Abstract
Nicotiana plumbaginifolia NpPDR1, a plasma membrane pleiotropic drug resistance-type ATP-binding cassette transporter formerly named NpABC1, has been suggested to transport the diterpene sclareol, an antifungal compound. However, direct evidence for a role of pleiotropic drug resistance transporters in the plant defense is still lacking. In situ immunolocalization and histochemical analysis using the gusA reporter gene showed that NpPDR1 was constitutively expressed in the whole root, in the leaf glandular trichomes, and in the flower petals. However, NpPDR1 expression was induced in the whole leaf following infection with the fungus Botrytis cinerea, and the bacteria Pseudomonas syringae pv tabaci, Pseudomonas fluorescens, and Pseudomonas marginalis pv marginalis, which do not induce a hypersensitive response in N. plumbaginifolia, whereas a weaker response was observed using P. syringae pv syringae, which does induce a hypersensitive response. Induced NpPDR1 expression was more associated with the jasmonic acid than the salicylic acid signaling pathway. These data suggest that NpPDR1 is involved in both constitutive and jasmonic acid-dependent induced defense. Transgenic plants in which NpPDR1 expression was prevented by RNA interference showed increased sensitivity to sclareol and reduced resistance to B. cinerea. These data show that NpPDR1 is involved in pathogen resistance and thus demonstrate a new role for the ATP-binding cassette transporter family.
ATP-binding cassette (ABC) transporters, which belong to a protein superfamily found in all living organisms, mediate the translocation of a wide range of structurally unrelated molecules across biological membranes (Higgins, 1992; Holland et al., 2002). All functional ABC proteins have a basic structural organization consisting of a hydrophobic transmembrane domain (TMD), usually made up of six transmembrane spans, and a hydrophilic nucleotide binding fold (NBF). The latter contains a conserved 200 amino acid region with three motifs, the Walker A and Walker B boxes (Walker et al., 1982), which are separated by approximately 120 amino acids that contain the ABC signature motif (Bairoch, 1992). The TMD and NBF are usually arranged in a duplicated forward (TMD-NBD-TMD-NBD) or reverse (NBD-TMD-NBD-TMD) configuration, and these proteins are known as full-size ABCs. However, half-size transporters, which can act as dimers, have also been reported (see Pighin et al., 2004 for a recent example in plants). In some cases, especially in prokaryotes, the different structural domains can be encoded as separate subunits, referred to as quarter molecules (Higgins, 1992).
The complete sequencing of the Arabidopsis (Arabidopsis thaliana) genome allowed the identification of 131 ABC transporter coding sequences (Sanchez-Fernandez et al., 2001; Martinoia et al., 2002), 54 of which belong to the full-size category, while analysis of the rice (Oryza sativa) genome, most of which has been sequenced, allowed the identification of a similar number of genes (Jasinski et al., 2003; Garcia et al., 2004). This abundance may be associated with the large variety of secondary metabolites that plants have to cope with and the absence of a specialized excretory structure. Most of the plant full-size transporters are classified according to the configuration of their TMD and NBF domains into three groups, multidrug resistance-associated proteins (MRP), multidrug resistance (MDR) transporters, and pleiotropic drug resistance (PDR) transporters (for review, see Theodoulou, 2000; Sanchez-Fernandez et al., 2001; Martinoia et al., 2002; Rea et al., 2002; van den Brûle and Smart, 2002; Jasinski et al., 2003).
The MRP subfamily contains the best-characterized plant ABC transporters. Some of these are responsible for the vacuolar import of chlorophyll catabolites and xenobiotics or endogenous metabolites conjugated to glutathione, glucoside, or glucuronate (for review, see Rea, 1999; Martinoia et al., 2002; Rea et al., 2002), and are suggested to play a role in cellular detoxification by vacuolar sequestration of endogenous or exogenous toxic compounds. This is consistent with the induction of AtMRP expression seen when an Arabidopsis cell suspension is treated with different chemicals, including herbicide safeners (Sanchez-Fernandez et al., 1998). Plant MRPs also have other functions; for example, AtMRP5 is suggested to function as an ion channel regulator (Gaedeke et al., 2001) and is involved in the regulation of the stomatal aperture (Klein et al., 2003).
The MDR subfamily contains Arabidopsis AtPGP1 (P-glycoprotein 1), the first plant ABC transporter to be cloned (Dudler and Hertig, 1992). The physiological implication of this subfamily in plants is still a matter of debate, since marked differences have been observed in phenotypes associated with a given AtMDR gene. The use of AtPGP1 sense and antisense constructs in transgenic plants suggests its involvement, via the transport of a hypothetical hormone, in the regulation of hypocotyl growth (Sidler et al., 1998), while reverse genetics analysis allowed the isolation of Arabidopsis AtMDR1 and AtPGP1 mutants characterized by a phenotype that could result from limited auxin transport (Noh et al., 2001). It was recently shown that this phenotype results from a disruption of the normal accumulation of PIN1, an auxin transporter, at the basal end of the hypocotyl cells (Noh et al., 2003). In the maize (Zea mays) br2 and sorghum (Sorghum bicolor) dw3 mutants, compact stalks result from the loss of an MDR transporter homologous to AtPGP1 (Multani et al., 2003). Other studies showed that, using an ATP gradient resulting from ectophosphatase activity, AtPGP1 might confer xenobiotic resistance via secretion of toxic metabolites, such as herbicides (Thomas et al., 2000; Windsor et al., 2003). Another interesting member of the plant MDR subfamily is the Coptis japonica CjMDR1, which is involved in the uptake of berberine, an alkaloid that accumulates in the rhizomes of this species (Shitan et al., 2003).
The plant PDR subfamily has been poorly investigated. For some of its members, a link between the ABC transporter and a physiological substrate has been demonstrated. The plasma membrane transporter NpABC1 (from now on, NpABC1 will be called NpPDR1 to specify the subfamily it belongs to) was identified in Nicotiana plumbaginifolia culture cells treated with sclareol, an antifungal diterpene, and was indirectly shown to transport a sclareol analogue, suggesting, but not demonstrating, the involvement of this transporter in plant defense (Jasinski et al., 2001). Expression of SpTUR2, a Spirodela polyrrhiza protein showing high homology with NpPDR1, is also induced by sclareol (van den Brûle and Smart, 2002), and its overexpression in Arabidopsis improves growth in the presence of toxic concentrations of sclareol (van den Brûle et al., 2002). These data suggest the involvement of plant PDR-like proteins in the secretion of antimicrobial terpenes.
Plant defense mechanisms are varied, depending on the plant and pathogen involved. In most cases, the plant response is complex, including both constitutive and pathogen-induced defense mechanisms. The latter generally involves either the salicylic acid (SA)- or jasmonic acid (JA)-controlled pathway. In addition to defense proteins, such as various lytic enzymes, secondary metabolites participate in constitutive or induced defense (Feys and Parker, 2000; Dangl and Jones, 2001; Cheong et al., 2002; Kunkel and Brooks, 2002). Sclareol belongs to the terpenoid group of secondary metabolites, a large class of molecules involved in various physiological processes, including plant-pathogen (Kennedy et al., 1992) and plant-insect (De Moraes et al., 2001) interactions. Sclareol, which also inhibits the growth of certain pathogenic fungi (Bailey et al., 1975), is synthesized in tobacco leaf trichomes (Guo and Wagner, 1995) and is, like some other diterpenes, a major constituent of the exudate covering the leaf surface (Colledge and Reid, 1975).
