Abstract
Importance
There is no antifungal otic drug for the treatment of otomycosis approved in the United States. Some current clotrimazole formulations in the market are used off-label.
Objective
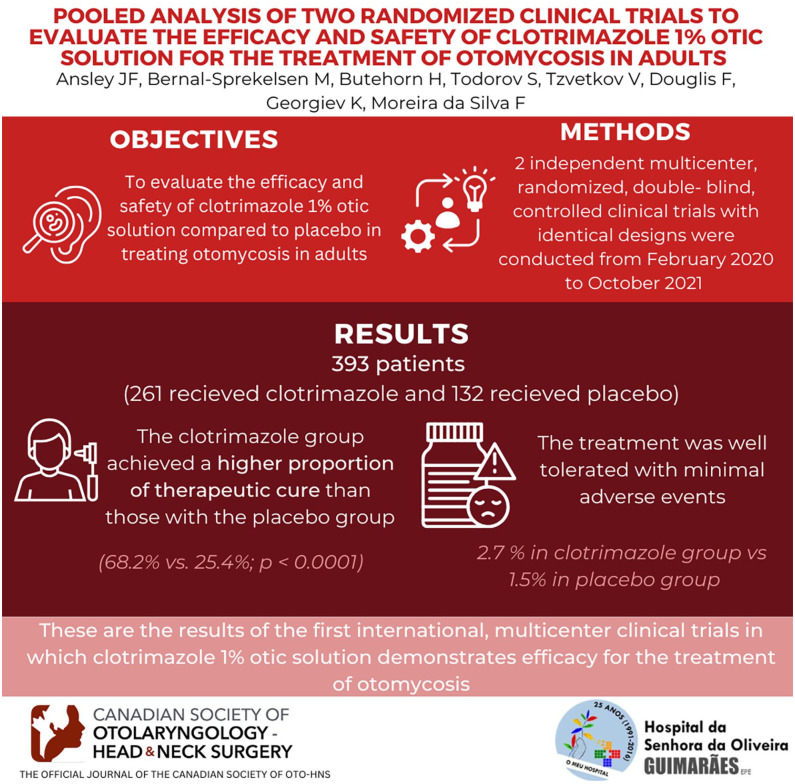
To evaluate the efficacy and safety of clotrimazole 1% otic solution compared to placebo in treating otomycosis in adults.
Design
Two independent twin multicenter, randomized (2:1), double-blind, controlled clinical trials with identical designs were conducted from February 2020 to October 2021.
Setting
Fifty-three sites located in the United States, Mexico, and Europe.
Participants
Adults with uncomplicated otomycosis presented with symptoms, debris, and drainage clinically consistent with fungal infection.
Intervention
Patients received clotrimazole or placebo twice daily for 14 days and were evaluated on visit 1 (day 1), visit 2 (day 8-10), visit 3 (day 15), and follow-up visit 4 (day 24-26).
Main Outcome Measures
At each visit, pruritus, otalgia, otorrhea, and ear fullness were assessed. Ear exudate was taken for a mycological and microbiological evaluation at baseline and, if present, at subsequent visits. The primary endpoint was a therapeutic cure (mycological and clinical) at visit 4 in the randomized population with positive fungal culture at baseline [mycological intent-to-treat (MITT)].
Results
Three hundred ninety-three patients received study medication (261 clotrimazole and 132 placebo). Efficacy data from the 228 patients (157 clotrimazole and 71 placebo) included in the MITT were analyzed. The clotrimazole group achieved a higher proportion for the primary endpoint than those with the placebo group (68.2% vs 25.4%; P < .0001), with a 42.8 difference in response rate (95% confidence interval: 30.3, 55.3). The treatment was safe and well tolerated, with 2.7% of related adverse events in the clotrimazole versus 1.5% in the placebo group.
Conclusions
Clotrimazole 1% otic solution has demonstrated its superiority over the placebo in each study and the pooled analysis. These are the results of the first international, multicenter clinical trials in which clotrimazole 1% otic solution demonstrates efficacy for the treatment of otomycosis.
Keywords: clotrimazole, otic solution, otomycosis, antifungal
Graphical Abstract.
