Abstract
Current understanding of floral development is mainly based on what we know from Arabidopsis (Arabidopsis thaliana) and Antirrhinum majus. However, we can learn more by comparing developmental mechanisms that may explain morphological differences between species. A good example comes from the analysis of genes controlling flower development in pea (Pisum sativum), a plant with more complex leaves and inflorescences than Arabidopsis and Antirrhinum, and a different floral ontogeny. The analysis of UNIFOLIATA (UNI) and STAMINA PISTILLOIDA (STP), the pea orthologs of LEAFY and UNUSUAL FLORAL ORGANS, has revealed a common link in the regulation of flower and leaf development not apparent in Arabidopsis. While the Arabidopsis genes mainly behave as key regulators of flower development, where they control the expression of B-function genes, UNI and STP also contribute to the development of the pea compound leaf. Here, we describe the characterization of P. sativum PISTILLATA (PsPI), a pea MADS-box gene homologous to B-function genes like PI and GLOBOSA (GLO), from Arabidopsis and Antirrhinum, respectively. PsPI encodes for an atypical PI-type polypeptide that lacks the highly conserved C-terminal PI motif. Nevertheless, constitutive expression of PsPI in tobacco (Nicotiana tabacum) and Arabidopsis shows that it can specifically replace the function of PI, being able to complement the strong pi-1 mutant. Accordingly, PsPI expression in pea flowers, which is dependent on STP, is identical to PI and GLO. Interestingly, PsPI is also transiently expressed in young leaves, suggesting a role of PsPI in pea leaf development, a possibility that fits with the established role of UNI and STP in the control of this process.
A huge variety of inflorescence and floral morphologies are found among higher plants. Increasing attention is currently being paid to the study of the mechanisms responsible for this natural diversity. Recent advances in plant molecular genetics have allowed detailed comparative studies on how the development of equivalent organs and structures is regulated in different plants. An important conclusion of these studies is that most of the genetic functions that establish the general pattern for organ specification are executed by orthologous genes in the different species and, more importantly, that the observed morphological variation is achieved in a large degree through the modification of the function of those regulators in each particular species (Theissen et al., 2000).
A clear example of conservation is the specification of the identity of floral organs. The classical ABC model proposes that floral organ identity results from the action of three genetic functions, A, B, and C, each of them active in two adjacent whorls of organs. A-function alone specifies sepal identity, A plus B petal identity, B plus C stamens, and C alone carpels. The ABC model was developed on the basis of studies carried out in the model plants Arabidopsis (Arabidopsis thaliana) and Antirrhinum majus (Coen and Meyerowitz, 1991), two species with a similar pattern of flower development, where the first A, B, and C genes were isolated. Later on, genes homologous to the ABC genes were isolated from a variety of plants (for review, see Theissen and Saedler, 1995; Theissen et al., 2000).
We are using pea (Pisum sativum) for comparative studies on the development of inflorescence and flowers. The inflorescence and flowers of pea are different from those of the models such as Antirrhinum and Arabidopsis. While the inflorescences of these two species are single racemes, pea has a more complex inflorescence, a compound raceme (Singer et al., 1999). Floral ontogeny in pea also differs to that in Arabidopsis and Antirrhinum. In these plants, the initiation of the whorls of floral organs is a sequential process. First, sepal primordia are formed, then petal primordia, immediately followed by stamens, and finally the carpels (Smyth et al., 1990; Carpenter et al., 1995). In pea, sepals also appear first, but petal and stamen primordia are not produced directly by the floral meristem. Instead, petals and stamens differentiate from four ephemeral meristems, called common primordia, that appear between sepal and carpel primordia. Each of these four common primordia divides, initiating a different number of floral organs in a stereotyped specific pattern. Another difference is that, in pea, the carpel primordium emerges at the same time as the common primordia, before petal and stamen primordia are initiated (Tucker, 1989; Ferrándiz et al., 1999). Due to these particularities in the development of the pea flower, an obvious question is whether the genes controlling flower development in pea are the same as in Arabidopsis and Antirrhinum and, if so, whether they work in the same way or whether they play additional roles in pea. In fact, the analysis of floral ontogeny in pea floral homeotic mutants has lead to the suggestion that A-, B-, and C-function genes also control the development of the common primordia (Ferrándiz et al., 1999).
The characterization of only three key genes regulating pea flower development has been reported: PROLIFERATING INFLORESCENCE MERISTEMS (PIM, also known as PEAM4; Berbel et al., 2001; Taylor et al., 2002), UNIFOLIATA (UNI; Hofer et al., 1997), and STAMINA PISTILLOIDA (STP; Taylor et al., 2001); these are the proposed pea orthologs of the orthologous gene pairs from Arabidopsis and Antirrhinum APETALA1/SQUAMOSA (AP1/SQUA; Huijser et al., 1992; Mandel et al., 1992), LEAFY/FLORICAULA (LFY/FLO; Coen et al., 1990; Weigel et al., 1992), and UNUSUAL FLORAL ORGANS/FIMBRIATA (UFO/FIM; Simon et al., 1994; Ingram et al., 1995), respectively. Like their orthologs from the model plants, PIM, UNI, and STP are mainly involved in floral meristem identity; however, a striking difference is that, in contrast to LFY/FLO and UFO/FIM, UNI and STP also play a key role in the regulation of leaf development in pea. The wild-type pea leaf is compound odd-pinnate; proximal pinnae are leaflets and distal ones tendrils. In addition to that, two leafy stipules flank the petiole base (Marx, 1987). UNI and STP regulate the complexity of the leaf, and mutations in either of them decrease the number of pinnae (Hofer et al., 1997; Taylor et al., 2001). In the case of the uni mutant, complexity is so reduced that they are transformed into leaves with just one leaflet.
The specification of petals and stamens (B-function) in Antirrhinum and Arabidopsis is controlled by the combined activity of two phylogenetically related MADS-box genes: DEFICIENS (DEF) and GLOBOSA (GLO) in Antirrhinum and their homologs APETALA3 (AP3) and PISTILLATA (PI) in Arabidopsis. In both species, mutations in either of these genes lead to very similar floral homeotic phenotypes, with petals being replaced by sepals and stamens by carpels (Bowman et al., 1989; Sommer et al., 1990; Tröbner et al., 1992).
