Abstract
A theory of fruit climacteric ethylene emission was developed and used as the basis of a simulation model called ETHY. According to the theory, the biosynthetic pathway of ethylene is supplied by ATP and is regulated by 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase. The conjugation of ACC with malonate to form MACC was taken into account as a way to decrease the availability of ACC. Because of the seasonal increase of fruit volume, the dilution of biochemical compounds used in ETHY was taken into account. Finally, the ethylene diffusion across the skin was considered. The theory took into account the effect of temperature and O2 and CO2 internal concentrations on ethylene. The model was applied to peach (Prunus persica) fruit over 3 years, several leaf:fruit ratios, and irrigation conditions. An adequate ethylene increase was predicted without considering any increase in respiration during the ripening period, which suggests that the respiratory climacteric may not be required for ripening. Another important result of this study is the high sensitivity of ETHY to the parameters involved in the calculation of ACC oxidase and ACC synthase activities, ATP production, and skin surface and permeability. ETHY was also highly sensitive to changes in fruit growth and temperature.
The plant hormone ethylene (C2H4) plays a major role in the ripening process of climacteric fruits. Ripening parameters such as flesh softening (Haji et al., 2003; Hiwasa et al., 2003), color change (Flores et al., 2001), and production of aromas depend strongly on C2H4 production (Rupasinghe et al., 2000; Alexander and Grierson, 2002; Flores et al., 2002).
C2H4 production in plant tissues has been studied extensively (Arshad and Frankenberger, 2002). It proceeds via a biosynthetic pathway, which was first established in apple (Malus domestica) fruit by Adams and Yang (1977). This pathway proceeds from Met, through S-adenosylmethionine (SAM) and 1-aminocyclopropane-1-carboxylic acid (ACC) to C2H4. The pathway enables high rates of C2H4 production without high intracellular concentrations of Met. This is achieved by recycling 5′-methylthioadenosine (MTA) to Met through the Yang cycle. The respiration produces the ATP needed for the Yang cycle. Thanks to this recycling process, high levels of C2H4 can be produced with a constant pool of Met (Arshad and Frankenberger, 2002). It is known that C2H4 production rates in ripening fruit are controlled by the tissue's capability to synthesize ACC and to convert ACC to C2H4. The two key controls are ACC synthase (ACCs) and ACC oxidase (ACCo; Tucker, 1993). The C2H4 is diluted in fruit tissue and then diffused into the atmosphere. Thus, fruit volume and permeability of skin to gas are important biophysical traits of fruit to consider when analyzing C2H4 emission (Ben-Yehoshua and Cameron, 1989).
The aim of this work was to develop a theory of C2H4 emission, based on a mathematical representation of the respiration process and C2H4 pathway. This representation was chosen to be as simple as possible because a lot of quantitative information about the regulation of this pathway is missing. The theory relates C2H4 production to fruit growth and environmental conditions. Indeed, fruit C2H4 production is known to vary during the development of the fruit depending on environmental conditions such as temperature; levels of O2 and CO2 in the air (Lelièvre et al., 1997); and fruit growth manipulated by changing leaf:fruit ratio (Poll et al., 1996; Souty et al., 1999), fruit thinning (Johnson, 1995), or branch ringing (Agusti et al., 1998). The theory was the basis of the ETHY simulation model, which predicts the seasonal C2H4 production. The model was tested by comparing simulations to experimental data recorded on peach (Prunus persica) fruit during 3 years under various leaf:fruit ratios and irrigation conditions. The importance of the different processes and environmental factors involved in C2H4 production was analyzed through a sensitivity analysis.
THEORETICAL ANALYSIS OF THE SYSTEM
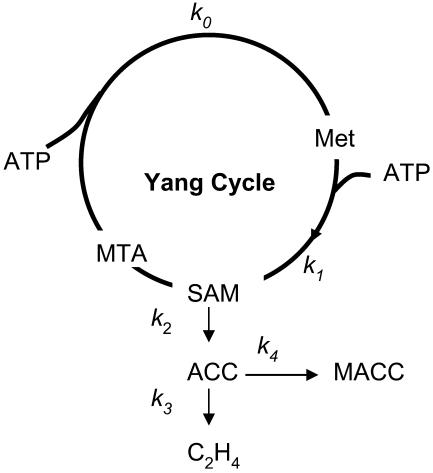
The theory concerns C2H4 emission during the last period of fruit development (for a peach, this is the last 2–3 months before maturity). It concerns (1) the biosynthetic pathway of C2H4, which includes the Yang cycle and the SAM-C2H4 pathway schematized in Figure 1, and (2) dilution and diffusion of C2H4 through the fruit skin.
Figure 1.
Schematic representation of the Yang cycle and SAM-C2H4 pathway. The metabolites considered are ATP, Met, SAM, MTA, MACC, and C2H4. Chains of reactions involved in their conversions are represented as simple reactions. The ki are the rate constants of the reactions.
It is known that Met and SAM pools are too low in plant tissues to sustain normal rates of ACC production, implying that they must be continuously regenerated (Baur and Yang, 1972). This is the role of the Yang cycle. As this cycle is supplied by ATP (Kushad et al., 1983; Theologis, 1992), ATP production through respiration is an important aspect of ETHY. The SAM-C2H4 pathway is regulated by ACCs and ACCo, which are the two key enzymes considered in ETHY. The stress was laid on the autocatalytic C2H4 production, which results in up-regulation by C2H4 of these two enzymes. The conjugation of ACC with malonate to form malonyl-ACC (MACC) was taken into account in ETHY as a way to decrease the availability of ACC for C2H4 production. Because of the seasonal increase of fruit volume, the dilution of biochemical compounds used in ETHY was taken into account. Finally, C2H4 diffusion across the skin was considered.
Metabolic and biophysical processes are strongly connected in ETHY. For example, the diffusion of C2H4 in the ambient atmosphere depends on the internal C2H4 concentration, which depends itself on the intensity of the diffusion and on other processes (dilution, metabolism). Yang cycle, respiration, production of MACC, regulation of ACCs and ACCo, dilution, and C2H4 release in ambient atmosphere are the basic processes represented in ETHY. The fruit is described as one compartment separated from the exterior by the skin. The ambient atmosphere plays the role of the exterior compartment. Gas exchange occurs by diffusion through the fruit skin. The temperature of the fruit is assumed to be equal to that of the ambient atmosphere.
Fruit growth is considered to influence C2H4 production because it is related to respiration, dilution, and to the skin area through which C2H4 is released. The temperature is an important external factor controlling respiration in ETHY. The model also takes into account the effect of O2 and CO2 internal concentrations on C2H4 through their action on ACCo, which requires O2 and is inhibited by high concentrations of CO2 (Lelièvre et al., 1997).
The main state variables of the system are MACC, ACC, CO2, O2, and C2H4 concentrations in the fruit. The hourly information coming from the external compartment is the temperature and the concentrations of O2 and CO2 in the ambient atmosphere. This information and the fruit growth components (dry and fresh mass, and dry growth rate) represent the ETHY inputs. Using the inputs together with a theoretical analysis quantified by the governing equations, we compute the main reaction rates of the C2H4 biosynthesis pathway and gas transfer processes. The lists of the ETHY variables and parameters are presented in “Materials and Methods.”
Governing Equations
Dilution versus Metabolism and/or Diffusion
In the following, the amount of a reactant X per fruit will be noted X and its concentration [X] (mol m−3).
As [X] = X/V, with V (m−3) ≈ 10−6 Mfresh and Mfresh (g) the fruit fresh mass, we get:
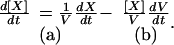 |
(1, a and b) |
This equation, which shows that the rate of variation of the concentration of a reactant, depends on two components related to metabolism and/or diffusion of X outside the fruit (a) and to dilution (b), will be used in the following to take into account the increase of fruit fresh mass during growth.
Yang Cycle, and the SAM-C2H4 and ACC-MACC Pathways
The Yang cycle and the SAM-C2H4 pathway (Fig. 1) are described according to the “rate law” of chemical kinetics (Chang, 2000), which states that the rate of a reaction is proportional to the reactant X in the fruit. The rate constant is denoted ki. The following set of equations was used for the Yang cycle:
 |
(2) |
In keeping with the recycling scheme, 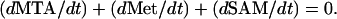
The variation of ACC results from the balance between its synthesis, its degradation in C2H4 controlled by ACCo (k3; h−1), and its conjugation with malonate to form MACC catalyzed by the ACC N-malonyltransferase (k4; h−1). The rate law equation for ACC is then:
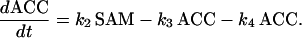 |
(3) |
Assuming the Yang cycle at steady-state 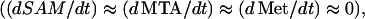 Equation 2 shows that the rate of synthesis of ACC from SAM is proportional to the amount of ATP present:
Equation 2 shows that the rate of synthesis of ACC from SAM is proportional to the amount of ATP present:
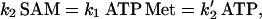 |
where k′2 (h−1) is a rate constant related to the activity of ACCs.
The ACC concentration depends on the amount of ACC per fruit and on dilution by water according to Equations 1 and 3. The rate law equation for ACC concentration is then:
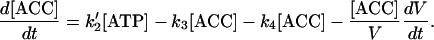 |
(4) |
The variation of MACC concentration is:
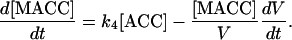 |
(5) |
The C2H4 concentration in the fruit depends on the one hand on the balance between C2H4 biosynthesis and diffusion to the external atmosphere (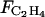 ; mol h−1), and on the other hand on dilution:
; mol h−1), and on the other hand on dilution:
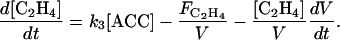 |
(6) |
Enzyme Regulations
Although the ACC N-malonyltransferase is developmentally regulated (Arshad and Frankenberger, 2002), the rate constant k4 was considered invariant for the sake of simplicity. Both ACCs and ACCo are encoded by multigene families (Lelièvre et al., 1997) and are regulated by a number of regulating factors. The values of the rate constants k′2 and k3 depend on the level of regulating factors, which influence the activities of both enzymes. The rate constants were considered to be the product of a parameter (respectively, kS and kO; h−1) by functions of regulating factors. Both enzymes are regulated by C2H4 (Lelièvre et al., 1997). For the sake of simplicity, the effect was considered to be proportional to the C2H4 concentration. The oxygen concentration in the tissues plays an important role in C2H4 biosynthesis since oxygen is a cosubstrate of ACCo. It has been assumed that ACCo is inhibited by carbon dioxide (Rothan and Nicolas, 1994). To describe this regulation of ACCo, a generalized form of the Michaelis-Menten equation used by de Wild et al. (1999) for pear (Pyrus communis) was applied.
