Abstract
Transcript levels of mRNA from 1-deoxy-d-xylulose 5-phosphate reductoisomerase (PcDXR), isoprene synthase (PcISPS), and phytoene synthase (PcPSY) showed strong seasonal variations in leaves of Grey poplar (Populus × canescens [Aiton] Sm.). These changes were dependent on the developmental stage and were strongly correlated to temperature and light. The expression rates of the genes PcDXR and PcISPS were found to be significantly correlated to each other, whereas the expression of the PcPSY gene showed a different seasonal pattern. Protein concentration and enzyme activity of PcISPS showed distinct seasonal patterns peaking in late summer, whereas highest transcription levels of PcISPS were observed in early summer. Moreover, correlation between PcISPS protein concentration and enzyme activity changed, in particular in autumn, when PcISPS protein levels remained high while enzyme activity declined, indicating posttranslational modifications of the enzyme. The positive correlation between dimethylallyl diphosphate levels and PcISPS protein content was found to be consistent with the demonstrated synchronized regulation of PcDXR and PcISPS, suggesting that metabolic flux through the 1-deoxy-d-xylulose 5-phosphate pathway and isoprene emission capacity are closely intercoordinated. Transcript levels of PcISPS showed strong diurnal variation with maximal values before midday in contrast to PcDXR, whose gene expression exhibited no clear intraday changes. During the course of a day, in vitro PcISPS activities did not change, whereas leaf dimethylallyl diphosphate levels and isoprene emission showed strong diurnal variations depending on actual temperature and light profiles on the respective day. These results illustrate that the regulation of isoprene biosynthesis in Grey poplar leaves seems to happen on transcriptional, posttranslational, and metabolic levels and is highly variable with respect to seasonal and diurnal changes in relation to temperature and light.
Isoprene (2-methyl-1,3-butadiene) is a volatile organic compound naturally emitted by many tree species and has a significant influence on atmospheric chemistry (Thompson, 1992; Biesenthal et al., 1997; Derwent et al., 1998). Different hypotheses are discussed to explain the functional role of isoprene emission for the plant itself, either to prevent leaf metabolic processes from thermal (Sharkey and Singsaas, 1995; Singsaas et al., 1997; for overview, see Sharkey and Yeh, 2001) and oxidative stress (Loreto and Velikova, 2001; Loreto et al., 2001) or to serve as an overflow mechanism for excess of carbon intermediates or photosynthetic energy (Logan et al., 2000; Rosenstiel et al., 2004).
Isoprene emission is controlled at least at two different levels. Intraday variations of isoprene emissions can be explained on the one hand by the synthesis of isoprene synthase (ISPS) substrate, mainly originating from recently fixed CO2. On the other hand, they are caused by the temperature dependence of ISPS activity (Eisenreich et al., 2001; Brüggemann and Schnitzler, 2002a), reflecting the temperature response of isoprene emission (Monson et al., 1992). The correlation with photosynthetic photon flux densities (PPFDs) is consistent with the light saturation response of photosynthetic processes (Sharkey and Loreto, 1993). Other environmental factors have minor influence on short-term variations of leaf isoprene emission (Guenther et al., 1993).
So far, the unraveling of the control of isoprene formation has been mainly focused on biochemical and physiological investigations. They show that long-term, seasonal variations in isoprene emission capacity (Monson et al., 1994, 1995) can be explained by variations in the amount of active ISPS (Kuzma and Fall, 1993; Schnitzler et al., 1997; Lehning et al., 2001). This basal emission capacity depends on physiological adaptations, e.g. leaf expansion and developmental stage, or leaf position within the canopy (sun or shade; Sharkey et al., 1996). It seems to be triggered by temperature (Monson et al., 1994), nutrition (Rosenstiel et al., 2004), atmospheric CO2 (Rosenstiel et al., 2003; Scholefield et al., 2004), or genetic disposition (Isebrands et al., 1999; Lehning et al., 2001).
The identification of the regulatory steps of the 1-deoxy-d-xylulose 5-phosphate (DOXP) pathway is of major importance in the study of isoprene biosynthesis. Currently, not much is known about the regulation of genes involved in isoprene biosynthesis. Very recently, Wiberley et al. (2005) found that transcription of ISPS and translation of this gene began at the same time in developing leaves of kudzu (Pueraria montana [Willd.] Maesen and S. Almeida) as isoprene emission starts to increase. Furthermore, the same study showed that high temperature stimulated not only gene expression of ISPS and isoprene emission but also accelerated the development of photosynthetic competence of the leaves.
In plastids, isopentenyl diphosphate and dimethylallyl diphosphate (DMADP) are formed via the DOXP pathway. The first step is catalyzed by 1-deoxy-d-xylulose 5-phosphate synthase (DXS), which is encoded by the DXS gene. The following conversion of DOXP to methyl erythritol 5-phosphate is catalyzed by 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR), the product of the DXR gene. DOXP is a precursor not only for isoprenoids but also for thiamine diphosphate and pyridoxal phosphate (Julliard and Douce, 1991; Julliard, 1992). The reaction catalyzed by DXR is actually the first specific step of the DOXP pathway. Therefore, DXR could play an important role in the control of plastidic isoprenoid biosynthesis.
However, detailed information on transcriptional regulation at different stages of plastidic isoprenoid biosynthesis during leaf development and the growing season is still lacking. In particular, comprehensive analysis of gene expression of ISPS related to protein level, enzyme activity, concentration of DMADP, and isoprene emission has not been investigated.
This study was therefore conducted in Grey poplar (Populus × canescens [Aiton] Sm.) leaves to examine whether transcript levels of PcDXR and PcISPS follow diurnal and seasonal changes. Another aim was to determine if these changes are related to diurnal and seasonal variations of PcISPS activity and the level of its substrate DMADP. In addition, we investigated their relationship to the diurnal variations of isoprene emission and seasonal variation of phytoene synthase gene (PcPSY) expression (encoding a key enzyme in carotenoid biosynthesis [Von Lintig et al., 1997], a pathway competing for DOXP products).
RESULTS
Isolation and Functional Analysis of PcDXR cDNA from Grey Poplar
To isolate a full-length cDNA clone encoding DXR from Grey poplar, initially a 309-bp segment was amplified by PCR using heterologous oligonucleotide primers for conserved sequences of other known DXR genes from plants. The resulting sequence of the PCR product showed highest homology to DXR from Lycopersicon esculentum Mill. (EMBL AF331705: 82.03% identity) on the nucleic acid level. Using this segment as a probe for hybridization with a poplar cDNA library, a cDNA clone was isolated harboring an open reading frame (ORF) of 1,724 bp with high homology to other known DXR genes, e.g. Arabidopsis (Arabidopsis thaliana; EMBL AF148852: 86.23%), Catharanthus roseus L. G. Don (EMBL AF250235: 86.23%), L. esculentum (EMBL AF331705: 84.75%), and Zea mays (EMBL AJ297566: 82.20%). The deduced amino acid sequence consisted of 472 amino acids and a putative molecular mass of about 51 kD. The EMBL accession number of the complete cDNA is AJ574852.