The B-class genes have been the subject of many phylogenetic studies. The current view resulting from these studies is that the AP3 and PI gene lineages derive from a duplication event before the origin of angiosperms. The AP3 lineage would have gone through another duplication at the base of the higher eudicots giving rise to two lineages: euAP3, to which AP3 belongs, and TM6 (TOMATO MADS-BOX GENE 6). The polypeptides encoded by genes from the three groups can be easily distinguished on the basis of their highly conserved C-terminal domains. The proteins from the PI group contain a C-terminal hydrophobic region called the PI motif, while the C terminus of the proteins from the euAP3 and the TM6 lineages contain the euAP3 or the paleoAP3 motif, respectively. The high degree of conservation of the C-terminal domains in each lineage has lead to the suggestion that the divergence in the sequence of these motifs has contributed to the evolution of distinct functions for these floral homeotic gene products (Kramer et al., 1998; Vandenbussche et al., 2004). The critical role of these lineage-specific motifs in the function of the products of the B-class genes has recently received strong support from the results of analysis of the function of truncated and chimeric versions in Arabidopsis (Lamb and Irish, 2003).
In general, the expression patterns of all PI and AP3 homologs in the different species analyzed are very similar to those seen for the Arabidopsis and Antirrhinum genes: they are specifically expressed in flowers, and their mRNAs are found at high levels in the developing second and third whorl organ primordia (for review, see Theissen et al., 1996; Irish, 1999). Regulation of the expression of the B-function genes in Arabidopsis and Antirrhinum takes place in two steps: the establishment of their initial expression and its maintenance. The induction of B-function gene expression seems to be controlled to a great extent by the combined action of the meristem identity genes LFY and UFO in Arabidopsis and FLO and FIM in Antirrhinum (Weigel and Meyerowitz, 1993; Simon et al., 1994; Hantke et al., 1995; Ingram et al., 1995; Levin and Meyerowitz, 1995; Lee et al., 1997; Parcy et al., 1998). On the other hand, maintenance seems to be autoregulated by the activity of the heterodimer of the two B proteins and occurs in the second and third whorls, where their expression overlaps (Schwarz-Sommer et al., 1992; Tröbner et al., 1992; Goto and Meyerowitz, 1994).
The characterization of the pea genes responsible for the B- and C-functions is a question that remains to be addressed. In this article, we describe the isolation and functional characterization of the pea MADS-box gene P. sativum PI (PsPI). We show that, although PsPI encodes an atypical PI-like protein, which lacks the PI motif, it behaves as a PI functional homolog and is able to complement specifically the floral defects of pi-1 mutants. We also show that, in addition to being expressed in the developing second and third whorls of floral organ primordia, PsPI is also expressed in young leaf primordia, suggesting a possible role of PsPI in the development of the pea compound leaf.
RESULTS
Isolation and Sequence Analysis of PsPI
To identify genes involved in pea floral organ development, a pea floral cDNA library was screened under low stringency conditions with the cDNA of the Antirrhinum MADS-box gene DEF as a probe. Out of 20 clones isolated, seven derived from the same gene, which we named PsPI. All seven clones contained a short 5′ untranslated region and a 3′ untranslated region of variable length followed by a poly(A) tail, flanking an identical 543-bp open reading frame that encodes a polypeptide with a predicted amino acid sequence with highest sequence similarity to B-class MADS-box genes. Figure 1A shows a neighbor-joining tree constructed by comparing the MIK domains of PsPI and members of the B-class MADS-box family from different species. PIM, the pea functional homolog of AP1, was used as an outgroup. This analysis shows that, within the B-class group, PsPI is most closely related to members of the PI/GLO subfamily (Theissen et al., 2000; Fig. 1A).
Figure 1.
A, Neighbor-joining tree of B-class MADS-box deduced polypeptides from selected species: PsPI (this study; AY842491); MsNGL9 (Zucchero et al., 2001; accession no. AAK77938); AmGLO (Tröbner et al., 1992; accession no. Q03378); PhGLO1 (Angenent et al., 1992; accession no. Q03488); PhGLO2 (Kush et al., 1993; accession no. Q07474); NtGLO (Hansen et al., 1993; accession no. Q03416); AtPI (Goto and Meyerowitz, 1994; accession no. P48007); AmDEF (Sommer et al., 1990; accession no. P23706); NtDEF (Davies et al., 1996; accession no. CAA65288); PhDEF (Kush et al., 1993; accession no. CAA49567); MsNMH7 (Heard and Dunn, 1995; accession no. AAC15419); AtAP3 (Jack et al., 1992; accession no. P35632). The tree was rooted with PEAM4, a pea ortholog of AP1/SQUA (Berbel et al., 2001; accession no. AJ279089). Species names are indicated as follows: Ps, Pisum sativum; Ms, Medicago sativa; Am, Antirrhinum majus; Ph, Petunia hybrida; Nt, Nicotiana tabacum; At, Arabidopsis. B, Multiple sequence alignment of PsPI, PhGLO2, AmGLO, PhGLO1, MsNGL9, NtGLO, and AtPI. Dark gray boxes indicate fully conserved residues. Light gray boxes indicate similar and partially conserved residues.
Full-length multiple sequence alignments confirmed that there is extensive sequence similarity, ranging from 52% to 63% identity, between PsPI and other PI-related proteins (Fig. 1B). The most similar sequence is NGL9, a PI-like protein from a closely related legume, Medicago sativa. Sequence identity with members of the AP3 subclade is lower, around 30%, consistent with PsPI as a pea ortholog of PI/GLO B-class genes. Strikingly, the PsPI-deduced polypeptide is significantly shorter than its proposed orthologs. PsPI lacks the PI motif, a conserved domain of 19 amino acids with a consensus core sequence of MPFxFRVQPxQPNLQE present in the C terminus of virtually all members of the core eudicot PI lineage and considered to play a key role in the function of the proteins of this family (Kramer et al., 1998; Lamb and Irish, 2003; Fig. 1B). To address the role of PsPI in pea flower development and to test whether the absence of the PI motif compromises PsPI functionality, we analyzed PsPI expression during pea development and performed a functional characterization of PsPI in heterologous systems.
Expression of PsPI during the Development of Pea Flowers
RNA from different pea tissues (roots, stem, mature leaves, and flowers) was probed with the PsPI cDNA. This detected the PsPI transcript in RNA extracted from flowers only (data not shown). A more detailed analysis of PsPI expression in pea floral apices by in situ hybridization showed that PsPI transcripts were detected in the floral meristem from very early stages of development. In late stage 2 flowers, where organ primordia have yet to differentiate, PsPI expression marks the domains of the floral meristem that will generate the common primordia, from which second and third whorl organs will develop (Fig. 2A). Figure 2B shows how, in stage 4 flowers, PsPI is strongly expressed throughout the whole common primordium. Expression of PsPI continues in much later stages of the development of the pea flower and is always confined to the second and third whorl organs. Figure 2, C and D, shows PsPI expression in petal and stamen primordia in stage 5 and stage 7 pea flowers, respectively. Northern hybridization carried out on young pea flowers showed that PsPI continues to be expressed at high levels at least up to the anthesis stage (data not shown). The pattern of expression of PsPI, therefore, is like that of the B-class genes PI and GLO.
Figure 2.
