Abstract
TPK1 (formerly KCO1) is the founding member of the family of two-pore domain K+ channels in Arabidopsis (Arabidopsis thaliana), which originally was described following expression in Sf9 insect cells as a Ca2+- and voltage-dependent outwardly rectifying plasma membrane K+ channel. In plants, this channel has been shown by green fluorescent protein fusion to localize to the vacuolar membrane, which led to speculations that the TPK1 gene product would be a component of the nonselective, Ca2+ and voltage-dependent slow-vacuolar (SV) cation channel found in many plants species. Using yeast (Saccharomyces cerevisiae) as an expression system for TPK1, we show functional expression of the channel in the vacuolar membrane. In isolated vacuoles of yeast yvc1 disruption mutants, the TPK1 gene product shows ion channel activity with some characteristics very similar to the SV-type channel. The open channel conductance of TPK1 in symmetrically 100 mm KCl is slightly asymmetric with roughly 40 pS at positive membrane voltages and 75 pS at negative voltages. Similar to the SV-type channel, TPK1 is activated by cytosolic Ca2+, requiring micromolar concentration for activation. However, in contrast to the SV-type channel, TPK1 exhibits strong selectivity for K+ over Na+, and its activity turned out to be independent of the membrane voltage over the range of ±80 mV. Our data clearly demonstrate that TPK1 is a voltage-independent, Ca2+-activated, K+-selective ion channel in the vacuolar membrane that does not mediate SV-type ionic currents.
The Arabidopsis (Arabidopsis thaliana) genome contains 15 genes encoding K+ ion channels, grouped into three structurally different families. Functionally best characterized are members of the family of shaker-type channels, consisting of nine members. These channels are characterized by six transmembrane (TM) helices plus one hydrophobic pore (P) loop between TM5 and TM6. The less well-characterized family of the TPK/KCO channels consists of five so-called tandem-pore channels (4TM-2P) and one Kir-type channel (2TM-1P).
Of the two-pore channel family, TPK1 (formerly KCO1) and TPK4 (formerly KCO4) have been functionally described. TPK4 has been recently reported to be a K+-selective leak channel in the plasma membrane of pollen (Becker et al., 2004). TPK1 was originally described in Sf9 and Sf21 insect cells as a plasma membrane outward-rectifying potassium channel with a steep Ca2+ dependency. However, Ca2+-activated background currents found in Sf21 cells strongly indicated that further proof of function in another expression system would be necessary (Czempinski et al., 1997). In two parallel studies, TPK1 was shown to express in the vacuolar membrane in tobacco (Nicotiana tabacum) cells and in Arabidopsis (Czempinski et al., 2002; Schönknecht et al., 2002). It was therefore tempting to speculate that the TPK1 gene product would actually be responsible for the slow-vacuolar (SV)-type channel activity found in many plant vacuolar membranes. If this were the case, inactivation of the TPK1 gene should have a significant effect on the observed vacuolar SV-type channel activity, and, indeed, vacuoles of tpk1 knockout plants were reported to display ionic current densities that were 75% smaller than those of wild-type plants. However, the remaining currents still showed the typical kinetics, Ca2+ requirement, and voltage dependence of SV-type channels. This residual SV-type channel current in vacuoles from tpk1 knock-out plants was proposed to result from the activity of the TPK3 (formerly KCO6) gene product (Schönknecht et al., 2002). It should be noted that both TPK1 and TPK3 show high transcriptional activity in leaf mesophyll cells and in guard cells of Arabidopsis. Since the TPK1 gene product shows all the structural elements of a K+ channel α-subunit, it seems unlikely that the TPK1 gene product would be a regulatory subunit, contributing to the SV-type current by modulating the TPK3 channel. Then, the only explanation for the above report (Schönknecht et al., 2002) is that the SV channel can function as a heterodimer, consisting of a TPK1 and a TPK3 subunit. Since both TPK1 and TPK3 display the typical K+ channel signature sequence (GYG), determining K+ selectivity, it is hard to imagine that such a heterodimeric pore composed of four GYG motifs would form the nonselective conduction pathway of the SV channel.