The coding sequence, including the sequence for the putative plastidic transit peptide at the 5′-end, was amplified by PCR, introducing Gateway recombination sequences, and cloned into the expression vector pDEST17 to transform Escherichia coli BL21-Star. The heterologously expressed PcDXR enzyme was purified under native conditions. Protein extracts were assayed indirectly for PcDXR activity by measuring spectrophotometrically the oxidation of NADPH to NADP. The apparent PcDXR activity of the heterologously expressed protein was 6.87 mkat kg−1 protein. Enzyme specificity was further tested by the addition of fosmidomycin (1 mm), a competitive inhibitor of DXR, to the enzyme assay. This application resulted in an inhibition of approximately 85% of PcDXR activity, confirming data on DXR from other species (e.g. the cyanobacterium Synechococcus leopoliensis; Miller et al., 2000).
Seasonal Variation of Transcript Levels of Plastidic Isoprenoid Genes in Relation to ISPS Activity, Levels of DMADP, and Levels of Photosynthetic Pigments
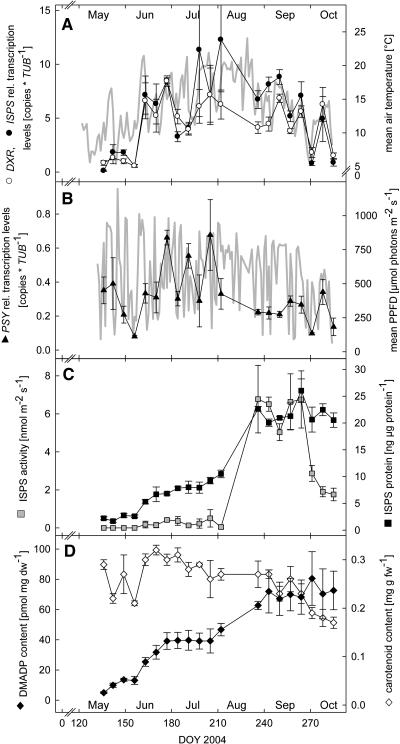
The present molecular biological and biochemical data on poplar show a clear seasonal variation (Fig. 1) of PcDXR and PcISPS transcript levels, PcISPS protein amount, enzyme activity, and total leaf DMADP levels dividing the growing season into four periods: springtime, early summer, late summer, and autumn.
Figure 1.
Variations of isoprenoid gene expression, PcISPS, leaf DMADP, and carotenoid contents during the growing season 2004. A, Transcript levels of PcDXR and PcISPS; B, transcript level of PcPSY; C, PcISPS protein amount and enzyme activity; D, DMADP and carotenoid contents in poplar leaves (n = 3 ± se). Daily means of air temperature and light-period means of PPFD are depicted in A and B, respectively (bold gray lines).
During the first 4 weeks of the growing season, transcript levels of PcISPS and PcDXR were low. However, levels increased very rapidly during the first strong temperature increase at beginning of June, such that values 8-fold higher were found (Fig. 1A). Over the early summer period, transcript levels of both genes remained high but fluctuated strongly, correlating with temperature and light variations (Fig. 1, A and B). In late summer and autumn, the daily mean air temperature and transcript levels correlate most obviously (Fig. 1A). The variation among individual trees was smaller in these periods as compared to in early summer. Regression analysis (Table I) reveals that transcript levels of PcDXR and PcISPS are significantly correlated to each other, indicating a synchronized regulation of gene expression. Also, expression levels of both genes showed a positive linear correlation (P ≤ 0.002) to actual mean air temperature values on the respective sampling days (Table I). Moreover, PcISPS gene expression correlated with light period means of PPFD.
Table I.
Statistical analysis of seasonal variations of gene expression and biochemical parametersa
| Seasonal Changes
| |||
|---|---|---|---|
| Parameters | R2 | Pb | Regression Function |
| Mean air temperature versus: | |||
| PcISPS transcript level | 0.698 | 0.000 | Linear |
| PcDXR transcript level | 0.493 | 0.002 | Linear |
| PcPSY transcript level | 0.200 | 0.072 | Linear |
| PcISPS protein | 0.109 | 0.197 | Linear |
| PcISPS activity | 0.192 | 0.163 | Square |
| Daily mean PPFD versus: | |||
| PcISPS transcript level | 0.525 | 0.001 | Linear |
| PcDXR transcript level | 0.178 | 0.091 | Linear |
| PcPSY transcript level | 0.001 | 0.915 | Linear |
| PcISPS protein | 0.112 | 0.189 | Linear |
| PcISPS activity | 0.217 | 0.059 | Linear |
| DMADP versus: | |||
| PcISPS protein | 0.903 | 0.000 | Linear |
| PcISPS activity | 0.576 | 0.001 | Square |
| PcISPS transcript level versus: | |||
| PcDXR transcript level | 0.697 | 0.000 | Linear |
| PcPSY transcript level | 0.096 | 0.185 | Linear |
| PcISPS protein | 0.125 | 0.322 | Square |
| PcISPS activity | 0.192 | 0.163 | Square |
| PcISPS protein versus: | |||
| PcISPS activity | 0.763 | 0.000 | Square |
For the regression analysis, the values obtained during leaf development were omitted.
Significant correlations between parameters are marked in bold.
Transcript levels of PcPSY, encoding a key enzyme in carotenoid biosynthesis (Von Lintig et al., 1997), were one order of magnitude lower and different concerning the seasonal trend when compared with transcript levels of PcDXR and PcISPS (Fig. 1B). High transcript levels were present during leaf development, decreasing for 3 weeks and rising again with the temperature increase at beginning of June. Over the summer period, the gene expression pattern of PcPSY was different than that of PcDXR and PcISPS. In autumn, however, the values varied in a similar way to PcDXR and PcISPS with respect to temperature. No correlation of PcPSY transcript levels with seasonal changes of light and temperature was detectable (Table I).
Amounts of PcISPS protein and enzyme activity (Fig. 1C) also showed a strong seasonal dependency, which can again be divided into four periods. During leaf development in May, only minimal amounts of PcISPS but no PcISPS activity were detectable. This observation coincides with the very low PcISPS transcript levels at that time point. Parallel to the strong increase of PcISPS transcript copy numbers in June, the amount of PcISPS protein increased constantly, reaching values of approximately 10 ng ISPS μg protein−1. However, PcISPS activity became detectable but remained constant at low values of approximately 0.5 nmol m−2 s−1. During late summer, the amount of PcISPS protein and PcISPS activity increased strongly within a few days. Following this increase, the level of PcISPS protein remained constant until the last sampling in October 2004. In contrast to this, PcISPS activity decreased in autumn in parallel to the decline of photosynthetic pigments (Fig. 1D). Determination of the turnover number (kcat [s−1]) of PcISPS confirms that protein content and enzyme activity of ISPS are not strictly related to each other (Fig. 1C). In early summer, the kcat was low (approximately 0.004 s−1), whereas in late summer, when protein and activity levels reached their maximum, a mean kcat of 0.022 s−1 was determined. In autumn, the kcat of ISPS dropped again to approximately 0.007 s−1 due to the decline of PcISPS activity.
Midday levels of total leaf DMADP concentration (Fig. 1D) correlate well with changes in PcISPS protein (Fig. 1C; see also Table I), with low DMADP contents, and with PcISPS protein (activity) levels during springtime. All values increased over summer, and DMADP as well as PcISPS protein concentrations remained high in autumn. Total leaf DMADP amount was found to be highly variable (Figs. 3 and 4), correlating with light and temperature during daytime, even if the values represent the total amount of DMADP of the leaves not distinguishing between cytosolic and plastidic pools. Hence, the present midday levels of DMADP only represent snapshots depending on the intrinsic climatic and physiological conditions at the sampling date (see also Brüggemann and Schnitzler, 2002b). It is therefore not possible to draw a direct conclusion on isoprene emission rates from midday DMADP contents and the PcISPS activities from the respective date.
