Abstract
Background
Hydrometrocolpos (HMC) is a rare prenatal ultrasound abnormality, presenting in two distinct types: urinary-type and secretory-type. The urinary variation is intricately linked to cloacal malformation (CM), thereby posing a heightened risk of perinatal adverse events. Additionally, children affected by this type often face long-term challenges that impact their quality of life. Therefore, predicting the presence of CM in HMC fetuses is of great clinical significance.
Case presentation
We present a case involving hydrometrocolpos accompanied by oligohydramnios. Prenatally, the condition was strongly indicative of CM based on imaging manifestations and intrauterine procedures, a suspicion that was later confirmed through postnatal autopsy.
Conclusion
Due to the extremely low incidence of HMC, current literature primarily consists of case reports, and there are no studies that comprehensively analyze the prognosis of the disease. This research fills this gap by statistically analyzing the present case as well as 164 prenatal HMC cases reported in the previous literature. Gestational age (GA) at initial detection, the presence of fetal ascites, urinary tract dilatation, anorectal imaging abnormalities, and Müllerian anomalies are meaningful predictors of CM in HMC fetuses. Intrauterine procedures can be used to preserve organ function in complete lower urinary tract obstruction and oligohydramnios secondary to HMC. In addition, biochemical analysis of HMC fluid can differentiate between urinary-type HMC and secretory-type HMC. This study provides valuable insights into the prognostic factors and management strategies for HMC, which could guide clinical decision-making in prenatal care.
Supplementary Information
The online version contains supplementary material available at10.1007/s00404-025-08004-8.
Keywords: Hydrometrocolpos, Cloacal malformation, Fetal ascites, Intrauterine procedures
What does this study add to the clinical work
| This study aims to utilize non-invasive and invasive methods to gain comprehensive prenatal information to better understand the anticipated challenges HMC fetuses may face postnatally, preparing for potential extended and multifaceted treatment. |
Background
Hydrometrocolpos (HMC) is a rare prenatal ultrasound abnormality characterized by a bilobed cystic structure positioned posterior to the fetal bladder, anterior to the sacrum, and deep within the pelvic floor. Its core manifestation involves the accumulation of fluid in the vagina and the uterus. The incidence of congenital HMC in full-term female newborns ranges from 0.0014 to 0.1% [1].
Some cases of HMC result from vaginal atresia, imperforate hymen, or a transverse vaginal membrane, primarily confined to the genital tract. This variant, termed secretory HMC, involves the accumulation of mucus secreted by the uterine and cervix glands. The condition is uncomplicated and in most cases can be cured with a relatively straightforward treatment approach after delivery [2].
Conversely, another subset of HMC is intricately linked with cloacal malformation (CM), extending beyond genital tract anomalies to include issues like rectal and anal developmental abnormalities, urethrovaginal and rectovaginal fistulae, or ambiguous genitalia. In this form, the HMC fluid component is a mix source of secretions from the genital and urinary tracts, and sometimes from the gastrointestinal (GI) tract. Termed urinary-type HMC, this form is frequently associated with a heightened risk of perinatal adverse events, necessitating multiple postnatal surgical interventions that significantly impact the child's long-term quality of life [3]. Consequently, parents often seek comprehensive prenatal information to better understand the anticipated challenges their fetus may face after birth, preparing for potential extended and multifaceted treatments.
While some reports on the prenatal diagnosis of HMC exist, a comprehensive summary addressing both the ultrasound abnormality and postpartum status, as well as the value of intrauterine therapy, has been lacking. This report presents a case of HMC combined with oligohydramnios, which was initially suspected prenatally to be associated with CM based on imaging manifestations and the impact of intrauterine procedures, and later confirmed by postnatal autopsy. This study also summarized previous HMC cases with detailed perinatal data and further analyzed the prenatal imaging factors that can help clarify the severity of HMC prenatally.”
