Abstract
To identify the region in which a root perceives a decrease in the ambient water potential and changes its elongation rate, we applied two agar blocks (1 × 1 × 1 mm3) with low water potential bilaterally to primary roots of maize (Zea mays) at various positions along the root. When agar blocks with a water potential of −1.60 MPa (−1.60-MPa blocks) or lower were attached to a root tip, the rate of elongation decreased. This decrease did not result from any changes in the water status of elongating cells and was not reversed when the −1.60-MPa blocks were replaced by −0.03-MPa blocks. The rate decreased slightly and was unaffected, respectively, when −1.60-MPa blocks were applied to the so-called decelerating region of the elongating zone and the mature region. However, the rate decreased markedly and did not recover for several hours at least when such blocks were attached to the accelerating region. In this case, the turgor pressure of the elongating cells decreased immediately after the application of the blocks and recovered thereafter. The decrease in elongation rate caused by −1.60-MPa blocks applied to the root tip was unaffected by additional −0.03-MPa blocks applied to the accelerating region and vice versa. We concluded that a significant reduction in root growth could be induced by water stress at the root tip, as well as in the accelerating region of the elongating zone, and that transmission of some signal from these regions to the decelerating region might contribute to the suppression of cell elongation in the elongation region.
Plants respond to water stress in a variety of ways. Decreased soil moisture can result in various responses, such as decreased cellular growth, suppressed leaf expansion, stomatal closure, a reduction in the rate of photosynthesis, and the accumulation of various osmolytes within cells (Hsiao, 1973; Taiz and Zeiger, 2002). Since cell growth is the most sensitive indicator of water stress in plants (Hsiao, 1973), the growth of plants under water stress has been studied extensively. When water is limited, shoot growth is inhibited more strongly than root growth (Westgate and Boyer, 1985). Consequently, the ratio of root to shoot of plants under water stress increases (Saab et al., 1990; Hsiao and Xu, 2000). Similarly, root growth is also strongly influenced by soil moisture (Sharp et al., 1988; Pardales and Kono, 1990; Mian et al., 1993; Nakamura et al., 2003), but the maintenance of root growth under water stress is important for plant survival because root elongation in drying soil has the added advantage of relieving water stress via the uptake of water from deeper and, thus, moister soil (Serraj and Sinclair, 2002).
Longitudinal root growth is insensitive to low water potential in the more apical regions of roots, which are referred to as the accelerating region of the elongating zone, and it is inhibited to a greater extent in more basal regions, which are referred to as the decelerating region of the elongating zone. Thus, a decrease in water potential is associated with a decrease in the length of the growing zone (Sharp et al., 1988; Pritchard et al., 1991; Akmal and Hirasawa, 2004). In water-stressed wheat (Triticum aestivum) roots, cell turgor returns to levels similar to those in nonstressed plants as a result of osmotic adjustment and remains constant throughout the region of expansion, even in the decelerating region of the elongating zone (Pritchard et al., 1991). In such cases, the loss of turgor pressure is not responsible for the reduction in cell elongation in the root. Loss of susceptibility to expansins is reportedly associated with the cessation of growth in the basal region of the elongation zone in water-stressed primary roots of maize (Zea mays) seedlings (Wu et al., 1996). Cell walls become more extensible (Wu et al., 1996) when the turgor pressure of elongating cells falls slightly, and the increase in observed extensibility has been attributed to the activity of expansins (Wu and Cosgrove, 2000; Wu et al., 2001).
While there have been many studies of the initial stages of the response of roots to water stress, relatively few researchers have examined where and how roots perceive water stress and the mechanisms that affect changes in elongation rates. Changes in levels of gene expression and activity of protein kinase, however, have been reported by Saab et al. (1995) and Conley et al. (1997). As reported in soybean (Glycine max) hypocotyls (Nonami and Boyer, 1990), it is possible that, in the initial growth response to water stress, a reduction in the surrounding water potential affects the turgor pressure of elongating cells, and this reduction causes a change in the elongation rate of the cells. However, it has also been proposed that the survival of a plant depends upon the capacity of root tips to sense and move toward water and other nutrients in the soil (Hawes et al., 2000). In gravitropism and hydrotropism, a root starts to bend after it perceives the direction of gravity or a gradient in water potential. Roots might similarly perceive a reduction in the water potential in the vicinity of the root tip. However, very little is known about the perception of water potential by plants, most likely because of the technical difficulties associated with attempts at imposing water stress at a specific site on a growing root.
Research on root hydrotropism by Takano et al. (1995) demonstrated that hydrotropism can be induced by applying small agar blocks with and without sorbitol unilaterally to the tip of a root. In this study, we employed this system to impose water stress on a specific region of a root by bilaterally applying two small agar blocks with decreased water potential to the primary roots of maize seedlings. In addition, using agar blocks applied at various positions on the root, we examined the effects of water potential on the rate of root elongation. Specifically, we attempted to identify the region of the root that is responsible for the reduction of root elongation that occurs in response to perception of a decrease in water potential. We also investigated the mechanism by which the plant decreases the rate of root elongation in response to the application of agar blocks with decreased water potential by measuring the water status of rapidly elongating and mature cells.
