Abstract
Introduction
Bartonella spp. are highly fastidious gram-negative facultative intracellular bacteria which can cause a culture-negative infective endocarditis (IE) with unique clinicopathologic features.
Methods
In this study, we assembled 20 cases of glomerulonephritis (GN) due to Bartonella IE from 3 institutions and compared them with 49 cases of culture-positive IEGN and 30 cases of non-endocarditis infection-related GN (IRGN).
Results
IEGN was seen in approximately 0.15% to 0.4% of native renal biopsies, with Bartonella causing 8% to 21% of IEGN. Patients with Bartonella IEGN had preexisting cardiac valve alterations (75%); antineutrophil cytoplasmic autoantibody (ANCA) positivity (67%); hypocomplementemia (75%); antinuclear antibody positivity (53%); cryoglobulinemia (45%); and hematologic manifestations, including B-symptoms (79%), splenomegaly (59%), thrombocytopenia (83%), and pancytopenia (44%). In 75% of the cases, Bartonella endocarditis was not diagnosed until after kidney biopsy. Pathologically, Bartonella IEGN presented as a focally crescentic GN, which was C3 codominant (80%) with strong IgM (65%) and/or C1q (55%), or pauci-immune (10%), with predominantly mesangial deposits and limited exudative features. At a median follow-up time of 15 months, progression to end-stage kidney disease (ESKD) for all-comers with IEGN was associated with higher creatinine levels at diagnosis, presence of nephrotic syndrome, female sex, and C1q staining intensity. Although delayed diagnosis of infection and immunosuppressive therapy for presumed autoimmune disease before kidney biopsy were more common in Bartonella IEGN than in culture-positive IEGN, neither were associated with ESKD.
Conclusion
IEGNs share laboratory and biopsy features with autoimmunity, which may obfuscate identification of underlying bacterial infection.
Keywords: Bartonella, Coxiella, endocarditis, infection-related GN, IRGN, vasculitis
Graphical abstract
Bartonella spp. are fastidious, facultative intracellular gram-negative bacteria first recognized as causes of endocarditis in 1993.1, 2, 3, 4 Bartonella is estimated to cause 28% of culture-negative endocarditis in the USA.5 Of the many members of genus Bartonella recognized as human pathogens, the two species most prominently associated with culture-negative endocarditis are B. henselae, associated with previous valvulopathy and cat contact; and B. quintana, the agent of trench fever and associated with poor sanitation, homelessness, and body louse infestation.1 In the USA, approximately 22,000 people per year develop cat scratch disease, and 28% of domesticated cats are chronically infected with B. henselae without clinical symptoms.6 The cat flea (Ctenocephalides felis) is the primary vector for transmission of B. henselae between cats, and human inoculation by B. henselae–contaminated flea feces usually occurs from cat scratches.7
Bartonella IE-associated GN was first described in 2004 by Bookman et al.8,9 and in multiple subsequent case reports and small series. The most recent series by Kitamura et al. described 4 cases with an extensive review and pooled analysis of 89 previously published cases of Bartonella IEGN (including 54 case reports and series and 18 abstracts) and compared them with 51 cases of culture-positive (culture+) IEGN from Ohio State University.10 Among patients with IE, those with Bartonella are significantly more likely to develop a GN, compared with IE because of other organisms.11 In this multi-institutional study, we sought to delineate clinical and pathologic features, and outcomes of glomerulonephritides associated with various infections, particularly focusing on the unique findings in Bartonella IEGN compared with culture-positive IEGN and non–endocarditis IRGN, especially those seen in skin and soft tissue infections.
Methods
Renal pathology biopsy databases from the Oregon Health and Science University (OHSU), Cedars-Sinai Medical Center, and Cleveland Clinic were searched for previously unpublished cases with a diagnosis, comment, or known follow-up suggesting blood culture-positive or culture-negative IE between 2015 and 2023. Culture-negative versus culture-positive designations were used to reflect diagnostic information commonly available at the time of nephrology consultation and kidney biopsy. However, because all culture-negative IEGN cases in this cohort were found to be Bartonella, the results could instead be considered as Bartonella IEGN versus non-Bartonella IEGN. All patients with IEGN and renal biopsies were included from OHSU and Cedars Sinai. Only Bartonella-associated IEGN cases were included from Cleveland Clinic. A cohort of patients with non-IE IRGN was obtained from Oregon Health and Science University for comparison. Non-IE IRGN was associated with skin and soft tissue infections with or without osteomyelitis in 16 of 30, pneumonia in 3, infected hardware in 2, spontaneous bacterial peritonitis in 2, dental infection in 1, and an unknown site of infection in 6.
Biopsies were cut at multiple levels and stained with Jones methenamine silver, periodic acid-Schiff, hematoxylin and eosin, and Masson's trichrome. For immunofluorescence (IF), frozen tissue was stained with antibodies against IgG, IgA, IgM, C3, C1q, fibrin/fibrinogen, κ, λ, and albumin. The dominant and/or codominant immunoreactants were noted; these and any other listed as “strong” staining were required to have a staining intensity of 2+ (on a scale of 0–4+) or greater. In this study, cases with < 2+ staining intensity for the brightest immunoreactants were considered in the pauci-immune spectrum. Clinical history was obtained through discussions with nephrologists and review of medical records.
Descriptive statistics were summarized as median and range for continuous variables, which were compared between groups using Mann-Whitney U-tests, whereas categorical variables were compared between groups using Fisher exact test using GraphPad Prism 8 (San Diego, CA). Regression analysis for the overall cohort was performed using the available outcome data (n = 47, 68% of the overall cohort and 95% of the Bartonella cohort). Survival analyses with Cox proportional hazard models and multivariate analyses were performed using Stata 13 (College Station, TX).
