the AIF were decay-corrected, and then, used to perform the 2TC. In the 2TC model, the assumptions are: 1- the transfer rate constant of the blood from the artery to the first tissue compartment, representing the un-bind18F-FAZA concentration (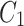 ), and also includes a perfusion-dependent component; and 2- the transfer rate between the first and second compartment represents the bind18F-FAZA concentration (
), and also includes a perfusion-dependent component; and 2- the transfer rate between the first and second compartment represents the bind18F-FAZA concentration ( ). The 2TC model is computed using the following system of linear differential Eqs. 2 and 3:
). The 2TC model is computed using the following system of linear differential Eqs. 2 and 3:
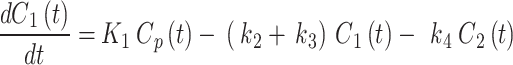 |
2 |
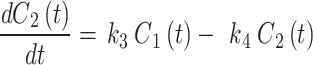 |
3 |
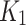 (ml/cm3/min) is the rate constant of perfusion from the blood to the first tissue compartment,
(ml/cm3/min) is the rate constant of perfusion from the blood to the first tissue compartment,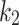 (min− 1) is the rate constant representing the transport rate from the interstitium to the blood,
(min− 1) is the rate constant representing the transport rate from the interstitium to the blood, 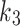 (min− 1) is the tracer accumulation rate constant (phosphatization) in the second compartment, and
(min− 1) is the tracer accumulation rate constant (phosphatization) in the second compartment, and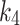 (min− 1) is the dissociation rate constant (de-phosphatization) of the trapped 18F-FAZA. When 18F-FAZA is trapped, an irreversible condition (will be referred here as 2TC3K) can be considered, and hence,
(min− 1) is the dissociation rate constant (de-phosphatization) of the trapped 18F-FAZA. When 18F-FAZA is trapped, an irreversible condition (will be referred here as 2TC3K) can be considered, and hence, 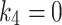 . In addition to performing the reversable (will be referred here as 2TC4K) and irreversible conditions, a Patlak analysis was also performed so that the best model representing the 18F-FAZA kinetic in non-cancerous hypoxic tissue can be determined [13].
. In addition to performing the reversable (will be referred here as 2TC4K) and irreversible conditions, a Patlak analysis was also performed so that the best model representing the 18F-FAZA kinetic in non-cancerous hypoxic tissue can be determined [13].
As part of weighing the AIF and model suitability, the goodness-of-fit (GoF) was assessed by observing the metrics of Akaike Information Criterion (AIC), ChiSquare, and root mean square error (RMSE) between the model and time-activity. Additionally, the 2TC model parameters, 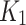 ,
, 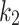 ,
, 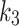 ,
,  ,
, 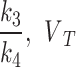 (total volume distribution - mL·cm− 3) for reversable model, and
(total volume distribution - mL·cm− 3) for reversable model, and 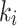 (total tracer flux - mL·cm− 3·min− 1) for irreversible model, were compared for the index limb and the contralateral limb, as well as for each AIF scenario. Furthermore, the fractional hypoxia volume
(total tracer flux - mL·cm− 3·min− 1) for irreversible model, were compared for the index limb and the contralateral limb, as well as for each AIF scenario. Furthermore, the fractional hypoxia volume 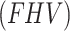 was measured, and the muscle-VOIs’ standardized uptake value (SUV) from the static PET scans was measured and normalized to the SUV from the mediastinal scan (SUVmed). Then, both the SUV and
was measured, and the muscle-VOIs’ standardized uptake value (SUV) from the static PET scans was measured and normalized to the SUV from the mediastinal scan (SUVmed). Then, both the SUV and  correlation with the kinetic parameters were examined.
correlation with the kinetic parameters were examined.
Statistical analysis
Wilcoxon signed-rank test was performed to compare the GoF metrics for each of the compartmental models, 2TC3K and 2TC4K. The same test was also used to compare the kinetic parameters (SUV, 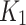 ,
, 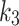 ,
, 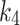 ,
, estimated from each AIF type (joint AIF vs. independent AIF), and for contralateral and index limb data sets. Spearman’s rank correlation coefficient (rs) between each model, as well as, between kinetic parameters and static SUV-to-SUVmed ratio for index and contralateral was calculated at significant level < 0.05. Additionally, Pearson correlation between ABI and the kinetic parameters were performed.
estimated from each AIF type (joint AIF vs. independent AIF), and for contralateral and index limb data sets. Spearman’s rank correlation coefficient (rs) between each model, as well as, between kinetic parameters and static SUV-to-SUVmed ratio for index and contralateral was calculated at significant level < 0.05. Additionally, Pearson correlation between ABI and the kinetic parameters were performed.
Results
Patient study
Ten of the twelve patients had confirmed type-2 diabetes, one had confirmed type-1 diabetes, and one patient was non-diabetic (Table 1).
Table 1.
