Abstract
In the cuticular wax mixtures from leaves of pea (Pisum sativum) cv Avanta, cv Lincoln, and cv Maiperle, more than 70 individual compounds were identified. The adaxial wax was characterized by very high amounts of primary alcohols (71%), while the abaxial wax consisted mainly of alkanes (73%). An aqueous adhesive of gum arabic was employed to selectively sample the epicuticular wax layer on pea leaves and hence to analyze the composition of epicuticular crystals exposed at the outermost surface of leaves. The epicuticular layer was found to contain 74% and 83% of the total wax on adaxial and abaxial surfaces, respectively. The platelet-shaped crystals on the adaxial leaf surface consisted of a mixture dominated by hexacosanol, accompanied by substantial amounts of octacosanol and hentriacontane. In contrast, the ribbon-shaped wax crystals on the abaxial surface consisted mainly of hentriacontane (63%), with approximately 5% each of hexacosanol and octacosanol being present. Based on this detailed chemical analysis of the wax exposed at the leaf surface, their importance for early events in the interaction with host-specific pathogenic fungi can now be evaluated. On adaxial surfaces, approximately 80% of Erysiphe pisi spores germinated and 70% differentiated appressoria. In contrast, significantly lower germination efficiencies (57%) and appressoria formation rates (49%) were found for abaxial surfaces. In conclusion, the influence of the physical structure and the chemical composition of the host surface, and especially of epicuticular leaf waxes, on the prepenetration processes of biotrophic fungi is discussed.
The primary physiological function of aboveground plant surfaces is to seal the tissue against a relatively dry atmosphere, preventing desiccation by minimizing nonstomatal water loss (Riederer and Schreiber, 1995; Kerstiens, 1996). Besides, plant surfaces are also of ecological importance as they necessarily represent the first line of contact with other organisms. There is growing evidence that insects can probe the surface composition of a substratum and the compatibility of many plant-herbivore relationships is first determined shortly after contact (Eigenbrode and Espelie, 1995). It has been suggested that plant surfaces also contain signals that influence germination of biotrophic fungi (Hegde and Kolattukudy, 1997) already in early infection stages stipulating host specificity of plant pathogens, such as powdery mildews (Carver et al., 1990; Tsuba et al., 2002). Specifically, germination (formation of a germ tube) and the subsequent differentiation of an appressorium might be triggered by surface signals. But the plant surface factors involved in these steps cannot be judged to date, as systematic investigations linking leaf surface composition to pathogen susceptibility are missing.
Primary plant tissues are covered with a cuticle, i.e. an extracellular lipid structure up to several micrometers thick. Two separate layers are generally recognized within plant cuticles—an inner portion characterized by intracuticular wax associated with a polyester matrix of cutin and a continuous surface layer of epicuticular wax without cutin (Jeffree, 1996). In many plant species, this wax surface is smooth, being formed by a relatively thin film of epicuticular material. In contrast, the surface of certain species is microscopically rough due to wax crystals protruding from the film. These epicuticular crystals exhibit a wide range of forms, e.g. platelets, ribbons, or rodlets (Barthlott et al., 1998), and specific shapes were found to be correlated with various prominent wax constituents (Baker, 1982). This indirect evidence suggested that these compounds both initiate crystallization and accumulate in the crystals. It was, for instance, assumed that platelet-shaped crystals on pea (Pisum sativum) leaves contained primary alcohols (Holloway et al., 1977). Hence, the presence of individual compounds at the very surface could qualitatively be inferred. But, as neither the crystal-forming compounds nor the possible admixtures could be quantified, the exact surface composition remained unknown.
Numerous reports have also directly addressed the composition of epicuticular waxes of diverse species. It was assumed that short extraction of intact tissue, typically yielding very-long-chain aliphatics and variable portions of triterpenoids, sterols, or phenolics, is specific for the outermost parts of the cuticle, i.e. the epicuticular wax layer (Silva Fernandes et al., 1964; Baker and Procopiou, 1975). But evidence is accumulating that even short extraction periods allow solvent molecules to enter deep into the cuticle and, therefore, release both epicuticular and intracuticular wax components (Jetter et al., 2000). The bulk wax extracts might consequently reflect surface composition only if both wax layers had similar composition. But this second assumption has repeatedly been challenged by studies that showed compositional gradients between intracuticular and epicuticular waxes of diverse species (Haas and Rentschler, 1984; Svenningsson, 1988). Hence, all those previous wax analyses relying on extraction lacked the necessary spatial resolution to accurately assess surface composition.
In a recent novel approach, gum arabic was employed as aqueous glue to mechanically remove surface compounds (Jetter and Schäffer, 2001). Quantitative analyses showed that the adhesive treatment was exhaustive and selective for epicuticular wax, while a consecutive extraction step released the remaining intracuticular wax. This sampling strategy proved to be very versatile and has yielded direct information on the surface composition of diverse plant species. In one application, the smooth epicuticular wax film on Prunus laurocerasus leaves was found to be dominated by aliphatic acetates, alcohols, and alkanes, depending on leaf developmental stages, respectively, while the intracuticular compartment was characterized by triterpenoids (Jetter et al., 2000; Jetter and Schäffer, 2001). In a second application, epicuticular crystals on leaves of the pitcher plant Nepenthes alata were reported to contain large concentrations of aldehydes, whereas other aliphatics accumulated in the underlying intracuticular wax (Riedel et al., 2003).
Using the greatly improved spatial resolution in cuticular wax sampling, surfaces of model plant species can now be analyzed in order to assess contact cues important in early stages of plant-pathogen interactions. Using this approach, we focused on leaves of pea and the host-specific powdery mildew fungus, Erysiphe pisi DC. Although total leaf wax analyses for two pea cultivars had been reported (Baker et al., 1963; Silva Fernandes, 1965; Macey and Barber, 1970), direct evidence for epicuticular crystal composition was lacking and, hence, the surface constituents relevant for plant-pathogen interaction were unknown. We selected three commercial pea cultivars to answer the following questions: (1) What is the quantitative composition of pea leaf epicuticular crystals; (2) how do crystal compositions differ between adaxial and abaxial leaf surfaces of various cultivars; and (3) do these differences affect early events in the infection by E. pisi?
