Abstract
Possible mechanical and hydraulic costs to increased cavitation resistance were examined among six co-occurring species of chaparral shrubs in southern California. We measured cavitation resistance (xylem pressure at 50% loss of hydraulic conductivity), seasonal low pressure potential (Pmin), xylem conductive efficiency (specific conductivity), mechanical strength of stems (modulus of elasticity and modulus of rupture), and xylem density. At the cellular level, we measured vessel and fiber wall thickness and lumen diameter, transverse fiber wall and total lumen area, and estimated vessel implosion resistance using (t/b)h2, where t is the thickness of adjoining vessel walls and b is the vessel lumen diameter. Increased cavitation resistance was correlated with increased mechanical strength (r2 = 0.74 and 0.76 for modulus of elasticity and modulus of rupture, respectively), xylem density (r2 = 0.88), and Pmin (r2 = 0.96). In contrast, cavitation resistance and Pmin were not correlated with decreased specific conductivity, suggesting no tradeoff between these traits. At the cellular level, increased cavitation resistance was correlated with increased (t/b)h2 (r2 = 0.95), increased transverse fiber wall area (r2 = 0.89), and decreased fiber lumen area (r2 = 0.76). To our knowledge, the correlation between cavitation resistance and fiber wall area has not been shown previously and suggests a mechanical role for fibers in cavitation resistance. Fiber efficacy in prevention of vessel implosion, defined as inward bending or collapse of vessels, is discussed.
Among vascular plants, water is transported through xylem under negative pressure. Xylem must withstand both the mechanical stresses associated with negative pressure as well as the risk of air entering the hydraulic pathway. Failure to do so may lead to cavitation of water columns and blockage of water transport. Failure may occur when gas is pulled into water-filled xylem conduits from gas-filled cells or intercellular spaces through pores in the xylem pit membrane in a process referred to as air-seeding (Zimmermann, 1983; Sperry and Tyree, 1988; Baas et al., 2004). Failure may also occur when negative pressures overcome the ability of the xylem conduit walls to resist implosion (i.e. inward bending or collapse; Carlquist, 1975; Hacke et al., 2001a; Donaldson, 2002; Cochard et al., 2004; Brodribb and Holbrook, 2005). Implosion may trigger cavitation or, in leaves, may restrict hydraulic transport by reducing conduit diameter (Brodribb and Holbrook, 2005). In addition to negative pressures, freezing can lead to failure when sap in the xylem freezes and the gas dissolved in the sap comes out of solution. This can lead to cavitation upon thawing if the bubbles do not go back into solution but instead expand (Yang and Tyree, 1992). The result of cavitation is embolism or gas blockage, which reduces hydraulic transport and can result in reduced stomatal conductance (Pratt et al., 2005), reduced photosynthesis (Brodribb and Feild, 2000), and dieback of branchlets (Rood et al., 2000; Davis et al., 2002).
Natural selection favors increased cavitation resistance among woody evergreen plants occurring in drought-prone environments; however, not all plants adapted to arid environments have high cavitation resistance (Pockman and Sperry, 2000; Maherali et al., 2004). One explanation for this is that increased cavitation resistance may come at the cost of decreased xylem conductive efficiency, preventing plants from developing high levels of cavitation resistance. Additionally, a plant may have to develop thicker xylem walls and/or smaller conduit diameters to strengthen the resistance of a conduit to implosion under negative pressure (Hacke et al., 2001a). Such changes may increase xylem construction costs or decrease conductive efficiency.
Selection to resist freezing-induced cavitation is also linked to decreased xylem conductive efficiency. To resist freezing-induced cavitation, a plant must have narrow vessel or tracheid diameters because resistance to freezing-induced cavitation is directly related to conduit diameter (Langan et al., 1997; Davis et al., 1999; Pittermann and Sperry, 2003; Pratt et al., 2005). Narrowing of conduits to resist freezing-induced cavitation would lead to a decrease in hydraulic conductivity. Therefore, attempts to examine the relationship between resistance to water-stress-induced cavitation and hydraulic efficiency may be confounded by selection to resist freezing-induced cavitation in environments that experience freezing temperatures.
In this study, we examine the cost of increased cavitation resistance at a nonfreezing site using a combined physiological and anatomical approach. We tested for several tradeoffs to increased cavitation resistance, including decreased conductive efficiency and increased xylem construction cost. Additionally, we expanded these traditional tradeoffs to include examination of a possible role for fibers in cavitation resistance. We sampled six species, examining cavitation resistance (xylem pressure at 50% loss of hydraulic conductivity [P50]), minimum seasonal pressure potential (Pmin), xylem biomechanics (modulus of elasticity [MOE] and modulus of rupture [MOR]), xylem density, vessel and fiber anatomy, and xylem conductive efficiency (specific conductivity [ks]). These species correspond to three species pairs of chaparral shrubs from three angiosperm families all growing at the same coastal site where freezing temperatures and, presumably, freezing-induced cavitation do not occur. Using the species at this site allowed us to examine possible costs to water-stress-induced cavitation relatively independent of environmental differences, including any limitations on vessel diameter resulting from selection to resist freezing-induced cavitation.
We hypothesized that increased mechanical strength (MOE and MOR) as well as xylem density would be correlated with increased cavitation resistance. We further hypothesized that the mechanical strength of stems would decrease with increasing water stress, since tension in the water column should exacerbate external mechanical stresses (Sperry and Hacke, 2004). We hypothesized that increased cavitation resistance would come at the cost of decreased hydraulic efficiency. At the cellular level, we hypothesized that cavitation resistance would come at the cost of decreased vessel lumen diameter and increased thickness of xylem cell walls. Changes in either or both of these cellular dimensions would result in a greater thickness-to-diameter ratio [(t/b)h2, where t is the thickness of adjoining vessel walls and b is the vessel lumen diameter] and would result in increased strength of cells against implosion under negative pressure (Hacke et al., 2001a).