Chronic limb ischemia patients’ characteristics, including the index limb and selection criterion used in the study
| n | Gender | Ethnicity | Weight | Age | Index Limb | Diabetes Type | ABI |
|---|---|---|---|---|---|---|---|
| 1 | Male | African | 63 | 55 | right | Type 2 | 0.52 |
| 2 | Male | Caucasian | 115 | 73 | left | Type 2 | 0.81 |
| 3 | Male | Caucasian | 94 | 73 | left | Type 2 | 0.46 |
| 4 | Male | Caucasian | 98 | 73 | left | Type 2 | * |
| 5 | Male | Caucasian | 79 | 66 | left | Type 2 | 0.71 |
| 6 | Male | Caucasian | 73 | 63 | right | Type 2 | 0.53 |
| 7 | Male | Caucasian | 84 | 72 | right | Type 2 | 0.69 |
| 8 | Female | Caucasian | 114 | 67 | right | none | 0.76 |
| 9 | Male | Middle-Eastern | 73 | 62 | right | Type 2 | 0.73 |
| 10 | Female | Caucasian | 81 | 69 | right | Type 2 | 0.55 |
| 11 | Male | Caucasian | 73 | 65 | left | Type 1 | 0.53 |
| 12 | Male | Caucasian | 72 | 91 | left | Type 2 | 0.68 |
| mean ± SD | 84.9 ± 16.9 | 69.1 ± 8.8 | 0.63 ± 0.12 | ||||
| Range | 63–115 | 55–91 | 0.46–0.81 |
*Ankle pressures was not measured
Independent AIF vs. joint AIF
As a sample, one patient is selected to demonstrate the AIF for each scenario and the TACs with the fitted model, as seen in Fig. 2. The joint AIF and independent AIF (indexed-limb), together with the TAC of the muscles, are shown in Fig. 2 under Independant AIF vs. joint AIF section, is not needed. Fig. 2-a and 2-b. The AIF overall maximum activity was higher for the joint AIF than for the independently evaluated AIF. The fitted 2TC model is also shown in (Fig. 2-c).
Fig. 2.
Arterial input function (AIF) from joint limb evaluation in a), and from independent index limb in b), evaluation of the fitted models 2TC3K in (c) and evaluation of the Patlak model in (d)
The comparison of GoF metrics from modelling all of the patients for each AIF scenario is shown in Fig. 3. The AIC metric from the joint AIF and the independent AIF modelling are well in agreement for the contralateral limbs, with a fitting slope of 1. For the index (hypoxic) limbs, a fitting slope of 0.91 (Fig. 3-a) was found. The RMSE evaluation exhibited agreement between the jointly and independently evaluated AIF for the index and contralateral limbs, with slopes of 1.00 and 0.99, respectively (Fig. 3-b). In the meantime, the ChiSquare metric correlation between the joint and independent AIF resulted in slopes of 1.20 and 0.97 for the index and contralateral limbs, respectively (Fig. 3-c). The mean and the standard deviation (SD) of GoF metrics (AIC, RMSE, and Chisquare) for modelling using joint AIF and independent AIF are shown in Table 2, where the SD were the lowest for independent AIF, indicating stability of the independent AIF approach over the joint AIF approach. An example patient with CLTI in the right limb is provided in Fig. 4 for each AIF scenario using the 2TC4K model. As shown in Fig. 5-a, the measured total volume distribution using the joint AIF was not distinguishable between the index and contralateral limbs, whereas the independent AIF clearly distinguished between the  of the index and contralateral limbs, as shown in Fig. 5-b. For other kinetic parameters, the independent AIF was also able to distinguish between the index and contralateral limbs. The
of the index and contralateral limbs, as shown in Fig. 5-b. For other kinetic parameters, the independent AIF was also able to distinguish between the index and contralateral limbs. The 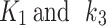 parameters were found to be significantly different (p = 0.02) for contralateral limbs when evaluated with the joint AIF approach and the independent AIF approach, and the same was found to be true for index limbs. Thus, indicating that the independent AIF represents the true localized blood volume within immediate proximity to the diseased tissues, providing an accurate volume for the modelling inputs and it therefore preferable over the combined AIF.
parameters were found to be significantly different (p = 0.02) for contralateral limbs when evaluated with the joint AIF approach and the independent AIF approach, and the same was found to be true for index limbs. Thus, indicating that the independent AIF represents the true localized blood volume within immediate proximity to the diseased tissues, providing an accurate volume for the modelling inputs and it therefore preferable over the combined AIF.
Fig. 3.
Scatter plot for goodness-of-fit metrics (a) AIC, (b) RMSE, and (c) ChiSquare, from modelling kinetics in contralateral and index limbs with independent and joint AIFs. The differences between the index and contralateral slopes for the AIC metric was 0.09, while it was 0.01 for the RMSE, and 0.23 for the ChiSquare
Table 2.
Goodness of fit (GoF) metrics mean ± standard deviation for 12 patient kinetic modelling with two-tissue compartment, reversable (2TC4k) and irreversible (2TC3k)
| Joint-AIF | Independent-AIF | |
|---|---|---|
| AIC | -78.54 ± 33.24 | -73.69 ± 27.21 |
| RMSE | 9.32 ± 2.27 | 9.40 ± 2.01 |
| ChiSquare | 0.14 ± 0.11 | 0.14 ± 0.09 |
| Index K 1 | 0.41 ± 0.18 | 0.17 ± 0.08 |
Fig. 4.