Introduction
Otomycosis is a superficial infection involving the external auditory canal caused by yeasts and filamentous fungi. The incidence of the disease and the pathogens involved vary in the different geographical areas. Several studies have reported Aspergillus and Candida species as the most common isolated organisms in otomycosis, with Aspergillus species being the predominant fungi. 1 Fungi grow rapidly on the corneous stratum of the skin and give rise to inflammation accompanied by unspecific symptoms such as itching, pruritus, otorrhea, hypoacusis, tinnitus, otalgia, and scaling of the epithelium. The infection is typically unilateral. The prevalence of otomycosis has been reported to be as low as 9% of the otitis externa cases and as high as 30.4% in patients presenting with symptoms of otitis or inflammatory conditions of the ear. Prevalence is also influenced by the geographical area, as otomycosis is most commonly present in tropical and subtropical humid climates. Otomycosis affects all age groups, but it is a condition that occurs most frequently in adults and less in children. 2
Clotrimazole is a broad-spectrum antifungal belonging to the triazole drug family and is used worldwide for treating fungal infections, including fungal otitis externa. In a review of the literature on otomycosis studies, clotrimazole was shown to be effective and well-tolerated treating otomycosis at different doses. 3
Although clotrimazole 1% is currently marketed in the United States in the form of oral, topical, and vaginal drug products, 4 there is no specific otic formulation. Hence, this article presents the results of 2 phase III clinical studies (CLEAR-1 and CLEAR-2) designed to evaluate the efficacy and safety of clotrimazole 1% otic solution and placebo in patients with otomycosis.5,6
Material and Methods
Study Design and Treatment Administration
Data from 2 multicenter, randomized, double-blind, phase III clinical trials conducted in individuals with otomycosis between February 2020 and October 2021 were pooled together for this analysis. Both studies were identically designed: CLEAR-1 5 (NCT03686384) included populations from the United States and Mexico, and CLEAR-2 6 (NCT03686397) from Europe. The design of the studies was discussed and approved by the FDA as pivotal studies for the regulatory approval of the formulation. Subjects were centrally randomized, and an identification number was assigned. After that, they received either clotrimazole 1% otic solution or placebo (saline solution 0.9%), at a 2:1 ratio, respectively, instilling 1 vial (0.20 ml) in the affected ear canal twice daily (preferably 12 hours apart) for 14 consecutive days.
The studies consisted of 4 visits: baseline (visit 1), during treatment at day 8-10 (visit 2), end of treatment at day 15-17 (visit 3), and test of cure at day 24-26 (visit 4). Mycological assessments comprised ear discharge samples that were taken prior to the debridement of the affected ear at baseline. In the case of positive fungal culture, samples were also taken at visits 2 to 4. Symptoms such as pruritus, otalgia, otorrhea, and ear fullness were assessed at each visit using a 0 (absent) to 3 (severe) score; adverse events (AEs) were also recorded.
Both studies were conducted according to Good Clinical Practices, the Declaration of Helsinki, and the country’s local rules and regulations. The protocols were approved by the corresponding central or local institutional committees, such as Advarra Institutional Review Board (Pro00038853). All participants provided written informed consent before inclusion in each study.
Study Population
We selected for the study males or females over 18 years of age with uncomplicated otomycosis in at least 1 ear requiring topical treatment and who presented with debris and/or drainage clinically consistent with fungal infection and at least 2 of the following symptoms: pruritus, otalgia, and ear fullness. Exclusion criteria included the presence of bacterial or malignant otitis externa, tympanic perforation, tympanostomy tubes, postmastoid surgery or structural ear anomalies, any recent systemic infection, and other concomitant disorders that may affect study interpretation (e.g., diabetes mellitus and immunosuppressive disorders), disease conditions contraindicating the use of an investigational drug, as well as prior or current treatment that could interfere with study evaluations (e.g., antifungal, corticoid, antipruritic, and analgesic).
Efficacy analyses were performed on the mycological intent-to-treat (MITT) population, composed of all randomized individuals with a positive baseline fungal culture for Aspergillus and/or Candida species. The safety population, consisting of all those who received any study drug, was considered for safety analyses.
Study Outcomes
The primary efficacy endpoint was defined as the proportion of patients with therapeutic cure at visit 4. The therapeutic cure was a combined assessment of the mycological outcome and clinical outcome based on the clinical symptoms.
Mycological assessments were performed at baseline and during subsequent visits if exudate was present. Samples of ear discharge using exudate sampling kits with standard instructions for sample preparation provided by a central laboratory were taken prior to the debridement of the affected ear. Susceptibility testing on fungal isolates was performed in a blinded central laboratory according to their procedures. In the case of positive fungal culture at visit 1, mycological outcomes at visits 2 to 4 were classified as mycological cure if eradication (culture did not show growth of any fungal pathogen) or presumed eradication (there was no material to culture) were present.
Clinical variables defined in the protocol were assessed at each visit and scored from 0 to 3. In the case of pruritus or ear fullness, it was scored as severe (3) if marked or intense; moderate (2) if definitely present; mild (1) if slight; or absent (0) if completely absent. Otalgia was scored following a previously published description 7 as severe (3) if it interfered with activities of daily living; moderate (2) if it caused discomfort but did not interfere with activities of daily living; mild (1) if there was awareness of pain but not much discomfort; or absent (0) in the total absence of pain. Otorrhea was assessed as severe (3) if copious discharge prevented visualization of the tympanic membrane unless the fluid was suctioned; moderate (2) if the anterior sulcus was full, but the tympanic membrane was still clearly visible; mild (1) if little fluid accumulated on the anterior tympanic sulcus and the tympanic membrane was still visible; or absent (0) if there was no fluid. Subsequently, the total signs/symptoms score (TSSS) was defined as the sum of scores. If TSSS was zero, then the clinical cure definition was reached.