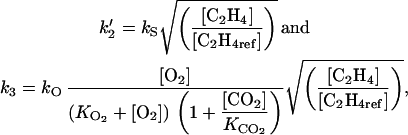 |
(7) |
with [C2H4ref] a reference concentration equal to 1 mol m−3 and 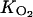 (mol m−3) and
(mol m−3) and 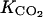 (mol m−3) Kms.
(mol m−3) Kms.
C2H4 Diffusion to the External Atmosphere
C2H4 diffusion to the external atmosphere has been described by Fick's first law for flat surfaces, which has often been applied to the study of gas exchange in bulky organs (Ben-Yehoshua and Cameron, 1989). Considering that C2H4 concentration in the ambient atmosphere is nil, C2H4 diffusion to the external atmosphere is:
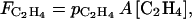 |
(8) |
where 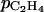 (m h−1) is the apparent skin permeability and A (m2) is the skin area.
(m h−1) is the apparent skin permeability and A (m2) is the skin area.
A is estimated from fruit mass through an empirical equation:
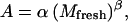 |
(9) |
where α and β are parameters.
According to Lescourret et al. (2001), skin permeability can vary with fruit size. It was assumed to vary linearly with fruit size:
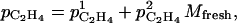 |
(10) |
where 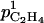 (m h−1) and
(m h−1) and 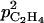 (m g−1 h−1) are parameters.
(m g−1 h−1) are parameters.
ACC and C2H4 Concentrations, and ACCs and ACCo Activities
The rate of variation of ACC and C2H4 concentration may be obtained by combining Equations 4 and 6 to 8:
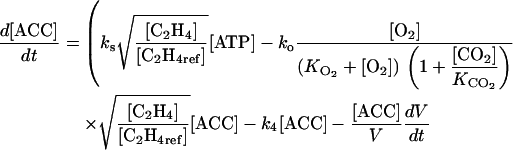 |
(11) |
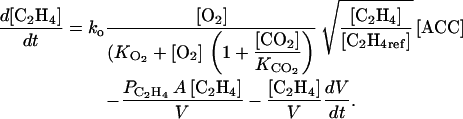 |
(12) |
The activity of ACCs is represented by ACC synthesis for a given concentration of ATP ([ATP0]). The activity of ACCo is represented by C2H4 synthesis for a given concentration of 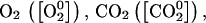 and ACC ([ACC0]).
and ACC ([ACC0]).
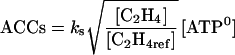 |
(13) |
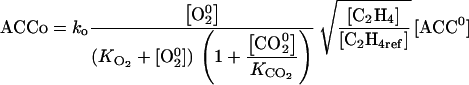 |
(14) |
ATP, O2, and CO2 Concentrations
The concentration of ATP in the fruit results from the balance between ATP production during the respiration process and consumption for energy-requiring processes with a rate constant λ (h−1) and dilution in water. One mol of CO2 produced by respiration was assumed to be coupled with the production of 5 mol of ATP (Cannell and Thornley, 2000). Accordingly, the variation of ATP concentration is:
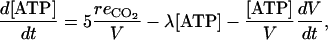 |
(15) |
where 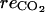 is the rate of CO2 production caused by respiration.
is the rate of CO2 production caused by respiration.
Spoelstra et al. (2002) have shown in tomato (Lycopersicon esculentum) embryos that the change of ATP concentration is negligible compared to the actual amount of ATP synthesized and turned over (i.e. 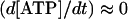 ). Assuming that such a property is valid for the fruit, the ATP concentration can be approximated by the following:
). Assuming that such a property is valid for the fruit, the ATP concentration can be approximated by the following:
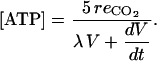 |
(16) |
This equation means that the ATP concentration is proportional to the respiration per unit of fruit mass when the fruit growth is negligible, which has been shown in ripening avocado (Persea americana) fruit (Bennett et al., 1987).
The oxygen and carbon dioxide concentrations are calculated assuming that they are under steady-state condition [i.e. respiration rates for O2 (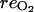 ) and CO2 are equal to the fluxes of these gases through the skin to the external atmosphere], and the gas diffusion to the external atmosphere can be described by Fick's first law:
) and CO2 are equal to the fluxes of these gases through the skin to the external atmosphere], and the gas diffusion to the external atmosphere can be described by Fick's first law:
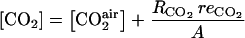 |
(17) |
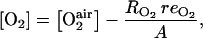 |
(18) |
where 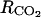 (h m−1) and
(h m−1) and 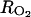 (h m−1) are resistances to diffusion of CO2 and O2, respectively. The air CO2 and O2 concentrations in mol m−3 are calculated from the percentage of O2 in the air using the ideal-gas equation.
(h m−1) are resistances to diffusion of CO2 and O2, respectively. The air CO2 and O2 concentrations in mol m−3 are calculated from the percentage of O2 in the air using the ideal-gas equation.
The respired oxygen is calculated from the CO2 produced by the respiration using the respiratory quotient (RQ) concept:
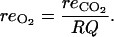 |
(19) |
The growth-maintenance paradigm (Cannell and Thornley, 2000) was used to calculate the respiration in terms of CO2. The growth respiration is considered proportional to fruit growth rate and the maintenance respiration to dry mass and temperature (Penning de Vries and van Laar, 1982; Thornley and Johnson, 1990). The effect of temperature is described with the Q10 concept. The fruit respiration in terms of CO2 production (mol h−1) is then calculated as:
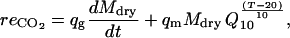 |
(20) |
where Mdry (g) is fruit dry mass, qg (mol g−1) the growth respiration coefficient, qm (mol g−1 h−1) the maintenance respiration coefficient at 20°C, Q10 the temperature ratio of maintenance respiration, and T (°C) the temperature.
Equations 16 to 20 are combined to calculate ATP, CO2, and O2 concentrations as a function of fruit dry mass, volume, area, and temperature:
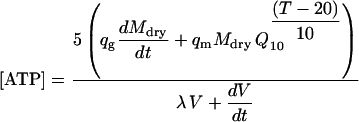 |
(21) |
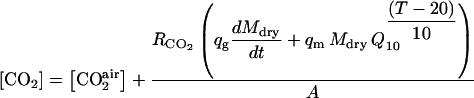 |
(22) |
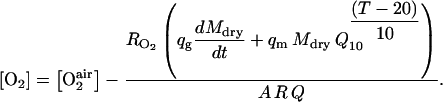 |
(23) |
Equations 5, 8 to 12, and 21 to 23 form a system of equations for [ACC], [MACC], [C2H4], [ATP], [CO2], [O2], and 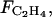 which can be solved numerically with given (inputted) functions of time Mdry(t), Mfresh(t) and T(t); O2 and CO2 concentrations in the air (assumed to be constant); and the initial values of respective variables.
which can be solved numerically with given (inputted) functions of time Mdry(t), Mfresh(t) and T(t); O2 and CO2 concentrations in the air (assumed to be constant); and the initial values of respective variables.
Initial Conditions and Parameterization
The MACC concentration reported in the literature during the early growth of fruits is very variable, from 3 10−5 to 8 10−3 mol m−3 according to the authors (Amoros et al., 1989; Tonutti et al., 1991; Lara and Vendrell, 2000). We chose an initial value of 5 10−4 mol m−3 to run the model. The ACC concentration can be very low before the rise of C2H4 production. ACC concentrations lower than 10−5 mol m−3 have been reported (Amoros et al., 1989; Lara and Vendrell, 2000). An initial value of 10−5 mol m−3 was chosen to run the model. The internal C2H4 concentration was reported to stay between almost zero and 2 10−6 mol m−3 before the C2H4 crisis for different fruit species (Lyons et al., 1962; Lau et al., 1986; Saltveit, 1993; Johnston et al., 2002). An initial value of 10−6 mol m−3 was chosen for the internal C2H4 concentration.