RESULTS AND DISCUSSION
The central goal of this study was to analyze the leaf cuticular waxes of pea, with special emphasis on those components exposed at the outermost surface of the leaves. To evaluate the genetic variability of wax compositions, pea cv Avanta, cv Lincoln, and cv Maiperle were selected for comparative chemical analyses. On each of these three lines, a series of three independent experiments giving different spatial resolution was performed.
Total Leaf Wax Coverage and Composition
In the initial experiment, the overall leaf wax composition was analyzed for the three cultivars. To provide results comparable with previous reports from other cultivars, the standard protocol for cuticular wax sampling had to be employed, i.e. extraction by dipping intact leaves into organic solvent. Preliminary experiments had shown that reproducible maximum yields of waxes were achieved by two consecutive washes with warm chloroform. Hence, it can be assumed that this treatment was exhaustive for the soluble lipophilic components and that resulting extracts contained all the cuticular waxes. It should be noted that this approach, used in most published analyses of pea leaf cuticles, does not allow resolution of any possible differences between adaxial and abaxial leaf wax composition.
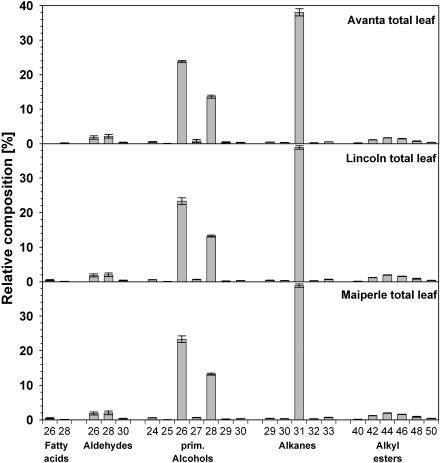
Total leaf wax coverage ranged from 14.7 ± 0.9 μg cm−2 for pea cv Maiperle to 24.3 ± 2.9 μg cm−2 for cv Avanta, differing significantly (P = 0.05) for all three lines (Table I). The overall results for these cultivars are in good accordance with previous reports indicating wax coverage of approximately 15 μg cm−2 for pea cv Surprise (Baker et al., 1963; Silva Fernandes, 1965). Pea cv Avanta and cv Lincoln were characterized by nearly identical qualitative wax compositions (Table I), their total leaf wax mixtures being dominated by 40% to 41% alkanes and 39% to 40% primary alcohols. Free fatty acids, aldehydes, alkyl esters, and secondary alcohols contributed between 0.2% and 6% to the wax mixtures. Only 6% of the total leaf cuticular waxes of these cultivars could not be identified. The cv Maiperle differed in composition, having significantly lower concentrations of alkanes and secondary alcohols that were compensated for by slightly increased amounts of all other compound classes.
Table I.
Composition of wax mixtures on leaves of three pea cultivars
Relative compound class compositions (%) and overall wax coverage (μg/cm2), analyzed in extracts from entire leaves or from individual leaf sides, are given as means from five replications with 95% confidence intervals.
| Avanta
|
Lincoln
|
Maiperle
|
|||
|---|---|---|---|---|---|
| Total Leaf | Adaxial | Abaxial | Total Leaf | Total Leaf | |
| Compound Class (%) | |||||
| Fatty acids | 0.2 ± 0.03 | 0.4 ± 0.04 | 0.1 ± 0.1 | 0.6 ± 0.2 | 0.3 ± 0.1 |
| Aldehydes | 4.4 ± 1.0 | 7.9 ± 0.5 | 3.8 ± 0.4 | 4.4 ± 1.1 | 5.8 ± 0.4 |
| Primary alcohols | 39.5 ± 0.4 | 67.6 ± 4.5 | 11.5 ± 1.0 | 38.4 ± 1.1 | 42.9 ± 0.6 |
| Esters | 5.6 ± 0.2 | 6.6 ± 0.3 | 2.3 ± 0.7 | 6.2 ± 0.2 | 5.5 ± 0.2 |
| Alkanes | 39.7 ± 1.1 | 14.3 ± 4.4 | 70.8 ± 2.6 | 40.6 ± 0.5 | 36.9 ± 0.5 |
| Secondary alcohols | 4.7 ± 0.1 | 0.3 ± 0.01 | 7.3 ± 0.5 | 4.4 ± 0.1 | 3.5 ± 0.2 |
| Unidentified | 5.9 ± 0.9 | 2.9 ± 0.2 | 4.3 ± 0.8 | 5.7 ± 0.7 | 5.4 ± 0.3 |
| Wax coverage (μg/cm2) | 24.3 ± 2.9 | 16.9 ± 1.0 | 25.3 ± 1.2 | 17.0 ± 0.9 | 14.7 ± 0.9 |
In the leaf waxes of all three pea cultivars, the n-alkane fraction comprised the complete series of chain lengths between C29 and C33, with a strong predominance of odd numbers and a maximum at C31 (Fig. 1). In addition, trace amounts of unbranched C25 to C28 alkane homologs could be identified, but not quantified. In the series of primary alcohols and aldehydes, chain lengths ranged between C24 and C32, while fatty acids C22 to C30 were identified. In all three compound classes, even-numbered homologs prevailed, with a maximum at C26, C28, and C26, respectively (Fig. 1). Esters with chain lengths between C40 and C50 could be identified and were found to contain C14 to C24 fatty acids condensed mainly with C26 primary alcohol, resulting almost exclusively in even-numbered homologs (Fig. 1). Thus, all the quantitative results of this investigation are in good accordance with earlier analyses of the total leaf wax mixtures from other pea cultivars (Kolattukudy, 1970; Macey and Barber, 1970).
Figure 1.
Relative composition of total leaf cuticular waxes of three cultivars of pea obtained by immersion of intact leaf blades into chloroform. Data are given as means from five replications with 95% confidence intervals (different letters differ significantly [P < 0.05] as determined by one-way ANOVA). Numbers on the x axis refer to the numbers of carbons in the chain of respective compounds.
In addition to the above, all the pea cultivars investigated here contained small amounts of secondary alcohols. Based on α-fragment abundances in the mass spectra of respective trimethylsilyl derivatives (data not shown), hentriacontan-16-ol and hentriacontan-15-ol constituted approximately two-thirds and one-third of this compound class in various cultivars, respectively. Trace amounts of hentriacontan-14-ol and of nonacosan-13-ol, -14-ol, and -15-ol were also detected. All of these secondary alcohols had previously been reported for another pea cultivar (Holloway et al., 1976). In summary, more than 70 individual compounds could be identified in the total leaf wax mixtures from pea cv Avanta, cv Lincoln, and cv Maiperle.