Recently, loss of SV-type currents in Arabidopsis mesophyll vacuoles was reported to be associated with disruption of the TPC1 gene, and currents were restored upon transformation of the tpc1 knockout mutant with green fluorescent protein-labeled TPC1 or by constitutive overexpression of TPC1 under the control of the cauliflower mosaic virus 35S promoter (Peiter et al., 2005). The SV-type channel was shown to be permeable to K+ and Ca2+, as determined in patch-clamp experiments from isolated Arabidopsis mesophyll vacuoles of wild-type plants. This is consistent with the structural characteristics of the two-pore domain cation channel TPC1, not exhibiting the GYG motif. It therefore seems likely that the SV-type currents described in many plants result from the activity of the TPC1 gene product, both of which are strongly outward rectifying with similar open channel conductances. The TPC1 channel is believed to be involved in physiological processes such as abscisic acid-sensitive seed germination or stomatal movement, both of which involve cytosolic Ca2+ signals. However, the involvement of the SV-type channel in mediating cytosolic Ca2+ signals by functioning as a Ca2+-induced Ca2+ release channel has been a matter of debate in the past (Ward and Schroeder, 1994; Pottosin et al., 1997; Miedema et al., 2003), and, since no TPC1-dependent Ca2+ inward current (into the cytosol) has been recorded (Peiter et al., 2005), this question still remains unanswered.
Here, we show that the plant vacuolar ion channel TPK1 expresses in the yeast (Saccharomyces cerevisiae) vacuolar membrane and displays characteristics different from both the TPC1 channel described recently and the SV-type channel found in many plants.
RESULTS
Ion Channels in the Yeast Vacuolar Membrane
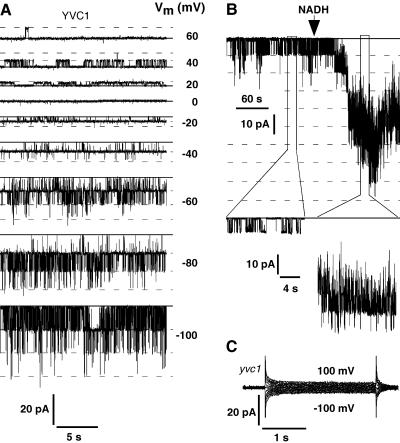
Yeast vacuoles have been shown to harbor a prominent cation-selective ion channel that is modulated by membrane voltage, cytosolic calcium, pH, and redox state (Bertl and Slayman, 1990). This ion channel has a single channel conductance of about 120 pS to 160 pS in symmetrical 100 mm KCl and displays inward rectification, favoring cation fluxes from the vacuole into the cytosol (Bertl and Slayman, 1990; Palmer et al., 2001). Channel activity in the yeast vacuolar membrane and its dependency on membrane voltage and redox state is demonstrated in Figure 1, showing single channel recordings from isolated tonoplast patches with the cytosolic side of the membrane exposed to the bath solution. Although the current-voltage characteristic of the open channel is rather linear over the voltage range of +60 mV to −100 mV, the open probability is much higher at negative membrane voltages, which renders this channel a clear inward rectifier. Vacuoles from a yvc1 disruption strain did not show any channel activity under our experimental conditions, as shown in the whole-vacuole experiment of Figure 1C, which is consistent with the report of Palmer et al. (2001) identifying the yeast vacuolar cation channel as the YVC1 gene product. We could not detect channel activity in any of the 20 vacuoles from yvc1 cells tested.
Figure 1.
Activity of the yeast vacuolar cation channel is modulated by membrane voltage and cytosolic redox state. A, Single channel currents from the yeast tonoplast in an excised membrane patch with 100 mm K+ in pipette and bath. The bath (cytosolic side) contained 10 μm Ca2+. The solid line indicates the baseline with all channels closed, and the dashed lines mark the open states with the corresponding membrane voltages to the right of each trace. B, Continuous current recording (top row) from an excised tonoplast patch demonstrating activation of at least nine channels by addition of 1 mm NADH to the bath (cytosolic) solution (150 mm KCl, 50 μm Ca2+, Vm = −40 mV). Baseline with all channels closed is marked by the solid line; open channel levels are indicated by the dashed lines. Two segments at higher time resolution are shown in the second and bottom rows, respectively. All data were filtered at 400 Hz and sampled at 1 kHz. C, Whole-vacuole currents from the yvc1 deletion strain in response to 2.5-s voltage pulses in the range of ±100 mV in 20-mV increments with ionic conditions as in A, but with 100 μm Ca2+ in the bath (cytosolic side). Data were filtered at 100 Hz and sampled at 1 kHz.