Case presentation
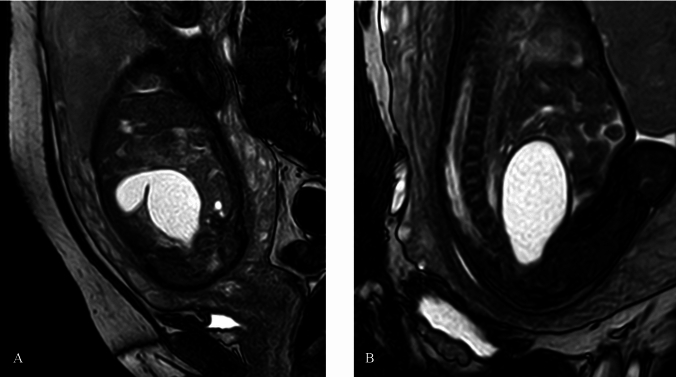
A 30-year-old woman, gravida 3 para 0, conceived spontaneously. The patient had a history of two previous embryonic arrests and was subsequently diagnosed with low-level 45,X/46,XX mosaicism. For this pregnancy, fetal nuchal translucency (NT) measurement and non-invasive prenatal testing (NIPT) results were normal. Fetal growth and amniotic fluid levels remained within normal range during ultrasound examinations at 22 and 26 weeks. However, at 29 + 6 weeks, the patient was referred to our center due to a suspected fetal anomaly. Ultrasonography revealed oligohydramnios (amniotic fluid depth [AFD]: 1.2 cm; amniotic fluid index [AFI]: 3.0 cm), bilateral hydronephrosis, right hydroureter, a pelvic cyst (4.1 × 2.1 × 3.3 cm), and a potential fistula between the bladder and the cyst as shown in Fig. 1. Magnetic resonance imaging (MRI) examination further confirmed persistent oligohydramnios and a gourd-shaped cyst in the lower abdomen of the fetus, measuring a maximum of 5.7 × 3.1 × 3.2 cm. The cystic lesion, originating from the pelvic floor and extending up to the umbilicus, compressed the rectum posteriorly, although the intestinal meconium signal remained normal (Fig. 2). These detailed findings were consistent with a diagnosis of HMC, which was hypothesized to be caused by CM based on imaging characteristics.
Fig. 1.

Sonographic image demonstrating HMC posterior to the fetal bladder (B), with fluid accumulation in the vagina (V) and uterus (U). No amniotic fluid is observed around the fetus
Fig. 2.
MRI of HMC (A: coronal image; B: sagittal image). A gourd-shaped cyst in the lower fetal abdomen demonstrated high signal on T2-weighted images. MRI can show the location information of the cyst
Following a 2-week interval, ultrasonography indicated a further reduction in amniotic fluid levels (AFD: 0.8cm, AFI: 1.6cm) and an enlargement of the fetal abdominal cyst (7.9 × 6.8 × 6.8cm). Additionally, fetal short femur length (-2.9 SD for Asian fetuses at the same gestational week) and lateral ventriculomegaly were observed. In response, a multidisciplinary consultation was convened to discuss the prognosis of CM, the potential for pulmonary hypoplasia, details of pediatric surgery, and the risk of genetic disorders. An invasive test for fetal karyotyping was recommended but declined by the mother. The parents expressed hesitation regarding the prognosis of fetal CM and requested cystic fluid aspiration to determine whether the HMC was of the secretory- or urinary-type.
At 33 weeks of gestation, ultrasound-guided aspiration of 80 mL of cystic fluid was performed. Upon complete aspiration, the cyst collapsed, theoretically releasing external pressure obstruction of the urinary tract. Interestingly, a few minutes later, bladder emptying was observed, followed by rapid reaccumulation of fluid in the cyst and the absence of additional amniotic fluid around the fetus. This confirmed the presence of a vesicovaginal fistula. Laboratory examination of clear and cloudy cystic fluid revealed creatinine levels of 607 and 620 µmol/L, urea nitrogen levels of 18.7 and 19.7 mmol/L, adenosine deaminase levels of 2.3 and 4.0 U/L, and lactate dehydrogenase levels of 303 and 729 U/L, respectively. Based on these results, a diagnosis of urinary-type HMC and suspected CM was established.