RESULTS
Mechanical Properties of Stems
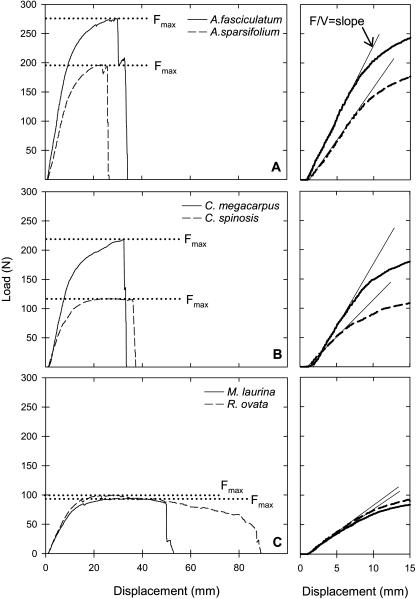
Adenostoma fasciculatum, Adenostoma sparsifolium, and Ceanothus megacarpus showed great resistance to bending (high load required for displacement) followed by very sharp drop-offs, indicating sudden complete failure of stems (Fig. 1, A and B). In contrast, Malosma laurina and Rhus ovata required less force for displacement, and the resistance to displacement soon flattened out such that there was an extended period before complete failure; thus, both partial and complete failure of stems occurred at very low load levels (Fig. 1C). Ceanothus spinosus had intermediate results compared to the other species (Fig. 1B).
Figure 1.
Load versus displacement in four-point bending tests for stems of six species of chaparral shrubs. The graphs on the left show the entire test for representative stems; the graphs on the right are a close-up of the earlier part of the bending tests. The slope of the initial, linear portion of the function (F/V) is used to calculate flexural stiffness and MOE, whereas the maximum load (Fmax) is used to calculate the MOR.
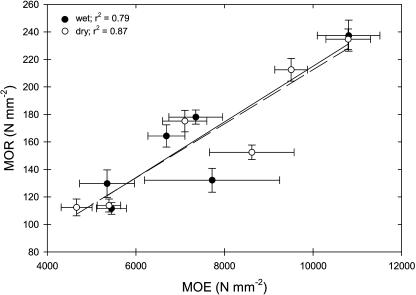
The MOE and MOR were not different between our wet and dry treatments with two exceptions: the dry treatment did result in significantly greater MOR values in C. megacarpus and in significantly greater MOE in C. spinosus (Table I). Stems of C. spinosus were dried down below the Pmin measured in the field (−10.1 MPa compared to a Pmin of −5.3 MPa); however, this value is not different from the Pmin measured in previous years on this species (Pmin < −9 MPa in 2002; data not shown) and is therefore still within the physiological range of this species. MOR was positively correlated to MOE, both for the wet (r2 = 0.79) and dry treatments (r2 = 0.87; Fig. 2). Linear regressions of MOE and MOR did not differ for wet and dry treatments in slope (P = 0.72) or intercept (P = 0.84; Fig. 2); therefore, we used averaged results from the wet and dry treatments in our comparisons with other xylem parameters.
Table I.
Xylem functional properties for six chaparral shrub species
Xylem-specific hydraulic conductivity (ks), xylem density, stem MOR, MOE, xylem pressure potential (Px) of the dry and wet treatments and at 50% loss of hydraulic conductivity (P50), and the 2004 seasonal low xylem pressure potential (Pmin) measured in six co-occurring chaparral species. All values are means ± 1 se, and sample sizes are given in column n. An asterisk indicates significant difference (P < 0.05) between fully saturated wet stems and dry stems dehydrated to approximate minimum water potentials experienced in the field. Letters indicate significant difference between species for a given parameter.
| Species Family | n | A. fasciculatum Rosaceae | A. sparsifolium Rosaceae | C. megacarpus Rhamnaceae | C. spinosus Rhamnaceae | M. laurina Anacardiaceae | R. ovata Anacardiaceae |
|---|---|---|---|---|---|---|---|
| ks (10−3 m2 MPa−1 s−1) | 6 | 0.88 ± 0.01a | 1.60 ± 0.12a | 1.54 ± 0.12a | 1.82 ± 0.29a | 6.46 ± 1.36b | 1.95 ± 0.15a |
| Xylem density (kg m−3) | 12 | 891 ± 65.0a | 684 ± 26.4b,c | 779 ± 34.6b | 659 ± 27.4c | 489 ± 6.2d | 529 ± 21.1d |
| MOR (N mm−2) | |||||||
| Dry | 12 | 235 ± 7.5a | 175 ± 7.8b | 212 ± 8.3c,* | 152 ± 5.3d | 114 ± 4.7e | 112 ± 6.1e |
| Wet | 12 | 237 ± 11.2a | 178 ± 5.2b | 164 ± 8.1b | 132 ± 8.7c | 112 ± 4.2c | 130 ± 10.0c |
| MOE (N mm−2) | |||||||
| Dry | 12 | 10,794 ± 505a,c | 7,104 ± 498b | 8,617 ± 955b,c | 9,505 ± 369c,* | 5,392 ± 265d | 4,661 ± 349d |
| Wet | 12 | 10,804 ± 707a | 7,353 ± 608b,c | 7,722 ± 1,526b | 6,689 ± 417c | 5,450 ± 339c | 5,349 ± 620c |
| Px (MPa) | |||||||
| Dry | 12 | −8.13 ± 0.39a,* | −4.45 ± 0.20b,* | −10.10 ± 0.31c,* | −10.05 ± 0.62c,* | −2.53 ± 0.96d,* | −2.85 ± 0.50d,* |
| Wet | 3–12 | −0.18 ± 0.06a | −0.14 ± 0.03a | −0.26 ± 0.05a,b | −0.16 ± 0.02a | −0.43 ± 0.09b | −0.43 ± 0.10b |
| P50 | 6 | −8.23 ± 0.19a | −5.09 ± 0.34b | −9.30 ± 0.66c | −5.38 ± 0.46b | −0.98 ± 0.31d | −1.23 ± 0.08d |
| Pmin | 6 | −6.53 ± 0.15a | −4.06 ± 0.18b | −7.99 ± 0.44c | −5.34 ± 0.31d | −2.34 ± 0.09e | −2.65 ± 0.10e |
Figure 2.
MOR versus MOE for wet and dry treatments of stems of six species of chaparral shrubs (n = 12 plants per treatment per species).