SV Channels in Arabidopsis Vacuoles
Plant vacuolar membranes exhibit prominent channel activity, described as SV-type channels. These channels display characteristics similar to those of the yeast channel Yvc1p, such as modulation by cytosolic calcium, pH, and redox state (Hedrich and Neher, 1987; Reifarth et al., 1994; Schulz-Lessdorf and Hedrich, 1995; Carpaneto et al., 1999; Pottosin et al., 2001). However, the voltage dependence of the SV cation channel (SV channel) is reverse to the voltage dependence of the yeast Yvc1p cation channel (Hedrich and Neher, 1987; Bertl and Slayman, 1990).
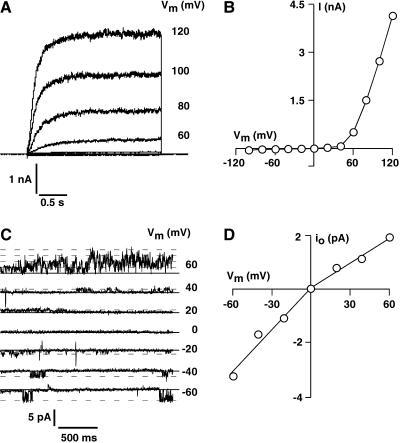
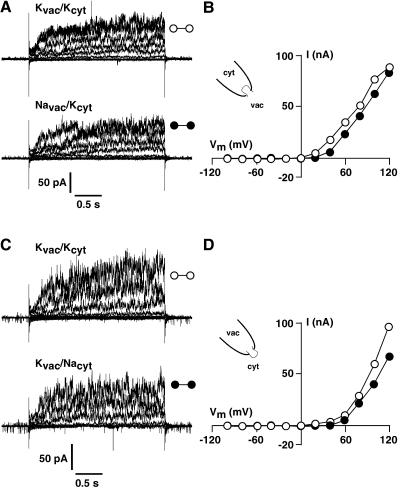
Patch-clamp experiments performed in the whole-vacuole mode revealed typical SV-type channel currents in isolated vacuoles of Arabidopsis leaf mesophyll cells (Fig. 2A). Macroscopic currents recorded in symmetrical ionic conditions (100 mm KCl) showed slow activation with activation time constants of about 150 ms at +120 mV and strong outward rectification of the steady-state currents (Fig. 2B). The open-channel conductance of the Arabidopsis SV channel displayed a weak asymmetry in symmetrical 100 mm KCl, with inward currents being larger than the outward currents. Open channel conductances determined from isolated tonoplast membrane patches as shown in Figure 2C were 60 pS at negative voltages and 35 pS in the positive voltage range (Fig. 2D). Note that occasional channel openings could be detected at negative membrane voltages, but channel activity increased dramatically at membrane voltages of +60 mV and above, as indicated by mutiple simultaneous channel openings in the +60 mV trace. Both macroscopic and single channel currents exhibited all the characteristics described for typical SV-type channels (Hedrich and Neher, 1987; Reifarth et al., 1994; Schulz-Lessdorf and Hedrich, 1995; Carpaneto et al., 1999; Pottosin et al., 2001): outward rectification, calcium dependency, redox modulation, pH sensitivity, and a permeability ratio of PNa:PK = 1. Selectivity of the SV channels was tested in isolated patches from Arabidopsis as shown in Figure 3. Under symmetrical ionic conditions with 100 mm KCl on both sides of the membrane, isolated membrane patches with the vacuolar side exposed to the bath displayed slowly activating outward currents in response to 2.5-s voltage steps, ranging from +120 mV to −100 mV in 20-mV decrements (Fig. 3A, top). Upon replacement of the vacuolar solution (bath, 100 mm KCl) by a solution containing 100 mm NaCl, the outward current remained largely unchanged (Fig. 3A, bottom). A comparison of the I-V curves in Figure 3B reveals that this maneuver had only minor effects on the steady-state outward currents. In isolated patches with the cytosolic side exposed to the bath (Fig. 3C) currents similar to those shown in Figure 3A were recorded under symmetrical ionic conditions (100 mm KCl on both sides). Replacement of cytosolic (bath) KCl by 100 mm NaCl had also only minor effects on the kinetics and steady-state current amplitudes of the outward currents, as can be seen from the original traces in Figure 3C, bottom, and from the I-V plots in Figure 3D. This clearly demonstrates that the SV-type channel in the vacuolar membrane of Arabidopsis mesophyll cells does poorly discriminate between K+ and Na+ as the substrate.
Figure 2.
