Abstract
The phototropic response of Arabidopsis (Arabidopsis thaliana) is induced by the phototropin photoreceptors and modulated by the cryptochrome and phytochrome photoreceptors. Downstream of these photoreceptors, asymmetric lateral redistribution of auxin underlies the differential growth, which results in phototropism. Historical physiological evidence and recent analysis of hormone-induced gene expression demonstrate that auxin and brassinosteroid signaling function interdependently. Similarly, in this study we report evidence that interactions between brassinosteroids and auxin signaling modulate phototropic responsiveness. We found that elongated, a previously identified photomorphogenesis mutant, enhances high-light phototropism and represents a unique allele of BAK1/SERK3, a receptor kinase implicated in brassinosteroid perception. Altogether, our results support the hypothesis that phototropic responsiveness is modulated by inputs that influence control of auxin response factor-mediated transcription.
For successful establishment, emerging seedlings rely upon etiolated and tropic growth to maximize light interception. Once the seedling reaches sufficient light to support growth, there is less apparent need to continue elongating rapidly, so photomorphogenesis decreases shoot elongation, expands cotyledons, and promotes chloroplast biogenesis. How changes in signaling associated with photomorphogenesis effect phototropism remains open for investigation. It is clear, however, that photomorphogenesis is not dependent upon phototropism as Arabidopsis (Arabidopsis thaliana) nonphototropic hypocotyl 3 (nph3) mutants display a normal photomorphogenic growth response (Motchoulski and Liscum, 1999; Folta and Spalding, 2001a).
Both phototropism and photomorphogenesis are modulated by a common set of photoreceptors. Under unilateral low blue-light conditions (≤1.0 μmol m−2 s−1), coaction between phototropin (phot1), cryptochrome (cry1 and cry2), and phytochrome (phyA, phyB, and phyD) photoreceptors stimulates or enhances the phototropic response of etiolated seedlings (Whippo and Hangarter, 2003, 2004). Under higher intensity blue light (>1 μmol m−2 s−1) from above, these same photoreceptors control growth during photomorphogenesis (Casal, 2000; Folta and Spalding, 2001a, 2001b).
We previously found that higher intensities of unilateral blue light (≥10 μmol m−2 s−1) delay the phototropic response of etiolated seedlings (Whippo and Hangarter, 2003). This attenuation of high-light phototropism is mediated by the activities of phot1, phot2, cry1, cry2, and phyA (Whippo and Hangarter, 2003, 2004). Initially, we hypothesized that the slower phototropic response to high-fluence rates of blue light was a secondary consequence of hypocotyl growth inhibition associated with photomorphogenesis (Whippo and Hangarter, 2003). However, we later found that the enhanced high-light phototropic response of specific phytochrome mutants does not always correlate to the inhibition of hypocotyl elongation (Whippo and Hangarter, 2004). This finding raises the possibility that the attenuation of high-light phototropism is caused by desensitization of the phototropism-signaling pathway.
Little is known about how phototropic signaling becomes desensitized to higher fluence rates of light. The most important photoreceptors promoting phototropism, phot1 and phot2 (Sakai et al., 2001), contain two chromophore-binding domains, LOV1 and LOV2 (Christie et al., 1998, 1999), each with different signaling capacities (Christie et al., 2002). The LOV2 domain is essential for phot1-dependent phototropism, whereas the physiological significance of the phot1 LOV1 domain in light signaling remains unknown (Christie et al., 2002). Since the LOV1 domain is less sensitive to light than the LOV2 domain (Salomon et al., 2000), high-light intensities might desensitize phototropism through stimulation of the LOV1 domain. Alternatively, regulation of NPH3 or its homolog RPT2 may desensitize phototropism (Liscum and Stowe-Evans, 2000; Sakai et al., 2000). The NPH3 protein binds to phot1 and is critical for the phototropic response of etiolated seedlings under low and high light (Motchoulski and Liscum, 1999; Sakai et al., 2000). Exposure to blue light causes NPH3 to dissociate from phot1 (Motchoulski and Liscum, 1999), therefore high blue light might desensitize phototropism through this dissociation (Liscum and Stowe-Evans, 2000). However, desensitization could also be related to aspects of photomorphogenesis that lead to changes in auxin transport or signaling.
Photomorphogenesis is repressed under darkness by the nuclear accumulation of COP1 (von Arnim and Deng, 1994), which interacts with and targets light-mediated transcriptional activators such as HY5 and HYH for proteosome-mediated degradation (Osterlund et al., 2000; Holm et al., 2002). Light, via the phytochromes and cryptochromes, causes COP1 levels to decrease in the nucleus, leading to derepression of the light-mediated transcriptional activators (von Arnim and Deng, 1994; von Arnim et al., 1997). If aspects of photomorphogenesis are involved in the attenuation of high-light phototropism, then hy5 mutants might display an enhanced phototropic response under high-fluence rates of light.
In dark-grown plants, hormones such as auxin, brassinosteroids, ethylene, and gibberellin contribute to the repression of photomorphogenesis, whereas cytokinins promote it (Nemhauser and Chory, 2002). Of these hormones, auxin and ethylene demonstrably control or modify phototropism (Harper et al., 2000), and other reports indicate that gibberellins (Phillips, 1972; Kang and Burg, 1974) might also influence the response. In a review citing unpublished results, Briggs and Liscum (1997) suggested that the nph5 mutants, which are reportedly allelic to amp1/cop2, have diminished gravitropism and phototropic responses. As amp1/cop2 display increased cytokinin levels and have a constitutive photomorphogenic development (Hou et al., 1993; Nogue et al., 2000) the diminished tropic responses of nph5/amp1/cop2 mutants might be either a consequence of increased photomorphogenesis or a function of the antagonistic relationship between cytokinins and auxin.