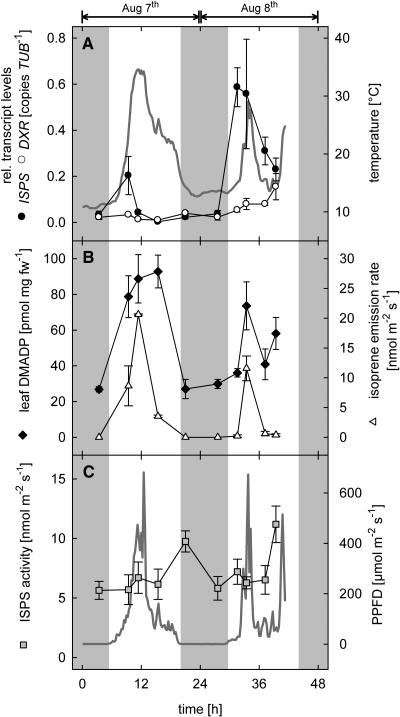
Figure 3.
Diurnal changes of isoprene biosynthesis-related parameters in leaves of 4-month-old poplar trees during two consecutive days in August 2001. A, Transcript levels of PcDXR, PcISPS, and leaf temperature (bold gray line); B, DMADP levels and isoprene emission rate; C, PcISPS activity and PPFD (bold gray line; n = 4 ± se; gas exchange measurements n = 2 ± range). Shaded regions symbolize dark periods.
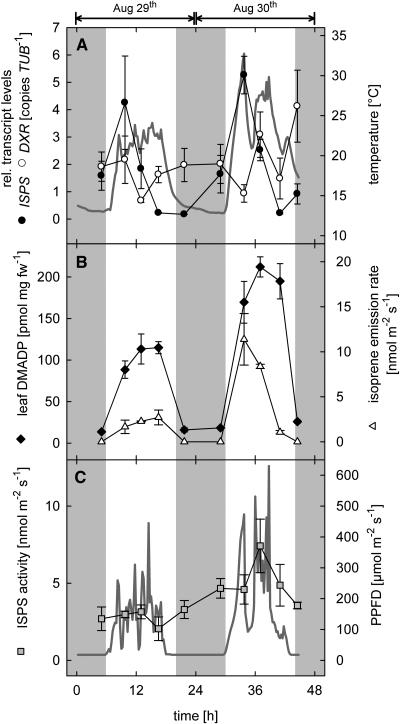
Figure 4.
Diurnal changes of isoprene biosynthesis-related parameters in leaves of 4-month-old poplar trees during two consecutive days in August 2002. A, Transcript levels of PcDXR, PcISPS, and leaf temperature (bold gray line); B, DMADP levels and isoprene emission rate; C, PcISPS activity and PPFD (bold gray line; n = 4 ± se; gas exchange measurements n = 2 ± range). Shaded regions symbolize dark periods.
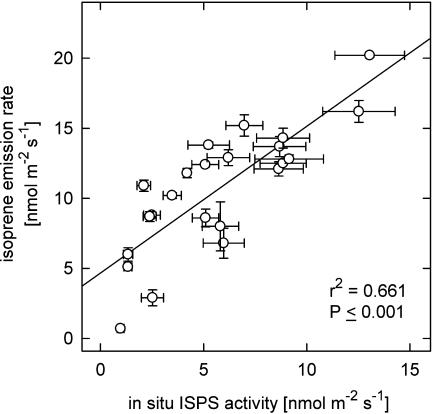
The importance of ISPS activity for the magnitude of isoprene emission is shown in Figure 2. In the experiment, mature poplar leaves had been acclimated in leaf cuvettes to different leaf temperatures (15°C–38°C) under saturating PPFD. The comparison of isoprene emission rate and ISPS activity adjusted to the respective leaf temperature using the temperature profile of poplar ISPS activity (Schnitzler et al., 2005) demonstrates the clear dependence of isoprene emission on ISPS activity actually present in the individual leaf.
Figure 2.
Correlation between PcISPS activity and isoprene emission rates in 24 leaves of 4-month-old poplar trees. Isoprene emission rates were determined under stable conditions at different leaf temperatures. PcISPS activities of the respective 24 leaves were determined under constant conditions and related to the respective leaf temperature during emission measurement.
Daily Variation of Transcript Levels of PcDXR and PcISPS in Relation to PcISPS Activity and Isoprene Emission
On two consecutive days in August 2001 (Fig. 3) and 2002 (Fig. 4), diurnal changes in isoprene emission, transcript levels of important genes in the DOXP pathway, and PcISPS activity were acquired twice. Emission measurements were performed on two trees, while leaf material for the molecular and biochemical characterization was collected from four different comparable trees. Five sampling points each day (predawn, morning, noon, afternoon, and night) were chosen for analysis (for experimental details, see Mayrhofer et al., 2004).
Isoprene emission showed a typical daily variation with a light- and temperature-dependent increase in the morning and a decline of the emission rate during the night (Figs. 3B and 4B). A similar trend could be observed for the leaf DMADP pool. Maximum daily values were reached by noon with 74 to 93 pmol mg dry weight−1 in 2001 (Fig. 3B) and 115 to 212 pmol mg dry weight−1 in 2002 (Fig. 4B).
Transcript levels of PcISPS showed a remarkable intraday variation with a clear maximum in the morning on all four investigated days, although the absolute amounts varied from day to day (Figs. 3A and 4A). In 2001, relative transcript levels of PcISPS peaked on August 7th at approximately 0.2 copies β-tubulin (PcTUB)−1, whereas during the second day maximum values of approximately 0.6 copies PcTUB−1 were reached. The relative PcISPS transcript level in the morning was therefore on average at least 6 times higher than the corresponding predawn value. In 2002, relative expression levels of PcISPS were 9 times higher than in 2001 with a relative transcript level of approximately 5.3 copies PcTUB−1, within the peak of the second day.
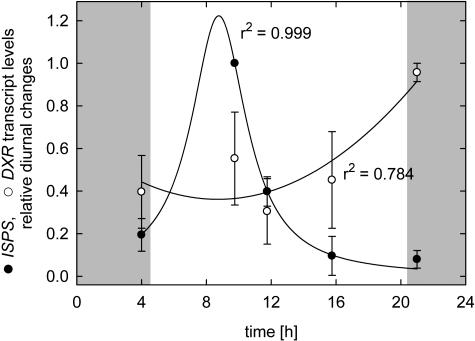
Normalization of maximum (set to 1) and minimum (set to 0) levels of each day (Fig. 5) revealed that PcISPS transcript levels significantly peak in the morning and decrease continuously.
Figure 5.
Relative diurnal changes in transcript levels of PcDXR and PcISPS obtained from four diurnal curves. Minimum and maximum values of each individual day were set to 0 and 1 for normalization, respectively (n = 4 d ± se). Regression analysis was performed with Sigmaplot 2000 for Windows (Version 6.10; SPSS). Shaded regions symbolize dark periods.
The increase in the PcISPS transcript level was not reflected directly in an increase of ISPS activity. In fact, in vitro activity of PcISPS did not change dramatically during the course of the day. During the first sampling period in 2001, in vitro ISPS activities were slightly enhanced at night (Figs. 3C and 4C).