In situ hybridization of PsPI mRNA in wild-type pea inflorescences. A, Longitudinal section through a young inflorescence. PsPI expression is detected in an early stage 2 floral meristem (F) in narrow stripes of cells corresponding to the domains that will differentiate later on as common primordia of petals and stamens. PsPI is also expressed in a developing axillary bud (Ax). B, Stage 4 flower. PsPI is expressed uniformly throughout common primordia (CP) and not detected in sepal (Sp) or carpel (C) primordia. C, Stage 5 flower. The common primordia have divided to differentiate petal (P) and stamen (St) primordia that strongly express PsPI. D, Stage 7 flower. PsPI mRNA is also detected at later stages of petal and stamen development. Developmental stages were defined according to Ferrándiz et al. (1999).
Constitutive Expression of PsPI in Tobacco
Protocols for pea transformation are currently inefficient, making transgenic approaches to study gene function in pea practically unfeasible. In order to obtain information about the function of PsPI in flower development, we generated transgenic lines of Arabidopsis and tobacco, both easily transformable plant species for which similar studies have been used extensively to investigate gene function. The effect of the constitutive expression of PsPI was analyzed by introducing a construct where the PsPI cDNA was fused to a double 35S promoter of cauliflower mosaic virus.
Of 19 tobacco plants transformed with PsPI, 17 showed a similar altered phenotype. The vegetative organs of these plants were normal, and no effect in flowering time was detected. Phenotypic alterations were observed only in flowers. While their second and third whorl organs were normal, the sepals of the 35S::PsPI flowers were pale and elongated and contrasted with the dark green globular calyx of wild-type flowers (Fig. 3, A and B). The most marked alterations were observed in the fourth whorl, where a variety of carpel morphologies was observed (Fig. 3G). These ranged from weakly affected carpels, with deformed ovaries and normal style, to strongly altered carpels, with morphologies more similar to stamens. In those carpels, the abnormal ovary was found at the top of a tubular structure, instead of being located at the base of the pistil, as in the wild type, and the style was very short and deformed (Fig. 3, F, G, and I). Analysis by scanning electron microscopy (SEM) revealed striking transformations in 35S::PsPI epidermal cell shape. As shown in Figure 3, J to O, the epidermis of the tubular structure that subtends the ovary in one of these fourth-whorl organs (Fig. 3, J, L, and N) is made up of elongated cells and trichomes, both typical features of the wild-type stamen filament (Fig. 3, K, M, and O). On the top of the tubular structure, the modified ovary has a central groove, which is strongly reminiscent of the two thecae of wild-type anthers (Fig. 3P). Moreover, the epidermal cell type of the transgenic ovary corresponds to that of the wild-type anthers (Fig. 3, Q and R).
Figure 3.
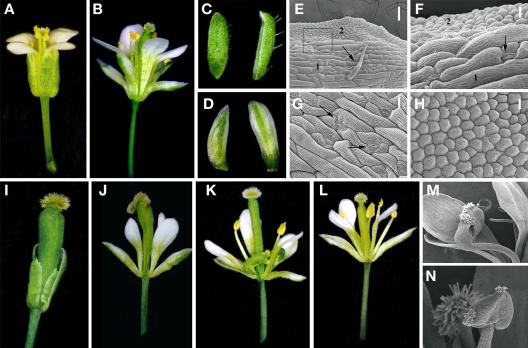
Phenotypic effects of constitutive expression of PsPI in tobacco plants. A, Wild-type (left) and 35S::PsPI flowers (right). Mild effects of transgenic 35S::PsPI expression on sepal morphology are visible. B, Sepals from a wild-type (left) and a 35S::PsPI flower (right). 35S::PsPI sepals are longer and paler than the wild type. C to E, SEM micrographs of the adaxial side of first or second whorl organs to reveal epidermal cell morphology. C, 35S::PsPI sepal. D, Wild-type sepal. E, Wild-type petal at the basal part of the corolla tube. F to I, Phenotypic alterations in fourth whorl organ morphology found in 35S::PsPI flowers. F, Wild-type carpel. G, Fourth whorl organs of 35S::PsPI flowers with different levels of homeotic transformations. In these organs, the ovary develops abnormally, shifting toward apical positions (arrowheads). H, Wild-type stamen. I, A strongly affected 35S::PsPI carpel dissected to reveal the developing ovules. The ovary occupies an apical position in the organ, and the style is short and deformed. J to S, SEM micrographs of different cellular types. J, Epidermal cells at the basal region of a 35S::PsPI carpel. K, Epidermal cells at the basal part of the filament of a wild-type stamen, densely covered by trichomes. L, Epidermal cells at intermediate positions of a 35S::PsPI carpel. M, Epidermal cells at intermediate positions of the filament of a wild-type stamen. Trichome number gradually decreases toward the apical positions of the filament. N, Close-up of the cells in L. O, Close-up of the cells in M. P, Apical structure in a 35S::PsPI carpel. The ovary develops a central groove, resembling the morphology of an anther divided in two thecae. Q, Epidermal cells of a wild-type anther, dome-shaped and crenellated. R, Epidermal cells of the abnormal ovary of a 35S::PsPI carpel, resembling wild-type anther cell types. S, Epidermal cells of a wild-type ovary, flat-shaped and not crenellated.
In summary, the constitutive expression of PsPI in tobacco causes homeotic alterations in the flowers consisting of sepal-to-petal and carpel-to-stamen transformations, the transformation of the carpel apparently being more severe than that of the sepals. These transformations are similar to those caused by ectopic expression of the Antirrhinum GLO gene in tobacco (Davies et al., 1996), indicating that PsPI functions as a GLO/PI-type B-function gene in tobacco.
Constitutive Expression of PsPI in Arabidopsis
The 35S::PsPI construct was also used to transform Arabidopsis plants. We obtained 21 Arabidopsis transgenic plants, 18 of which displayed a floral morphology phenotype clearly different from the wild type. The appearance of the vegetative organs was normal, and the plants flowered at the same time as the wild type (data not shown). The phenotypic alterations of 35S::PsPI Arabidopsis flowers were restricted to the first whorl organs. These organs showed white sectors and were inserted in the pedicel at a wider angle than wild-type sepals (Fig. 4, A–D). Further analysis of 35S::PsPI first whorl organs by SEM showed that they had a chimeric nature, being composed of a mosaic of sepal and petal tissue (Fig. 4, E–H). This phenotype is very similar to that caused by constitutive expression of PI in Arabidopsis (Krizek and Meyerowitz, 1996).
Figure 4.
