Abstract
Transgenic cassava (Manihot esculenta Crantz, cv MCol22) plants with a 92% reduction in cyanogenic glucoside content in tubers and acyanogenic (<1% of wild type) leaves were obtained by RNA interference to block expression of CYP79D1 and CYP79D2, the two paralogous genes encoding the first committed enzymes in linamarin and lotaustralin synthesis. About 180 independent lines with acyanogenic (<1% of wild type) leaves were obtained. Only a few of these were depleted with respect to cyanogenic glucoside content in tubers. In agreement with this observation, girdling experiments demonstrated that cyanogenic glucosides are synthesized in the shoot apex and transported to the root, resulting in a negative concentration gradient basipetal in the plant with the concentration of cyanogenic glucosides being highest in the shoot apex and the petiole of the first unfolded leaf. Supply of nitrogen increased the cyanogenic glucoside concentration in the shoot apex. In situ polymerase chain reaction studies demonstrated that CYP79D1 and CYP79D2 were preferentially expressed in leaf mesophyll cells positioned adjacent to the epidermis. In young petioles, preferential expression was observed in the epidermis, in the two first cortex cell layers, and in the endodermis together with pericycle cells and specific parenchymatic cells around the laticifers. These data demonstrate that it is possible to drastically reduce the linamarin and lotaustralin content in cassava tubers by blockage of cyanogenic glucoside synthesis in leaves and petioles. The reduced flux to the roots of reduced nitrogen in the form of cyanogenic glucosides did not prevent tuber formation.
Cassava (Manihot esculenta Crantz) is the most important root crop in the world and ranks second among African staple crops (Nweke et al., 2002). Cassava is vegetatively propagated through stem cuttings and produces well on poor soils. The tubers may be kept in the soil for extended time periods. This secures rural farmers a carbohydrate source in years with adverse growth conditions where other crops fail and famine would otherwise prevail. These features and high crop yield contribute to the importance of cassava in Africa, South East Asia, and South America (Nweke et al., 2002). The average annual per capita consumption of cassava in 2002 was 287 kg in the Democratic Republic of Congo and 127 kg in Nigeria (http://faostat.fao.org/ [nutrition data from 2002]). Industrial cassava starch production is important, especially in South East Asia (Bokanga, 1994). Cassava leaves are not widely used as a food source despite their high protein content (Ngudi et al., 2003).
Major drawbacks of the cassava crop are the low tuber protein content, rapid tuber perishability following harvest, and high content of the cyanogenic glucosides linamarin and lotaustralin in all tissues. Upon tissue disruption, the cyanogenic glucosides are brought in contact with β-glucosidases and α-hydroxynitrile lyases and degraded into cyanohydrins, hydrogen cyanide, and ketones (Conn, 1980). When cassava products are used as a primary staple food, careful processing to remove these toxic constituents is required to avoid chronic cyanide intoxication (Onabolu et al., 2002). Incomplete processing may result in high cyanide exposure and give rise to severe diseases like tropical ataxic neuropathy and konzo, especially in populations with poor nutritional status. Unfortunately, careful processing generally results in loss of proteins, vitamins, and minerals, i.e. in products with low nutritional value. In spite of the availability of efficient processing procedures, cyanide exposure from cassava diets prevails (Oluwole et al., 2000). Traditional breeding has generated cassava cultivars with a high to low cyanide potential, but has not succeeded in providing cassava cultivars totally devoid of cyanogenic glucosides (Nweke et al., 2002).
The first committed step in biosynthesis of the Val- and Ile-derived cyanogenic glucosides linamarin and lotaustralin in cassava is catalyzed by the two cytochromes P450 CYP79D1 and CYP79D2 (Fig. 1; Andersen et al., 2000). The presence of two CYP79 paralogs in cassava is in accordance with cassava being allopolyploid (Fregene et al., 1997). Mapping of the CYP79D1 and CYP79D2 genes would provide the necessary molecular tools in a marker-assisted classical breeding program to select acyanogenic cassava lines. Such an approach is hampered by the allopolyploidity, the long generation time, and the small number of seeds produced in each generation.
Figure 1.
The biosynthetic pathway for the cyanogenic glucosides linamarin and lotaustralin in cassava. The enzymatic step blocked by RNAi technology is indicated.
In this study, we have obtained transgenic cassava plants with >99% reduction in cyanide potential in leaves and a 92% reduction in tubers using RNA interference (RNAi) technology. The distribution and transport of cyanogenic glucosides in cassava was investigated and the site of expression of CYP79D1 and CYP79D2 at the tissue and cellular levels determined.
RESULTS
Down-Regulation of Cyanogenic Glucoside Synthesis Using Anti-Sense and RNAi Technology
Two anti-sense constructs were used to produce a large number of independent cassava lines with reduced cyanide potential. One construct specifically targeted CYP79D1 and gave rise to the transgenic line designated AS17A. The second construct was designed to target both CYP79D1 and CYP79D2 and is represented by the transgenic line designated AS17B. The highest reduction of cyanide potential, obtained in transgenic plants produced with either of these two constructs, was 80% as monitored in the first fully unfolded leaf of both in vitro and in vivo-grown plants. In the tubers, the content of linamarin measured by liquid chromatography-mass spectrometry (LC-MS) was reduced by 60%.
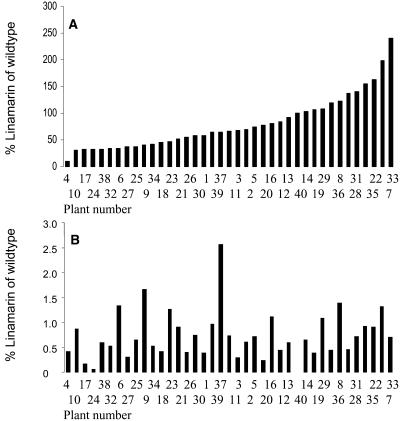
To obtain a more significant reduction in cyanide potential, a new strategy based on RNAi technology was employed. An RNAi hairpin construct targeting both CYP79D1 and CYP79D2 was developed (Fig. 1). Approximately 300 independent transformants were selected using β-glucuronidase (GUS) staining. The cyanide potential of each transformed line was determined to identify those lines with the most marked reduction. About 180 transgenic lines were obtained in which the cyanide potential in the first fully unfolded leaf was reduced to <1% of the level in wild-type control plants (65 μmol per g fresh weight). This was confirmed by LC-MS analysis (Fig. 2). Ninety of these lines were transferred to soil and grown for 6 months to obtain tuber formation. In about 80 of these lines, the cyanogenic glucoside content in leaves remained below 1% of the wild-type level (Fig. 2A). In a subset of these RNAi plants (approximately 40 independent lines), the linamarin content of 6-month-old cassava tubers was determined (Fig. 2B). In one transformed line, the linamarin content in the tuber was reduced to 8% of the wild-type level. In six independent lines, the linamarin content was below 35% of the wild-type level. In another six of the transformants, the linamarin content was below 50% of the wild-type level, whereas the linamarin content of the remaining lines approached and in a few lines was even higher than the wild-type level.
Figure 2.
LC-MS analysis of the content of linamarin in tubers (A) and the first unfolded leaf (B) in 6-month-old in vivo-grown cassava RNAi plants. For all lines included in the analyses, the cyanide potential in the first leaf was below 3% of the wild-type level. The linamarin content of wild-type MCol22 tubers was 65 μmol CN/g fresh weight.