The parents made the decision to terminate the pregnancy, resulting in the delivery of a female fetus weighing 1830 g (17th percentile for Asian newborns at the same gestational week). Physical examination revealed two openings in the perineum (Fig. 3). The anterior orifice was confirmed as the opening of the common urogenital channel through urethrogram and autopsy. The posterior orifice, stained with meconium, was identified as an anterior anus. At this juncture, the fetus was conclusively diagnosed with a cloaca variant, one type of the spectrum of CM. Anatomy of abdominal organs revealed a slender fallopian tube, which may explain the absence of fetal ascites (Fig. 4). Both placental pathology and fetal whole-exome sequencing (WES) yielded unremarkable results.
Fig. 3.

Appearance of the perineum of the fetus (long arrow: common urogenital orifice; short arrow: anterior anus)
Fig. 4.
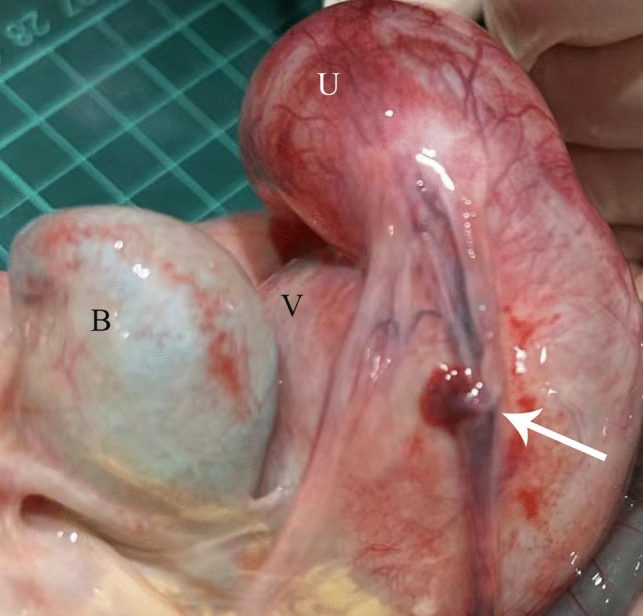
Fetal autopsy. A enlarged uterus (U), vagina (V), bladder (B) and slim fallopian tubes (arrow) can be seen
Literature review
In this case, we successfully established a prenatal diagnosis of CM through invasive examination. However, recognizing the potential risks associated with invasive procedures during pregnancy, we sought to investigate the viability of non-invasive imaging methods for identifying CM in HMC fetuses, aiming to address the following two questions:
1. How can the risk of intrauterine and postnatal mortality in HMC fetuses be predicted?
2. How can the risk of combined urinary tract, gastrointestinal (GI) tract, and genital anomalies be predicted, and how can clinicians prepare for subsequent multiple reconstructive surgeries?
To achieve this, we conducted an electronic search of Medline and Embase from 1980 to December 2024, using combinations of the following terms: hydrometrocolpos, hydrocolpos, cloacal malformation, cloaca, urorectal septum malformation, urogenital sinus, vaginal septum, vaginal atresia, imperforate hymen, hydrocolpos, dilated uterus, dilated vagina, congenital vaginal obstruction, prenatal diagnosis, MURCS, and MRKH. The search and selection criteria were limited to English language publications, and only full-text articles were considered eligible for inclusion. Articles not relevant to the topic were excluded. Studies exclusively featuring cases detected postnatally were excluded as were those reviews and retrospective researches that did not have detailed case reports. Additionally, 122 articles without detailed perinatal data or descriptions of postnatal outcomes as well as 19 articles involving severe fetal anomalies such as cloacal exstrophy were also excluded. After exclusion, 108 articles consisting of 19 case series and 89 case reports were reviewed.