Isolated vacuoles of Arabidopsis mesophyll cells show typical SV-type channel activity. A, Macroscopic SV-type currents recorded from an isolated mesophyll cell vacuole from a wild-type Arabidopsis plant. Currents were recorded in symmetrical standard recording solutions (see “Materials and Methods”) in response to 2.5-s voltage pulses. Over the voltage range of +120 mV to −100 mV, large, slowly activating outward currents and only negligible inward currents were detected. Strong outward rectification is also evident from the corresponding current-voltage plot in B, showing the steady-state currents, as averaged over the last 150 ms for each trace and plotted versus the applied membrane voltage. C, SV-type currents recorded in an isolated membrane patch with the cytosolic side of the membrane facing the bath solution. Conditions were as in A. Channel activity is high at positive voltages and channel openings are very rare at negative voltages, giving rise to the strong outward rectification of the macroscopic current. D, The open channel current-voltage curve corresponding to the original traces in C shows clear asymmetry with smaller open channel amplitudes at positive membrane voltages. All data were filtered at 400 Hz and sampled at 1 kHz.
Figure 3.
The SV-type channels from Arabidopsis mesophyll cells discriminate poorly between K+ and Na+. Slowly activating outward currents were recorded from isolated tonoplast patches in response to 2.5-s voltage pulses ranging from +120 mV to −100 mV. Holding voltage between the test pulses was 0 mV. A, Current recordings from an isolated tonoplast patch with the vacuolar side of the membrane exposed to the bath solution. Replacement of the bath (vacuolar) KCl with 100 mm NaCl had only little effect on current amplitude, activation kinetics, and rectification. B, Steady-state current-voltage curves corresponding to the data in A. C, Current recordings from an isolated tonoplast patch with the inverse geometry (cytosolic side exposed to the bath). As above, current amplitudes, activation kinetics, and current-voltage characteristic (D) were largely unaffected by replacement of cytosolic KCl by NaCl. Data were filtered at 400 Hz and sampled at 1kHz. Steady-state currents were determined by averaging the last 250 ms of each current trace.
Function of TPK1 in the Yeast Vacuolar Membrane
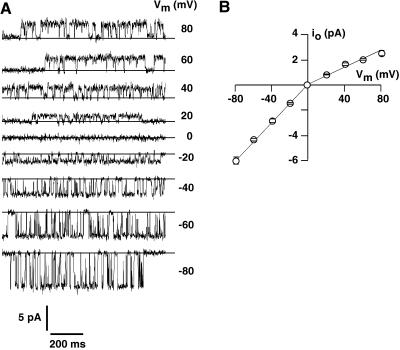
To test expression and localization of the plant TPK1 channel in yeast and to compare the electrical properties of the TPK1 gene product with the properties of the Arabidopsis SV-type channel, yeast cells that had the endogenous cation channel Yvc1p deleted were transformed with the p112A1XE plasmid carrying the TPK1 cDNA. In isolated vacuoles from this yeast strain, clear TPK1-related channel activity with up to eight channels per vacuole was observed in 60 out of 75 vacuoles tested. The open channel currents of TPK1 displayed weak asymmetry with larger inward currents and smaller, saturating outward currents (Fig. 4A). The open channel conductance of TPK1 in symmetrically 100 mm KCl was similar to that of the SV-type channel from Arabidopsis mesophyll vacuoles, being about 75 pS for the inward currents and about 40 pS for the outward currents (Fig. 4B). As expected from the presence of two C-terminal Ca2+-binding EF-hands, TPK1 channels were modulated by cytosolic Ca2+ (Fig. 5), but channel activity was recorded only in the presence of high concentrations of cytosolic Ca2+ (>5 μm), which is also characteristic for SV-type channels (Hedrich and Neher, 1987; Reifarth et al., 1994). However, in contrast to the SV-type channel, the apparent open probability of TPK1 seems to be voltage independent, which is shown in Figure 6 together with the rather linear time-averaged I-V relation, resulting from multiplying open channel current amplitudes (Fig. 4) with the respective open probability. This lack of voltage dependency suggests that the TPK1 gene product does not mediate the SV-type currents described in plant vacuoles. However, expression of an ion channel in a heterologous system can easily result in loss or alteration of some regulatory mechanisms, which could explain the difference between the characteristics reported for SV-type currents and the properties of the TPK1 channel in yeast vacuoles, such as the lack of sensitivity of TPK1 to voltage, redox agents, and cytosolic pH (not shown here).