Adaxial and Abaxial Wax Coverage and Composition
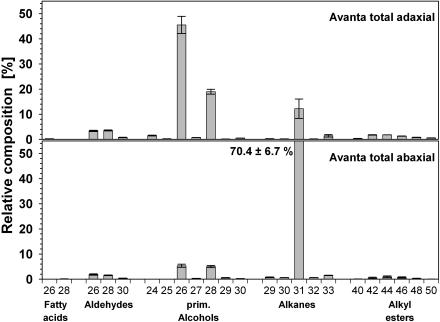
In a second experiment, the wax mixtures were extracted separately from adaxial and abaxial sides of leaves. The cuticular wax coverage on the adaxial side of cv Avanta was significantly lower than on the abaxial surface (Table I). Waxes from each side contained all those compound classes and homologs that had been detected in the total leaf wax extracts. The adaxial wax was characterized by very high amounts of primary alcohols (68%), with a strong predominance of hexacosanol (C26; Fig. 2). In contrast, the abaxial wax consisted mainly of alkanes (71%), with the dominating compound being hentriacontane (C31). The adaxial wax was further characterized by relatively high proportions of fatty acids, aldehydes, and alkyl esters, whereas the abaxial wax contained higher percentages of secondary alcohols. The homolog distributions in all these compound classes were very similar for both leaf sides, also matching the chain length patterns found for total leaf waxes (Fig. 2). The only notable difference between the adaxial and abaxial homolog distributions was the relatively high amount of C28 primary alcohol on the abaxial side. While the adaxial wax contained alcohol chain lengths C26 and C28 in a ratio of approximately 2:1, the wax from the abaxial side showed a ratio of about 1:1. Similar results were found for cv Lincoln and cv Maiperle (data not shown).
Figure 2.
Relative composition of cuticular waxes on adaxial and abaxial leaf sides of pea cv Avanta. Data are given as means from five replications with 95% confidence intervals (different letters differ significantly [P < 0.05] as determined by one-way ANOVA).
These results are in close accordance with the two previous reports distinguishing between wax mixtures from the different leaf sides of pea cv Meteor (Holloway et al., 1977) and the line WEL (wax eliminator; Eigenbrode et al., 1998). Unlike these earlier investigations, however, the two experiments described above also allow a direct comparison between the constitution of total leaf wax and that of the adaxial and abaxial waxes, since all extractions were performed under identical conditions (solvent used, extraction time, extraction temperature). The total wax coverage determined by entire leaf extraction did not differ significantly (P = 0.1) from the summed wax load of upper and lower leaf sides. Similarly, the coverage of compound classes and of individual constituents, when averaged for adaxial and abaxial surfaces, largely resembled those of the total leaf extracts. Hence, this investigation presents independent datasets that mutually confirm quantitative details of pea leaf wax composition and, furthermore, resolves differences between the adaxial and abaxial constituents.
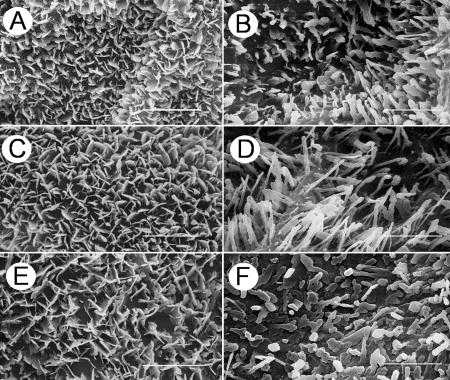
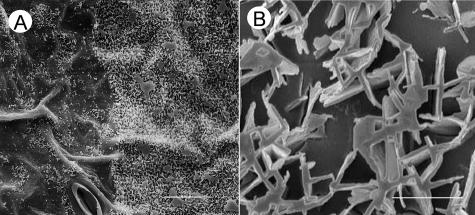
Both leaf surfaces of pea were densely covered with epicuticular wax crystals, which could be visualized by scanning electron microscopy (SEM; Fig. 3). On the adaxial leaf surface of all three cultivars, platelet-shaped crystals protruded perpendicularly from the leaf plane (Fig. 3, A, C, and E). No fine structural differences could be detected among the cultivars, the platelets in all cases exhibiting crenate margins and fusions at approximately right angles. The smooth surface of the cuticle proper was visible only in small portions of the adaxial side because the crystals formed a dense, homogeneous, and relatively thick network. The abaxial surfaces of all three cultivars were covered less densely with crystals. On cv Avanta and cv Lincoln, most of these crystals were upright, straight ribbons with length:width ratios ranging between 3:1 and 5:1, and with crenate margins (Fig. 3, B and D), although a few cylindrical rods were also present. The abaxial surface of cv Maiperle was characterized by short crystals with variably rounded cross-sections and a flat upper surface (Fig. 3F). Similar structures and marked differences between adaxial and abaxial wax structure had previously been described for other pea cultivars (Juniper, 1959; Holloway et al., 1977; Hunt and Baker, 1982; Ditsch et al., 1995; Eigenbrode et al., 1998).
Figure 3.
SEMs of pea leaves. Comparison between adaxial (left column) and abaxial (right column) leaf surfaces of cv Avanta (A and B), cv Lincoln (C and D), and cv Maiperle (E and F). Bars = 3 μm.
Selective Sampling of Epicuticular and Intracuticular Waxes
Based on the high percentages of hexacosanol and hentriacontane in the adaxial and abaxial wax extracts, respectively, it seemed likely that these compounds are involved in determining the crystalline structures of epicuticular waxes present on the different surfaces. To test this hypothesis, and to gain further direct evidence on the quantitative chemical composition of the surface structures on different sides of pea leaves, a third experiment was performed. A combination of mechanical and chemical sampling strategies was employed to selectively probe both epicuticular and intracuticular waxes. It had previously been shown that aqueous solutions of gum arabic can be used to mechanically remove both epicuticular wax films (Jetter and Schäffer, 2001) and epicuticular crystals (Riedel et al., 2003) from plant surfaces, and this approach, followed by chemical extraction, was adopted here.