All RNAi plants in which the cyanogenic glucoside content was reduced to <25% of the average cyanide potential of the GUS negative lines exhibited a distinct morphological phenotype when grown in vitro (Fig. 3). The plants had long and slender stems with long internodes. The leaves at the nodes rarely developed, and those that did develop were small and withered quickly. The majority of these plants did not produce roots when grown in vitro. In those plants that did, the roots were shorter and thicker compared to roots of wild-type plants. When the growth medium was changed to full Murashige and Skoog (MS), growth was partly restored and transfer of the shoots to soil in all cases restored a normal wild-type phenotype (data not shown).
Figure 3.
The phenotype of transgenic RNAi plants in vitro. A, Transgenic line T30 with a linamarin content equivalent to 0.3% of the wild type in the first unfolded leaf. B, Wild-type cassava plant. C, Transgenic line T3 with a linamarin content equivalent to 0.4% of the wild type in the first unfolded leaf. The plastic container used for growth is 10-cm wide, and all plants were 2 months old.
Cyanide Potential of Leaves, Petioles, and Stems throughout an Entire Cassava Plant
To investigate the distribution of cyanogenic glucosides within a cassava plant, the cyanide potential in shoot apex and in all individual leaves, petioles, and internodes of an entire cassava plant was determined. The experiment was carried out with five different plants, and the same overall distribution pattern was obtained as illustrated in Figure 4 with the dataset from a single plant. When the cyanide potential was measured per gram fresh weight in leaves, petioles, and internodes, a gradient basipetal in the plant was observed. The negative gradient was most pronounced in petioles and least pronounced in leaves (Fig. 4A). The youngest petiole had the highest cyanide potential per gram fresh weight of all tissues examined. In petioles and internodes, the decrease in cyanide potential per gram fresh weight was most remarkable in the upper part of the plant. In all experiments, the total content of cyanogenic glucosides reached a maximum in the third or fourth leaf, petiole, and internode (Fig. 4B). The leaves contained about 75% of the total cyanogenic glucoside content of 12-week-old plants.
Figure 4.
Cyanide potential of individual leaves, petioles, and stems of an entire in vivo-grown cassava plant. A, Cyanide potential measured per gram fresh weight (μmol HCN per g fresh weight). B, Total cyanide potential in the different plant parts (μmol HCN).
Girdling
In wild-type plants, the cyanide potential of the internode segments showed a strong basipetal reduction (Fig. 4A). By stem girdling, it is possible to remove the outer cell layers of the stem from the epidermis to the cambium, including the phloem (Fig. 5). This technology was used to monitor whether the cyanogenic glucoside gradient partly reflected synthesis at the shoot apex and transport of cyanogenic glucosides from upper parts toward the root. Blockage of phloem-mediated transport by stem girdling would be expected to result in an increased cyanide potential in the internode section above the incision zone. This was indeed observed in wild-type as well as in two transgenic lines (AS17A, anti-sense against CYP79D1; and AS17B, anti-sense against both CYP79D1 and CYP79D2) that contain about one-fifth the amount of cyanogenic glucosides in leaves in comparison to wild type. Stem girdling was performed between leaf positions five and six (Fig. 5). In wild type, the cyanide potential of a stem segment above the incision zone was approximately 75-fold higher than below (Table I). In the two transgenic lines examined, accumulation above the incision point was much less pronounced (approximately 2-fold) but still substantial considering that the overall synthesis of cyanogenic glucosides was strongly reduced in these plants (Table I). The accumulation of cyanogenic glucosides above the incision zone combined with the observation that the total content of cyanogenic glucosides peaks at the third or fourth leaf position indicated that the synthesis of cyanogenic glucosides is mainly in the top of the plant and that cyanogenic glucosides are transported basipetal in the plant.
Figure 5.
Stem girdling of a cassava plant. A, Ten-week-old in vivo-grown cassava plant; the leaf numbers are indicated (18-cm pot diameter). B, Stem (0.5 cm in diameter) before girdling between the fifth and sixth leaves. C, Stem after girdling. D and E show light microscopy of transverse sections (50 μm) of the stem before and after girdling, respectively. Bars = 200 μm.
Table I.
Transport of cyanogenic glucosides in in vivo-grown cassava plants as visualized by girdling of two transgenic lines (anti-sense), AS17A and AS17B, and wild-type plants below the fifth leaf
Cyanide potential of the internode and leaf above and below the incision zone was determined. ND, Not determined.
| Cassava Line | AS17A | AS17B | MCol22 |
|---|---|---|---|
| mmol HCN per g fresh weight | |||
| Fifth leaf | 26 | ND | 59 |
| Stem above incision | 0.5 | 0.6 | 15 |
| Stem below incision | 0.3 | 0.3 | 0.2 |
| Sixth leaf | 29 | 19 | 55 |
Variation in Cyanide Potential of Cassava Cultivated in Vivo and in Vitro
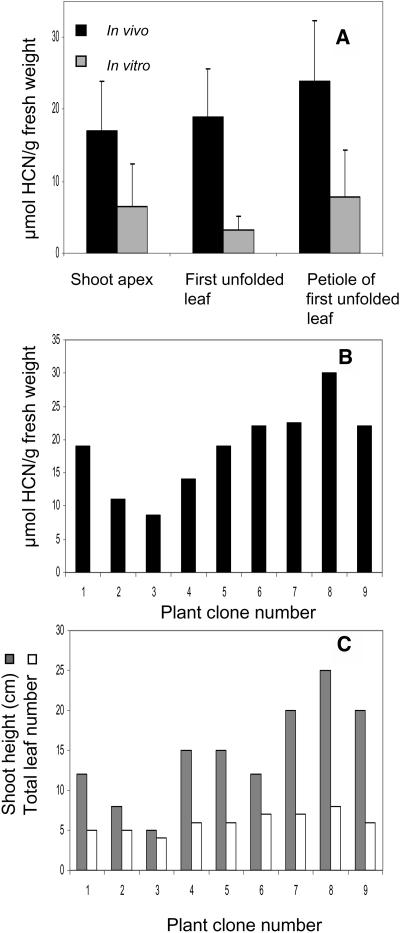
The cyanide potential of cloned cassava plants derived by vegetative propagation of the cultivar MCol22 was found to vary considerably when grown in vivo (greenhouse in soil) as well as in vitro (climate chamber on half-strength MS agar), in spite of all efforts to treat the clones from each type of plant material as identically as possible (Fig. 6A). In general, the cyanide potential of in vitro-grown material was about one-third of the cyanide potential of plant material grown in vivo. The variation among the in vitro-grown plant clones was more pronounced than among the in vivo-grown clones, although in vitro the growth conditions were fully controlled and interaction with biotic factors such as insects was excluded. In an attempt to identify parameters to reduce sd, an apparent positive correlation was observed between plant height and cyanide potential of in vivo-grown plants (Fig. 6B). No correlation between total leaf number of in vivo-grown plants and cyanide potential was observed (Fig. 6C). For in vivo-grown plants, petioles had the highest cyanide potential per gram fresh weight, whereas no significant difference in cyanide potential was observed between the shoot apex and the first fully unfolded leaf (Fig. 6A). In in vitro-grown plant material, no significant differences in cyanide potential between these plant parts were observed.
Figure 6.
