Abstract
The effect of water stress on respiration and mitochondrial electron transport has been studied in soybean (Glycine max) leaves, using the oxygen-isotope-fractionation technique. Treatments with three levels of water stress were applied by irrigation to replace 100%, 50%, and 0% of daily water use by transpiration. The levels of water stress were characterized in terms of light-saturated stomatal conductance (gs): well irrigated (gs > 0.2 mol H2O m−2 s−1), mildly water stressed (gs between 0.1 and 0.2 mol H2O m−2 s−1), and severely water stressed (gs < 0.1 mol H2O m−2 s−1). Although net photosynthesis decreased by 40% and 70% under mild and severe water stress, respectively, the total respiratory oxygen uptake (Vt) was not significantly different at any water-stress level. However, severe water stress caused a significant shift of electrons from the cytochrome to the alternative pathway. The electron partitioning through the alternative pathway increased from 10% to 12% under well-watered or mild water-stress conditions to near 40% under severe water stress. Consequently, the calculated rate of mitochondrial ATP synthesis decreased by 32% under severe water stress. Unlike many other stresses, water stress did not affect the levels of mitochondrial alternative oxidase protein. This suggests a biochemical regulation (other than protein synthesis) that causes this mitochondrial electron shift.
Water stress is considered one of the most important factors limiting plant performance and yield worldwide (Boyer, 1982). Effects of water stress on a plant's physiology, including growth (McDonald and Davies, 1996), signaling pathways (Chaves et al., 2003), gene expression (Bray, 1997, 2002), and leaf photosynthesis (Lawlor and Cornic, 2002; Flexas et al., 2004), have been studied extensively. Surprisingly, compared with other physiological processes, studies examining the effects of water stress on respiration are few (Hsiao, 1973; Amthor, 1989), despite the importance of respiration in ecosystem annual net productivity (Valentini et al., 1999) and the fact that ecosystem respiration is strongly affected by water availability (Bowling et al., 2002). Another important point to consider is the effect of water stress on the electron partitioning between the cytochrome and the cyanide-resistant, alternative pathway and its consequences for ATP synthesis. Unfortunately, the few studies that have focused on alternative respiration as affected by water stress (Zagdańska, 1995; Collier and Cummins, 1996; González-Meler et al., 1997) used specific inhibitors for the cytochrome (KCN) and alternative (salicylhydroxamic acid [SHAM]) respiratory pathways; this methodology is now known to be invalid (Millar et al., 1995; Day et al., 1996; Lambers et al., 2005). Currently, the only available system to measure electron partitioning between the two respiratory pathways and their actual activities is the use of the oxygen-isotope-fractionation technique (Day et al., 1996; McDonald et al., 2003; Ribas-Carbo et al., 2005). This technique is based on the fact that the two terminal oxidases fractionate 18O differently, with the cytochrome oxidase discriminating less than the alternative oxidase (AOX; Guy et al., 1989). This differential fractionation is the basis of a methodology that allows the assessment of the electron partitioning between the cytochrome and alternative respiratory pathways in the absence of added inhibitors (Guy et al., 1989; Robinson et al., 1992, 1995; Ribas-Carbo et al., 1995, 1997; Gastón et al., 2003). Using this technique, it has been observed that the electron transport through the alternative pathway increases under phosphate limitation (González-Meler et al., 2001), during chilling recovery (Ribas-Carbo et al., 2000), and by the application of allelochemicals (Peñuelas et al., 1996) or herbicides inhibiting branched-chain amino acid synthesis (Gastón et al., 2003).
The biochemical regulation of the electron partitioning between the cytochrome and alternative pathways is quite complex because the two pathways that compete for electrons from the ubiquinone pool have their own regulation (Finnegan et al., 2004; Sluse and Jarmuszkiewicz, 2004; Lambers et al., 2005). The cytochrome pathway, including Complex III and Complex IV, is strongly regulated by the proton gradient between the matrix and the intermembrane space, which in turn is directly affected by the mitochondrial “respiratory control” or ATP/ADP ratio (Millenaar and Lambers, 2003). Changes in the ATP/ADP ratio could result from a change in the kinetics of the ATPase or from a change in the balance between ATP synthesis and demand. Furthermore, the cytochrome c oxidase can be inhibited by natural inhibitors such as carbon monoxide, cyanide, sulfide, or nitric oxide among others (Moore and Siedow, 1991; Millar and Day, 1996). On the other hand, the alternative pathway has several mechanisms of biochemical regulation. AOX can be activated by pyruvate and other α-ketoacids (Millar et al., 1993). Furthermore, the AOX in plants is present as a dimer and is regulated by a disulfide/sulfhydryl system (Umbach and Siedow, 1993; Lennon et al., 1995; Hiser et al., 1996; Vanlerberghe et al., 1999), with the two subunits linked by disulfide bridges and with the reduced form being the active form and the oxidized form being almost inactive (Umbach and Siedow, 1993, 1996; Ribas-Carbo et al., 1997; Vanlerberghe et al., 1999). It has been suggested that a mitochondrial thioredoxin might be involved in the regulation of the redox status of this disulfide bridge (Umbach and Siedow, 1993). Finally, the maximum capacity of the AOX is determined by the total amount of AOX protein present (Vanlerberghe et al., 1994). The expression of the AOX has previously been shown to increase under several stress situations (Millenaar and Lambers, 2003; Lambers et al., 2005), such as low temperature (Stewart et al., 1990; Vanlerberghe and McIntosh, 1992), low phosphate availability (Parsons et al., 1999; Juszczuk et al., 2001), inhibition of the cytochrome pathway (Wagner et al., 1992), the application of inhibitors of mitochondrial protein synthesis (Day et al., 1996), and herbicides inhibiting branched-chain amino acid synthesis (Aubert et al., 1997). This increase is remarkably strong under conditions where the production of reactive oxygen species (ROS), especially superoxide, occurs (Wagner and Krab, 1995). Water stress, particularly combined with light, increases the risk of oxidative stress by increasing the presence of ROS in different cell compartments (Sgherri et al., 1993; Sgherri and Navari-Izzo, 1995; Bartoli et al., 2004). Under these conditions, the alternative respiratory path could play a role in preventing the formation of damaging ROS. A possible role of the alternative pathway is in protecting plants from ROS or in sustaining respiration under situations where the cytochrome pathway is restricted (Wagner and Krab, 1995; Lennon et al., 1997; Lambers et al., 2005). It has also been proposed that the cyanide-resistant respiratory pathway could be involved in the prevention of the formation of ROS (Purvis, 1997; Maxwell et al., 1999) and programmed cell death (Vanlerberghe et al., 2002). Finally, it has been suggested that ROS could be part of a signal or communication pathway indicating that there is a restriction of the activity of the cytochrome pathway (Wagner and Krab, 1995; Foyer and Noctor, 2003).