A total of 164 articles meeting the specified criteria were identified, and details are presented in Table S1. The analyzed antenatal factors included the gestational age at which HMC was initially detected, the maximum diameter of HMC, the presence of fluid debris within HMC, combined fetal ascites or oligohydramnios, urinary tract dilatation (UTD: hydronephrosis or hydroureter), unilateral or bilateral dysplastic kidney, digestive tract dilatation, anorectal imaging abnormalities (e.g., invisible rectum or anus, high rectum, meconium not reaching the perineum, or enterolithiasis), Müllerian malformations (e.g., uterus didelphis, or vaginal septum). The postnatal confirmation of anatomical etiology served as the outcome of the study, leading to the division of the 164 HMC cases into two groups: ORT (obstruction of the reproductive tract) group and CM group, the latter group including urogenital sinus, cloacal dysgenesis, persistent cloaca/classic cloaca, posterior cloaca, cloacal variant, and posterior cloacal variant [4].
Data were analyzed using IBM SPSS Statistics 26 and R software Version R 4.2.1. Normality tests using the Kolmogorov–Smirnov test were conducted for continuous variables. The GA at initial detection was proved to be a non-normalized distribution, and max HMC diameter had normality characteristics, both of the 2 factors were represented as median (interquartile range). The Kruskal–Wallis test statistics was used for analysis the significant of GA at initial detection. One-way ANOVA was used to study the differences of max HMC diameter. For clinical data with categorical variables, it was represented as absolute numbers and percentages (%). The chi-square test (cross-tabulation) was used to examine the differences these categorical variables of between 2 groups. In univariate analysis, each potential predictor was assessed for its association with the outcome, and significant variables were included in the multivariate model. We constructed a clinical predictive model using multivariate ordinal logistic regression analysis. Patients with missing data were excluded from the analyses. All reported P values were two-sided, and significance was set at P < 0.05. Model performance was evaluated using the ROC curve, and area under the curve (AUC).
Anorectal imaging abnormalities, such as invisible rectum or anus, high rectum, meconium not reaching the perineum, and enterolithiasis, were present exclusively in the CM group and demonstrated a strong positive predictive value for CM. Univariate analysis of other variables indicated that GA at initial detection, fetal ascites, oligohydramnios, UTD, and Müllerian anomalies showed statistically significant differences. No statistically significant differences were observed for the remaining factors. After fully eliminating confounding factors, the multivariate logistic analysis further showed that the GA at initial detection (p = 0.009), fetal ascites (p = 0.011), UTD (p = 0.020), and Müllerian anomalies (p = 0.027) were independent predict factors for CM (Table 1). Figure 5 showed that the predictive efficiency of the logistic regression model was adequate, with an area under the receiver operating characteristic curve (AUROC) of 0.8601 (95% CI [confidence interval]: 0.79630.9239).
Table 1.
The univariate analysis of HMC ultrasound characterization with or without CM
| Variables | ORT group (N = 42) |
CM group (N = 122) |
Univariate analysis (P value) |
Multivariate logistic regression analysis (P value/RC) |
|---|---|---|---|---|
| GA at initial detection | 34 (30, 36) | 30 (27,32) | 0.0001* | 0.009/-0.166* |
| Maximum HMC diameter | 7.7(6.1, 8.6) | 7.8 (5.5, 9.6) | 0.8015 | |
| Fluid-debris in HMC | 8/42(19.05%) | 23/122 (18.85%) | 0.9007 | |
| Fetal ascites | 5/42(11.90%) | 63/122 (51.64%) | < 0.0001* | 0.011/1.517* |
| Oligohydramnios | 4/42(9.52%) | 38/122 (31.15%) | 0.0108* | 0.114/1.161 |
| UTD | 18/42(42.86%) | 89/122 (72.95%) | 0.0007* | 0.020/1.198* |
| Dysplastic kidney | 4/42(9.52%) | 24/122 (19.67%) | 0.1312 | |
| Digestive tract dilatation | 1/42(2.38%) | 15/122 (12.30%) | 0.0937 | |
| Anorectal imaging abnormalities | 0/42(0.00%) | 21/122 (17.21%) | # | # |
| Müllerian anomalies | 3/42(7.14%) | 46/122 (37.70%) | 0.0009* | 0.027/1.535* |
#This variable was positive only in CM-URSMS subgroup, making the analysis not fit a logistic regression due to perfect separation. GA gestational age, HMC hydrometrocolpos, GI gastrointestinal, UTD urinary tract dilatation, ORT obstruction of the reproductive tract, CM cloacal malformation, RC regression coefficient. *P < 0.05
Fig. 5.