To better understand the photomorphogenic and hormonal modulation of phototropism, we measured the phototropic response of a number of known photomorphogenic and hormone mutants. One of these mutants, elongated (elg) (Halliday et al., 1996), displayed a robust enhancement of high-light phototropism compared to wild-type plants but retained a normal very-low-light response. The elg mutant was initially identified as a suppressor of the gibberellin biosynthesis mutant, ga4 (Halliday et al., 1996). Indicative of a photomorphogenesis mutant, elg plants have long hypocotyls compared to wild type when grown under white, blue, red, and far-red light conditions, but when grown in darkness the hypocotyls of elg mutants are no different than wild type (Halliday et al., 1996). We found that elg plants contain a mutation in a previously described receptor kinase implicated in brassinosteroid perception, BAK1/SERK3 (Li et al., 2002; Nam and Li, 2002). As elg mutants have greater sensitivity to exogenous brassinosteroid, this mutation probably confers this hypersensitivity to brassinosteroids. Our results suggest the possibility that brassinosteroids modulate phototropism of etiolated seedlings under high-light conditions by modulating auxin signaling.
RESULTS
High-Light Phototropic Response Is Altered in Photomorphogenesis and Auxin Response Mutants
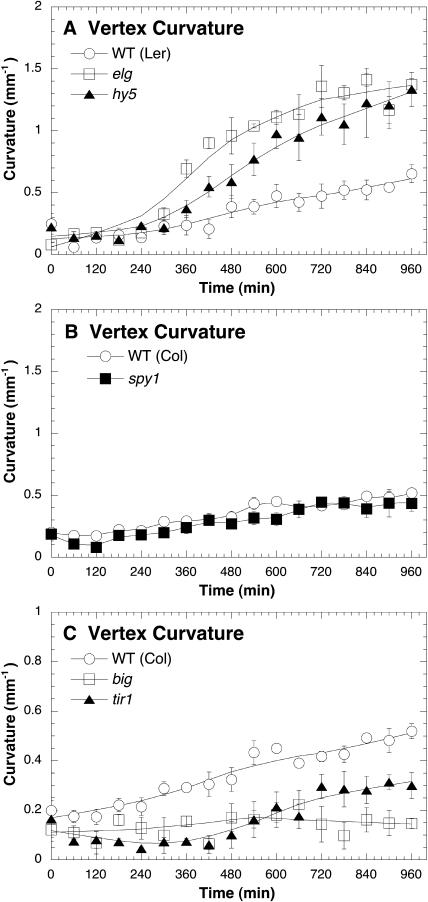
In previous reports we found that etiolated hypocotyls display a slower phototropic response to high-fluence rates of unilateral blue light than to low-fluence rates of light. This attenuation in response to high light is mediated by phot1, phot2, cry1, cry2, and phyA (Whippo and Hangarter, 2003, 2004). As phot1, cry1, cry2, and phyA are involved in the early stages of light-mediated growth inhibition associated with photomorphogenesis (Folta and Spalding, 2001a, 2001b), we wanted to determine whether other mutants with diminished photomorphogenic responses, specifically hy5 and elg (Koornneef et al., 1980; Halliday et al., 1996), would display an enhanced high-light phototropic response. In support of the hypothesis that some aspect of photomorphogenesis slows phototropism under high-light conditions, both the hy5 and elg mutants displayed an enhanced high-light response (Fig. 1A).
Figure 1.
Photomorphogenic and hormonal mutants showing altered phototropic response under high-fluence rate unilateral blue light. The fluence rate of blue light was 100.0 μmol m−2 s−1. Vertex curvature (mm−1) was used to measure the magnitude of phototropism. Error bars represent se.
Since elg partially suppresses the phenotype of gibberellin biosynthesis mutants (Halliday et al., 1996), we initially hypothesized that the enhanced high-light response of elg mutants could be caused by the constitutive activation of gibberellin signaling. To test this possibility, we measured the high-light response of spy mutants, which, like elg, can suppress gibberellin biosynthesis mutants by conferring a constitutive gibberellin response (Wilson and Somerville, 1995). Indicating that a constitutive gibberellin response does not enhance the high-light response, spy mutants responded like wild-type plants (Fig. 1B).
Auxin signaling relies upon degradation of Aux/IAA transcriptional repressors (Dharmasiri et al., 2005). Auxin binds to TIR1 (Dharmasiri et al., 2005; Kepinski and Leyser, 2005), which is an F-box protein that targets Aux/IAA proteins for polyubiquitiation (Gray et al., 2001; Dharmasiri and Estelle, 2002). To determine how auxin signaling impacts high-light phototropism, we measured the high-light response of tir1 mutants. Demonstrating that auxin signaling through TIR1 is important for high-light phototropism, tir1 mutants displayed a reduced phototropic response (Fig. 1C). Results from Jensen et al. (1998) indicate that polar auxin transport becomes more important for hypocotyl growth under higher fluence rates of light. To see if this also held for phototropism, we observed the high-light phototropic response of big mutants, which have reduced polar auxin transport (Gil et al., 2001). Our results suggest that disrupting polar auxin transport might limit the high-light response as big mutants were nonphototropic under high-intensity light (Fig. 1C).