RESULTS
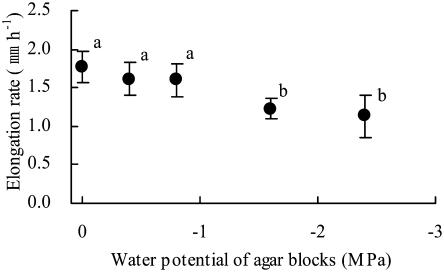
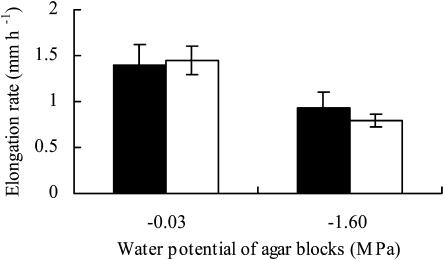
We initially applied agar blocks with various low water potentials to the tips of primary roots (0.5 mm from the apex) of maize (Z. mays L. cv Golden Cross Bantam) seedlings for 5 h and examined their effects on elongation rates (Fig. 1). The application of agar blocks with a water potential of −0.40 or −0.80 MPa did not affect the root elongation rate. However, the rate of elongation decreased significantly when the water potential of agar blocks was −1.60 MPa or lower. When mannitol was used as the osmoticum instead of sorbitol, the effect of agar blocks with a water potential of −1.60 MPa on the rate of root elongation was the same as that when sorbitol was used (data not shown). These results indicated that the elongation rate of maize primary roots was affected by the water potential of agar blocks applied directly to the root tip.
Figure 1.
Growth responses of primary roots to the water potential of pairs of agar blocks applied to the root tip. The graph shows the average elongation rate for 5 h after agar blocks had been applied to the root tip. Bars represent sds (n = 6–7). Letters (a and b) indicate statistically significant differences between treatments (Tukey-Kramer test; P = 0.05).
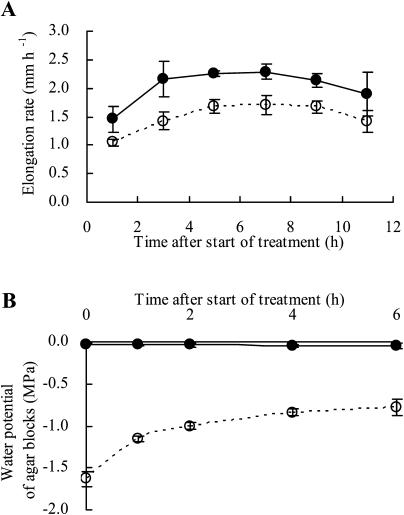
Figure 2 shows elongation rates and water potentials of agar blocks after agar blocks with water potentials of −0.03 and −1.60 MPa (referred to as −0.03- and −1.60-MPa agar blocks, respectively) were applied to root tips. The rate of root elongation increased for a few hours after the application of agar blocks to the root tip, after which the higher rate was maintained for a further 6 h irrespective of the water potential of the agar blocks (Fig. 2A). There were no differences in rates of elongation between roots with and without −0.03-MPa agar blocks (data not shown). The rate was always lower in the case of roots treated with −1.60-MPa agar blocks than with −0.03-MPa blocks. The water potential of −0.03-MPa agar blocks did not change for 6 h after they have been applied to the root tip (Fig. 2B). Conversely, the water potential of the −1.60-MPa agar blocks increased rapidly, before stabilizing, after they had been applied to root tips. The water potential of agar blocks reached −0.80 MPa after 4 h and then hardly increased any further. These findings indicate that the influx of water from the roots to the agar blocks ceased almost completely 4 h after application because the water potential of the agar block was close to that of elongating cells. Given that the −0.80-MPa agar blocks did not affect the root elongation rate (Fig. 1), the observed decrease in the root elongation rate 4 h after the application of −1.60-MPa agar blocks was not due exclusively to the decreased water potential of agar blocks at the root tip.
Figure 2.
Changes in rates of elongation of primary roots (A) and in the water potentials of agar blocks (B) after agar blocks had been applied to the root tip. Eight to 10 agar blocks from four or five roots were used for each measurement. Bars represent sds (n = 3 in A and n = 3–5 in B). Closed and open circles represent water potentials of −0.03 and −1.6 MPa, respectively, for agar blocks applied to roots at the beginning of the treatment.