The involvement of PDR-type transporters in plant defense is supported by the observations that a pathogen-produced elicitor induces NtPDR1 expression in an Nicotiana tabacum suspension culture (Sasabe et al., 2002) and that AtPDR12 is up-regulated in Arabidopsis inoculated with compatible and incompatible fungi (Campbell et al., 2003). However, despite an enhanced susceptibility to sclareol, Arabidopsis insertion lines disrupted in AtPDR12 do not display any altered sensitivity to pathogens such as Fusarium oxysporum or P. syringae pv Tomato (Campbell et al., 2003), thus questioning the role of this transporter in resistance to pathogens. The possible involvement of NpPDR1 in plant defense was therefore investigated in this study in that we showed that NpPDR1 was constitutively expressed in the leaf trichomes and the root, but was induced in the whole leaf following pathogen attack. Silencing of NpPDR1 by RNA interference resulted in increased sensitivity to sclareol and the plant pathogen Botrytis cinerea, thus demonstrating its involvement in a defense system.
RESULTS
NpPDR1 Expression in N. plumbaginifolia
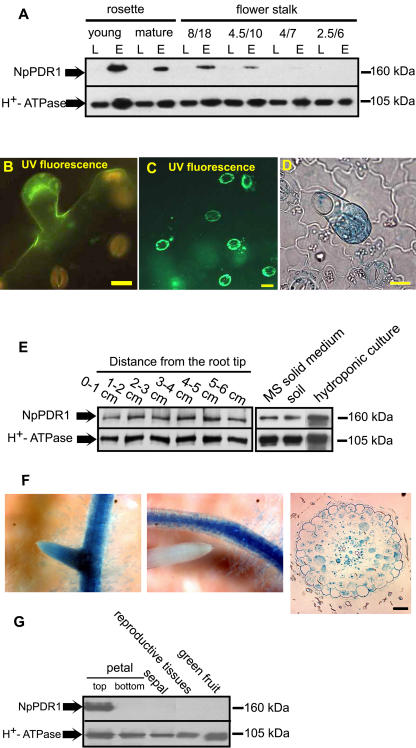
NpPDR1 has been previously immunodetected in N. plumbaginifolia peeled leaf epidermis (Jasinski et al., 2001), a tissue localization in agreement with its possible involvement in terpene secretion at the leaf surface. Biosynthesis of secondary metabolites, including monoterpenes (Turner et al., 1999), sesquiterpenes (Crock et al., 1997), and diterpenes (Guo and Wagner, 1995), occurs in the secretory head cells of the leaf glandular trichomes. Ultrastructural analysis of tobacco short (unicellular stalk and pluricellular head) and long (multicellular stalk and uni/pluricellular head) glandular trichomes showed that both have secretory properties (Akers et al., 1978). Since the secretion of secondary metabolites changes during plant development, we used western blotting to follow NpPDR1 expression in N. plumbaginifolia during leaf development and in leaves of different sizes and stalk positions; in this and subsequent western blotting and in situ immunolocalization studies, two different anti-NpPDR1 antisera (see “Materials and Methods”) were used, with identical results. As shown in Figure 1A, in contrast to the constitutive expression of plasma membrane H+-ATPase, NpPDR1 was not detected in the microsomal fraction of whole leaf, but was detected in the same fraction from the peeled epidermis of rosette and stalk leaves, provided the latter were at least one-half of the maximal size observed for a fully expanded leaf. The amount of the control protein, plasma membrane H+-ATPase, did not vary significantly.
Figure 1.
NpPDR1 is expressed in the leaf trichomes, the root, and the petals. A, Microsomal fractions were extracted from the whole leaf (L) or leaf epidermis (E) of N. plumbaginifolia rosette or flower-stalk leaves at different developmental stages or of the indicated size (width [cm]/length [cm]) and subjected to western blotting using anti-NpPDR1 or anti-H+-ATPase antibodies. Five to ten leaves were pooled per stage. The apparent molecular masses of the bands are indicated on the right. B and C, The epidermis was peeled from mature N. plumbaginifolia stalk leaves and prepared for in situ immunolocalization (see "Materials and Methods") using anti-NpPDR1 (B) or anti-H+-ATPase antibodies (C). D, The epidermis of a transgenic N. tabacum plant expressing NpPDR1-gusA was peeled off and processed for histochemical analysis; GUS-positive structures are stained blue. Bars = 20 μm. E, Microsomal fractions extracted from the indicated root sections of a flowering plant grown in the soil (left section) or from the whole root of a plant grown in vitro in Murashige and Skoog solid medium, soil, or hydroponic culture (right section) were submitted to western blotting using the indicated antibodies. The apparent molecular masses of the bands are indicated on the right. F, Roots of a transgenic N. tabacum plant expressing the NpPDR1-gusA construct were processed for histochemical analysis. The left section shows a secondary root emerging from a primary root. The middle section shows absence of GUS expression in the tip. GUS-positive structures are stained blue. The right section shows a root cross section after GUS staining. G, Microsomal fractions were extracted from the indicated flower organs and submitted to western blotting using the indicated antibodies. The petal top (colored) and bottom parts were separated and individually tested.
In situ immunolocalization studies on N. plumbaginifolia peeled leaf epidermis localized NpPDR1 to the glandular trichomes (Fig. 1B), whereas plasma membrane H+-ATPase was mainly found in the stomatal guard cells (Fig. 1C). To confirm this, we introduced a DNA construct containing the gusA reporter gene under the control of the NpPDR1 transcription promoter region into N. tabacum; this construct has been shown to function in N. tabacum BY2 cells but has not been tested in plants (Grec et al., 2003). β-Glucuronidase (GUS) histochemical analysis of the epidermis of the transgenic plants confirmed that NpPDR1 was expressed in the leaf trichomes (Fig. 1D).
NpPDR1 expression analysis was extended to other organs. Western blotting of the root of a plant grown in soil showed that NpPDR1 was expressed throughout the entire length of this organ and that expression was also observed in hydroponic culture and in sterile in vitro culture (Fig. 1E). In situ immunolocalization failed to detect NpPDR1 in the root, probably because the antigenic determinants were lost during the harsh embedding conditions. However, in transgenic plants expressing NpPDR1-gusA, GUS expression was seen in the whole root, except for the tip (Fig. 1F). Cross section showed expression in most of the root cells.
Western blotting with flower organs showed that NpPDR1 expression was exclusively found in the upper part of the petal (Fig. 1G).