Very-Low-Light Phototropism Is Modulated by hy5, spy, and Auxin
Although phot1, phot2, cry1, cry2, and phyA attenuate phototropism under high-light conditions, they also promote phototropism under low-fluence rates (Whippo and Hangarter, 2003, 2004). The basis for the differential function of these photoreceptors during phototropism under different light conditions is not clear. In an attempt to identify if or where the attenuating and promoting pathways might diverge, we looked at the very-low-light response of several downstream-signaling mutants.
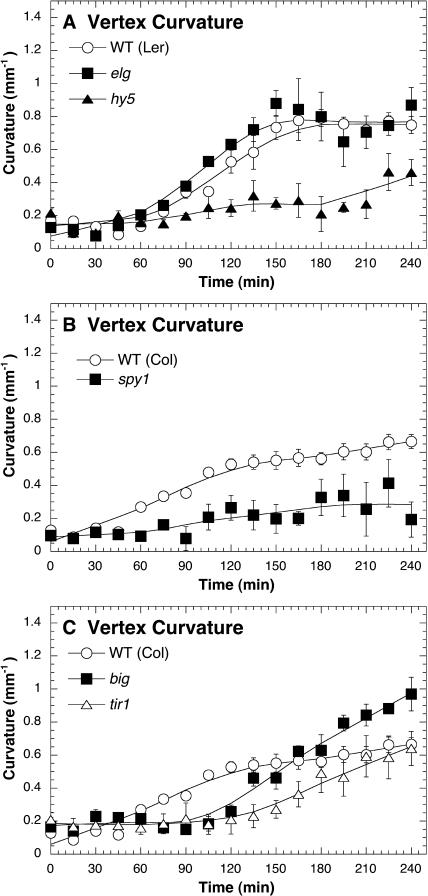
Suggestive of a branch point between the attenuating and promoting signaling pathways, elg mutants display a normal very-low-light response (Fig. 2A), despite having an enhanced high-light response (Fig. 1A). As a photomorphogenesis mutant, the normal low-light response of elg mutants supports the notion that phototropic curvature is not dependent upon photomorphogenesis. However, it appears that the stabilization of HY5 during light signaling could have an enhancing effect upon phototropism because hy5 mutants have a slower very-low-light response (Fig. 2A). Surprisingly, spy mutants also displayed a reduced very-low-light response compared to wild type (Fig. 2B). Since SPY is an N-acetyl glucosamine transferase (Thornton et al., 1999), this result suggests a role for glycosylation during phototropism under very-low-light conditions.
Figure 2.
Photomorphogenic and hormonal mutants showing altered phototropic response under very-low-fluence rate unilateral blue light. The fluence rate of blue light was 0.01 μmol m−2 s−1. Vertex curvature (mm−1) was used to measure the magnitude of phototropism. Error bars represent se.
It was important to know if the reduced phototropic response of tir1 mutants and the aphototropic response of big mutants to high-fluence rates of blue light (Fig. 1C) were caused by a stronger relative attenuating response or by a general inability to mount a phototropic response because of diminished auxin transport and signaling. Although displaying a longer latent period than wild type, tir1 and big mutants showed a strong overall response after 4 h (Fig. 2C). Therefore, it appears that as the light intensity increases, auxin transport and auxin signaling become more constrained in the promotion of phototropism.
elg Is an Allele of BAK1, a Leu-Rich Repeat Receptor Kinase Implicated in Brassinosteroid Signaling
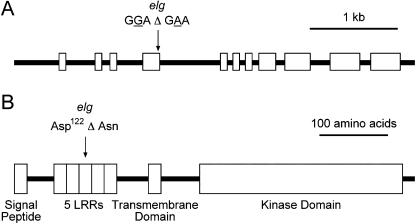
To better understand why the elg mutant displayed an enhanced high-light response, we wanted to identify the genetic basis of the elg mutation. We narrowed down the position of elg as originally reported by Halliday et al. (1996) to a 170-kb interval on chromosome 4. Of the genes in this interval, BAK1, which encodes a Leu-rich receptor kinase implicated in brassinosteroid perception (Li et al., 2002; Nam and Li, 2002), was an attractive candidate because elg mutants display phenotypes consistent with plants treated with brassinosteroids or plants overexpressing BAK1 (i.e. long hypocotyls, long inflorescence, early flowering time, and curled leaves; Halliday et al., 1996; Bishop and Koncz, 2002; Nam and Li, 2002). Sequencing of the BAK1/SERK3 (At4g33430) locus of elg plants revealed a point mutation (GGA to GAA) 1,370 bp downstream from the transcriptional start site that changes an Asp (Asp-122) to an Asn in the third Leu-rich repeat (LRR; Fig. 3, A and B). Suggestive of functional importance, this Asp is conserved in the LRRs of the SERK family (Baudino et al., 2001).
Figure 3.
elg is an allele of BAK1/SERK3 (At4g33430). A, elg plants have a point mutation (GGA to GAA) in the fourth exon, 1,370 bp from the transcriptional start site. B, This mutation is predicted to change Asp-122 to an Asn residue in the third LRR of BAK1.