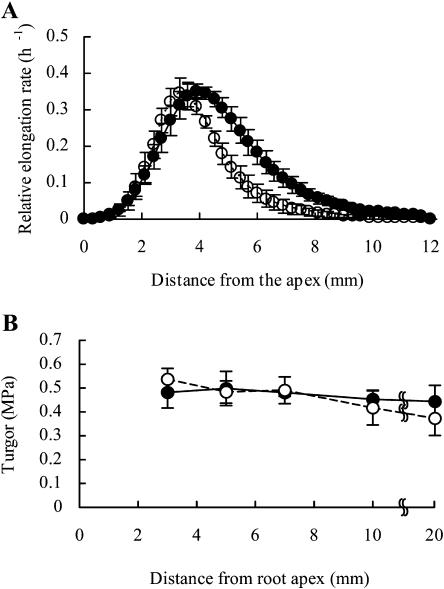
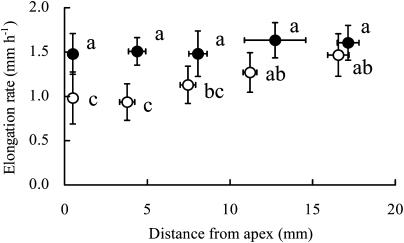
The spatial distribution of the elongation rate (Fig. 3A) and the cell turgor pressure (Fig. 3B) along the axis of a root from 2 to 4 h after agar blocks had been applied to the tip is shown in Figure 3. Upon application of the −0.03-MPa agar blocks, longitudinal elongation occurred from 1 to 9 mm from the tip, and the rate was highest approximately 4 mm from the root apex. By contrast, upon application of the −1.60-MPa agar blocks to the root tip, the relative root elongation rate decreased markedly in the so-called decelerating region of the elongation zone. Extremely limited elongation was observed more than 7 mm from the apex, and the region of maximum elongation shifted to approximately 3 mm from the root apex. However, a high elongation rate was maintained in the accelerating region of the elongating zone. Upon application of −0.03-MPa agar blocks, the turgor pressure along the axis of the root was approximately 0.5 MPa, with minor longitudinal variation (Fig. 3B). There were no differences in cell turgor between roots subjected to treatment with −0.03- and −1.60-MPa agar blocks, not only in the decelerating region of the elongating zone but also in the accelerating region of this zone. However, cell turgor tended to decrease gradually as the distance from the tip increased in roots treated with −1.60-MPa agar blocks. The turgor pressure of elongating and mature cells did not decrease even 30 min after −1.60-MPa agar blocks had been applied to the root tips (Table I).
Figure 3.
Spatial distribution of elongation rates (A) and cell turgor pressures (B) in primary roots. Each root was marked with black ink at 1-mm intervals from the root apex to a distance of 15 to 20 mm from the apex. The elongation rate was derived from the measurements of the displacement of each mark from the apex from 2 to 4 h after the agar blocks had been applied to root tips. Cell turgor pressure was measured at the exodermis or most-external cortex 2 h after the application of agar blocks. Closed and open circles represent water potentials of the agar blocks applied to roots of −0.03 and −1.6 MPa, respectively. Bars represent the sds (n = 3 in A and n = 7–8 in B).
Table I.
Change in turgor pressure (MPa) after agar blocks were applied to root tips
Turgor was measured in the epidermis or exodermis. Measurements were made approximately 5 and 20 mm from the root apex for the elongating and mature regions, respectively. Data represent means ± sd (n = 4–8).
| Region
|
Water Potential of Agar Blocks
|
Time after Start of Treatment
|
||
|---|---|---|---|---|
| 0.5 | 2 | 4 | ||
| Mpa | h | |||
| Elongating | −0.03 | 0.47 ± 0.05 | 0.53 ± 0.03 | 0.44 ± 0.02 |
| −1.60 | 0.42 ± 0.06 | 0.54 ± 0.03 | 0.46 ± 0.07 | |
| Mature | −0.03 | 0.44 ± 0.08 | 0.40 ± 0.05 | 0.33 ± 0.08 |
| −1.60 | 0.39 ± 0.06 | 0.50 ± 0.02 | 0.31 ± 0.08 | |
We observed no differences in the osmotic potential of elongating and mature cells in roots to which −0.03- and −1.60-MPa agar blocks had been applied (Table II). Consequently, there were no differences in calculated water potential between the roots in the case of the elongating cells, as well as the mature cells. Additionally, we measured the water potential of the mature region of a root at 15 to 25 mm from the apex with the isopiestic psychrometer 4 h after −0.03-MPa agar blocks had been applied to the tip and obtained the water potential of −0.24 ± 0.09 MPa (n = 4). The difference between the water potential measured with the psychrometer and that with the cell pressure probe (Table II) was not statistically significant. We also observed with the psychrometer that the water potential of the root tip from the apex to 1 mm was −0.91 ± 0.08 MPa (n = 3) 2 h after −1.6 MPa-agar blocks had been applied to the tip. These results confirm that the values measured with the cell pressure probe indicate the actual water status of cells of a root.
Table II.
Turgor, osmotic potential, and water potential of root tips to which agar blocks had been applied
Turgor and osmotic potential were measured in the mature region (approximately 20 mm from the root apex) and in the elongating region (approximately 5 mm from the root apex) 4 h after agar blocks had been applied to the apex. Data represent means ± sd (n = 4–5).