Effects of constitutive expression of PsPI in Arabidopsis. A to H, Phenotypic effects of 35S::PsPI in Arabidopsis. A, Wild-type flower at anthesis. B, 35S::PsPI flowers at anthesis. White sectors appear on the edges of first whorl organs. Chimeric sepals are inserted at the base of the flowers at a wider angle when compared to the wild type. C, Wild-type sepals. D, 35S::PsPI first whorl organs. Patches of petal-like tissue are clearly visible. E to H, SEM micrographs of the abaxial side of first and second whorl organs. E, Epidermal cells of 35S::PsPI first whorl organs show mixed morphologies. Cells in region 1 are elongated and contain trichomes (arrow) and stomata, resembling cell morphologies of wild-type sepals shown in G. Cells in region 2 are smaller and round, similar to wild-type petal cells shown in H. F, Close-up of boxed area in E. Arrow indicates the presence of a stoma. G, Abaxial epidermal cells of a wild-type sepal. H, Abaxial epidermal cells of a wild-type petal. I to N, Phenotypic complementation of the Arabidopsis pi-1 mutant by 35S::PsPI. I, A pi-1 mutant flower, showing the typical homeotic transformations of B-class mutants. One sepal has been removed to reveal the sepal-like second whorl organs. Third whorl organs have acquired carpel identity and have incorporated into the central gynoecium. J to L, Flowers from different 35S::PsPI pi-1 lines exhibiting different degrees of phenotypic rescue. J, A partially complemented 35S::PsPI pi-1 flower. Petal formation has been fully restored, but no stamens have formed. K, A 35S::PsPI pi-1 flower in which different types of third whorl organs have developed. Two stamens have formed in lateral positions, while two carpel-like structures are visible in medial positions. L, 35S::PsPI pi-1 flower with a full complement of petals and four nearly wild-type stamens. M and N, Mosaic organs with mixed characteristics of carpel and stamens developing at the third whorl of 35S::PsPI pi-1 flowers.
Mutation in the B-type gene PI of Arabidopsis results in flowers with petals transformed into sepals and carpelloid organs or filaments in place of stamens (Bowman et al., 1989; Hill and Lord, 1989; Fig. 4I). To test whether PsPI can functionally replace the Arabidopsis PI gene, 35S::PsPI was introduced into the strong pi-1 mutant by crossing a homozygous plant from a transgenic line strongly expressing PsPI (35S::PsPI.16) with a homozygous pi-1 plant. In the F2 population (n = 235), apart from plants with (1) wild-type (n = 45), (2) pi (n = 18), and (3) 35S:: PsPI (n = 130) phenotypes, a fourth phenotypical class appeared (n = 42). The flowers of the plants from this new class were similar to 35S::PsPI, with sepal-to-petal transformations and normal petal and carpel development (Fig. 4, J–L), but third whorl organs were abnormal stamens displaying different degrees of carpelloidy. These ranged from carpelloid structures or filaments to near normal stamens that showed traces of carpelloid tissue (Fig. 4, M and N). These features suggested that this phenotypic class corresponded to homozygous pi plants carrying the 35S::PsPI transgene, for which petal and most of stamen development had been restored. The expected segregation of 35S::PsPI; wild-type; pi; 35S::PsPI pi phenotypes in a 9:3:1:3 ratio was confirmed by χ2 assessment of F2 generation plants (n = 235, P < 0.05), validating the hypothesis of pi-1 being rescued by 35S::PsPI. To further confirm the segregation data, we genotyped 10 individuals that showed the highest degree of stamen complementation. All of them were shown to be homozygous for the pi-1 allele and to bear at least one copy of the 35S::PsPI transgene. This proved that constitutive PsPI expression was able to rescue the defects caused by pi-1 mutations.
In order to test whether PsPI was able to complement the phenotype of mutations in the other Arabidopsis B-function gene, AP3, homozygous plants for two mutant alleles of this gene, ap3-1 and ap3-3 (Bowman et al., 1989; Jack et al., 1992), which present a phenotype indistinguishable from that of pi mutants, were also crossed with homozygous plants from the same 35S::PsPI.16 line. The F2 progenies of both crosses were comprised of only three phenotypic classes: (1) wild-type plants, (2) ap3 plants, and (3) plants with the phenotype of the 35S::PsPI transgenic parental. In both F2 populations, those classes appeared in a ratio very close to 9:4:3 (3:2:1), as expected if 35S::PsPI ap3 plants had the same phenotype as ap3 mutants. These data indicate that PsPI is unable to rescue the phenotype of ap3 mutants and, moreover, that 35S::PsPI phenotypic effects in the first whorl are dependent on a functional AP3 gene, which has been shown to be transiently expressed in first whorl organs at early stages of flower development (Jack et al., 1992; Weigel and Meyerowitz, 1993; Ng and Yanofsky, 2001).
In conclusion, the 35S::PsPI construct is able to complement the Arabidopsis pi-1 mutation but not ap3-1 or ap3-3, showing that PsPI works as a functional homolog to the PI gene.
Expression of PsPI in Pea Homeotic Mutants
To gain further insights into the role and regulation of PsPI during pea flower development, we studied PsPI expression in the flowers of pea floral homeotic mutants. The phenotype of these mutants has been described in detail elsewhere (Ferrándiz et al., 1999).
The frondosus (brac) mutation affects floral meristem identity and floral organ identity. The flowers of brac are surrounded by an extra organ of leafy nature (Fig. 5) and have altered petals and sepals. Sepals show bracteoid morphology and frequently contain sectors of petaloid tissue. Northern hybridization on RNA extracted from organs of brac flowers showed that, in this mutant, the expression of PsPI is not restricted to petals and stamens but is also extended to the modified sepals, correlating with the presence of petaloid tissue in these organs (Fig. 5B).
Figure 5.
Expression of PsPI in flowers and floral organs of wild-type and pea mutant plants. A, Phenotype of pea floral mutants. Top left, wild-type (WT) flower; top right, brac mutant flower; bottom left, stp-2 mutant flower; bottom right, first whorl organs of a brac mutant flower, showing a sector of petaloid tissue. B and C, northern analysis of PsPI expression in floral organs of the brac mutant (B) or flowers of pea mutant plants (C). Blots were loaded with samples of 10 μg RNA or 15 μg RNA, respectively. As a control for equal RNA loading, a picture of each gel, stained with ethidium bromide, is shown below the blots. Br, Bract; Sp, sepal; P, petal; St, stamen; C, carpel.
The STP gene has been shown to be the pea ortholog of UFO and FIM, which regulate the expression of B-function genes in the flowers of Arabidopsis and Antirrhinum, respectively (Taylor et al., 2001). Accordingly, the strong stp-2 mutation (Fig. 5A) results in a floral phenotype that resembles that of B-function mutants in these model plants, where all petals and stamens are fully transformed into sepals and carpels, respectively. The stp-1 mutation results in a much weaker phenotype, with only a small number of second and third whorl organs of the flowers exhibiting homeotic transformations (Ferrándiz et al., 1999; Taylor et al., 2001). Analysis of PsPI expression in mature stp mutant flowers showed a dramatic reduction in the level of the PsPI transcript in the strong stp-2 mutant (Fig. 5C). However, PsPI expression in the stp-1 mutant did not differ significantly from the wild type, either in the level of expression or in the size of the PsPI transcript. The reduction of PsPI expression in stp-2 flowers suggests that STP acts in the pea flower as a regulator of the B-function genes.