Cavitation Resistance
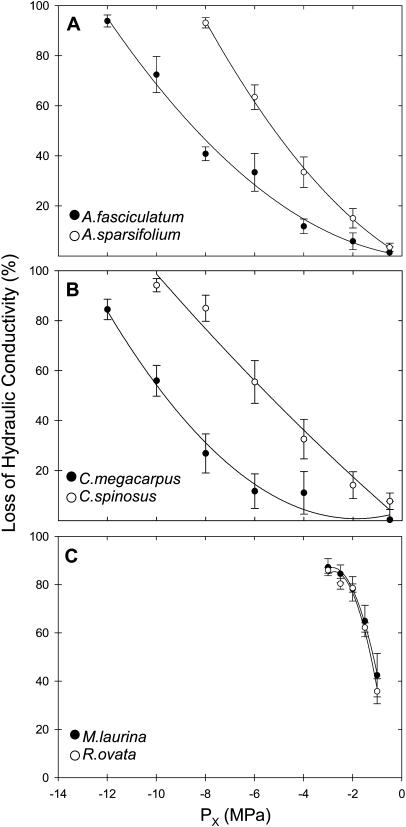
The vulnerability curves are simplified down to one value, P50, shown for each species in Table I. The stems of A. fasciculatum (Fig. 3A) and C. megacarpus (Fig. 3B) were the most resistant to water-stress-induced cavitation, followed by C. spinosus and A. sparsifolium. The species most vulnerable to cavitation were M. laurina and R. ovata (Fig. 3C). M. laurina and R. ovata both displayed high levels of embolism at relatively high pressures (29% and 28% loss of hydraulic conductivity [PLC] at ≥−0.5 MPa) compared to the remaining four species (3.2 ± 1.6 PLC at ≥−0.5 MPa). This indicates that cavitation fatigue (see Hacke et al., 2001b) may have been present in M. laurina and R. ovata.
Figure 3.
Percentage of loss of hydraulic conductivity of stems as a function of xylem pressure potential (Px). Curves were fit with a second order polynomial (r2 ≥ 0.99 and P ≤ 0.01, for all curves). Means ± 1 se are shown with n = 6 plants per species. The graphs were used to calculate for each species the xylem pressure at which there was 50% loss of conductivity (P50 in Figs. 4 and 5).
Stem Traits
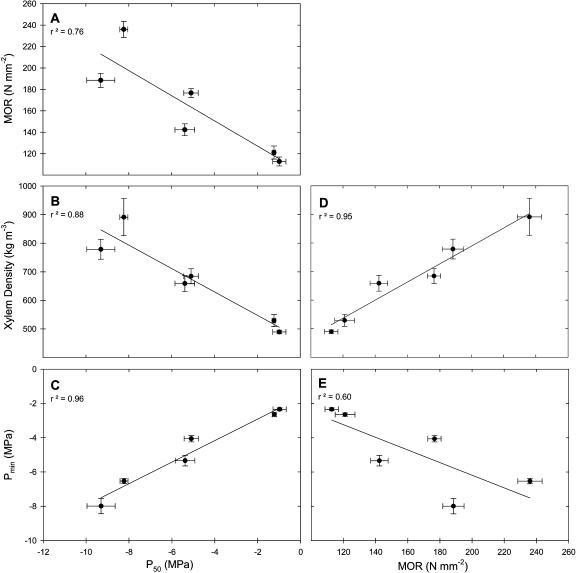
Stems that were more resistant to cavitation had stiffer (greater MOE) and stronger (greater MOR) stem tissue and experienced lower seasonal pressure potentials. Greater cavitation resistance was correlated to increased MOE (r2 = 0.74; data not shown), MOR (r2 = 0.76; Fig. 4A), and xylem density (r2 = 0.88; Fig. 4B). Resistance to cavitation (P50) was positively correlated with Pmin (r2 = 0.96; Fig. 4C). Similarly, MOR was positively correlated with xylem density (r2 = 0.95; Fig. 4D) and negatively correlated with Pmin (r2 = 0.60; Fig. 4E).
Figure 4.
MOR (A), xylem density (B), and minimum seasonal pressure potential (C; Pmin) as functions of xylem pressure at 50% loss of conductivity (P50). Xylem density (D) and Pmin (E) as functions of MOR for six species of chaparral shrubs. Means ± 1 se are shown, and n = 12 plants per species, except for P50, where n = 6 plants per species.
In contrast, xylem conductive efficiency (ks) was not related to cavitation resistance or to measures of stem mechanical strength. Xylem conductive efficiency varied independently of P50 (P = 0.15), Pmin (P = 0.19), MOE (P = 0.20), MOR (P = 0.15), and xylem density (P = 0.11; data not shown).
Cellular Traits
Percentage of transverse fiber wall area per total transverse xylem area ranged from 41.0% in M. laurina to 60.7% in C. megacarpus, with an across species mean of 53.3% ± 3.1%. Percentage of transverse vessel wall area per transverse xylem area occupied considerably less area and ranged from 3.5% to 5.5%, with an across species mean of 4.5% ± 0.3%.
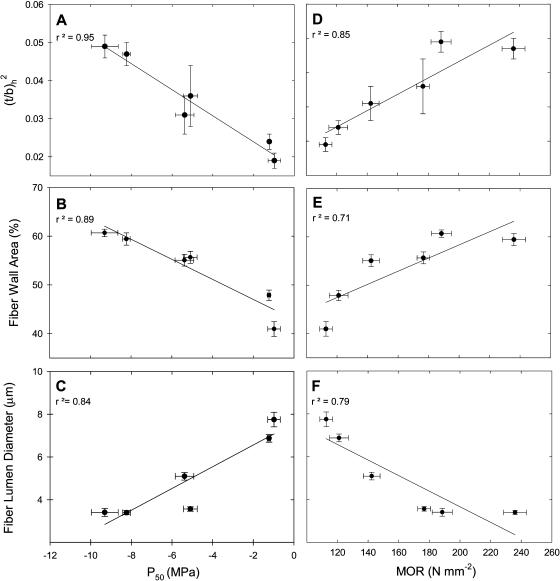
At the cellular level, increased cavitation resistance and stem mechanical strength were associated with increased thickness of fiber cell walls. Resistance to cavitation (P50) and MOR were correlated with increased strength against implosion of xylem vessel walls [(t/b)h2; r2 = 0.95 and 0.85, respectively; Fig. 5, A and D], increased percentage of transverse fiber wall area (r2 = 0.89 and 0.71, respectively; Fig. 5, B and E), decreased fiber lumen diameter (r2 = 0.84 and 0.79, respectively; Fig. 5, C and F), and decreased percentage of transverse lumen area (r2 = 0.76 and 0.86, respectively; data not shown). MOR was also correlated with an increase in fiber wall thickness (r2 = 0.86; data not shown).