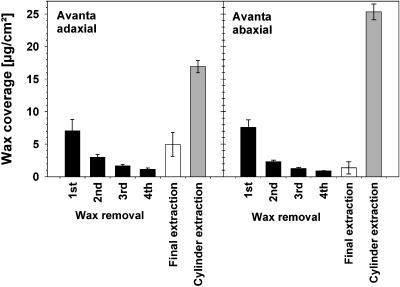
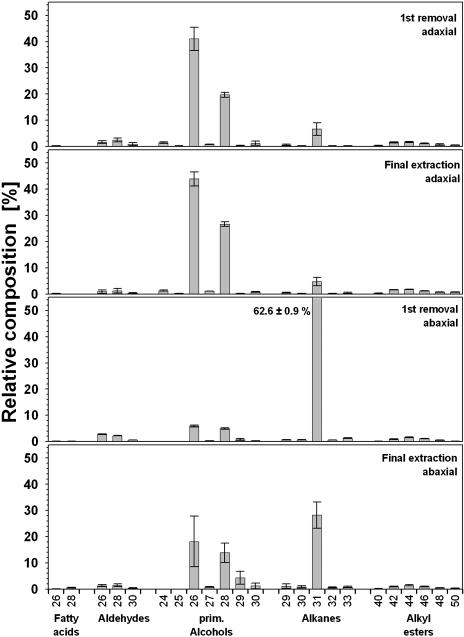
Repeated gum arabic treatments of adaxial and abaxial surfaces of cv Avanta yielded steadily decreasing amounts of waxes (Fig. 4) and it was found that, after four rounds of this mechanical procedure, no more material was collected. However, subsequent chloroform extraction of the gum arabic-treated surfaces yielded relatively high wax quantities, especially from adaxial surfaces. As previously shown (Jetter et al., 2000), this indicated that the gum Arabic sampling collected only those waxes that had been located outside the mechanical barrier formed by the outer surface of the cutin matrix that delineates the epicuticular wax layers. By contrast, the solvent was able to penetrate the cutin matrix. Hence, mechanical wax removal by gum arabic was selective for epicuticular material on pea leaves and repeated gum arabic application ensured the exhaustive removal of epicuticular material. Consequently, the remaining material released in the final extraction step can be interpreted as intracuticular wax.
Figure 4.
Yields of waxes removed from pea cv Avanta leaves by a series of mechanical and extractive treatments. In one experiment, four consecutive applications of gum arabic and a final extraction with chloroform were performed for adaxial and abaxial surfaces. Data from an independent experiment in which both leaf surfaces were separately extracted without prior mechanical treatment are given for comparison. Results are shown as means from five replications with 95% confidence intervals.
For cv Avanta, the total quantity of epicuticular plus intracuticular wax from the adaxial leaf side (Fig. 4) was nearly identical to the total wax coverage found in the extraction experiment described above. The epicuticular wax layer contained 74% of total wax. The sum of abaxial epicuticular and intracuticular wax did not match the total wax coverage determined for this side of the leaf (Fig. 4), possibly due to subtle differences between the leaf batches used for both experiments (gum arabic plus final extraction versus cylinder extraction). Nonetheless, the relative portions of both wax layers could also tentatively be interpreted for this leaf surface, and the epicuticular wax was found to contain 83% of the total yield of the mechanical plus extractive treatments. Very similar quantitative results were found for the other pea cultivars investigated (data not shown).
The effects of mechanical wax removal from pea leaves could be visualized by SEM (Fig. 5). After the first gum arabic treatment of the adaxial side, the immediate epidermal cell surfaces became visible (Fig. 5A) as the majority of platelet-shaped crystals had been removed. The area treated with gum arabic was delineated by a distinct boundary from the adjacent area where epicuticular crystals remained in their original arrangement. After removal, the lower side of the gum arabic films contained discrete wax platelets attached to the adhesive (Fig. 5B). In their arrangement, size, and individual shapes, they resembled the structures seen on the native leaf surfaces. Hence, it can be concluded that the epicuticular crystals on the adaxial leaf surface of pea have homogeneous forms starting at their outermost margins and extending to the points near the flat surface of the cuticle proper. At the same time, these intact crystal structures on the gum arabic preparations also showed that the mechanical treatment removed the crystals without disturbing the intracuticular layer, thus further confirming the selectivity of the collection method. Based on the crystal numbers visible both on the native surface and the gum arabic film, a space fill in the range of 5% to 10% can be estimated for the epicuticular structures. Further assuming wax densities near 1 g/cm3 and a thickness of the epicuticular crystal network of approximately 1 μm, the first gum arabic preparation should yield approximately 5 to 10 μg/cm2. This value is in good accordance with the chemical results (compare with Fig. 4).
Figure 5.
SEMs of pea epicuticular wax crystals. A, Adaxial leaf surface of cv Avanta after one locally restricted treatment with gum arabic. A clear borderline is visible between the treated leaf area (left side) and the adjacent untreated area (right side). Bar = 20 μm. B, Lower surface of gum arabic film containing removed epicuticular wax crystals. Bar = 2 μm.
Composition of Epicuticular and Intracuticular Waxes
In the selective preparations of epicuticular and intracuticular wax layers from both the adaxial and abaxial sides of cv Avanta, the same constituents were detected as in previous extraction experiments. In the adaxial wax mixture, fatty acids, esters, and secondary alcohols were found in similar percentages for both wax layers (Table II). In contrast, the primary alcohols occurred at much lower concentrations in the epicuticular wax than in the intracuticular layer (P = 0.01), while the aldehydes (P = 0.05) and the alkanes (P = 0.2) showed the opposite distribution. Due to the small sample amounts, relatively large portions of the material collected from both wax layers could not be identified. For the abaxial surface, similar differences between the compound class compositions of the epicuticular and intracuticular wax layers were found (Table II). The alkanes, predominating in the total extract from this side of the leaf, accumulated predominantly in the epicuticular wax (P = 0.001). In contrast, the primary alcohols predominated in the intracuticular layer (P = 0.002) even though their overall concentration was relatively low (compare with Table I). Within compound classes, the epicuticular and intracuticular wax mixtures from both sides of cv Avanta leaves showed chain length distributions similar to the corresponding total wax extracts (Fig. 6). Only the ratio between C26 and C28 primary alcohols was slightly shifted between layers on the adaxial surface. The intracuticular wax from this tissue contained relatively high percentages of the C28 homolog, as compared with the adjacent intracuticular mixture.
Table II.