NpPDR1 Expression Is Induced by Biotic Stresses
The antimicrobial properties of sclareol and other terpenes (Bailey et al., 1975; Kennedy et al., 1992), which are possible NpPDR1 substrates, suggest that NpPDR1 is involved in the plant response to pathogens. Thus far, we had demonstrated constitutive expression of NpPDR1 in the root and in leaf glandular trichomes, which are known to secrete terpenoid molecules as a constitutive first barrier of defense. However, since plant defense also involves induced responses following pathogen attack, we studied the response of NpPDR1 to pathogen infection. N. plumbaginifolia fully expanded stalk leaves were infiltrated with water (control), or with P. syringae pv tabaci Laboratorium voor Microbiologie van Gent (LMG) 5393 or P. syringae pv syringae PsP2. Both have hrp genes that encode for the type III secretion system and avirulence (avr) proteins, which provide a selective advantage in the pathogen infection process (Alfano and Collmer, 1997). However, the avr proteins from P. syringae pv syringae are recognized by tobacco Resistance proteins, which act as receptors and trigger programmed cell death, known as the hypersensitive response (HR), whereas P. syringae pv tabaci avr proteins are not recognized by the host and HR is not initiated, resulting in strong pathogenesis. We also tested the response to the nonpathogenic P. fluorescens LMG 1794 and to P. marginalis pv marginalis LMG 5177, which produces pectolytic enzymes but is not known as a pathogen for Nicotiana species. Western-blot analysis (Fig. 2A) of microsomal fractions extracted from different leaf regions and after increasing periods of infection revealed that all the tested Pseudomonas strains, except P. syringae pv syringae, induced strong expression of NpPDR1 in the whole leaf. NpPDR1 was first detected in the infiltration zone (zone 1) and, in some cases, to a lesser extent in the surrounding zone (zone 2), while a longer period of infection resulted in its appearance in more remote zones of the infiltrated leaf (zone 3). However, in a leaf infiltrated with HR-inducing P. syringae pv syringae, little NpPDR1 expression was observed. This was not due to lack of infection, since the infiltrated tissues displayed necrosis (data not shown). GUS histochemical analysis of P. syringae pv tabaci-infiltrated transgenic N. tabacum plants expressing the NpPDR1-GUS reporter gene confirmed it was expressed in the trichomes in the absence of the pathogen and showed that NpPDR1 was expressed in the leaf mesophyll following infection (Fig. 2B), suggesting its involvement in an induced plant pathogen response.
Figure 2.
NpPDR1 expression in the leaf following bacterial infection. A, Mature stalk leaves from a flowering N. plumbaginifolia plant were infiltrated through the stomata with the indicated Pseudomonas strain. After the indicated period of time, the infiltration site (lanes marked 1), a 5 mm zone surrounding the infiltration site (lanes marked 2), or a zone remote from the site (lanes marked 3) was used to prepare a microsomal fraction, which was used for western blotting using the indicated antibodies. B, Mature leaves of a transgenic plant expressing NpPDR1-gusA were left untreated (−) or infiltrated (+) with P. syringae pv tabaci LMG 5393, then, after 52 h, a leaf section remote from the infiltration site was examined by GUS histochemical analysis. C, Total RNA extracted from whole leaves treated as in A (28 h for P. syringae pv syringae and P. syringae pv tabaci and 52 h for P. fluorescens and P. marginalis) was subjected to RT-PCR analysis using primers specific for the indicated genes. D, Mature leaves of a wild-type plant were infiltrated with water (H2O), 800 μm JA, 1 mm SA, or 400 μm amino cyclopropane carboxylic acid (ACC), an ethylene precursor, then, after 24 h, the infiltrated zones were used to prepare a microsomal fraction which was used for western blotting using the indicated antibodies.
The difference in the responses seen using P. syringae pv syringae, which induces HR, and the other strains, which do not, prompted us to examine markers of the SA and JA signaling pathways involved in plant defense. Using reverse transcription (RT)-PCR (Fig. 2C), we found that the PR1a gene, an SA pathway-dependent marker, was only induced following infection with P. syringae pv syringae PsP2, while the PR2b gene, a JA pathway-dependent marker, was up-regulated to a greater extent following infection with P. syringae pv tabaci LMG 5393, P. fluorescens LMG 1794, or P. marginalis pv marginalis LMG 5177, all of which induced strong NpPDR1 expression. Examination of the effect of JA, SA, and ethylene infiltration confirmed these data; NpPDR1 expression was more induced by JA than by the other two chemicals (Fig. 2D), in agreement with previous data obtained with N. tabacum suspension cells (Grec et al., 2003). All together, these data suggest that NpPDR1 is predominantly involved in an induced defense pathway distinct from the SA-dependent HR.
NpPDR1 Silencing Confers Increased Susceptibility of N. plumbaginifolia to Sclareol and the Pathogen B. cinerea
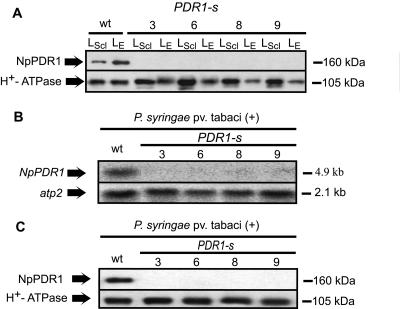
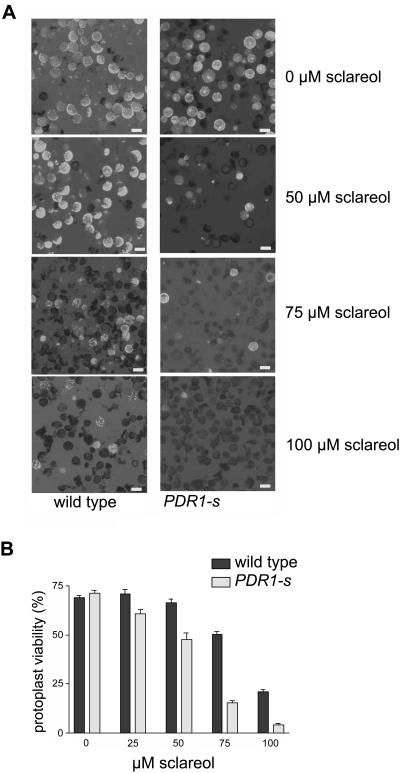
In order to decipher the physiological function of NpPDR1 and investigate its possible involvement in the plant-pathogen response, we prevented its expression by RNA interference. We thus obtained transgenic plants expressing a double-stranded RNA corresponding to a region encompassing nucleotides 12 to 434 of the NpPDR1 cDNA. Several transgenic plants showed no NpPDR1 expression (western blotting) in the leaf epidermis or in the whole leaf upon induction by sclareol infiltration (four independent lines are shown in Figure 3A). Induction of NpPDR1 expression in the whole leaf by P. syringae pv tabaci was prevented as found by northern (Fig. 3B) and western blotting (Fig. 3C). In order to investigate in more detail the involvement of NpPDR1 in sclareol transport, we took advantage of the toxic properties of this diterpene (Cutler et al., 1977) and compared its toxicity on protoplasts isolated from wild-type and NpPDR1-silenced plants. Protoplast viability assays (Fig. 4) showed an increased sensitivity of the transgenic material with impaired NpPDR1 expression compared to the wild type, supporting the involvement of the PDR protein in sclareol extrusion. The increased sclareol susceptibility of the NpPDR1 expression-impaired cells was confirmed by a bud regeneration assay performed on leaf disks grown on a regeneration medium supplemented with sclareol ranging from 75 to 200 μm (data not shown).
Figure 3.
NpPDR1 silencing by RNA interference. A, Microsomal fractions prepared from the leaf epidermis (LE) or from a leaf section 16 h after sclareol infiltration (LScl) of wild-type or NpPDR1-silenced plants were subjected to western blotting using the indicated antibodies. wt, Wild-type; 3, 6, 8, 9, PDR1-silenced lines. B, RNA was prepared from a leaf section 96 h after P. syringae pv tabaci infiltration and subjected to northern-blot analysis using primers specific for the indicated genes. C, Microsomal fractions prepared from the samples analyzed in B were subjected to western blotting using the indicated antibodies.
Figure 4.