Variation in cyanide potential among individual plants belonging to the same cassava clone. A, Variation in cyanide potential (μmol HCN per g fresh weight) in apex, first unfolded leaf, and its corresponding petiole as monitored in 9-week-old plants grown in vitro and in vivo. sd is shown by error bars. B, Cyanide potential of the first unfolded leaf of nine randomly selected individual in vivo-grown cassava plants all belonging to the same clone. C, Plant height and total leaf number of the same in vivo-grown cassava plants. An apparent correlation between plant height and cyanide potential is observed.
Influence of Nitrogen and Potassium Fertilizer on Cyanogenic Glucoside Content
The influence of nutrient supply on the content of cyanogenic glucosides was investigated using cuttings from 6-week-old cassava plants carrying apex and three fully expanded leaves. When supplied with 25 mm KNO3 for 7 d, the cyanogenic glucoside content of the apex showed a 5-fold increase in the amount of cyanogenic glucosides per gram fresh weight in comparison to cuttings supplied with either water or 25 mm KCl (Fig. 7). The cyanogenic glucoside content of leaves and internodes was not significantly changed by nutrient supply.
Figure 7.
Influence of nitrate and potassium fertilizer on cyanogenic glucoside content in cuttings from 6-week-old cassava plants as determined by LC-MS. sd is shown by error bars.
Localization of mRNA for CYP79D1 and CYP79D2
The expression of CYP79D1 and CYP79D2 in 3-month-old cassava plants was monitored by in tube in situ PCR on tissue sections of the first unfolded leaf and its petiole. These two tissues were chosen because they have the highest content of cyanogenic glucosides per gram fresh weight (Fig. 8). CYP79D1 and CYP79D2 transcripts colocalized in all tissues examined. Accordingly, data presented on the location of one transcript also applies for the other transcript. Light microscopy of a transverse section of the petiole revealed the position of the different cell types (Fig. 8A). Laticifers are recognized as dense cells scattered among the phloem cells. When subjected to the in tube in situ PCR procedure, the tissue section in between the endodermis and the phloem/laticifers was prone to rupture. It is the same type of cell layers that are broken when the stem is girdled (Fig. 5). In control experiments without specific primers, weak green fluorescence representing unspecific fluorescein isothiocyanate (FITC) labeling of cell walls was apparent in some sections (Fig. 8B). Strong green fluorescence representing expression of CYP79D1/CYP79D2 was observed in the epidermis and the next two cortex cell layers and in the cell layers corresponding to endodermis and pericycle. Strong expression was also observed around the laticifers among the phloem cells, in regions between the vascular bundles and in parenchymatic cells in the vascular tissue, especially in between the protoxylem and metaxylem cells (Fig. 8, C and D). The same expression pattern was obtained using staining with alkaline phosphatase, which did not give any unspecific labeling of the cell walls (Fig. 8E). Light microscopy of a transverse section of the leaf blade after treatment according to the in tube in situ PCR procedure revealed the positions of the different cell types (Fig. 8F). In control experiments without specific primers, no green FITC fluorescence was observed (Fig. 8G). CYP79D1 and CYP79D2 were preferentially expressed in specific layers of mesophyll cells situated close to the epidermis. The zone of expression positioned toward the upper epidermis was the first cell layer in the mesophyll palisade parenchyma (Fig. 8, H and I). At the abaxial part of the leaf, expression was observed in the first row of cells in the spongy parenchyma directly beneath the epidermis. Strong expression was also observed in parenchymatic cells in the vascular tissue, most pronounced in the vascular tissue in the midvein area of the leaf (Fig. 8H).
Figure 8.
Cellular localization of CYP79D1and CYP79D2 in the first fully unfolded cassava leaf and the corresponding petiole determined by in tube in situ RT-PCR analysis using light microscopy of 100-μm transverse sections. A, Petiole control to visualize the different cell types. B, Petiole labeling background control with FITC as observed without primers in the RT reaction. C, Expression of CYP79D2 in petiole as monitored by FITC labeling. D, Expression of CYP79D1 in petiole as monitored by FITC labeling. E, Expression of CYP79D1 in petiole as monitored by alkaline phosphatase labeling. F, Leaf blade control to visualize the different cell types. G, Midvein of the leaf blade labeling background control with FITC as observed without primers in the RT reaction. H, Expression of CYP79D1 in midvein of the leaf blade as monitored by FITC labeling. I, Expression of CYP79D1in leaf blade. Mp, Palisade parenchyma; ms, spongy parenchyma; v, vascular tissue; e, epidermis; p, phloem; l, laticifers; en, endodermis. Bars = 200 μm.
DISCUSSION
The data presented in this article demonstrate that cassava lines that have acyanogenic (<1% of wild type) leaves and a depleted content of cyanogenic glucosides in tubers may be obtained using RNAi technology to block synthesis of the two committed enzymes in cyanogenic glucoside synthesis in cassava (Andersen et al., 2000). A similar reduction in cyanogenic glucoside synthesis was not achievable using classical anti-sense technology. In other plant species, RNAi technology has also proved more effective to knock out gene function (Smith et al., 2000).
More than 300 independent transgenic cassava RNAi lines were generated in this study. In about 180 of these lines, the content of cyanogenic glucosides in leaves was reduced to less that 1% of wild type, whereas it proved a lot more difficult to obtain a similar strong reduction in tubers. In cassava seedlings, cyanogenic glucoside synthesis takes place in the cotyledons and in the upper part of the hypocotyl, with a major portion of the cyanogenic glucosides being transported to the fibrous roots (Nartey, 1968; Koch et al., 1992). In tuber-producing cassava plants, the main site of synthesis is the shoot apex (de Bruijn, 1973; Andersen et al., 2000). In young cassava plants derived from stakes, the highest concentration of cyanogenic glucosides has generally been found in young leaves (de Bruijn, 1973; Ayanru and Sharma, 1984/1985). In plants with a high cyanide potential, the cyanogenic glucoside content of leaves has been reported to increase with plant age, whereas the cyanogenic glucoside content in leaves decreased with plant age in cassava plants with low cyanide potential (Indira and Ramanujam, 1987). The cassava cultivar MCol22 used in this study has a relatively low cyanide potential (Wheatley et al., 1992). The observed developmental differences may be related to differences in the balance between the rates of synthesis in the shoot apex in comparison to transport to the tubers. In this study, this balance was studied in girdling experiments using wild-type cassava plants and anti-sense plants in which cyanogenic glucoside accumulation in leaves was reduced by 80%. The girdling experiments clearly demonstrated accumulation of cyanogenic glucosides above the incision zone as strong evidence for transport from shoot apex toward the tuber and that the accumulation was dependent on the biosynthetic capacity in the leaves. Similar conclusions have been reached from earlier girdling experiments (de Bruijn, 1973; Ramanujam and Indira, 1984). de Bruijn (1973) also observed that when leaves were shaded, the content of cyanogenic glucosides increased in leaves and the amount of cyanogenic glucosides transported to the tubers decreased. Pruning of cassava also served to decrease the cyanide content in tubers. Previously, de novo synthesis of linamarin in cassava tubers has been reported (Du et al., 1995). Likewise, grafting experiments have shown that the cyanide potential of tubers is not solely determined by the cyanide potential of the scion (Makame et al., 1987). The conclusion from these observations is that a major portion of the cyanogenic glucoside content in tubers is derived from transport from the shoot. A search for promoters related to storage root formation in cassava identified two cDNAs, c15 and c54, by differential screening (Zhang et al., 2003). c15 may encode a putative CYP71E1 paralog. It is noticeable that the expression pattern of the c15 promoter is strong in cell types where expression of the 35S promoter is weak or absent. If c15 turns out to encode a CYP71E1 paralog, the use of the 35S promoter may not have resulted in complete suppression of endogenous cyanogenic glucoside synthesis in the cassava tuber.