The Michaelis-Menten constant for O2 and CO2 inhibition of C2H4 production were deduced from the experiment of de Wild et al. (1999) on pear fruit (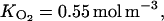
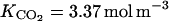 ). The apparent peach skin permeability to C2H4 was measured by Cameron and Reid (1982). Considering at first approximation that it was unvarying with fruit development, we took their estimation (2 10−3 m h−1) for
). The apparent peach skin permeability to C2H4 was measured by Cameron and Reid (1982). Considering at first approximation that it was unvarying with fruit development, we took their estimation (2 10−3 m h−1) for 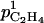 and zero for
and zero for 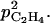 Parameters α = 6.049 10−4 and β = 0.601 in Equation 9, relating the fruit surface area to the fruit mass, were taken from Fishman and Génard (1998) for the peach cultivar Suncrest. To estimate λ, the rate constant of ATP consumption, we considered that the mean ATP concentration in the fruit tissues was 0.015 mol m−3 according to the data of Saquet et al. (2000, 2003a, 2003b). Then Equation 21 was used to get an estimation of λ = 500 h−1. The temperature ratio of maintenance respiration Q10 was taken equal to 2 as estimated by Pavel and DeJong (1993). The qg and qm were estimated using nonlinear least squares regression, Equation 20, and respiration measurements. The growth respiration coefficient was estimated to be equal to qg = 0.0025 ± 2 10−4 mol g−1, which is lower than the value (0.007 mol g−1) obtained by DeJong and Goudriaan (1989) for peach fruits but close to coefficients obtained for fleshy fruits such as mango (Mangifera indica; 0.0033 mol g−1) and cucumber (Cucumis sativus; 0.0036 mol g−1) by Léchaudel et al. (2005) and Marcelis and Baan Hofman-Eijer (1995). The maintenance respiration coefficient at 20°C was estimated to be equal to qm = 12 10−5 ± 5 10−6 mol g−1 h−1, a value higher than that estimated for peach (5.6 10−5 mol g−1 d−1) by DeJong and Goudriaan (1989), lower than that measured on cucumber (34 10−5 mol g−1 d−1) by Marcelis and Baan Hofman-Eijer (1995) and on tomato (27 10−5 mol g−1 d−1) by Walker and Thornley (1977), but comparable to that obtained on mango (9.6 10−5 mol g−1 d−1) by Léchaudel et al. (2005). The respiratory quotient (RQ) was calculated as the mean of the measured ratio
Parameters α = 6.049 10−4 and β = 0.601 in Equation 9, relating the fruit surface area to the fruit mass, were taken from Fishman and Génard (1998) for the peach cultivar Suncrest. To estimate λ, the rate constant of ATP consumption, we considered that the mean ATP concentration in the fruit tissues was 0.015 mol m−3 according to the data of Saquet et al. (2000, 2003a, 2003b). Then Equation 21 was used to get an estimation of λ = 500 h−1. The temperature ratio of maintenance respiration Q10 was taken equal to 2 as estimated by Pavel and DeJong (1993). The qg and qm were estimated using nonlinear least squares regression, Equation 20, and respiration measurements. The growth respiration coefficient was estimated to be equal to qg = 0.0025 ± 2 10−4 mol g−1, which is lower than the value (0.007 mol g−1) obtained by DeJong and Goudriaan (1989) for peach fruits but close to coefficients obtained for fleshy fruits such as mango (Mangifera indica; 0.0033 mol g−1) and cucumber (Cucumis sativus; 0.0036 mol g−1) by Léchaudel et al. (2005) and Marcelis and Baan Hofman-Eijer (1995). The maintenance respiration coefficient at 20°C was estimated to be equal to qm = 12 10−5 ± 5 10−6 mol g−1 h−1, a value higher than that estimated for peach (5.6 10−5 mol g−1 d−1) by DeJong and Goudriaan (1989), lower than that measured on cucumber (34 10−5 mol g−1 d−1) by Marcelis and Baan Hofman-Eijer (1995) and on tomato (27 10−5 mol g−1 d−1) by Walker and Thornley (1977), but comparable to that obtained on mango (9.6 10−5 mol g−1 d−1) by Léchaudel et al. (2005). The respiratory quotient (RQ) was calculated as the mean of the measured ratio 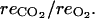 The estimation was RQ = 0.834 ± 0.006. The ranges of CO2 and O2 concentrations for fleshy fruits are 0.4% to 8% and 15% to 19%, respectively, according to measurements obtained for cantaloupe (Cucumis melo; Lyons et al., 1962), orange (Citrus sinensis; Ben-Yehoshua et al., 1985), avocado (Ben-Yehoshua et al., 1963), pear (Williams and Patterson, 1962), and tomato (Saltveit, 1993). To estimate the CO2 and O2 resistances to diffusion (
The estimation was RQ = 0.834 ± 0.006. The ranges of CO2 and O2 concentrations for fleshy fruits are 0.4% to 8% and 15% to 19%, respectively, according to measurements obtained for cantaloupe (Cucumis melo; Lyons et al., 1962), orange (Citrus sinensis; Ben-Yehoshua et al., 1985), avocado (Ben-Yehoshua et al., 1963), pear (Williams and Patterson, 1962), and tomato (Saltveit, 1993). To estimate the CO2 and O2 resistances to diffusion (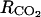 and
and 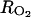 ), we considered that the mean concentrations in the fruit tissues have to be in these ranges. Using Equations 22 and 23, we obtained
), we considered that the mean concentrations in the fruit tissues have to be in these ranges. Using Equations 22 and 23, we obtained 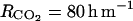 and
and 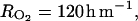 which are close to the minimal resistance values obtained by Cameron and Reid (1982) for different fruit species (RCO2 = 117–583 h m−1, RO2 = 80–833 h m−1). The air O2 and CO2 concentrations used in Equations 22 and 23 were 20.97% and 0.03%, respectively. The rate constant for ACC malonyltransferase was taken at k4 = 0.001 h−1 in order to obtain coherent MACC concentrations with what is mentioned in the literature with regard to peach (3 10−5 to 10−2 mol m−3; Amoros et al., 1989; Tonutti et al., 1991). The other parameters of the model (ks and ko) were estimated using nonlinear least squares, the model (to predict the C2H4 emission), and C2H4 measurements.
which are close to the minimal resistance values obtained by Cameron and Reid (1982) for different fruit species (RCO2 = 117–583 h m−1, RO2 = 80–833 h m−1). The air O2 and CO2 concentrations used in Equations 22 and 23 were 20.97% and 0.03%, respectively. The rate constant for ACC malonyltransferase was taken at k4 = 0.001 h−1 in order to obtain coherent MACC concentrations with what is mentioned in the literature with regard to peach (3 10−5 to 10−2 mol m−3; Amoros et al., 1989; Tonutti et al., 1991). The other parameters of the model (ks and ko) were estimated using nonlinear least squares, the model (to predict the C2H4 emission), and C2H4 measurements.
ETHY Inputs
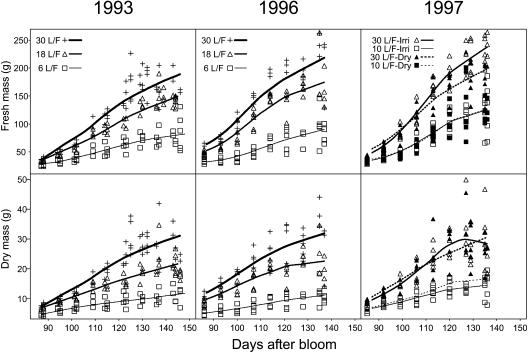
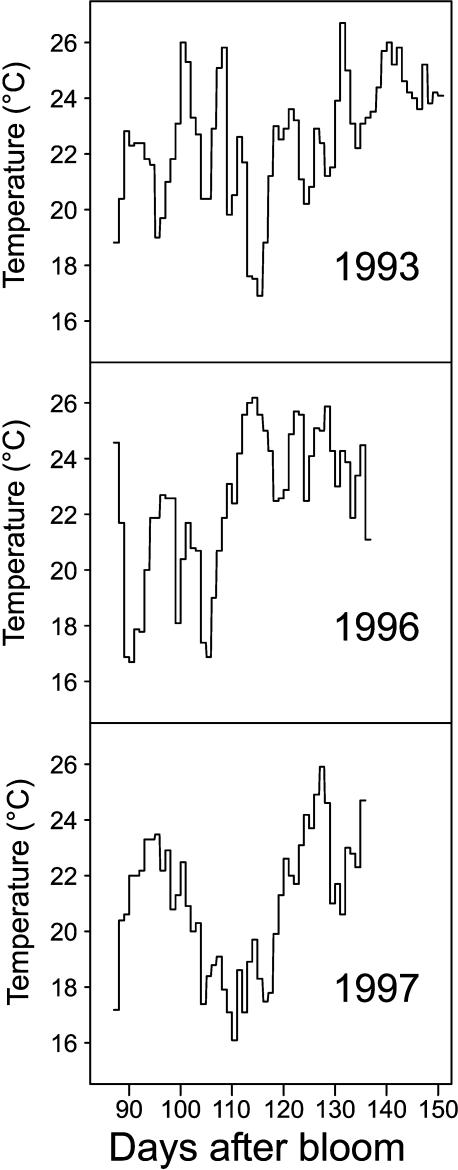
The seasonal trends of fruit masses are shown in Figure 2. Fresh and dry masses increased with both fruit age and leaf:fruit ratio. The suppression of tree irrigation in 1997 had only a minor negative effect on fresh mass for the 30 leaf:fruit ratio. There was high variability between fruit masses for a given treatment and date. That is why the mean, maximal, and minimal growth curves (Fig. 3) were used for each treatment and year as inputs of ETHY. The mean temperature was 22.5°C in 1993 and 1996, and 21°C in 1997. The mean daily temperature fluctuated greatly each year between 16°C and 26°C (Fig. 4).
Figure 2.
Seasonal variation of peach fruit (cv Suncrest) fresh and dry mass over the 3-year experimental period. Points are experimental data and lines smooth curves. The leaf:fruit and irrigation treatments are indicated on the graphs.
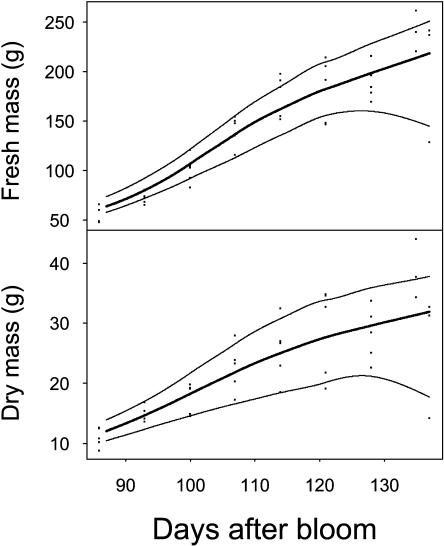
Figure 3.
Seasonal variation of peach fruit (cv Suncrest) fresh and dry mass in 1996 for the treatment involving 30 leaves per fruit. The maximal, mean, and minimum curves are drawn. Points are experimental data and lines smooth curves.
Figure 4.
Seasonal variation of mean daily temperature over the 3-year experimental period.
RESULTS
ETHY Goodness-of-Fit and Predictive Quality
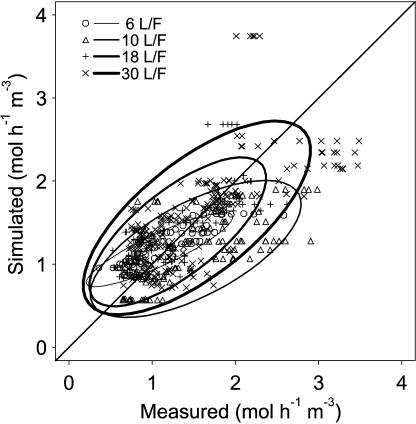
The model was able to simulate the order of magnitude of fruit respiration, the lack of irrigation effect (data not shown), and the increase of maximal respiration with the leaf:fruit ratio (Fig. 5). Root mean squared errors (RMSE) varied from 0.16 to 0.38 mol h−1 m−3 according to year and leaf:fruit ratio treatment, and the mean value of the error was 0.27 mol h−1 m−3. The predictive quality of the model was quite correct: the root mean squared errors of prediction (RMSEP), which ranged from 0.16 to 0.54 mol h−1 m−3 with a mean equal to 0.3 mol h−1 m−3, were close to the RMSE.