| Region | Water Potential of Agar Blocks | Turgor | Osmotic Potential | Water Potential |
|---|---|---|---|---|
| MPa | Mpa | Mpa | Mpa | |
| Elongating | −0.03 | 0.44 ± 0.02 | −1.05 ± 0.09 | −0.61 ± 0.10 |
| −1.60 | 0.46 ± 0.07 | −1.16 ± 0.10 | −0.70 ± 0.12 | |
| Mature | −0.03 | 0.33 ± 0.08 | −0.66 ± 0.16 | −0.33 ± 0.17 |
| −1.60 | 0.31 ± 0.08 | −0.62 ± 0.06 | −0.32 ± 0.12 |
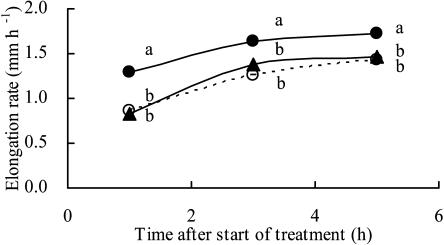
The results in Figure 2 indicated that the effect of −1.60-MPa agar blocks applied to the root tip on the rate of root elongation was maintained even when the water potential of the agar blocks was increased. To confirm this finding, we replaced the −1.60-MPa agar blocks with −0.03-MPa blocks 2 h after the −1.60-MPa agar blocks had been applied to the root tip (Fig. 4). Even when the −0.03-MPa agar blocks were applied to the tip, rates of root elongation did not reach rates similar to those observed when the agar blocks were applied at the start of the measurement.
Figure 4.
Effects of the water potential of agar blocks applied to root tips on the elongation rate of the primary root. Closed and open circles represent root elongation rates for roots with agar blocks with water potentials of −0.03 and −1.60 MPa, respectively. Closed triangles represent the elongation rate of roots to which agar blocks with a water potential of −1.60 MPa were applied for the first 2 h of the experiment. Thereafter, these agar blocks were replaced by blocks with a water potential of −0.03 MPa. Letters (a and b) indicate significant differences between treatments at each time point according to lsd at P = 0.05 (n = 12–22).
We can conclude that root elongation in maize seedlings is suppressed by low water potential due to agar blocks applied to the root tip without any decrease in turgor pressure in elongating cells. Furthermore, it seems likely that the effect of the low water potential of agar blocks on the elongation rate was maintained and could not be reversed in a short period of time if the original agar blocks were replaced by those at a higher water potential.
To identify the sites at which a root perceives low water potential and reduces its elongation rate, we examined the growth response of the primary root to the application of agar blocks to the root surface at various positions along the root axis (Fig. 5). No inhibitory or stimulatory effects on the elongation rate were associated with the application of −0.03-MPa agar blocks. However, the elongation rate decreased significantly when −1.60-MPa agar blocks were applied approximately 0.5 mm (root tip) and 4 mm from the apex. When −1.60-MPa agar blocks were applied 7 mm from the apex, the elongation rate decreased slightly. There was no reduction in the elongation rate when −1.60-MPa agar blocks were applied 13 and 17 mm from the apex. The effect of the application of −1.60-MPa agar blocks at a position of 4 mm from the apex on the spatial distribution of elongation rates along the root axis was similar (data not shown) to the effect of the application of similar blocks to the root tip, as shown in Figure 3. These results indicate that the primary root of a maize seedling perceives the low water potential of agar blocks at both the root tip and in the elongating region, but not in the mature region of the root, and decreases its rate of elongation. It is noteworthy that both the perception of water stress and the inhibitory effects of root growth were not marked in the decelerating region, as compared with those in the accelerating region, although the reduction in the elongation rate was more marked in the decelerating region than it was in the accelerating region. Immediately after −1.60-MPa agar blocks had been applied to the accelerating region of the elongating zone, the turgor pressure of the cells that were elongating at that position decreased (Table III) but then recovered completely. The application of −1.60-MPa agar blocks did not reduce the turgor pressure of the cells in the decelerating and mature regions. These results indicate that the marked reduction in cell elongation in the decelerating region was not caused by the reduction in the turgor pressure of cells when −1.60-MPa agar blocks were applied to the accelerating region. Furthermore, the effect associated with the application of −1.60-MPa agar blocks on the turgor pressure of cells was limited to the position at which the agar blocks were applied and the effect was temporary. The same results were obtained when −1.60-MPa agar blocks were applied to the root tip (data not shown).
Figure 5.
Growth responses of primary roots to the water potential of agar blocks applied to the root surface at various positions along the root axis. Elongation rates were averaged for the first 3 h after the start of treatment. Closed and open circles represent −0.03- and −1.60-MPa agar blocks, respectively. Bars represent sds (n = 4–15). Letters (a, b, and c) indicate statistically significant differences between treatments (Tukey-Kramer test; P = 0.01).
Table III.
Changes in turgor pressure (MPa) when agar blocks were applied to the surface of the accelerating region of the elongating zone of the root (3 to 4 mm from the apex at the start of the experiment)
Turgor was measured in either the epidermis or exodermis. Measurements were made at the sites at which the agar blocks had been applied, 6 and 20 mm (mature region) from the root apex. Data represent means ± sd (n = 4–8). The asterisk indicates significant difference at the 1% level between treatments (Student's t test).
| Region
|
Water Potential of Agar Blocks
|
Time after Start of Treatment
|
||
|---|---|---|---|---|
| 0.5 | 2 | 4 | ||
| Mpa | h | |||
| 4 mm from the apex | −0.03 | 0.51 ± 0.05 | 0.44 ± 0.08 | 0.51 ± 0.04 |
| −1.60 | 0.29 ± 0.05* | 0.47 ± 0.08 | 0.46 ± 0.09 | |
| 6 mm from the apex | −0.03 | 0.50 ± 0.02 | 0.50 ± 0.04 | 0.48 ± 0.02 |
| −1.60 | 0.49 ± 0.02 | 0.52 ± 0.03 | 0.48 ± 0.02 | |
| Mature | −0.03 | 0.46 ± 0.06 | 0.41 ± 0.03 | 0.46 ± 0.03 |
| −1.60 | 0.39 ± 0.06 | 0.46 ± 0.05 | 0.43 ± 0.03 | |
When agar blocks with a low water potential were applied to a decapped root tip, the rate of elongation decreased to the same extent as it did when the root had a root cap (Fig. 6).