Oxygen isotope fractionation measurements were combined with a suite of other measurements (respiration rate, photosynthetic rate, stomatal conductance, analysis of AOX, and porin proteins) to obtain a comprehensive view of the effect of water stress on leaf respiration at the protein and mitochondrial electron transport level.
RESULTS AND DISCUSSION
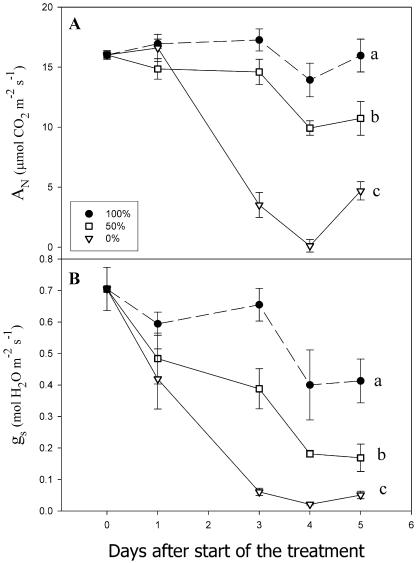
Water stress, induced by controlled watering, caused a progressive and concomitant decrease in net photosynthesis (AN) and light-saturated stomatal conductance (gs) in soybean (Glycine max) leaves (Fig. 1, A and B). The severity of water stress has often been assessed by its effect on relative water content (RWC), leaf water potential (ψ), gs, or even AN. However, Flexas et al. (2004) demonstrated that gs would be a more sensitive indicator of the severity of water stress in leaves, at least for comparative purposes in studies of photosynthesis. Generally, most metabolic down-regulation of photosynthesis occurs before any change in RWC can be detected (Tardieu and Simonneau, 1998). Therefore, to study the effects of water stress on respiration and mitochondrial electron partitioning and on AOX protein abundance, three different degrees of irrigation (100%, 50%, and 0%) were selected to achieve three gs intervals defined by Flexas et al. (2004): control (gs > 0.2 mol H2O m−2 s−1), mild water stress (gs between 0.1 and 0.2 mol H2O m−2 s−1), and severe water stress (gs < 0.1 mol H2O m−2 s−1).
Figure 1.
Response of photosynthesis (A) and stomatal conductance (B) to water withdrawal. One-month-old soybean plants were watered with 100% (black circles), 50% (white squares), and 0% (white triangles) of the daily weight loss. Values are the average and errors bars are se of five to 15 plants. Different letters denote significant differences (P < 0.05), at day 5, according to Duncan's multiple range test.
The Effect of Water Stress on Leaf Respiration
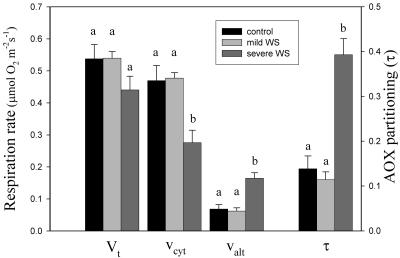
Respiration rates are presented per unit leaf area for comparison with rates of AN (Fig. 2). Respiration averaged 0.54 μmol O2 m−2 s−1 in control and mildly water-stressed plants. Under severe water stress, respiration was slightly lower (0.44 μmol O2 m−2 s−1), although not significantly different. To the best of our knowledge, no previous study has analyzed the response of leaf respiration to water stress in soybean plants. Nevertheless, this observation agrees with some published studies in other species (Lawlor, 1976; Loboda, 1993; Flexas et al., 2005). However, several other authors have found different results on the effect of water stress on respiration, ranging from decrease (Brix, 1962; Brown and Thomas, 1980; Palta and Nobel, 1989; Escalona et al., 1999; Ghashghaie et al., 2001; Haupt-Herting et al., 2001) to stimulation (Upchurch et al., 1955; Shearmann et al., 1972; Zagdańska, 1995). In all cases, as in this study, changes in respiration were much less than those in photosynthesis, causing a significant increase in the respiration/photosynthesis ratio under water stress, indicating that the role of respiration becomes more important as water stress develops.
Figure 2.
Effect of different levels of water stress on total respiration (Vt), the activities of the cytochrome (vcyt), and alternative (valt) pathways and the partitioning through the alternative pathway (τa).