Figure 5.
Simulated versus measured respiration rate over the 3-year experimental period. Variance ellipses indicating 90% of the data for a given leaf:fruit ratio are drawn (the thickness of the ellipse line is proportional to the leaf:fruit ratio).
With regard to C2H4, the RMSE varied from 8 to 234 μmol h−1 m−3, according to year and leaf:fruit ratio, and the mean value of the error was 89 μmol h−1 m−3. The RMSEP ranged from 7 to 260 μmol h−1 m−3 with an average of 107 μmol h−1 m−3. The parameters related to ACCs and ACCo were estimated at ks = 0.778 ± 0.042 h−1, ko = 0.268 ± 0.017 h−1.
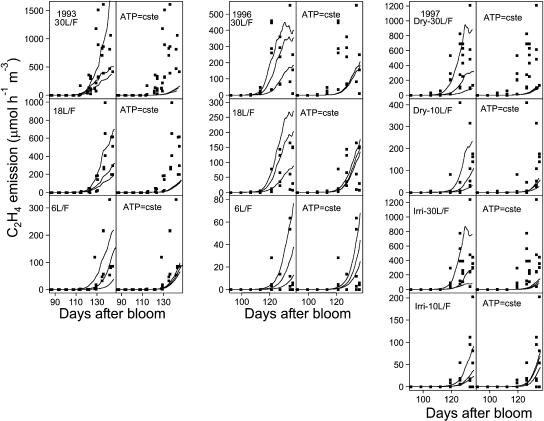
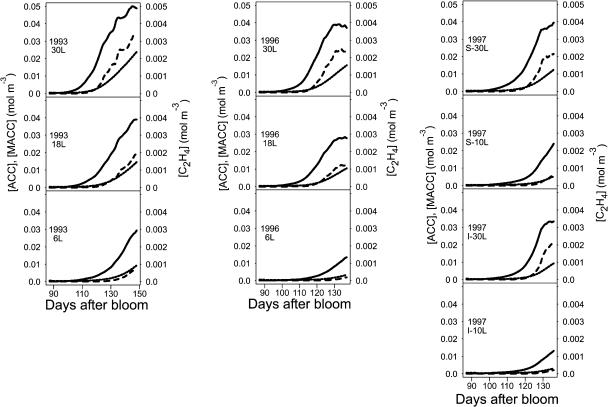
In accordance with the measured data, the model predicted an increase in C2H4 production with the leaf:fruit ratio, no effect of irrigation treatment, and lower C2H4 production in 1996 than in the other two years (Fig. 6). The intratreatment variation of C2H4 production was high (Fig. 6), which was fairly well depicted by the model using as input the maximal and minimal growth curves of each treatment.
Figure 6.
Observed (points) and simulated (lines) C2H4 emission for each treatment over the 3-year experimental period. The three lines per graph originate from three simulations corresponding to the maximum, mean, and minimum masses of fruits. For each year and treatment, the case where the ATP concentration varies as a function of respiration according to the model assumption (graphs on the left for each experiment) and the case where it is constant were considered (graphs on the right).
Seasonal Variation of ATP, Internal O2 and CO2, ACC, MACC, and C2H4 Concentrations in Response to Year and Fruit Growth
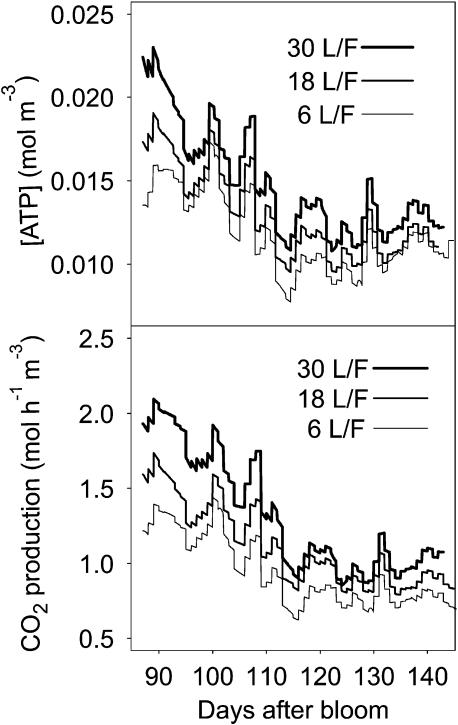
The model predicted significant variations of ATP concentration from 0.008 to 0.023 mol m−3 with a general decrease following that of fruit respiration per unit of fruit volume (Fig. 7). The increase of fruit growth from six to 30 leaves per fruit has for consequence a 25% increase of ATP concentration. At a short time step, fairly high fluctuations of ATP concentrations are simulated, which results mainly from the effect of temperature on fruit respiration (Figs. 4 and 7).
Figure 7.
Seasonal variation of ATP concentration and respiration rate simulated by ETHY over the three leaf:fruit ratios studied in 1993.
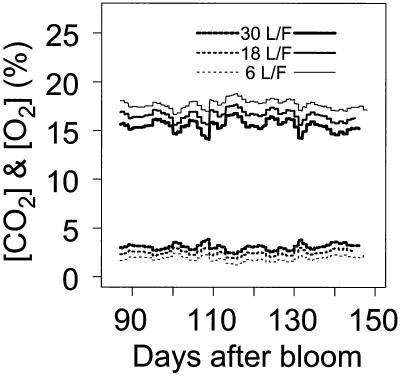
The predicted concentrations of O2 in the fruit for the different treatments and years were in the14% to 18.7% range, which were slightly lower than in the air (21%). The predicted CO2 concentrations in the fruit (1.3%–3.9%) were much higher than in the air (0.03%). Under the steady-state assumption, the O2 and CO2 concentrations in the fruit were fairly stable during the season, but short time variations were observed in response to temperature fluctuations (Fig. 8). The increase of fruit growth with leaf:fruit ratio resulted in an [O2] decrease and [CO2] increase in the fruit.
Figure 8.
Seasonal variation of O2 (continuous lines) and CO2 (dashed lines) concentrations simulated by ETHY over the three leaf:fruit ratios studied in 1993.
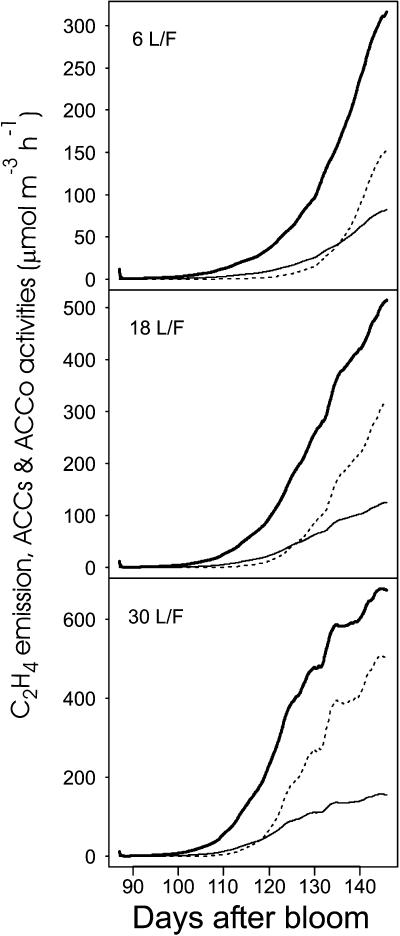
The model predicted a seasonal increase of ACC, MACC, and C2H4 concentrations and a positive effect of leaf number per fruit (Fig. 9). The concentration of C2H4 was 10 times lower than that of MACC and ACC, and MACC concentrations were always lower than those of ACC. The ACC reached its maximal concentration before MACC and C2H4, especially in the case of high leaf:fruit ratio. The activity of ACCs and ACCo was simulated for [ATP0] = 0.015 mol m−3, [ACC] = 0.015 mol m−3, and ambient [O2] and [CO2] for the treatment with six and 30 leaves per fruit in 1993. The activity of the two enzymes increased from 100 to 140 d after bloom (DAB) and this all the more strongly as the leaf:fruit ratio was high (Fig. 10). The C2H4 production started much later and this more especially as the leaf:fruit ratio was low.
Figure 9.
Seasonal variation of ACC (continuous lines), MACC (dotted line), and C2H4 (dashed lines) concentrations simulated by ETHY over the treatments and years studied.
Figure 10.
Seasonal variation of ACCo (continuous thin lines) and ACCs (continuous thick lines) activities, and C2H4 (dashed lines) emission simulated by ETHY over the treatments in 1993.
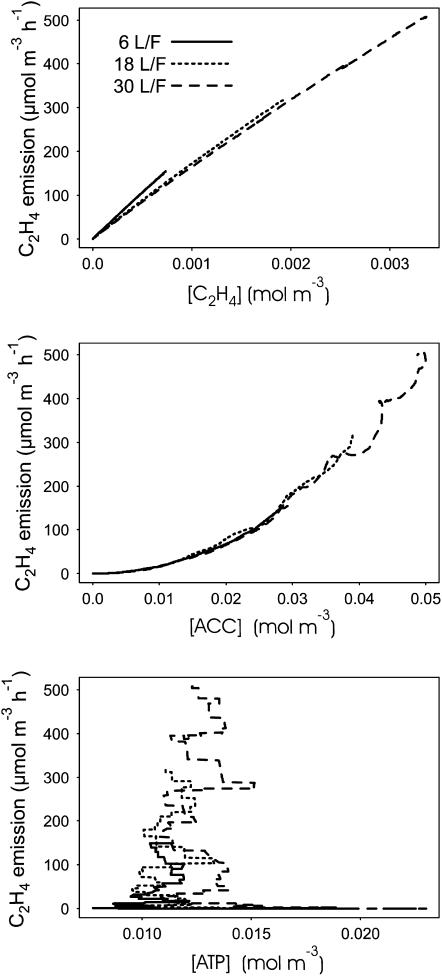
Key Internal Variables for C2H4 Production
According to ETHY, C2H4 emission depends on three main variables, the concentration of ATP on which ACCs depends, the concentration of ACC on which C2H4 synthesis depends, and the concentration of C2H4 itself on which the intensity of the diffusion in the external atmosphere depends. The link between C2H4 emission and these three variables was analyzed using the simulations of the leaf:fruit ratio done in 1993. As expected, there was, whatever the leaf:fruit ratio, a unique very strong positive linear link between internal C2H4 concentration and C2H4 emission (Fig. 11). A strong positive exponential link was also found with ACC concentration. There was no obvious connection between C2H4 emission and the ATP concentration, which shows that there is no link between C2H4 emission at a given time and ATP concentration at this time. This could be due to the low ATP cost of C2H4 production. The amount of ATP needed for C2H4 production was computed considering that two ATP are needed to produce one ACC molecule (Fig. 1). The computed ATP cost of C2H4 production was always below 0.04% of ATP production by respiration. Nevertheless, ATP concentration is a key variable for C2H4 production. Indeed, keeping ATP concentration as a constant (0.013 mol m−3), whatever the leaf:fruit ratio, and re-estimating the parameters (ks = 0.5 ± 0.08 h−1 and ko = 0.36 ± 0.06 h−1) led to a poor adjustment to the experimental data (RMSE = 131 μmol h−1 m−3). With a constant concentration of ATP, the model was able to simulate correct C2H4 production for low leaf:fruit ratio but predicted too low the C2H4 production for high leaf:fruit ratio (Fig. 6).