NpPDR1-silenced cells display increased susceptibility to sclareol. A, Protoplasts were isolated from stalk leaves of wild-type and NpPDR1-silenced (PDR1-s) plants and incubated for 9 h with the indicated sclareol concentrations, then protoplast viability was examined by fluorescence microscopy using fluorescein diacetate. The figure shows representative pictures. Bars = 10 μm. B, Group data for four independent experiments examining protoplast viability for each treatment. Protoplast viability is expressed as the percentage (mean ± standard error) of fluorescent protoplasts compared to the total number of protoplasts.
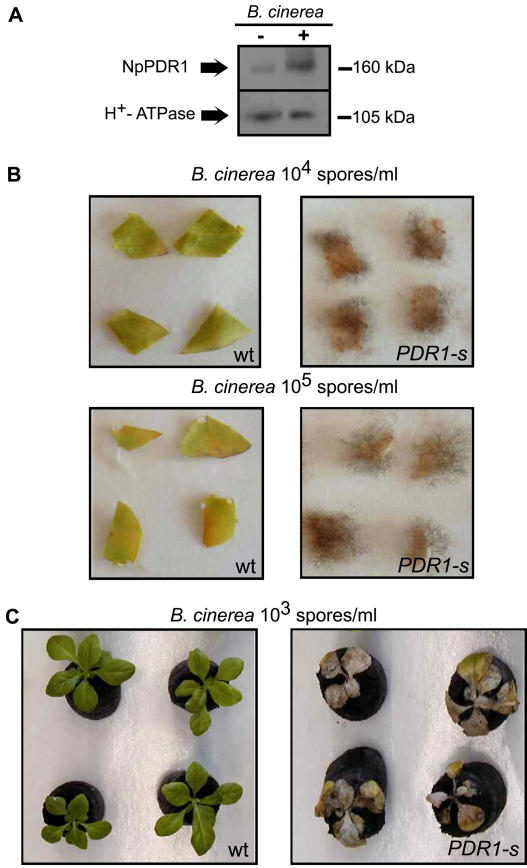
The NpPDR1-silenced plants did not show any particular modification of growth and development compared to the wild type. Since the antimicrobial properties of sclareol and the response of NpPDR1 to biotic stress suggest the involvement of NpPDR1 in the plant-pathogen response, the resistance of wild-type and NpPDR1-silenced plants to P. syringae pv tabaci was tested by leaf infiltration. Although, in some experiments we observed that the transgenic plants were more susceptible to the pathogen and showed a faster burnishing of the infiltrated zone (data not shown), these data were not systematically observed and were not investigated further. On the other hand, increased pathogen susceptibility of NpPDR1-silenced plants was evidenced by their frequent spontaneous infection by a fungus identified as B. cinerea (Fig. 5), which never occurred in wild-type plants. B. cinerea infection led to rotting at the root-stem junction and, eventually, plant death sometimes before the flowering stage. This was observed for the four lines tested but not for the wild-type plants (Fig. 5D). Fungal growth was frequently observed along the stem and on the lower stalk leaves. Western blotting showed that NpPDR1 expression was induced in wild-type leaf disks infected by B. cinerea (Fig. 6A). We then challenged the resistance of leaf disks of wild-type and four silenced plants to B. cinerea and found that silenced plants were clearly more susceptible to the pathogen; for 38 out of 48 leaf disks (divided in three independent experiments) of silenced plants, clear signs of pathogen development (Fig. 6B) were observed, while none was detected for the wild-type plant. B. cinerea susceptibility was also challenged on 14 plantlets in soil. All of the NpPDR1-silenced plantlets clearly showed severe infection development, while this was not the case for the wild-type (Fig. 6C).
Figure 5.
Spontaneous infection of NpPDR1-silenced plants by B. cinerea. A, A silenced plant (PDR1-s) spontaneously infected by B. cinerea is shown. The frame shows the region enlarged in B. C, An age-matched wild-type (wt) plant is shown for comparison. D, Quantitative data showing for four silenced lines the number of plants that spontaneously became infected and died before seed production. For each of the three experiments (numbered 1, 2, and 3), six plants of the wild-type and the indicated lines were grown in soil pots in a growth chamber.
Figure 6.
NpPDR1-silenced plants are more susceptible to B. cinerea infection. A, Microsomal fractions extracted from a leaf disk from an NpPDR1-silenced plant incubated for 10 d with or without B. cinerea (106 spores/mL) were subjected to western blotting using the indicated antibodies. The apparent masses of the bands are indicated on the right. B, Sixteen leaf disks were cut from 2-month-old in vitro wild-type (wt) and NpPDR1-silenced (PDR1-s) plants, and infected in vitro by spotting with 5 μL of the indicated concentration of B. cinerea spores. Leaf disks were observed after 14 d. This experiment was performed three times. C, After 2 weeks of acclimation to the compost substrate, 14 wild-type (wt) and 14 NpPDR1-silenced (PDR1-s) plantlets were infected with 1 mL of B. cinerea spores (103/mL) and observed after 14 d.
DISCUSSION
The previous characterization of NpPDR1, performed on a N. plumbaginifolia suspension cell culture (Jasinski et al., 2001), suggested that it is involved in sclareol transport, and the antimicrobial properties of this diterpenoid (Bailey et al., 1975) led to the conclusion that NpPDR1 might be involved in plant defense. However, this hypothesis is not yet supported by any experimental data and is even questioned by the observation that Arabidopsis plants knocked out for AtPDR12, the closest homolog of NpPDR1, do not show increased susceptibility to the pathogens tested (Campbell et al., 2003). As the expression profile at the cellular level of NpPDR1 or its S. polyrrhiza and Arabidopsis homologs had not been reported, we first determined NpPDR1 expression using the gusA reporter gene. We showed that NpPDR1 was detected in the leaf glandular trichomes, consistent with its involvement in the secretion of the sclareol-related diterpenes found in the leaf exudates of Nicotiana species (Colledge and Reid, 1975; Severson et al., 1984). In the root, expression was found in most cell types. We could eliminate the possible up-regulation of NpPDR1 expression in response to soil microorganisms since the protein was also immunodetected in roots of plants grown under sterile conditions. This is in agreement with the constitutive presence of antimicrobial terpenes and other metabolites in this organ (Oberlies et al., 2001; Ulubelen et al., 2001; Chen et al., 2002; Tan et al., 2002).
NpPDR1 expression in the upper part of the petal might also be related to plant defense like in other organs. However, petals are involved in the biosynthesis of flower volatile metabolites (Dudareva and Pichersky, 2000), and we cannot exclude the hypothesis that NpPDR1 might be involved in volatile metabolite secretion. The floral fragrances are dominated by monoterpenoid, sesquiterpenoid, phenylpropanoid, and benzenoid compounds (Dudareva and Pichersky, 2000) that are structurally distant from sclareol. However, a broad substrate specificity for NpPDR1 is possible, considering the large range of substrates presented by some ABC transporters of the PDR family, such as the yeast Pdr5p (Kolaczkowski et al., 1998).