The Effect of Water Stress on the Mitochondrial Electron Partitioning
Severe water stress induced large and significant changes on oxygen-isotope fractionation and, consequently, on the partitioning of electrons between the cytochrome and alternative pathways (Table I; Fig. 2). This shift in fractionation shows that the participation of the cyanide-resistant alternative respiratory pathway (τa) increased from 0.14 under well-watered conditions to 0.39 under severe water stress (Fig. 2). Both pathways responded in opposite ways to severe water stress; while the cytochrome pathway declined significantly from 0.47 to 0.28 μmol O2 m−2 s−1 (Fig. 2), the alternative pathway increased from 0.07 to 0.17 μmol O2 m−2 s−1 (Fig. 2).
Table I.
Changes in RWC and oxygen-isotope fractionation during respiration (Δ 18O) in soybean leaves under well-irrigated (gs > 0.2 mol H2O m−2 s−1), mild water-stress (0.1 < gs < 0.2 mol H2O m−2 s−1), and severe water-stress conditions (gs < 0.1 mol H2O m−2 s−1)
Means followed by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
| Well Watered | Mild Water Stress | Severe Water Stress | |
|---|---|---|---|
| RWC | 90.3 ± 0.8 a | 87.3 ± 2.2 a | 75.9 ± 2.6 b |
| Δ 18O (‰) | 20.5 ± 0.3 a | 20.2 ± 0.2 a | 23.5 ± 0.4 b |
Previous observations on the effect of water stress on the partitioning between the two respiratory pathways using specific inhibitors showed inconclusive results. Zagdańska (1995) observed in wheat that the SHAM-resistant cytochrome pathway increased under water stress, while Collier and Cummins (1996) showed in Saxifraga cernua that the CN-resistant component of respiration decreased as water deficit increased. On the other hand, Gonzalez-Meler et al. (1997) showed in bean and pepper leaves that water stress decreased SHAM-resistant respiration, with no effect on KCN-resistant respiration. However, it is now well known that specific inhibitors cannot be used to assess the electron partitioning between the two respiratory pathways (Millar et al., 1995; Day et al., 1996; Lambers et al., 2005). Consequently, many of these discrepancies might be due to a shift of electrons from one pathway to the other upon addition of a specific inhibitor of either of the two pathways. Furthermore, several authors have shown that, under different stress situations, changes in electron partitioning between the two respiratory pathways were mostly due to a decrease in the activity of the cytochrome pathway rather than an increase in the activity of the alternative pathway (Peñuelas et al., 1996; Lambers et al., 2005). However, this was not the case in this study, where an important increase in the activity of the alternative pathway coincided with a decrease in the cytochrome pathway.
The Effect of Water Stress on AOX Protein Levels
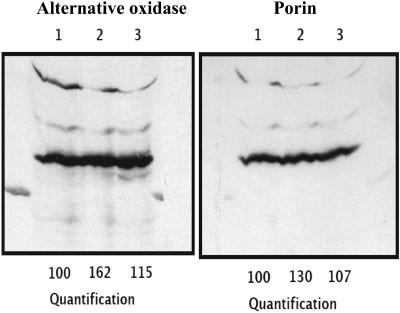
In a parallel experiment, carried out at a different time and location, the effect of water stress on mitochondrial AOX protein abundance was studied both in soybean leaves and in mitochondria isolated from soybean leaves under control conditions and at two levels of water stress (mild and severe). Although in leaves it appeared that a mild water stress caused a slight increase in AOX protein abundance (Fig. 3), this increase was mainly due to a minor increase in total mitochondrial protein, as shown by the anti-porin western blot (Fig. 3). Therefore, from these results it can be concluded that water stress did not significantly affect AOX protein amount. This finding is supported by the results obtained with mitochondria isolated from leaves, where no significant changes were observed between well-watered and water-stressed plants (data not shown).
Figure 3.
Immunoblots of AOX protein and porin in leaves from soybean under different watering regimes. Lane 1, control, 100% watering; lane 2, mild water stress, 50% watering; lane 3, severe water stress, 0% watering. Three leaves per treatment were collected at day 5 after the start of the treatment (Fig. 1). Seventy-five microliters of extracted protein (approximately 18.75 mg FW) were loaded onto each lane. Quantified data are also shown.
The present results seem to contradict previous observations that indicate that stresses, in general, increase AOX protein abundance (Millenaar and Lambers, 2003; Lambers et al., 2005), especially under conditions where the production of ROS occurs (Wagner and Krab, 1995), as is the case under water stress (Sgherri et al., 1993; Sgherri and Navari-Izzo, 1995). The effects observed under severe water stress only resemble those described in studies of the effects of different light intensities. In these studies, the observed increases in the electron partitioning between the two respiratory pathways under high light (Noguchi et al., 2001) also were not accompanied by increases in AOX protein abundance (Noguchi et al., 2005).
ATP Yield and Production
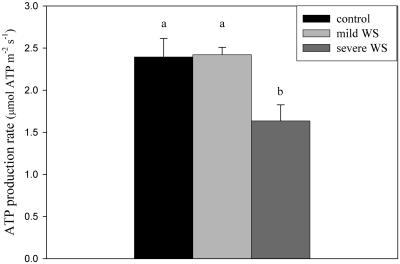
An important aspect of the effect of water stress on the activity of the two respiratory pathways is the change in ATP production. There is no ATP production when electrons are transported from the ubiquinone pool to the AOX (Moore and Siedow, 1991). Therefore, any increase of the cyanide-resistant alternative pathway activity at the expense of the cytochrome path implies a lower ATP yield per oxygen consumed. Figure 4 shows the results of a calculation of the rate of ATP synthesis, based on measured activities of the two respiratory pathways. This calculation assumes that Complex I consumes all mitochondrial NADH and that there are no leaks across the inner membrane (Amthor, 1994; Noguchi et al., 2001). Figure 4 shows that there was a significant decrease in mitochondrial ATP synthesis under severe water stress in soybean leaves. Under well-watered and mild water-stress conditions, the rate of ATP synthesis was about 2.4 μmol ATP m−2 s−1, while under severe water stress the rate of ATP production dropped to about 1.6 μmol ATP m−2 s−1.