Coronal view of a patient’s limbs, modelled with joint AIF (left), and independent index-AIF (right) (patient #1 in Table 1). The images under the independent AIF, are for the kinetic parameters computed for the index limb only, using independent index-AIF only. Here, the pixel-wise parametric maps for each kinetic parameter 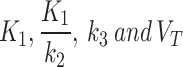 (top-to-bottom respectively), fused to the MR images are shown, as well as the SUV from the static PET acquisition (bottom row). Diffusion parameter
(top-to-bottom respectively), fused to the MR images are shown, as well as the SUV from the static PET acquisition (bottom row). Diffusion parameter 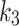 for independent-AIF indicates accumulation of the tracer in the index limb peripheries suggesting lack of oxygenation. In the SUV figure (bottom), a 18F-FAZA accumulation seen in the index limb is higher than the contralateral, indicating hypoxic conditions. SUV is also in agreement with the
for independent-AIF indicates accumulation of the tracer in the index limb peripheries suggesting lack of oxygenation. In the SUV figure (bottom), a 18F-FAZA accumulation seen in the index limb is higher than the contralateral, indicating hypoxic conditions. SUV is also in agreement with the 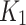 measured using the joint AIF (
measured using the joint AIF (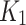 figure, top left). When using AIF from the index limb only, and compute the same parameter (
figure, top left). When using AIF from the index limb only, and compute the same parameter (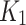 figure top right) for the index only, a similar and a better distribution can be seen. In general, the parametric maps for the index limb that were generated from the joint AIF and independent AIF were visually comparable
figure top right) for the index only, a similar and a better distribution can be seen. In general, the parametric maps for the index limb that were generated from the joint AIF and independent AIF were visually comparable
Fig. 5.
Total volume distribution (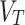 ) in the 12 patients measured, using joint-AIF a), and independent (index) AIF b). The dashed lines in the plots are the fitted line while the dotted lines are for upper and lowed 95% CI
) in the 12 patients measured, using joint-AIF a), and independent (index) AIF b). The dashed lines in the plots are the fitted line while the dotted lines are for upper and lowed 95% CI
All 12 patients’ total volume distributions were not significantly different when comparing the contralateral (p = 0.76) vs. index (p = 0.14) limbs, irrespective of using the joint AIF or the independent AIF.
Kinetic models analysis
The 2TC3K parameters, 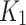 ,
, 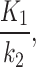
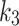 ,
, 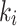 , SUV/SUVmed, and
, SUV/SUVmed, and 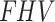 measured in the index and contralateral limbs for all patients are provided in Table 3. For all patients the AIC values were lower in the 2TC3K modelling than the 2TC4K.
measured in the index and contralateral limbs for all patients are provided in Table 3. For all patients the AIC values were lower in the 2TC3K modelling than the 2TC4K.
Table 3.
The kinetic parameters evaluated with the irreversible (2TC3k), for the index and contralateral limbs of all 12 patients individually
| K1 (ml/ccm/min) | K1/k2 (mL·cm− 3) | k3 (min− 1) | Ki (mL·cm− 3·min− 1) | SUV/SUVmed | FHV | |
|---|---|---|---|---|---|---|
| Index | 0.05 | 0.42 | 0.04 | 0.02 | 0.49 | 0.96 |
| 0.07 | 1.02 | 0.00 | 0.04 | 0.56 | 0.87 | |
| 0.08 | 0.34 | 0.03 | 0.02 | 0.50 | 0.94 | |
| 0.11 | 0.74 | 0.00 | 0.01 | 0.51 | 0.72 | |
| 0.16 | 0.75 | 0.02 | 0.04 | 0.53 | 0.54 | |
| 0.07 | 0.44 | 0.05 | 0.03 | 0.52 | 0.92 | |
| 0.13 | 0.40 | 0.06 | 0.03 | 0.72 | 0.88 | |
| 0.04 | 0.52 | 0.02 | 0.02 | 0.50 | 0.90 | |
| 0.77 | 0.90 | 0.02 | 0.03 | 0.51 | 0.82 | |
| 0.02 | 0.72 | 0.00 | 0.01 | 0.54 | 0.89 | |
| 0.23 | 0.77 | 0.03 | 0.05 | 0.47 | 0.82 | |
| 0.17 | 0.57 | 0.02 | 0.03 | 0.52 | 0.92 | |
| Contralateral | 0.05 | 0.37 | 0.04 | 0.02 | 0.48 | 0.89 |
| 0.06 | 0.87 | 0.00 | 0.03 | 0.55 | 0.87 | |
| 0.04 | 0.38 | 0.05 | 0.02 | 0.49 | 0.86 | |
| 0.03 | 0.37 | 0.09 | 0.02 | 0.51 | 0.79 | |
| 0.24 | 1.32 | 0.01 | 0.07 | 0.49 | 0.65 | |
| 0.05 | 0.58 | 0.02 | 0.03 | 0.53 | 0.89 | |
| 0.31 | 0.50 | 0.04 | 0.03 | 0.69 | 0.87 | |
| 0.04 | 0.30 | 0.10 | 0.02 | 0.49 | 0.94 | |
| 0.31 | 0.63 | 0.03 | 0.03 | 0.52 | 0.78 | |
| 0.03 | 0.32 | 0.06 | 0.02 | 0.53 | 0.79 | |
| 0.07 | 0.68 | 0.00 | 0.03 | 0.36 | 0.68 | |
| 0.13 | 0.65 | 0.02 | 0.03 | 0.56 | 0.87 |