Figure 6.
Effects of the removal of the root cap on the elongation rate of roots treated with −0.03- and −1.60-MPa agar blocks, respectively. Black and white columns represent capped and decapped roots, respectively. Bars represent the sds (n = 5–13). Differences are not statistically significant.
As shown in Table IV, we examined the effect of the water potential of agar blocks, applied bilaterally to both the root tip and the elongating region, on the elongation rate of the primary root. When −0.03- and −1.60-MPa agar blocks were applied to the root tip and 4 mm from the apex, respectively, the effect of −1.60-MPa agar blocks on the root elongation rate was not overcome by −0.03-MPa agar blocks. Similarly, when −1.60- and −0.03-MPa agar blocks were applied to the root tip and 4 mm from the root apex, respectively, the effect of −1.60-MPa agar blocks was also not overcome by the −0.03-MPa agar blocks. When −1.60-MPa agar blocks were applied both to the root tip and 4 mm from the apex, the rate of root elongation tended to decrease, although the extent of the reduction was not statistically significant.
Table IV.
Effects of the water potential of agar blocks applied to the root tip (0.5 mm from root apex) and the elongating region (4 mm from root apex) on the elongation rate of primary roots
Data represent means ± sd (n = 4–5). Letters (a and b) indicate statistically significant differences between treatments (Tukey-Kramer test; P = 0.05).
| Water Potential of Agar Blocks
|
Elongation Rate
|
|
|---|---|---|
| 0.5 mm | 4 mm | |
| Mpa | mm h−1 | |
| −0.03 | −0.03 | 1.63 ± 0.31 a |
| – | −1.60 | 1.01 ± 0.13 b |
| −0.03 | −1.60 | 0.98 ± 0.17 b |
| −1.60 | – | 1.07 ± 0.31 b |
| −1.60 | −0.03 | 1.12 ± 0.20 b |
| −1.60 | −1.60 | 0.80 ± 0.18 b |
DISCUSSION
Soil water influences root growth (Sharp et al., 1988; Pardales and Kono, 1990; Mian et al., 1993; Nakamura et al., 2003). However, little is known about the early phases of root growth in response to exposure to water stress. Consequently, our first goal was to identify the region responsible for the perception of a decrease in the ambient water potential by applying agar blocks with low water potential, bilaterally, at various positions on a root. We observed the largest reductions in rates of root elongation when we applied the blocks to the root tip and the accelerating region (Fig. 5). However, we observed very minor or no effects at all when we applied the blocks to the decelerating region and the mature region, respectively (Fig. 5). The decrease in the rate of root elongation was marked in the decelerating region even when agar blocks with low water potential were applied at the root tip (Fig. 3) or in the accelerating region (data not shown). These results imply that the cells in the accelerating region and at the root tip perceive the low water potential of agar blocks and somehow suppress the rate of elongation in the cells in the decelerating region. To our knowledge, this is the first report on the perception of water stress by a root and the effects of such perception on the rate of elongation, even though it is well known that root cap cells act as sensors of gravity and of moisture gradients.
The growth rate of roots is regulated by a combination of the expansion and the production of cells (Beemster and Baskin, 1998). Moreover, cell expansion appears to be more sensitive in general to water stress than cell production (Hsiao, 1973). The rate of root elongation decreased in the decelerating region of the elongating zone soon after agar blocks with a low water potential had been applied to the root tip or to the accelerating region (Fig. 2). However, a high rate of root elongation was maintained in the accelerating region close to the apical meristem where cell division is vigorous (Fig. 3). These findings suggest that the decrease in root elongation induced by agar blocks with a low water potential resulted from the suppression of cell elongation rather than the suppression of cell division.
Turgor pressure decreased temporarily in tissues when agar blocks with a low water potential were applied to the accelerating region (Table III) and the root tip (data not shown). This decrease was due to the large influx of water into agar blocks. The amount of water that flowed into agar blocks relative to the amount of water transported to the elongation region of the root was estimated to be about 45% during the hour that followed the application of the agar blocks (data not shown). The relative amount decreased markedly thereafter, and the actual flow of water stopped 4 h after the application (Fig. 2). The application of agar blocks with a low water potential did not affect the turgor pressure of cells located 2 mm away, in the basal direction, from the blocks (Table III). The turgor pressure of cells in the decelerating region was maintained while the rate of cell elongation decreased markedly (Table III; Fig. 3B). Therefore, it seems likely that a temporary decrease in cell turgor pressure at the root tip and in the accelerating region might act as a stimulus of water stress, and when this stimulus is transmitted to the decelerating region, elongation is suppressed.