PsPI Is Expressed in Developing Axillary Leaves
The pair of orthologous genes UFO/FIM and LFY/FLO play key roles in the initiation and development of flowers in Arabidopsis and Antirrhinum, respectively. One of their major roles in flower development is the regulation of the expression of B-function genes. In addition to these functions, the corresponding orthologs of those genes in pea, STP and UNI, also play a key role in the control of leaf development; accordingly, both genes are expressed in developing leaves (Hofer et al., 1997; Taylor et al., 2001).
In addition to being expressed in floral organ primordia, PsPI expression was also found in young axillary buds, although at lower levels (Fig. 6). These buds develop as secondary branches and differentiate several (three to four) vegetative nodes, each of them containing a stipule and a compound leaf, before they start producing flowers. The structure of a pea axillary bud in an early stage of development is shown in Figure 6C. Initially, PsPI is uniformly expressed in these axillary buds (Fig. 6, A, B, and D). Later on, the expression of PsPI disappears from the meristem and is restricted to the emerging primordia of the stipules and of the leaflets of the developing leaves (Fig. 6, E and F). Expression of PsPI has been observed in axillary buds from both inflorescence and vegetative apices (Fig. 6, A and B). As described above, we could not detect PsPI transcripts in mature leaves, indicating that PsPI expression in leaves is probably transient and restricted to early stages of leaf development.
Figure 6.
In situ hybridization analysis of PsPI expression during axillary bud development in wild-type pea plants. A, Longitudinal section of a young vegetative pea apex. PsPI expression is detected in axillary buds from early stages of development. B, Longitudinal section through a young pea inflorescence. PsPI is strongly expressed in common primordia of the stage 4 flower at the top. Lower levels of PsPI expression are also detected in axillary buds at lower nodes. C, SEM micrograph of a developing axillary bud, showing the morphology of this structure. D to F, PsPI expression during development of the axillary bud. PsPI mRNA is detected uniformly at inception of the axillary bud but becomes progressively restricted to the developing stipules and leaves, disappearing from the axillary meristem as the bud develops. SAM, Shoot apical meristem; Ax, axillary meristem; Spl, stipule; L, leaf.
This result suggests that, like STP and UNI, the B-function gene PsPI might also play a role in the development of the pea compound leaf. However, it is clear that much more detailed studies are needed to test this hypothesis.
DISCUSSION
An emerging question in developmental genetics is to determine to what extent homologous genes from different species function in a similar way as well as to what extent their roles have diversified. The answer to these questions should provide a basis for understanding how the huge variety of plant forms found in nature has been generated. We describe here the characterization of a pea MADS-box gene, PsPI, and present results that indicate that PsPI, in spite of lacking the conserved PI motif, is a functional homolog of PI/GLO genes in pea. Our data also suggest that PsPI could play a role in the development of the pea leaf.
PsPI Is a Pea Functional Homolog to the B-Function Genes PI/GLO
Sequence comparisons locate PsPI in the PI/GLO clade of the plant MADS-box gene family. The PsPI expression pattern is very similar to that described for B-function genes from other species, supporting the idea, suggested by the sequence homology data, that PsPI acts as a B-function gene in pea.
Constitutive expression of PsPI in tobacco causes homeotic changes in the flower. The organs of the first and fourth whorls of the 35S::PsPI tobacco flowers show phenotypic traits corresponding to sepal-to-petal and carpel-to-stamen transformations. This is similar to the transformations caused by the constitutive expression of the Antirrhinum B-function gene GLO in tobacco (Davies et al., 1996). The observed homeotic transformations can be explained by the pattern of expression of the endogenous NtDEF gene, present in all four whorls of the flower, thereby making NtDEF protein available for the formation of a functional heterodimer with PsPI (Davies et al., 1996; our unpublished results). Constitutive expression of PsPI also causes homeotic transformations in Arabidopsis flowers. In this case, PsPI only affects the first whorl and causes the appearance of petaloid sepals. This phenotype is essentially the same as that of 35S::PI Arabidopsis flowers (Krizek and Meyerowitz, 1996; Lamb and Irish, 2003) and is consistent with the described pattern of expression of the second Arabidopsis B-type gene, AP3, which in addition to being expressed in whorls 2 and 3, is also weakly expressed in whorl 1 (Jack et al., 1992; Weigel and Meyerowitz, 1993; Ng and Yanofsky, 2001).
The clearest demonstration that PsPI is a functional homolog of PI comes from the observation that 35S::PsPI rescues the floral defects caused by the strong pi-1 mutant allele. The extent to which PsPI complements the mutant phenotype of pi-1 is very high, so that in many cases 35S::PsPI pi-1 flowers show fully restored petal and stamen development, in addition to typical 35S::PsPI petaloid sepals in whorl 1. The complementation observed is specific to mutations in PI, since 35S::PsPI is unable to rescue the defects caused by mutations in the other Arabidopsis B-type gene AP3. Moreover, in 35S::PsPI ap3-3 flowers, in addition to ap3 derived petal-to-sepal and stamen-to-carpel transformations, no ectopic petal formation is observed in whorl 1. This result indicates that 35S::PsPI ectopic petals are dependent on the presence of a functional AP3 gene and provides further supporting evidence for the role of PsPI as a PI/GLO-type B-function gene.
While sequence analysis strongly supports the pea gene PsPI as an ortholog of PI/GLO genes, the predicted PsPI polypeptide lacks the highly conserved C-terminal domain known as the PI motif. This motif is found in virtually all the PI homologs so far analyzed and has been considered to play a key role in the function of the PI polypeptide (Kramer et al., 1998; Lamb and Irish, 2003). Our results of constitutive expression in transgenic plants unequivocally show that PsPI works as a functional homolog to PI both in tobacco and Arabidopsis, demonstrating that, at least in the context of the PsPI protein, the PI motif is not absolutely required for functional specificity. The outcome of our experiments differs from the results of the studies performed by Lamb and Irish (2003). In their experiments, they showed that a truncated version of the Arabidopsis PI protein, lacking the last 22 amino acids that contain the PI motif, did not produce any floral phenotype when expressed under the control of the 35S promoter in the wild type and was also unable to rescue the phenotype of the pi1-1 mutant. Our experimental design is similar to the experiments described by Lamb and Irish. PsPI encodes a polypeptide essentially equivalent to a truncated version of PI lacking the last 26 amino acids. When expressed from the 35S promoter, PsPI did cause a typical 35S::PI phenotype and rescued the pi-1 phenotype. While further detailed analyses would be required to explain this apparent discrepancy, we hypothesize that differences in the rest of the PsPI polypeptide could functionally compensate for the lack of the PI motif.