In contrast to PcISPS expression, the relative transcription rates of PcDXR of all 4 d investigated gave no clear evidence for a higher/induced transcription during light periods (Figs. 3A, 4A, and 5). In fact, PcDXR expression rates were found to oscillate over the day, but in three of the four days values increased to the maximum at the latest sampling point of the day (Fig. 5).
DISCUSSION
Several studies (Monson et al., 1994; Kempf et al., 1996; Funk et al., 2005) have observed a strong seasonal variation of isoprene emission rates, with increases in springtime that rapidly decline in autumn. Kuzma and Fall (1993) and Lehning et al. (2001) correlated these changes mainly to the seasonal development of ISPS activity, adjusting the constitutive isoprene emission capacity (Fall, 1999). This study on poplar, which employed molecular biological and biochemical experiments, also showed clear seasonal variation during the growing season.
Gene expression rates of isoprenoid biosynthesis-related genes in poplar leaves are highly variable over the growing season, with temperature and light as the most obvious factors controlling transcript levels in fully developed leaves.
During leaf expansion, transcript levels of PcISPS and PcDXR were low; however, levels increased very rapidly after approximately 4 weeks at the beginning of June. The delayed increase in transcript level of poplar ISPS is consistent with recent data on kudzu leaves, where it was found that ISPS gene expression also increased after a certain lag phase (Wiberley et al., 2005). Depending on temperature conditions (growth at 20°C or 30°C), the lag phase of the onset of ISPS gene expression and isoprene emission varied by about 2 weeks in kudzu leaves. It seems that the achievement of full photosynthetic competence is a criterion for the onset of isoprene biosynthesis, even when leaf expansion is not completely finished. This is consistent with results found for the velvet bean (Mucuna sp.; Grinspoon et al., 1991) that show isoprene emission starts about 3 d after leaves become photosynthetically competent. Since kudzu leaves grow faster under higher temperature, Wiberley et al. (2005) concluded that onset of isoprene biosynthesis is governed more by plant growth conditions than by developmental stages.
This assumption is confirmed by the study of Lehning et al. (2001). They found that ISPS activity in oak (Quercus robur) leaves becomes measurable when carotenoid contents reach a stable level. This study for Grey poplar gives further approval by the finding that transcript levels of PcDXR and PcISPS increased when the leaf carotenoid content reached its maximum. Transcript levels of PcPSY were different when compared with PcDXR and PcISPS transcript levels. In addition, the seasonal trend was different. The missing correlation to transcript levels of PcDXR and PcISPS indicates that later steps in the plastidic isoprenoid biosynthesis, i.e. formation of accessory photosynthetic pigments, might be regulated differently to isoprene biosynthesis. This is particularly true for very young leaves where gene expression rates of PcPSY were relatively high in comparison to that of PcDXR and PcISPS. This indicates that young and, thus, photosynthetically noncompetent poplar leaves have an intensive plastidic carotenoid biosynthesis, without metabolic branching to isoprene. Therefore, it can be hypothesized that, during leaf ontogenesis, intermediates of the DOXP pathway are mostly channeled to carotenoids, while in fully developed leaves isoprene emission is an important sink for plastidic DMADP.
ISPS gene expression in poplar undergoes a strong diurnal variation with maximum levels before midday, when the temperature has not necessarily reached its daily maximum. This observation confirms an earlier study from Arimura et al. (2004) where northern-blot analysis on poplar leaves indicated that PcISPS transcript levels differ between day and night time. Recently, Dudareva et al. (2005) demonstrated for snapdragon flowers (Antirrhinum majus) that DXS gene expression also followed a diurnal variation. Similar to the present data, they also found no diurnal changes of DXR gene expression rates. Moreover, they showed that the DOXP pathway in snapdragon petals operates in a circadian manner, which is reflected in a rhythmic terpenoid emission under constant darkness. Hence, it has to be tested whether ISPS gene expression is also controlled by a circadian clock. Providing that there is no circadian rhythm, the diurnal course of PcISPS transcript levels gives a strong indication that PcISPS gene expression is light regulated, as has already been shown for phytochrome genes (Hauser et al., 1998) as well as for many other genes related to light-dependent processes (e.g. nitrate reductase; Hänsch et al., 2001).
It has to be analyzed in future experiments to which extent gene expression of PcISPS is triggered by photoperiod and light intensity. In addition, the role of temperature has to be elucidated in this process. Does temperature influence the intraday variation, or does it serve as an important trigger that determines the maximum level of transcript? The significant correlation between daily mean temperatures and PcISPS transcript levels observed during the long-term study supports this hypothesis.
In addition to the seasonal variation of PcISPS transcript level, a strong seasonal dependence of PcISPS and enzyme activity, which divided the growing season into four periods, could be demonstrated. Indeed, transcript, protein, and enzyme activity levels were not completely identical over the growing season. Therefore, no statistically significant correlation between both parameters could be found, indicating that besides transcriptional control other parameters influence isoprene biosynthesis in poplar leaves.
Changes of ISPS activity over the growing season have also been reported for different species by Kuzma and Fall (1993) and Lehning et al. (2001), who correlated these changes with isoprene emission. In this work, no seasonal isoprene emission rates were determined. Nevertheless, in additional experiments we could clearly demonstrate that isoprene emission rates of poplar leaves are correlated to PcISPS activity. A similar positive correlation of ISPS activity and isoprene emission was demonstrated earlier for European oaks (Lehning et al., 1999; Brüggemann and Schnitzler, 2001), velvet bean (Kuzma and Fall, 1993), and Phragmites australis (Scholefield et al., 2004). Also, Wiberley et al. (2005) recently found that increasing levels of ISPS protein during leaf development in kudzu positively correlates to the increase of basal isoprene emission capacity. Therefore, we conclude that seasonal changes in PcISPS activity are reflected in changes of seasonal basal emission capacity.
There did not appear to be a strict correlation between the enzyme activity and the PcISPS protein levels in poplar leaves over the growing season. Therefore, different apparent turnover numbers (kcat [s−1]) of PcISPS were determined. In contrast to Silver and Fall (1995), who described a higher kcat of 1.7 s−1 for the purified ISPS from Populus tremuloides Michx., our actual values are much lower. However, the present apparent kcat values are in the same range as the numbers described for heterologously expressed ISPS (0.088 s−1) from kudzu (Sharkey et al., 2005) and other terpene synthases. For example, for γ-terpinene synthase from Thymus vulgaris (Alonso and Croteau, 1991) and for 4S-limonene synthase from Mentha spicata (Alonso et al., 1992), kcat values of 0.01 s−1 and 0.3 s−1, respectively, have been described (for overview, see Kuhn et al., 2004).
The changes in the apparent kcat values indicate that effects on the protein level influence PcISPS activity. Consequently, a variable portion of active and inactive PcISPS protein is building up in the leaves over the vegetation period, in particular in autumn after the onset of senescence. At that time, ISPS protein levels remained high while ISPS activity was declining.
In accordance with this assumption, Schnitzler et al. (2005) recently localized ISPS in poplar chloroplasts, attached to thylakoid membranes as well as in the stroma, using a ISPS-specific polyclonal antibody. On native isoelectric focusing gels, four to five bands were immunologically detected. Therefore, it is likely that at least two ISPS forms with different binding affinities to membranes exist in poplar leaves. In future experiments, attention should be drawn to the regulation of ISPS activity versus protein content. Whether these different immunodetectable proteins reflect posttranslationally modified active or inactive ISPS or whether they are concerning, rather, ISPS proteins under degradation should be further investigated.