In both gravitropism and hydrotropism, after the perception of gravity or a water potential gradient in the root cap, a stimulus is transmitted to the elongating zone that results in differential growth (Takahashi, 1997; Blancaflor and Masson, 2003). Gravitropism and hydrotropism are not observed when root caps have been removed (Shaw and Wilkins, 1973; Jaffe et al., 1985; Takahashi and Scott, 1993). However, in this study, when agar blocks with a low water potential were applied to a decapped root tip, the rate of elongation was observed to decrease to the same extent as it did in the case of roots with a root cap (Fig. 6). This observation indicates that the perception of water stress by a root involves a mechanism that is different from the mechanisms of gravitropism and hydrotropism. Ishikawa and Evans (1995) found that cells in the distal elongation zone have special physiological properties, that is, they respond to a variety of signals such as gravistimulation and thigmostimulation. Our results indicate that the cells that correspond to the distal elongation zone also function in the perception of hydrostimulation but do not change their elongation rates.
The growth rate of plant cells is often assessed in terms of extensibility of the cell wall, yield threshold, turgor pressure, hydraulic conductivity, and growth-induced water potential (Lockhart, 1965; Cosgrove, 1993; Boyer, 2001). Differential growth is induced by a change in cell wall extensibility and hydraulic conductivity in both gravitropism (Muday, 2001; Miyamoto et al., 2005) and hydrotropism (Hirasawa et al., 1997; Miyamoto et al., 2002). In a root grown under water stress, the rate of cell elongation decreases via a reduction in turgor pressure (Spollen and Sharp, 1991) or in cell wall extensibility (Pritchard et al., 1991; Akmal and Hirasawa, 2004). In this research, we observed no differences in turgor pressure and growth-induced water potential, as expressed by the difference in water potential between mature and elongating cells, between roots to which agar blocks at −0.03 and −1.60 MPa had been applied bilaterally (Tables II and III). We can conclude, therefore, that the decrease in root elongation induced by agar blocks with a low water potential might be caused by changes in cell wall extensibility and/or yield threshold as well as by a reduction in the hydraulic conductivity of the cells or tissues. Further research is required if we are to understand the mechanism involved in the decrease of root elongation.
The mechanism of signal transmission in this study can be compared with the mechanism responsible for transmission of the signal in gravitropism. Starch-containing amyloplasts in the columella region of the root cap are important for the sensing of gravity (Blancaflor and Masson, 2003). The sedimentation of amyloplasts in the statocysts is transformed into a chemical signal that is mediated by calcium ions and auxin (Sinclair and Trewavas, 1997; Muday, 2001) and moves along the actin cytoskeleton (Blancaflor and Masson, 2003; Hou et al., 2003). In hydrotropism, when a moisture gradient is perceived by the root cap (Jaffe et al., 1985; Takahashi and Scott, 1993), signal transmission might involve calcium ions, auxin, and abscisic acid (Takahashi, 1997; Takahashi et al., 2002). These signals might induce changes in cell wall extensibility and hydraulic conductivity, resulting finally in differential growth. When agar blocks with a low water potential are used to induce a decrease in root elongation, it seems plausible that some signal might be mediated by the temporary reduction in the turgor pressure of the cells at the root tip or in the accelerating region and that the signal might be transmitted to the cells in the decelerating region. As a result, the properties of cell walls and hydraulic conductivity might change, with consequent suppression of cell elongation in the decelerating region. However, little is known about the nature of such a signal. It is noteworthy that the suppressive effect of the temporary reduction in turgor pressure on elongation was maintained for several hours during our experiments (Fig. 2A). Furthermore, once the suppression of growth had been induced, the effect could not be overcome by simply replacing the original agar blocks with blocks at a higher water potential (Fig. 4; Table IV). These observations indicate that cells at the tip and in the accelerating region of a root can sense water stress and that they might emit some elongation-inhibitory signal to the cells in the decelerating region via a temporary decrease in the turgor pressure of the cells at the tip and in the accelerating region. Further research is required to characterize the mechanisms of signal transduction and their effects on cell elongation.
Finally, let us consider the regulation of root elongation and the maintenance of cell turgor pressure in the soil. When a root grows into soil with a decreased water potential, the turgor pressure of the root tip might decrease, causing a reduction in the rate of root elongation. This reduction might lead to accumulation of solutes in the elongation region, as well as in the mature region, and might maintain the turgor of the cells, as noted by Akmal and Hirasawa (2004). This function of the root tip might be important for roots that are growing in soil with a moisture deficit, protecting cells from a marked reduction in turgor pressure.