Figure 4.
Calculated rate of mitochondrial ATP synthesis under well-watered, mild water-stress, and severe water-stress conditions in soybean leaves. The rate of ATP synthesis was calculated according to the following formula (Amthor, 1994; Noguchi et al., 2001): ATP synthesis = (29/6 × vcyt) + (11/6 × valt). Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan's multiple range test.
Final Considerations
The relative importance of respiration in the plant's overall biochemistry becomes relatively more important under water-stress situations, when photosynthesis declines. As shown in Figure 1A, photosynthesis decreased by about 70% while the respiration rate was not significantly affected under severe water stress (Fig. 2). This relative importance of respiration is even more significant because severe water stress changes the electron partitioning between the two respiratory pathways (Fig. 2), and, as a consequence, the rate of mitochondrial ATP synthesis decreases by 32% (Fig. 4). The response of ATP synthesis to severe water stress in this study agrees with a similar response of the ATP concentration in leaves, as presented by Flexas et al. (2004), using data compiled from the literature. Tezara et al. (1999) interpreted the decline in leaf ATP concentration during water stress as an indicator of impaired photophosphorylation, which was considered the main factor limiting photosynthesis under water stress. However, the close agreement of the present results with those published by Flexas et al. (2004) suggests an additional interpretation: Reduced leaf ATP concentrations in leaves under severe water stress may, at least partially, reflect changes in the partitioning of electrons between the cytochrome and the alternative pathways in the mitochondria.
The cytochrome and AOX are at the end of two branches in the respiratory electron transport chain. As reviewed above, this electron transport can be regulated by (1) the input to the chain from glycolysis and other catabolic pathways; (2) the kinetics of the AOX; and (3) the kinetics of the cytochrome pathway. If any one of these factors were to change, the rate of electron flow through the system and the partitioning to the two oxidases could all change. Total respiration (Fig. 2), total soluble sugar concentrations (data not shown), and AOX protein content (Fig. 3) were not affected by water stress. It is likely that starch levels declined due to the decrease in rate of photosynthesis under severe water stress, and this decrease could account for the decrease in the activity of the cytochrome pathway. However, it cannot explain the increase in the activity of the alternative pathway. Therefore, the change in mitochondrial electron partitioning must be the result of subtle biochemical controls operating on the kinetics of the enzymes in these pathways. In principle, the observed increase in the activity of the alternative pathway with a concomitant decrease in the activity of the cytochrome pathway could be caused by either an activation of the alternative pathway that would allow AOX to withdraw electrons from the cytochrome pathway by competition or by an inhibition of the cytochrome pathway that would induce an increase on the redox status of the ubiquinone pool to a level that would be able to donate electrons to the alternative pathway. In isolated mitochondria, activation of the AOX by pyruvate or by an increase on the reduction status of the disulfide bridge caused an increase in AOX activity but also an increase in total oxygen uptake (Ribas-Carbo et al., 1995, 1997). The unchanged rate of total respiration (Fig. 2) could indicate that in intact tissues there is a strong biochemical control upstream of the ubiquinone pool, even under severe water stress. On the other hand, the observed increase in the activity of the alternative pathway without any increase in total AOX protein could also be due to an increase in the redox status of the ubiquinone pool (Qr/Qt). In general, an increase in Qr/Qt would result in an increase of the activities of both cytochrome and alternative pathways (Dry et al., 1989; Moore and Siedow, 1991; Millenaar et al., 1998). However, this was not the case under severe water stress, where the increase in alternative pathway activity was concomitant with a decrease in the cytochrome pathway activity. Such differential decrease of the cytochrome pathway activity might be explained by two mechanisms: a direct inhibition of the cytochrome oxidase, as observed by Peñuelas et al. (1996) in studies with allelochemicals, or an increase in the ATP/ADP ratio due to a significant decrease in ATP demand, which would somehow simulate a transition from state 3 to state 4 in the mitochondrial electron transport (Krab, 1995). From our results, the latter mechanism seems the most likely to occur because a decreased demand for ATP is to be expected under severe water stress as a consequence of the observed decreases in growth, photosynthesis, and photorespiration (Flexas and Medrano, 2002). This increase in alternative path activity should certainly maintain a lower redox status of the ubiquinone pool and, consequently, reduce the formation of ROS, which has been described one of the main negative effects of severe water stress (Hsiao, 1973).
In this study, we measured respiration in the dark during the day, which might change substrate supply and sink demand compared with normal nocturnal conditions. However, the present results do emphasize the important role that respiration plays in the plant's carbon balance and, consequently, in ecosystem annual net productivity (Valentini et al., 1999) and crop production (Amthor, 1989), and that the efficiency of respiration is modified under severe water stress.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Soybean (Glycine max) seeds were treated with 0.5% (v/v) NaOCl for 10 min and allowed to swell in distilled water for 2 h with continuous bubbling of air. Seeds were planted in separate trays of a mixture of sand and perlite (1:1) and placed in a growth chamber at constant temperature (25°C) and 600 μmol m−2 s−1 with a 14:10 light:dark regime. Seedlings were watered twice a day. Five days after germination, plants were placed in 2-L pots and watered daily with standard nutrient solution for 30 d. Plants were then watered to field capacity, weighed, and divided into three groups with different watering regimes. Thereafter, they were watered with 100%, 50%, and 0% of the daily consumed water to achieve different degrees of water stress.