Figure 5.
Xylem anatomical measures as functions of the xylem pressure at which there is a 50% loss of conductivity (P50) and MOR, including estimated vessel implosion resistance [(t/b)h2; A and D], percentage of fiber wall area (B and E), and fiber lumen diameter (C and F) in six species of chaparral shrubs. Means ± 1 se are shown, and n = 6 plants per species for P50. n = 12 for MOR.
Increased vessel wall thickness was not correlated with increased cavitation resistance and mechanical strength. Vessel wall thickness was not correlated to P50 (P = 0.56), Pmin (P = 0.80), MOE (P = 0.37), MOR (0.37), or xylem density (P = 0.40). Vessel wall thickness also was not correlated with fiber wall thickness (P = 0.15). Hydraulic vessel lumen diameter was correlated with xylem density (r2 = 0.67) but was not correlated with cavitation resistance (P = 0.07; data not shown).
Xylem conductive efficiency (ks) was correlated with increased hydraulic vessel lumen diameter (r2 = 0.75; data not shown) and decreased fiber wall area (r2 = 0.75; data not shown). Xylem conductive efficiency was not correlated with total lumen area (P = 0.06; data not shown).
DISCUSSION
Water-stress-induced cavitation in woody plants occurs when air is seeded into a functional conduit from an adjacent gas-filled cell or intercellular space (Jarbeau et al., 1995; Sperry et al., 1996; Tyree and Zimmermann, 2002). Conduits may also cavitate following collapse under negative pressure. Conduit collapse may be reversible, depending on conduit structure and biomechanics (Cochard et al., 2004; Brodribb and Holbrook, 2005). Although some have questioned the existence of these extreme negative pressures in the water transport system of woody plants (Zimmermann et al., 2004), the existence of tensions in the xylem is widely supported (see Angeles et al., 2004), and, presumably, plants have adapted to tolerate such stresses.
Hacke et al. (2001a) suggested that to resist collapse of conduits, plants that exhibit a high degree of cavitation resistance and that experience greater negative pressures display thicker vessel walls relative to their lumen diameter [i.e. higher (t/b)h2] and that such plants also have denser xylem. The link between vessel (t/b)h2 and xylem density is apparently due to correlated changes in vessel- and fiber-lumen-to-wall ratios (Hacke et al., 2001a; Sperry and Hacke, 2004). Although data are lacking on the correlated nature of vessel- and fiber-lumen-to-wall ratios, its seems that the link between vessel (t/b)h2 and xylem density is dependent on this correlation, since the density of xylem in angiosperms is primarily affected by the abundance and properties of fibers and only minimally affected by changes in vessel (t/b)h2. Our data support the suggestion by Hacke et al. (2001a) that fiber and vessel properties are indeed correlated. Additionally, our results point to a possible role for fibers in increased vessel implosion resistance, an idea that was hypothesized but not previously tested (Hacke et al., 2001a). Previous studies have not analyzed fiber anatomical properties independently and have relied on assumptions of fiber anatomical measures and mechanical properties. Here, we examine the role of changes in vessel and fiber anatomy and mechanical strength on cavitation resistance.
Vessel implosion resistance can be increased by a decrease in vessel lumen diameter, an increase in wall thickness, or both; however, the species included in this study did not appear to utilize all of these anatomical options. The variation in (t/b)h2 among species was largely due to changes in vessel lumen diameter and not to changes in vessel wall thickness. The range of vessel wall thicknesses among our sampled species was narrow (2.3–3.8 μm) and was not correlated with cavitation resistance (P50) or minimum seasonal pressure potential (Pmin). One interpretation would be that the sampled species were limited in their ability to alter their vessel wall thickness, limiting changes in vessel mechanics to changes in the lumen diameter. However, this would suggest that increased (t/b)h2 would correlate with decreased conduit efficiency, a tradeoff that was not observed. A second interpretation would be that other surrounding tissues, such as fibers, may act to strengthen vessel walls, somehow increasing resistance to cavitation without a necessary change in either vessel wall thickness or lumen diameter. Our data support this second interpretation and suggest that viewing vessels as isolated pipes [i.e. (t/b)h2] may not fully describe their ability to resist mechanical stresses imposed by negative pressures. Indeed, strong correlations between fiber characters, cavitation resistance, and xylem density suggest that fibers may be involved in cavitation resistance of woody plants. In this study, fiber wall area was, on average, 53.3% of the total transverse xylem area, compared to <5% vessel wall area per transverse xylem area. Since >10 times the biomass goes into fiber walls than vessel walls, the cost of fiber support for mechanical safety may be considerable in plants that experience very low xylem pressure potentials.
Direct evidence for fibers in preventing vessel collapse in stems is lacking; however, recent studies in leaves have demonstrated the importance of supportive tissue in the prevention of collapse. Under severe water stress, tracheids in conifer needles in Pinus spp. collapse preferentially when xylem conduits are adjacent to nonstrengthening parenchyma (Cochard et al., 2004), suggesting that the cells adjacent to a xylem conduit are important in preventing conduit collapse under negative pressure. The importance of the strength of the surrounding tissue is further supported by the finding that conifer stem tracheids, which are surrounded by a mechanically supportive tracheid matrix, did not exhibit collapse (Cochard et al., 2004). Brodribb and Holbrook (2005) similarly found that tracheids occurring outside leaf midveins exhibited collapse, whereas tracheids within the more supported midveins did not display evidence of collapse. Vessel implosion has also been observed in stems of genetically modified angiosperms with weakened cell walls (Turner and Somerville, 1997; Franke et al., 2002), demonstrating that mechanical strength of supportive tissue may be important in preventing implosion. The idea that the mechanical strength of stems is related to vessel implosion resistance contrasts with the idea that stem mechanical strength is mainly the result of selection for resistance to stem breakage due to wind or fruit load (Wagner et al., 1998).