Figure 11.
Relationships between C2H4 emission and C2H4, ACC, and ATP concentrations simulated by ETHY over the three leaf:fruit ratios studied in 1993.
Key Parameters of Fruit Metabolism
A sensitivity analysis to the model parameters was performed to look for the key parameters, i.e. the parameters whose variation has a strong effect on the model outputs. The analysis was performed using the inputs of 1993. To see if the model was sensitive to different degrees to small or large parameter variations, each parameter was varied from X = ±1%, 5%, 10%, 20%, 40%, 60%, 80% and 100% around the value reported in “Materials and Methods.” The effect of this variation on the average value of (1) O2, CO2, ATP, ACC, MACC, and C2H4 concentrations and (2) fruit respiration and C2H4 emission was assessed. The model was considered sensitive to a parameter when an X percentage of variation of this parameter resulted in at least an X/2 percentage of variation of one or more of the above-mentioned variables. As the model was similarly sensitive to parameters whatever the level of X (data not shown), only the results obtained for X = ±20% are presented (Table I). The sensitivity increased from six to 30 leaves per fruit. However, as the general trends were very similar, only the results obtained for 18 leaf:fruit ratio in 1993 were presented (Table I). The oxygen internal concentration was only slightly sensitive to parameter β relating fruit surface area to fruit mass. The higher the fruit surface was, the higher the internal O2 concentration was. The carbon dioxide internal concentration was sensitive to β but also to α (Eq. 9), to the resistance to CO2 diffusion (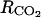 ), and to the maintenance respiration coefficient (qm). The lower the fruit surface and the higher
), and to the maintenance respiration coefficient (qm). The lower the fruit surface and the higher 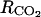 and qm were, the higher the carbon dioxide internal concentration was. The respiration was only sensitive to qm. The ATP concentration was only slightly sensitive to the maintenance respiration coefficient and to λ, which is the rate constant of ATP consumption. The ACC, MACC, and C2H4 concentrations and C2H4 emission were sensitive to the same set of parameters, which are growth and maintenance respiration coefficients; λ; parameters ks and ko, which are involved in the activities of ACCs and ACCo; the skin permeability to C2H4
and qm were, the higher the carbon dioxide internal concentration was. The respiration was only sensitive to qm. The ATP concentration was only slightly sensitive to the maintenance respiration coefficient and to λ, which is the rate constant of ATP consumption. The ACC, MACC, and C2H4 concentrations and C2H4 emission were sensitive to the same set of parameters, which are growth and maintenance respiration coefficients; λ; parameters ks and ko, which are involved in the activities of ACCs and ACCo; the skin permeability to C2H4 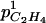 ; and parameters α and β. They were sensitive to a lesser degree to
; and parameters α and β. They were sensitive to a lesser degree to 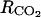 and
and 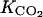 involved in CO2 diffusion and in ACCo inhibition by CO2. The ACC, MACC, and C2H4 concentrations and C2H4 emission were only slightly sensitive to the rate constant k4, which determines MACC synthesis. This means that the possible variation of ACC malonyltransferase activity would have a small impact on C2H4 synthesis, except if this variation is large enough to compensate for the low sensitivity.
involved in CO2 diffusion and in ACCo inhibition by CO2. The ACC, MACC, and C2H4 concentrations and C2H4 emission were only slightly sensitive to the rate constant k4, which determines MACC synthesis. This means that the possible variation of ACC malonyltransferase activity would have a small impact on C2H4 synthesis, except if this variation is large enough to compensate for the low sensitivity.
Table I.
Effect of a ± 20% variation of the ETHY parameters on the average value of O2, CO2, ATP, ACC, MACC, and C2H4 concentrations, and fruit respiration and C2H4 emission
Values are expressed as a percentage of the reference condition. The analysis was performed using the inputs of treatment 18 leaves per fruit in 1993.
| Parameters | [O2] | [CO2] | [ATP] | [MACC] | [ACC] | [C2H4] | Respiration | C2H4 Emission | |
|---|---|---|---|---|---|---|---|---|---|
| Respiration parameters | |||||||||
| qg | +20 | −1 | 5 | 5 | 21 | 17 | 26 | 5 | 26 |
| −20 | 1 | −5 | −5 | −20 | −16 | −24 | −5 | −24 | |
| qm | +20 | −4 | 15 | 15 | 50 | 45 | 81 | 15 | 82 |
| −20 | 4 | −15 | −15 | −40 | −40 | −57 | −15 | −58 | |
| Q10 | +20 | −1 | 4 | 4 | 8 | 10 | 17 | 4 | 17 |
| −20 | 1 | −4 | −4 | −9 | −10 | −17 | −5 | −17 | |
| RQ | +20 | 4 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| −20 | −7 | 0 | 0 | −1 | −1 | −2 | 0 | −2 | |
| ATP parameter | |||||||||
| λ | +20 | 0 | 0 | −17 | −54 | −52 | −74 | 0 | −74 |
| −20 | 0 | 0 | 25 | 118 | 89 | 186 | 0 | 191 | |
| MACC, ACC, and C2H4 metabolic parameters | |||||||||
| k4 | +20 | 0 | 0 | 0 | 9 | −7 | −12 | 0 | −12 |
| −20 | 0 | 0 | 0 | −12 | 7 | 13 | 0 | 13 | |
| ks | +20 | 0 | 0 | 0 | 92 | 71 | 143 | 0 | 146 |
| −20 | 0 | 0 | 0 | −61 | −61 | −82 | 0 | −82 | |
| ko | +20 | 0 | 0 | 0 | 44 | 23 | 76 | 0 | 79 |
| −20 | 0 | 0 | 0 | −52 | −47 | −78 | 0 | −78 | |
| KO2 | +20 | 0 | 0 | 0 | −4 | −3 | −6 | 0 | −6 |
| −20 | 0 | 0 | 0 | 4 | 3 | 6 | 0 | 7 | |
| KCO2 | +20 | 0 | 0 | 0 | 10 | 6 | 17 | 0 | 17 |
| −20 | 0 | 0 | 0 | −15 | −11 | −24 | 0 | −24 | |
| Permeability and resistances to gas | |||||||||
| RO2 | +20 | −5 | 0 | 0 | −1 | −1 | −2 | 0 | −2 |
| −20 | 5 | 0 | 0 | 1 | 1 | 2 | 0 | 2 | |
| RCO2 | +20 | 0 | 20 | 0 | −12 | −8 | −19 | 0 | −19 |
| −20 | 0 | −20 | 0 | 12 | 8 | 20 | 0 | 21 | |
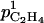 |
+20 | 0 | 0 | 0 | −50 | −47 | −76 | 0 | −71 |
| −20 | 0 | 0 | 0 | 81 | 54 | 188 | 0 | 134 | |
| Allometric parameters | |||||||||
| α | +20 | 4 | −16 | 0 | −42 | −38 | −66 | 0 | −60 |
| −20 | −7 | 25 | 0 | 65 | 47 | 150 | 0 | 102 | |
| β | +20 | 11 | −41 | 0 | −81 | −85 | −99 | 0 | −98 |
| −20 | −19 | 70 | 0 | 153 | 108 | 582 | 0 | 291 | |
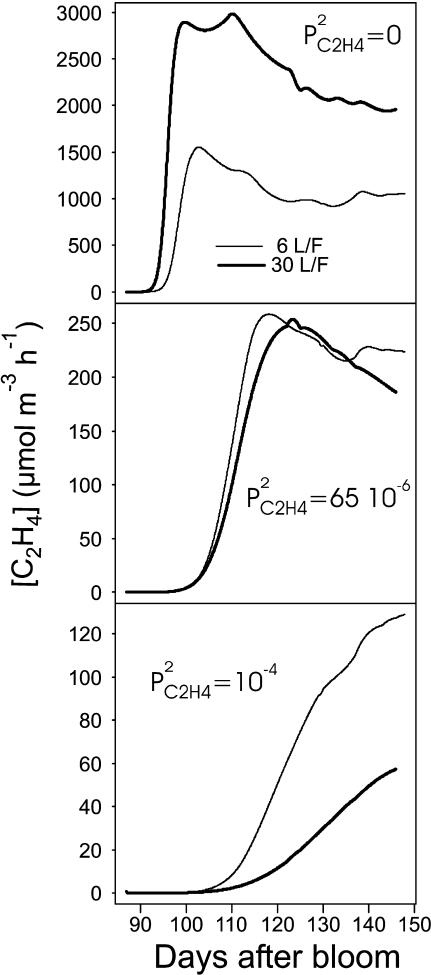
It is interesting to note that an increase in skin permeability to C2H4 induced a decrease in ACC, MACC, and C2H4 concentrations as well as in C2H4 emission. Equation 10 allows an increase of skin permeability to C2H4 with fruit mass if 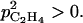 For small values of
For small values of 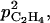 the C2H4 emission started earlier and reached a higher level for high than for low leaf:fruit ratio as observed in our experiments. But for high values of
the C2H4 emission started earlier and reached a higher level for high than for low leaf:fruit ratio as observed in our experiments. But for high values of 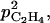 the opposite situation was predicted by the model. In the latter case, the increase of C2H4 synthesis with the leaf:fruit ratio was overcompensated by the increase of C2H4 diffusion to the external atmosphere (Fig. 12). For medium values of
the opposite situation was predicted by the model. In the latter case, the increase of C2H4 synthesis with the leaf:fruit ratio was overcompensated by the increase of C2H4 diffusion to the external atmosphere (Fig. 12). For medium values of 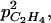 no effect of leaf:fruit ratios was predicted.
no effect of leaf:fruit ratios was predicted.