Previous studies have not revealed a correlation between the content of cyanogenic glucosides, plant morphology, and crop yield (Mahungu, 1994). The cassava lines transformed with the RNAi construct and in which cyanogenic glucoside accumulation was reduced to <25% of wild-type levels in leaves exhibited a distinct morphological phenotype when grown in vitro on half-strength MS medium. The phenotype was characterized by long internodes with few if any leaves, barely any roots, and slow growth especially following apex transfer to new medium. When the transgenic lines were transferred to full MS medium, leaves and roots started to develop, but the plants were long and slender. Transfer of the shoots to soil restored the wild-type phenotype. An inability of the transgenic plants to develop roots on low-nitrogen-containing media has likewise been described by Siritunga and Sayre (2004) and was interpreted to indicate a physiological function of cyanogenic glucosides, e.g. as a nitrogen source during root development. In the transgenic line with acyanogenic (<1% of wild type) leaves and a 92% reduction of cyanogenic glucosides in tubers, tuber formation was not prevented in soil-grown plants.
The sprouting efficiency of cassava stakes is dependent on the physiological condition of the mother plant from which the stakes were acquired (Molina and El-Sharkawy, 1995). The potassium content in the soil in which the mother plant was grown has been reported to exert a stronger effect on stake growth in comparison to direct application of fertilizer to the stakes (Molina and El-Sharkawy, 1995). In this study, we observed that the variability of cyanide potential was more pronounced among in vitro plant material cultivated under sterile conditions on agar in comparison to greenhouse-soil-grown in vivo plants. Nevertheless, the variability in cyanide potential between soil-grown plants derived from stakes was substantial. Differences observed with respect to sprouting period and plant growth rate may rely on different physiological states of the stakes and on the position on the mother plant from which the stake was taken. These parameters may explain why in this study a close correlation was observed between height of plants developed from stakes and cyanide potential.
The cyanide potential of a specific cassava line varies depending on soil type and nutrient supply (de Bruijn, 1973; Bokanga et al., 1994b). In sorghum (Sorghum bicolor), dhurrin synthesis is induced by nitrogen supply in plants above the age of 8 weeks (Busk and Møller, 2002). In a similar series of experiments, we determined that the cyanide potential was raised in 6-week-old cassava plants following administration of nitrogen. The cyanide potential in leaves and tubers from the same plant may also differ (de Bruijn, 1973; Riis et al., 2003). To minimize the experimental work to identify a transgenic cassava line that does not contain cyanogenic glucosides in leaves and tubers, it was desirable to be able to define the earliest plant stage and the type of plant tissue suitable for robust screening to avoid growing all transgenic lines to the tuber stage. A good correlation between leaf and tuber cyanide potential in cassava grown in the field has been reported (Cooke et al., 1978; Mahungu, 1994), but the existence of an association between root and leaf cyanide potential has subsequently been refuted (Ayanru and Sharma, 1984/1985; Makame et al., 1987; Mkong et al., 1990; Bokanga et al., 1994). In tissue culture of cassava, cyanogenesis arises late in the regeneration process (Joseph et al., 2001). No cyanide potential was detectable in the embryos, and the cyanide potential rises slowly during the development of plantlets. In contrast, seedlings derived from cassava seeds are cyanogenic from the onset of germination (Koch et al., 1992). Accordingly, cyanogenic glucoside synthesis in shoots is initiated differently whether the shoot arises from a seed or from a stake. In the selection of cassava lines with greatly reduced cyanide potential, it is pivotal to be certain that cyanogenic glucoside synthesis is not initiated at a subsequent developmental stage. To gain confidence in the identification of lines with low cyanide potential, it was necessary to follow a quite laborious approach where analysis of the cyanogenic glucoside content was carried out at several developmental stages. The first measurements served to identify plantlets with acyanogenic (<1% of wild type) leaves, the second measurement on leaves was carried out after the presumed acyanogenic (<1% of wild type) lines had grown 1 to 2 months in soil, and the final set of measurements was performed on tubers of 6-month-old plants. The data obtained in this study demonstrate that even a low rate of cyanogenic glucoside synthesis in leaves results in transport and accumulation of significant amounts of cyanogenic glucosides in tubers.
Biosynthetic studies using radiolabeled Val as precursor showed active synthesis of linamarin in petioles, midrib of the leaf, and the shoot apex (Bediako et al., 1981). In this study, we demonstrated that mRNAs encoding CYP79D1/D2 are expressed in specific cells situated between the laticifers in the petiole. In the leaves, preferential expression of CYP79D1/D2 was observed in specific layers of mesophyll cells and in the parenchyma cells in the vascular bundles. Thus, the cells harboring biosynthetic and catabolic enzymes are located separately but in close proximity. The processes resulting in degradation of linamarin and lotaustralin are well documented (Pancoro and Hughes, 1992; White et al., 1998). Degradation proceeds in two steps. Initially, linamarin and lotaustralin are hydrolyzed into the corresponding cyanohydrins by the β-glucosidase linamarase (Pancoro and Hughes, 1992). Degradation of the cyanohydrins may proceed nonenzymatically or catalyzed by α-hydroxynitrile lyase, and in both cases results in release of hydrogen cyanide and ketones (Conn, 1980; White et al., 1998). Linamarase is synthesized in the laticifers (Pancoro and Hughes, 1992) and found in the latex in the apoplast and in the laticifers (Gruhnert et al., 1994; Elias et al., 1997; Santana et al., 2002). α-Hydroxynitrile lyase has primarily been found in the cell walls in the leaves (White et al., 1998). Overexpression of α-hydroxynitrile lyase in root tissue of cassava lowered the amount of acetone cyanohydrin present and helped to reduce tuber toxicity by enhancing formation of volatile degradation products (Siritunga et al., 2004). An advantage of this approach to reduce tuber toxicity is that a putative protective function toward pests that the cyanogenic glucosides may exert during plant growth would not be lost. However, different rates of tuber tissue drying and different degrees of tissue disruption are encountered during the vast number of cassava-processing procedures that are in use. This renders the efficiency of this approach to secure conversion of the cyanogenic glucoside content into volatile products highly variable because enzymatic degradation requires an aqueous phase to proceed. Overexpression of the α-hydroxynitrile lyase should therefore not be envisioned as a substitute to traditional cassava processing but as a complementary tool to lower the residual amounts of cyanogens following traditional processing.
In a recent study using transgenic cassava plants harboring an anti-sense construct against CYP79D1/D2 driven by a leaf-specific promoter (CAB1) to specifically down-regulate cyanogenic glucoside synthesis in leaves, no cyanide potential was found in the roots of 4-month-old in vitro plants (Siritunga and Sayre, 2003). Data on soil-grown cassava plants were not included. In our studies, we have never been able to produce tubers in vitro even after extended growth for 6 months; tuber formation required 3 months of growth in soil in the greenhouse. In the anti-sense lines produced in this study, the 80% decrease in cyanogenic glucoside content in leaves resulted in a less pronounced decrease in roots (approximately 60%). Numerous RNAi lines with <1% of cyanogenic glucosides in leaves were obtained, but some of these lines had wild-type levels of cyanogenic glucosides in their tubers. Considering these data and the fact that Du et al. (1995) have demonstrated de novo synthesis of cyanogenic glucosides in cassava tubers, it is difficult to envision that acyanogenic cassava tubers could be obtained simply by down-regulation of cyanogenic glucoside synthesis in leaves.