Results
Clinical Presentation
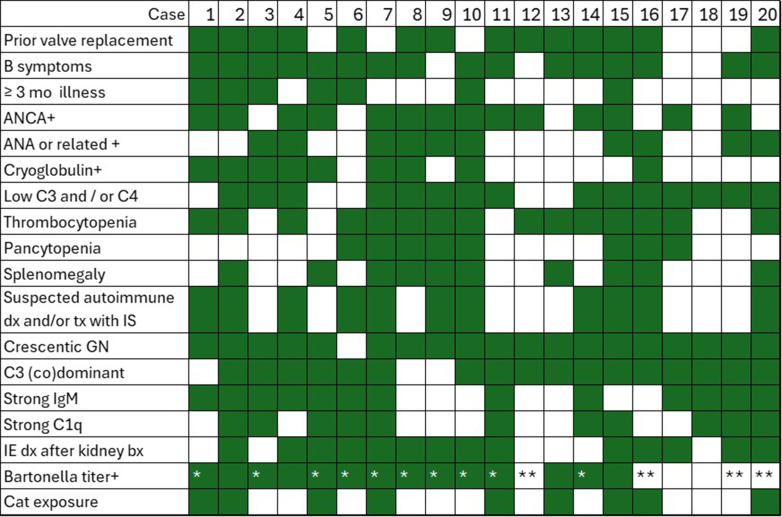
IEGN was seen in approximately 0.15% to 0.4% of native renal biopsies, and Bartonella represented 8% to 21% of IEGN. Patients with Bartonella IEGN (n = 20; Table 1, Figure 1) were predominantly male (80%) and presented with a median age of 58 (range: 12–79) years. B-symptoms, including fatigue, weight loss, fever, and night sweats, were significantly more common in Bartonella IEGN (79%) than in culture+ IEGN (41%, P = 0.01) or IRGN (3%, P < 0.01). Splenomegaly was significantly more common in Bartonella (59%) than in the culture+ IEGN group (18%, P = 0.02). One patient had Bartonella-associated lymphadenopathy (confirmed with polymerase chain reaction on lymph node biopsy), approximately 2 years before the diagnosis of endocarditis. The rates of underlying hepatitis C viral infection (54% vs. 5%, P < 0.01) and intravenous (i.v.) or injection drug use(62% vs. 0%, P<0.01) were significantly higher in the culture+ IEGN group than Bartonella IEGN group.
Table 1.
Presenting clinical and laboratory features of Bartonella IEGN, culture+ IEGN, and non-IE IRGN
| Variable | Bartonella IEGN (n = 20) | Culture+ IEGN (n = 49) | Bartonella vs. culture+ IEGN | Non-IE IRGN (n = 30) | Bartonella IEGN vs. non-IE IRGN |
|---|---|---|---|---|---|
| Median age, yrs | 58 (range: 12–79) | 47 (range: 25–80) | P = 0.07 | 58 (range: 31–85) | P = 0.62 |
| Male, % | 80% (16/20) | 65% (32/49) | P = 0.26 | 63% (19/30) | P = 0.35 |
| Diabetes, % | 10% (2/20) | 16% (8/49) | P = 0.71 | 33% (10/30) | P = 0.09 |
| HTN, % | 65% (13/20) | 45% (21/47) | P = 0.18 | 81% (17/21) | P = 0.31 |
| B-symptoms, % | 79% (15/19) | 41% (15/37) | P = 0.01 | 3% (1/29) | P < 0.01 |
| Rash, % | 25% (5/20) | 8% (4/49) | P = 0.11 | 10% (3/30) | P = 0.24 |
| Splenomegaly, % | 59% (10/17) | 18% (4/22) | P = 0.02 | NA | NA |
| Hx IVDU, % | 0% (0/20) | 62% (28/45) | P < 0.01 | 13% (4/30) | P = 0.14 |
| Hx HCV, % | 5% (1/20) | 54% (19/35) | P < 0.01 | 15% (3/20) | P = 0.61 |
| Hx cirrhosis, % | 0% (0/20) | 2% (1/49) | P > 0.99 | 20% (6/30) | P = 0.07 |
| Laboratory results | |||||
| Median creatinine at presentation (mg/dl) | 4.2 (range: 2.2–8.9) | 3.6 (range: 1.7–14.3) | P = 0.77 | 3.6 (range: 0.6–5) | P = 0.31 |
| Median proteinuria, % nephrotic range | 1.28 (0.76–4.3), 13% (2/16) | 3 (range: 0.4–15), 47% (9/19) | P = 0.04, P = 0.04 | 2.4 (range: 1.8–7.6), 27% (3/11) | P < 0.01, P = 0.37 |
| Hematuria, % | 100% (19/19) | 88% (30/34) | P = 0.28 | 95% (18/19) | P>0.99 |
| Cytopenias: Any, % | 100% (18/18) | 93% (37/40) | P = 0.55 | NA | NA |
| Thrombocytopenia, % | 83% (15/18) | 33% (13/40) | P < 0.01 | NA | NA |
| Pancytopenia, % | 44% (8/18) | 3% (1/40) | P < 0.01 | NA | NA |
| Anemia only, % | 11% (2/18) | 60% (24/40) | P < 0.01 | NA | NA |
| ANCA positive, % | 67% (14/20) | 33% (7/28) | P < 0.01 | 4% (1/26) | P < 0.01 |
| ANA and/or related autoantibody positive, % | 53% (10/19) | 22% (5/23) | P = 0.05 | 4% (1/26) | P < 0.01 |
| Hypocomplementemia: any, low C3, C4, both , % | 75% (15/20), 65% (13/20), 35%(7/20), 25% (5/20) | 56% (18/32), 56% (18/32), 35% (11/32), 35% (11/32) | P = 0.24, 0.57, > 0.99, 0.55 | 25% (4/16), 25% (4/16), 6% (1/16), 6% (1/16) | P < 0.01, 0.02, 0.05, 0.20 |
| Cryoglobulin positive, % | 45% (9/20) | 8% (4/49) | P < 0.01 | NA | NA |
| Initially suspected autoimmune disease and/or tx with steroids, % | 55% (11/20) | 10% (5/49) | P < 0.01 | 7% (2/30) | P < 0.01 |
ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; HCV, hepatitis C virus infection; HTN, hypertension; Hx, history; IEGN, infective endocarditis–associated glomerulonephritis; IRGN, infection–related GN; IVDU, i.v. drug use; NA, not available; tx, treated.