Recent studies by Litt and Irish (2003) and Vandenbussche et al. (2003) on the evolution of the large MADS-box gene family in plants suggest that one possible mechanism driving evolution and acquisition of de novo function could have been gene duplication followed by frameshift mutations in the 3′ end of the coding region. This would originate novel highly conserved motifs in the C terminus that have been related to the acquisition of new functions. Thus, these motifs have been identified in a number of gene lineages, such as the PI motif, the euAP3 and paleoAP3 motifs, or the euAP1 and paleoAP1 motifs. Pea provides intriguing exceptions to this rule. We have previously shown that the polypeptide encoded by PIM, a pea AP1 ortholog, lacks the highly conserved prenylation motif found in AP1-related proteins. This motif was shown to be necessary for posttranslational modification of the AP1 protein and its function (Yalofsky et al., 2000). Like the PI motif in PsPI, we showed that the lack of prenylation motif in the PIM protein did not compromise its function, since a 35S::PIM construct introduced in Arabidopsis recapitulated the 35S::AP1 phenotypes and was able to rescue the ap1-1 mutation in Arabidopsis (Berbel et al., 2001). pim mutants are phenotypically similar to Arabidopsis ap1 mutants, therefore demonstrating the function of PIM as an AP1 gene even though lacking a functional prenylation motif (Taylor et al., 2002). Our functional analysis of PsPI indicates that the PI motif is also dispensable for PsPI function, although further confirmation of the role of PsPI in pea development waits for the identification of pspi loss-of-function mutants. As discussed above, it is possible that changes in the rest of the polypeptide could balance the loss of these motifs. An alternative scenario is that the requirement for these motifs could be less strict than proposed, as also suggested by the results described by Whipple et al. (2005), where Silky, a maize AP3 homolog that encodes a protein lacking the euAP3 motif, was shown to rescue the ap3 mutation in Arabidopsis. It would be interesting to see whether there are other examples of absence of lineage-specific motifs in legume MADS-box genes and how they affect protein function.
PsPI in the Development of the Pea Flower
Though the transformation of tobacco and Arabidopsis are informative about the likely function of PsPI, the function of this gene in pea cannot be definitively established due to the lack of pspi mutants.
However, our analyses of PsPI expression in different mutant backgrounds support the idea that PsPI works as a B-function gene also in pea. Thus, in the pea brac mutant flowers, the PsPI transcript is also found in organs of the first whorl, correlating with the presence of petaloid sectors in these organs and, therefore, with a role of PsPI in petal identity specification.
The expression pattern of PsPI during pea flower development indicates that its expression is regulated in a similar way to that of PI/GLO in model species. B-gene activation in Arabidopsis is set by LFY and UFO and in Antirrhinum by FLO and FIM. Orthologs for both genes have been identified in pea, UNI and STP. Our results and data from other labs indicate that the onset of B-gene expression in pea floral meristems is controlled by this pair of genes. Thus, the phenotype of the flowers of the uni mutants, lacking petals and stamens, suggests that UNI activates the expression of B-class genes in the pea floral meristem (Hofer et al., 1997). Likewise, the phenotype of stp flowers, which show petal-to-sepal and stamen-to-carpel transformations, is typical of B loss-of-function mutations (Ferrándiz et al., 1999; Taylor et al., 2001). Accordingly, we have shown that PsPI expression is dramatically reduced in the flowers of the stp-2 mutant.
An unanswered question remains about how the development of common primordia is controlled. A role for B-function genes in this process has been suggested previously. This was based on the observation that, in the flowers of the stp-2 mutant, which show a loss of B-function phenotype, the common primordia are larger and continue growing longer than in wild-type flowers before the differentiation of floral organ primordia (Ferrándiz et al., 1999). The onset of PsPI expression in the floral meristem precedes the formation of the common primordia and marks the group of cells that will originate these structures. This suggests that PsPI plays a role in the specification of common primordia identity; however, this expression pattern is not informative enough to identify more precisely the role of PsPI in the development of these structures. The understanding of the contribution of PsPI in the control of the particular ontogeny of the pea flower is limited by the lack of mutants affected in this gene.
A Role of PsPI in the Development of the Pea Leaf?
While LFY/FLO and UFO/FIM, in Arabidopsis and Antirrhinum, are mainly involved in flower development, their pea orthologs also play a key role in controlling the development of the leaf. In addition to being expressed in flowers, STP and UNI are also expressed in the pea developing leaf and promote the complexity of this organ, so that stp mutants exhibit leaves with reduced complexity, and strong uni mutations cause the development of unifoliate leaves (Hofer et al., 1997; Taylor et al., 2001).
Our in situ hybridization experiments show that PsPI is expressed not only in flowers but also in young axillary buds. The expression of PsPI initially encompasses the whole axillary meristem, but when this meristem starts developing, PsPI expression is confined to the developing leaf. This suggests that PsPI could play a role also in the control of leaf development in pea. This would fit with the key role played by STP and UNI, regulators of B-function gene expression in flowers, in controlling the development of the pea leaf and highlights the parallelisms between the development of the pea compound leaf and the flower previously indicated by other authors (Hofer and Ellis, 2002). To our knowledge, our data represent the first indication that a B-function gene could be involved in controlling the development of leaves. It is clear that our results can only be considered as a suggestion and that the validation of this attractive hypothesis would require further studies that should include the isolation and analysis of pspi mutant plants or the use of Medicago truncatula or Lotus japonicus, two species now emerging as models in legumes, where a variety of reverse genetic methods are being developed (Cook, 1999; Young et al., 2003).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis plants were grown in cabinets at 21°C under long-day (16 h light) conditions, illuminated by cool-white fluorescent lamps (150 μE m−2 s−1), in a 1:1:1 mixture of sphagnum:perlite:vermiculite. The Arabidopsis (Arabidopsis thaliana) plants used in this work were from the Columbia (wild type and derivates) or Landsberg erecta (the pi-1, ap3-1, and ap3-3 mutants) ecotypes. Pea (Pisum sativum) plants were grown in a greenhouse at 22°C (day) and 18°C (night); 16-h photoperiods were maintained with supplementary lighting (400 W Phillips HDK/400 HPI. The pea mutants used in this work have been described previously (Ferrándiz et al., 1999); the wild-type pea used was cv Dippes Gelbe Viktoria. Tobacco plants (Nicotiana tabacum cv Petite Havana SR1) were grown under the same conditions as pea, in a mixture (1:1) of sphagnum:vermiculite. Pea and tobacco were irrigated with a Hoagland No. 1 solution supplemented with oligoelements (Hewitt, 1966). Arabidopsis plants were irrigated with water and, once a week, with the same mineral solution.