Two experiments were carried out in parallel, one for the respiration measurements and the other for AOX protein analysis in intact leaves and isolated mitochondria. Although both experiments were separated in time and place, plants were grown under similar conditions and watered according to the same procedure. AN and gs were measured in both parallel experiments, to determine the degree of water stress.
Gas-Exchange Measurements
AN and gs were measured with an open gas-exchange system (Li-6400; LI-COR). Analyses were done on the youngest, fully mature leaves. Measuring conditions were 1,500 μmol photons m−2 s−1, 25°C, and a relative humidity near 50%.
Respiratory Measurements on Intact Tissues
For respiratory analyses, a plant was placed in the dark for 30 min after gas-exchange analysis to avoid any light-enhanced dark respiration. Then, the same leaf area used for photosynthesis measurements was cut into a leaf disc, immediately weighed (fresh weight), and placed in the closed respiration chamber for respiration analysis.
Total respiration as well as cytochrome and alternative respiratory activities were determined using a gas-phase system connected to a dual-inlet mass spectrometer. Leaf discs (0.2–0.3 g fresh weight) were placed in a 3-mL stainless-steel, closed cuvette. All experiments were carried out at controlled room temperature (25°C).
Oxygen-isotope fractionation during respiration was measured as described by Gastón et al. (2003). The measuring system consisted of a 3-mL stainless-steel closed cuvette from which 200 μL of air was sequentially withdrawn and fed into a dual-inlet mass spectrometer sample bellows. The mass spectrometer (Finnigan Delta S; Thermo Finnigan) simultaneously measured m/z 34/32 (18O2/16O2) and m/z 32/28 (O2/N2) ratios. These values were obtained from the sample and the standard air with a dual-inlet analysis with four replicate cycles of each respiration measurement.
During inhibitory treatments, either 1.0 mm KCN (in 1 mm TES, pH = 8) or 10 mm SHAM (in water from a 1.0 m stock in dimethylsulfoxide) was applied by sandwiching the plant tissues between medical wipes soaked with the corresponding inhibitor. No recovery from inhibitor treatment was observed, as respiratory rates were constant throughout the experiment. All stocks were freshly prepared before measurement. In addition, for KCN experiments, a piece of tissue wetted with KCN was present in the cuvette. Calculations of isotopic fractionation were made as described by Guy et al. (1989) and Ribas-Carbo et al. (1995), without argon correction, and electron partitioning between the two pathways in the absence of inhibitors was calculated as described by Guy et al. (1989). The r2 values of all unconstrained linear regressions between −ln f and ln (R/Ro), with a minimum of five data points, were at least 0.995, considered minimally acceptable (Ribas-Carbo et al., 1995, 1997; Lennon et al., 1997).
The electron partitioning through the alternative pathway (τa) was calculated as follows:
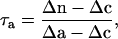 |
where Δn, Δc, and Δa are the isotope fractionation in the absence of inhibitors, in the presence of SHAM, and in the presence of KCN, respectively. Δc and Δa were 18.9‰ and 30.7‰, respectively. The individual activities of the cytochrome (vcyt) and alternative pathway (valt) were obtained from multiplying the total oxygen uptake (Vt) by the partitioning to each pathway as follows.
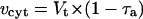 |
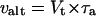 |
The formula used for calculating mitochondrial ATP production assumes that there is no proton leak across the inner mitochondrial membrane and that all mitochondrial NADH is oxidized by Complex I (Amthor, 1994; Noguchi et al., 2001).
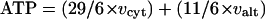 |
Isolation of Mitochondria
Mitochondria were isolated according to the methods of Day et al. (1985) and Taylor et al. (2002), from 20 to 60 g of leaves. Leaves were disrupted with a Polytron (Kinematica) in 250 mL of cold extraction medium (0.3 m Suc, 25 mm tetra-sodium pyrophosphate, 10 mm KH2PO4, 2 mm EDTA, 1% [w/v] PVP-40, 1% [w/v] bovine serum albumin [BSA], 20 mm ascorbate, pH 7.5). The homogenate was filtered through four layers of Miracloth and centrifuged for 5 min at 1,100g. The supernatant was centrifuged for 20 min at 18,000g, and the pellet was resuspended in 200 mL of wash medium (0.3 m Suc, 10 mm TES, 0.1% [w/v] BSA, pH 7.5), and centrifuged for 5 min at 1,100g. The supernatant was centrifuged for 20 min at 18,000g, and the pellet was resuspended in 10 mL of wash medium. Aliquots of 5 mL were then layered over 27.5 mL of solution (0.3 m Suc, 10 mm TES, 0.1% [w/v] BSA, 28% and 32% [v/v] Percoll, and a linear gradient of 0%–4.4% [w/v] PVP-40, pH 7.5) in a centrifuge tube, and centrifuged for 40 min at 40,000g. The mitochondria were found as a tight, light-yellow-brown band near the bottom of the tube. The mitochondrial fraction was removed and diluted in 250 mL of wash medium, and centrifuged at 31,000g for 15 min. The supernatant was removed, and this wash was repeated. The final mitochondrial pellet was resuspended in approximately 1 mL of wash medium.