Relative composition of epicuticular and intracuticular wax mixtures from leaves of pea cultivar Avanta
Compound class percentages are given as means from five replications with 95% confidence intervals.
| Compound Class (%)
|
Avanta Adaxial
|
Avanta Abaxial
|
||
|---|---|---|---|---|
| First Removal | Final Extraction | First Removal | Final Extraction | |
| Fatty acids | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.6 ± 0.2 |
| Aldehydes | 5.2 ± 1.4 | 2.6 ± 1.7 | 5.6 ± 0.2 | 3.2 ± 0.9 |
| Primary alcohols | 64.9 ± 4.6 | 74.3 ± 2.7 | 12.3 ± 1.0 | 38.4 ± 10.8 |
| Esters | 6.1 ± 0.8 | 6.5 ± 0.3 | 4.6 ± 0.4 | 4.3 ± 0.6 |
| Alkanes | 8.3 ± 2.0 | 6.4 ± 2.0 | 66.0 ± 0.9 | 31.7 ± 6.1 |
| Secondary alcohols | 1.9 ± 0.6 | 1.9 ± 0.4 | 6.6 ± 0.9 | 4.6 ± 0.8 |
| Unidentified | 12.7 ± 4.9 | 7.4 ± 1.1 | 4.0 ± 1.2 | 16.4 ± 5.3 |
Figure 6.
Relative composition of cuticular waxes obtained either by mechanical removal or by extraction from the adaxial and abaxial surface of pea cv Avanta. Results are shown as means from five replications with 95% confidence intervals. As mechanical wax removal proved to be specific for epicuticular wax crystals, respective results can be interpreted as composition of the very surface of the leaf relevant for interaction with fungal spores.
The first gum arabic treatment of the adaxial leaf surface yielded a mixture dominated by the C26 primary alcohol hexacosanol (Fig. 6A). It was accompanied by substantial amounts of octacosanol and hentriacontane, while diverse other very-long-chain compounds were present only in relative amounts below 2%. These components therefore appear to constitute the adaxial epicuticular wax crystals that are selectively removed in gum arabic (see above). Thus, these results provide direct evidence confirming inferences that could previously only be drawn from comparative studies: (1) Primary alcohols are involved in the formation of epicuticular crystals in the shape of platelets (Baker, 1982); and (2) one chain length, in this case C26, accumulates in the crystals to a relatively high level. In addition to these general conclusions, the gum arabic preparations allowed assessments of the percentages of minor compounds within the crystals. A second homologous alcohol plus a longer chain alkane most notably contribute to the epicuticular layer, and all three major crystal constituents thus share similar molecular geometry. Consequently, it is plausible that these compounds mix on a molecular scale, assembling within individual crystals instead of separating into adjacent platelets.
The epicuticular wax on the abaxial leaf surface of pea cv Avanta was strongly dominated by a single compound, hentriacontane, representing approximately 63% of this layer (Fig. 6C). In addition, approximately 5% of both hexacosanol and octacosanol were detected, together with even smaller percentages of all other constituents. Hence, the ribbon-shaped crystals on the lower leaf surfaces contained the same compounds as the platelets on the upper surfaces, albeit in very different proportions. Based on similar results for gum Arabic preparations from pea cv Lincoln and cv Maiperle (data not shown), similar compositions of epicuticular crystals on both leaf sides of these cultivars can be inferred.
In summary, these results quantify the chemical composition of the epicuticular crystals on both sides of the leaf and, therefore, of the interfaces relevant for the initial contact between pathogen spores and the host leaf. Similar details on the composition of epicuticular crystals on plant leaves had previously only been reported for N. alata (Riedel et al., 2003). In this case, platelets, appearing very similar to the crystals on adaxial pea leaf surfaces, contained high percentages of a very-long-chain aldehyde, triacontanal, together with other homologs and compound classes. The authors concluded that crystal formation was due to the high amounts of this aldehyde and that admixed compounds cocrystallize if they have sufficiently similar molecular characteristics. These results are in close accord with this interpretation.
Effects of Pea Leaf Surfaces on the Early Stages of E. pisi Development
The second goal of this investigation was to correlate variations in pea leaf cuticular composition with early events in the infection by E. pisi. Hence, we made a quantitative analysis of the two major stages of prepenetration fungal development, i.e. spore germination (as evidenced by germ tube emergence) and appressorium differentiation. This was first applied to the adaxial and abaxial leaf surfaces of the three pea cultivars, cv Avanta, cv Lincoln, and cv Maiperle. In order to control incubation conditions, detached leaflets were used as substrate for fungal spore development. Similar systems for E. pisi had been described (Ayres, 1983; Reeser et al., 1983), and detached leaf systems are routinely used for culture and analysis of the cereal powdery mildew fungi (Brown and Wolfe, 1990; Lyngkjær and Carver, 1999; Tsuba et al., 2002). Previous studies (Smith et al., 1996), as well as our own preliminary experiments (data not shown), further showed that E. pisi develops normally on detached leaflets where colonies also formed with frequencies similar to those seen on whole plants.
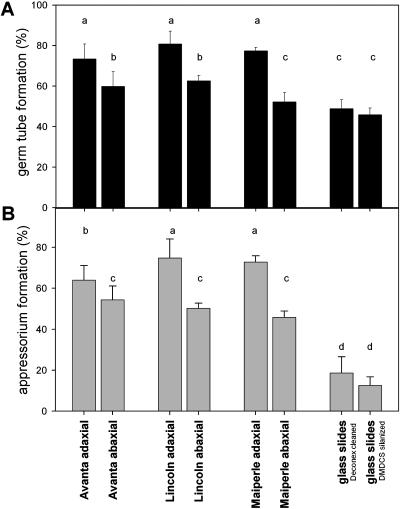
There were no significant differences between the three pea cultivars in frequencies of either spore germination or appressorium differentiation by E. pisi (Fig. 7). On adaxial surfaces, approximately 80% of the inoculated spores germinated and 70% differentiated appressoria. In contrast, significantly lower (P < 0.05) germination efficiencies (57%) and appressorium formation rates (49%) were found for abaxial surfaces. These differences between leaf surfaces are put into perspective by comparison with fungal behavior on artificial substrates. Fungal spores also develop on glass slides, but on these smooth polar surfaces only 49% of the E. pisi spores formed germ tubes and less than 20% developed appressoria. Very similar results were found for glass surfaces that had either been cleaned by various detergent applications or silanized to decrease surface polarity.
Figure 7.