MATERIALS AND METHODS
Plant Materials
Seeds of maize (Zea mays L. cv Golden Cross Bantam) were allowed to germinate on wet filter paper in a moisture-saturated petri dish at 25°C in darkness. Seedlings with straight primary seminal roots of 35 to 50 mm in length were used in all experiments. Each seedling was installed in a glass chamber (40 × 120 × 20 mm3). The root was mounted vertically in the chamber. Each seed was wrapped with wet absorbent paper (Kimwipe S-200; Jyujo Kimberly) to keep the seedling hydrated. Wet filter paper was affixed to the inner walls of the chamber to maintain a water-saturated atmosphere in the chamber. The chamber was placed in a vapor-saturated cooler box. In some experiments, several seedlings were suspended vertically from a wet urethane block (140 × 105 × 50 mm3) with an insect pin in the seed, and the urethane block was suspended in the vapor-saturated acrylic chamber (200 × 250 ×210 mm3). All experiments were performed in a constant-environment room at 25°C.
Hydrostimulation of Roots
A pair of agar blocks (1 × 1 × 1 mm3) with known water potential was applied bilaterally and directly to the surface of individual roots (Takano et al., 1995; Miyamoto et al., 2002). Agar for plant culture (Wako Chemicals) at a concentration of 1% (w/v) was prepared in 2 mm MES buffer. The water potential of agar blocks was decreased by adding a given amount of sorbitol to the agar solution. Before the application of the agar blocks to a root, the mucigel on the root surface was gently blotted with absorbent paper (Kimwipe S-200). The absorption of mucigel from the root had no effect on the root elongation rate.
Water potentials of agar blocks were measured with an isopiestic psychrometer (Boyer and Knipling, 1965). More than eight agar blocks were used for each series of measurements, as described by Miyamoto et al. (2002).
Measurement of Root Elongation Rate
A root was marked gently with black ink (YMSCRI-BK; Zebra) at a distance of approximately 11 to 15 mm from root apex in the mature region. Images of the marked root were made using a Quick microscope (VH-5000; Keyence) at 2-h intervals. Digital images were stored on a personal computer (DynaBook T3; Toshiba). The distance from the mark to the root tip was measured with image analysis software (SigmaScan; Jandel Scientific), and the rate of root elongation was calculated.
For the determination of the root elongation profile, a root was marked gently with black ink at intervals of approximately 1 mm from the root apex in a vapor-saturated cooler box. Pictures of the root were taken at 2-h intervals with the Quick microscope. The displacement of marks from the root apex was measured with the image analysis software. The relative elongation rates along a root were calculated as described by Morris and Silk (1992). We performed preliminary experiments to compare the rate of root elongation upon exposure to light during photography with the rate of root elongation in the absence of light. We also compared the elongation of roots with and without marks on their root surfaces. Neither light nor marking of roots had any effect on root elongation.
Measurements of the Turgor Pressure and Osmotic Potential of Individual Cells
Cell turgor and osmotic potential were determined in either the epidermis or the exodermis. A pressure probe was used to measure cell turgor (Hüsken et al., 1978). For the measurement of cell turgor pressure, we used a glass chamber with a small slit on the right side for insertion of the capillary tube of the probe into the chamber. After the insertion of the capillary, the slit was covered with wet absorbent cotton to keep the humidity inside the chamber at saturation level. Fiber optic illumination (LA-150SAE; Hayashi Watch Works) was employed to monitor the position of a meniscus between the cell solution and the oil in the microcapillary of the pressure probe.
After cell turgor had been determined, the pressure in the capillary tube was decreased rapidly to about 0 MPa for extraction of cell sap. The osmotic potential of the cell sap was determined with a nanoliter freezing point osmometer as described by Malone et al. (1989) and Malone and Tomos (1992). The cell water potential was obtained as the algebraic sum of the turgor pressure and osmotic potential for a given cell.
This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 14656006) and by a Grant-in-Aid (Bio Cosmos Program) from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062091.
References
- Akmal M, Hirasawa T (2004) Growth responses of seminal roots of wheat seedlings to a reduction in the water potential of vermiculite. Plant Soil 267: 319–328 [Google Scholar]
- Beemster GTS, Baskin TI (1998) Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116: 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol 133: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS (2001) Growth-induced water potentials originate from wall yielding during growth. J Exp Bot 52(360): 1483–1488 [DOI] [PubMed] [Google Scholar]
- Boyer JS, Knipling EB (1965) Isopiestic technique for measuring leaf water potentials with a thermocouple psychrometer. Proc Natl Acad Sci USA 54: 1044–1051 [PMC free article] [PubMed] [Google Scholar]
- Conley TR, Sharp RE, Walker JC (1997) Water deficit rapidly stimulates the activity of a protein kinase in the elongation zone of the maize primary root. Plant Physiol 113: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ (1993) Wall extensibility: its nature, measurement and relationship to plant cell growth. New Phytol 124: 1–23 [DOI] [PubMed] [Google Scholar]