There are two possibilities as to how the activation of plastidic PcISPS could be achieved. Activation can be driven by a light-dependent shift of stromal conditions (pH, Mg2+ concentration) to the optimal range of PcISPS activity (for details about poplar PcISPS, see Schnitzler et al., 2005). The other possibility is activation by posttranslational modifications, such as redox-controlled protein phosphorylation or redox signaling via the ferredoxin/thioredoxin system (Allen, 2002; Schürmann, 2003). However, the present data on poplar ISPS and previous studies on oaks (Lehning et al., 1999; Brüggemann and Schnitzler, 2002a) showed that ISPS activity undergoes no intraday variation. Therefore, it is more likely that the light-dependent increase in DMADP concentration demonstrated for poplar and other species (Brüggemann and Schnitzler, 2002b; Rosenstiel et al., 2003; Wolfertz et al., 2004) is the main factor regulating short-term changes in isoprene biosynthesis.
DMADP levels on the daily scale show that dark-adapted leaves contained approximately 30% of the maximum DMADP level of light-adapted leaves, as was found by Fisher et al. (2001) and Brüggemann and Schnitzler (2002b). Isoprene emission rates respond very rapidly to changes in light intensity. Under conditions of no light, isoprene emission in poplar leaves dropped to zero in less than 20 min (Graus et al., 2004). Equal to the isoprene emission development, leaf DMADP levels of poplar declined to a new steady state, which accounted for approximately 45% of the DMADP concentration in the light (data not shown). That observation indicates that the light-labile DMADP pools rapidly reach values comparable to the day/night ratio. However, the distribution of DMADP between cytosolic and plastidic pools can vary between 30% (Rosenstiel et al., 2002; Brüggemann and Schnitzler, 2002b) and 70% (Loreto et al., 2004). In addition, the rate of possible exchange of C5-precursors (isopentenyl diphosphate and DMADP) between the cytosol and the plastid is unknown. Therefore, it must be assumed that the ratio between cytosolic and plastidic DMADP varies over the growing season, and, hence, the actual plastidic substrate pool for ISPS activity at specific dates remains unknown.
The significant positive correlation between PcISPS protein and leaf DMADP levels indicates that the metabolic flux through the plastidic DOXP pathway (Eisenreich et al., 2001) might be greater the higher the PcISPS activity present in the leaves. The synchronized regulation of PcDXR and PcISPS transcript accumulation (Fig. 1A) also confirms this hypothesis of a seasonal up-regulation of isoprene formation and of the DOXP pathway, which seemed to be highly coordinated and/or complex regulated. Indeed, it has been shown that the Arabidopsis DXR gene is under the control of both developmental and environmental (particularly light) factors (Carretero-Paulet et al., 2002). However, less is known about the regulative flux limiting steps in the DOXP pathway.
With respect to this assumption, future studies will have to ascertain how the different fluxes of metabolic intermediates during certain stages of leaf ontogenesis are regulated on a molecular and/or biochemical level to sustain the individual demands of the different branches of plastidic isoprenoid biosynthesis. Several investigations support that DXS and DXR play an important role in the control of plant isoprenoid biosynthesis. To date, the rate-limiting step is unknown, although molecular engineering revealed that overexpression of DXS (Estévez et al., 2001) or of DXR (Mahmoud and Croteau, 2001) resulted in an enhanced accumulation of isoprenoid end products. A positive correlation between DXS and DXR transcript levels was also found in mycorrhizal roots from monocots (Walter et al., 2000) and C. roseus cell suspension cultures (Veau et al., 2000). However, analysis of DXR during fruit ripening in L. esculentum indicated that DXR plays a nonlimiting role in this system (Rodríguez-Concepción et al., 2001). Hence, for a conclusive interpretation of the regulating roles of DXS and/or DXR on isoprene emission, both genes and proteins have to be studied in parallel in future experiments.
Although the present data manifest that gene expression of PcDXR and PcISPS vary in relation to day-to-day changes of temperature and light intensity, further efforts are needed to clarify the linkage between gene expression of PcDXS, PcDXR, and PcISPS, actual protein levels, and enzyme activities. In particular, laboratory studies under controlled conditions are necessary to analyze the chronological interrelation of gene expression, protein biosynthesis, posttranslational modifications of PcISPS protein, and protein turnover to manifest isoprene emission rates.
MATERIALS AND METHODS
Plant Material and Experimental Design
Intraday experiments were performed in special greenhouses (solar domes) with 4-month-old Grey poplar plants (Populus×canescens [Aiton] Sm.), cultivated by micropropagation (Leplé et al., 1992) at the University of Freiburg, Germany. Growing conditions and climate parameters were as described by Mayrhofer et al. (2004). The experiments in 2001 and 2002 were performed on August 7th and 8th and 29th and 30th, respectively. At each time point, four plants were completely harvested. For biochemical analysis, seven fully developed mature leaves from the middle part of the twigs were pooled, quick frozen in liquid nitrogen, and stored at −80°C.
Temperature experiments under controlled conditions were performed in addition. Poplar leaves were adjusted to different leaf temperatures (15°C−38°C) under saturating PPFD (400–700 μmol photons m−2 s−1) in leaf cuvettes. After reaching a stable leaf temperature, gas exchange and isoprene emission rates were measured for 45 to 60 min. Leaves from inside the cuvette were then rapidly removed and shock frozen in liquid N2 for biochemical analysis.
The seasonal study was performed with 4-year-old poplar plants of the same origin. In spring 2001, saplings were planted in 10-L pots containing commercial garden soil and cultivated in the garden of the Institut für Meteorologie und Klimaforschung, Atmosphärische Umweltforschung in Garmisch-Partenkirchen, Germany. Plants were irrigated regularly, trimmed each spring, and fertilized in May by applying 20 g Osmocote (Spiess). From May to October 2004, mixed leaf samples were taken from three trees simultaneously in intervals of 1 to 3 weeks. Sampling was performed exactly at 12:30 pm CET by shock freezing four comparable mature leaves per tree in liquid nitrogen. During the entire growing season, half-hour means of air temperature (HP-100-A; Imko) and PPFD (quantum sensor Li-190SA; LI-COR) were monitored.
Cuvette Measurements of Photosynthetic Gas Exchange
Photosynthetic gas exchange and rates of isoprene emission of leaves were measured in real time with a leaf cuvette system on twigs of two plants simultaneously during each sampling period according to Mayrhofer et al. (2004). The isoprene concentration at the outlet of the cuvette was analyzed using a Fast Isoprene Sensor (Hills Scientific) according to Brüggemann and Schnitzler (2002a).
Determination of PcISPS Activity and Cellular Metabolites
ISPS activity was assayed as previously described by Lehning et al. (1999) with minor changes. A poplar-adapted plant extraction buffer (100 mm Tris/HCl, pH 8.0, 20 mm MgCl2, 100 mm CaCl2, 5% [v/v] glycerol, 0.1% [v/v] Tween 80, 20 mm dithiothreitol) with 250 mg polyvinylpolypyrrolidone was added prior to use and stirred for 15 min. Protein concentrations were determined using the Bradford assay with bovine serum albumin as a standard. In situ PcISPS activities in the leaves were calculated by correcting the enzyme activities at 30°C according to the actual measured leaf temperatures using the temperature dependency profile of PcISPS (Bachl, 2005).