In parallel, changes in individual signs and symptoms in TSSS at visits 2, 3, and 4 were part of the secondary objectives of both clinical trials.
Safety was assessed by the incidence of AEs. The severity of each AE (mild, moderate, or severe) and its association with the study medication (not related, possibly, probably, or definitely related) were assessed and recorded by the investigator using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0.
Statistical Analysis
Continuous data were summarized by the treatment group using descriptive statistics [n, mean, standard deviation (SD), standard error of the mean, median, minimum, maximum]. Categorical variables were tabulated by treatment group and category (frequency and percentage). Efficacy analyses were performed in the MITT population. Differences between groups for the proportion of responders were determined with the Chi-square test. Statistical significance was defined as P < .05. Statistical analyses were performed using SAS software (SAS Institute, Cary, SC, USA) for Windows version 9.4.
Results
Baseline Characteristics
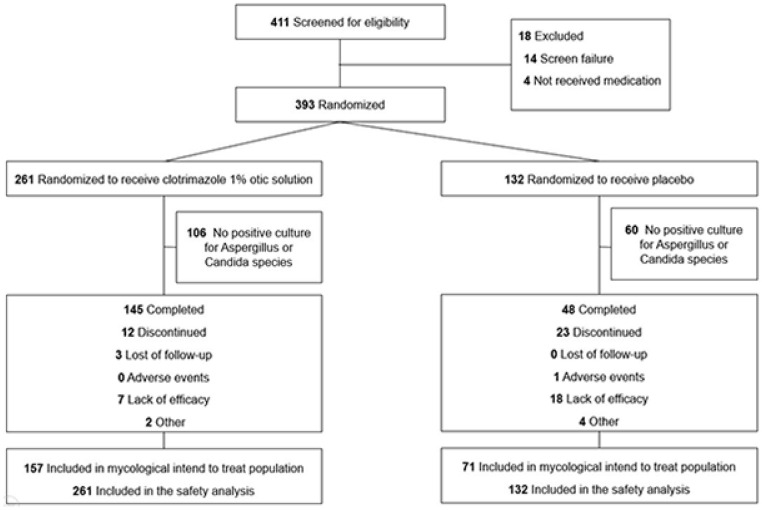
In total, 393 patients were enrolled in the clinical trials (Figure 1). The MITT population included 228 participants (157 receiving clotrimazole and 71 placebo), and the safety population included 393 individuals (261 in the clotrimazole group and 132 in the placebo group).
Figure 1.
Patient flow.
No marked differences were observed between placebo and treatment groups in terms of demographic and baseline data in either the efficacy or safety population. Males represented 55.5% of the safety population, the mean age of the sample was 51.5 years (SD = 17.22), and 71% were under 65 years of age. Regarding ethnicity, 86.5% of the population were Caucasian, and nearly 50% of the pooled analysis population were from the United States.
The most common risk factor for otomycosis in the safety population was exposure to excess moisture (48.6%), and more than 75% of participants presented unilateral otomycosis. Half of the patients had baseline fungal and bacterial organisms. Only 2 (0.5%) patients (1 in each group) had an ear wick placement. Safety and MITT populations obtained similar results regarding sociodemographic and baseline data. Detailed results for the MITT population are shown in Table 1.
Table 1.
Demographic and Baseline Characteristics (MITT Population).