It has been hypothesized that stronger stems may be needed to resist whole stem bending, which would exacerbate the internal stresses that occur during severe water stress (Sperry, 2003; Sperry and Hacke, 2004); however, our data do not support this. We found that stem mechanical strength did not vary between hydrated stems, and stems dried down to their seasonal low pressure potential. Although C. megacarpus had greater MOR and C. spinosus had greater MOE in the dry treatment compared to the wet treatment, for five out of six species, there was no difference in MOE or MOR with the treatments. This finding suggests that negative internal stresses within the stem do not affect whole stem bending strength. A caveat of these findings is that we do not know the xylem pressures while stems were being bent. Bending of vessels may have reduced vessel volume, thereby reducing the negative pressures within the xylem. Regardless, the experiment did recreate the stresses that would occur with bending in intact hydrated and water-stressed stems in the field. A further criticism would be that we do not have evidence as to whether the fibers were embolized during the wet or dry treatments and, therefore, did not fully test the mechanism suggested by Sperry and Hacke (2004) of exacerbated bending stress with water stress. Thus, while we found no evidence that pressures are intensified in stems experiencing both water and bending stresses, this mechanism has not yet been tested fully.
It has also been hypothesized that stiffer and denser stems, which may be more resistant to vessel implosion, would be a mechanical liability because they are less flexible and more likely to break (Hacke et al., 2001a). Our data do not support this suggestion. For the species in this study, stems that were stiffer (i.e. less flexible; high MOE) and denser displayed the greatest strength (i.e. resistance to breakage; high MOR). This relationship did not vary with stem water stress, suggesting that even under extreme water stress the stiffest stems remain the strongest and least likely to break.
Stem mechanical properties and cavitation resistance were unrelated to xylem conductive efficiency in this study. This is consistent with previous studies that have surveyed many angiosperm families and found that there is only a weak tradeoff between hydraulic conductivity and cavitation resistance at best (Tyree et al., 1994; Maherali et al., 2004) and weak evidence for a tradeoff between conductivity and mechanical strength (Wagner et al., 1998). Tradeoffs may be present in the case of freezing-induced embolism where vessel diameter is directly related to susceptibility to cavitation (Cochard and Tyree, 1990; Langan et al., 1997; Davis et al., 1999). The lack of a tradeoff between stem biomechanical traits and conductivity is perhaps because stem mechanics are largely dependent on fiber properties, while conductivity is controlled by vessel properties. Xylem can be constructed in many different ways such that it can have a relatively high mechanical strength and simultaneously have high transport efficiency (Woodrum et al., 2003; Kern et al., 2005). In addition, certain factors impact mechanical properties of xylem but are independent of the number and diameter of vessels, including the amount of lignin in the fibers and the cellulose microfibril angle (Panshin and deZeeuw, 1980; Niklas, 1992).
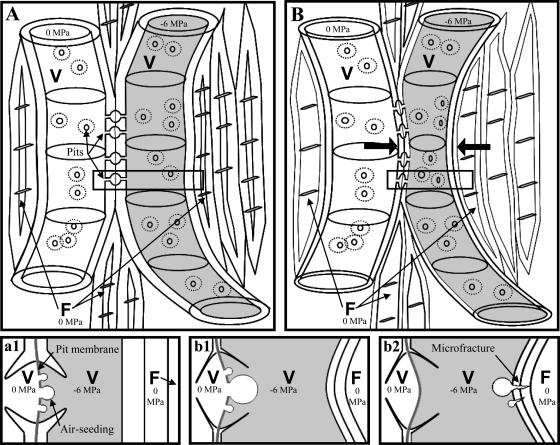
Reinforcement of xylem tissue may represent a significant cost limiting increased cavitation resistance. MOE, MOR, and xylem density showed strong correlations with P50, suggesting that stems that are mechanically stronger and denser are apparently able to withstand more negative pressures. This suggests that the fiber matrix may be important in increased resistance to cavitation. Partial implosion of vessel walls under negative pressure could lead to increased likelihood of air-seeding due to stretching or rupture of pit membranes (Zimmermann, 1983; Sperry and Tyree, 1988) or microfractures in other parts of the wall that could initiate cavitation (Pickard, 1981; Fig. 6). The correlation between increased allocation to the fiber matrix and P50 supports the suggestion by Niklas (1997) and Hacke et al. (2001a) that fibers play a key role in buttressing vessel walls against implosion under extreme negative pressure.
Figure 6.
Some possible causes of embolism in a woody angiosperm stem. Depicted in the diagram is a segment of an embolized vessel represented by several vessel elements (V; 0 MPa) adjacent a functional water-filled vessel (V; −6 MPa) and surrounded by a matrix of fibers (F). In this diagram, we have assumed that the fibers are embolized (0 MPa). The bottom panels represent more detailed diagrams of an intervessel pit and are close-ups of the rectangular areas indicated in A and B. With thick fiber and vessel walls (A), membrane deflection may occur, but it is not exacerbated by wall buckling, limiting possible causes of hydraulic failure to air-seeding at the pit pore or membrane rupture (a1). With thin vessel and fiber cell walls (B), there is partial implosion of the water-filled vessel (indicated by arrows). With partial implosion, there is stretching of the pit membrane with water stress, stretching the pores in the pit membrane and increasing the likelihood that air-seeding will occur either at the pit pore or following membrane rupture (b1). Additionally, implosion of vessel walls could lead to microfractures, another source of nucleation that would lead to cavitation of the water column (b2).
Pit membrane tears and microfracture of vessel walls may be more common than previously realized. Permanent implosion of stem tracheary elements has been observed only in plants with weakened cell walls that were deficient in lignin (Donaldson, 2002; Franke et al., 2002) or cellulose (Turner and Somerville, 1997). The implosion observed by Cochard et al. (2004) and Brodribb and Holbrook (2005) was temporary and completely reversible, either with rehydration of the tissue or upon cavitation of the water columns, either of which would relieve tension on the walls. The reversibility of implosion means that implosion and resultant tears in the pit membrane or microfracture of the lignified cell walls might not be visible with conventional light microscopy and would be difficult to distinguish from artifacts with electron microscopy. However, some authors consider checks that are often seen in wood to be damage caused by negative pressure (Hunter, 2001; Donaldson, 2002).