Protein Analysis
Protein concentrations were determined by the method of Peterson (1977), using BSA as a standard.
SDS-PAGE and Western Blotting
For isolated mitochondria, aliquots containing 60 μg protein were solubilized in sample buffer (2% [w/v] SDS, 62.5 mm Tris-HCl, pH 6.8, 10% [v/v] glycerol, 0.002% [w/v] bromphenol blue, 10% [v/v] β-mercaptoethanol) and boiled for 5 min and loaded per lane for SDS-PAGE. For whole-tissue extracts, 250 mg fresh weight of leaves was snap frozen in liquid nitrogen, and the sample was crushed to a fine powder with a mortar and pestle. Samples were then solubilized in 1 mL of the sample buffer, boiled for 5 min, and centrifuged at 10,000g for 5 min; 75 μL of the supernatant was loaded per lane for SDS-PAGE.
Prepared samples were separated by electrophoresis under denaturing reducing conditions on 0.1% (w/v) SDS, 12% (w/v) polyacrylamide gels according to Laemmli (1970). For immunoreaction experiments, proteins were electroblotted from SDS-PAGE gels onto Hybond-C+ extra membrane (Amersham Pharmacia Biotech) using a Hoefer Semi-Phor semidry blotting apparatus (Amersham Pharmacia Biotech) and blocked. Mitochondrial proteins were probed with the primary antibodies, anti-AOX serum (Elthon et al., 1989), and 1/100 dilution of anti-porin serum. Chemiluminescence was used for detection of horseradish peroxidase-conjugated secondary antibodies and visualized using LAS 1000 (Fuji). The blots were quantified using the Image Gauge v3.0 software (Fuji) with the control band denoted as 100%; other bands were calculated relative to that value.
Statistical Analysis
The results presented are the means with standard errors of five to 15 replicates. Means were compared by one-way ANOVA and Duncan's multiple range test at the 5% level of significance using SPSS (version 10.0).
Acknowledgments
We thank Pepi Martín and Beth Guy for their great technical assistance and continuous support of this research, Dr. Mike Shane for his unconditional support and help at the University of Western Australia, Dr. Tim Colmer for the analysis of leaf soluble sugars, and Dr. Josep Cifre for the statistical analysis. We are grateful to our colleagues Drs. Josep Argilés and Sharon A. Robinson for their helpful discussion, critical comments, and sustained support.
This work was supported by the Spanish Ministry of Science and Technology (MCyT grant no. BFI2002–00772) and the Australian Research Council. This is Carnegie Institution of Washington Department of Global Ecology publication number 96.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.065565.
References
- Amthor J (1989) Respiration and Crop Productivity. Springer-Verlag, New York
- Amthor J (1994) Respiration and carbon assimilate use. In KJ Boote, JM Bennett, TR Sinclair, GM Paulsen, eds, Physiology and Determination of Crop Yield. American Society of Agronomy, Madison, WI, pp 221–250
- Aubert S, Bligny R, Day DA, Whelan J, Douce R (1997) Induction of alternative oxidase synthesis by herbicides inhibiting branched-chain amino acid synthesis. Plant J 11: 649–657 [Google Scholar]
- Bartoli CG, Gomez F, Martinez DE, Guiamet JJ (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55: 1663–1669 [DOI] [PubMed] [Google Scholar]
- Bowling DR, McDowell NG, Bond BJ, Law BE, Ehleringer JR (2002) C-13 content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia 131: 113–124 [DOI] [PubMed] [Google Scholar]
- Boyer JS (1982) Plant productivity and environment. Science 218: 443–448 [DOI] [PubMed] [Google Scholar]
- Bray E (1997) Plant responses to water deficit. Trends Plant Sci 2: 48–54 [Google Scholar]
- Bray E (2002) Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ 25: 153–161 [DOI] [PubMed] [Google Scholar]
- Brix H (1962) The effect of water stress on the rates of photosynthesis and respiration in tomato plants and loblolly pine seedlings. Physiol Plant 15: 10–20 [Google Scholar]
- Brown KW, Thomas JC (1980) The influence of water stress preconditioning on dark respiration. Physiol Plant 49: 205–209 [Google Scholar]
- Chaves MM, Maroco JP, Pereira J (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30: 239–264 [DOI] [PubMed] [Google Scholar]
- Collier DE, Cummins WR (1996) The rate of development of water deficits affects Saxifraga cernua leaf respiration. Physiol Plant 96: 291–297 [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT (1996) The cyanide-resistant oxidase: To inhibit or not to inhibit, that is the question. Plant Physiol 110: 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Neuburger M, Douce R (1985) Biochemical-characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol 3: 219–228 [Google Scholar]
- Dry IB, Moore AL, Day DA, Wiskich JT (1989) Regulation of alternative pathway activity in plant mitochondria: nonlinear relationship between electron flux and redox poise of the ubiquinone pool. Arch Biochem Biophys 273: 148–157 [DOI] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L (1989) Monoclonal-antibodies to the alternative oxidase of higher-plant mitochondria. Plant Physiol 89: 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalona JM, Flexas J, Medrano H (1999) Stomatal and non-stomatal limitations of photosynthesis under water stress in field grown grapevine. Aust J Plant Physiol 26: 421–433 [Google Scholar]
- Finnegan PM, Soole KL, Umbach AL (2004) Alternative mitochondrial electron transport proteins in higher plants. Chapter 9. In DA Day, AH Millar, J Whelan, eds, Plant Mitochondria: From Genome to Function, Vol 17. Advances in Photosynthesis and Respiration Series. Kluwer Academics Publishers, Dordrecht, The Netherlands, pp 163–230
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 6: 269–279 [DOI] [PubMed] [Google Scholar]