| Demographic/baseline characteristic statistic/category | Clotrimazole (N = 157) | Placebo (N = 71) | Total (N = 228) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 52.9 (16.54) | 52.5 (17.89) | 52.8 (16.93) |
| Median | 52.0 | 55.0 | 52.5 |
| Min, Max | 21, 83 | 19, 89 | 19, 89 |
| Age category, n (%) | |||
| <65 years | 106 (67.5) | 48 (67.6) | 154 (67.5) |
| ≥65 years | 51 (32.5) | 23 (32.4) | 74 (32.5) |
| Sex, n (%) | |||
| Male | 82 (52.2) | 41 (57.7) | 123 (53.9) |
| Female | 75 (47.8) | 30 (42.3) | 105 (46.1) |
| Childbearing potential, n (%) | |||
| Yes | 29 (38.7) | 13 (43.3) | 42 (40.0) |
| No | 46 (61.3) | 17 (56.7) | 63 (60.0) |
| Race, n (%) | |||
| American Indian or Alaska Native | 3 (1.9) | 1 (1.4) | 4 (1.8) |
| Asian | 5 (3.2) | 1 (1.4) | 6 (2.6) |
| Black or African-American | 10 (6.4) | 4 (5.6) | 14 (6.1) |
| White | 135 (86.0) | 60 (84.5) | 195 (85.5) |
| Other | 2 (1.3) | 5 (7.0) | 7 (3.1) |
| Missing | 2 (1.3) | 0 | 2 (0.9) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 40 (25.5) | 24 (33.8) | 64 (28.1) |
| Not Hispanic or Latino | 117 (74.5) | 47 (66.2) | 164 (71.9) |
| Geographical region, n (%) | |||
| U.S. | 76 (48.4) | 32 (45.1) | 108 (47.4) |
| Non-U.S. | 81 (51.6) | 39 (54.9) | 120 (52.6) |
| Risk factors for otomycosis, n (%) | |||
| Exposure to excess moisture | 67 (42.7) | 33 (46.5) | 100 (43.9) |
| Trauma | 4 (2.5) | 1 (1.4) | 5 (2.2) |
| Use of hearing aids | 15 (9.6) | 4 (5.6) | 19 (8.3) |
| Prior topical otic antibacterial | 12 (7.6) | 3 (4.2) | 15 (6.6) |
| Quantitative or qualitative changes in earwax | 13 (8.3) | 8 (11.3) | 21 (9.2) |
| Other | 19 (12.1) | 13 (18.3) | 32 (14.0) |
| Missing | 27 (17.2) | 9 (12.7) | 36 (15.8) |
| Bilateral otomycosis, n (%) | |||
| Yes | 29 (18.5) | 18 (25.4) | 47 (20.6) |
| No | 128 (81.5) | 53 (74.6) | 181 (79.4) |
| Evaluable ear, n (%) | |||
| Left | 81 (51.6) | 33 (46.5) | 114 (50.0) |
| Right | 76 (48.4) | 38 (53.5) | 114 (50.0) |
| Baseline pathogen, n (%) | |||
| Fungal only | 35 (22.3) | 12 (16.9) | 47 (20.6) |
| Fungal+Bacterial | 122 (77.7) | 59 (83.1) | 181 (79.4) |
| Ear wick placement, n (%) | |||
| No | 157 (100) | 71 (100) | 228 (100) |
Percentages for childbearing potential are based on the number of female subjects. Otherwise, % = 100 × n/N.
Abbreviations: MITT, mycological intent-to-treat; SD, standard deviation.
Efficacy Results
The primary efficacy endpoint was the proportion of patients with therapeutic cure (mycological and clinical cure) in the MITT population at visit 4. The results demonstrated the superiority of clotrimazole compared with placebo (68.2% vs 25.4%, P < .0001). Results are summarized in Table 2.
Table 2.
Proportion of Patients with Therapeutic Cure (Mycological and Clinical) at Visit 4 (MITT Population).
| Results | Clotrimazole (N = 157) | Placebo (N = 71) |
|---|---|---|
| Therapeutic cure, n (%) a | 107 (68.2) | 18 (25.4) |
| Therapeutic failure, n (%) a | 50 (31.8) | 53 (74.6) |
| 95% CI of response rate b | 60.3, 75.4 | 15.8, 37.1 |
| Difference in response rate (95% CI) c | 42.8 (30.3, 55.3) | |
| Miettinen-Nurminen 95% CI of the difference in response rate | 29.4, 54.2 | |
| P-Value d | <.0001 |
Abbreviations: CI, confidence interval; MITT, mycological intent-to-treat; TSSS, total signs/symptoms score.
Therapeutic cure is defined as both mycological and clinical cure. The mycological cure is defined as eradication (culture does not show growth of any fungal pathogen) or presumed eradication (there is no material to culture, and the overall clinical outcome is the clinical cure). The clinical cure is defined as a TSSS of zero on a 4-point scale for pruritus, otalgia, ear fullness, and otorrhea. Subjects who received rescue medication or with missing endpoints are considered therapeutic failures.
Ninety-five percent CI for the cure rate in each treatment group based on the Clopper–Pearson method.
Ninety-five percent for between-treatment group differences based on the normal approximation to a binomial distribution.
P-value based on the chi-square test.
Considering the findings of each study independently, clotrimazole showed superiority compared to placebo in both trials. In CLEAR-1, clotrimazole reached a therapeutic cure at visit 4 in 58.5% of patients compared to 27.8% in the placebo group (P = .0021). In CLEAR-2, 78.7% of individuals in the clotrimazole group versus 22.9% in the placebo group (P < .0001) presented a therapeutic cure at visit 4.
The following secondary endpoints showed significant differences between groups (P < .0001 in all cases). Therapeutic cure at the end of treatment visit (visit 3) reached 46.5% in the clotrimazole group compared to 19.7% in the placebo group. Mycological cure was also greater in the clotrimazole versus placebo group at visit 3 (61.8% vs 28.2%) and visit 4 (73.9% vs 28.2%).