For some species, once a tracheary element cavitates due to water stress it becomes more susceptible to cavitation following refilling and with subsequent water stress, i.e. a tracheary element is more susceptible to cavitation after a prior cavitation event. The phenomenon has been called cavitation fatigue (Hacke et al., 2001b). It has been suggested that cavitation fatigue may be the result of pit membrane damage or tearing (Hacke et al., 2001b). Consistent with our suggestion that the fiber matrix may play an important role in the prevention of implosion and possible permanent damage or weakening of vessels, we found evidence for fatigue in two species, M. laurina and R. ovata. Both of these species have weak stems (low MOR), low transverse fiber wall area, and vessels that are predominantly surrounded by fibers. Additionally, the fiber walls are much thinner in these species (1.9 μm for both) compared to their vessel walls (3.8 and 3.4 μm, respectively), meaning that risk of implosion [(t/b)2] may be greatest between a vessel and its surrounding fibers rather than between two vessels. It is possible that the support of fibers helps to minimize cavitation fatigue; however, the mechanism for this remains to be elucidated.
Previous studies have found correlations between wood density and resistance to cavitation (Hacke et al., 2001a; Baas et al., 2004); however, to our knowledge, no previous study has tested the relationship between stem mechanical strength and cavitation resistance. The correlations we found between wood anatomical traits and cavitation resistance suggest a greater role for fibers in imparting conductive safety than previously considered. While it is thought that resistance to cavitation is primarily a function of pore size in pit membranes (Jarbeau et al., 1995; Sperry et al., 1996), it may be that the mechanical properties of xylem fibers, as well as of other cell types associated with vessels, including xylem parenchyma and, in some species, tracheids, are important in determining resistance to cavitation. Fibers may impact vessel implosion risk by reducing stresses that exacerbate pit membrane deflection or stresses that lead to vessel collapse and microfracture of the cell wall.
MATERIALS AND METHODS
Study Site
All plant material was collected at a site in the Santa Monica Mountains in California, 0.5 km south of Encinal Canyon Road, at an elevation of 480 m (34° 05′ 27″ N, 118° 50′ 29″ W). This site, described as site 3 in Wagner et al. (1998), is a mixed stand of chaparral with a coastal exposure. Wildfires occurred at the site in 1956 and 1978, meaning that at the time of the study, in 2003, this was a mature chaparral stand with aboveground shoots up to 26 years old. Measurements were made on six species of chaparral shrub, including Adenostoma fasciculatum Hook. and Arn., Adenostoma sparsifolium Torrey, Ceanothus megacarpus Nutt., Ceanothus spinosus Nutt., Malosma laurina (Nutt.) Abrams (formerly Rhus laurina), and R. ovata S. Watson (nomenclature follows Hickman, 1993).
Mechanical Measurements
Twelve plants per species were marked and used for all measures. For comparison between stems dehydrated to their seasonal low water potentials and well hydrated stems, on each plant, two stems were identified that were very similar in size, appearance, exposure to the sun, and angle of inclination. The shoots, 1.5 to 2.0 m in length, were cut from the plant, immediately placed into plastic bags, and transported to the laboratory at Pepperdine University. In the laboratory, one shoot of each pair was recut at the base under water, and the base was kept in water overnight with the aerial portions covered with a plastic bag. For the wet treatment, twigs or leaves were sampled the next morning to measure the xylem pressure potential (Px) using a pressure chamber (PMS Instrument Company) in order to confirm that the stems were rehydrated. The stems were then recut under water to a length of between 0.26 and 0.29 m and kept entirely submerged for another 24 h. The dry treatment shoot of each pair was allowed to dehydrate in an air conditioned laboratory until their Px values were close to the lowest pressures that the plants experience in the field. The shoots were then double bagged and allowed to equilibrate overnight. The next morning, branchlets or leaves were sampled for Px, and the stems were cut to a length of slightly over 0.3 m. Stems of the wet and dry treatments were tightly wrapped in plastic and placed in separate bags before being shipped via overnight express to Michigan State University, where an Instron Universal Machine (model 4202, Instron Corporation) was used to measure MOE and MOR.
Stem segments were kept at approximately 10°C until MOE and MOR were measured. A four-point bending test with a compression load cell of 500 n was conducted as described by Woodrum et al. (2003). However, the stem segments used were longer (about 0.275 m) than were used in previous studies, so that most of the vessels would be intact during the bending experiments on wet- versus dry-treated stems. The span length (L), the distance between the two supported ends, was 0.21 m. The load was applied at two points along the span length with a crosshead speed of 20 mm/min, and stems were stressed until the load reached a maximum value (Fmax), determined as the maximum force obtained in the bending test prior to stem failure (Fig. 1). The distance between one supported end and the nearest loading point (a in the equations below) was 0.07 m. The elastic limit was always exceeded with the bending test; thus, stems did not return to their original shape.
Flexural rigidity (EI) was calculated using slope (F/V) of the linear (elastic) portion of the curve (Fig. 1) and the equation EI = (F/V)(a2/12)(3L − 4a) (modified from Gere and Timoshenko, 1997). Flexural rigidity was divided by I, the second moment of area, to determine MOE.
MOR was estimated from the equation MOR = (Fmax×a×Rmajor)/I, modified from Ugural (1991), where Fmax is the load at stem failure and Rmajor is the radius of the xylem. The second moment of area, I, was calculated as π (Rmajor4 − Rminor4)/4, with Rminor as the radius of the pith, based upon I of a hollow cylinder (Niklas, 1992).
Adjacent stem segments were used to estimate xylem density (in kg m−3) as the dry mass per saturated volume of xylem. For this measurement, the pith and bark (including the phloem and vascular cambium) were removed from the xylem.