- Hawes MC, Gunawardena U, Miyasaka S, Zhao X (2000) The role of root border cells in plant defence. Trends Plant Sci 5: 128–132 [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Takahashi H, Suge H, Ishihara K (1997) Water potential, turgor and cell wall properties in elongating tissues of the hydrotropically bending roots of pea (Pisum sativum L.). Plant Cell Environ 20: 381–386 [Google Scholar]
- Hou G, Mohamalawari DR, Blancaflor EB (2003) Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol 131: 1360–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24: 519–570 [Google Scholar]
- Hsiao TC, Xu L (2000) Sensitivity of growth of roots versus leaves to water stress: biophysical analysis and relation to water transport. J Exp Bot 51: 1595–1616 [DOI] [PubMed] [Google Scholar]
- Hüsken D, Steudle E, Zimmermann U (1978) Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol 61: 158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML (1995) Specialized zones of development in roots. Plant Physiol 109: 725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe MJ, Takahashi H, Biro RL (1985) A pea mutant for the study of hydrotropism in roots. Science 230: 445–447 [DOI] [PubMed] [Google Scholar]
- Lockhart JA (1965) An analysis of irreversible plant cell elongation. J Theor Biol 8: 264–275 [DOI] [PubMed] [Google Scholar]
- Malone M, Leigh RA, Tomos AD (1989) Extraction and analysis of sap from individual wheat leaf cells: the effect of sampling speed on the osmotic pressure of extracted sap. Plant Cell Environ 12: 919–926 [Google Scholar]
- Malone M, Tomos AD (1992) Measurement of gradients of water potential in elongating pea stem by pressure probe and picolitre osmometry. J Exp Bot 43: 1325–1331 [Google Scholar]
- Mian MAR, Nafziger ED, Kolb FL, Teyker RH (1993) Root growth of wheat genotypes in hydroponic culture and in the greenhouse under different soil moisture regimes. Crop Sci 33: 283–286 [Google Scholar]
- Miyamoto N, Katsuhara M, Ookawa T, Kasamo K, Hirasawa T (2005) Hydraulic conductivity and aquaporins of cortical cells in gravitropically bending roots of Pisum sativum L. Plant Prod Sci 8 (in press)
- Miyamoto N, Ookawa T, Takahashi H, Hirasawa T (2002) Water uptake and hydraulic properties of elongation cells in hydrotropically bending roots of Pisum sativum L. Plant Cell Physiol 43: 393–401 [DOI] [PubMed] [Google Scholar]
- Morris AK, Silk WK (1992) Use of a flexible logistic function to describe axial growth of plants. Bull Math Biol 54: 1069–1081 [Google Scholar]
- Muday GK (2001) Auxins and tropisms. J Plant Growth Regul 20: 226–243 [DOI] [PubMed] [Google Scholar]
- Nakamura E, Ookawa T, Ishihara K, Hirasawa T (2003) Effects of soil moisture depletion for one month before flowering on dry matter production and ecophysiological characteristics of wheat plants in wet soil during grain filling. Plant Prod Sci 6: 195–205 [Google Scholar]
- Nonami H, Boyer JS (1990) Primary events regulating stem growth at low water potentials. Plant Physiol 94: 1601–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardales JR Jr, Kono Y (1990) Development of sorghum root system under increasing drought stress. Jpn J Crop Sci 59: 752–761 [Google Scholar]
- Pritchard J, Jones RGW, Tomos AD (1991) Turgor, growth and rheological gradients of wheat roots following osmotic stress. J Exp Bot 42: 1043–1049 [Google Scholar]
- Saab IN, Ho T-HD, Sharp RE (1995) Translatable RNA populations associated with maintenance of primary root elongation and inhibition of mesocotyl elongation by abscisic acid in maize seedlings at low water potentials. Plant Physiol 109: 593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J, Voetberg GS (1990) Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol 93: 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR (2002) Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant Cell Environ 25: 333–341 [DOI] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC (1988) Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiol 87: 50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S, Wilkins MB (1973) The source and lateral transport of growth inhibitors in geotropically stimulated roots of Zea mays and Pisum sativum. Planta 109: 11–26 [DOI] [PubMed] [Google Scholar]
- Sinclair W, Trewavas AJ (1997) Calcium in gravitropism. A re-examination. Planta Suppl 203: S85–S90 [DOI] [PubMed] [Google Scholar]
- Spollen WG, Sharp RE (1991) Spatial distribution of turgor and root growth at low water potentials. Plant Physiol 96: 438–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E (2002) Plant Physiology, Ed 3. Sinauer Associates, Sunderland, MA, pp 591–623
- Takahashi H (1997) Hydrotropism: the current state of our knowledge. J Plant Res 110: 163–169 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Goto N, Okada K, Takahashi H (2002) Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta 216: 203–211 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Scott TK (1993) Intensity of hydrostimulation for the induction of root hydrotropism and its sensing by the root cap. Plant Cell Environ 16: 99–103 [DOI] [PubMed] [Google Scholar]
- Takano M, Takahashi H, Hirasawa T, Suge H (1995) Hydrotropism in roots: sensing of a gradient in water potential by the root cap. Planta 197: 410–413 [Google Scholar]
- Westgate ME, Boyer JS (1985) Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize. Planta 164: 540–549 [DOI] [PubMed] [Google Scholar]
- Wu Y, Cosgrove DJ (2000) Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot 51: 1543–1553 [DOI] [PubMed] [Google Scholar]
- Wu Y, Sharp RE, Durachko DM, Cosgrove DJ (1996) Growth maintenance of the maize primary root at low water potentials involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiol 111: 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Thorne ET, Sharp RE, Cosgrove DJ (2001) Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiol 126: 1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]