Statistically significant results are shown with P values < 0.05.
Figure 1.
Details of patients with Bartonella-endocarditis associated glomerulonephritis. Green shading indicates variable is present in the patient. ∗ indicates polymerase chain reaction positive in blood and/or valve tissue. ∗∗ indicates positive microbial cell-free DNA sequencing (Karius). Bartonella test details were not available for patients 17 and 18.
All patients with Bartonella IEGN had acute kidney injury or progressive renal insufficiency (median Cr: 4.2 mg/dl), with a previously normal creatinine level in at least 12 (60%). All had proteinuria (median: 1.28 g/g, 13% in the nephrotic range) and hematuria. Cytopenias (in 100%), particularly thrombocytopenia (83% vs. 33%, P < 0.01) and pancytopenia (44% vs. 3%, P < 0.01), were common and significantly enriched in patients with Bartonella compared with those with culture+ IEGN. Hematologic abnormalities prompted bone marrow biopsy before kidney biopsy in 7 patients with Bartonella IEGN (35%). These were negative in all but 1 patient in whom an atypical CD8+ T-cell infiltrate suspicious for large granular T-cell lymphoma was identified. Subsequent T-cell clonality studies were negative, and the findings were attributed in retrospect to Bartonella infection.
Fourteen patients (67%) with Bartonella IEGN had a positive ANCA, particularly directed to proteinase 3 (PR3, 11 patients, 55%), which was accompanied by a cANCA in 6 (30%); one anti–PR3 positive patient was dual anti-myeloperoxidase (MPO) positive, and the second had equivocal anti-MPO reactivity. One patient tested positive for MPO and pANCA positive. Two patients had only cANCA or pANCA (1 each) with negative MPO and PR3 studies results. Before presenting with Bartonella endocarditis, none of the patients had an established autoimmune disease; however, 53% had positive autoimmune serology during the workup. These included a positive antinuclear antibody (8, 40%), anti-double stranded DNA (2, 10%), ribonucleoprotein antibody (1, 5%) and combinations of anti-cardiolipin and β2 glycoprotein-1 Ig (2, 10%), anti-Ro/SSA and anti-La/SSB (1), anti-chromatin and anti-SCL-70 (topoisomerase 1) antibodies (1), rheumatoid factor and poly-specific Coombs (1).
Rates of malignancy and paraproteinemia were not significantly different between the 2 IEGN groups. Previous malignancies were present in 3 patients with culture+ IEGN (6%) and consisted of polycythemia vera, carcinoid, and colon and lung cancer. Two patients (10%) with Bartonella IEGN had a previous history of malignancy, consisting of non-Hodgkin’s lymphoma and cutaneous squamous cell carcinoma. Monoclonal gammopathy of undetermined significance (MGUS) was present in 1 patient with culture+ IEGN (2%) and 1 patient with Bartonella IEGN (5%). Two additional patients with Bartonella IEGN had serum protein electrophoresis (SPEP) suggestive of a monoclonal protein; in 1 case, immunofixation was negative with a normal k/l ratio. In the second patient, there was a possible band in IgG kappa that was too small to quantify with a normal k/l ratio. Bone marrow biopsies in all 3 patients with Bartonella IEGN and MGUS, or possible MGUS were negative for lymphoproliferative disorders. If patients with MGUS and indeterminate SPEP results were considered together, 15% of patients with Bartonella IEGN had serum protein electrophoresis alteration raising the possibility of a monoclonal protein, which showed a trend toward enrichment (P = 0.07) compared with culture+ IEGN.
Pathology
Light microscopy revealed that 90% of Bartonella IEGN cases displayed crescents (vs. 65% of culture+ IEGN, P = 0.04; and 53% of IRGN, P = 0.01) (Table 2, Figure 2). These were generally focal (median: 16%, range: 1%–65%), with only 1 case having more than 50% crescents. A variable mesangial proliferative pattern was present in 7 (35%), and rates of endocapillary hypercellularity (60%) and exudative features (35%) were similar to those of culture+ IEGN and significantly less than those of IRGN (93%, P = 0.01, and 87%, P < 0.01, respectively). None of the patients had a background of diabetic nephropathy, which was again more comparable to culture+ IEGN (12%) than IRGN (30%, P = 0.01). A relatively similar proportion of cases in all 3 cohorts had accompanying acute tubulointerstitial inflammation (50%–85%), including neutrophils (30%–33%) and eosinophils (27%–33%). Tubular atrophy and interstitial fibrosis were significantly lower in patients with culture+ IEGN (median: 10% vs. 23% in Bartonella GN, P < 0.01), which may partly reflect the trend of younger age in this cohort (median age: 47 years in culture+ IEGN versus 58 years in both Bartonella and IRGN, P = 0.07), time to diagnosis, or other factors.
Table 2.