Figure 12.
Effect of 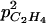 increase on seasonal variation of C2H4 emission simulated by ETHY at 23°C over six (thin lines) and 30 (thick lines) leaves per fruit.
increase on seasonal variation of C2H4 emission simulated by ETHY at 23°C over six (thin lines) and 30 (thick lines) leaves per fruit.
Effect of O2 and CO2 Air Concentration and Temperature on C2H4 Production
Sensitivity to environmental variables was analyzed using, as previously, the results obtained with an 18 leaf:fruit ratio in 1993. The environmental variables had a variation of ±X% throughout the season, and the effect of this variation on the average value of C2H4 emission was assessed. The values of X considered in the analysis were the same as those used when examining the sensitivity to parameters. The model was similarly sensitive whatever the level of X. Considering a ±20% variation, the effect was very small for CO2 (±0.2%), and no effect of the natural variation of CO2 concentration in the air seemed possible. Although larger, the effect of O2 remained negligible (−11% to +7%). On the contrary, sensitivity to temperature was high (−71% to +170%). Indeed, a temperature increase as low as +5% had a large effect on C2H4 production (+31%).
DISCUSSION
The theoretical framework developed here enables us to provide a description of the relationship between environment, fruit growth, and C2H4 emission. It pieces together elements of present knowledge on autocatalytic C2H4 in a biophysical and metabolic theory focusing on processes of regulation by C2H4 itself, ATP, CO2, O2, and temperature. The simulation model based on this theory was able to predict the effect of fruit growth intensity on C2H4 emission for several years in peach.
ETHY predicted significant variations of ATP concentration from 0.007 to 0.023 mol m−3 with a general decrease following that of fruit respiration per fruit mass. Such a variation of more than 100% of ATP concentration is commonly observed during postharvest fruit storage (Saquet et al., 2003a, 2003b). The internal O2 and CO2 concentrations were sensitive to parameters relating fruit surface area to fruit mass and much less to parameters implicated in fruit respiration and permeability to gas. This means that changes in fruit shape (e.g. between cultivars) might have important consequences. The predicted internal O2 and CO2 concentrations were fairly stable during the season, which is in conflict with the results on tomato and cantaloupe of Lyons et al. (1962), Saltveit (1993), and Shellie and Saltveit (1993), who found a regular increase of CO2 and decrease of O2 concentrations before and during ripening. This discrepancy was not ascribable to our steady-state assumption as indicated by additional simulations done in no steady-state conditions (data not shown). On the other hand, it could be explained by the higher maintenance respiration coefficient of these fruits. Indeed, when the model was run using the maintenance respiration coefficient of tomato in place of that of peach, a seasonal increase of CO2 and decrease of O2 concentrations were obtained (data not shown). As predicted by the model, Tonutti et al. (1991, 1997) and Agusti et al. (1999) observed a sharp increase of ACC and C2H4 in peach during maturation and an increase of ACCs and ACCo. Similar trends are observed in species such as apple (Suzuki et al., 1999), which means that our model could be extended to species other than peach. The model also predicts that the ACC concentration reaches a plateau before the final increase in C2H4 emission, as usually reported (Tonutti et al., 1997; Agusti et al., 1999). When the fruit growth is promoted, Johnson (1995) and Agusti et al. (1999) found that ACCs, ACCo, and C2H4 and ACC biosynthesis occur earlier, which is in accordance with our experimental results and model. As predicted by the model, they also found that the increase of the enzyme activities began earlier than the C2H4 burst. As shown by Amoros et al. (1989) on peach and Lara and Vendrell (2000) on apple, our model predicts an increase of MACC during ripening. However, a decrease of MACC has also been observed by Tonutti et al. (1991) on peach fruit. This plasticity in MACC seasonal variation reflects the effect of regulations of the rate constant k4 in Equation 5 that are not considered in the model. As the model is not very sensitive to k4, only strong variations of this rate constant could explain these different patterns of MACC kinetics. Future research could focus more on the regulation of ACC N-malonyltransferase, which generates MACC.
The different steps leading to C2H4 production have been considered, with a focus on ACCs and ACCo. The strong sensitivity to parameters related to these enzymes shows the importance of metabolism in the control of C2H4 production. In agreement with our results, studies on gene expression showed the importance of these enzymes in controlling the rate of endogenous C2H4 production (Lelièvre et al., 1997; Alexander and Grierson, 2002).
Our main assumption is that C2H4 production is highly dependent on ATP availability and thus on fruit respiration. This assumption is in agreement with results of Saquet et al. (2003b) on stored apples. They observed simultaneous variations in ATP and CO2 concentrations and C2H4 production when the composition of the controlled atmosphere changed. This aspect is not often considered in the works on autocatalytic C2H4. Our simulations showed that the control of C2H4 production by ATP availability makes it possible to account for the differences observed between treatments leading to contrasted growths. The activities of ACCs and ACCo were different between treatments, but this was primarily due to differences in ATP availability. Surprisingly, the model was not very sensitive to the respiration parameters, whereas ATP production was directly dependent on respiration. Indeed, there is no simple connection between respiration or ATP concentration and C2H4 concentration because the ATP concentrations decrease during the season when the C2H4 concentration increases in response to the autocatalytic process.
Another important assumption of the model is that the diffusion process through the skin has to be considered as shown by the high sensitivity of the model to the permeability to C2H4 and to the skin area. The importance of such a process has been put forward by postharvest scientists studying the effect of film permeability of packaging on gas concentrations (Fishman et al., 1995). This is all the more important as permeability and skin area are highly variable between species and within the same species (Cameron and Reid, 1982). Considering that skin permeability could vary during fruit development, the model predicted either no effect of fruit size (or leaf number per fruit) on C2H4 or a decrease of this C2H4 with increasing fruit size. This lack of effect has been observed on apple by Johnston et al. (2002). A negative effect has also been observed on apple (Poll et al., 1996) and on cantaloupe by Valantin (1998).
It was also assumed that C2H4 production was regulated by factors such as O2 and CO2 concentrations in the tissues and temperature. Effects of O2, CO2, and temperature have been well established, especially in postharvest studies. Our model is only slightly sensitive to air O2 concentrations and almost insensitive to CO2 concentrations. Only strong variations, such as those encountered during postharvest fruit storage in packages, can be effective. On the contrary, the temperature has a strong effect on C2H4 emission, and small variations can have a significant effect.
One interesting result of our study is that it was possible to simulate the onset of C2H4 production without considering any initial event such as the increase of endogenous concentrations of jasmonates (Fan et al., 1998), auxins (Miller et al., 1987; Agusti et al., 1999), or abscisic acid as mentioned above. Moreover, adequate C2H4 increase for different years and treatments was predicted without considering any increase in respiration during the ripening period. The respiration was only calculated as a function of temperature, fruit mass, and growth rate. These results are in agreement with those of Saltveit (1993) and Shellie and Saltveit (1993), who suggest that the respiratory climacteric may not be required for the ripening of climacteric fruit. Indeed, the increase of energy demand for the climacteric rise of C2H4 is not so strong that a simultaneous increase of respiration is needed. According to our predictions, the ATP requirement for C2H4 production in peach fruit would represent less than 0.04% of the available ATP.
An interesting perspective of this work is based on the high ETHY sensitivity to parameters involved in calculating ACCo and ACCs activities, ATP production, and skin surface and permeability. This would enable the use of ETHY to analyze comprehensively the high genetic variability in C2H4 production (Miccolis and Saltveit, 1991; Klozenbucher et al., 1994; Xu et al., 1998) following the approach initiated by Quilot et al. (2004) for sugar concentrations.
MATERIALS AND METHODS
Plant Material and Experimental Design
The ETHY model was parameterized, calibrated, and validated for the late-maturing peach (Prunus persica L. Batsch) cv Suncrest/GF 677. The measurements were performed on peach trees planted in 1981 in the orchard of the Institut National de la Recherche Agronomique (INRA) Avignon Centre. Trees were goblet trained and received routine horticultural care suitable for commercial orchards. This care included winter and summer pruning, weekly irrigation from June to harvest, hand thinning in April, and pest control. Fungicides and insecticides were applied every 2 weeks from February to July. Weeds were destroyed by weed-killer.
The first experiments in 1993 and 1996 varied assimilate supply to the fruits. Treatments were applied to fruit-bearing shoots located on the southern part of each tree and isolated from the tree by girdling. Three treatments set leaf:fruit ratios at six, 18, and 30 leaves per fruit to obtain minimum, mean, and maximum growth curves that were representative of the Suncrest cultivar. Shoots with six or 18 leaves per fruit were thinned to four fruits, and shoots with 30 leaves per fruit were thinned to two or three fruits. In 1993 and 1996, 240 fruit-bearing shoots were prepared on 64 and 36 trees, respectively, to provide 80 sets of three neighboring shoots with leaf:fruit ratios of six, 18, and 30.
Fruits were harvested from five replicates per treatment each week from mid-June to the beginning of fruit maturation. A replicate was made up of fruits from two shoots when fruits were small and from one shoot later in the season. The last harvests were on August 16, 1993, and August 9, 1996.
Experiment 2, performed in 1997, varied both assimilate and water supply to the fruits. The treatments were applied to 240 fruit-bearing shoots isolated from 40 trees by girdling and located on the southern part of each tree. Half of the trees were irrigated in June and July, whereas irrigation was withheld from the remaining trees. For each irrigated treatment, leaf:fruit ratio of the selected fruit-bearing shoots was 10 or 30. Sixty pairs of neighboring shoots with leaf:fruit ratios of 10 and 30 were prepared for each irrigation treatment. Fruits from five replicates per treatment were harvested each week from mid-June to fruit maturation (July 24).