Sorghum is the only plant from which all three genes (CYP79A1, CYP71E1, and UGT85B1) encoding conversion of a parent amino acid into a cyanogenic glucoside have been identified (Tattersall et al., 2001). Because synthesis of cyanogenic glucosides follows a common biosynthetic scheme, orthologs of CYP71E1 and UGT85B1 constitute other target genes to block cyanogenic glucoside synthesis in cassava. Blockage of expression of the CYP71E1 ortholog would result in accumulation of toxic oximes and detoxification products thereof (Bak et al., 2000; Kristensen et al., 2005). Blockage of UGT85B1 expression would result in accumulation of cyanohydrins that upon dissociation would liberate hydrogen cyanide and ketones and give rise to plants with stunted growth (Bak et al., 2000; Kristensen et al., 2005). Thus, even if these genes were available in cassava, they would not be as good of targets for blocking of cyanogenic glucoside synthesis as CYP79D1 and CYP79D2. An alternative approach to block cyanogenic glucoside synthesis would be to target transcription factors or other regulatory genes controlling the expression level of the biosynthetic genes. In the synthesis of indole-3-acetic acid and indole glucosinolates in Arabidopsis (Arabidopsis thaliana), the Myb transcription factor ALTERED TRYPTOPHAN REGULATION1 has been shown to control indole glucosinolate homeostasis by regulating the transcript level of Trp synthesizing genes and of CYP79B2/CYP79B3 and CYP83B1 of indole glucosinolate synthesis (Bender and Fink, 1998; Celenza et al., 2005). No equivalent regulatory genes are available in cassava, reflecting the fact that cassava is an understudied crop of primary interest only for the Third World.
Yet, a third approach to reduce the accumulation of cyanogenic glucosides in tubers would be to block transport from the shoot apex. In Hevea braziliensis, linamarin has been suggested to be transported as a linamarase-insensitive transport form, the diglucoside linustatin (Selmar, 1993). The presence of minute amounts of linustatin has indeed been detected in cassava using radiolabeled precursors (Lykkesfeldt and Møller, 1994), which might suggest the operation of a similar apoplast-based transport system for cyanogenic glucosides in cassava. The UDP-glycosyltransferase putatively involved in the conversion of linamarin to linustatin has not been identified, but knock out of the corresponding gene would be an obvious approach to reduce linamarin accumulation in tubers. In this study, it was not possible to detect linustatin in the LC-MS spectra (data not shown). This most likely reflects the transient nature of the transport form.
The role of cyanogenesis in plants has been widely discussed. Riis et al. (2003) have investigated pest attack on cassava varieties possessing different cyanide potential and concluded that a larger pest outbreak or damage level in acyanogenic clones was not to be expected. Insect generalists such as the grasshopper Zonocerus variegatus and the burrowing bug Cyrtomenus bergi do not accept high levels of cyanogenic glucosides. The specialist hornworm Erinnyis ello and green mite Mononychellus tanajoa have coevolved with cassava and therefore have no preference for high- or low-cyanide-containing varieties (Bellotti and Riis, 1994). In the rubber tree H. braziliensis, the presence of high amounts of cyanogenic glucosides has been demonstrated to increase the sensitivity of the plant to attack by the fungus Microcyclis ulei, most likely because the cyanide released inhibits simultaneous synthesis of protective phytoalexins (Lieberei, 1986; Lieberei et al., 1989). White clover (Trifolium repens) is polymorphic with respect to cyanogenesis (Corkill, 1942; Hughes, 1991). Acyanogenic white clover plants may be divided into three different groups: those that lack the cyanogenic glucosides, those that lack linamarase, and those that lack both the cyanogenic glucosides and the linamarase. A selective advantage of the cyanogenic white clover phenotype against feeding by the slug Deroceras reticulatum appears to be manifested only when the frequency of cyanogenic plants is low (Burgess and Ennos, 1987). On the other hand, acyanogenic white clover sets more flowers, especially at lower temperature (Daday, 1965). The balancing between metabolic costs of cyanogenic glucoside formation and benefits related to reduced herbivory and pathogen attack in white clover thus remains to be established. The existence of the acyanogenic white clover varieties argues against a basic role of cyanogenic glucosides in primary metabolism. Some insects preferentially feed on plants producing cyanogenic glucosides. The best-studied example is the moth Zygaena trifolii, which has acquired the ability to sequester the cyanogenic glucosides linamarin and lotaustralin present in its cyanogenic host plant birdsfoot trefoil Lotus corniculatus for use in its own defense against predators (Zagrobelny et al., 2004). This insect is also able to de novo synthesize the two cyanogenic glucosides (Holzkamp and Nahrstedt, 1994). These datasets demonstrate that attempts to assign a specific biological role to cyanogenic glucosides are not meaningful. The function varies dependent on plant species, ecosystem, and abiotic and biotic stress factors. Accordingly, only careful and extensive field trials can decide on the overall fitness of acyanogenic cassava plants.
MATERIALS AND METHODS
Plant Material
Cassava (Manihot esculenta Crantz) plants of the Columbian cultivar MCol22, derived from stem cuttings provided by Centro Internacional de Agricultura Tropical, Cali, Colombia, were grown in a greenhouse at 16-h light/28°C and 8-h dark/25°C in 2-L pots in Pindstrup soil. The plants were kept under constant humidity at 70%. For each series of experiments, plants (1.5–3 months old) derived from stakes obtained from plants of similar age (4–6 months) and of similar stem diameter (5 mm) were used. Throughout this study, greenhouse-grown plants are referred to as in vivo plants.
Constructs for Plant Transformation
Anti-Sense Constructs
Two constructs were assembled in the pPZP111 plasmid (Hajdukiewicz et al., 1994). One contained the first exon of CYP79D1 (accession no. AF140613) in anti-sense direction positioned between the enhanced 35S cauliflower mosaic virus promoter and a nos terminator. The second construct contained the first exon of CYP79D1 (accession no. AF140613) and the second exon of CYP79D2 (accession no. AF140614), both positioned in anti-sense direction between an enhanced 35S cauliflower mosaic virus promoter and a nos terminator (E35S∷anti-sense CYP79D1/E35S∷anti-sense CYP79D2).
RNAi Construct
A hairpin loop construct was generated from 300 bp of CYP79D1 (accession no. AY834391), position 1329-1629, and 330 bp of CYP79D2 (accession no. AY834390), position 998-1328 (accession no. AF140614), including a 345-bp fragment of CYP79D1 intron (S. Bak and P.K. Busk, unpublished data) and positioned between the enhanced 35S cauliflower mosaic virus promoter and 35S terminator (E35S∷anti-sense CYP79D1/anti-sense CYP79D2/intron CYP79D1/sense CYP79D2/sense CYP79D1:35S) in pPS48 harboring GUS and NPTII (Odell et al., 1985; Kay et al., 1987). The hairpin construct was ligated into pCAMBIA 2301.