Table 4 shows the SUV-to-SUVmed ratio, as well as the fractional hypoxic volume, where a small 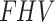 difference is observed between the contralateral (0.82) and the index (0.85) limbs. The difference between the SUV ratio of the contralateral (0.517) and the index (0.531) limbs was also small. In the same Table 4, the average of each kinetic parameter from the 2TC4K, 2TC3K, and Patlak model evaluation for all of the patients is provided. The
difference is observed between the contralateral (0.82) and the index (0.85) limbs. The difference between the SUV ratio of the contralateral (0.517) and the index (0.531) limbs was also small. In the same Table 4, the average of each kinetic parameter from the 2TC4K, 2TC3K, and Patlak model evaluation for all of the patients is provided. The  parameters calculated by the 2TC4K and 2TC3K models were significantly higher (p = 0.001 and p = 0.01, respectively) for the index limbs, compared to the contralateral limbs. However, the averages of the parameters,
parameters calculated by the 2TC4K and 2TC3K models were significantly higher (p = 0.001 and p = 0.01, respectively) for the index limbs, compared to the contralateral limbs. However, the averages of the parameters, 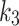 ,
, 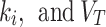 were lower for the index limbs, compared to the contralateral limbs. The Wilcoxon test revealed that the
were lower for the index limbs, compared to the contralateral limbs. The Wilcoxon test revealed that the parameter, in particular, was significantly (p = 0.045) different when comparing the 2TC3K and 2TC4K models for either the contralateral, or the index limbs. Meanwhile, the same parameters, when compared between the compartmental models using the Spearman correlation test, were strongly positively correlated, except for the
parameter, in particular, was significantly (p = 0.045) different when comparing the 2TC3K and 2TC4K models for either the contralateral, or the index limbs. Meanwhile, the same parameters, when compared between the compartmental models using the Spearman correlation test, were strongly positively correlated, except for the 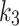 values in the contralateral case, was negatively correlated (Table 5).
values in the contralateral case, was negatively correlated (Table 5).
Table 4.
The average of the kinetic parameters, estimated for each kinetic modelling two-tissue compartment, reversible (2TC4k) and irreversible (2TC3k), for index and contralateral limbs of 12 patients. Binding potential (BP). K1 is higher than k3 when using the optimum kinetic irreversible 2TC3k model
| (n = 12) | 2TC4k - reversable independent AIF | 2TC3k - irreversible independent AIF | Patlak | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K 1 | k 2 | k 3 | k 4 | BP | V T | K 1 | k 2 | k 3 | K 1 /k 2 | K i | Pf | *t | K i | intercept | FHV | SUV/SUV med | |
| Contralateral | 0.116 | 0.241 | 0.162 | 0.224 | 2.741 | 4.048 | 0.113 | 0.193 | 0.038 | 0.581 | 0.012 | 0.194 | 1.111 | 0.029 | 0.13 | 0.82 | 0.517 |
| Index | 0.175 | 0.385 | 0.128 | 0.305 | 1.355 | 3.268 | 0.158 | 0.237 | 0.024 | 0.632 | 0.011 | 0.101 | 1.621 | 0.027 | 0.21 | 0.85 | 0.531 |
Table 5.
Spearman test results for correlation between two-tissue compartment models, reversible 2TC4K and irreversible 2TC3K parameters using independent AIF for both the contralateral and index limbs
| Contralateral | Index | |
|---|---|---|
| K 1 | rs= 0.99, p = 0.001 | rs = 1, p = 0.001 |
| k 2 | rs= 0.72, p = 0.01 | rs = 0.94, p = 0.001 |
| k 3 | rs= -0.45, p = 0.14 | rs = 0.82, p = 0.001 |
| VT - Ki | rs= 0.65, p = 0.02 | rs = 0.39, p = 0.21 |
Figure 6-a shows that the Patlak  values were in good agreement with the 2TC3K
values were in good agreement with the 2TC3K 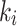 values with a strong correlation between the measurements for the index limbs (rs = 0.58, p = 0.04), whereas correlation was weak for the contralateral limbs (rs = 0.15, p = 0.65). In Fig. 6-b, a statistically significant positive correlation (rs = 0.62, p = 0.03) was measured between
values with a strong correlation between the measurements for the index limbs (rs = 0.58, p = 0.04), whereas correlation was weak for the contralateral limbs (rs = 0.15, p = 0.65). In Fig. 6-b, a statistically significant positive correlation (rs = 0.62, p = 0.03) was measured between  (Patlak) and
(Patlak) and 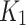 (2TC3K) for the index case. Based on the lower AIC values, and the significant correlation of
(2TC3K) for the index case. Based on the lower AIC values, and the significant correlation of 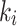 between Patlak and 2TC3K models, the irreversible 2TC3K model was determined to be best among the compared models and selected to further assessing the hypoxia in limb ischemia.
between Patlak and 2TC3K models, the irreversible 2TC3K model was determined to be best among the compared models and selected to further assessing the hypoxia in limb ischemia.