- Flexas J, Galmes J, Ribas-Carbo M, Medrano H (2005) The effects of water stress on plant respiration. Chapter 6. In H Lambers, M Ribas-Carbo, eds, Plant Respiration: From Cell to Ecosystem, Vol 18. Advances in Photosynthesis and Respiration Series. Springer, Dordrecht, The Netherlands, pp 85–94
- Flexas J, Medrano H (2002) Energy dissipation in C3 plants under drought. Funct Plant Biol 29: 1209–1215 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 199: 355–364 [Google Scholar]
- Gastón S, Ribas-Carbo M, Busquets S, Berry JA, Zabalza A, Royuela M (2003) Changes in mitochondrial electron partitioning in response to herbicides inhibiting branched-chain amino acid biosynthesis in soybean. Plant Physiol 133: 1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaie J, Duranceau M, Badeck FW, Cornic G, Adeline M-T, Deleens E (2001) δ13C of CO2 respired in the dark in relation to δ13C of leaf metabolites: comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ 24: 505–515 [Google Scholar]
- González-Meler MA, Giles L, Thomas RB, Siedow JN (2001) Metabolic regulation of leaf respiration and alternative pathway activity in response to phosphate supply. Plant Cell Environ 24: 205–215 [Google Scholar]
- González-Meler MA, Matamala R, Peñuelas J (1997) Effects of prolonged drought stress and nitrogen deficiency on the respiratory O2 uptake of bean and pepper leaves. Photosynthetica 34: 505–512 [Google Scholar]
- Guy RD, Berry JA, Fogel ML, Hoering TC (1989) Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 177: 483–491 [DOI] [PubMed] [Google Scholar]
- Haupt-Herting S, Klug K, Fock HP (2001) A new approach to measure gross CO2 fluxes in leaves. Gross CO2 assimilation, photorespiration, and mitochondrial respiration in the light in tomato under drought stress. Plant Physiol 126: 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser C, Kapranov P, McIntosh L (1996) Genetic modification of respiratory capacity in potato. Plant Physiol 110: 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao T (1973) Plant responses to water stress. Annu Rev Plant Physiol 24: 519–570 [Google Scholar]
- Juszczuk IM, Malusa E, Rychter AM (2001) Oxidative stress during phosphate deficiency in roots of bean plants (Phaseolus vulgaris). J Plant Physiol 158: 1299–1305 [Google Scholar]
- Krab K (1995) Kinetic and regulatory aspects of the function of the alternative oxidase in plant respiration. J Bioenerg Biomembr 27: 387–396 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lambers H, Robinson SA, Ribas-Carbo M (2005) Regulation of respiration in vivo. Chapter 1. In H Lambers, M Ribas-Carbo, eds, Plant Respiration: From Cell to Ecosystem, Vol 18. Advances in Photosynthesis and Respiration Series. Springer, Dordrecht, The Netherlands, pp 1–15
- Lawlor DW (1976) Water stress induced changes in photosynthesis, photorespiration, respiration and CO2 compensation concentration of wheat. Photosynthetica 10: 378–387 [Google Scholar]
- Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25: 275–294 [DOI] [PubMed] [Google Scholar]
- Lennon AM, Neueschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN (1997) The effects of salicylic acid and TMV infection upon the alternative oxidase of tobacco. Plant Physiol 115: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon AM, Pratt J, Leach G, Moore AL (1995) Developmental regulation of respiratory activity in pea leaves. Plant Physiol 107: 925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loboda T (1993) Gas exchange of different spring cereal genotypes under normal and drought conditions. Photosynthetica 29: 567–572 [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96: 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AE, Sieger SM, Vanlerberghe GC (2003) Methods and approaches to study plant mitochondrial alternative oxidase. Physiol Plant 116: 135–143 [DOI] [PubMed] [Google Scholar]
- McDonald AJS, Davies WJ (1996) Keeping in touch: responses of the whole plant to deficits in water and nitrogen supply. Adv Bot Res 22: 229–300 [Google Scholar]
- Millar AH, Atkin OK, Lambers H, Wiskich JT, Day DA (1995) A critique of the use of inhibitors to estimate partitioning of electrons between mitochondrial respiratory pathways in plants. Physiol Plant 95: 523–532 [Google Scholar]
- Millar AH, Day DA (1996) Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Lett 398: 155–158 [DOI] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA (1993) Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett 329: 259–262 [DOI] [PubMed] [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H (1998) The role of the alternative oxidase in stabilizing the in vivo reduction of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol 118: 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Lambers H (2003) The alternative oxidase: in vivo regulation and function. Plant Biol 5: 2–15 [Google Scholar]
- Moore AL, Siedow JN (1991) The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta 1059: 121–140 [DOI] [PubMed] [Google Scholar]
- Noguchi K, Go CS, Terashima I, Ueda S, Yoshinari T (2001) Activities of the cyanide-resistant respiratory pathway in leaves of sun and shade species. Aust J Plant Physiol 28: 27–35 [Google Scholar]
- Noguchi K, Taylor NL, Millar AH, Lambers H, Day DA (2005) Response of mitochondria to light intensity in the leaves of sun and shade species. Plant Cell Environ 28: 760–771 [Google Scholar]
- Palta JA, Nobel P (1989) Root respiration for Agave deserti: influence of temperature, water status and root age on daily patterns. J Exp Bot 40: 181–186 [Google Scholar]