The elg Mutation Confers Hypersensitivity to Brassinosteroids and Alters Auxin Signaling
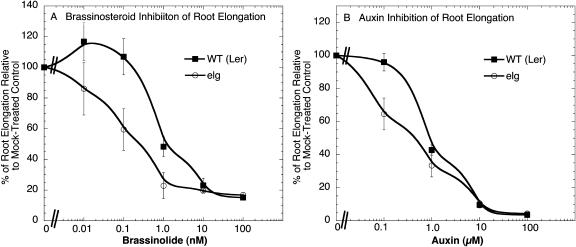
The partially dominant nature of the elg mutation (Halliday et al., 1996) and traits associated with an enhanced brassinosteroid response suggested that the elg allele of BAK1 may confer hypersensitivity to brassinosteroids. To test this, we measured the root growth response of light-grown seedlings treated with exogenous brassionolide. Indeed, root elongation of elg mutants showed greater sensitivity to brassinolide than wild-type plants (Fig. 4A). Since brassinosteroid and auxin signaling are interdependent (Nemhauser et al., 2004), we also measured how supplemental auxin affected the root growth of elg mutants. We found that root growth was slightly more sensitive to IAA in light-grown elg mutants compared to wild-type seedlings (Fig. 4B).
Figure 4.
The elg mutant shows increased sensitivity to brassinolide and IAA. A, Root growth of wild-type seedlings (7 d old) grown on supplemental brassinolide. B, Root growth of 7-d-old wild-type seedlings grown on supplemental IAA. Error bars represent se.
Brassinosteroids Enhance the High-Light Phototropic Response
The increased brassinosteroid sensitivity and enhanced high-light phototropic response of elg mutants indicates that the attenuation of high-light phototropism might be caused by a light-mediated reduction in brassinosteroid levels or sensitivity. To test if brassinosteroids could enhance the high-light response, we measured the high-light response of wild-type seedlings grown on supplemental brassinolide. Compared to mock-treated seedlings, addition of brassinolide resulted in a strongly enhanced high-light response (Fig. 5A). This, along with the enhanced high-light response of elg mutants, supports the hypothesis that high-intensity light might attenuate phototropism in part by decreasing active brassinosteroid levels or sensitivity. In contrast, it appears that high levels of brassinosteroids may hinder phototropism under very-low-light conditions as brassinolide-treated seedlings showed a slower and less robust response than wild-type seedlings (Fig. 5B).
Figure 5.
Supplemental brassinolide enhances high-light phototropism and slightly reduces very-low-light phototropism. A and B, High-light (A; 100 μmol m−2 s−1) and very-low-light (B; 0.01 μmol m−2 s−1) phototropic responses of brassionolide (1.0 nm) and mock-treated (ethanol) wild-type (Ler) seedlings. Vertex curvature (mm−1) was used to measure the magnitude of phototropism. Error bars represent se.
BAK1 Is Not Required for a Normal Phototropic Response
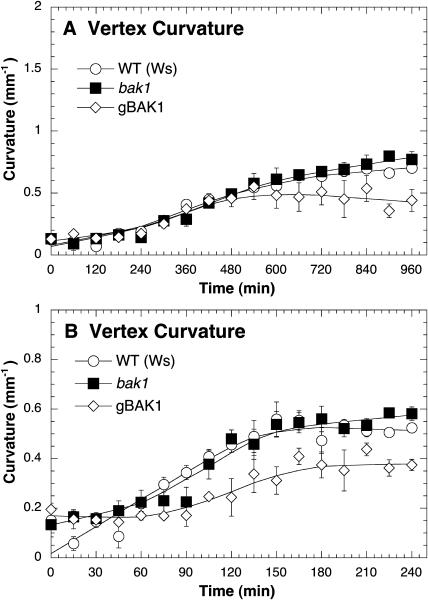
Because our results suggest that increasing brassinosteroid responsiveness via the elg allele of BAK1 confers an enhanced high-light response, we predicted that bak1 null mutants, which show a slightly reduced sensitivity to brassinolide (Li et al., 2002), would have an even slower response than wild-type plants. However, this does not appear to be the case as bak1 mutants showed a normal high-light response (Fig. 6A). This indicates that BAK1 itself is not a limiting factor of high-light phototropism. Likewise, BAK1 does not appear to be essential for low-light phototropism since bak1 mutants displayed a normal very-low-light response (Fig. 6B).
Figure 6.
The phototropic responses of bak1-1 mutants and BAK1 overexpressing plants (gBAK1). A, Phototropic responses to high-fluence rates of unilateral blue light (100 μmol m−2 s−1). B, Phototropic responses to very-low-fluence rates of unilateral blue light (0.01 μmol m−2 s−1). Vertex curvature (mm−1) was used to measure the magnitude of phototropism. Error bars represent se.
Overexpression of BAK1 Causes a Reduced Very-Low-Light Response
Since elg mutants displayed phenotypes similar to BAK1 overexpressors (Halliday et al., 1996; Nam and Li, 2002), we expected that BAK1 overexpressors would have a similarly enhanced high-light response. However, the high-light response of gBAK1 seedlings, which overexpress BAK1 (Nam and Li, 2002), was similar to wild-type seedlings (Fig. 6A). This result also supports the hypothesis that BAK1 is not a limiting factor during high-light phototropism. However, similar to seedlings treated with exogenous brassinolide (Fig. 5B), gBAK1 seedlings displayed a reduced very-low-light response (Fig. 6B).