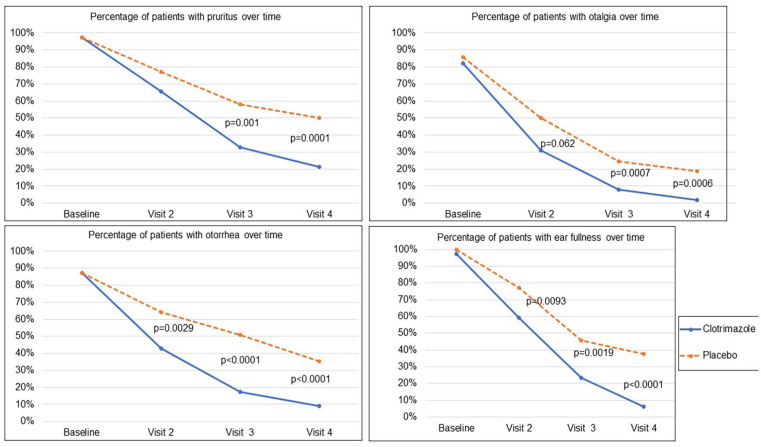
When analyzing each symptom individually, the proportion of patients presenting symptoms consistently decreased over time. The absence of pruritus, otalgia, ear fullness, or otorrhea at visit 4 was significantly higher in the clotrimazole group compared to the placebo group: pruritus (78.6% vs 50%, P < .0001), otalgia (97.2% vs 81.3%, P = .0006), ear fullness (93.8% vs 62.5%, P < .0001), and otorrhea (91% vs 64.6%, P < .0001). The percentages of patients with each sign and symptom over time are shown in Figure 2.
Figure 2.
Percentage of patients with each sign and symptom over time (MITT population). MITT, mycological intent-to-treat.
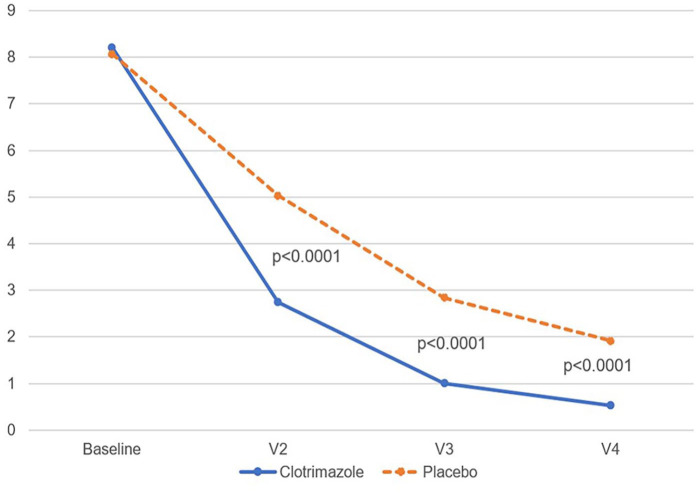
In parallel, the mean value of the TSSS decreased over time (Figure 3), performing better in the clotrimazole group (0.5 vs 1.90 at visit 4, P < .0001).
Figure 3.
Mean value of total signs and symptoms score changes over time (MITT population). MITT, mycological intent-to-treat.
Safety Results
As shown in Table 3, 9 (2.3%) of the 393 patients experienced a total of 10 mild to moderate related AEs during the study: 7 (2.7%) patients experienced 8 related AEs in the clotrimazole group, and 2 (1.5%) patients experienced 2 related AEs in the placebo group.
Table 3.
Study Drug-Related Adverse Events.
| Clotrimazole | Placebo | Total | ||||
|---|---|---|---|---|---|---|
| N = 261 | N = 132 | N = 393 | ||||
| Results | n (%) | E | n (%) | E | n (%) | E |
| Any study drug-related adverse event | 7 (2.7) | 8 | 2 (1.5) | 2 | 9 (2.3) | 10 |
| Otic events | ||||||
| Application site pain | 2 (0.8) | 3 | 0 | 0 | 2 (0.5) | 3 |
| Ear pain | 1 (0.4) | 1 | 1 (0.8) | 1 | 2 (0.5) | 2 |
| Tympanic membrane perforation | 1 (0.4) | 1 | 0 | 0 | 1 (0.3) | 1 |
| Nonotic events | ||||||
| Dizziness | 1 (0.4) | 1 | 1 (0.8) | 1 | 2 (0.5) | 2 |
| Headache | 1 (0.4) | 1 | 0 | 0 | 1 (0.3) | 1 |
| Paresthesia | 1 (0.4) | 1 | 0 | 0 | 1 (0.3) | 1 |
Abbreviations: %, percentage of subjects; E, number of events; n, number of subjects.