Logistic regression model and the receiver operating characteristic (ROC) curves for predicting CM in HMC fetuses with the GA at initial detection and imaging variables
Discussion
The spectrum of CM, primarily occurring in female fetuses, represents the most severe form of anorectal and urogenital malformation. It is hypothesized to result from differential timing of arrest in the development of the urorectal septum and urogenital sinus [5]. The estimated incidence is 1 in 50,000 births [6]. Affected fetuses often require multiple complex surgical procedures for reconstruction after birth, with a potential for long-term impacts on quality of life. Versteegh’s systematic review revealed frequent functional disturbances in all three systems (anorectal, urinary, and gynecologic): only 57% of patients achieved voluntary bowel movements, less than half were able to void spontaneously, and only 35% exhibited normal menstruation upon reaching puberty [3]. Therefore, prenatal diagnosis of CM is crucial as it provides parents with more time to understand the condition and prepare psychologically.
In a retrospective analysis at Cincinnati Children’s Hospital Medical Center in 2010, prenatal ultrasound reports of 95 patients born with CM were examine [7]. Abdominal or pelvic cystic/mass was the most frequent ultrasound abnormality (39/95). Five years later, another report from the same center suggested that CM could be identified through prenatal ultrasound, with suspicious signs in order of frequency being abdominal cystic mass, bilateral hydronephrosis, oligohydramnios, ascites, and distended bowel [6]. Therefore, accurate interpretation of HMC features on prenatal ultrasound is particularly important.
Our literature review revealed that diagnosing HMC was more challenging when ultrasound was the sole prenatal imaging method as it primarily relied on identifying a retrovesical cyst. In recent years, the introduction of MRI technology in prenatal imaging has unveiled more detailed features of HMC and surrounding tissues, significantly facilitating diagnosis. MRI offers superior visualization of the location and fluid signal intensity of fetal abdominal cysts, the distribution of meconium in fetal intestinal segments, and genitalia morphology, regardless of fetal position. When used in conjunction with ultrasound, MRI provides comprehensive prenatal imaging data, enhancing multifactorial analysis. As a result, prenatal MRI plays a pivotal role in evaluating HMC, identifying its likely etiology, and supporting multidisciplinary consultation, counseling, prognostication, and perinatal care for affected families [8, 9].
Newman and Capito proposed a simplified diagnostic decision tree algorithm to utilize prenatal information for distinguishing CM from isolated genital obstructions in HMC fetuses [9, 10]. Their algorithm relies on evaluating the signal intensity of HMC fluid and meconium, as well as genital morphology. However, the small sample sizes in both studies limit their applicability. In contrast, our study aims to enhance prenatal differentiation between CM and isolated genital obstructions by analyzing a larger sample of retrospective cases and incorporating more comprehensive imaging data.