Photomorphogenic Development Influences Phototropism
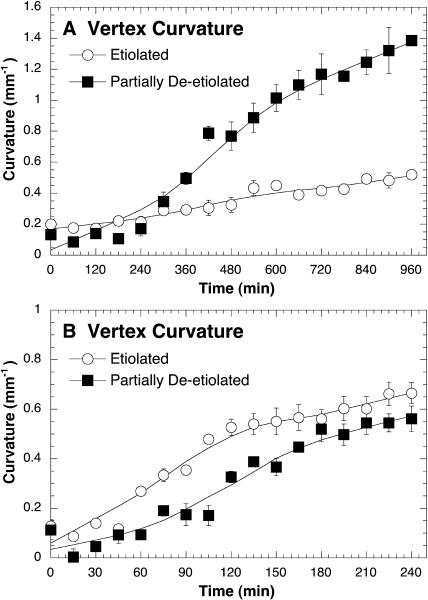
Seeing as the photomorphogenesis mutants hy5 and elg displayed abnormal phototropic responses (Figs. 1A and 2A), we compared the phototropic response of 3-d-old partially de-etiolated seedlings to dark-grown seedlings to determine how photomorphogenic development impacts phototropic responsiveness. The partially de-etiolated seedlings used in this study had open hooks, had closed but green cotyledons (data not shown), and retained relatively long hypocotyls (5.58 ± 0.30 mm). When exposed to a high-fluence rate (100 μmol m−2 s−1) unilateral blue light, the partially de-etiolated seedlings displayed a stronger phototropic response than etiolated seedlings (Fig. 7A), but, when exposed to a very-low-intensity light (0.01 μmol m−2 s−1), partially de-etiolated seedlings showed a slower response than etiolated seedlings (Fig. 7B). These results indicate that sensitivity and responsiveness to unilateral blue light increases from lower to higher light intensities during photomorphogenesis.
Figure 7.
Partially de-etiolated seedlings have an enhanced high-light phototropic response and slower very-low-light response compared to etiolated seedlings. Partially de-etiolated seedlings were grown under continuous white light (1.0 μmol m−2 s−1) for 3 d at 23°C prior to blue-light treatment (450 nm ± 50). The responses of etiolated seedlings shown here have been previously reported (Whippo and Hangarter, 2003). A and B, Vertex curvature (mm−1) of etiolated and partially de-etiolated wild-type (Col) hypocotyls under (A) high-fluence rates of unilateral blue light (100.0 μmol m−2 s−1) or (B) under very-low-fluence rates of unilateral blue light (0.01 μmol m−2 s−1). Error bars represent se.
DISCUSSION
Part of the complexity of phototropism stems from the dynamic establishment of differential growth gradients during the tropic response (Silk, 1984; Whippo and Hangarter, 2003). Another component of the complexity stems from the regulatory control of the response. In etiolated Arabidopsis hypocotyls, we have observed that higher intensities of unilateral blue light elicit a slower phototropic response than lower fluence rates (Whippo and Hangarter, 2003). Moreover, many of the photoreceptors involved in the promotion of low-light-induced phototropism are also involved in the attenuation of phototropism by high light (Whippo and Hangarter, 2003, 2004). How these photoreceptors elicit both promoting and attenuating responses remains uncertain. We previously postulated a phytochrome-dependent low-fluence response promotes phototropism, whereas a phyA-dependent high-irradiance response attenuates phototropism (Whippo and Hangarter, 2004). To better understand the interaction between hormonal and photomorphogenic signaling pathways during very-low-light and high-light phototropism, we examined the phototropic behavior of several hormone and photomorphogenic-signaling mutants.
Auxin Modulation of Phototropism
Auxin signaling functions by targeting Aux/IAA proteins to the ubiquitin-proteosome pathway, which in turn regulates the transcriptional activity of auxin response factors (ARFs; Gray et al., 2001; Liscum and Reed, 2002). With respect to phototropism, auxin specifically destabilizes IAA19, and this increases ARF7 activity (Tatematsu et al., 2004). Therefore, loss-of-function mutations in the ARF7 gene or gain-of-function mutations in the IAA19 gene result in a weaker phototropic response (Harper et al., 2000; Tatematsu et al., 2004). Our results support the model that auxin-mediated degradation of Aux/IAA proteins is important for phototropism because tir1 mutants display slower high-light phototropism (Fig. 1C) and longer latency under very-low-light conditions (Fig. 2C). The observation, however, that tir1 mutants display a strong overall very-low-light response (Fig. 1C) suggests that other auxin-binding F-box proteins, such as ABF1, ABF2, and ABF3 (Dharmasiri et al., 2005), function redundantly to TIR1 during phototropism.
Our results indicate that polar auxin transport can be a limiting factor of phototropism. This is based on the observation that big mutants, which have reduced polar auxin transport (Gil et al., 2001), display a longer latent period during very-low-light phototropism and are aphototropic in response to unilateral highlight (Figs. 1C and 2C). Furthermore, the aphototropic response of big mutants under high-light conditions suggests that as the light intensity increases, phototropism becomes more sensitive to disruptions in polar auxin transport. In a similar manner, hypocotyl elongation becomes more sensitive to polar auxin transport inhibitors as light intensity increases (Jensen et al., 1998). The more pronounced reduction of phototropism in the tir1 and big mutants under high-light conditions may be due to an increase in abundance of IAA proteins under high-light conditions.
Brassinosteroid Regulation of Phototropism
Brassinosteroids have been shown to enhance root gravitropism (Kim et al., 2000), and our results demonstrate that brassinosteroids also influence hypocotyl phototropism. Seedlings treated with exogenous brassinosteroids or overexpressing BAK1 display a slightly reduced very-low-light phototropic response (Figs. 5B and 6B). In addition, elg mutants, which are hypersensitive to brassinolide, have an enhanced high-light phototropic response (Fig. 2A) as do wild-type seedlings treated with brassinolide (Fig. 5A). Thus, as light intensity increases, decreases in brassinosteroid levels and/or sensitivity might constrict the promotion of phototropism.