Transformation
Primary embryos were obtained by dissection of nodes from plants (3–4 month old) grown under sterile conditions in transparent growth containers. These plants are referred to as in vitro plants throughout this study. The nodes were incubated on MS medium (Murashige and Skoog, 1962; Sigma) supplemented with 2% Suc, 0.9% agar, and 2,4-dichlorophenoxyacetic acid (10 mg/L). After 3 weeks, primary embryos were induced. The globular- to torpedo-shaped embryos were matured on MS medium (Murashige and Skoog, 1962; Sigma) supplemented with 2% Suc, 0.9% agar, and 6-benzylaminopurine (0.1 mg/L). When embryos reached the cotyledonary stage, the cotyledons were harvested and placed on MS medium (Murashige and Skoog, 1962; Sigma) supplemented with 2% Suc, 0.9% agar, and 2,4-dichlorophenoxyacetic acid (6 mg/L) to induce formation of secondary embryos.
Secondary embryos obtained from the cassava cultivar MCol22 were used for transformation according to Li et al. (1996) with NPTII resistance as the selection marker gene. The shoots were selected with G418. The selected shoots were harvested onto medium composed of half-strength MS (Murashige and Skoog, 1962; Sigma) supplemented with 2% Suc and 0.9% agar. RNAi plantlets were identified by GUS histochemical staining (Jefferson et al., 1987) and anti-sense plants by NPTII ELISA assays (Agdia). GUS-positive and NPTII-positive shoots were analyzed for cyanide potential to detect lines with low content of cyanogenic glucosides. Transgenic shoots with a cyanide potential below one-half the average value of the tested wild-type lines were further analyzed by LC-MS. Shoots with a significantly reduced content of linamarin and lotaustralin were maintained and cultivated in vivo and in vitro.
Linamarase Preparation
A crude preparation of linamarase was obtained from cassava latex (approximately 500 μL) collected from leaf, petiole, and internode incisions of MCol22. The latex was diluted (1,000 times) with sodium phosphate buffer (0.1 m, pH 8.0), filtered, and dialyzed overnight against sodium phosphate buffer (0.1 m, pH 8.0). The linamarase preparation was divided into aliquots, frozen in liquid nitrogen, and stored at −80°C.
Determination of Cyanide Potential
A small plant sample (5–10 mg) was submerged in boiling tricine buffer (200 μL 50 mm, pH 7.9) and boiled (15 min). An aliquot (5–25 μL) was incubated (28°C, 2 h) with linamarase (20 μL) in tricine buffer (50mm, pH 7.9, total volume 200 μL) in a closed Eppendorf tube to completely degrade the cyanogenic glucoside content. The incubation was stopped by freezing the samples in liquid nitrogen and addition of NaOH (40 μL 6 m) to the frozen samples. After thawing, samples were left at room temperature (20 min), and the cyanide potential was determined (Halkier and Møller, 1989) by addition of glacial HOAc (50 μL) immediately followed by 200 μL reagent A (50 mg succinimide and 125 mg N-chlorosuccinimide in 50 mL water) and 200 μL reagent B (3 g barbituric acid and 15 mL pyridine in 35 mL water; Halkier and Møller, 1989). After thorough mixing and incubation (5 min), the cyanide content was determined spectrophotometrically (580-nm to 750-nm scan; UV/VIS spectrometer, Lambda 800; Perkin-Elmer).
LC-MS Analysis of Cyanogenic Glucoside Content
To determine the content of cyanogenic glucosides directly, the plant samples were immersed into boiling methanol (80%, 1 mL) and boiled (15 min). The MeOH extract was transferred to a new tube, lyophilized to dryness, resuspended in water (total volume 200 μL), and filtered through a 0.45-μm filter.
Analytical LC-MS was carried out using an Agilent 1100 Series LC (Agilent Technologies) coupled to a Bruker Esquire 3000+ ion trap mass spectrometer (Bruker Daltonics) fitted with an XTerra MS C18 column (Waters; 3.5 μm, 2.1×100 mm, flow rate 0.2 mL min−1). The mobile phases were as follows: A, 0.1% (v/v) HCOOH and 50 μm NaCl; and B, 0.1% (v/v) HCOOH and 80% (v/v) MeCN. The gradient program was as follows: 0 to 4 min, isocratic 2% (v/v) B; 4 to 10 min, linear gradient 2% to 8% B; 10 to 30 min, linear gradient 8% to 50% (v/v) B; 30 to 35 min, linear gradient 50% to 100% (v/v) B; and 35 to 40 min, isocratic 100% B. The mass spectrometer was run in positive ion mode. Traces of total ion current and of extracted ion currents for specific [M + Na]+ adduct ions were used to identify selected peaks. The retention time for linamarin and for lotaustralin was 5.5 and 15.8 min, respectively.
Variation in Cyanide Potential
To investigate the variation in cyanide potential among vegetatively propagated cassava plants derived from the same parent plant and grown under the same greenhouse conditions (in vivo plants), samples (10–20 mg) were taken from apex, first leaf, and first petiole of genetically identical clones of the same age (9-week-old plants) and the cyanide potential determined. A similar series of experiments were carried out using in vitro plants grown on half-strength MS (Murashige and Skoog, 1962; Sigma), 2% (w/v) Suc, and 0.9% (w/v) agar at 28°C under a 16/8-h light/dark regime in a controlled climate chamber. To avoid neighbor effects, each transparent growth container contained a single plant. For each series of experiments, all plants were initiated the same day using top shoots from 9-week-old plants.
The Cyanide Potential of Leaves, Petioles, and Internodes throughout an Entire Cassava Plant
Apex, all leaves, petioles, and internode sections were harvested from greenhouse-grown MCol22 plants (9 weeks old). Each plant part was weighed and its total cyanide potential determined from dissected segments (10–20 mg).
Girdling Experiments
Phloem transport of cyanogenic glucosides was monitored by girdling, i.e. removal of the outer layer of the stem (epidermis, cortex, and phloem; de Bruijn, 1973). The cassava plants (8–12 weeks old) were girdled above the sixth leaf. After 2 d, stem segments (1 cm) from the internode beneath the first unfolded leaf, from just above and beneath the incision zone as well as neighboring leaves, were collected and their cyanide potential determined. Three wild-type plants and two transgenic anti-sense lines (AS17A and AS17B) in which the cyanogenic glucoside content of the leaves was reduced to 20% of the wild-type level were used for the experiments.
Influence of Nitrogen and Potassium Fertilizer on Cyanogenic Glucoside Content
Cuttings from three MCol22 plants (8–12 weeks old) carrying apex and the three upper leaves were kept for 1 week in water, 25 mm KNO3, or 25 mm KCl in 50-mL Falcon tubes. The content of cyanogenic glucosides in apex, first leaf, and first petiole was determined by LC-MS. The cuttings were replenished with fresh growth medium every day.