Our findings suggest that as the GA at the time of initial detection increases, the likelihood of CM decreases. Additionally, certain ultrasound findings—such as abdominal fluid accumulation, hydronephrosis or hydroureter, and a longitudinal septum within the HMC—are strongly associated with CM. However, it is important to note that the potential impact of urine and meconium causing irritation and subsequent tube obstruction, which may lead to the disappearance of ascites, highlights the need for vigilance even in HMC cases without fetal ascites, as exemplified in our case [11]. Anorectal imaging abnormalities, such as an absent target ring, enterolithiasis, or meconium signals failing to reach the anus, were observed exclusively in our CM group. These findings demonstrated a strong positive predictive value, consistent with results from previous studies [9, 12]. However, it is important to acknowledge that this retrospective analysis of the literature may still be subject to biases, such as missing data. For earlier studies, we also considered whether limitations in imaging technology at the time could have contributed to false-negative results for the imaging variables.
Despite our findings, we are yet to identify a reliable negative predictive imaging indicator for CM. Predicting associated CM in cases of HMC without other imaging abnormalities or late-onset HMC remains challenging. Notably, six cases in the CM group presented no other fetal structural abnormalities, ascites, oligohydramnios, and exhibited normal external genitalia and anorectal anatomy. Therefore, in cases where parents seek prenatal clarification regarding the possibility of CM, interventional techniques may be considered to provide more definitive information.
Interventional procedures play a crucial role in clarifying the diagnosis of HMC and differentiating it from other fetal abdominal cysts. Chen proposed that cytological findings, such as mucus-like material and atrophied nuclei, indicate the presence of estrogen-affected vaginal squamous epithelium, suggesting that the fetal cyst was a dilated vagina. However, the absence of biochemical testing of the cystic fluid in their case report limited their ability to confirm a prenatal diagnosis of CM [13]. Lecarpentier further demonstrated that estradiol, digestive enzymes, and intestinal alkaline phosphatase isoenzymes could help differentiate HMC, ovarian cysts, and CM [14]. In our study, we performed urinary markers analysis of the fetal HMC cyst fluid after aspiration. The creatinine concentration in the cyst fluid was comparable to neonatal urine levels at the same GA but significantly higher than neonatal serum creatinine levels. This finding supported a diagnosis of urinary-type HMC, pointing toward CM. Cytological and biochemical analyses have also been applied to assess ascites secondary to HMC. Morikawa analyzed the cytology and biochemistry of ascitic fluid, including protein, bilirubin, bile acids, IgG, ammonia, and microglobulin, identifying the fluid as urinary ascites [15]. These diagnostic tools have proven instrumental in establishing more accurate prenatal diagnoses.
On the other hand, according to Peiro et al., intrauterine therapy for CM should be limited to preserving organ functions, mainly kidney and lung development [6]. Complete lower urinary tract obstruction can result in hydronephrosis, progressive renal dysfunction, and pulmonary hypoplasia secondary to oligohydramnios. This therapeutic principle is believed to extend to intrauterine therapy for HMC. Han recommended the decompression of HMC through prenatal ultrasound-guided fluid aspiration in cases of severe urinary tract obstruction [16]. Similarly, Chen proposed that prenatal drainage of fluid from HMC through ultrasound-guided aspiration can help decompress distended genital organs and alleviate the severity of urinary obstruction [13]. In our case, we pondered the prospect of treating highly suspicious CM in HMC fetuses through invasive intrauterine procedures. Our patient had a normal amount of amniotic fluid until 29 weeks of gestation, suggesting an unobstructed passage from the bladder to the common urogenital orifice. However, during our intrauterine procedure, we found that even though the HMC was emptied after aspiration and the lower urinary tract obstruction was relieved, urine could only flow to the HMC via the bladder, not reaching the common urogenital orifice and the amniotic cavity to alleviate oligohydramnios. This finding implies the gradual development of the urethrovaginal fistula into a unidirectional valve structure. In this scenario, a single aspiration may not suffice to preserve organ functions, prompting consideration of HMC-amniotic shunting as an alternative option for intrauterine therapy.