Prepenetration development of E. pisi on leaf surfaces of three pea cultivars and on glass surfaces (Deconex cleaned = polar; DMDCS silanized = apolar). Percentages of spore germination (A) and of appressorial differentiation (B) are given as mean values ± sd of five parallels (Student-Newman-Keuls procedure, SPSS 10.0; different letters differ significantly [P < 0.05] as determined by one-way ANOVA).
These correlative results suggest three conclusions: (1) Processes leading to germination and appressorium development are stimulated, but at very low frequency, by contact with artificial substrates; (2) specific leaf surface characteristics provide a stimulating signal or signals that augments both processes; and (3) adaxial surfaces apparently provide more stimulation than abaxial surfaces since they supported intermediate success rates for germination and appressorium differentiation.
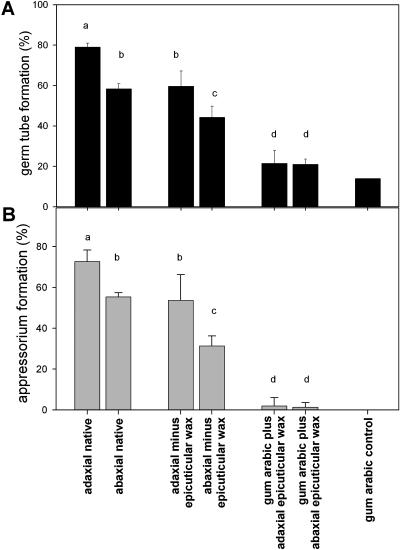
To further corroborate these inferences, fungal development was tested on artificial surfaces derived from adaxial and abaxial leaf sides of pea (Fig. 8). After mechanical removal of epicuticular wax crystals (using gum arabic as described above), the frequencies of germination and appressorium formation decreased significantly. The differences between fungal behavior on both leaf sides persisted after removal of the surface wax layers, with germination rates of 60% and 50% on the adaxial and abaxial leaf sides, and appressorium formation rates of 50% and 35%, respectively. These changes in fungal development reflect alterations in the physical structure of both surfaces (compare with Fig. 5), as well as changes in the chemical composition of the abaxial surface (compare with Fig. 6). Overall, the results confirm the conclusion that the epicuticular wax crystals contain stimuli for early steps in fungal development.
Figure 8.
Prepenetration development of E. pisi on native leaf surfaces of pea cv Avanta and on the same leaf surfaces after manipulation with gum arabic to remove the epicuticular wax layer. The gum arabic fractions with and without integrated epicuticular waxes were analyzed as substratum for spore development. Percentages of spore germination (A) and of appressorial differentiation (B) are given as mean values ± sd of three parallels (Student-Newman-Keuls procedure, SPSS 10.0; different letters differ significantly [P < 0.05] as determined by one-way ANOVA).
The gum arabic preparations, containing the epicuticular wax crystals after removal from the leaf surface, did not stimulate fungal development beyond control values for pure gum arabic or glass surfaces. Moreover, germination and appressorium formation frequencies were identical for the gum arabic preparations from both leaf sides. These seemingly contradictory results could be due to artifacts caused by the presence of gum arabic. The porous structure of this polymer likely absorbs fungal exudates necessary for successful development, thus preventing germination and appressorium differentiation. This physical effect of gum arabic may have superseded possible stimulatory effects of the epicuticular wax crystals.
The differences in strength of specific plant surface signals triggering early steps in fungus development can now be correlated with the physical and chemical properties of adaxial and abaxial pea leaf cuticles. As epicuticular wax crystals cover both sides of the leaves, they are characterized by similar micro-roughness and polarity. Accordingly, the leaf surfaces of all three pea lines were highly water repellent, with nearly identical contact angles of 144.7° ± 4.9° and 141.2° ± 4.3° for adaxial and abaxial surfaces, respectively. Since both sides of the leaf have such similar physical properties, it seems most likely that fungal adhesion forces will differ only slightly for adaxial and abaxial surfaces. But both leaf sides were found to have largely differing surface composition, the epicuticular crystals of the adaxial surface being dominated by the primary alcohols hexacosanol and octacosanol, whereas the abaxial surface consists mainly of the alkane hentriacontane. We therefore conclude that the crystal composition, rather than the surface geometry, carries the surface cues that stimulate early fungal development.
An influence of the chemical composition of the substrate surface, and especially of epicuticular leaf waxes, on the prepenetration processes of biotrophic fungi had been described in several investigations (Carver and Thomas, 1990; Podila et al., 1993; Hwang and Kolattukudy, 1995; Rubiales and Niks, 1996; Hegde and Kolattukudy, 1997; Iwamoto et al., 2002; Niks and Rubiales, 2002; Tsuba et al., 2002). Most interestingly, in one study comparable to this investigation, normal germling development of Erysiphe graminis (the grass powdery mildew fungus) was reported on the adaxial leaf surface of ryegrass (Lolium sp.), whereas conidia on the abaxial leaf surface generally produced only multiple short germ tubes and few formed normal appressoria (Carver et al., 1990). Amorphous wax sheets and wax crystals in the shape of platelets were described for the abaxial and adaxial leaf surfaces, respectively. This result clearly points to differences in the epicuticular wax composition of the different surfaces of ryegrass, but data on their composition are not available and the chemical cues for fungal development cannot be judged. Application of analytical approaches such as those described here offers the possibility of gaining detailed understanding of the early stages of plant-pathogen interactions. This will help toward understanding the control of intracellular signaling processes that drive germination and infection structure differentiation by plant pathogenic fungi and may reveal approaches for modifying plant surface features that can confer novel forms of disease resistance.
MATERIALS AND METHODS
Plant Material
Seeds of pea (Pisum sativum) cv Avanta, cv Lincoln, and cv Maiperle were obtained from IPK Gatersleben GenBank, Germany. Plants were grown in soil:sand (1:1) mixed with 50% vermiculite in plastic pots (diameter 9 cm). The plants were kept in growth chambers under the following conditions: day/night 14 h/10 h; light intensity 300 to 400 μmol photons m−2 s−1; temperature 22°C/18°C; relative humidity 70%. For analysis, fully expanded 13-d-old leaves were harvested randomly (BBCH macro stage 1, code 15). Leaf surface areas were determined gravimetrically using photocopies of the extracted leaves.