In Tube in Situ RT-PCR on Tissue Sections
RT-PCR on plant tissue (leaf, petiole, or stem) was carried out using a modification of the protocol described by Koltai and Bird (2000). The tissue was fixed in FAA (2% formalin, 60% ethanol, and 5% HOAc in PBS [8 g NaCL, 0.2 g KCl, 1.44 g Na2 HPO4, 0.24 g KH2PO4 in 1 L water]; 4–16 h, 4°C), washed (2 × 5 min) with washing buffer (60% ethanol, 5% HOAc in PBS), and transferred to PBS. Samples of fixed tissue (approximately 1 cm) were embedded in 5% low-melting-point agarose in PBS, and cut into small blocks with known orientation of the tissue and sectioned on a vibratome (Leica VT 1000S; Leica; 80–100 μm thickness). The sections (5–10 sections per tube) were immediately placed in sterile water (20 μL) containing RNase inhibitor (4 U) and DNase (40 U). After incubation (37°C, overnight), washing in sterile water (2×10 min), and treatment (15 min) with pepsin (2 ng in 1 μL 10 mm HCl), the sections were washed twice in sterile water. The pepsin treatment served to remove FAA-mediated cross-linking and thus to ease subsequent access of primers and polymerase.
After the sections were washed with sterile water, the reverse transcriptase reaction mixture (1× RT buffer, 2 mm dNTP, 1 mm specific primer [CYP79D1, rev, 5′-CTT CTT CAG GAT TTC TGG TTG ATT-3′; CYP79D2, rev, 5′-AGA TTA GGG ATG TCA GAT TCT TGC-3′]) was added (total volume 20 μL). After heat treatment (65°C, 5 min) and lowering of the temperature (4°C), RNase inhibitor (10 U/100 μL) and Sensiscript (Qiagen; 1 μL) were added to each tube, followed by incubation (45°C, 60 min) before heat treatment (97°C, 1 min) and completion of the cycle (4°C).
To initiate the PCR reaction, the RT reaction mixture was removed and the PCR reaction mixture added (1× ExTaQ buffer, 0.2 mm dNTP, 10 0 nm DIG-11-dUTP, 0.25 μL ExTaQ, 0.5 mm primer [CYP79D1, rev, 5′-CTT CTT CAG GAT TTC TGG TTG ATT-3′; for, 5′-AAT TTG TGC TTG ATG CAA ATA AGA-3′; CYP79D2, rev, 5′-AGA TTA GGG ATG TCA GAT TCT TGC-3′; for, 5′-AGA AGA AAG GAT TCA ACA ATG GAG-3′] [total volume 25 μL]). Thermocycling parameters were as follows: 2 min at 70°C, then 30 cycles at 92°C for 30 s, 60°C for 30 s, and 72°C for 1 min, and final lowering to 4°C.
The sections were washed in PBS and either labeled by fluorescent antibody enhancer set (1 768 506; Roche Diagnostics GmbH) using FITC as a fluorescent marker according to the manufacturer's protocol or by alkaline phophatase as described by Koltai and Bird (2000). The sections were examined in a Leica DMR fluorescence microscope and photographed with Leica DC 300F.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY834390, AY834391, and AF140614.
Acknowledgments
We thank Christina Mattson, Christine Ratke, and Susanne Jensen for skilful technical help, and Steen Malmmose for taking care of the cassava plants in the greenhouse.
This work was supported by the Danish International Development Agency (grant nos. 104Dan8/503 and 104.Dan.8/91125) and by a grant to the Center for Molecular Plant Physiology (PlaCe) from the Danish National Research Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.065904.
References
- Andersen MD, Busk PK, Svendsen I, Møller BL (2000) Cytochromes P-450 from cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin. J Biol Chem 275: 1966–1975 [DOI] [PubMed] [Google Scholar]
- Ayanru DKG, Sharma VC (1985 1984) Changes in total cyanide content of tissues from cassava plants infested by mites (Mononychellus tanajoa) and mealybugs (Phenacoccus manihoti). Agric Ecosyst Environ 12: 35–46 [Google Scholar]
- Bak S, Olsen CE, Halkier BA, Møller BL (2000) Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochromes P450, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiol 123: 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bediako MKM, Tapper BA, Pritchard GG (1981) Metabolism, synthetic site, and translocation of cyanogenic glycosides in cassava. In ER Terry, KO Oduro, F Caveness, eds, Tropical Root Crops. Proceedings of the First Triennial Symposium of the International Society for Tropical Root Crops—African Branch. International Development Research Centre, Ottawa, pp 143–148
- Bellotti AC, Riis L (1994) Cassava cyanogenic potential and resistance to pests and diseases. Acta Hortic 375: 141–151 [Google Scholar]
- Bender J, Fink GF (1998) A Myb homologue, ATR1, activates tryptophan gene expression in Arabidopsis. Proc Natl Acad Sci USA 95: 5655–5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokanga M (1994) Distribution of cyanogenic potential in cassava germplasm. Acta Hortic 375: 117–123 [Google Scholar]
- Bokanga M, Ekanayake IJ, Dixon AGO, Porto MCM (1994) Genotype-environment interactions for cyanogenic potential in cassava. Acta Hortic 375: 131–139 [Google Scholar]
- Burgess RSL, Ennos RA (1987) Selective grazing of acyanogenic white clover: variation in behaviour among populations of the slug Deroceras reticulatum. Oecologia 73: 432–435 [DOI] [PubMed] [Google Scholar]
- Busk PK, Møller BL (2002) Dhurrin synthesis in sorghum is regulated at the transcriptional level and induced by nitrogen fertilization in older plants. Plant Physiol 129: 1222–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Quiel JA, Smolen GA, Merrikh H, Silvestro AR, Normanly J, Bender J (2005) The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol 137: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn EE (1980) Cyanogenic glucosides. Annu Rev Plant Physiol 31: 433–451 [Google Scholar]
- Cooke RD, Howland AK, Hahn SK (1978) Screening cassava for low cyanide using an enzymatic assay. Exp Agric 14: 367–372 [Google Scholar]
- Corkill L (1942) Cyanogenesis in white clover (Trifolium repens L.). V. The inheritance of cyanogenesis. NZ J Sci Technol B23: 178–193 [Google Scholar]
- Daday H (1965) Gene frequencies in wild populations of Trifolium repens L. IV. Mechanism of natural selection. Heredity 20: 355–365 [Google Scholar]
- de Bruijn GH (1973) The cyanogenic character of cassava (Manihot esculenta). In B Nestel, R MacIntyre, eds, Chronic Cassava Toxicity: Proceedings of an Interdisciplinary Workshop, January 29–30, 1973, London. International Development Research Centre, Ottawa, pp 43–48
- Du L, Bokanga M, Møller BL, Halkier BA (1995) The biosynthesis of cyanogenic glucosides in roots of cassava. Phytochemistry 39: 323–326 [Google Scholar]
- Elias M, Nambisan B, Sudhakaran PR (1997) Characterization of linamarase of latex and its localization in petioles in cassava. Arch Biochem Biophys 341: 222–228 [DOI] [PubMed] [Google Scholar]
- Fregene M, Angel F, Gomez R, Rodriguez F, Chavarriaga P, Roca W, Tohme J, Bonierbale M (1997) A molecular genetic map of cassava (Manihot esculenta Crantz). Theor Appl Genet 95: 431–441 [Google Scholar]