Finally, HMC can be part of several syndromes, for example, McKusick–Kaufman (MKK) syndrome, Bardet–Biedl syndrome (BBS), the VACTERL association, Pallister-Hall syndrome (PHS), Langer–Giedion syndrome, Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome, or even Herlyn–Werner–Wunderlich (HWW) syndrome. It has also been reported that HMC may be associated with sacrococcygeal teratoma [8].In our literature review, we identified 19 cases of postaxial polydactyly (PAP), 18 cases of polyhydramnios, 16 cases of single umbilical artery (SUA), 14 cases of congenital heart disease (CHD), 12 cases of vertebral anomaly, 10 cases of fetal growth restriction (FGR), 6 cases of duodenal or esophageal malformation, 3 cases of ventriculomegaly, and 2 cases of HWW. Therefore, it is crucial to evaluate for additional structural malformations outside the genital, urinary, and GI tracts.
Conclusion
Due to the extremely low incidence of HMC, current literature primarily consists of case reports, with no comprehensive studies analyzing the prognosis of the disease. This research addresses that gap. GA at initial detection, as well as the presence of fetal ascites, hydronephrosis, hydroureter, anorectal imaging abnormalities, and a longitudinal septum within HMC, are significant predictors of CM in HMC fetuses. However, predicting CM in cases of HMC without other imaging abnormalities remains challenging. Intrauterine procedures offer a valuable means of prenatal diagnosis and can help preserve organ function in cases of complete lower urinary tract obstruction and oligohydramnios secondary to HMC. Additionally, biochemical analysis of HMC fluid is an effective tool for differentiating between urinary-type and secretory-type HMC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the doctors and staff who have been involved in this work. All persons that contributed to this study are listed as authors and meet the criteria for authorship.
Abbreviations
- HMC
Hydrometrocolpos
- CM
Cloacal malformation
- GA
Gestational age
- GI
Gastrointestinal
- NT
Nuchal translucency
- NIPT
Non-invasive prenatal testing
- AFD
Amniotic fluid depth
- AFI
Amniotic fluid index
- MRI
Magnetic resonance imaging
- WES
Whole-exome sequencing
- MURCS
Müllerian duct aplasia–renal aplasia–cervicothoracic somite dysplasia association
- MRKH
Mayer–Rokitansky–Küster–Hauser
- UTD
Urinary tract dilatation
- ORT
Obstruction of the reproductive tract
- UGS
Urogenital sinus
- URSMS
Urorectal septum malformation sequence
- AUC
Area under the curve
- IUD
Intrauterine death
- NND
Neonatal death
- TOP
Termination of pregnancy
- FGR
Fetal growth restriction
- SUA
Single umbilical artery
- CHD
Congenital heart disease
- PAP
Postaxial polydactyly
- MKS
McKusick–Kaufman syndrome
- BBS
Bardet–Biedl syndrome
- HWW
Herlyn–Werner–Wunderlich syndrome
- OHVIRA
Obstructed hemivagina and ipsilateral renal agenesis
- PHS
Pallister–Hall syndrome
Author contributions
ML participated in the analysis of cases and literature review. FK carried out the statistical analysis. RJ conceived anddesigned the whole study, supervised the work, and revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by Sichuan Science and Technology Program (2023ZYD0117).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
Approval was obtained from the Ethics Committee of West China Second Hospital, Sichuan University. Informed consent was obtained from all participants. All methods were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meylign Long and Kaiyu Fu have contributed equally to this work and are co first authors.