No AEs resulted in death during the study. No patients in the clotrimazole group experienced any serious AE, while 1 patient in the placebo group had 2 nonrelated serious AEs (labyrinthitis and cholesteatoma).
Discussion
The results of this analysis show that clotrimazole 1% otic solution is superior in efficacy compared to placebo and is safe and well-tolerated for treating otomycosis. These pooled results come from 2 identically designed multicenter, randomized, double-blind, phase III clinical trials conducted in the United States, Mexico, and Europe, which also demonstrate this individual superiority.
Although clotrimazole is available in various formulations 4 for a variety of fungal infections, none of them is approved in United States for the treatment of otomycosis. In Europe, there is no commercialized product based on data from randomized clinical trials. However, there are only a few approved products in a few countries. Globally, products containing clotrimazole are used off-label for treating otomycosis, representing a currently unmet medical need. Therefore, the studies presented in this report constitute the first evidence for the efficacy and safety of clotrimazole otic solution from randomized clinical trials.
Diseases of fungal origin can be treated using drugs with different mechanisms of action, such as antiseptics, azoles, nucleoside analogs, echinocandins, polyenes, and hydroxyquinolines. 8 Particularly, azole antifungal drugs inhibit the enzyme lanosterol 14 α-demethylase, preventing lanosterol from becoming ergosterol, which leads to fungal growth inhibition. 9 It has also been shown to have a potent broad-spectrum in vitro fungicidal activity for the most common species causing otomycosis, such as Aspergillus and Candida species. 10
Clotrimazole is the most widely used topical azole 8 and is considered one of the most efficient drugs due to its 95% to 100% efficacy in quickly reducing the signs and symptoms of otomycosis and the chances of relapse; hence, it can also be used in the prevention of disease recurrence. 4 These results derive, in part, from different observational real-world studies2,11 -14 or meta-analyses,8,15 highlighting the efficacy and safety of clotrimazole in the treatment of otomycosis. Due to the nature of these investigations, there is great heterogeneity between studies in terms of the objective for measuring treatment efficacy, the formulations used, the study design, and the sites where the studies were conducted. This makes it difficult to reach conclusions about the effect of clotrimazole on the clinical resolution of otomycosis.
On the other hand, some comparative clinical trials have been described,16,17 although there are no studies evaluating topical azoles against placebo or nontreatment. 8 In summary, current evidence regarding the effect of clotrimazole on the clinical resolution of otomycosis is uncertain, as well as the information regarding AEs when compared with other treatments, and it may be difficult to generalize these results. 8 However, results were recently published of a double-blind phase III noninferiority trial conducted in Mexico comparing eberconazole 1% otic solution with clotrimazole 1% solution in 190 patients with otomycosis. The treatment duration was 14 days, and the complete response rate (clinical and mycological response) was 81.8% with eberconazole and 83.5% with clotrimazole, which allows for a conclusion of high clinical efficacy of clotrimazole 1%. 18
In this pooled analysis of CLEAR-1 and CLEAR-2 studies, 393 individuals from different countries were recruited. Notably, only 228 (57.9%) of the patients were included in the efficacy analysis, as there was an unexpectedly high percentage of patients with no evidence of baseline pathogen, although they had been clinically diagnosed with otomycosis. This percentage of fungal infection evidence was consistent between both trials (58.7% in CLEAR-1 and 57% in CLEAR-2). The individual efficacy results of each clinical trial demonstrated the statistical superiority of clotrimazole versus placebo in each study, although the therapeutic cure rate was higher in CLEAR-2 (78.7%) than in CLEAR-1 (58.5%). The expected efficacy of clotrimazole was also shown in terms of fungal eradication at the last visit in both studies, with a mycological cure of 73.9% in the clotrimazole group versus 28.2% in the placebo (P < .0001). Interestingly, mycological cure increased in the clotrimazole group from visit 3 to visit 4, whereas the placebo group remained stable, meaning a lingering effect of the drug following its administration.
Regarding symptoms, the studies assessed changes in the individual’s signs and symptoms over time, defined as the difference between the global mean scores at visits 2, 3, and 4 from baseline and the change in the TSSS at each visit. The percentage of patients presenting signs and symptoms was also evaluated, which was similar in both groups at baseline. The rate of patients with otalgia, pruritus, otorrhea, and ear fullness decreased at each visit. It was significantly lower in the clotrimazole group at every visit for all evaluated symptoms (except pruritus at visit 2), showing a fast and sustained effect of clotrimazole on the clinical signs and symptoms associated with otomycosis.
Safety comparisons between studies are usually difficult due to the heterogeneity with which AEs are reported. 8 Nevertheless, the low number of AEs reported in the individual trials and their mild severity confirm the safety and tolerability of the product administration, with no differences between clotrimazole and placebo.