It is plausible that brassinosteroids reduce the low-light phototropic response while enhancing high-light phototropism by decreasing phot1 expression (Nemhauser et al., 2004) since the phototropic response of phot1 mutants is abolished under low-light conditions (Liscum and Briggs, 1995) and enhanced under high-light conditions (Whippo and Hangarter, 2003). Alternatively, brassinosteroids may reduce low-light phototropism by increasing the expression of Aux/IAA genes (Mussig et al., 2002; Nakamura et al., 2003; Nemhauser et al., 2004). In addition, brassinosteroids may enhance high-light phototropism by increasing ARF-mediated transcription since brassinosteroids and auxin have been reported to function interdependently to regulate ARF-mediated transcription (Nemhauser et al., 2004). Cycling between Aux/IAA protein production and ARF-mediated transcription has already been proposed to be important for the establishment of phototropic curvature (Tatematsu et al., 2004). Thus, brassinosteroids could modulate phototropic responsiveness (either promoting or attenuating responsiveness) by influencing the equilibrium between Aux/IAA proteins and ARF activity. However, it is important to note that how brassinosteroids enhance ARF-mediated transcription is not clear (Nemhauser et al., 2004) and may work by an indirect mechanism, possibly through brassinosteroid modulation of auxin transport (Bao et al., 2004; Wang et al., 2005).
Based on the observations that BAK1 overexpressors (Fig. 6B) and seedlings supplemented with brassinolide (Fig. 6B) display a diminished very-low-light phototropic response, the normal very-low-light response of elg mutants is somewhat surprising (Fig. 2A). The reason for this discrepancy is not immediately clear. Since a negative feedback loop regulates brassinosteroid levels (Mathur et al., 1998; Bancos et al., 2002; He et al., 2005; Tanaka et al., 2005), it is possibly that elg mutants might be able to maintain brassinosteroid homeostasis more like wild-type plants under darkness or dim-light conditions than BAK1 overexpressors or seedlings grown supplemented with brassinolide.
Our observation that BAK1-overexpressing lines fail to show an enhanced high-light response (Fig. 6A) like elg mutants (Fig. 1A) or seedlings grown on supplemental brassinolide is also unexpected (Fig. 5A). At a minimum, this result suggests that under high-light conditions, BAK1 itself is not a limiting factor in the promotion of phototropism under high-fluence rates of light. The elg mutants may be more sensitive to brassinosteroids than BAK1-overexpressing plants since previous reports indicated that plants overexpressing BAK1 are only weakly hypersensitive to brassinolide (Nam and Li, 2002). In addition, unlike other gain-of-function brassinosteroid mutants, overexpressing BAK1 does not suppress the effects of brassinosteroid biosynthesis mutants or strong bri1 mutant alleles (Li et al., 2002; Nemhauser and Chory, 2004). This may be particularly important under high-light conditions where light signaling leads to the inactivation of brassinosteroids (Neff et al., 1999; Turk et al., 2003). Consequently, under high-light conditions active brassinosteroids could become more limiting with respect to the promotion of phototropism. Under this scenario, elg mutants and wild-type seedlings grown on supplemental brassinolide would be expected to have an enhanced high-light response, whereas BAK1-overexpressing lines, which cannot suppress the effect of lower brassinosteroid levels, would not be expected to display an enhanced high-light response.
elg and Brassinosteroid Signaling
We have shown that the elg allele of BAK1 confers hypersensitivity to brassinolide (Fig. 4A). This hypersensitivity to brassinosteroids is probably responsible for the enhanced high-light phototropic response and greater hypocotyl elongation of light-grown elg seedlings. How the elg mutation confers increased sensitivity to brassinosteroids remains to be determined. BAK1 forms heterodimers with BRI1 (Li et al., 2002; Nam and Li, 2002), which is a receptor kinase involved in brassinosteroid perception (Friedrichsen et al., 2000; He et al., 2000). The elg mutation might alter localization of BAK1 because LRRs, the site of the elg mutation, are important for the proper localization of other SERK proteins (Shah et al., 2001). By altering BAK1 localization, the elg mutation may alter the equilibrium between BRI1 homodimers in the plasma membrane and BRI1/BAK1 heterodimers in endosomes (Russinova et al., 2004). Alternatively, the elg mutation may enhance brassinosteroid binding to BRI1 or enhance the signaling capacity of BAK1 or BRI1 in the absence of brassinosteroids.
Photomorphogenic Regulation of Phototropism
The results presented here show that HY5 signaling, which is typically associated with photomorphogenesis, also modulates phototropism of etiolated plants. The reduced phototropic response of hy5 mutants under very-low light indicates that light-mediated stabilization of HY5 enhances phototropism under very-low-light conditions (Fig. 2A). It is possible that enhancement of phototropism via the cryptochromes (Whippo and Hangarter, 2003) and phytochromes (Whippo and Hangarter, 2004) is a consequence of their function in stabilizing HY5. Somewhat paradoxically, hy5 mutants also display an enhanced high-light response (Fig. 1A). Recent evidence demonstrates that HY5 activates the transcription of Aux/IAA genes (Cluis et al., 2004); therefore, HY5 activity might attenuate phototropism by increasing the levels of Aux/IAA proteins.