Fig. 6.
Tracer flux ( ) from the Patlak and 2TC3k models using independent AIF positively correlate for the index limb, as seen in a). The flux also moderately correlates with perfusion parameter,
) from the Patlak and 2TC3k models using independent AIF positively correlate for the index limb, as seen in a). The flux also moderately correlates with perfusion parameter, 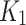 , for the index limb, and is better than in the contralateral limb in b). The SUV-to-SUVmed ratio correlation with the perfusion parameter is shown in c), and the diffusion parameter,
, for the index limb, and is better than in the contralateral limb in b). The SUV-to-SUVmed ratio correlation with the perfusion parameter is shown in c), and the diffusion parameter, 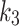 , in d)
, in d)
Hypoxia analysis
As seen in Fig. 6-c, the SUV-to-SUVmed ratio and the perfusion parameter, 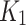 , from the 2TC3K model are displayed in a scatter plot. The Spearman correlations were measured to be weak negative-to-none for index (rs = -0.07, p = 0.84) and weak positive for contralateral (rs = 0.22, p = 0.49) and the index and contralateral limbs were distinguishable. The scatter relation between SUV-to-SUVmed ratio and diffusion parameter
, from the 2TC3K model are displayed in a scatter plot. The Spearman correlations were measured to be weak negative-to-none for index (rs = -0.07, p = 0.84) and weak positive for contralateral (rs = 0.22, p = 0.49) and the index and contralateral limbs were distinguishable. The scatter relation between SUV-to-SUVmed ratio and diffusion parameter 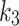 is also shown in Fig. 6-d. The correlation was measured to be for index (rs = -0.16, p = 0.63) and for contralateral (rs = -0.11, p = 0.73). The perfusion parameter,
is also shown in Fig. 6-d. The correlation was measured to be for index (rs = -0.16, p = 0.63) and for contralateral (rs = -0.11, p = 0.73). The perfusion parameter,  , significantly and strong-negatively correlated with ABI for contralateral (R = -0.71, p = 0.02), and moderately-negative for the index (R = -0.43, p = 0.19). The diffusion parameter
, significantly and strong-negatively correlated with ABI for contralateral (R = -0.71, p = 0.02), and moderately-negative for the index (R = -0.43, p = 0.19). The diffusion parameter 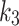 and SUV are also moderately-negative correlated with ABI (R = -0.45, p = 0.16, and R = -0.39, p = 0.27). The scatter plots for ABI against these parameters are provided in supplementary Fig. 1). When comparing the SUV and the
and SUV are also moderately-negative correlated with ABI (R = -0.45, p = 0.16, and R = -0.39, p = 0.27). The scatter plots for ABI against these parameters are provided in supplementary Fig. 1). When comparing the SUV and the 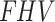 , the test shows no statistical significance (p = 0.14 and p = 0.24, respectively). The negative correlation between
, the test shows no statistical significance (p = 0.14 and p = 0.24, respectively). The negative correlation between 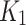 and the SUV ratio for the index limb might be used to distinguish between index and contralateral, while the
and the SUV ratio for the index limb might be used to distinguish between index and contralateral, while the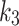 may be used as a metric for hypoxia.
may be used as a metric for hypoxia.
Discussion
Main findings
In this study, we used 18F-FAZA PET-MR to investigate the feasibility of kinetic modelling in distinguishing and measuring hypoxia in patients with CLTI. The study established a segmentation procedure to separate vessels, tissue, and bones in the limb, and compared different sources of image-based AIF. Thereafter, the 2TC4K, 2TC3K, and Patlak kinetic models were performed, and their respective results were compared to determine their suitability in modelling the 18F-FAZA kinetic in CLTI patients. These kinetic parameters were also compared to the conventional metrics used for hypoxia, such as the FHV and static SUV.
Independent-AIF vs. joint-AIF
The tissue TAC characteristic from both the index and contralateral limbs followed a build-up and accumulation of the 18F-FAZA uptake with no indication of wash-out or reaching equilibrium, even after a 20 min scan. The reason for this behavior is due to either slow perfusion / diffusion at the legs while the patient is in the lying position in the scanner, or just simply due to the muscle tissue having limited diffusion and structural hypoxia [24].
Overall, while the image-based AIF from joint limbs seemed to be generally adequate for modeling, its signal amplitude (blood volume) was higher in all cases, compared to the AIF from the independent-AIF (indexed-limb), as observed in Fig. 2. This may have affected the model and caused higher parameters, like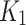 , to be produced by joint AIF, as opposed to those produced by independent-AIF, as reported in the study by Napieczynska [25]. Therefore, the independent-AIF represents the true localized blood volume with immediate proximity to the diseased tissues, providing an accurate volume for the modelling input. Thus, modelling with an independent-AIF appears to better describe the kinetic of 18F-FAZA in CLTI patients. This is potentially important from a clinical sense perspective in CLTI patients since both legs (and generally many vessels) are diseased but one leg is in a critical state and needs intervention.