The procedure for the determination of DMADP and photosynthetic pigments was as described by Brüggemann and Schnitzler (2002b) and Lichtenthaler and Wellburn (1983), respectively.
Quantification of PcISPS Protein with an ELISA
Quantification of ISPS protein was performed according to Schnitzler et al. (2005) using purified polyclonal anti-PcISPS-IgG generated against N-terminal 6× His-tagged PcISPS (Miller et al., 2001). Anti-PcISPS-IgG conjugated with horseradish peroxidase (HRP; BioGenes) was used as a second antibody in the ELISA.
The ELISA was set up according to the QIAexpress and Assay Handbook (Qiagen). Ninety-six-well microtiter plates (Greiner) with high protein binding capacity were precoated overnight at 4°C with anti-PcISPS-IgG diluted 1:500 in phosphate-buffered saline (PBS; 50 mm NaPi, pH 7.2, 140 mm NaCl). Before the next step and in between all following steps, the wells were washed four times with 200 μL PBS-Tween (0.1% [v/v] in PBS) for 1 min each. In the second step, residual protein binding sites were blocked with BSA (0.2% [w/v] in PBS) for 1 h. For binding of ISPS, protein samples with a total of 2.5 μg protein in 200 μL PBS per well were incubated at room temperature for 1 h and carefully agitated (Titramax 100; Heidolph Instruments). Thereafter, the plates were incubated for 1 h with the second anti-PcISPS-IgG antibody, which was conjugated with HRP. After the final washing, each well was filled with 200 μL of a ready-to-use staining solution containing the HRP substrate tetra-methylbenzidine. The plates were incubated at room temperature for 45 min. After that time the staining intensity was enhanced by the addition of 10 μL 2 n H2SO4. The resulting yellow-colored complex was analyzed with a plate reader (Tecan Spectra Image; SLT) at 450 nm. For calibration, purified 6× His-tagged PcISPS protein was used in a range of 10 to 250 ng protein well−1. Each sample and standard was analyzed in triplicates.
Screening of cDNA Library for Full-Length PcDXR
To isolate the DXR gene from Grey poplar, a λ-phage cDNA library (Miller et al., 2001) with 3 × 105 colony forming units was used to transform Escherichia coli XL1-Blue MRF′ cells (Stratagene). In vitro excision of the identified phage was performed by the use of E. coli SOLR and the helper phage ExAssist (Stratagene).
Oligonucleotide primers (Roth) were designed for conserved homologous regions of the deduced amino acid sequences of DXR genes from Oryza sativa (EMBL AF367205), Zea mays (EMBL AJ297566), Arabidopsis (Arabidopsis thaliana; EMBL AF148852), Lycopersicon esculentum (EMBL AF331705), and Mentha piperita (EMBL AF116825). The forward oligonucleotide primer [5′ gac atc gtc gc(agct) ga(ag) aa(tc) cc(agct) g 3′] and the reverse oligonucleotide primer [5′ gct atg tcc ttc cc(agct) gc(tc) tc(agt) at(agct) gc 3′] were used in PCR (PCR conditions: hot start at 95°C for 30 s; annealing, two cycles at 48°C for 30 s followed by 40 cycles at 52°C; extension at 72°C for 30 s; and denaturation 30 s at 95°C) to produce a 300-bp product. After cloning and sequencing, the amplified product was used for screening of the cDNA library. The product was labeled by the incorporation of digoxigenin-labeled nucleotides and used for hybridization according to the DIG Nucleic Acid labeling and detection kit (Roche Diagnostics).
Characterization of a Partial Sequence of the PcPSY Gene
PCR was performed with Grey poplar cDNA using degenerated oligonucleotide primer [forward oligonucleotide primer, 5′ (ac)(ag) a a(ag)(ag) gc(gt) (ag) t(act) tgg gc(at) at 3′; reverse oligonucleotide primer, 5′ tct c(acg) g c(cgt)(at) (ct)(ag) t c(ag) a aga ac 3′] designed by the alignment of diverse PSY gene sequences from the literature. Two clones of the 549-bp amplicon were sequenced from both directions, resulting in a unique sequence (EMBL AJ889824) with high homology to characterized PSY genes as the ones from Daucus carota (EMBL AB032797), Citrus unshiu Marc. (EMBL AF220218), Tagetes erecta (EMBL AF252015), and Citrus sinensis L. Osbeck (EMBL AY669084).
DNA Sequencing
Cycle sequencing dideoxy chain termination reactions with Big Dye Terminators (Applied Biosystems) were performed for both cDNA strands of all DNA segments investigated, using universal forward and reverse oligonucleotide primers (Invitrogen) or sequence-specific oligonucleotide primers. The sequences were analyzed by using an ABI PRISM System 310 (Applied Biosystems).
Heterologous Expression and Functional Analysis of Full-Length PcDXR cDNA
Cloning of the full-length PcDXR cDNA into an expression vector was performed using Gateway technology (Invitrogen). The PcDXR sequence including start and stop codons was amplified from the isolated cDNA clone by PCR using primers with attB sites (forward oligonucleotide primer, 5′ g ggg aca agt ttg tac aaa aaa gca ggc ttg atg gca ctt aat att cta tct cca g 3′; reverse oligonucleotide primer, 5′ gg gga cca ctt tgt aca aga aag ctg ggt tca agc aaa aac agg act tgg 3′). The underlined nucleotides are gene specific; italicized nucleotides have been integrated to get an ORF. PCR was performed with 1 unit Platinum Taq Polymerase High Fidelity (Invitrogen). PCR conditions were as follows: hot start at 96°C for 3 min; annealing, four cycles at 55°C for 45 s followed by 19 cycles at 65°C; extension at 72°C for 2 min; and denaturation 45 s at 96°C. The amplicon was recombined into the vector DONR 221 (Invitrogen) by BP recombination reaction according to the Gateway protocol and transformed into E. coli Top 10 (Invitrogen). After verification of the sequence, the ORF segment was transferred into pDEST17 expression vector with an N-terminal His-encoding region (Invitrogen) by a LR recombination reaction according to the Gateway protocol and transformed into E. coli BL21-Star (Invitrogen) for protein expression.
Purification of the His-tagged protein was performed according to the QIAexpress Type IV protocol (Qiagen), except that E. coli cells were disrupted by a French pressure cell press (SLM Instruments) at 140 MPa and 0°C to 4°C as described by Miller et al. (2000).
After affinity chromatography on a Ni-NTA column according the manufacturer's instructions (Qiagen), 2.5 mL of eluate was desalted on a PD-10 column (Pharmacia) into 3.5 mL assay buffer (150 mm Tris/HCl, pH 7.0, with 5 mm MgCl2, 1 mm thiamine diphosphate, 5 mm β-mercaptoethanol, 10% [v/v] glycerol) according to Miller et al. (2000). PcDXR enzyme assay was performed according to Grolle et al. (2000) and Miller et al. (2000). Fosmidomycin (1 mm final concentration) was used as specific inhibitor for DXR reaction.
RNA Isolation and cDNA Synthesis
Total RNA from fresh leaves was isolated with Tri-Reagent (Sigma) according to the manufacturer's protocol. From leaves stored for more than 1 year at −80°C, total RNA was prepared with Qiagen RNeasy minikit (Qiagen) following the Qiagen standard protocol. The amount and purity of isolated RNA were determined spectrophotometrically.