The strong up-regulation of NpPDR1 in the whole leaf after infection by the bacteria P. syringae pv tabaci, P. fluorescens, and P. marginalis pv marginalis and the fungus B. cinerea, suggests its involvement in induced plant defense resulting in the secretion of secondary metabolites, such as sclareol and other diterpenes, which inhibit the growth of the invading organism. The lower induction of NpPDR1 expression seen after P. syringae pv syringae infection might be explained by the inhibitory effect of SA on the JA-dependent defense pathway (Doares et al., 1995), since P. syringae pv syringae induces SA production in infected plants (Cameron et al., 1994). This was confirmed by RT-PCR analysis, which showed that expression of PR1a (Payne et al., 1988), a marker for the SA-dependent defense response, was only induced by P. syringae pv syringae. In contrast, expression of PR2b (Linthorst et al., 1990), a JA-dependent defense marker, was induced to a lesser extent by P. syringae pv syringae than by the other Pseudomonas strains, which all induced NpPDR1 expression. In agreement with these data, leaf infiltration with JA induced stronger NpPDR1 expression than with SA. A similar observation had been reported for N. tabacum suspension cells (Grec et al., 2003). These observations indicate that NpPDR1 is predominantly a JA-responsive defense-related gene that responds poorly to a pathogen that induces HR and the SA signaling pathway. It is interesting to note that bacterial strains like P. fluorescens and P. marginalis pv marginalis, which are not pathogenic to Nicotiana species, nevertheless induced NpPDR1 expression, suggesting that these strains induce a plant response in the absence of usual pathogenic symptoms.
A recent analysis of the response of NtPDR1, a N. tabacum gene related to, but not an ortholog of, NpPDR1, to microbial elicitors in tobacco suspension cells showed that its expression is induced by JA, but not SA, and that NtPDR1 expression is up-regulated by elicitors, some of which induce HR, linked in many instances to the SA pathway (Sasabe et al., 2002). Since no SA- and JA-specific markers were tested in the latter study, we cannot conclude whether NpPDR1 and NtPDR1 are linked to the same signaling pathway. In addition, since elicitin exhibits different biological activities in culture cells compared to leaves (Dorey et al., 1999), it is difficult to compare our data obtained using plants and the NtPDR1 data obtained using suspension cells.
These data contrast with those reported in a study on the pathogen response of AtPDR12, the closest NpPDR1 homolog in Arabidopsis (69.6% amino acid sequence identity). Campbell et al. (2003) showed that AtPDR12 is predominantly regulated by the SA signaling pathway, although the JA pathway is also required for a rapid response. This contrasts with the observation reported here for plants and previously for culture cells (Grec et al., 2003) that NpPDR1 is less dependent on the SA pathway and is poorly induced by an HR-inducing pathogen. Another major difference is that AtPDR12 insertion lines did not show any altered resistance to the fungal and bacterial pathogens tested, while the transgenic N. plumbaginifolia lines with impaired NpPDR1 expression as a result of RNA interference showed decreased resistance to spontaneous or controlled infection by the fungus B. cinerea. However, Botrytis sensitivity of the AtPDR12 insertion line has not been tested yet. In this line, insertion occurred within the last exon and it is likely that AtPDR12 may still be transcribed (Campbell et al., 2003). Therefore, we cannot exclude that the encoded protein is still partly functional even though this line showed increased sensitivity to sclareol. An additional AtPDR12 insertion line without any detectable PDR12 transcript should be obtained before comparing Botrytis sensitivity of NpPDR1-silenced N. plumbaginifolia plants and AtPDR12 insertion Arabidopsis lines. A third difference between NpPDR1 and AtPDR12 is their expression pattern. Although NtPDR12 expression at the tissue or cell level is still unknown, this gene is more highly expressed in the leaf than in the root, under normal conditions (Campbell et al., 2003), while we found the converse to be the case for NpPDR1. These discrepancies between the Arabidopsis and Nicotiana homologs possibly reflect differences in the regulatory and functional properties that diverged during evolution. Indeed, given the high variability in secondary metabolites produced by plant species, large variations in the genes coding for transporters of these metabolites might be expected. For instance, diterpenes, such as sclareol, are important defense molecules at the leaf surface of some Nicotiana species, but, so far, have not been identified in Arabidopsis. Another reason might be that the plant defense does not operate in a similar way in different species. For instance, the SA-dependent defense pathway induces resistance to B. cinerea in tomato (Lycopersicon esculentum) but not in tobacco (Achuo et al., 2004). The sclareol susceptibility of the transgenic material with impaired NpPDR1 expression supports a role of the PDR transporter in the secretion of this and possibly other antimicrobial or defense-related metabolites both as a constitutive defense barrier at the leaf surface and in the soil around the root and as part of the JA-dependent induced defense system following pathogen attack.
As P. syringae pv tabaci was shown to induce NpPDR1 expression, it could be hypothesized that preventing NpPDR1 expression would make the plant more sensitive to this bacteria. Actually we did not observe reproducible higher sensitivity of NpPDR1-silenced plants to this strain. This suggests that the substrates transported by NpPDR1 are not the only defense mechanism that protects the plant against this pathogen. On the contrary, preventing NpPDR1 expression clearly increased the plant's susceptibility to the fungus B. cinerea to such an extent that spontaneous infection was observed that eventually led to plant death. This observation demonstrates the major role of NpPDR1 in the plant defense.
The question of the substrates transported by NpPDR1 is still puzzling, like for many ABC transporters. Sclareol has been used as a model substrate for NpPDR1 (Jasinski et al., 2001), AtPDR12 (Campbell et al., 2003), and the S. polyrrhiza homolog, SpTUR2 (van den Brûle et al., 2002). The increased susceptibility of the NpPDR1-silenced plants to sclareol is a strong argument supporting that this diterpene is indeed an NpPDR1 substrate. However, there is no reason to believe that this chemical is the only or even the major substrate of these PDR-type transporters. Indeed, some ABC transporters are known to have broad substrate specificity. For instance, Pdr5p, the Saccharomyces cerevisiae ABC transporter showing highest identity with NpPDR1, transports a wide range of structurally unrelated molecules, such as flavonoids, steroids, and various antibiotics (Kolaczkowski et al., 1998). It is therefore conceivable that NpPDR1 might transport different classes of terpenes or other compounds synthesized in response to pathogen attack. A large range of substrates is likely to be also the case for AtPDR12 since this transporter was recently shown to contribute to lead resistance, presumably involving other substrates than sclareol (Lee et al., 2005). To try to identify NpPDR1 substrates, we attempted to express this transporter in a yeast S. cerevisiae strain in which the eight major ABC transporters had been deleted (Decottignies et al., 1998). Unfortunately, the protein was expressed at a low level and did not fold correctly in this heterologous system (J. Crouzet and M. Boutry, unpublished data). A phylogenetically closer expression system will therefore have to be developed.
In conclusion, the localization of NpPDR1 expression to the leaf trichomes and the roots and its response to biotic stress support its involvement in the secretion of defense-related metabolites, both as a constitutive defense barrier and as part of an inducible defense mechanism. The finding that NpPDR1 silencing resulted in increased susceptibility to sclareol and spontaneous infection by B. cinerea provides direct evidence for a role of NpPDR1 in plant defense and defines a new function for the ABC transporter family.
MATERIALS AND METHODS
Plant Material
Nicotiana plumbaginifolia seeds were treated with 500 μg/mL of gibberellic acid and germinated on humidified compost. After germination, the plants were grown in compost in a growth chamber under conditions of 16 h light (200 μmol photons s−1 m−2 at soil level) at 25°C and 8 h dark at 19°C.