Pathological features of Bartonella IEGN, culture+ IEGN, and non-IE IRGN
| Variable | Bartonella IEGN (n = 20) | Culture+ IEGN (n = 49) | Bartonella vs. Culture+ IEGN | Non-IE IRGN (n = 30) | Bartonella IEGN vs. non-IE IRGN |
|---|---|---|---|---|---|
| Light microscopy | |||||
| Crescents: % with any, median | 90% (18/20),16% (range: 1–65) | 65% (32/49), 6% (range: 2–75) | P = 0.04, P = 0.25 | 53% (16/30), 5% (range: 4–27) | P = 0.01, P < 0.01 |
| Endocapillary hypercellularity | 60% (12) | 63% (31) | P = 0.79 | 93% (28) | P = 0.01 |
| Exudative GN | 35% (7) | 31% (15) | P = 0.78 | 87% (26) | P<0.01 |
| Diabetic GS | 0% (0) | 12% (6) | P = 0.17 | 30% (9) | P = 0.01 |
| AIN | 65% (13) | 84% (41) | P = 0.11 | 50% (15) | P = 0.39 |
| With neutrophils | 30% (6) | 31% (15) | P > 0.99 | 33% (10) | P > 0.99 |
| With eosinophils | 30% (6) | 27% (13) | P = 0.77 | 33% (10) | P > 0.99 |
| Median %global GS | 9% (range: 0–57) | 4% (range: 0–70) | P = 0.17 | 15% (range: 0–69) | P = 0.11 |
| Median %IFTA | 23% (range: 0–60) | 10% (range: 0–60) | P < 0.01 | 28% (range: 5–80) | P = 0.15 |
| Vascular disease | as: 1, ah: 0.25 | as: 1, ah: 0.75 | P = 0.30, 0.03 | as: 1 ah: 0.25 | P = 0.85, 0.04 |
| Immunofluorescence | |||||
| C3 (co)dominant | 80% (16) | 82% (40) | P > 0.99 | 86% (24/28) | P = 0.70 |
| Strong IgG | 15% (3) | 8% (4) | P = 0.41 | 7% (2) | P = 0.38 |
| Strong IgA | 5% (1) | 16% (8) | P = 0.27 | 57% (17) | P < 0.01 |
| Strong IgM | 65% (13) | 22% (11) | P<0.01 | 0% (0) | P < 0.01 |
| Strong C1q | 55% (11) | 20% (10) | P<0.01 | 3% (1) | P < 0.01 |
| Pauci-immune | 10% (2) | 14% (7) | P = 0.71 | 7% (2) | P > 0.99 |
| Mes & PCW, mes only | 40% (8), 60% (12) | 57% (28), 39% (19) | P = 0.29, 0.12 | 70% (21), 30% (9) | P = 0.04, 0.04 |
| Extraglomerular deposits | 0% (0) | 6% (3) | P = 0.55 | 0% (0) | P = 0.26 |
| Electron microscopy | |||||
| Mes/subendo/subepi/ intramembranous | 100% (19/19), 47% (9), 0% (0), 0% (0), | 94% (45/48), 52% (25) 53% (26), 33% (16) | P = 0.55, 0.79, <0.01, < 0.01 | 93% (26/28), 54% (15), 36% (10), 14% (4) | P = 0.51, 0.77, < 0.01, 0.14 |
AIN, acute interstitial nephritis; GN, glomerulonephritis; GS, glomerulosclerosis; IEGN, infective endocarditis–associated glomerulonephritis; IRGN, infection–related GN; IFTA, tubular atrophy and interstitial fibrosis; Mes, mesangial; PCW, peripheral capillary wall.
For IRGN, an additional 11 cases (37%) had IgA as the strongest immunoglobulin staining; however, at an intensity below 2+ (scale 0–4+).
Twenty-two percent of patients with culture+ IEGN had moderate or severe arteriolar hyalinosis compared with none of the patients with moderate or severe hyalinosis with Bartonella IEGN.
Statistically significant results are shown with P values < 0.05.
Figure 2.
Pathologic features of Bartonella IEGN, with (a and b) segmental endocapillary hypercellularity, necrosis, and small crescent formation (Jones silver stain 200×), (c) mesangial immune deposits (transmission electron microscopy, 2900x), and granular mesangial with segmental capillary wall staining for (d) IgM, (e) C3, and (f) C1q. IEGN, infective endocarditis–associated glomerulonephritis.
By IF, C3 was the dominant or codominant immune reactant in all 3 cohorts for 80%–86% of cases. Bartonella IEGN was distinguished by the presence of strong IgM staining (65%, P < 0.01) and/or strong C1q staining (55%, P < 0.01). Strong IgA staining was primarily a feature of IRGN (57%), rather than Bartonella, (5%, P < 0.01) or culture+ IEGN (16%). IgG was strong in a minority of cases (7%–15%) across all 3 groups. A similar percentage of cases in all 3 groups (7–14%) had limited or pauci-immune complex staining.
The glomerular distribution of complement and immune complexes by IF staining in patients with Bartonella IEGN was more likely to be limited to the mesangium than in patients with IRGN (60% vs. 30%, P = 0.04). Electron microscopy revealed mesangial (100%) and subendothelial (47%) immune deposits; however, subepithelial and intramembranous deposits were significantly less common in Bartonella IEGN (not seen in any case) than in culture+ IEGN (53%, P < 0.01) and IRGN (36%, P < 0.01).
Culture+ IEGN and IRGN
Clinicopathologic features of culture+ IEGN are summarized in Tables 1, 2, and 3 and were not the primary focus of this investigation. Most culture+ IEGN were due to Staphylococcus aureus infection (76%). Organism information was not collected for the IRGN cohort; however, 53% of these were due to skin and soft tissue infections. Compared with non-endocarditis IRGN, patients with culture+ IEGN were more likely to have a history of i.v. drug use (P < 0.001), hepatitis C virus (P = 0.005), B-symptoms (P < 0.001), and trend toward a positive ANCA (P = 0.052), and less likely to have hypertension (P = 0.007). Histologically, biopsies in culture+ IEGN shared similarities with Bartonella IEGN in that they were less likely to have endocapillary hypercellularity (P = 0.003), exudative features (P < 0.001), or strong IgA staining (P < 0.001) and were more likely to have strong IgM (P = 0.005) and/or C1q (P = 0.044) staining than IRGN.