For first-strand cDNA synthesis, 3 μg of total RNA was reverse transcribed using oligo(dT) primers and Superscript II reverse transcriptase (Invitrogen) in a total volume of 20 μL according to the manufacturer's protocol. cDNA was stored at −20°C prior to analysis.
Quantitative Reverse Transcription-PCR
For quantitative PCR measurements of the transcription rates of PcISPS (EMBL AJ294819), PcDXR, and PcPSY, the following oligonucleotide primer sets were used: for PcISPS, forward 5′ ttt gcc tac ttt gcc gtg gtt caa aac 3′ and reverse 5′ tcc tca gaa atg cct ttt gta cgc atg 3′; for PcDXR, forward 5′ gca tat gtc ttt tcc agc ttc tat tgc 3′ and reverse 5′ gga ata gta ggt tgc gca ggc 3′; for PcPSY, forward 5′ atg cat cac ata tca cac cca aa 3′ and reverse 5′ ctc cta gca tct tct cca aca tct c 3′. The resulting PCR segment lengths were 197 bp (PcISPS), 66 bp (PcDXR), and 379 bp (PcPSY), respectively. SYBR Green was used as a fluorescent marker for the increasing amount of double-stranded DNA. The assays contained 12.5 μL 2× SYBR Green PCR Master Mix (Applied Biosystems), 300 nm of each primer, and 5 μL of total cDNA (diluted 7-fold) in a final volume of 25 μL. After a “hot start” (10 min, 95°C), 45 PCR cycles were performed with a 15-s melting step at 95°C and a 1-min annealing/extension step at 60°C on a GeneAmp 5700 sequence detection system (Applied Biosystems). For internal normalization, transcription rates of poplar PcTUB (EMBL AY353093) with forward primer 5′ gat ttg tcc ctc gcg ctg t 3′ and reverse primer 5′ tcg gta taa tga ccc ttg gcc 3′ were determined and used.
Statistical Analysis
Statistical and correlation analysis was performed with SPSS for Windows NT (release 8.0.0) and Sigmaplot 2000 for Windows (Version 6.10), both programs from SPSS.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AJ574852 and AJ889824.
Acknowledgments
We greatly acknowledge the provision of a PcTUB fragment of poplar by S. Kopriva (University of Freiburg, Germany, now Norwich Research Park, UK), and the critical reading of the manuscript by B. Miller (University of Freiburg, Germany). We further acknowledge the stylistic proofreading by I.H. Franke-Whittle (University of Innsbruck, Austria).
This work was supported by national (joint research project Atmosphären-Forschungsprogramm 2000 [Bundesministerium für Bildung und Forschung], research group “Poplar: A Model to Address Tree-Specific Questions” [Deutsche Forschungsgemeinschaft]) and international (EU Marie Curie Research Training Networks ISONET) research programs.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.066373.
References
- Allen JF (2002) Plastoquinone redox control of chloroplast thylakoid protein phosphorylation and distribution of excitation energy between photo systems: discovery, background, implications. Photosynth Res 73: 139–148 [DOI] [PubMed] [Google Scholar]
- Alonso WR, Croteau R (1991) Purification and characterisation of the monoterpene cyclase γ-terpinene synthase from Thymus vulgaris. Arch Biochem Biophys 286: 511–517 [DOI] [PubMed] [Google Scholar]
- Alonso WR, Rajaonarivony JIM, Gershenzon J, Croteau R (1992) Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha x piperita) and spearmint (Mentha spicata). J Biol Chem 267: 7582–7587 [PubMed] [Google Scholar]
- Arimura G, Huber DPW, Bohlmann J (2004) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterisation, and patterns of gene expression of (−) germacrene D synthase, PtdTPS1. Plant J 37: 603–616 [DOI] [PubMed] [Google Scholar]
- Bachl A (2005) Biochemical characterisation of isoprene synthase from Grey poplar and its expression in Arabidopsis thaliana. PhD thesis. Universität zu Köln, Germany
- Biesenthal TA, Wu Q, Shepson PB, Wiebe HA, Anlauf KG, MacKay GI (1997) A study of relationships between isoprene, its oxidation products, and ozone, in the lower Fraser valley, BC. Atmos Environ 31: 2049–2058 [Google Scholar]
- Brüggemann N, Schnitzler J-P (2001) Influence of powdery mildew (Microsphaera alphitoides) on isoprene biosynthesis and emission of pedunculate oak (Quercus robur L.) leaves. J Appl Bot 75: 91–96 [Google Scholar]
- Brüggemann N, Schnitzler J-P (2002. a) Comparison of isoprene emission, intercellular isoprene concentration and photosynthetic performance in water-limited oak (Quercus pubescens Willd. and Quercus robur L.) saplings. Plant Biol 4: 456–463 [Google Scholar]
- Brüggemann N, Schnitzler J-P (2002. b) Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur L.) leaves. Physiol Plant 115: 190–196 [DOI] [PubMed] [Google Scholar]
- Carretero-Paulet L, Ahumada I, Cunillera N, Rodríguez-Concepción M, Ferrer A, Boronat A, Campos N (2002) Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-d-erythritol 4-phosphate pathway. Plant Physiol 129: 1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwent RG, Jenkin ME, Saunders SM, Pilling MJ (1998) Photochemical ozone creation potentials for organic compounds in northwest Europe calculated with a master chemical mechanism. Atmos Environ 32: 2429–2441 [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102: 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway of terpenoids. Trends Pharmacol Sci 6: 78–84 [DOI] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Reindl A, Reichler S, Leon P (2001) 1-Deoxy-d-xylulose 5-phosphatase, a rate limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276: 22901–22909 [DOI] [PubMed] [Google Scholar]
- Fall R (1999) Biogenic emissions of volatile organic compounds from higher plants. In CN Hewitt ed, Reactive Hydrocarbons in the Atmosphere. Academic Press, San Diego, pp 41–95
- Fisher AJ, Rosenstiel TN, Shirk MC, Fall R (2001) Nonradioactive assay for cellular dimethylallyl diphosphate. Anal Biochem 292: 272–279 [DOI] [PubMed] [Google Scholar]
- Funk JL, Jones CG, Gray DW, Throop HL, Hyatt LA, Lerdau MT (2005) Variation in isoprene emission from Quercus rubra: sources, causes, and consequences for estimating fluxes. J Geophys Res 110: D04301, doi/10.1029/2004JD005229
- Graus M, Schnitzler JP, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser J (2004) Transient release of oxygenated VOC during light-dark transitions. Plant Physiol 135: 1967–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon J, Bowman WD, Fall R (1991) Delayed onset of isoprene emission in developing velvet bean (Mucuna sp.) leaves. Plant Physiol 97: 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolle S, Bringer-Meyer S, Sahm H (2000) Isolation of a dxr gene from Zymomonas mobilis and characterization of the 1-deoxy-d-xylulose 5-phosphate reductoisomerase. FEMS Microbiol Lett 191: 131–137 [DOI] [PubMed] [Google Scholar]
- Guenther AB, Zimmermann PR, Harley AC, Monson RK, Fall R (1993) Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. J Geophys Res 98: 12609–12617 [Google Scholar]
- Hänsch R, Gómez-Fessel D, Witt C, Hesberg C, Hoffmann G, Walch-Liu P, Engels C, Kruse J, Rennenberg H, Kaiser WM, et al (2001) Tobacco plants that lack expression of functional nitrate reductase in roots show changes in growth rates and metabolite accumulation. J Exp Bot 52: 1251–1258 [PubMed] [Google Scholar]
- Hauser BA, Cordonnier-Pratt M-M, Pratt LH (1998) Temporal and photoregulated expression of five tomato phytochrome genes. Plant J 14: 431–439 [DOI] [PubMed] [Google Scholar]