, to be produced by joint AIF, as opposed to those produced by independent-AIF, as reported in the study by Napieczynska [25]. Therefore, the independent-AIF represents the true localized blood volume with immediate proximity to the diseased tissues, providing an accurate volume for the modelling input. Thus, modelling with an independent-AIF appears to better describe the kinetic of 18F-FAZA in CLTI patients. This is potentially important from a clinical sense perspective in CLTI patients since both legs (and generally many vessels) are diseased but one leg is in a critical state and needs intervention.
2TC3K vs. 2TC4K models
The lowest values of the AIC and RSME metrics indicated that both 2TC3K and 2TC4K were suitable for modelling 18F-FAZA, however, AIC was found (Fig. 3) to be a more sensitive metric than RSME when evaluating hypoxic muscle regions. The average of the 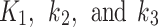 parameters calculated using the 2TC4K model were insignificantly higher than those calculated by 2TC3K (table-3), possibly due to the additional unknown fitting parameter in the 2TC4K model. Moreover,
parameters calculated using the 2TC4K model were insignificantly higher than those calculated by 2TC3K (table-3), possibly due to the additional unknown fitting parameter in the 2TC4K model. Moreover, 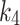 , was found to be high in both the index and the contralateral limbs in three cases compared to the overall average. An explanation for this finding could be that there are different diffusion times of the tracer (possibly secondary to differences in vascular architectures induced by ischemia), which could result in instability of
, was found to be high in both the index and the contralateral limbs in three cases compared to the overall average. An explanation for this finding could be that there are different diffusion times of the tracer (possibly secondary to differences in vascular architectures induced by ischemia), which could result in instability of 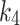 and consequently differences in total volume distribution [18]. On the other hand, the tracer flux,
and consequently differences in total volume distribution [18]. On the other hand, the tracer flux, 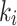 , calculated from the 2TC3K and Patlak models were in great agreement, supporting the stability of 2TC3K model in this case. This shows that the model itself appears to be appropriate, since it delivers similar results in tumours as well as non-tumorous tissue, and also for other hypoxia radiopharmaceutical (i.e. 18F-MISO) [24].
, calculated from the 2TC3K and Patlak models were in great agreement, supporting the stability of 2TC3K model in this case. This shows that the model itself appears to be appropriate, since it delivers similar results in tumours as well as non-tumorous tissue, and also for other hypoxia radiopharmaceutical (i.e. 18F-MISO) [24].
Measuring hypoxia
The no-to-weak negative and the weak positive correlation observed between 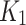 and the SUV-to-SUVmed ratio for index and contralateral cases, respectively, were not significant. yet, this result supports the hypothesis that there is a negative correlation between the tracer delivery and the perfusion for the index limb and that this inverse relation (between
and the SUV-to-SUVmed ratio for index and contralateral cases, respectively, were not significant. yet, this result supports the hypothesis that there is a negative correlation between the tracer delivery and the perfusion for the index limb and that this inverse relation (between 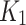 and SUV-to-SUVmed) can be used to distinguish between index and contralateral. However, further validation with bigger sample size is required. As for the positive correlation observed for contralateral limbs, it must be noted that patients under this study had both limbs diseased at different stages that could have affected the result. While not entirely comparable (since the evaluation was done in animal experiments) similar pattern of
and SUV-to-SUVmed) can be used to distinguish between index and contralateral. However, further validation with bigger sample size is required. As for the positive correlation observed for contralateral limbs, it must be noted that patients under this study had both limbs diseased at different stages that could have affected the result. While not entirely comparable (since the evaluation was done in animal experiments) similar pattern of 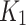 results were seen in hypoxic tumours i.e. in the publication by Choen et al. [26]. This again points towards the validity of the parameter to detect hypoxia even in non-tumorous tissue.
results were seen in hypoxic tumours i.e. in the publication by Choen et al. [26]. This again points towards the validity of the parameter to detect hypoxia even in non-tumorous tissue.
Other CLTI imaging methods
Novel imaging modalities for CLTI are relatively rare, and it is important to establish new quantitative methods since the classical thinking about the etiology of CLTI of flow, restoring flow and pressure measurements are insufficient to predict outcomes. As Reekers JA. pointed out, in other vascular sciences, new tests with improved predictive capabilities have been developed as well (i.e. coronary flow reserve (CFR) or index of microvascular resistance (IMR)) [4]. One could therefore view detecting and quantifying hypoxia is likely a functionality of the microcirculation.
A quantitative method to evaluate hypoxia in CLTI patients could potentially have several clinical applications or could support clinical decision making in different scenarios. For example, by quantifying the levels of hypoxia in anatomical areas of tissue loss (i.e. in diabetic patients), prognostication about the potential healing success might become possible. In more severe cases, the amount of tissue/limb which can still be recovered vs. what parts of the limb are too hypoxic to be recovered might be quantifiable and guide a respective surgical approach. While further prospective research is needed to answer those questions, such spatial and temporal quantification might offer additional information for clinical decision making for the entire leg like no other currently available method.