Sampling of Cuticular Waxes
Total leaf wax extracts were obtained by dipping entire leaves twice for 60 s into 100 mL CHCl3 that had been heated to approximately 40°C and contained n-tetracosane as internal standard. Selective extraction of total cuticular waxes from either the adaxial or the abaxial surface was achieved by placing the intact leaf onto a flexible rubber mat, gently pressing a glass cylinder with 10-mm diameter onto the exposed surface, and filling the cylinder with approximately 1.5 mL of warm chloroform (40°C). The solvent was agitated for 30 s by pumping with a Pasteur pipette and removed. This procedure was repeated once and both extracts were combined. Extracts from five individual leaves were pooled for further analysis, n-tetracosane was immediately added as internal standard, and the solvent was removed under reduced pressure.
A polymer film of gum arabic was employed for the selective mechanical collection and analysis of surface waxes. Prior to use, gum arabic (Roth) was extracted exhaustively with hot chloroform in order to remove impurities. Approximately 0.1 mL of a 90% (w/w) aqueous solution of gum arabic was applied per square centimeter of leaf surface using a small paint brush. After 1 to 2 h, the gum arabic solution had formed a dry and stable polymer film in which wax crystals were embedded. The film was broken away and the pieces were collected in a vial containing 7 mL each of chloroform and water. The polymer films from five leaflets were pooled into the same two-phase systems and n-tetracosane was added as internal standard. After vigorous agitation and phase separation, the organic solution was removed, concentrated under reduced pressure, and stored until analyzed. After treatment with gum arabic, the leaves were physically intact and subjected to further, sequential treatment with gum arabic to collect remaining epicuticular waxes and, ultimately, to a final solvent extraction of intracuticular waxes.
Chemical Analysis
Prior to gas chromatography (GC) analysis, hydroxyl-containing compounds in all samples were transformed into the corresponding trimethylsilyl derivatives by reaction with bis-N,O-trimethylsilyltrifluoroacetamide (Macherey-Nagel) in pyridine (30 min at 70°C). The qualitative composition of the mixtures was studied using capillary GC (6890N; Agilent Technologies) with on-column injection (30 m OV-1 WCOT, i.d. 320 μm; Chrompack) and mass spectrometric detection (70 eV, m/z 50–650; MSD 5973; Agilent Technologies). GC was carried out with oven temperature programmed for 2 min at 50°C, 40°C min−1 to 200°C, 2 min at 200°C, 3°C min−1 to 320°C, 30 min at 320°C, and He carrier gas inlet pressures programmed 5 min at 50 kPa, 3 kPa min−1 to 150 kPa, and 30 min at 150 kPa. Wax components were identified by comparison of their mass spectra with those of authentic standards and literature data. For quantification of individual compounds, GC was used under conditions as described above, but with carrier gas H2 (5 min at 5 kPa, 3 kPa min−1 to 50 kPa, and 30 min at 50 kPa) and a flame ionization detector (HP ChemStation software package).
Characterization of Surface Structures
Pieces of gum arabic film with embedded epicuticular wax crystals were placed bottom up on glass slides, thus allowing a view onto the lower parts of the crystals. The glass slides carrying the wax preparations, as well as leaf samples, were mounted on aluminum holders, sputter coated with gold palladium (Bal-Tec SCD005 sputter coater; 25 mA, 300 s), and examined by SEM (Zeiss DSM 962, 15 kV). The sputtering conditions, depositing approximately 20 nm of the alloy on the tissue samples, were optimized for the acceleration voltage used in the SEM.
To measure leaf surface hydrophobicity, contact angles using 3-μL droplets of distilled water were determined (contact angle system OCA15 using software system SCA20; Dataphysics Instruments). For each line, 10 independent measurements were carried out for both the adaxial and abaxial leaf surface.
Infection Studies and Characterization of Mildew Development
Detached 13-d-old healthy leaves of the different cultivars were fixed horizontally, either adaxial or abaxial surface up, at the base of a settling tower. The same arrangement was used in a second experiment with native leaf surfaces of cv Avanta, with the corresponding leaf surfaces after treatment with gum arabic, with the corresponding gum arabic preparations after the treatment, and with pure gum arabic. Conidia (asexual spores) from infected pea leaves were blown directly into the tower using pressurized air to ensure their even distribution. After inoculation, single leaves were laid in glass petri dishes lined with wet filter paper and incubated in a growth chamber for 14 h at 20°C in the dark. For fixation and chlorophyll removal, leaves were treated so as to avoid displacement of ungerminated conidia (Lyngkjær and Carver, 1999). Briefly, the leaves were placed, inoculated surface up, onto Whatman 3MM paper moistened with ethanol:acetic acid (3:1) until bleached, transferred for 4 h to filter paper moistened with water, and then for 24 h to filter paper moistened with lactoglycerol (lactic acid:glycerol:water [1:1:1]). Finally, fungal structures were stained for 3 min by carefully pipetting a few drops of lactophenol cotton blue (0.1%) onto their inoculated surface. On each surface, 500 individual spores were analyzed by light microscopy (Leica DMR with Leica IM1000 software package) to determine whether they had germinated (formed a visible germ tube) and, if so, whether a lobed appressorium (infection structure) had differentiated. For each cultivar and treatment, five independent replicates were examined.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permission will be the responsibility of the requester.
Acknowledgments
We thank Dr. C. Hart, Jealott's Hill Research Station, UK, for fruitful discussions. Technical assistance by Christina Specht, Bianka Pink, and the staff of the Botanical Garden of the University of Würzburg is gratefully acknowledged.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 567, and the Fonds der Chemischen Industrie.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.053579.