Table 3.
Endocarditis or infection details and follow-up
| Variable | Bartonella IEGN (n = 20) | Culture+ IEGN (n = 49) | Bartonella vs. culture+ IEGN |
|---|---|---|---|
| % preexisting cardiac valve replacement or alteration | 75% (15/20): AVR: 9, bicuspid or stenotic AV: 3, Tetralogy of Fallot: 3, LVAD: 1 |
18% (9/49): AVR: 4, tricuspid: 2, previous endocarditis: 2, MVR: 1 |
P < 0.01 |
| Organism | Bartonella spp.: 100% (20/20) | Staphylococcus aureus: 76% (29/38), Streptococcus spp.: 16% (6/38), Enterococcus spp.: 8% (3/38), Neisseria spp.: 3% (1/38), Pseudomonas aeruginosa: 3% (1/38), Escherichia coli: 3% (1/38) | NA |
| Valve involved: | |||
| Tricuspid | 5% (1/20) | 64% (23/36) | P < 0.01 |
| Pulmonic | 15% (3/20) | 0% (0) | P = 0.04 |
| Mitral | 15% (3/20) | 19% (7/36) | P > 0.99 |
| Aortic | 75% (15/20) | 25% (9/36) | P < 0.01 |
| Method of organism identification | Culture: 0, serology: 77% (14/18), PCR-based only: 22% (4/18) | Culture: 100% (39/39) | NA |
| Infection diagnosed after kidney biopsy | 75% (15/20) | 0% (0/49) | P < 0.01 |
| Short term dialysis required | 56% (10/18) | 57% (16/38) | P > 0.99 |
| ESKD or death at follow-up | 26% (5/19) | 18% (5/28) | P = 0.50 |
AV, aortic valve; AVR, aortic valve replacement; ESKD, end-stage kidney disease; LVAD, left ventricular assist device; MVR, mitral valve replacement; PCR, polymerase chain reaction.
For both Bartonella and culture+ IEGN, 3 patients had 2 valves.
For culture+ IEGN, polymicrobials were found in 3 patients.
One patient had gram-positive cocci with no species identified.
Statistically significant results are shown with P values < 0.05.
Notable case features not included in these summaries were the concurrent histological presence of concurrent necrotizing arteritis in 1 patient with a history of i.v. drug use (ANCA studies not available), membranous nephropathy in 1 patient with hepatitis C virus (PLA2R staining not available), and tubulointerstitial immune deposits in 2 patients, both of whom had a history of i.v. drug use.
Endocarditis Details and Bartonella Testing
In most cases (75%), Bartonella endocarditis was diagnosed after renal biopsy, whereas nearly all (P < 0.01) patients with culture+ IEGN had a confirmed or suspected diagnosis of endocarditis before renal biopsy. Indeed 7 patients with Bartonella endocarditis (35%) had ≥ 3 months of unexplained symptoms before diagnosis often prompting detailed rheumatologic evaluation, including 2 patients with 2 years of symptoms and 3 patients with ≥ 6 months of symptoms. Preexisting cardiac valve replacement or alteration was significantly more common in Bartonella GN (75%) than in culture+ IEGN (18%, P < 0.01) and often consisted of aortic valve replacement in 9 patients (45%) or bicuspid or stenotic aortic valve (15%) (Table 3). Bartonella IE usually involved the aortic valve (75%), whereas tricuspid IE was more common in the culture+ IEGN group (64%, P < 0.01). Bartonella exposure was identified in 9 patients (45%) and consisted of cat contact.
Identification of Bartonella infection was facilitated by positive IgG antibody titers against B. henselae in 14 of 18 patients with available information (77%), 1 of whom had high-titer antibodies (> 1:1024) against B. quintana. Of the 12 patients with detailed titer information available, 11 (92%) had a high-titer (>1:1024) antibody to B. henselae and 1 had a lower-titer antibody (1:256; positive ≥ 1:256). B. henselae IgM titers were available for 3 patients and ranged from 1:64 to 1:128 (positive ≥ 1:16). The 16S polymerase chain reaction was positive for Bartonella spp. in 10 of the 10 patients whose blood and/or resected valve tissues were tested. In 4 patients (22%), serologic testing for Bartonella was negative, and infection was identified through blood microbial cell-free DNA sequencing (Karius) (Figure 1 and Supplementary Table S1).
Treatment
Given the high rate of positivity for ANCA and/or autoimmune serologies, B-symptoms, and variable smoldering to accelerated disease tempo, 11 patients (55%) were suspected of having autoimmune disease and/or vasculitis before kidney biopsy, 9 (45%) of whom received immunosuppression (corticosteroids in 9, oral cyclophosphamide in 2, and hydroxychloroquine in 1). The exposure to prebiopsy immunosuppression ranged from a few days during hospitalization for suspected vasculitis or other GN to weeks or months of immunosuppression, including for: suspected lupus (for 3 weeks before biopsy), suspected giant cell arteritis (1 month before biopsy), autoimmune hemolytic anemia (2 months before biopsy), “membranoproliferative glomerulonephritis” on outside biopsy of unclear etiology (5 months before repeat biopsy and Bartonella diagnosis), and vague inflammatory disorder with leukocytoclastic vasculitis (intermittently for a year) (Supplementary Table S1). In 1 patient with Tetralogy of Fallot repair and suspected new lupus, fever did not develop until administration of cyclophosphamide; incomplete transthoracic echo did not visualize vegetation; Bartonella serologies performed after biopsy were negative; however, a DNA-based assay (Karius) identified Bartonella infection. Immunosuppression was stopped after correct diagnosis in all adult patients; 1 pediatric patient with no delay in the diagnosis of Bartonella IEGN was administered steroids with antibiotics after biopsy because of the degree of the crescents.