Limitations
This study has few limitations, notably, a major limitation is that a small number of patients were used, and a larger size sample (n > 52 at 95%CI) would be sufficient to confirm the findings before clinical translation. The small size sample was due to availability of subjects within the study time and strict inclusion criteria, and could have potential biases in the results such as bias due to selection and bias due to level of hypoxia from one patient to another. Here, it is worth noting that the selection criteria included only patients with confirmed CLTI, and therefore, selection does not affect the findings in this case. On the other hand, the study relies on the use of an established tracer, imaging and modelling techniques, as well as on discriminating the results against the conventional clinical parameter (ABI) related to CLTI. With this consideration, and the similarity of the findings to the literature, the sample size might have limited effect, and the difference in hypoxia levels might only affect the degree of significance. Nevertheless, the higher perfusion and diffusion parameters for the index vs. the contralateral limbs, even in a small size sample, suggest the suitability of the kinetic modelling in assessing CLTI. Another limitation is the duration of the dynamic scan being short, comparing to scanning duration reported in the literature [27]. The blood sampling for AIF was not obtained in this study which also was a limitation to improve the AIF accuracy. In any case, as shown in this study, the AIF on the index limb is different from the AIF on the healthy side. Therefore, image-based AIF is considered to be more useful, and accurate quantification of image-based AIF. This has already been demonstrated in brain vessels [28], which have a similar diameter to the lower limb vessels, so it is not a major limitation. Finally, there are different tissue contouring processes available in the literature which have not been compared in this study.
Conclusion
We showed the feasibility of hypoxia measurements with 18F-FAZA PET-MR in non-cancerous tissues in patients with CLTI. The use of AIF from independent indexed-limb was able to distinguish more precisely between the index and the contralateral limbs, when using 2TC3K. While the kinetic parameters (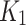 ,
, 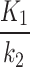 , and
, and  ) and the SUV may be used as metrics for detecting and measuring hypoxia, the clinical use case from interpreting these parameters is yet to be assessed.
) and the SUV may be used as metrics for detecting and measuring hypoxia, the clinical use case from interpreting these parameters is yet to be assessed.
Key points
QUESTION: Can CLTI be assessed using alternative non-invasive 18F-FAZA PET-MR imaging?
PERTINENT FINDINGS: 18F-FAZA PET-MR imaging can clearly distinguish the index from the contralateral limbs, and both static and dynamic PET imaging provided metrics to measure hypoxia. Hence, 18F-FAZA PET-MR imaging can potentially be used as a non-invasive method to assess CLTI.
IMPLICATIONS FOR PATIENT CARE: A quantitative method to evaluate hypoxia in CLTI patients could potentially have several clinical applications, and could support clinical decision-making in different scenarios. Results from such a method will ideally provide more accurate information in the future which muscle group is diseased the most and therefore could potentially guide specific intervention. It might even be possible to extract predictive values about success rate of intervention for this patient population. However, at this point, this remains an exploratory study, and further clinical validation is needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
PVH and SM: planned the image acquisition; TS and AK: image reading and contouring; AF: images processing, modelling and data analysis; AF and PVH: wrote the manuscript; AF, TS, PVH, AS, UM and KT: edited the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
Data will not be shared as per the institution policy.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Board of the University Health Network (ethics of antecedence without protocol identifiers). The research was conducted in accordance with the principles embodied in the 10 Declaration of Helsinki and in accordance with local statutory requirements.
Consent for publication
All enrolled patients gave written informed consent for the publication of this study and its accompanying images.
Informed consent
Informed consent was obtained from patients involved in this study. All procedures performed were in accordance with the research ethics protocol, approved by the University Health Network.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Santilli JD, Santilli SM. Chronic critical limb ischemia: diagnosis, treatment and prognosis. Am Fam Physician. 1999;59(7):1899–908. [PubMed] [Google Scholar]
- 2.Kayama T, Sano M, Inuzuka K, Katahashi K, Yata T, Yamanaka Y, et al. A pilot study investigating the use of regional oxygen saturation as a predictor of ischemic wound healing outcome after endovascular treatment in patients with chronic limb-threatening ischemia. Annals Vascular Dis. 2021;14(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery,* Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. circulation. 2006;113(11):e463-e654. [DOI] [PubMed]
- 4.Reekers JA. Stop turning a blind eye! CVIR Endovascular. 2021;4(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreuder SM, Nieuwdorp M, Koelemay MJ, Bipat S, Reekers JA. Testing the sympathetic nervous system of the foot has a high predictive value for early amputation in patients with diabetes with a neuroischemic ulcer. BMJ Open Diabetes Res Care. 2018;6(1):e000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höckel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991;51(22):6098–102. [PubMed] [Google Scholar]
- 7.Lewis DM, Park KM, Tang V, Xu Y, Pak K, Eisinger-Mathason TK et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proceedings of the National Academy of Sciences. 2016;113(33):9292-7. [DOI] [PMC free article] [PubMed]
- 8.Chapelin F, Gedaly R, Sweeney Z, Gossett LJ. Prognostic value of Fluorine-19 MRI oximetry monitoring in cancer. Mol Imag Biol. 2022:1–12. [DOI] [PubMed]
- 9.Hillestad T, Hompland T, Fjeldbo CS, Skingen VE, Salberg UB, Aarnes E-K, et al. MRI distinguishes tumor hypoxia levels of different prognostic and biological significance in cervical Cancer. Cancer Res. 2020;80(18):3993–4003. [DOI] [PubMed] [Google Scholar]
- 10.Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27(7):661–70. [DOI] [PubMed] [Google Scholar]
- 11.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations J Cereb Blood Flow Metabolism. 1985;5(4):584–90. [DOI] [PubMed] [Google Scholar]
- 12.Beck R, Röper B, Carlsen JM, Huisman MC, Lebschi JA, Andratschke N, et al. Pretreatment 18F-FAZA PET predicts success of hypoxia-directed radiochemotherapy using Tirapazamine. J Nucl Med. 2007;48(6):973–80. [DOI] [PubMed] [Google Scholar]
- 13.Shi K, Astner S, Souvatzoglou M, Miederer I, Wilkens J, Vaupel P, et al. editors. Quantitative assessment of hypoxia kinetic models by a cross-study of dynamic 18 F-FAZA and 15 OH 2 O in head and neck tumors. 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC); 2009: IEEE.