Figure 4.
Functional expression of the Arabidopsis TPK1 channel in yeast vacuoles. A, Single channel currents recorded in the whole-vacuole mode from the yvc1 deletion mutant expressing TPK1 from the p112A1XE plasmid. Data were filtered at 400 Hz and sampled at 1 kHz. This vacuole contained at least five TPK1 channels, but traces showing only single events were selected for display. Note the asymmetric current amplitude in symmetric 100 mm KCl, which is also evident from the open channel current-voltage plot in B. Data in B are the means of 12 independent experiments and error bars are se of the mean.
Figure 5.
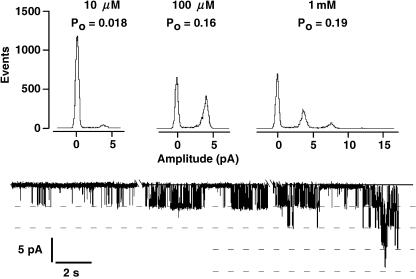
Activity of TPK1 is modulated by cytosolic calcium. Whole-vacuole experiment from a yvc1 deletion mutant expressing KCO1 from the p112A1XE plasmid. With 10 μm cytosolic Ca2+ and −60 mV holding voltage, only little channel activity can be recorded (lower trace). From an amplitude histogram (top), the apparent open probability was determined as 0.018, assuming four channels to be present in this vacuole. The open probability increased to 0.16 in 100 μm cytosolic Ca2+. In 1 mm cytosolic Ca2+, the apparent open probability increased further to 0.19 and mutiple channel openings (up to four channel openings simultaneously) became evident. Data were recorded in symmetrical 100 mm KCl with cytosolic Ca2+ as indicated. For complete exchange of the bath solution, the chamber was perfused with the new solution for 3 min, which is indicated by the gaps in the current trace. Filter frequency was 100 Hz and sampling rate was 1 kHz. Open probability for the displayed current traces were calculated from the all-points histograms as described by Bertl and Slayman (1990).
Figure 6.
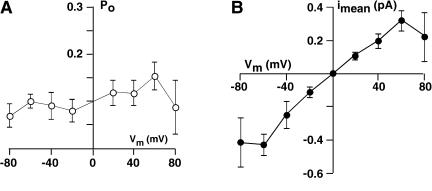
Apparent open probability of TPK1 and macroscopic current as a function of membrane voltage. A, The open probability (Po) was determined from all points histograms as described by Bertl and Slayman (1990). Data points are means (±se of the mean) from 12 independent experiments. B, The macroscopic current, calculated as the product of Po and open-channel current (Fig. 4B) for each experiment, does not show any rectification over the voltage range of ±80 mV.
Selectivity for certain ions is a fundamental property of an ion channel usually linked to intrinsic structures (pore loops) that form the narrowest part of the channel's central pore. Thus, one would not expect to see changes in ion selectivity of TPK1 by heterologous expression in yeast. As expected from the secondary structure of TPK1 with the conserved GYG motif in both of the two pore loops, the channel was clearly selective for K+, as demonstrated in Figure 7.
Figure 7.
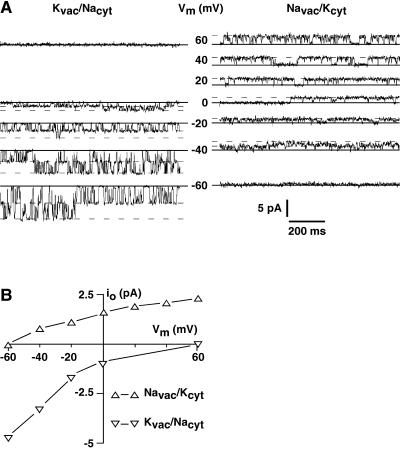
TPK1 exhibits strong selectivity for K+ over Na+. A, Under bi-ionic conditions with 100 mm KCl on the vacuolar side and 100 mm NaCl on the cytosolic side, only inward currents were detected (left section). With KCl on the cytosolic side and NaCl on the vacuolar side, outward currents were recorded at positive voltages. Even at voltages as negative as −40 mV, the channel currents were still outward (right section), indicating strong preference of the channel for K+ over Na+. B, Graphical representation of the data in A visualize the selectivity of the TPK1 channel with currents not reversing sign within the voltage range tested (±60 mV).