For in vitro culture, seeds of transgenic Nicotiana tabacum L. cv SRI and N. plumbaginifolia were sterilized for 1 min in 70% (v/v) ethanol and 3 min in 50% (v/v) commercial bleach, then washed five times in sterile water. N. plumbaginifolia seeds were further treated with 500 μg/mL of 0.22 μm-filtered gibberelic acid. Transformants were selected on solid Murashige and Skoog medium (4.4 g/L of Murashige and Skoog salts [ICN Biomedicals], pH 5.6, 3% Suc, 1% agar) supplemented with 100 mg/L of kanamycin, and grown at 25°C under conditions of 16 h light (50 μmol photons s−1 m−2) and 8 h dark. When rooted, the plants were transferred to soil and grown in growth chambers as described above.
Plant Transformation
The NpPDR1 cDNA 5′ region encompassing nucleotides 12 to 434 was amplified using two sets of oligonucleotides containing additional restriction sites (5′-ATAGGTCTCGAGTTTTCAGTTCATTTGATC-3′ and 5′-TGTGTGAATTCTTCTATTCTTGAGTTTCAG-3′ for the sense fragment, and 5′-ATTCGTGGATCCTTTTCAGTTCATTTGATC-3′ and 5′-TGTGTAAGCTTTTCTATTCTTGAGTTTCAG-3′ for the antisense fragment) and cloned in the sense and antisense orientation into the polylinkers flanking a pyruvate orthophosphate dikinase intron in the pKANNIBAL plasmid (Wesley et al., 2001) using the XhoI/EcoRI and BamHI/HindIII restriction sites, respectively. The corresponding expression cassette, under the control of the cauliflower mosaic virus 35S promoter, was excised from pKANNIBAL and cloned into the pART 27 binary plasmid (Gleave, 1992) using the NotI restriction site.
The 1,282-bp sequence upstream of the NpPDR1 transcription initiation site was fused to the gusA reporter gene and the construct inserted into the pBi 101.1 binary vector (Jefferson et al., 1987) using the BamHI and SmaI restriction sites (Grec et al., 2003). The binary vectors were transferred into Agrobacterium tumefaciens C58 GV3101:pMP90 (Koncz and Schell, 1986) for stable introduction into the N. tabacum L. cv SRI and N. plumbaginifolia genomes, as described previously (Horsch et al., 1985). Transformed plants were selected on solid medium (4.4 g/L of Murashige and Skoog salts [ICN Biomedicals], pH 5.6, 3% [w/v] Suc, 1% [w/v] agar) supplemented with 100 mg/L of kanamycin, and grown as described above. Four independent lines (3, 6, 8, and 9) were kept for further investigation and propagated up to the fifth generation by selecting them on kanamycin media. All experiments were performed on at least two (usually four) lines.
Pseudomonas Infiltration of N. plumbaginifolia Leaves
Pseudomonas syringae pv tabaci LMG 5393 (Laboratorium voor Microbiologie van Gent, collection no. 5393 [Belgian Coordinated Collections of Microorganisms, BCCM]), Pseudomonas fluorescens LMG 1794, Pseudomonas marginalis pv marginalis LMG 5177, and P. syringae pv syringae PsP2 were grown overnight at 28°C in King B medium (King et al., 1954), then suspended in water to concentrations of 108 colony forming units/mL, and infiltrated through the stomata into the mature stalk leaves of 3-month-old N. plumbaginifolia plants, using a syringe without a needle.
Botrytis cinerea Identification
The fungal strain isolated from two independent spontaneous infection events of NpPDR1-silenced plants was identified by the BCCM section of the Université catholique de Louvain (http://bccm.belspo.be/index.html) as Botrytis cinerea according to morphological properties of the mycelium and spores. The strain was indexed as Mycothèque Université catholique de Louvain 46725.
B. cinerea Infection Assays
Monospore cultures of B. cinerea were grown in petri dishes on solid yeast dextrose medium (2% [w/v] Glc, 2% [w/v] yeast extract, 2% [w/v] agar). Spores were collected in 1 mL of sterile 0.1% (w/v) Tween 80, filtered on Miracloth paper (Calbiochem), and diluted in water to the appropriate concentration after counting (Thomas cell). For in vitro leaf-disk infection assays, 1 cm2 leaf disks were cut from 2-month-old in vitro-grown plants and laid on two blotting papers (MN 218B, Macherey-NagelO) wetted with sterile water and were then spotted with 5 μL of the B. cinerea suspension (104 or 105 spores/mL). For in vitro root-infection assays, 2-month-old in vitro plants were transferred to sterile peat pellets (Jiffy Products International AS) in a closed box and infected 2 weeks later by addition of 1 mL of B. cinerea spores (103/mL) to the soil next to the stem-root junction.
Sclareol Toxicity Assays on Leaf Disks and Protoplasts
Leaves of growth-chamber-grown plants were soaked in tap water containing two drops of commercial detergent (3 min), rinsed under tap water, and dipped for 30 s in 70% (v/v) ethanol. After a 3-min sterilization in 50% (v/v) bleach, the leaves were rinsed for 5 × 30 s in sterile water, then 1 cm2 pieces were hand cut and incubated on regeneration medium (Murashige and Skoog solid medium supplemented with 0.2 mg/L of 3-indol-acetic acid and 2.2 mg/L of 6-benzylaminopurin) containing different concentrations of sclareol (0, 75, 100, 150, and 200 μm). Adventitious bud regeneration was then observed for 3 to 4 weeks, with renewal of the medium each week.
Protoplasts were isolated as described previously (Lukaszewicz et al., 1998) from growth-chamber-grown plant leaves and sterilized as described above, then 150 μL aliquots of the protoplast preparation were incubated for 9 h in a sterile 96-well microplate (Greiner bio-one) with different sclareol concentrations (0, 25, 50, 75, and 100 μm) and their viability estimated using 0.008% (w/v) fluorescein diacetate by fluorescence microscopy (Leica DMR).
Microsomal Fraction Preparation
Two hundred milligrams of plant material was ground in 1 mL of homogenization buffer (250 mm sorbitol, 50 mm Tris-HCl, pH 8.0, 2 mm EDTA, 7g/L of polyvinylpyrrolidone, 5 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, and 2 μg/mL each of leupeptin, pepstatin, aprotinin, antipain, and chymostatin) in a glass tissue grinder (Kontes) in ice. The homogenate was centrifuged for 5 min at 10,000g at 4°C and the supernatant centrifuged again under the same conditions, then the resulting supernatant was centrifuged for 1 h at 22,000g at 4°C, and the final pellet suspended in 30 μL of 5 mm KH2PO4, pH 7.8, 330 mm Suc, 3 mm KCl, and 2 μg/mL each of leupeptin, pepstatin, aprotinin, antipain, and chymostatin.
Antibody Preparation
Rabbit polyclonal antibodies were raised against a peptide corresponding to Ala-212-Arg-335 of NpPDR1 using a previously described method (Jasinski et al., 2001).