References
- Ayres PG (1983) Conidial germination and germ-tube growth of Erysiphe pisi in relation to visible light and its transmission through pea leaves. Trans Br Mycol Soc 81: 269–274 [Google Scholar]
- Baker EA (1982) Chemistry and morphology of plant epicuticular waxes. In DF Cutler, KL Alvin, CE Price, eds, The Plant Cuticle. Linnean Society Symposium Series, Vol 10. Academic Press, London, pp 139–165
- Baker EA, Batt RF, Silva Fernandes AMS, Martin JT (1963) Cuticular waxes of plant species and varieties. In Annual Report of the Agricultural and Horticultural Research Station, Long Ashton. University of Bristol, Bristol, UK, pp 106–110
- Baker EA, Procopiou J (1975) The cuticles of Citrus species. Composition of the intracuticular lipids of leaves and fruits. J Sci Food Agric 26: 1347–1352 [Google Scholar]
- Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H (1998) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126: 237–260 [Google Scholar]
- Brown JKM, Wolfe MS (1990) Structure and evolution of a population of Erysiphe graminis f. sp. hordei. Plant Pathol 39: 376–390 [Google Scholar]
- Carver TLW, Thomas BJ (1990) Normal germling development by Erysiphe graminis on cereal leaves freed of epicuticular wax. Plant Pathol 39: 367–375 [Google Scholar]
- Carver TLW, Thomas BJ, Ingersonmorris SM, Roderick HW (1990) The role of the abaxial leaf surface waxes of Lolium spp. in resistance to Erysiphe graminis. Plant Pathol 39: 573–583 [Google Scholar]
- Ditsch F, Patha H, Barthlott W (1995) Micromorphology of epicuticular waxes in Fabales s.l. and its systematic significance. Beitr Biol Pflanz 68: 297–310 [Google Scholar]
- Eigenbrode SD, Espelie KE (1995) Effects of plant epicuticular lipids on insect herbivores. Annu Rev Entomol 40: 171–194 [Google Scholar]
- Eigenbrode SD, White C, Rohde M, Simon CJ (1998) Epicuticular wax phenotype of the wel mutation and its effect on pea aphid populations in the greenhouse and in the field. Pisum Genet 20: 13–17 [Google Scholar]
- Haas K, Rentschler I (1984) Discrimination between epicuticular and intracuticular wax in blackberry leaves: ultrastructural and chemical evidence. Plant Sci Lett 36: 143–147 [Google Scholar]
- Hegde Y, Kolattukudy PE (1997) Cuticular waxes relieve self-inhibition of germination and appressorium formation by the conidia of Magnaporthe grisea. Physiol Mol Plant Pathol 51: 75–84 [Google Scholar]
- Holloway PJ, Hunt GM, Baker EA, Macey MJK (1977) Chemical composition and ultrastructure of the epicuticular wax in four mutants of Pisum sativum (L). Chem Phys Lipids 20: 141–155 [Google Scholar]
- Holloway PJ, Jeffree CE, Baker EA (1976) Structural determination of secondary alcohols from plant epicuticular waxes. Phytochemistry 15: 1768–1770 [Google Scholar]
- Hunt GM, Baker EA (1982) Developmental and environmental variations in plant epicuticular waxes: some effects on the penetration of naphthylacetic acid. In DF Cutler, KL Alvin, CE Price, eds, The Plant Cuticle. Linnean Society Symposium Series, Vol 10. Academic Press, London, pp 279–292
- Hwang C-S, Kolattukudy PE (1995) Isolation and characterization of genes expressed uniquely during appressorium formation by Colletotrichum gloeosporioides conidia induced by the host surface wax. Mol Gen Genet 247: 282–294 [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Takeuchi Y, Takada Y, Yamaoka N (2002) Coleoptile surface cuticle of barley is involved in survival and penetration of Blumeria graminis. Physiol Mol Plant Pathol 60: 31–38 [Google Scholar]
- Jeffree CE (1996) Structure and ontogeny of plant cuticles. In G Kerstiens, ed, Plant Cuticles. An Integrated Approach, Ed 1. BIOS Scientific, Oxford, pp 33–82
- Jetter R, Schäffer S (2001) Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol 126: 1725–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter R, Schäffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23: 619–628 [Google Scholar]
- Juniper BE (1959) Die Oberflächen von Pflanzen. Endeavour 18: 20–25 [Google Scholar]
- Kerstiens G (1996) Cuticular water permeability and its physiological significance. J Exp Bot 47: 1813–1832 [Google Scholar]
- Kolattukudy PE (1970) Composition of the surface lipids of pea leaves (Pisum sativum). Lipids 5: 398–402 [Google Scholar]
- Lyngkjær MF, Carver TLW (1999) Induced accessibility and inaccessibility to Blumeria graminis f. sp. hordei in barley epidermal cells attacked by a compatible isolate. Physiol Mol Plant Pathol 55: 151–162 [Google Scholar]
- Macey MJK, Barber HN (1970) Chemical genetics of wax formation on leaves of Pisum sativum. Phytochemistry 9: 5–12 [Google Scholar]
- Niks RE, Rubiales D (2002) Potentially durable resistance mechanisms in plants to specialised fungal pathogens. Euphytica 124: 201–216 [Google Scholar]
- Podila GK, Rogers LM, Kolattukudy PE (1993) Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol 103: 267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeser P, Hagedorn DJ, Rouse DI (1983) Quantitative inoculations with Erysiphe pisi to assess variation of infection efficiency on peas. Phytopathology 73: 1238–1240 [Google Scholar]
- Riedel M, Eichner A, Jetter R (2003) Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta 218: 87–97 [DOI] [PubMed] [Google Scholar]
- Riederer M, Schreiber L (1995) Waxes—the transport barriers of plant cuticles. In RJ Hamilton, Waxes: Chemistry, Molecular Biology and Functions, Ed 1, Vol 6. The Oily Press, Dundee, UK, pp 131–156
- Rubiales D, Niks RE (1996) Avoidance of rust infection by some genotypes of Hordeum chilense due to their relative inability to induce the formation of appressoria. Physiol Mol Plant Pathol 49: 89–101 [Google Scholar]
- Silva Fernandes AMS (1965) Studies on the plant cuticle VIII. Surface waxes in relation to water-repellency. Ann Appl Biol 56: 297–304 [Google Scholar]
- Silva Fernandes AMS, Baker EA, Martin JT (1964) Studies on the plant cuticle. VI. The isolation and fractionation of cuticular waxes. Ann Appl Biol 53: 43–58 [Google Scholar]
- Smith PH, Foster EM, Boyd LA, Brown JKM (1996) The early development of Erysiphe pisi on Pisum sativum L. Plant Pathol 45: 302–309 [Google Scholar]
- Svenningsson M (1988) Epi- and intracuticular lipids and cuticular transpiration rates of primary leaves of eight barley (Hordeum vulgare) cultivars. Physiol Plant 73: 512–517 [Google Scholar]
- Tsuba M, Katagiri C, Takeuchi Y, Takada Y, Yamaoka N (2002) Chemical factors of the leaf surface involved in the morphogenesis of Blumeria graminis. Physiol Mol Plant Pathol 60: 51–57 [Google Scholar]