At the time of biopsy, more than half of the patients with either Bartonella IEGN or culture+ IEGN required short-term dialysis (56% and 57%, respectively), ranging from 2 to 14 weeks. All patients with IE were treated with antibiotics. For those with Bartonella endocarditis, doxycycline (used in 95% of the cases) was the most common agent, often used in combination with rifampicin ( 63%) and/or ceftriaxone (58%), with treatment periods lasting weeks to months. Approximately 68% of patients with Bartonella IEGN underwent surgical valve repair compared with 36% of patients with culture+ IEGN (P = 0.04).
Outcomes
At a median follow-up time of 15 (range: 0.5–102) months, 10 of 47 patients with available data, including 26% of patients with Bartonella IEGN and 18% with culture+ IEGN progressed to ESKD, 2 of whom subsequently died. On univariate analysis for all-comers with IEGN, progression to ESKD was associated with female sex (hazard ratio [HR]: 7.22, P = 0.018, 95% confidence interval [CI]:1.40–37.29), higher creatinine at diagnosis (HR: 1.47, P = 0.002, 95% CI: 1.15–1.89), presence of nephrotic syndrome (HR: 6.29, P = 0.029, 95% CI: 1.20–32.84), and intensity of C1q staining (HR: 2.08, P = 0.024, 95% CI: 1.10–3.95), but not with any other tested clinical or pathologic variable, including Bartonella versus culture+ IEGN or receipt of immunosuppression (Figure 3 and Supplementary Figure S1). On multivariable analysis, both female sex (adjusted HR: 6.177, P = 0.031, 95% CI: 1.18–32.37), and higher creatinine at diagnosis (adjusted HR: 1.49, P = 0.004, 95% CI: 1.13–1.96) remained statistically significant. Among the tested variables, no statistically significant differences were found between males and females, including age, comorbid conditions, time to diagnosis, creatinine level at diagnosis, other laboratory findings, or treatment.
Figure 3.
Forest plot of variables associated with outcome of ESKD in all-comers with IEGN, Bartonella IEGN, and culture+ IEGN. ESKD, end stage kidney disease; IEGN, infective endocarditis–associated glomerulonephritis.
In the Bartonella cohort, 5 of 19 patients (26%) with available follow-up data progressed to ESKD. Of the tested variables, only the intensity of C1q deposition (HR: 6.48, P=0.045, 95% CI: 1.04–40.2) was associated with progression to ESKD (median C1q intensity 2.5+ in progressors vs. 1.5+ in those not progressing to ESKD, P = 0.01); variables significant in the overall cohort had a trend for significance in this smaller cohort, including female sex (P = 0.077), creatinine at diagnosis (P = 0.071), and nephrotic syndrome (P = 0.061). In patients with culture+ IEGN, only creatinine at diagnosis (HR: 1.46, P = 0.017, 95% CI: 1.07–2.0) was significantly associated with progression to ESKD, with trends for significance with the degree of arteriolar hyalinosis (P=0.054) and receipt of immunosuppression (P = 0.072) (Figure 3).
Discussion
In this kidney biopsy-based cohort, 75% of Bartonella endocarditis cases were diagnosed after kidney biopsy, highlighting the importance of interdisciplinary communication among pathology, nephrology, and infectious diseases in patients with unusual C3, IgM, or C1q dominant or lupus-like focally crescentic GNs and a constellation of newly positive serologies or cytopenias. Our study adds to the literature on the treatment, recovery timing, and outcomes of Bartonella and culture+ IEGN. Specifically, we demonstrated the prevalence of cardiac valve alterations (75%), ANCA positivity (67%), hypocomplementemia (75%), antinuclear antibody positivity (53%), and cryoglobulinemia (45%) in patients with Bartonella IEGN compared with those with culture+ IEGN or non-endocarditis IRGN. Pathologically, Bartonella IEGN presents as a focally crescentic GN, which is C3 (co)-dominant (80%) with strong IgM (65%) and/or strong C1q (55%) with mesangial-predominant deposits but fewer exudative features than IRGN. Approximately 10% of cases may be pauci-immune, leading to further confusion regarding ANCA-associated vasculitis. Even in cases with modest IF staining, the degree of crescentic activity was occasionally disproportionate relative to the number of immune complexes observed by IF and electron microscopy. Overall, the clinical and pathologic findings of patients with Bartonella IEGN presented here are highly concordant with those described in the robust pooled analysis by Kitamura and colleagues.10 We identified a significant enrichment of hematologic symptoms, including B-symptoms (79%), splenomegaly (59%), thrombocytopenia (83%), and/or pancytopenia (44%), in patients with Bartonella compared with culture+ IRGN.
On follow-up in their pooled analysis, Kitamura and colleagues10 found that of 45 of 89 patients with Bartonella IEGN were treated with immunosuppressants, similar to 55% in our cohort. They found a significant difference in timing to kidney biopsy between Bartonella IEGN (3 months) compared with culture+ IRGN (1 month)10; delay in diagnosis is reflected in our cohort, in which 35% of Bartonella IEGN had ≥ 3 months of unexplained symptoms before diagnosis. Our study provides additional details on the complex scenarios leading to diagnostic confusion with an autoimmune disease (initially suspected in 55% of cases) and reveals that the potential delay in diagnosis and/or receipt of immunosuppression was not statistically associated with ESKD.