- Parsons HL, Yip JYH, Vanlerberghe GC (1999) Increased respiratory restriction during phosphate-limited growth in transgenic tobacco lacking alternative oxidase. Plant Physiol 121: 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J, Ribas-Carbo M, Giles L (1996) Effects of allelochemicals on plant respiration and oxygen isotope fractionation by the alternative oxidase. J Chem Ecol 22: 801–805 [DOI] [PubMed] [Google Scholar]
- Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83: 346–356 [DOI] [PubMed] [Google Scholar]
- Purvis AC (1997) Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiol Plant 100: 165–170 [Google Scholar]
- Ribas-Carbo M, Aroca R, Gonzalez-Meler MA, Irigoyen JJ, Sanchez-Diaz M (2000) The electron partitioning between the cytochrome and alternative respiratory pathways during chilling recovery in two cultivars differing in chilling sensitivity. Plant Physiol 122: 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN (1995) Electron partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol 109: 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Lennon AM, Robinson SA, Giles L, Berry J, Siedow JN (1997) The regulation of the electron partitioning between the cytochrome and alternative pathways in soybean cotyledon and root mitochondria. Plant Physiol 113: 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M, Robinson SA, Giles L (2005) The application of the oxygen-isotope technique to assess respiratory pathway partitioning. Chapter 3. In H Lambers, M Ribas-Carbo, eds, Plant Respiration: From Cell to Ecosystem, Vol 18. Advances in Photosynthesis and Respiration Series. Springer, Dordrecht, The Netherlands, pp 31–42
- Robinson SA, Ribas-Carbo M, Yakir D, Giles L, Reuveni Y, Berry JA (1995) Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen isotope discrimination. Aust J Plant Physiol 22: 487–496 [Google Scholar]
- Robinson SA, Yakir D, Ribas-Carbo M, Giles L, Osmond CB, Siedow JN, Berry JA (1992) Measurements of the engagement of cyanide-resistant respiration in the Crassulacean acid metabolism plant Kalanchoë daigremontiana with the use of on-line oxygen isotope discrimination. Plant Physiol 100: 1087–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgherri CLM, Navari-Izzo F (1995) Sunflower seedlings subjected to increasing water deficit stress: oxidative stress and defence mechanisms. Physiol Plant 93: 25–30 [Google Scholar]
- Sgherri CLM, Pinzino C, Navari-Izzo F (1993) Chemical changes and O2− production in thylakoid membranes under water stress. Physiol Plant 87: 211–216 [Google Scholar]
- Shearmann LL, Esatin JD, Sullivan CY, Kinbacher EJ (1972) Carbon dioxide exchange in water-stressed maize sorghum. Crop Sci 12: 406–409 [Google Scholar]
- Sluse F, Jarmuszkiewicz W (2004) Regulation of electron transport in the respiratory chain of plant mitochondria. Chapter 10. In DA Day, AH Millar, J Whelan, eds, Plant Mitochondria: From Genome to Function, Vol 17. Advances in Photosynthesis and Respiration Series. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 231–246
- Stewart CR, Martin BA, Reding L, Cerwick S (1990) Respiration and alternative oxidase in corn seedlings tissues during germination at different temperatures. Plant Physiol 92: 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modeling isohydric and anisohydric behaviours. J Exp Bot 49: 419–432 [Google Scholar]
- Taylor NL, Day DA, Millar AH (2002) Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J Biol Chem 45: 42663–42668 [DOI] [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401: 914–917 [Google Scholar]
- Umbach AL, Siedow JN (1993) Covalent and noncovalent dimers of the cyanide-resistant oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol 103: 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN (1996) The reaction of the soybean cotyledon mitochondrial cyanide-resistant oxidase with sulfhydryl reagents suggests that the a-keto acid activation involves the formation of a thiohemiacetal. J Biol Chem 271: 25019–25026 [DOI] [PubMed] [Google Scholar]
- Upchurch RP, Peterson ML, Hagan RM (1955) Effect of soil-moisture content on the rate of photosynthesis and respiration in ladino clover (Trifolium repens L.). Plant Physiol 30: 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini R, Matteucci G, Dolman AJ, Schulze E-D, Rebmann C, Moore EJ, Granier A, Gross P, Jensen NO, Pilegaard K, et al (1999) Respiration as the main determinant of carbon balance in European forests. Nature 404: 861–865 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1992) Lower growth temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiol 100: 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Robson CA, Yip JYH (2002) Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiol 129: 1829–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Vanlerberghe AE, McIntosh L (1994) Molecular genetic alteration of plant respiration. Silencing and overexpression of alternative oxidase in transgenic tobacco. Plant Physiol 106: 1503–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Yip JYH, Parsons HL (1999) In organello and in vivo evidence of the importance of the regulatory sulfhydryl/disulfide system and pyruvate for alternative oxidase activity in tobacco. Plant Physiol 121: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Krab K (1995) The alternative respiration pathway in plants: role and regulation. Physiol Plant 95: 318–325 [Google Scholar]
- Wagner AM, Van Emmerik WAM, Zwiers JH, Kaagman HMCM (1992) Energy metabolism of Petunia hybrida cell suspensions growing in the presence of antimycin A. In H Lambers, LHW Van der Plas, eds, Molecular, Biochemical and Physiological Aspects of Plant Respiration. SPB Academic Publishing, The Hague, The Netherlands, pp 609–614
- Zagdańska B (1995) Respiratory energy demand for protein turnover and ion transport in wheat leaves upon water demand. Physiol Plant 95: 428–436 [Google Scholar]