Isolation and Sequence Analysis of the PSPI cDNA Clones
A λNM1149 cDNA library of pea (cv Dippes Gelbe Viktoria) inflorescences was screened at moderated stringency, 52°C, with washes at room temperature in 2× SSC (0.3 m and 0.1 m NaCl) and 0.1% (w/v) SDS, using the MADS domain of DEF cDNA (Sommer et al., 1990) as a probe.
Sequence Analysis
Protein sequences were aligned using the multiple alignment mode of the ClustalW software and visualized and edited with the GENEDOC utilities (version 2.3.000; www.psc.edu/biomed/genedoc). Phylogenetic trees were computed using the TREECON program according to the neighbor-joining algorithm (Saitou and Nei, 1987).
Plant Transformation
PsPI cDNA was cloned into the pBINJIT60 vector (Guerineau and Mullineaux, 1993), a pBIN19 derivate (CLONTECH Laboratories), which placed PsPI transcription under the control of a tandem repeat of the 35S promoter of cauliflower mosaic virus. Tobacco and Arabidopsis plants were transformed according to standard procedures (Horsch et al., 1985; Bechtold et al., 1993).
Northern Analysis
Total RNA was purified by phenol/chloroform extraction, followed by precipitation with 3 m LiCl. RNA was electrophoresed in formaldehyde-agarose gels, transferred to Hybond N+ membranes (Amersham), and hybridized with 32P-labeled probes under standard conditions. The PsPI probe was a 573-bp fragment of the 3′ end of the cDNA.
Genotypic Analysis
Homozygous transgenic lines were used as pollen donor for crosses to homozygous mutants (pi-1, ap3-1, and ap3-3). The resulting progeny were allowed to self-fertilize, and genotypic analyses were performed in the next generation. To check for the presence of the pi-1 mutation, the derived cleaved amplified polymorphic sequence (dCAPS) protocol in Lamb and Irish (2003) was followed. To confirm the presence of the 35S::PsPI transgene, kanamycin resistance was scored by plating seeds from individual F2 plants on antibiotic-containing medium.
SEM
For SEM, fresh floral organs and complete flowers were vacuum infiltrated with 4% formaldehyde (w/v) in 1× phosphate-buffered saline for 10 min and fixed with fresh solution for 16 h at 4°C. Samples were dehydrated in an ethanol series and critical point dried in liquid CO2 (Polaron E300 apparatus). Dried samples were mounted on stubs; when necessary, several outer whorl organs of individual flowers were removed manually and then were coated with gold- palladium (4:1) in a Sputter Coater SCD005 (BALTEC). SEM was performed with a JEOL JSM-5410 microscope (10 kV).
RNA In Situ Hybridization
RNA in situ hybridization with digoxigenin-labeled probes was performed on 8-μm longitudinal paraffin sections of pea inflorescences, as described by Ferrándiz et al. (2000). RNA antisense and sense probes were generated from 458 bp of the 3′ region of the PsPI cDNA and cloned into pBluescript KS II + vector (Stratagene) using SP6 and T7 polymerases, respectively. Signal was detected as a purple precipitate when viewed under the light microscope.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY842491 (PsPI); AAK77938 (MsNGL9); Q03378 (AmGLO); Q03488 (PhGLO1); Q07474 (PhGLO2); Q03416 (NtGLO); P48007 (AtPI); P23706 (AmDEF); CAA65288 (NtDEF); CAA49567 (PhDEF); AAC15419 (MsNMH7); P35632 (AtAP3); AJ279089 (PEAM4).
Acknowledgments
We thank M.A. Blazquez, D. Bradley, and J. Hofer for critical reading of the manuscript and acknowledge the collaboration of R. Martínez Pardo and A. Millán in the greenhouse.
This work was supported by grant number BIO2000–0940 of the Secretaría General del Plan Nacional de Investigación Científica y Desarrollo Tecnológico. A.B. was supported by a fellowship of the Conselleria de Cultura, Educación, y Ciencia (Generalitat Valenciana). C.N. and C.F. were supported by fellowships of the Ministerio de Educación y Ciencia (Spain).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057687.
References
- Angenent GC, Busscher M, Franken J, Mol JNM, van Tunen AJ (1992) Differential expression of two MADS box genes in wild-type and mutant petunia flowers. Plant Cell 4: 983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Ser III Life Sci 316: 1194–1199 [Google Scholar]
- Berbel A, Navarro C, Ferrándiz C, Cañas LA, Madueño F, Beltrán JP (2001) Analysis of PEAM4, the pea AP1 functional homologue, supports a model for AP1-like genes controlling both floral meristem and floral organ identity in different plant species. Plant J 25: 441–451 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell 1: 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter R, Copsey L, Vincent C, Doyle S, Magrath R, Coen ES (1995) Control of flower development and phyllotaxy by meristem identity genes in Antirrhinum. Plant Cell 7: 2001–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353: 31–37 [DOI] [PubMed] [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliot R, Murphy G, Carpenter R (1990) floricaula: a homeotic gene required for flower development in Antirrhinum majus. Cell 63: 1311–1322 [DOI] [PubMed] [Google Scholar]
- Cook DR (1999) Medicago truncatula-a model in the making! Curr Opin Plant Biol 2: 301–304 [DOI] [PubMed] [Google Scholar]
- Davies B, Di Rosa A, Eneva T, Saedler H, Sommer H (1996) Alteration of tobacco floral organ identity by expression of combinations of Antirrhinum MADS-box genes. Plant J 10: 663–677 [DOI] [PubMed] [Google Scholar]
- Ferrándiz C, Gu Q, Martienssen R, Yanofsky M (2000) Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127: 725–734 [DOI] [PubMed] [Google Scholar]
- Ferrándiz C, Navarro C, Gómez MD, Cañas LA, Beltrán JP (1999) Flower development in Pisum sativum: from the war of the whorls to the battle of the common primordia. Dev Genet 25: 280–290 [DOI] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM (1994) Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev 8: 1548–1560 [DOI] [PubMed] [Google Scholar]
- Guerineau F, Mullineaux P (1993) Plant transformation and expression vectors. In B Croy, ed, Plant Molecular Biology Labfax. BIOS Scientific Publishers, Oxford, pp 121–148
- Hansen G, Estruch JJ, Sommer H, Spena A (1993) NTGLO: a tobacco homologue of the GLOBOSA floral homeotic gene of Antirrhinum majus: cDNA sequence and expression pattern. Mol Gen Genet 239: 310–312 [DOI] [PubMed] [Google Scholar]
- Hantke SS, Carpenter R, Coen ES (1995) Expression of floricaula in single cell layers of periclinal chimeras activates downstream homeotic genes in all layers of floral meristems. Development 121: 27–35 [DOI] [PubMed] [Google Scholar]
- Heard J, Dunn K (1995) Symbiotic induction of a MADS-box gene during development of alfalfa root nodules. Proc Natl Acad Sci USA 92: 5273–5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt Y (1966) Sand and Water Culture Methods Used in the Study of Plant Nutrition, Ed 2. Commonwealth Agricultural Bureau, Farnham, UK