Hydraulic Conductivity and Cavitation Resistance
Six plants per species were sampled for hydraulic conductivity (kh), specific conductivity (kh/sapwood area; ks), and cavitation resistance of their stem xylem. One branch per plant, approximately 2 m in length, was excised, bagged in the field, and taken to the laboratory. The branch was recut under water to obtain one distal stem segment 6 to 8 mm in diameter and 0.10 m in length for measurements of ks and xylem anatomical features. An adjacent proximal segment was used for measurement of resistance to cavitation using the centrifuge technique.
Both the proximal and distal stems were connected to a tubing system and flushed with water that had been passed through a 0.1-μm filter and adjusted to pH 2 with HCl in order to discourage microbial growth. The stems were flushed at a pressure of 100 kPa for 1 h to remove gas emboli from the xylem vessels. The kh (in m4 MPa−1 s−1) was then measured gravimetrically and the high-pressure perfusion process repeated until a maximum value (kmax) was obtained for each segment (Sperry et al., 1988).
The distal segments were then attached to a tubing system that allowed uptake of a 0.1% (mass/volume) dye solution of crystal violet under a suction of 5 to 6 kPa for 25 min. The dye solution had been passed through a 0.1-μm filter. The midpoints of the distal segments were transversely sectioned at a thickness of 40 μm with a sliding microtome. The active sapwood area, indicated by the dye, was measured with a light microscope (Nikon Microphot-FX microscope and Diagnostic Instruments Spot RT color camera) and analyzed using image analysis software (Image v.1.61; National Institutes of Health). The ks value (in m2 MPa−1 s−1) was then calculated as kmax/active sapwood area.
The proximal stem segments, following determination of their kmax, were spun in a centrifuge (RC5G Plus, Sorvall, Kendro Laboratory Products) using a modified rotor to accommodate stem segments. Stems were spun at a prescribed rpm in order to generate a known negative pressure on the water column in the xylem vessels (Alder et al., 1997). The proximal segments were 0.271 m long (for use with a large centrifuge rotor) in the case of C. megacarpus and A. fasciculatum and 0.14 m long (for use with a smaller rotor) in the case of the other four species. The larger rotor was needed to create greater centrifugal force for the species that, based upon previous studies, required greater tensions (measured as lower Px in previous studies) to induce embolism (Kolb and Davis, 1994; Redtfeldt and Davis, 1996; Davis et al., 1999). Vulnerability curves were constructed by plotting decreasing values of xylem pressure potential versus the percentage loss in hydraulic conductivity (PLC). Two species, M. laurina and R. ovata, demonstrated evidence of cavitation fatigue (Hacke et al., 2001b). For these species, a correction for fatigue was done by calculating PLC using the kh measure from a centrifuge spin at ≥−0.5 MPa instead of kmax. For each stem, the PLC values were fitted with a polynomial model, which was used to predict the pressure potential at 50 PLC (P50). The mean P50 value was thus determined for each species.
While this is a relatively arbitrary value, it is one that is widely used and can be objectively applied to a wide range of vulnerability curves.
Minimum Field Pressure Potentials
The lowest xylem pressure potentials (Pmin) occurred following the summer drought but before the first rains in autumn (Kolb and Davis, 1994; Redtfeldt and Davis, 1996; Davis et al., 1999). On October 13, 2004, we sampled six of the marked plants of each species for midday xylem pressure potential, measured from branchlets with the pressure chamber technique (Scholander et al., 1965). Since the first rain of the fall season fell on October 17, 2004, ending a >7 month period with no rain, we assumed the October 13th measurements were representative of the lowest pressure potentials that the plants would experience in 2004.
Xylem Anatomical Measures
Images were taken of wedge-shaped sectors, using vascular rays as the borders, to sample for vessel and fiber features. Vessel lumen diameter (in μm), fiber lumen diameter, fiber wall thickness, total transverse lumen area (vessel + fiber lumen area/sapwood area), and transverse fiber wall area/sapwood area were measured with these images. All of the vessels and fibers in sectors were measured until a sample size of 200 vessels and 100 fibers was obtained for a stem. The hydraulic vessel diameter (dh) was calculated with the formula dh = (Σd5)/(Σd4), based upon all the sampled vessels in a stem. The vessel implosion resistance [(t/b)h2; Hacke et al., 2001a] was determined for those vessels, within the sampled 200 vessels per stem, that formed pairs in which one or both vessels fell within ±5 μm of the calculated dh, with t as the thickness of adjoining vessel walls and b as the lumen diameter of the vessel.
Data Analysis
MOE and MOR of wet and dry treatments were compared within a species using t tests. An ANCOVA was used to compare the relationship between MOE and MOR for wet and dry treatments. Parameters were compared across species using an ANOVA followed by a Fisher's lsd post-hoc analysis. Linear regression analysis was used to examine the correlations between parameters as predicted in our a priori hypotheses (StatView, SAS Institute). We used an α of 0.05 to determine statistical significance.
This work was supported by the National Science Foundation (grants DBI–0243788, IBN–0130870, and IBN–0131247). We thank Raymond Sauvajot of the National Park Service for logistic support and Brad Marks and Steve Marquie for Instron Universal Machine use.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.058404.