Gas Measurements
At each harvest, O2 consumption and CO2 and C2H4 production were measured individually at a constant room temperature (23°C) by confining intact fruit in a gas-tight 400-mL jar. The internal atmosphere of the jar was analyzed by gas chromatography. CO2 and O2 were separated on a Porapak Q column at 110°C, followed by a 13× molecular sieve column at room temperature (Chambroy et al., 1984), and quantified using a catharometric detector. The C2H4 released by the fruits was determined by a flame ionization detector gas chromatograph with an activated alumina column at 120°C.
Inputs of the Model
After C2H4 measurement, the stone was removed and the fresh mass of fruit flesh was measured. The dry mass was measured by drying the stone and the flesh at 70°C for 72 h. The hourly dry and fresh fruit masses, which were inputs of the model, were interpolated from our measurements by local regression (Chambers and Hastie, 1992). For each treatment, a local regression was applied to the whole set of data, to the upper range of data (i.e. the set of maximal masses per measurement date), and to the lower range of data (i.e. the set of minimal masses per measurement date) to get mean, maximal, and minimal growth curves, respectively. Hourly temperatures of the air were collected in INRA weather stations close to the experimental fields.
Modeling Technique
Simulation of both CO2 and C2H4 emission were based on an hourly scale. Accordingly, the results were displayed hourly on the figures, using DAB as units (e.g. for hour 12 of DAB 110, the x value was 110.5). An hour was also used as the time frame in the numerical integration. The computer program was written using Splus simulation language (Becker et al., 1988). The differential equations were solved numerically by the first-order Runge-Kutta method. The “nonlinear least squares regression” Splus function was used for model calibration to estimate parameters that could not be determined in independent experiments. Parameters were estimated by minimizing the sum of squared differences between the response and the prediction using the Gauss-Newton algorithm.
For testing model quality, a goodness-of-fit criterion was calculated separately for C2H4 and CO2 emission in each of the 10 year×treatment combinations. The basic criterion was a RMSE, a very common criterion describing the mean distance between simulation and measurement (Kobayashi and Us Salam, 2000), here computed as:
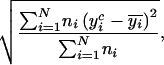 |
where N is the number of measurement dates over the growing period and ni the number of measures at date i. 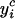 is the C2H4 (or the CO2) emission at date i calculated by the model, and
is the C2H4 (or the CO2) emission at date i calculated by the model, and 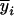 the average of the ni measures.
the average of the ni measures.
The smaller the RMSE in comparison to measurements, the better the goodness-of-fit.
The predictive quality of the model, which is not the same as adjustment quality (Wallach et al., 2001), is very important to assess to be able to use the model outside the range of its development. For C2H4 and CO2 emission, we computed the classical RMSEP using the 10 year × treatment combinations, by cross-validation as did Batchelor et al. (1994) and Wallach et al. (2001). The principles of cross-validation are as follows (Wallach et al., 2001). A situation being one year × treatment combination, the data are split into two parts: one part includes a single situation (the target situation), and the other part includes all the data independent of the target situation, i.e. in our case, not from the same year. Parameters are adjusted using the second part of the data. The RMSE of the resulting model is calculated on the target situation, as presented before. The procedure is repeated using every situation in turn as the target situation, and averaging the RMSE over all the target situations gives the estimate of prediction error.
ETHY Variables
The ETHY variables are as follows: Mfresh (g), fruit fresh mass; Mdry (g), fruit dry mass; V (m−3), fruit volume; A (m2), skin area; T (°C), temperature; 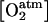 (mol m−3), atmospheric O2 concentration;
(mol m−3), atmospheric O2 concentration; 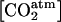 (mol m−3), atmospheric CO2 concentration;
(mol m−3), atmospheric CO2 concentration; 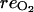 (mol h−1), respired oxygen;
(mol h−1), respired oxygen; 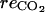 (mol h−1), CO2 produced through respiration; [O2] (mol m−3), internal O2 concentration; [CO2] (mol m−3), internal CO2 concentration; [ATP] (mol m−3), ATP concentration; [MACC] (mol m−3), MACC concentration; [ACC] (mol m−3), ACC concentration; [C2H4] (mol m−3), C2H4 concentration; and
(mol h−1), CO2 produced through respiration; [O2] (mol m−3), internal O2 concentration; [CO2] (mol m−3), internal CO2 concentration; [ATP] (mol m−3), ATP concentration; [MACC] (mol m−3), MACC concentration; [ACC] (mol m−3), ACC concentration; [C2H4] (mol m−3), C2H4 concentration; and 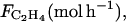 C2H4 diffusion to the external atmosphere.
C2H4 diffusion to the external atmosphere.
ETHY Parameters
The ETHY parameters are as follows: qg = 0.0025 (mol g−1), growth respiration coefficient; qm = 12 10−5 (mol g−1 h−1), maintenance respiration coefficient at 20°C; Q10 = 2, temperature ratio of maintenance respiration; RQ = 0.834, respiratory quotient; λ = 500 (h−1), ATP parameter; k4 = 0.001 (h−1), MACC parameter; ks = 0.778 (h−1), ACCs parameter; ko = 0.268 (h−1), ACCo parameter; 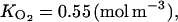 Michaelis constant involved in effect of O2 on ACCo;
Michaelis constant involved in effect of O2 on ACCo; 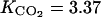 (mol m−3), Michaelis constant involved in effect of CO2 on ACCo;
(mol m−3), Michaelis constant involved in effect of CO2 on ACCo; 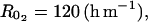 resistances to diffusion of O2;
resistances to diffusion of O2; 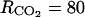 (h m−1), resistances to diffusion of CO2;
(h m−1), resistances to diffusion of CO2; 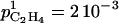 (m h−1), skin permeability; and α = 6.049 10−4 and β = 0.601, empirical parameter relating fruit area (m2) to fruit mass (g).
(m h−1), skin permeability; and α = 6.049 10−4 and β = 0.601, empirical parameter relating fruit area (m2) to fruit mass (g).
Acknowledgments
We thank J. Hostallery and R. Laurent for their assistance during the field experiments, G. Jacquemin for the gas exchange analysis, and M. Reich for mass measurements. We thank F. Lescourret and M.G. Grotte for helpful comments on this paper, and C. Young (INRA Translation Unit, Unité Centrale de Documentation, Jouy-en-Josas) for revising the manuscript.
This work was supported by Région Provence-Alpes-Côte d'Azur (grant nos. 2002–06290 and 2003–10048).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.063339.
References
- Adams DO, Yang SF (1977) Methionine metabolism in apple tissue. Implication of S-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol 60: 892–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti M, Almela V, Andreu I, Juan M, Zacarias L (1999) Synthetic auxin 3,5,6-TPA promotes fruit development and climacteric in Prunus persica L. Batsch. J Hortic Sci Biotechnol 74: 556–560 [Google Scholar]
- Agusti M, Andreu I, Juan M, Almela V, Zacarias L (1998) Effects of ringing branches on fruit size and maturity of peach and nectarine cultivars. J Hortic Sci Biotechnol 73: 537–540 [Google Scholar]
- Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53: 2039–2055 [DOI] [PubMed] [Google Scholar]
- Amoros A, Serrano M, Riquelme F, Romojaro F (1989) Levels of ACC and physical and chemical parameters in peach development. J Hortic Sci 64: 673–677 [Google Scholar]
- Arshad M, Frankenberger JWT (2002) Ethylene in plant physiology. In M Arshad, JWT Frankenberger, eds, Ethylene. Agricultural Sources and Applications. Kluwer Academic/Plenum Publishers, New York, pp 11–50
- Batchelor WD, Jones JW, Boote KJ, Hoogenboom G (1994) Carbon-based models to predict peanut-pod detachment. Trans ASAE 37: 1639–1646 [Google Scholar]
- Baur AH, Yang SF (1972) Methionine metabolism in apple tissue in relation to ethylene biosynthesis. Phytochemistry 11: 3207–3214 [Google Scholar]
- Becker RA, Chambers JM, Wilks AR (1988) The New S Language. Wadsworth & Brooks/Cole, Pacific Grove, CA
- Ben-Yehoshua S, Burg SP, Young R (1985) Resistance of citrus fruit to mass transport of water vapor and other gases. Plant Physiol 79: 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehoshua S, Cameron AC (1989) Exchange determination of water vapor, carbon dioxide, oxygen, ethylene, and other gases of fruits and vegetables. In HF Linskens, JF Jackson, eds, Modern Methods of Plant Analysis. New Series, Vol. 9. Gases in Plant and Microbial Cells. Springer-Verlag, Berlin, pp 177–193
- Ben-Yehoshua S, Robertson RN, Biale JB (1963) Respiration and internal atmosphere of avocado fruit. Plant Physiol 28: 194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AB, Smith GM, Brenda GN (1987) Regulation of climacteric respiration in ripening avocado fruit. Plant Physiol 83: 973–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AC, Reid SR (1982) Diffusive resistance: importance and measurement in controlled atmosphere storage. In DG Richardson, M Meherink, eds, Controlled Atmospheres for Storage Transport of Perishable Agricultural Commodities. Timber Press, Portland, OR, pp 171–180
- Cannell MGR, Thornley JHM (2000) Modelling the components of plant respiration: representation and realism. Ann Bot (Lond) 85: 55–67 [Google Scholar]