Under bi-ionic conditions with 100 mm KCl in the pipette (vacuolar side) and 100 mm NaCl in the bath (cytosolic side) no Na+ outward currents were detected at positive membrane voltages, but clear K+ inward currents were evident at 0 mV and at negative voltages (Fig. 7A, left).
Using inverse ionic conditions with 100 mm NaCl in the pipette and 100 mm KCl in the bath, only outward currents (K+) were visible at membrane voltages positive to −40 mV (Fig. 7A, right). This indicates strong selectivity of TPK1 for K+ over Na+, which is evident also from the I-V plot shown in Figure 7B. Furthermore, it should be noted that in contrast to the SV-type channel (Ivashikina and Hedrich, 2005), the TPK1 channel does not allow Na+ fluxes in either direction, which makes it very unlikely that the unspecific SV-type cation currents are determined by the activity of the TPK1 gene product.
DISCUSSION
Plant cells contain a central acidic vacuole that can occupy up to 90% of the cell volume, playing a key role in ion homeostasis, pH regulation, Ca2+ signaling, and protein degradation. The membrane enclosing this prominent compartment contains a variety of ion channels, of which the SV channel certainly is the most widely characterized ion channel in plants in general. Basic transport properties, such as unitary conductance and selectivity, have been worked out in great detail, as have been the regulatory properties of this cation channel. Despite this vast amount of information, the physiological role of the SV-type channel has been controversially discussed and the gene encoding for this cation channel has not been identified until recently (Peiter et al., 2005).
The identification of a new class of ion channels in Arabidopsis and demonstration of the vacuolar localization of its founding member TPK1 (KCO1 in Czempinski et al., 2002; Schönknecht et al., 2002) were indeed followed by an attempt to identify the SV-type channel activity with the TPK1 gene product (Schönknecht et al., 2002). In addition to the vacuolar localization, the TPK1 channel also contains two putative Ca2+-binding EF-hands, which could explain the Ca2+ dependency of the SV-type currents. However, the experimental evidence that linked the SV-type currents with the TPK1 gene product was not very convincing, since disruption of the TPK1 gene did not abolish SV-type currents. Disruption of the TPK1 gene rather resulted in a reduction of the total tonoplast current density, but the typical slowly activating SV-type component persisted. In addition, several structural features of the TPK1 gene product are not compatible with the characteristics of the SV-type currents. SV-type channels are voltage dependent (Hedrich and Neher, 1987) and display strong outward rectification under most experimental conditions (Ivashikina and Hedrich, 2005). But the TPK1 gene does not show any structural elements that could be identified as a voltage-sensing domain. In fact, most K+ channels containing four TM domains and two pore regions do lack any voltage-sensing structural elements, and these channels have been shown to be rather voltage independent (O'Connell et al., 2002), including TPK4, a TPK1-related ion channel from Arabidopsis (Becker et al., 2004).
Consistent with this observation, our work clearly demonstrates that in contrast to the SV-type channel, TPK1, when expressed in yeast, does not show any voltage dependency or rectification of the time-averaged current (Fig. 6). Structural elements reminiscent of the voltage sensor in shaker-type ion channels do exist in the TPC1 gene product, and clear voltage dependency is evident from the currents related to TPC1 (Peiter et al., 2005).
More striking, however, is the incompatibility of the TPK1 pore structure with the lack of K+ selectivity of the SV channel. TPK1 displays in both pore loops the conserved GYG motif, known to determine K+ selectivity (Doyle et al., 1998). In contrast to the expected high selectivity for K+, SV-type channels do not discriminate between Na+ and K+ and even conduct divalent cations and anions (Hedrich and Kurkdjian, 1988; Pottosin et al., 2001). Our data on the TPK1 channel, however, show clear selectivity for K+ over Na+ (Fig. 7), as expected from the sequence homology of the two pore loops to other K+-selective channels. Although selectivity of the TPC1 gene product has not been tested explicitly (Peiter et al., 2005), the missing GYG motif indicates TPC1 to be an ion channel not selective for K+, which renders the TPC1 gene a very likely candidate to encode for the SV-type channel.
Our data clearly demonstrate that TPK1 is a vacuolar cation channel that shows characteristics different from the SV-type channel. The reduction in total current density with the slowly activating currents prevailing, as observed in mesophyll vacuoles from tpk1 mutant of Arabidopsis (Schönknecht et al., 2002), could well be compatible with removal of an instantaneous, voltage-independent current like the TPK1-related current described here, which in whole-cell (or whole-vacuole) patch-clamp analyses is often erratically treated as leak conductance.