References
- Alder NN, Pockman WT, Sperry JS, Nuismer S (1997) Use of centrifugal force in the study of xylem cavitation. J Exp Bot 48: 665–674 [Google Scholar]
- Angeles G, Bond B, Boyer JS, Brodribb T, Brooks JR, Burns MJ, Cavender-Bares J, Clearwater M, Cochard H, Comstock J, et al (2004) The cohesion-tension theory. New Phytol 163: 451–452 [DOI] [PubMed] [Google Scholar]
- Baas P, Ewers FW, Davis SD, Wheeler EA (2004) Evolution of xylem physiology. In I Poole, A Hemsley, eds, Evolution of Plant Physiology. Linnaean Society Symposium Series. Elsevier Academic Press, London, pp 273–295
- Brodribb TJ, Feild TS (2000) Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant Cell Environ 23: 1381–1388 [Google Scholar]
- Brodribb TJ, Holbrook NM (2005) Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiol 137: 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlquist S (1975) Ecological Strategies of Xylem Evolution. University of California Press, Berkley, CA
- Cochard H, Froux F, Mayr S, Coutand C (2004) Xylem collapse in water-stressed pine needles. Plant Physiol 134: 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Tyree MT (1990) Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal-changes in embolism. Tree Physiol 6: 393–407 [DOI] [PubMed] [Google Scholar]
- Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC (2002) Shoot dieback during prolonged drought in Ceanothus chaparral of California: a possible case of hydraulic failure. Am J Bot 89: 820–838 [DOI] [PubMed] [Google Scholar]
- Davis SD, Ewers FW, Wood J, Reeves JJ, Kolb KJ (1999) Differential susceptibility to xylem cavitation among three pairs of Ceanothus species in the Transverse Mountain Ranges of southern California. Ecoscience 6: 180–186 [Google Scholar]
- Donaldson LA (2002) Abnormal lignin distribution in wood from severely drought stressed Pinus radiata trees. IAWA J 23: 161–178 [Google Scholar]
- Franke R, Hemm MR, Denault JW, Ruegger MO, Humphreys JM, Chapple C (2002) Changes in secondary metabolism and deposition of an unusual lignin in the ref8 mutant of Arabidopsis. Plant J 30: 47–59 [DOI] [PubMed] [Google Scholar]
- Gere JM, Timoshenko SP (1997) Mechanics of Materials. International Thomson Publishing Company, Boston
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001. a) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA (2001. b) Cavitation fatigue. Embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol 125: 770–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman JC (1993) The Jepson Manual. University of California Press, Berkeley, CA
- Hunter AJ (2001) The distribution of mechanical stresses in the cell wall of wood induced by capillary tension in the lumen water—an approximate analysis. Wood Sci Technol 35: 283–296 [Google Scholar]
- Jarbeau JA, Ewers FW, Davis SD (1995) The mechanism of water-stress-induced embolism in two species of chaparral shrubs. Plant Cell Environ 18: 189–196 [Google Scholar]
- Kern KA, Ewers FW, Telewski FW, Köhler L (2005) Mechanical perturbation affects conductivity, mechanical properties, and aboveground biomass of hybrid poplars. Tree Physiol 25: 1243–1251 [DOI] [PubMed] [Google Scholar]
- Kolb KJ, Davis SD (1994) Drought tolerance and xylem embolism in co-occurring species of coastal sage and chaparral. Ecology 75: 648–659 [Google Scholar]
- Langan SJ, Ewers FW, Davis SD (1997) Xylem dysfunction caused by water stress and freezing in two species of co-occurring chaparral shrubs. Plant Cell Environ 20: 425–437 [Google Scholar]
- Maherali H, Pockman WT, Jackson RB (2004) Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85: 2184–2199 [Google Scholar]
- Niklas KJ (1992) Plant Biomechanics: An Engineering Approach to Plant Form and Function. University of Chicago Press, Chicago
- Niklas KJ (1997) Mechanical properties of Black Locust (Robinia pseudoacacia) wood: correlations among elastic and rupture moduli, proportional limit, and tissue density and specific gravity. Ann Bot (Lond) 79: 479–485 [Google Scholar]
- Panshin AJ, deZeeuw C (1980) Textbook of Wood Technology: Structure, Identification, Properties, and Uses of Commercial Woods of the United States and Canada. McGraw-Hill, New York
- Pickard WF (1981) The ascent of sap in plants. Prog Biophys Mol Biol 37: 181–229 [Google Scholar]
- Pittermann J, Sperry JS (2003) Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiol 23: 907–914 [DOI] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS (2000) Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am J Bot 87: 1287–1299 [PubMed] [Google Scholar]
- Pratt RB, Ewers FW, Lawson MC, Jacobsen AL, Brediger M, Davis SD (2005) Mechanisms for tolerating freeze-thaw stress of two evergreen chaparral species: Rhus ovata and Malosma laurina (Anacardiaceae). Am J Bot 92: 1102–1113 [DOI] [PubMed] [Google Scholar]
- Redtfeldt RA, Davis SD (1996) Physiological and morphological evidence of niche segregation between two co-occurring species of Adenostoma in California chaparral. Ecoscience 3: 290–296 [Google Scholar]
- Rood SB, Patiño S, Coombs K (2000) Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14: 248–257 [Google Scholar]
- Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148: 339–346 [DOI] [PubMed] [Google Scholar]
- Sperry JS (2003) Evolution of water transport and xylem structure. Int J Plant Sci 164: S115–S127 [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT (1988) A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ 11: 35–40 [Google Scholar]
- Sperry JS, Hacke UG (2004) Analysis of circular bordered pit function. I. Angiosperm vessels with homogenous pit membranes. Am J Bot 91(3): 369–385 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Saliendra NZ, Pockman WT, Cochard H, Cruiziat P, Davis SD, Ewers FW, Tyree MT (1996) New evidence for large negative xylem pressures and their measurement by the pressure chamber method. Plant Cell Environ 19: 427–436 [Google Scholar]
- Sperry JS, Tyree MT (1988) Mechanism of water stress-induced xylem embolism. Plant Physiol 88: 581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SR, Somerville CR (1997) Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9: 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: Is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA J 15: 335–360 [Google Scholar]
- Tyree MT, Zimmermann MH (2002) Xylem Structure and the Ascent of Sap, Ed 2. Springer-Verlag, New York
- Ugural AC (1991) Mechanics of Materials. McGraw-Hill, New York
- Wagner KR, Ewers FW, Davis SD (1998) Tradeoffs between hydraulic efficiency and mechanical strength in the stems of co-occurring species of chaparral shrubs. Oecologia 117: 53–62 [DOI] [PubMed] [Google Scholar]
- Woodrum CL, Ewers FW, Telewski FW (2003) Hydraulic, biomechanical, and anatomical interactions of xylem from five species of Acer (Aceraceae). Am J Bot 90: 693–699 [DOI] [PubMed] [Google Scholar]
- Yang S, Tyree MT (1992) A theoretical model of hydraulic conductivity recovery from embolism with comparison to experimental data on Acer saccharum. Plant Cell Environ 15: 633–643 [Google Scholar]
- Zimmermann MH (1983) Xylem Structure and the Ascent of Sap. Springer-Verlag, New York
- Zimmermann U, Schneider H, Wegner LH, Haase A (2004) Water ascent in tall trees: Does evolution of land plants rely on a highly metastable state? New Phytol 162: 575–615 [DOI] [PubMed] [Google Scholar]