- Isebrands JG, Guenther AB, Harley P, Helmig D, Klinger L, Vierling L, Zimmerman P, Geron C (1999) Volatile organic compound emission rates from mixed deciduous and coniferous forests in Northern Wisconsin, USA. Atmos Environ 33: 2527–2536 [Google Scholar]
- Julliard JH (1992) Biosynthesis of the pyridoxal ring (vitamin B6) in higher plant chloroplasts and its relationship with the biosynthesis of the thiazole ring (vitamin B1). CR Acad Sci III 314: 285–290 [Google Scholar]
- Julliard JH, Douce R (1991) Biosynthesis of the thiazole moiety of thiamin (vitamin B1) in higher plant chloroplasts. Proc Natl Acad Sci USA 88: 2042–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf K, Allwine E, Westberg H, Claiborn C, Lamb B (1996) Hydrocarbon emissions from spruce species using environmental chamber and branch enclosure methods. Atmos Environ 30: 1381–1389 [Google Scholar]
- Kuhn U, Rottenberger S, Biesenthal T, Wolf A, Schebeske G, Ciccioli P, Kesselmeier J (2004) Strong correlation between isoprene emission and gross photosynthetic capacity during leaf phenology of the tropical tree species Hymenaea courbaril with fundamental changes in volatile organic compounds emission composition during early leaf development. Plant Cell Environ 27: 1469–1485 [Google Scholar]
- Kuzma J, Fall R (1993) Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol 101: 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Brüggemann N, Schnitzler JP (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur L. leaves. Plant Cell Environ 22: 495–504 [Google Scholar]
- Lehning A, Zimmer W, Zimmer I, Schnitzler JP (2001) Modeling of annual variations of oak (Quercus robur L.) isoprene synthase activity to predict isoprene emission rates. J Geophys Res 106: 3157–3166 [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L (1992) Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep 11: 137–141 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans 603: 591–592 [Google Scholar]
- Logan BA, Monson RK, Potosnak MJ (2000) Biochemistry and physiology of foliar isoprene production. Trends Pharmacol Sci 5: 477–481 [DOI] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126: 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Brancaleoni E, Ciccioli P (2004) 13C labeling reveals chloroplastic and extra-chloroplastic pools of dimethylallyl diphosphate and their contribution to isoprene formation. Plant Physiol 135: 1903–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA 98: 8915–8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer S, Heizmann U, Magel E, Eiblmeier M, Müller A, Rennenberg H, Hampp R, Schnitzler J-P, Kreuzwieser J (2004) Carbon balance in the leaves of young poplar trees. Plant Biol 6: 730–745 [DOI] [PubMed] [Google Scholar]
- Miller B, Heuser T, Zimmer W (2000) Functional involvement of a deoxy-D-xylulose 5-phosphate reductoisomerase gene harboring locus of Synechococcus leopoliensis in isoprenoid biosynthesis. FEBS Lett 481: 221–226 [DOI] [PubMed] [Google Scholar]
- Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213: 483–487 [DOI] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R (1994) Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia 99: 260–270 [DOI] [PubMed] [Google Scholar]
- Monson RK, Jaeger CH, Adams WW III, Driggers EM, Silver GM, Fall R (1992) Relationship among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol 98: 1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Lerdau MT, Sharkey TD, Schimel DS, Fall R (1995) Biological aspects of constructing volatile organic compound emission inventories. Atmos Environ 29: 2989–3002 [Google Scholar]
- Rodríguez-Concepción M, Ahumada I, Diez-Juez E, Sauret-Gueto S, Lois LM, Gallego F, Carretero-Paulet L, Campos N, Boronat A (2001) 1-Deoxy-d-xylulose 5-phosphate reductoisomerase and plastidic isoprenoid biosynthesis during tomato fruit ripening. Plant J 27: 213–222 [DOI] [PubMed] [Google Scholar]
- Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK (2004) Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biol 6: 12–21 [DOI] [PubMed] [Google Scholar]
- Rosenstiel TN, Fisher AJ, Fall R, Monson RK (2002) Differential accumulation of dimethylallyl diphosphate in leaves and needles of isoprene- and methylbutenol-emitting and nonemitting species. Plant Physiol 129: 1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK (2003) Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421: 256–259 [DOI] [PubMed] [Google Scholar]
- Schnitzler J-P, Lehning A, Steinbrecher R (1997) Seasonal pattern of isoprene synthase activity in Quercus robur leaves and its significance for modeling isoprene emission rates. Bot Acta 110: 240–243 [Google Scholar]
- Schnitzler J-P, Zimmer I, Bachl A, Arend M, Fromm J, Fischbach RJ (2005) Biochemical properties of isoprene synthase from poplar (Populus x canescens). Planta doi/10.1007/s00425-005-0022-1 [DOI] [PubMed]
- Scholefield PA, Kieron J, Doick K-J, Herbert B, Hewitt CNS, Schnitzler JP, Pinelli P, Loreto F (2004) Impact of rising CO2 on VOC emissions: isoprene emission from Phragmites australis growing at elevated CO2 in a natural carbon dioxide spring. Plant Cell Environ 27: 381–392 [Google Scholar]
- Schürmann P (2003) Redox signalling in the chloroplast: the ferredoxin/thioredoxin system. Antioxid Redox Signal 5: 69–78 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F (1993) Water stress, temperature and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 343: 1–6 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374: 769 [Google Scholar]
- Sharkey TD, Singsaas EL, Vanderveer PJ, Geron C (1996) Field measurements of isoprene emission from trees in response to temperature and light. Tree Physiol 16: 649–654 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52: 407–436 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Yeh S, Wiberley AE, Falbel TG, Gong D, Fernandez DE (2005) Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol 137: 700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver GM, Fall R (1995) Characterisation of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J Biol Chem 270: 13010–13016 [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115: 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM (1992) The oxidizing capacity of the Earth's atmosphere: probable past and future changes. Science 256: 1157–1165 [DOI] [PubMed] [Google Scholar]
- Veau BMC, Oudin A, Chénieux J-C, Rideau M, Clastre M (2000) Cloning and expression of cDNAs encoding two enzymes of the MEP pathway in Catharanthus roseus. Biochim Biophys Acta 1517: 159–163 [DOI] [PubMed] [Google Scholar]
- Von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A, Kleinig H (1997) Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapsis alba and Arabidopsis thaliana seedlings. Plant J 12: 625–634 [DOI] [PubMed] [Google Scholar]
- Walter MH, Fester T, Strack D (2000) Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the “yellow pigment” and other apocarotenoids. Plant J 21: 571–578 [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Linskey AR, Falbel TG, Sharkey TD (2005) Development of the capacity for isoprene emission in kudzu. Plant Cell Environ 28: 898–905 [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F (2004) Rapid regulation of the methylerythritol 4-pathway during isoprene synthesis. Plant Physiol 135: 1939–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]