- 14.Fleming IN, Manavaki R, Blower PJ, West C, Williams K, Harris A, et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2015;112(2):238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gammon ST, Pisaneschi F, Bandi ML, Smith MG, Sun Y, Rao Y, et al. Mechanism-specific pharmacodynamics of a novel complex-I inhibitor quantified by imaging reversal of consumptive hypoxia with [18F] FAZA PET in vivo. Cells. 2019;8(12):1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Reviews Clin Oncol. 2012;9(12):674–87. [DOI] [PubMed] [Google Scholar]
- 17.Lopci E, Grassi I, Chiti A, Nanni C, Cicoria G, Toschi L, et al. PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am J Nucl Med Mol Imaging. 2014;4(4):365. [PMC free article] [PubMed] [Google Scholar]
- 18.Verwer EE, Bahce I, van Velden FH, Yaqub M, Schuit RC, Windhorst AD, et al. Parametric methods for quantification of 18F-FAZA kinetics in non–small cell lung cancer patients. J Nucl Med. 2014;55(11):1772–7. [DOI] [PubMed] [Google Scholar]
- 19.Chang E, Liu H, Unterschemmann K, Ellinghaus P, Liu S, Gekeler V, et al. 18F-FAZA PET imaging response tracks the reoxygenation of tumors in mice upon treatment with the mitochondrial complex I inhibitor BAY 87-2243. Clin Cancer Res. 2015;21(2):335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, et al. A comparative study of the hypoxia PET tracers [18F] HX4,[18F] FAZA, and [18F] FMISO in a preclinical tumor model. Int J Radiation Oncology* Biology* Phys. 2015;91(2):351–9. [DOI] [PubMed] [Google Scholar]
- 21.Metran-Nascente C, Yeung I, Vines DC, Metser U, Dhani NC, Green D, et al. Measurement of tumor hypoxia in patients with advanced pancreatic cancer based on 18F-fluoroazomyin arabinoside uptake. J Nucl Med. 2016;57(3):361–6. [DOI] [PubMed] [Google Scholar]
- 22.Christensen NL, Sørensen J, Bouchelouche K, Madsen MA, Buhl CS, Tolbod LP. Repeatability of [15O] H2O PET imaging for lower extremity skeletal muscle perfusion: a test–retest study. EJNMMI Res. 2024;14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou TH, Nabavinia M, Tram NK, Rimmerman ET, Patel S, Musini KN, et al. Quantification of skeletal muscle perfusion in peripheral artery disease using 18F-Sodium fluoride positron emission tomography imaging. J Am Heart Association. 2024;13(4):e031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorwarth D, Eschmann SM, Paulsen F, Alber M. A kinetic model for dynamic [18F]-Fmiso PET data to analyse tumour hypoxia. Phys Med Biol. 2005;50(10):2209. [DOI] [PubMed] [Google Scholar]
- 25.Napieczynska H, Kolb A, Katiyar P, Tonietto M, Ud-Dean M, Stumm R, et al. Impact of the arterial input function recording method on kinetic parameters in small-animal PET. J Nucl Med. 2018;59(7):1159–64. [DOI] [PubMed] [Google Scholar]
- 26.Choen S, Kent MS, Chaudhari AJ, Cherry SR, Krtolica A, Zwingenberger AL. Kinetic evaluation of the hypoxia radiotracers [18F] FMISO and [18F] FAZA in dogs with spontaneous tumors using dynamic PET/CT imaging. Nuclear Med Mol Imaging. 2023;57(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savi A, Incerti E, Fallanca F, Bettinardi V, Rossetti F, Monterisi C, et al. First evaluation of PET-based human biodistribution and dosimetry of 18F-FAZA, a tracer for imaging tumor hypoxia. J Nucl Med. 2017;58(8):1224–9. [DOI] [PubMed] [Google Scholar]
- 28.Little PV, Arnberg F, Jussing E, Lu L, Ingemann Jensen A, Mitsios N, et al. The cellular basis of increased PET hypoxia tracer uptake in focal cerebral ischemia with comparison between [18F] FMISO and [64Cu] CuATSM. J Cereb Blood Flow Metabolism. 2021;41(3):617–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will not be shared as per the institution policy.