In addition to the nonselective SV- and FV-type cation channels, K+-selective channel activity has been reported from plant vacuoles occasionally (Gradmann and Bertl, 1989; Ward and Schroeder, 1994). The K+-selective channel from Vicia faba guard cell vacuoles (VK channel) has been described as a Ca2+-activated, voltage-independent, K+-selective ion channel with an open channel conductance of 70 pS in symmetrical 100 mm KCl (Ward and Schroeder, 1994), thus exhibiting all the hallmark properties of the TPK1 channel described above. Whether or not the TPK1 gene product is responsible for the VK channel activity has to be clarified in further studies involving tpk1 mutant plants.
MATERIALS AND METHODS
General methods for growing, handling, and protoplasting yeast (Saccharomyces cerevisiae) and for isolating yeast vacuoles were as described (Bertl et al., 1998). Isolation of protoplasts from Arabidopsis (Arabidopsis thaliana) mesophyll cells was done similar to the method described previously (Spalding et al., 1992). Briefly, three to five leafs of 5-week-old plants were excised and the epidermis was stripped off. The leaves were incubated for 60 min in an osmotically protective enzyme solution containing 0.6 m sorbitol, 5 mg/mL Cellulase Onozuka R-10 (Serva), 5 mg/mL Macerozym R-10 (Serva), 5 mg/mL BSA (Serva), 1 mm CaCl2, 10 mm KCl, pH 5.8 adjusted with Tris/MES. Large pieces of undigested leaves were removed using forceps, and the solution containing the protoplasts was transferred to an Eppendorf tube, centrifuged at 500g for 3 min, and resuspended in storage solution (150 mm KCl, 10 mm CaCl2, 5 mm MgCl2, pH 7). Aliquots of Arabidopsis mesophyll protoplasts were pipetted into the recording chamber and allowed to settle and adhere for about 5 min. The chamber was then slowly perfused with the recording solution, which ruptured the plasma membrane of some protoplasts giving access to the central vacuole.
For electrophysiological analysis, TPK1 was expressed from the p112A1XE plasmid in yeast strain CEN.SR36-3C (Euroscarf, Frankfurt), which has the gene encoding for endogenous yeast vacuolar cation channel, Yvc1p, deleted. This strain does not show any intrinsic vacuolar channel activity and is therefore an excellent system for expression and functional analysis of heterologous vacuolar ion channels. Current recordings from isolated yeast vacuoles were performed in the whole-vacuole mode as described by Bertl et al. (1998).
TPK1 channel currents recorded from isolated vacuoles of the transgenic yeast were compared with the SV-type channel from Arabidopsis mesophyll cells. For that purpose, we recorded channel activity from isolated vacuoles of Arabidopsis mesophyll cells in the whole-vacuole mode and from isolated patches.
For data recording, we used the HEKA software package Pulse/Pulsefit 8.5 for MacIntosh in combination with the EPC-9/ITC-16 amplifier/data aquisition system (HEKA, Lamprecht, Germany). Data were filtered at 400 Hz, using a built-in eight-pole Bessel filter, sampled at 1 kHz, and stored on the computer hard drive.
The sign convention proposed by Bertl et al. (1992) for membrane voltage and current was used throughout. According to this sign convention, the flow of positive charges from the cytosolic side of the membrane to the extracytosolic side (here vacuolar lumen) is defined positive and designated outward current (out of the cytosol).
For recordings from yeast and Arabidopsis vacuoles, standard composition of the pipette solution, representing the vacuolar content, and of the bath solution, facing the cytosolic side of the membrane, was 100 mm KCl, 5 mm MgCl2, 10 μm CaCl2, pH 7. Recordings from yeast vacuoles expressing the TPK1 channel were done in 1 mm cytosolic calcium. Deviations from these conditions are given in the respective figure legends.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number 407825.
Acknowledgments
We thank Dr. C.L. Slayman for critical reading of the manuscript and helpful comments, and S. Wörner for technical assistance. Dedicated to Professors F.-W. Bentrup and M.H. Weisenseel on the occasion of their retirements.
This work was supported by the Deutsche Forschungsgemeinschaft (grant nos. Be1181/6–1 to A.B. and Cz87/1–1 to K.C.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.065599.