To the best of our knowledge, this pooled analysis is the first to assess the efficacy and safety of clotrimazole 1% otic solution versus placebo, demonstrating a statistically significant superior rate of mycological eradication, therapeutic cure, and symptom control compared with placebo, with a low rate of AEs.
Conclusions
The primary study objective of this pooled analysis was met by clotrimazole 1% otic solution, demonstrating statistical superiority over placebo, and the administration was observed to be safe and well tolerated. In our opinion, the results of these well-designed clinical trials showed very consistent evidence of the efficacy and safety of clotrimazole in the treatment of otomycosis and that clotrimazole 1% otic solution can cover the current medical need of having no specific available therapy for this disease.
Acknowledgments
The authors wish to thank the investigators, staff, and participants in the CLEAR-1 (NCT03686384) and CLEAR-2 (NCT03686397) studies. Medical writing support was provided by Cristina Puig from Trialance SCCL (Spain).
Footnotes
Author Contributions: John F. Ansley: substantial contributions to the conception, design, and supervision of the study. He also contributed to the acquisition, analysis, interpretation of data, and critical revision of the manuscript for important intellectual content. He had full access to all study data and took responsibility for the integrity of the data and the accuracy of the data analysis.
Manuel Bernal-Sprekelsen: substantial contributions to the conception and design of the study. He also contributed to the acquisition, analysis, interpretation of data, and critical revision of the manuscript for important intellectual content. He had full access to all study data and took responsibility for the integrity of the data and the accuracy of the data analysis.
Henry F. Butehorn: substantial contributions to the acquisition, analysis, or interpretation of data, and critical revision of the manuscript for important intellectual content.
Spiridon Todorov: substantial contributions to the acquisition, analysis, or interpretation of data, and critical revision of the manuscript for important intellectual content.
Ventsislav Tzvetkov: substantial contributions to the acquisition, analysis, or interpretation of data, and critical revision of the manuscript for important intellectual content.
Franklin Douglis: substantial contributions to the acquisition, analysis, or interpretation of data, and critical revision of the manuscript for important intellectual content.
Konstantin Georgiev: substantial contributions to the acquisition, analysis, or interpretation of data, and critical revision of the manuscript for important intellectual content.
Francisco Moreira da Silva: substantial contributions to the acquisition, analysis, or interpretation of data, and critical revision of the manuscript for important intellectual content.
Additional Contributions: Enrique Jimenez, MD and Patricia Lois from Medical Department at Laboratorios Salvat, S.A. contributed to the study design, analysis data, and revision of the manuscript. The following investigators participated in the trial: John Ansley, Henry F. Butehorn III, Magdalena Chirila; Laurence Chu, Madalina Criveanu, Franklin Douglis, G. Paul Doxey, Ann L. Edmunds, Javier Gavilanes, Konstantin Georgiev, Maria Julia Hernández, Rafael Hijano, Amy Ingram, Ivaylo Ivanov, Curtis Johnson, Brian Kellermeyer, Johan Lanza, Francisco Larrosa, Antonio Larroude, Christopher Larsen, Maria Teresa Llopiz, David Lobo Duro, Kenneth Maxwell, Daniela Medzhidieva, Ritvik Mehta, Corina Mella, Elias Michaelides, Sean Miller, Michaela Mitroi, Victoria Montoro, Francisco Moreira da Silva, José Juan Narváez, Geoffrey B. Pitzer, Lyuben Popov, Serafin Sánchez, Eric Slattery, Melanie Smith, Zorik Spektor, Catalina Stefan, Evelina Stoyanova, Spiridon Todorov, Ana G. Torres, Ventzislav Tzevetkov, Hristo Zlatanov.
Data Availability Statement: The data that support the findings of these studies will not be available.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ansley J.F., Butehorn H.F., Todorov S., Tzvetkov V., Douglis F., Georgiev K., Moreira da Silva F.: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Bernal-Sprekelsen: The author declared speaker honorarium for GSK Spain, Sanofi Spain, Consultant for Bionorica Germany.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Laboratorios Salvat, S.A.
This report represents the results of the clinical trials sponsored by Laboratorios Salvat S.A. as part of the U.S. Food and Drug Administration NDA of clotrimazole 1% otic solution for the treatment of otomycosis.
Ethical Considerations: CLEAR-1 and CLEAR-2 clinical trials had an identical design that was discussed and agreed with the U.S. Food and Drug Administration (FDA). Both studies were by approved by the institutional review boards of each participating institution (Advarra Institutional Review Board (Pro00038853) approved the study on November 19, 2019). All participants provided written informed consent before inclusion in each study.