- Hill JP, Lord EM (1989) Floral development in Arabidopsis thaliana: comparison of the wild type and the homeotic pistillata mutant. Can J Bot 67: 2922–2936 [Google Scholar]
- Hofer J, Ellis N (2002) Conservation and diversification of gene function in plant development. Curr Opin Plant Biol 5: 56–61 [DOI] [PubMed] [Google Scholar]
- Hofer J, Turner L, Hellens R, Ambrose M, Matthews P, Michael A, Ellis N (1997) UNIFOLIATA regulates leaf and flower morphogenesis in pea. Curr Biol 7: 581–587 [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Huijser P, Klein J, Lönnig W-E, Meijer H, Saedler H, Sommer H (1992) Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS-box gene squamosa in Antirrhinum. EMBO J 11: 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram GC, Goodrich J, Wilkinson MD, Simon R, Haughn GW, Coen ES (1995) Parallels between UNUSUAL FLORAL ORGANS and FIMBRIATA, genes controlling flower development in Arabidopsis and Antirrhinum. Plant Cell 7: 1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish V (1999) Petal and stamen development. Curr Top Dev Biol 41: 133–161 [DOI] [PubMed] [Google Scholar]
- Jack T, Brockman LL, Meyerowitz EM (1992) The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS-box and is expressed in petals and stamens. Cell 68: 683–697 [DOI] [PubMed] [Google Scholar]
- Kramer EM, Dorit RL, Irish VF (1998) Molecular evolution of genes controlling petal and stamen development: duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics 149: 765–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA, Meyerowitz EM (1996) The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122: 11–22 [DOI] [PubMed] [Google Scholar]
- Kush A, Brunelle A, Shevell D, Chua NH (1993) The cDNA sequence of two MADS box proteins in petunia. Plant Physiol 102: 1051–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RS, Irish VF (2003) Functional divergence within the APETALA3/PISTILLATA floral homeotic gene lineages. Proc Natl Acad Sci USA 100: 6558–6563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Wolfe DS, Nilsson O, Weigel D (1997) A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr Biol 7: 95–104 [DOI] [PubMed] [Google Scholar]
- Levin JZ, Meyerowitz EM (1995) UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 7: 529–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Irish VF (2003) Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics 165: 821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360: 273–277 [DOI] [PubMed] [Google Scholar]
- Marx GA (1987) A suite of mutants that modify pattern formation on pea leaves. Plant Mol Biol Rep 5: 311–355 [Google Scholar]
- Ng M, Yanofsky MF (2001) Activation of the Arabidopsis B class homeotic genes by APETALA1. Plant Cell 13: 739–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Nilsson O, Lee I, Busch M, Weigel D (1998) A genetic framework for floral patterning. Nature 395: 561–566 [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425 [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z, Hue I, Huijser P, Flor PJ, Hansen R, Tetens F, Lönnig W-E, Saedler H, Sommer H (1992) Characterization of the Antirrhinum floral homeotic MADS-box gene deficiens: evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J 11: 251–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Carpenter R, Doyle S, Coen E (1994) Fimbriata controls flower development by mediating between meristem and organ identity genes. Cell 78: 99–107 [DOI] [PubMed] [Google Scholar]
- Singer SR, Sollinger J, Maki S, Fishbach J, Short B, Reinke C, Fick J, Cox L, McCall A, Mullen H (1999) Inflorescence architecture: a developmental genetics approach. Bot Rev 65: 385–410 [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H, Beltrán JP, Huijser P, Pape H, Lönnig W-E, Saedler H, Schwarz-Sommer Z (1990) Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J 9: 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Hofer J, Murfet I (2001) Stamina pistilloida, the pea ortholog of Fim and UFO, is required for normal development of flowers, inflorescences, and leaves. Plant Cell 13: 31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SA, Hofer JM, Murfet IC, Sollinger JD, Singer SR, Knox MR, Ellis TH (2002) PROLIFERATING INFLORESCENCE MERISTEM, a MADS-box gene that regulates floral meristem identity in pea. Plant Physiol 129: 1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Munster T, Winter KU, Saedler H (2000) A short history of MADS-box genes in plants. Plant Mol Biol 42: 115–149 [PubMed] [Google Scholar]
- Theissen G, Kim JT, Saedler H (1996) Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J Mol Evol 43: 484–516 [DOI] [PubMed] [Google Scholar]
- Theissen G, Saedler H (1995) MADS-box genes in plant ontogeny and phylogeny: Haeckel's ‘biogenetic law’ revisited. Curr Opin Genet Dev 5: 628–639 [DOI] [PubMed] [Google Scholar]
- Tröbner W, Ramirez L, Motte P, Hue I, Huijser P, Lönnig W-E, Saedler H, Sommer H, Schwarz-Sommer Z (1992) GLOBOSA: a homeotic gene which interacts with DEFICIENS in the control of Antirrhinum floral organogenesis. EMBO J 11: 4693–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S (1989) Overlapping organ initiation and common primordia in flowers of Pisum sativum (Leguminosae: Mimosoidae). Am J Bot 75: 204–224 [Google Scholar]
- Vandenbussche M, Theissen G, Van der Peer Y, Gerats T (2003) Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations. Nucleic Acids Res 31: 4401–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche M, Zethof J, Royaert S, Weterings K, Gerats T (2004) The duplicated B-class heterodimer model: whorl-specific effects and complex genetic interactions in Petunia hybrida flower development. Plant Cell 16: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69: 843–859 [DOI] [PubMed] [Google Scholar]
- Weigel D, Meyerowitz EM (1993) Activation of floral homeotic genes in Arabidopsis. Science 261: 1723–1726 [DOI] [PubMed] [Google Scholar]
- Whipple CJ, Ciceri P, Padilla CM, Ambrose BA, Bandong SL, Schmidt RJ (2005) Conservation of B-class floral homeotic gene function between maize and Arabidopsis. Development 131: 6083–6091 [DOI] [PubMed] [Google Scholar]
- Yalofsky S, Rodriguez-Concepcion M, Bracha K, Toledo-Ortiz G, Gruissem W (2000) Prenylation of the floral transcription factor APETALA1 modulates its function. Plant Cell 12: 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ND, Mudge J, Ellis TN (2003) Legume genomes: more than peas in a pod. Curr Opin Plant Biol 6: 199–204 [DOI] [PubMed] [Google Scholar]
- Zucchero JC, Caspi M, Dunn K (2001) ngl9: a third MADS box gene expressed in alfalfa root nodules. Mol Plant Microbe Interact 14: 1463–1467 [DOI] [PubMed] [Google Scholar]