- Chambers JM, Hastie TJ (1992) Statistical Models in S. Chapman & Hall, Pacific Grove, CA
- Chambroy Y, Flanzy C, Jacquemin G, Tacchini M (1984) Maîtrise expérimentale du comportement anaérobie de la baie de raisin. CR Acad Agric Fr 70: 53–61 [Google Scholar]
- Chang R (2000) Physical Chemistry for the Chemical and Biological Sciences. University Science Books, Sausalito, CA
- de Wild HPJ, Woltering EJ, Peppelenbos HW (1999) Carbon dioxide and 1-MCP inhibit ethylene production and respiration of pear fruit by different mechanisms. J Exp Bot 50: 837–844 [Google Scholar]
- DeJong TM, Goudriaan J (1989) Modeling peach fruit growth and carbohydrate requirement: reevaluation of the double-sigmoid growth pattern. J Am Soc Hortic Sci 114: 800–804 [Google Scholar]
- Fan XT, Mattheis JP, Fellman JK (1998) A role for jasmonates in climacteric fruit ripening. Planta 204: 444–449 [Google Scholar]
- Fishman S, Génard M (1998) A biophysical model of fruit growth: simulation of seasonal and diurnal dynamics of mass. Plant Cell Environ 21: 739–752 [Google Scholar]
- Fishman S, Rodov V, Peretz J, Ben-Yehoshua S (1995) Model for gas-exchange dynamics in modified-atmosphere packages of fruits and vegetables. J Food Sci 60: 1078–1087 [Google Scholar]
- Flores F, Ben Amor M, Jones B, Pech JC, Bouzayen M, Latché A, Romojaro F (2001) The use of ethylene-suppressed lines to assess differential sensitivity to ethylene of the various ripening pathways in Cantaloupe melons. Physiol Plant 113: 128–133 [Google Scholar]
- Flores F, El-Yahyaoui F, de Billerbeck G, Romojaro F, Latché A, Bouzayen M, Pech JC, Ambid C (2002) Role of ethylene in the biosynthetic pathway of aliphatic ester aroma volatiles in Charentais Cantaloupe melons. J Exp Bot 53: 201–206 [DOI] [PubMed] [Google Scholar]
- Haji T, Yaegaki H, Yamaguchi M (2003) Softening of stony hard peach by ethylene and the induction of endogenous ethylene by 1-aminocyclopropane-1-carboxylic acid (ACC). J Jpn Soc Hortic Sci 72: 212–217 [Google Scholar]
- Hiwasa K, Kinugasa Y, Amano S, Hashimoto A, Nakano R, Inaba A, Kubo Y (2003) Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. J Exp Bot 54: 771–779 [DOI] [PubMed] [Google Scholar]
- Johnson DS (1995) Effect of flower and fruit thinning on the maturity of Coxs-Orange-Pippin apples at harvest. J Hortic Sci 70: 541–548 [Google Scholar]
- Johnston JW, Hewett EW, Hertog M, Harker FR (2002) Harvest date and fruit size affect postharvest softening of apple fruit. J Hortic Sci Biotechnol 77: 355–360 [Google Scholar]
- Klozenbucher KA, Altman SA, McIntosh MS, Walsh CS (1994) Effect of cultivar on endogenous ethylene evolution and its relationship to increases of soluble protein in peach mesocarp tissue. Fruit Var J 48: 20–26 [Google Scholar]
- Kobayashi K, Us Salam M (2000) Comparing simulated and measured values using mean squared deviation and its component. Agron J 92: 345–352 [Google Scholar]
- Kushad MM, Richardson DG, Ferro AJ (1983) Intermediates in the recycling of 5-methylthioribose to methionine in fruits. Plant Physiol 73: 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara I, Vendrell M (2000) Changes in abscisic acid levels, ethylene biosynthesis, and protein patterns during fruit maturation of “Granny Smith” apples. J Am Soc Hortic Sci 125: 183–189 [Google Scholar]
- Lau OL, Liu Y, Yang SF (1986) Effects of fruit detachment on ethylene biosynthesis and loss of flesh firmness, skin color, and starch in ripening ‘Golden Delicious’ apples. J Am Soc Hortic Sci 111: 731–734 [Google Scholar]
- Léchaudel M, Génard M, Lescourret F, Urban L, Jannoyer M (2005) Modelling effects of weather and source-sink relationships on mango fruit growth. Tree Physiol 25: 583–597 [DOI] [PubMed] [Google Scholar]
- Lelièvre JM, Latché A, Jones B, Bouzayen M, Pech JC (1997) Ethylene and fruit ripening. Physiol Plant 101: 727–739 [Google Scholar]
- Lescourret F, Génard M, Habib R, Fishman S (2001) Variation in surface conductance to water vapor diffusion in peach fruit and its effects on fruit growth assessed by a simulation model. Tree Physiol 21: 735–741 [DOI] [PubMed] [Google Scholar]
- Lyons JM, McGlasson WB, Pratt HK (1962) Ethylene production, respiration, and internal gas concentrations in cantaloupe fruits at various stages of maturity. Plant Physiol 37: 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelis LEM, Baan Hofman-Eijer LR (1995) Growth and maintenance respiratory costs of cucumber fruits as affected by temperature, and ontogeny and size of the fruits. Physiol Plant 93: 484–492 [Google Scholar]
- Miccolis V, Saltveit MEJ (1991) Morphological and physiological changes during fruit growth and maturation of seven melon cultivars. J Am Soc Hortic Sci 116: 1025–1029 [Google Scholar]
- Miller AN, Walsh CS, Cohen JD (1987) Measurement of indole-3-acetic acid in peach fruits (Prunus persica L. Batsch cv Redhaven) during development. Plant Physiol 84: 491–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel EW, DeJong TM (1993) Seasonal CO2 exchange patterns of developing peach (Prunus persica) fruits in response to temperature, light and CO2 concentration. Physiol Plant 88: 322–330 [Google Scholar]
- Penning de Vries FWT, van Laar HH (1982) Simulation of plant growth and crop production. Pudoc, Wageningen, The Netherlands
- Poll L, Rindom A, Toldam-Andersen TB, Hansen P (1996) Availability of assimilates and formation of aroma compounds in apples as affected by the fruit/leaf ratio. Physiol Plant 97: 223–227 [Google Scholar]
- Quilot B, Génard M, Kervella J, Lescourret F (2004) Analysis of genotypic variation in fruit flesh total sugar content via an ecophysiological model applied to peach. Theor Appl Genet 109: 440–449 [DOI] [PubMed] [Google Scholar]
- Rothan C, Nicolas J (1994) High CO2 levels reduce ethylene production in kiwifruit. Physiol Plant 92: 1–8 [Google Scholar]
- Rupasinghe HPV, Murr DP, Paliyath G, Skog L (2000) Inhibitory effect of 1-MCP on ripening and superficial scald development in ‘McIntosh’ and ‘Delicious’ apples. J Hortic Sci Biotechnol 75: 271–276 [Google Scholar]
- Saltveit ME (1993) Internal carbon-dioxide and ethylene levels in ripening tomato fruit attached to or detached from the plant. Physiol Plant 89: 204–210 [Google Scholar]
- Saquet AA, Streif J, Bangerth F (2000) Changes in ATP, ADP and pyridine nucleotide levels related to the incidence of physiological disorders in ‘Conference’ pears and ‘Jonagold’ apples during controlled atmosphere storage. J Hortic Sci Biotechnol 75: 243–249 [Google Scholar]
- Saquet AA, Streif J, Bangerth F (2003. a) Energy metabolism and membrane lipid alterations in relation to brown heart development in ‘Conference’ pears during delayed controlled atmosphere storage. Postharvest Biol Technol 30: 123–132 [Google Scholar]
- Saquet AA, Streif J, Bangerth F (2003. b) Impaired aroma production of CA-stored ‘Jonagold’ apples as affected by adenine and pyridine nucleotide levels and fatty acid concentrations. J Hortic Sci Biotechnol 78: 695–705 [Google Scholar]
- Shellie KC, Saltveit ME (1993) The lack of a respiratory rise in muskmelon fruit ripening on the plant challenges the definition of climacteric behavior. J Exp Bot 44: 1403–1406 [Google Scholar]
- Souty M, Génard M, Reich M, Albagnac G (1999) Effect of assimilate supply on peach (Prunus persica L. ‘Suncrest’) fruit maturation and quality. Can J Plant Sci 79: 259–268 [Google Scholar]
- Spoelstra P, Joosen RVL, Van der Plas LHW, Hilhorst HWM (2002) The distribution of ATP within tomato (Lycopersicon esculentum Mill.) embryos correlates with germination whereas total ATP concentration does not. Seed Sci Res 12: 231–238 [Google Scholar]
- Suzuki A, Takahashi A, Aoba K, Masuda T, Kashimura Y (1999) The effects of plant growth regulators, amino acids and minerals on ethylene biosynthesis and evolution in ‘Tsugaru’ and ‘Akane’ apple fruit. J Jpn Soc Hortic Sci 68: 327–335 [Google Scholar]
- Theologis A (1992) One rotten apple spoils the whole bushel—the role of ethylene in fruit ripening. Cell 70: 181–184 [DOI] [PubMed] [Google Scholar]
- Thornley JHM, Johnson IR (1990) Plant and crop modelling. Oxford University Press, Oxford
- Tonutti P, Bonghi C, Ruperti B, Tornielli GB, Ramina A (1997) Ethylene evolution and 1-aminocyclopropane-1-carboxylate oxidase gene expression during early development and ripening of peach fruit. J Am Soc Hortic Sci 122: 642–647 [Google Scholar]
- Tonutti P, Casson P, Ramina A (1991) Ethylene biosynthesis during peach fruit development. J Am Soc Hortic Sci 116: 274–279 [Google Scholar]
- Tucker GA (1993) Introduction. In GB Seymour, JE Taylor, GA Tucker, eds, Biochemistry of Fruit Ripening. Chapman & Hall, London, pp 1–51
- Valantin M (1998) Fécondation, environnement climatique, équilibre source-puits et qualité du melon cantaloup charentais (Cucumis melo L.). PhD thesis. Université Aix-Marseille III, Marseille, France
- Walker AJ, Thornley JHM (1977) The tomato fruit: import, growth, respiration and carbon metabolism at different fruit sizes and temperatures. Ann Bot (Lond) 41: 977–985 [Google Scholar]
- Wallach D, Goffinet B, Bergez JE, Debaeke P, Leenhardt D, Aubertot JN (2001) Parameter estimation for crop models: a new approach and application to a corn model. Agron J 93: 757–766 [Google Scholar]
- Williams MW, Patterson ME (1962) Internal atmosphere in Bartlett pears stored in controlled atmosphere. Proc Am Soc Hortic Sci 81: 129–136 [Google Scholar]
- Xu ZC, Ikoma Y, Yano M, Ogawa K, Hyodo H (1998) Varietal differences in the potential to produce ethylene and gene expression of ACC synthase and ACC oxidase between ‘Kui mi’ and ‘Hong xin’ of chinese kiwifruit. J Jpn Soc Hortic Sci 67: 204–209 [Google Scholar]