Western Blotting
For immunoblotting, 5 μg of each protein sample solubilized for 15 min at 37°C in an SDS cocktail (2% [w/v] SDS, 10% [w/v] glycerol, 1% [w/v] dithiothreitol, 0.005% [w/v] bromophenol blue, and 80 mm Tris-HCl, pH 6.8, containing the protease inhibitors mentioned above) was subjected to SDS-PAGE (7% polyacrylamide) and transferred electrophoretically to a polyvinylidene difluoride membrane (Millipore), which was then blocked with 3% (w/v) nonfat milk powder in 20 mm Tris-HCl, pH 7.6, 137 mm NaCl, and 0.5% (w/v) Tween 80. The membrane was then incubated for 1 h at 20°C with a 1:500 dilution of one of two rabbit anti-NpPDR1 antisera raised, respectively, against a C-terminal peptide (Jasinski et al., 2001) or against a peptide corresponding to the less conserved region of residues 212 to 335, raised as described above, or with a 1:30,000 dilution of a rabbit anti-plasma membrane H+-ATPase antiserum (Morsomme et al., 1998) in 20 mm Tris-HCl, pH 7.6, 137 mm NaCl, 0.1% (w/v) Tween 80, and 0.5% nonfat milk. The secondary antibodies (peroxidase-conjugated sheep anti-rabbit IgG antibodies; Chemicon) were diluted in the same buffer and used as indicated by the manufacturer, and then bound antibody was detected by chemiluminescence (Roche Applied Science).
In Situ Immunolocalization
The leaf abaxial epidermis was pealed off and fixed for 20 min on ice in 100 mm sodium phosphate (SP), pH 7.2 (SP buffer), containing 4% (w/v) paraformaldehyde. After two washes in SP buffer containing 0.2% (v/v) Nonidet and one wash in SP buffer, the cell walls were digested by incubation for 30 min at 20°C in 5 mm CaCl2, 500 mm Suc, 0.6% (w/v) cellulase, 0.2% (w/v) macerozyme (Yakult Pharmaceuticals), and 0.1% (w/v) bovine serum albumin, pH 5.2. The tissue was then blocked for 1 h in blocking buffer (phosphate-buffered saline [136 mm NaCl, 2.7 mm KCl, 10 mm NaHPO4, 1.76 mm KH2PO4, pH 7.4] containing 0.4% [w/v] nonfat milk, 0.04% [w/v] Tween 80), then incubated for 1 h at 20°C in blocking buffer containing antibodies against NpPDR1 (1:150 dilution) or H+-ATPase (1:80 dilution). After four washes in blocking buffer, the samples were incubated for 1 h at 37°C with fluorescein isothiocyanate-coupled goat anti-rabbit IgG antibodies (Molecular Probes), washed 4 × 10 min in blocking buffer and once in phosphate-buffered saline, and examined using a Leica DMR fluorescence microscope.
Histochemical Analysis
GUS activity in the leaf epidermis of N. tabacum plants was measured as described previously (Moriau et al., 1999).
RT-PCR Analysis
Twenty micrograms of total RNA was reverse transcribed using dT-18 and M-MLV reverse transcriptase (Promega), following the manufacturer's instructions. Using 1 μL of different dilutions (1:10, 1:50, and 1:100) of the sample, a 294-bp fragment of NpPDR1 cDNA, and, as a control, a 650-bp fragment of the atp2-1 cDNA (Boutry and Chua, 1985), were amplified by PCR using the primers described above. A 369-bp fragment of tobacco PR1a (Payne et al., 1988) and a 427-bp fragment of tobacco PR2b (Linthorst et al., 1990) were amplified using, respectively, the primers 5′-TGCCTTCATTTCTTCTTGTC-3′ plus 5′-AAACCACCTGAGTATAGTGTCC-3′, and 5′-ACATTGCTTCCGGAATGG-3′ plus 5′-ACCATCTTGTACCACCAC-3′. The number of cycles varied according to the gene to prevent signal saturation; 22 (atp2-1), 30 (PR1a), 26 (PR2-b), and 30 (NpPDR1).
Acknowledgments
We thank A.M. Faber and P. Gosselin (Université catholique de Louvain) for their excellent technical assistance and C. Decock (Belgian Coordinated Collections of Microorganisms) for the B. cinerea identification.
This work was supported by grants from the Belgian National Fund for Scientific Research, the Interuniversity Attraction Poles Program-Belgian Science Policy, and the European Community (IHP-RTN). Y.S. was a recipient of a fellowship from the Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture, and A.B. is funded by the Ministère de la Région Wallonne.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062372.
References
- Achuo EA, Audenaert K, Meziane H, Höfte M (2004) The salicylic acid-dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant Pathol 53: 65–72 [PubMed] [Google Scholar]
- Akers CP, Weybrew JA, Long RC (1978) Ultrastructure of glandular trichomes of leaves of Nicotiana tabacum L., cv Xanthi. Am J Bot 65: 282–292 [Google Scholar]
- Alfano JR, Collmer A (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol 179: 5655–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JA, Carter GA, Burden RS, Wain RL (1975) Control of rust diseases by diterpenes from Nicotiana glutinosa. Nature 255: 328–329 [Google Scholar]
- Bairoch A (1992) PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res (Suppl) 20: 2013–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M, Chua NH (1985) A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J 4: 2159–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron RK, Dixon RA, Lamb CJ (1994) Biologically induced systemic acquired-resistance in Arabidopsis thaliana. Plant J 5: 715–725 [Google Scholar]
- Campbell EJ, Schenk PM, Kazan K, Penninckx IAMA, Anderson JP, Maclean DJ, Cammue BPA, Ebert PR, Manners JM (2003) Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol 133: 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ding J, Ye YM, Zhang JS (2002) Bioactive abietane and seco-abietane diterpenoids from Salvia prionitis. J Nat Prod 65: 1016–1020 [DOI] [PubMed] [Google Scholar]
- Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129: 661–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge A, Reid WW (1975) The diterpenoids of Nicotiana species and their potential technological significance. Chem Ind 5: 570–571 [Google Scholar]
- Crock J, Wildung M, Croteau R (1997) Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha x piperita, L.) that produces the aphid alarm pheromone (E)-beta-farnesene. Proc Natl Acad Sci USA 94: 12833–12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler HG, Reid W, Deletang J (1977) Plant growth inhibiting properties of diterpene from tobacco. Plant Cell Physiol 18: 711–714 [Google Scholar]
- Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411: 826–833 [DOI] [PubMed] [Google Scholar]
- Decottignies A, Grant AM, Nichols JW, de Wet H, McIntosh DB, Goffeau A (1998) ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem 273: 12612–12622 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 410: 577–580 [DOI] [PubMed] [Google Scholar]
- Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA (1995) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108: 1741–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S (1999) Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol 121: 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E (2000) Biochemical and molecular genetic aspects of floral scents. Plant Physiol 122: 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler R, Hertig C (1992) Structure of an mdr-like gene from Arabidopsis thaliana. Evolutionary implications. J Biol Chem 267: 5882–5888 [PubMed] [Google Scholar]
- Feys BJ, Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16: 449–455 [DOI] [PubMed] [Google Scholar]
- Gaedeke N, Klein M, Kolukisaoglu U, Forestier C, Muller A, Ansorge M, Becker D, Mamnun Y, Kuchler K, Schulz B, et al (2001) The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J 20: 1875–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia O, Bouige P, Forestier C, Dassa E (2004) Inventory and comparative analysis of rice and Arabidopsis ATP-binding cassette (ABC) systems. J Mol Biol 343: 249–265 [DOI] [PubMed] [Google Scholar]
- Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Grec S, Vanham D, Christyn de Ribaucourt J, Purnelle B, Boutry M (2003) Identification of regulatory sequence elements within the transcription promoter region of NpABC1, a gene encoding a plant ABC transporter induced by diterpenes. Plant J 35: 237–250 [DOI] [PubMed] [Google Scholar]
- Guo ZH, Wagner GJ (1995) Biosynthesis of labdenediol and sclareol in cell-free-extracts from trichomes of Nicotiana glutinosa. Planta 197: 627–632 [Google Scholar]
- Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8: 67–113 [DOI] [PubMed] [Google Scholar]
- Holland BI, Cole SPC, Kuchler K, Higgins CF (2002) ABC Proteins, from Bacteria to Man. Elsevier, Amsterdam
- Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Jasinski M, Ducos E, Martinoia E, Boutry M (2003) The ATP-binding cassette transporters: structure, function, and gene family comparison between rice and Arabidopsis. Plant Physiol 131: 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski M, Stukkens Y, Degand H, Purnelle B, Marchand-Brynaert J, Boutry M (2001) A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13: 1095–1107 [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BS, Nielsen MT, Severson RF, Sisson VA, Stephenson MK, Jackson DM (1992) Leaf surface chemicals from Nicotiana affecting germination of Peronospora tabacina (Adam) sporangia. J Chem Ecol 18: 1467–1479 [DOI] [PubMed] [Google Scholar]
- King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44: 301–307 [PubMed] [Google Scholar]
- Klein M, Perfus-Barbeoch L, Frelet A, Gaedeke N, Reinhardt D, Mueller-Roeber B, Martinoia E, Forestier C (2003) The plant multidrug resistance ABC transporter AtMRP5 is involved in guard cell hormonal signalling and water use. Plant J 33: 119–129 [DOI] [PubMed] [Google Scholar]
- Kolaczkowski M, Kolaczowska A, Luczynski J, Witek S, Goffeau A (1998) In vivo characterization of the drug resistance profile of the major ABC transporters and other components of the yeast pleiotropic drug resistance network. Microb Drug Resist 4: 143–158 [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 [Google Scholar]
- Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Lee M, Lee K, Lee J, Noh EW, Lee Y (2005) AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiol 138: 827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst HJM, Melchers LS, Mayer A, Vanroekel JSC, Cornelissen BJC, Bol JF (1990) Analysis of gene families encoding acidic and basic beta-1,3-glucanases of tobacco. Proc Natl Acad Sci USA 87: 8756–8760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz M, Jerouville B, Boutry M (1998) Signs of translational regulation within the transcript leader of a plant plasma membrane H+-ATPase gene. Plant J 14: 413–423 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Muller-Rober B, Schulz B (2002) Multifunctionality of plant ABC transporters—more than just detoxifiers. Planta 214: 345–355 [DOI] [PubMed] [Google Scholar]
- Moriau L, Michelet B, Bogaerts P, Lambert L, Michel A, Oufattole M, Boutry M (1999) Expression analysis of two gene subfamilies encoding the plasma membrane H+-ATPase in Nicotiana plumbaginifolia reveals the major transport functions of this enzyme. Plant J 19: 31–41 [DOI] [PubMed] [Google Scholar]
- Morsomme P, Dambly S, Maudoux O, Boutry M (1998) Single point mutations distributed in 10 soluble and membrane regions of the Nicotiana plumbaginifolia plasma membrane PMA2 H+-ATPase activate the enzyme and modify the structure of the C-terminal region. J Biol Chem 273: 34837–34842 [DOI] [PubMed] [Google Scholar]
- Multani DS, Briggs SP, Chamberlin MA, Blakeslee JJ, Murphy AS, Johal GS (2003) Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science 302: 81–84 [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS (2003) Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423: 999–1002 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlies NH, Burgess JP, Navarro HA, Pinos RE, Soejarto DD, Farnsworth NR, Kinghorn AD, Wani MC, Wall ME (2001) Bioactive constituents of the roots of Licania intrapetiolaris. J Nat Prod 64: 497–501 [DOI] [PubMed] [Google Scholar]
- Payne G, Parks TD, Burkhart W, Dincher S, Ahl P, Metraux JP, Ryals J (1988) Isolation of the genomic clone for pathogenesis-related protein-1a from Nicotiana tabacum cv Xanthi-Nc. Plant Mol Biol 11: 89–94 [DOI] [PubMed] [Google Scholar]
- Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL (2004) Plant cuticular lipid export requires an ABC transporter. Science 306: 702–704 [DOI] [PubMed] [Google Scholar]
- Rea PA (1999) MRP subfamily ABC transporters from plants and yeast. J Exp Bot 50: 895–913 [Google Scholar]
- Rea PA, Sanchez-Fernandez R, Chen S, Peng M, Klein M, Geisler M, Martinoia E (2002) The plant ABC transporter superfamily: the functions of a few and the identity of many. In SP Cole, K Kuchler, C Higgins, and B Holland, eds, ABC Transporters: From Bacteria to Man. Elsevier, Amsterdam, pp 335–355
- Sanchez-Fernandez R, Ardiles-Diaz W, Van Montagu M, Inze D, May MJ (1998) Cloning and expression analyses of AtMRP4, a novel MRP-like gene from Arabidopsis thaliana. Mol Gen Genet 258: 655–662 [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez R, Davies TG, Coleman JO, Rea PA (2001) The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 276: 30231–30244 [DOI] [PubMed] [Google Scholar]
- Sasabe M, Toyoda K, Shiraishi T, Inagaki Y, Ichinose Y (2002) cDNA cloning and characterization of tobacco ABC transporter: NtPDR1 is a novel elicitor-responsive gene. FEBS Lett 518: 164–168 [DOI] [PubMed] [Google Scholar]
- Severson RF, Arrendale RF, Chortyk OT, Johnson AW, Jackson DM, Gwynn GR, Chaplin JF, Stephenson MG (1984) Quantification of the major cuticular components from green leaf of different tobacco types. J Agric Food Chem 32: 566–570 [Google Scholar]
- Shitan N, Bazin I, Dan K, Obata K, Kigawa K, Ueda K, Sato F, Forestier C, Yazaki K (2003) Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci USA 100: 751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidler M, Hassa P, Hasan S, Ringli C, Dudler R (1998) Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell 10: 1623–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan N, Kaloga M, Radtke OA, Kiderlen AF, Oksuz S, Ulubelen A, Kolodziej H (2002) Abietane diterpenoids and triterpenoic acids from Salvia cilicica and their antileishmanial activities. Phytochemistry 61: 881–884 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL (2000) Plant ABC transporters. Biochim Biophys Acta 1465: 79–103 [DOI] [PubMed] [Google Scholar]
- Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ (2000) A role for ectophosphatase in xenobiotic resistance. Plant Cell 12: 519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R (1999) Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol 120: 879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulubelen A, Oksuz S, Topcu G, Goren AC, Voelter W (2001) Antibacterial diterpenes from the roots of Salvia blepharochlaena. J Nat Prod 64: 549–551 [DOI] [PubMed] [Google Scholar]
- van den Brûle S, Muller A, Fleming AJ, Smart CC (2002) The ABC transporter SpTUR2 confers resistance to the antifungal diterpene sclareol. Plant J 30: 649–662 [DOI] [PubMed] [Google Scholar]
- van den Brûle S, Smart CC (2002) The plant PDR family of ABC transporters. Planta 216: 95–106 [DOI] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ (1982) Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1: 945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590 [DOI] [PubMed] [Google Scholar]
- Windsor B, Roux SJ, Lloyd A (2003) Multiherbicide tolerance conferred by AtPgp1 and apyrase overexpression in Arabidopsis thaliana. Nat Biotechnol 21: 428–433 [DOI] [PubMed] [Google Scholar]