ORCID iDs: Bernal-Sprekelsen M.  https://orcid.org/0000-0001-8191-9833
https://orcid.org/0000-0001-8191-9833
Georgiev K.  https://orcid.org/0000-0001-5587-0853
https://orcid.org/0000-0001-5587-0853
References
- 1. Westby D, O’Connell N, Powell J, Fenton JE. The changing nature of paediatric otomycosis in the mid-west of Ireland. J Laryngol Otol. 2020;134(7):592-596. doi: 10.1017/S0022215120001164 [DOI] [PubMed] [Google Scholar]
- 2. Khan F, Muhammad R, Khan MR, et al. Efficacy of topical clotrimazole in treatment of otomycosis. J Ayub Med Coll Abbottabad. 2013;25(1-2):78-80. [PubMed] [Google Scholar]
- 3. Munguia R, Daniel SJ. Ototopical antifungals and otomycosis: a review. Int J Pediatr Otorhinolaryngol. 2008;72(4):453-459. doi: 10.1016/j.ijporl.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 4. Haq M, Deshmukh P. Review of recurrent otomycosis and clotrimazole in its treatment. Cureus. 2022;14(10):e30098. doi: 10.7759/CUREUS.30098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salvat. SVT-15652 Otic Solution for the Treatment of Otomycosis NCT03686384. U.S. National Library of Medecine. Published 2021. Accessed July 26, 2023. https://www.clinicaltrials.gov/study/NCT03686384?term=SVT-15652&cond=Otomycosis&rank=2 [Google Scholar]
- 6. Salvat. SVT-15652 Otic Solution for the Treatment of Otomycosis NCT03686397. National Library of Medicine. Published 2021. Accessed July 26, 2023. https://www.clinicaltrials.gov/study/NCT03686397?term=SVT-15652&cond=Otomycosis&rank=1 [Google Scholar]
- 7. Claros Blanch P, Sanchez Garcia F, Rodriguez Leal A, Gomez Vida J, Llanes Domingo S. Estudio clinico para evaluar la efectividad de ciprofloxacino otico al 0.3% en ninos con otitis externa difusa. Acta Pediatr Esp. 2000;58(6):358-362. [Google Scholar]
- 8. Lee A, Tysome JR, Saeed SR. Topical azole treatments for otomycosis. Cochrane Database Syst Rev. 2021(5):CD009289. doi: 10.1002/14651858.CD009289.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12(1):40-79. doi: 10.1128/cmr.12.1.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stern JC, Shah MK, Lucente FE. In vitro effectiveness of 13 agents in otomycosis and review of the literature. Laryngoscope. 1988;98(11):1173-1177. doi: 10.1288/00005537-198811000-00005 [DOI] [PubMed] [Google Scholar]
- 11. Hamza A, Khan Q, Khan M. Efficacy of topical clotrimazole in otomycosis. PJMHS. 2011;5(4):738–740. [Google Scholar]
- 12. Navaneethan N, YaadhavaKrishnan RPD. Type of antifungals: does it matter in empirical treatment of otomycosis? Indian J Otolaryngol Head Neck Surg. 2015;67(1):64-67. doi: 10.1007/s12070-014-0780-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chavan RP, Ingole SM, Kanchewad Resident GS. Single topical application of 1% clotrimazole cream in otomycosis. Indian J Otolaryngol Head Neck Surg. 2023;75(Suppl 1):147-154. doi: 10.1007/s12070-022-03206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dundar R, Iynen I. Single dose topical application of clotrimazole for the treatment of otomycosis: is this enough? J Audiol Otol. 2019;23(1):15. doi: 10.7874/JAO.2018.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herasym K, Bonaparte JP, Kilty S. A comparison of Locacorten-Vioform and clotrimazole in otomycosis: a systematic review and one-way meta-analysis. Laryngoscope. 2016;126(6):1411-1419. doi: 10.1002/LARY.25761 [DOI] [PubMed] [Google Scholar]
- 16. Prabhakaran P, Navin N, Srinivasan R, et al. A comparative study of efficacy of clotrimazole and fluconazole ear drops in otomycosis. J Evol Med Dent Sci. 2018;7(17):2058-2061. doi: 10.14260/jemds/2018/462 [DOI] [Google Scholar]
- 17. Mofatteh MR, Naseripour Yazdi Z, Yousefi M, Namaei MH. Comparison of the recovery rate of otomycosis using betadine and clotrimazole topical treatment. Braz J Otorhinolaryngol. 2018;84(4):404-409. doi: 10.1016/j.bjorl.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de la Paz Cota BR, Cepero Vega PP, Matus Navarrete JJ, et al. Efficacy and safety of eberconazole 1% otic solution compared to clotrimazole 1% solution in patients with otomycosis. Am J Otolaryngol Head Neck Med Surg. 2018;39(3):307-312. doi: 10.1016/j.amjoto.2018.03.017 [DOI] [PubMed] [Google Scholar]