This multi-institutional cohort included 4 cases (22%) of Bartonella IEGN in which molecular techniques were required for diagnosis, because conventional serologic testing was negative. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis12 added newer nucleic acid–based microbiologic techniques for blood culture negative endocarditis as a major criterion, specifically: “positive polymerase chain reaction or other nucleic acid–based techniques for Coxiella burnetii, Bartonella spp., or Tropheryma whipplei from blood.” Before this addition, in one study of 106 patients with Bartonella endocarditis, Eduoard et al. demonstrated that whereas all tested by Western blot were positive for Bartonella, indirect IF assay was negative (in 9%) or positive only at low titer (IgG 1:100–1:800, in 37%) in a substantial minority of patients, and more strongly positive (≥ 1:800) in only 58% of cases, with a serum RT-polymerase chain reaction sensitivity of 36%.1 Approximately 2% to 20% of persons experiencing homelessness have antibodies to B. quintana.13 Thus, low-level positives and false negatives are challenges in the serologic assessment of Bartonella spp. infection when assessing for active endocarditis and/or the etiology of a GN.
Mechanistically, intracellular Bartonella spp. survive and avoid the immune system by producing biofilms, inducing angiogenesis, avoiding neutrophil phagocytosis (assisted by Bartonella adhesin A in B. henselae), resisting fusion and thus, degradation by lysosomes, and increasing production of the anti-inflammatory interleukin (IL)-10.14 As an antipyretic, IL-10 may contribute to delay in diagnosis by inhibiting fever production15 in earlier stages of infection. IL-10 decreases platelet production,16 potentially accounting for the increase in thrombocytopenia in patients with Bartonella IEGN. Patients with cat scratch disease have increased levels of IL-6,17 a cytokine associated with B-cell lymphomas and B-symptoms,18 which may contribute to the high incidence of B-symptoms seen in Bartonella but not in culture+ IEGN or IRGN.
Bartonella causes 0% to 15.6% of IE in Europe, and along with C. burnetii, is among the most common causes of culture-negative endocarditis.1,5 Bartonella and Coxiella are both intracellular bacteria, which can evoke a similar cytokine response including IL-6 and IL-10,19 and mimic a systemic vasculitis.20 A recent series of 7 patients with C. burnetii (Q-fever) associated GN showed substantial similarities with Bartonella IEGN, specifically, a male predominance, longer duration of kidney disease before diagnosis (> 6 months in 4/7), splenomegaly (4/7), pancytopenia (2/7), positive PR3 ANCA (3/7), cryoglobulinemia (in all), endocarditis or aortic arch infection (4/7) and preexisting cardiac valve abnormality, replacement, and/or aortic dissection (in all). Biopsies from patients with Q-fever show a mesangial proliferative to membranoproliferative pattern of glomerular injury with endocapillary hypercellularity, focal crescents, and frequent IgM and C3 staining.21 Neither Bartonella nor Coxiella DNA has been found in affected tissue biopsies from patients with associated vasculitis.20 Thus, Bartonella- and Coxiella-associated GN can have substantial overlap in clinical and pathologic features, and it is prudent to test for both agents serologically and/or molecularly in the setting of high clinical concern or with the described biopsy findings.
We identify C1q intensity as associated with ESKD in patients with Bartonella IEGN. Mesangial C1q deposition has been associated with a worse prognosis in IgA nephropathy,22,23 and persistent C1q positivity in repeat biopsies from patients with lupus nephritis may be associated with a worse outcome.24 Both the alternative and classical complement pathways are involved in host response to Bartonella,7 and in this case, differences in C1q intensity may reflect host complement system differences, duration or severity of infection among others.
Weaknesses of this study include the low number of patients progressing to ESKD, limiting the strength of the conclusions on the association with outcomes. However, our outcome analysis revealing creatinine at diagnosis as a variable associated with progression to ESKD was concordant with the study by Boils et al.,25 previously the largest case series of IEGN (n = 49) composed largely of culture-positive endocarditis (90%) due to staphylococcal (53%) or streptococcal (23%) infections with 8% having Bartonella IEGN. Unexpectedly, we identified female sex as associated with progression to ESKD in the overall IEGN cohort. This is discordant with trends for worse prognosis in males reported for lupus nephritis,26 membranous nephropathy, and focal segmental glomerulosclerosis,27 but not for IgA nephropathy or ANCA GN,27,28 and is likely multifactorial and requires further study. In addition, for patients with culture+ IEGN versus IRGN, we examined differences by location of infection, but not by organism type or other factors. With this caveat, certain features were significantly more common in IEGN—strong IgM and C1q staining, with less IgA deposition or exudative features and a trend toward ANCA positivity—compared with non-endocarditis IRGN. In a review of published cases of ANCA-positive endocarditis, 18% to 43% of patients with endocarditis developed a positive ANCA, which subsequently became negative in 69%.29 It has been suggested that ANCA may develop in patients with endocarditis because of molecular mimicry and/ or infection-induced neutrophil activation, degranulation, and NETosis, or production of net-like traps composed of chromatin and bactericidal proteins from neutrophil granules and cytoplasm which have been associated with ANCA development.29, 30, 31
In conclusion, IRGNs are heterogeneous. Different pathological patterns and clinical presentations of IRGN reflect how the kidney, and glomerular inflammation and injury in particular, offer a window into the immune responses to different inciting microbes. Because gram-negative intracellular bacteria produce a subacute to chronic systemic infection, Bartonella spp. elicit a different host response than the robust and acute immune activation typically triggered by gram-positive extracellular bacteria such as S aureus. Likely at least in part reflecting these differences, we showed that the clinicopathological features of Bartonella IEGN significantly differ from those of predominantly S. aureus–related culture-positive IEGN and non-endocarditis IRGN, although certain clinicopathological features are more common in IE than in other IRGNs, regardless of organism. Our findings corroborate the hallmark features of Bartonella IEGN and underscore the need for further studies on the diverse glomerular manifestations of host responses to infection and secondary autoimmunity.
Disclosure
All the authors declared no competing interests.
Acknowledgment
RSA was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award number K23DK135855.
Footnotes
Figure S1. Forest plot of tested variables associated with end stage kidney disease in all-comers with endocarditis associated glomerulonephritis.