Confirming previous reports (Pringheim, 1912; Ellis, 1987), we found that, compared to etiolated seedlings, light-grown seedlings display a stronger high-light (100 μmol m−2 s−1) phototropic response (Fig. 7A) and weaker very-low-light phototropic response (Fig. 7B). On the surface, this appears to be inconsistent with the hypothesis that aspects of photomorphogenesis contribute to the attenuated high-light phototropic response of etiolated hypocotyls. However, light-grown seedlings have already acclimated to light, and the hormonal- and light-signaling pathways are undoubtedly altered after some period of photomorphogenic development. This light-dependent reconfiguration of signaling processes during photomorphogenesis may lead to changes in phototropic responsiveness. In any case, it appears that the relationship between photomorphogenic signaling and phototropism is dynamic and influenced by the life history of the seedling.
CONCLUSION
In this work, we show that photomorphogenesis modulates the phototropic response of Arabidopsis seedlings. HY5, which is an important transcriptional promoter of photomorphogenesis, is involved in the promotion of very-low-light phototropism and the attenuation high-light phototropism. Conversely, brassinosteroids, which are hormonal repressors of photomorphogenesis, are involved in the repression of very-low-light phototropism and the enhancement of high-light phototropism. We also found that elg is an allele of BAK1 that affects phototropism and confers a brassinosteroid-hypersensitive phenotype. The effects of brassinosteroids and mutations in TIR and BIG on phototropism suggests the possibility that changes in the equilibrium of Aux/IAA protein activity modulates phototropism. From these results, we hypothesize that control of photomorphogenic signaling via HY5 and brassinosteroids modulates phototropism by impinging on ARF-mediated gene transcription.
MATERIALS AND METHODS
Genotypes
Arabidopsis (Arabidopsis thaliana) seeds of hy5-1 (Landsberg erecta [Ler] background), elg (Ler background), spy1-3, (Columbia [Col] background), and bak1-1 (Wassilewskija background) were provided by the Arabidopsis Stock Center (Ohio State University). The auxin mutants used in this study, tir1-1 and big1-2, are in the Col background and were provided by Dr. Mark Estelle (Indiana University). The BAK1 overexpressor, gBAK1 (Wassilewskija background), was donated by Dr. Jianming Li (University of Michigan). The phototropic responses of the Ler and Col ecotypes used as wild-type controls in this study were previously reported (Whippo and Hangarter, 2003, 2004).
Phototropism Experiments
The phototropism experiments were carried out as previously described with 3-d-old seedlings (Whippo and Hangarter, 2003). When used in this study, partially de-etiolated seedlings were grown under continuous low-intensity white light (1.0 μmol m−2 s−1). The phototropic responses were recorded through time-lapse imaging via an infrared-sensitive digital camera. Hypocotyl shapes were fitted to polynomial equations via nonlinear regression of pixel xy-coordinates. The polynomial equations were used in calculating vertex curvature, which is proportional to the differential growth at the position of greatest bending along the hypocotyl. To measure the effects of brassinosteroids upon phototropism, wild-type (Ler) seedlings were grown as above with supplemental brassinolide (Sigma). The brassinolide was dissolved in ethanol before addition to the growth medium. For controls in the brassinolide experiments, wild-type (Ler) seedlings were also mock treated with equivalent amounts of ethanol.
Analysis of Growth on Brassinolide and Auxin
Seedlings where grown on 1% agar containing 0.5× Murashige and Skoog, 1% Suc and supplemented with brassinolide (Sigma) or IAA (Sigma), under 40 μmol m−2 s−1 of white light. Since the hormones were dissolved in ethanol prior to addition to the growth medium, controls were mock treated with equivalent amounts of ethanol. After 7 d, images of the seedlings were captured using a digital camera, and root and lengths were measured using the public domain NIH Image program (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image).
Mapping of elg and Sequencing BAK1
To map elg, a segregating F2 mapping population was created from a cross between elg (Ler background) with Col. F2s with elongated light-grown hypocotyls typical of elg mutants were selected for recombinant mapping using small insertions/deletions between Col and Ler ecotypes as annotated in the Cereon database for the chromosome 4 map interval originally identified by Halliday et al. (1997). Because the elg mutant is partially dominant, progeny testing of F2 plants with heterozygotic genotypes was carried out. F2s whose F3 progeny segregated with wild-type plants were classified as heterozygotic at the elg loci. The mapping interval was narrowed down to between bacterial artificial chromosomes T4I10 and T16L1 on chromosome 4, which contains BAK1 (At4g33430). We sequenced the BAK1 allele of elg mutants and Ler plants using an Applied Biosystems 3730 automatic DNA sequencer.
This work was supported by the National Science Foundation (grant no. IBN–0080783) and the Department of Energy (grant no. DE–FG02–01ER15223).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.064444.