- Gruhnert C, Biehl B, Selmar D (1994) Compartmentation of cyanogenic glucosides and their degrading enzymes. Planta 195: 36–42 [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Halkier BA, Møller BL (1989) Biosynthesis of the cyanogenic glucoside dhurrin in seedlings of Sorghum bicolor (L.) Moench and partial purification of the enzyme system involved. Plant Physiol 90: 1552–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzkamp G, Nahrstedt A (1994) Biosynthesis of cyanogenic glucosides in the Lepidoptera. Incorporation of [U-14C]-2-methylpropanealdoxime, 2S-[U-14C]-methylbutanealdoxime and D,L-[U-14C]-N-hydroxyisoleucine into linamarin and lotaustralin by the larvae of Zygaena trifolii. Insect Biochem Mol Biol 24: 161–165 [Google Scholar]
- Hughes MA (1991) The cyanogenic polymorphism in Trifolium repens (L.) (white clover). Heredity 66: 105–115 [Google Scholar]
- Indira P, Ramanujam T (1987) Distribution of hydrocyanic acid in high-cyanide and a low-cyanide variety of cassava in relation to the age of the plant. Indian J Agric Sci 57: 436–437 [Google Scholar]
- Jefferson RA, Kavangh T, Bevan M (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph T, Yeoh H-H, Loh C-S (2001) Linamarin content and genetic stability of cassava plants derived by somatic embryogenesis. Euphytica 120: 7–13 [Google Scholar]
- Kay R, Shan A, Daly M, McPherson J (1987) Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236: 1299–1302 [DOI] [PubMed] [Google Scholar]
- Koch B, Nielsen VS, Halkier BA, Olsen CE, Møller BL (1992) The biosynthesis of cyanogenic glucosides in seedlings of cassava (Manihot esculenta Crantz). Arch Biochem Biophys 292: 141–150 [DOI] [PubMed] [Google Scholar]
- Koltai H, Bird MD (2000) High throughput cellular localization of specific plant mRNAs by liquid-phase in situ reverse transcription-polymerase chain reaction of tissue sections. Plant Physiol 123: 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen C, Morant M, Olsen CE, Ekstrøm CT, Galbraith DW, Møller BL, Bak S (2005) Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc Natl Acad Sci USA 102: 1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HQ, Sautter C, Potrykus I, Pounti-Kaerlas J (1996) Genetic transformation of cassava (Manihot esculenta Crantz). Nat Biotechnol 14: 736–740 [DOI] [PubMed] [Google Scholar]
- Lieberei R (1986) Cyanogenesis of Hevea braziliensis during infection with Microcyclus ulei. J Phytopathol 115: 134–146 [Google Scholar]
- Lieberei R, Biehl B, Giesemann A, Junqueira NTV (1989) Cyanogenesis inhibits active defense reactions in plants. Plant Physiol 90: 33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkesfeldt J, Møller BL (1994) Cyanogenic glycosides in cassava, Manihot esculenta Crantz. Acta Chem Scand 48: 178–180 [Google Scholar]
- Mahungu M (1994) Relationships between cyanogenic potential of cassava and other agronomic traits. Acta Hortic 375: 125–130 [Google Scholar]
- Makame M, Akoroda MO, Hahn SK (1987) Effects of reciprocal stem grafts on cyanide translocation. J Agric Sci Camb 109: 605–608 [Google Scholar]
- Mkong OE, Yan H, Chism G, Sayre RT (1990) Purification, characterization, and localization of linamarase in cassava. Plant Physiol 93: 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JL, El-Sharkawy MA (1995) Increasing crop productivity of planting material. Field Crops Res 44: 151–157 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nartey F (1968) Studies on cassava, Manihot utilissima Pohl. I. Cyanogenesis: the biosynthesis of linamarin and lotaustralin in etiolated seedlings. Phytochemistry 7: 1307–1312 [Google Scholar]
- Ngudi DD, Kuo YH, Lambein F (2003) Cassava cyanogens and free amino acids in raw and cooked leaves. Food Chem Toxicol 41: 1193–1197 [DOI] [PubMed] [Google Scholar]
- Nweke FI, Spencer DSC, Lynam JK (2002) The Cassava Transformation. Africa's Best Kept Secret. Michigan State University Press, East Lansing, MI
- Odell JT, Nagy F, Chua NH (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313: 810–812 [DOI] [PubMed] [Google Scholar]
- Oluwole OSA, Onabolu AO, Link H, Rosling H (2000) Persistence of tropical ataxic neuropathy in a Nigerian community. J Neurol Neurosurg Psychiatry 69: 96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onabolu AO, Oluwole OSA, Rosling H, Bokanga M (2002) Processing factors affecting the level of residual cyanohydrins in gari. J Sci Food Agric 82: 966–969 [Google Scholar]
- Pancoro A, Hughes MA (1992) In-situ localization of cyanogenic β-glucosidase (linamarase) gene expression in leaves of cassava (Manihot esculenta Crantz) using non-isotopic riboprobes. Plant J 2: 821–827 [Google Scholar]
- Ramanujam T, Indira P (1984) Effect of girdling on the distribution of total carbohydrates and hydrocyanic acid in cassava. Indian J Plant Physiol 27: 355–360 [Google Scholar]
- Riis L, Bellotti AC, Bonierbale M, O'Brien GM (2003) Cyanogenic potential in cassava and its influence on a generalist insect herbivore Cyrtomenus bergi (Hemiptera:Cydnidae). J Econ Entomol 96: 1905–1914 [DOI] [PubMed] [Google Scholar]
- Santana MA, Vásquez V, Matehus J, Aldao RR (2002) Linamarase expression in cassava cultivars with roots of low- and high-cyanide content. Plant Physiol 129: 1686–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D (1993) Transport of cyanogenic glucosides: linustatin uptake by Hevea cotyledons. Planta 191: 191–199 [Google Scholar]
- Siritunga D, Arias-Garzon D, White W, Sayre RT (2004) Over-expression of hydroxynitrile lyase in transgenic cassava roots accelerates cyanogenesis and food detoxification. Plant Biotechnol J 2: 37–43 [DOI] [PubMed] [Google Scholar]
- Siritunga D, Sayre R (2004) Engineering cyanogen synthesis and turnover in cassava (Manihot esculenta). Plant Mol Biol 56: 661–669 [DOI] [PubMed] [Google Scholar]
- Siritunga D, Sayre RT (2003) Generation of cyanogen-free transgenic cassava. Planta 217: 367–373 [DOI] [PubMed] [Google Scholar]
- Smith NA, Singh SP, Wang M-B, Stoutjesdjik PA, Green GA, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407: 319–320 [DOI] [PubMed] [Google Scholar]
- Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Høj PB, Møller BL (2001) Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293: 1826–1828 [DOI] [PubMed] [Google Scholar]
- Wheatley CC, Orrego JI, Sanchez T, Granados E (1992) Quality evaluation of the cassava core collection at CIAT. In WM Roca, AM Thro, eds, Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network, August 25–28, 1992, Cartogana, Colombia. Centro Internacional de Agricultura Tropical, Cali, Columbia, pp 255–264
- White WLB, Arias-Garzon DI, McMahon JM, Sayre RT (1998) Cyanogenesis in cassava. The role of hydroxynitrile lyase in root cyanide production. Plant Physiol 116: 1219–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Rasmussen AV, Jørgensen B, Naumann CM, Møller BL (2004) Cyanogenic glucosides and plant-insect interactions. Phytochemistry 65: 293–306 [DOI] [PubMed] [Google Scholar]
- Zhang P, Bohl-Zenger S, Puonti-Kaerlas J, Potrykus I, Gruissem W (2003) Two cassava promoters related to vascular expression and storage root formation. Planta 218: 192–203 [DOI] [PubMed] [Google Scholar]