References
- Becker D, Geiger D, Dunkel M, Roller A, Bertl A, Latz A, Carpaneto A, Dietrich P, Roelfsema MR, Voelker C, et al (2004) AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc Natl Acad Sci USA 101: 15621–15626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A, Bihler H, Kettner C, Slayman CL (1998) Electrophysiology in the eukaryotic model cell Saccharomyces cerevisiae. Pflugers Arch 436: 999–1013 [DOI] [PubMed] [Google Scholar]
- Bertl A, Blumwald E, Coronado R, Eisenberg R, Findlay G, Gradmann D, Hille B, Köhler K, Kolb H-A, MacRobbie E, et al (1992) Electrical measurements on endomembranes. Science 258: 873–874 [DOI] [PubMed] [Google Scholar]
- Bertl A, Slayman CL (1990) Cation-selective channels in the vacuolar membrane of Saccharomyces: dependence on calcium, redox state, and voltage. Proc Natl Acad Sci USA 87: 7824–7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpaneto A, Cantu AM, Gambale F (1999) Redox agents regulate ion channel activity in vacuoles from higher plant cells. FEBS Lett 442: 129–132 [DOI] [PubMed] [Google Scholar]
- Czempinski K, Frachisse JM, Maurel C, Barbier-Brygoo H, Müller-Röber B (2002) Vacuolar membrane localization of the Arabidopsis “two-pore” K+ channel KCO1. Plant J 29: 809–820 [DOI] [PubMed] [Google Scholar]
- Czempinski K, Zimmermann S, Ehrhardt T, Müller-Röber B (1997) New structure and function in plant K+ channels: KCO1, an outward rectifier with a steep Ca2+ dependency. EMBO J 16: 2565–2575; erratum Czempinski K, Zimmermann S, Ehrhardt T, Müller-Röber B (1997) EMBO J 16: 6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77 [DOI] [PubMed] [Google Scholar]
- Gradmann D, Bertl A (1989) Physiological control of membrane currents in plants. Plant Physiol Biochem 27: 587–593 [Google Scholar]
- Hedrich R, Kurkdjian A (1988) Characterization of an anion permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J 7: 3661–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Neher E (1987) Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329: 833–836 [Google Scholar]
- Ivashikina N, Hedrich R (2005) K+ currents through SV-type vacuolar channels are sensitive to elevated luminal sodium levels. Plant J 41: 606–614 [DOI] [PubMed] [Google Scholar]
- Miedema H, de Boer AH, Pantoja O (2003) The gating kinetics of the slow vacuolar channel: a novel mechanism for SV channel functioning? J Membr Biol 194: 11–20 [DOI] [PubMed] [Google Scholar]
- O'Connell AD, Morton MJ, Hunter M (2002) Two-pore domain K+ channels–molecular sensors. Biochim Biophys Acta 1566: 152–161 [DOI] [PubMed] [Google Scholar]
- Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y (2001) A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci USA 98: 7801–7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Pottosin II, Dobrovinskaya OR, Muniz J (2001) Conduction of monovalent and divalent cations in the slow vacuolar channel. J Membr Biol 181: 55–65 [DOI] [PubMed] [Google Scholar]
- Pottosin II, Tikhonova LI, Hedrich R, Schönknecht G (1997) Slow activating vacuolar channels can not mediate Ca2+-induced Ca2+ release. Plant J 12: 1387–1398 [Google Scholar]
- Reifarth FW, Weiser T, Bentrup FW (1994) Voltage- and Ca2+-dependence of the K+ channel in the vacuolar membrane of Chenopodium rubrum L. suspension cells. Biochim Biophys Acta 1192: 79–87 [DOI] [PubMed] [Google Scholar]
- Schönknecht H, Spoormaker P, Steinmeyer R, Brüggeman L, Ache P, Dutta R, Reintanz B, Godde M, Hedrich R, Palme K (2002) KCO1 is a component of the slow-vacuolar (SV) ion channel. FEBS Lett 511: 28–32 [DOI] [PubMed] [Google Scholar]
- Schulz-Lessdorf B, Hedrich R (1995) Protons and calcium modulate SV type channels in the vacuolar-lysosomal compartment-channel interaction with calmodulin inhibitors. Planta 197: 655–671 [Google Scholar]
- Spalding E, Slayman CL, Goldsmith MHM, Gradmann D, Bertl A (1992) Ion channels in Arabidopsis plasma membrane: transport characteristics and involvement in light-induced voltage changes. Plant Physiol 99: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6: 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]