References
- 1.Sharma D, Murki S, Pratap OT et al (2015) A case of hydrometrocolpos and polydactyly. Clin Med Insights Pediatr 9:7–11. 10.4137/CMPed.S20787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen MC, Chang YL, Chao HC (2022) Hydrometrocolpos in infants: etiologies and clinical presentations. Children (Basel). 10.3390/children9020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Versteegh HP, van Rooij IALM, Levitt MA et al (2013) Long-term follow-up of functional outcome in patients with a cloacal malformation: a systematic review. J Pediatr Surg 48(11):2343–2350. 10.1016/j.jpedsurg.2013.08.027 [DOI] [PubMed] [Google Scholar]
- 4.Dannull KA, Browne LP, Meyers MZ (2019) The spectrum of cloacal malformations: how to differentiate each entity prenatally with fetal MRI. Pediatr Radiol 49(3):387–398. 10.1007/s00247-018-4302-x [DOI] [PubMed] [Google Scholar]
- 5.Vilanova-Sanchez A, Halleran DR, Reck CA et al (2020) Factors predicting the need for vaginal replacement at the time of primary reconstruction of a cloacal malformation. J Pediatr Surg 55(1):71–74. 10.1016/j.jpedsurg.2019.09.054 [DOI] [PubMed] [Google Scholar]
- 6.Peiro JL, Scorletti F, Sbragia L (2016) Prenatal diagnosis of cloacal malformation. Semin Pediatr Surg 25(2):71–75. 10.1053/j.sempedsurg.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Bischoff A, Levitt MA, Lim FY et al (2010) Prenatal diagnosis of cloacal malformations. Pediatr Surg Int 26(11):1071–1075. 10.1007/s00383-010-2685-3 [DOI] [PubMed] [Google Scholar]
- 8.Mallmann MR, Reutter H, Mack-Detlefsen B et al (2019) Prenatal diagnosis of hydro(metro)colpos: a series of 20 cases. Fetal Diagn Ther 45(1):62–68. 10.1159/000486781 [DOI] [PubMed] [Google Scholar]
- 9.Newman CL, Forbes-Amrhein MM, Brown BP et al (2024) Prenatal hydrocolpos: imaging findings and differential diagnosis. Pediatr Radiol 54(10):1618–1630. 10.1007/s00247-024-05990-w [DOI] [PubMed] [Google Scholar]
- 10.Capito C, Belarbi N, Paye Jaouen A et al (2014) Prenatal pelvic MRI: additional clues for assessment of urogenital obstructive anomalies. J Pediatr Urol 10(1):162–166. 10.1016/j.jpurol.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 11.Hung YH, Tsai CC, Ou CY et al (2008) Late prenatal diagnosis of hydrometrocolpos secondary to a cloacal anomaly by abdominal ultrasonography with complementary magnetic resonance imaging. Taiwan J Obstet Gynecol 47(1):79–83. 10.1016/S1028-4559(08)60059-5 [DOI] [PubMed] [Google Scholar]
- 12.Dhombres F, Jouannic JM, Brodaty G et al (2007) Contribution of prenatal imaging to the anatomical assessment of fetal hydrocolpos. Ultrasound Obst Gyn 30(1):101–104. 10.1002/uog.3998 [DOI] [PubMed] [Google Scholar]
- 13.Chen CP, Liu FF, Jan SW et al (1996) Ultrasound-guided fluid aspiration and prenatal diagnosis of duplicated hydrometrocolpos with uterus didelphys and septate vagina. Prenat Diagn 16(6):572–576. 10.1002/(SICI)1097-0223(199606)16:6%3c572::AID-PD913%3e3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 14.Lecarpentier E, Dreux S, Blanc T et al (2012) Biochemical analysis of cystic fluid in the diagnosis of fetal intra-abdominal masses. Prenatal Diag 32(7):627–631. 10.1002/pd.3871 [DOI] [PubMed] [Google Scholar]
- 15.Morikawa M, Yamada T, Cho K et al (2006) Prenatal diagnosis and therapy of persistent cloaca: a case report. Fetal Diagn Ther 21(4):343–347. 10.1159/000092463 [DOI] [PubMed] [Google Scholar]
- 16.Han BH, Park SB, Lee YJ et al (2013) Uterus didelphys with blind hemivagina and ipsilateral renal agenesis (Herlyn-Werner-Wunderlich syndrome) suspected on the presence of hydrocolpos on prenatal sonography. J Clin Ultrasound 41(6):380–382. 10.1002/jcu.21950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.