References
- Bancos S, Nomura T, Sato T, Molnar G, Bishop GJ, Koncz C, Yokota T, Nagy F, Szekeres M (2002) Regulation of transcript levels of the Arabidopsis cytochrome p450 genes involved in brassinosteroid biosynthesis. Plant Physiol 130: 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z (2004) Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 134: 1624–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudino S, Hansen S, Brettschneider R, Hecht VF, Dresselhaus T, Lorz H, Dumas C, Rogowsky PM (2001) Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta 213: 1–10 [DOI] [PubMed] [Google Scholar]
- Bishop GJ, Koncz C (2002) Brassinosteroids and plant steroid hormone signaling. Plant Cell (Suppl) 14: S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Liscum E (1997) Blue light-activated signal transduction in higher plants. In P Aducci, ed, Signal Transduction in Plants. Verlag, Basel, Switzerland, pp 107–135
- Casal JJ (2000) Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 71: 1–11 [DOI] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Christie JM, Salomon M, Nozue K, Wada M, Briggs WR (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA 96: 8779–8783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Swartz TE, Bogomolni RA, Briggs WR (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J 32: 205–219 [DOI] [PubMed] [Google Scholar]
- Cluis CP, Mouchel CF, Hardtke CS (2004) The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 38: 332–347 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Estelle M (2002) The role of regulated protein degradation in auxin response. Plant Mol Biol 49: 401–409 [PubMed] [Google Scholar]
- Ellis RJ (1987) Comparison of fluence response relationships of phototropism in light-grown and dark-grown buckwheat. Plant Physiol 85: 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001. a) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001. b) Opposing roles of phytochrome A and phytochrome B in early cryptochrome-mediated growth inhibition. Plant J 28: 333–340 [DOI] [PubMed] [Google Scholar]
- Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J (2000) Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol 123: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Halliday K, Devlin PF, Whitelam GC, Hanhart C, Koornneef M (1996) The ELONGATED gene of Arabidopsis acts independently of light and gibberellins in the control of elongation growth. Plant J 9: 305–312 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288: 2360–2363 [DOI] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Von Arnim AG, Deng XW (1993) A new class of Arabidopsis constitutive photomorphogenic genes involved in regulating cotyledon development. Plant Cell 5: 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BG, Burg SP (1974) Red light enhancement of the phototropic response in pea stems. Plant Physiol 53: 445–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Kim SK, Chang SC, Lee EJ, Chung WS, Kim YS, Hwang S, Lee JS (2000) Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol 123: 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP (1980) Genetic control of light-inhbited hypocotyl elongation in Arabidopsis thaliana (L) Heynh. Z Pflanzenphysiol 100: 147–160 [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Reed JW (2002) Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol 49: 387–400 [PubMed] [Google Scholar]
- Liscum E, Stowe-Evans EL (2000) Phototropism: a “simple” physiological response modulated by multiple interacting photosensory-response pathways. Photochem Photobiol 72: 273–282 [DOI] [PubMed] [Google Scholar]
- Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C, et al (1998) Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J 14: 593–602 [DOI] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Mussig C, Fischer S, Altmann T (2002) Brassinosteroid-regulated gene expression. Plant Physiol 129: 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Higuchi K, Goda H, Fujiwara MT, Sawa S, Koshiba T, Shimada Y, Yoshida S (2003) Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in Arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol 133: 1843–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, Chory J (1999) BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA 96: 15316–15323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser J, Chory J (2002) Photomorphogenesis. In CR Somerville, EM Meyerwitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD [DOI] [PMC free article] [PubMed]
- Nemhauser JL, Chory J (2004) BRing it on: new insights into the mechanism of brassinosteroid action. J Exp Bot 55: 265–270 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Mockler TC, Chory J (2004) Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol 2: E258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogue F, Hocart CH, Letham DS, Dennis E, Chaudhury A (2000) Cytokinin biosythesis is higher in the Arabidopsis amp1 mutant. Plant Growth Regul 32: 275–283 [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Phillips IDJ (1972) Diffusible gibberellins and phototropism in helianthus annuus. Planta 106: 363–367 [DOI] [PubMed] [Google Scholar]
- Pringheim EG (1912) Die Reizbewegungen der Planzen. Springer-Verlag, Berlin
- Russinova E, Borst JW, Kwaaitaal M, Cano-Delgado A, Yin Y, Chory J, de Vries SC (2004) Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16: 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M, Christie JM, Knieb E, Lempert U, Briggs WR (2000) Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39: 9401–9410 [DOI] [PubMed] [Google Scholar]
- Shah K, Gadella TW Jr, van Erp H, Hecht V, de Vries SC (2001) Subcellular localization and oligomerization of the Arabidopsis thaliana somatic embryogenesis receptor kinase 1 protein. J Mol Biol 309: 641–655 [DOI] [PubMed] [Google Scholar]
- Silk WK (1984) Quantitative descriptions of development. Annu Rev Plant Physiol 35: 479–518 [Google Scholar]
- Tanaka K, Asami T, Yoshida S, Nakamura Y, Matsuo T, Okamoto S (2005) Brassinosteroid homeostasis in Arabidopsis is ensured by feedback expressions of multiple genes involved in its metabolism. Plant Physiol 138: 1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton TM, Swain SM, Olszewski NE (1999) Gibberellin signal transduction presents…the SPY who O-GlcNAc'd me. Trends Plant Sci 4: 424–428 [DOI] [PubMed] [Google Scholar]
- Turk EM, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Denzel MA, Torres QI, Neff MM (2003) CYP72B1 inactivates brassinosteroid hormones: an intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiol 133: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim AG, Deng XW (1994) Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79: 1035–1045 [DOI] [PubMed] [Google Scholar]
- von Arnim AG, Osterlund MT, Kwok SF, Deng XW (1997) Genetic and developmental control of nuclear accumulation of COP1, a repressor of photomorphogenesis in Arabidopsis. Plant Physiol 114: 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tiwari S, Hagen G, Guilfoyle T (2005) AUXIN RESPONSE FACTOR7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell 17: 1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP (2003) Second positive phototropism results from coordinated co-action of the phototropins and cryptochromes. Plant Physiol 132: 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP (2004) Phytochrome modulation of blue-light phototropism. Plant Cell Environ 27: 1223–1228 [Google Scholar]
- Wilson RN, Somerville CR (1995) Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol 108: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]