Table S1. Details of patients with Bartonella-endocarditis associated glomerulonephritis.
Supplementary Material
Figure S1. Forest plot of tested variables associated with end stage kidney disease in all-comers with endocarditis associated glomerulonephritis. Table S1. Details of patients with Bartonella-endocarditis associated glomerulonephritis.
References
- 1.Edouard S., Nabet C., Lepidi H., Fournier P.E., Raoult D. Bartonella, a common cause of endocarditis: A report on 106 cases and review. J Clin Microbiol. 2015;53:824–829. doi: 10.1128/JCM.02827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spach D.H., Callis K.P., Paauw D.S., et al. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol. 1993;31:692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly J.S., Worthington M.G., Brenner D.J., et al. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadfield T.L., Warren R., Kass M., Brun E., Levy C. Endocarditis caused by Rochalimaea henselae. Hum Pathol. 1993;24:1140–1141. doi: 10.1016/0046-8177(93)90196-n. [DOI] [PubMed] [Google Scholar]
- 5.Houpikian P., Raoult D. Blood culture-negative endocarditis in a reference center: Etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84:162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- 6.McCool T.L., Hoey J.G., Montileone F., et al. Discovery and analysis of Bartonella henselae antigens for use in clinical serologic assays. Diagn Microbiol Infect Dis. 2008;60:17–23. doi: 10.1016/j.diagmicrobio.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Jin X., Gou Y., Xin Y., et al. Advancements in understanding the molecular and immune mechanisms of Bartonella pathogenicity. Front Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1196700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bookman I., Scholey J.W., Jassal S.V., Lajoie G., Herzenberg A.M. Necrotizing glomerulonephritis caused by Bartonella henselae endocarditis. Am J Kidney Dis. 2004;43:e25–e30. doi: 10.1053/j.ajkd.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Khalighi M.A., Nguyen S., Wiedeman J.A., Palma Diaz M.F. Bartonella endocarditis-associated glomerulonephritis: A case report and review of the literature. Am J Kidney Dis. 2014;63:1060–1065. doi: 10.1053/j.ajkd.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura M., Dasgupta A., Henricks J., et al. Clinicopathological differences between Bartonella and other bacterial endocarditis-related glomerulonephritis - our experience and a pooled analysis. Front Nephrol. 2024;3 doi: 10.3389/fneph.2023.1322741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrestha N.K., Kanyo E.C., Nakhoul G.N., Herlitz L.C., Gordon S.M. Association between causative pathogen and occurrence of infection-related glomerulonephritis in infective endocarditis. Clin Infect Dis. 2024;78:1551–1553. doi: 10.1093/cid/ciae213. [DOI] [PubMed] [Google Scholar]
- 12.Fowler V.G., Durack D.T., Selton-Suty C., et al. The 2023 Duke-International Society for cardiovascular infectious diseases criteria for infective endocarditis: Updating the modified Duke criteria. Clin Infect Dis. 2023;77:518–526. doi: 10.1093/cid/ciad271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonilla D.L., Kabeya H., Henn J., Kramer V.L., Kosoy M.Y. Bartonella quintana in body lice and head lice from homeless persons, San Francisco, California, USA. Emerg Infect Dis. 2009;15:912–915. doi: 10.3201/eid1506.090054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi Y., Li X., Liu L., et al. Sneaky tactics: Ingenious immune evasion mechanisms of Bartonella. Virulence. 2024;15 doi: 10.1080/21505594.2024.2322961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leon L.R. Invited Review: Cytokine regulation of fever: Studies using gene knockout mice. J Appl Physiol (1985) 2002;92:2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- 16.Sosman J.A., Verma A., Moss S., et al. Interleukin 10-induced thrombocytopenia in normal healthy adult volunteers: Evidence for decreased platelet production. Br J Haematol. 2000;111:104–111. doi: 10.1046/j.1365-2141.2000.02314.x. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos N.G., Gourgiotis D., Bossios A., et al. Circulating cytokines in patients with cat scratch disease. Clin Infect Dis. 2001;33:e54–e56. doi: 10.1086/322596. [DOI] [PubMed] [Google Scholar]
- 18.Kato H., Kinoshita T., Suzuki S., et al. Production and effects of interleukin-6 and other cytokines in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 1998;29:71–79. doi: 10.3109/10428199809058383. [DOI] [PubMed] [Google Scholar]
- 19.Honstettre A., Imbert G., Ghigo E., et al. Dysregulation of cytokines in acute Q fever: Role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. J Infect Dis. 2003;187:956–962. doi: 10.1086/368129. [DOI] [PubMed] [Google Scholar]
- 20.Beydon M., Rodriguez C., Karras A., et al. Bartonella and Coxiella infections presenting as systemic vasculitis: Case series and review of literature. Rheumatoogyl (Oxford) 2022;61:2609–2618. doi: 10.1093/rheumatology/keab691. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Shen Y., Yang H., et al. Q fever-related glomerulonephritis unveiled by metagenomic next-generation sequencing. Kidney Int Rep. 2024;9:3062–3066. doi: 10.1016/j.ekir.2024.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan L., Tang Y., Pei G., et al. A multicenter, prospective, observational study to determine association of mesangial C1q deposition with renal outcomes in IgA nephropathy. Sci Rep. 2021;11:5467. doi: 10.1038/s41598-021-84715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian S., Yang X., Luo J., Guo H. Clinical and prognostic significance of C1q deposition in IgAN patients-a retrospective study. Int Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106896. [DOI] [PubMed] [Google Scholar]
- 24.Alsuwaida A., Husain S., Al Ghonaim M., et al. Prognostic significance of C1q deposition in serial biopsies for predicating the long-term outcome in patients with proliferative lupus nephritis. Saudi J Kidney Dis Transplant. 2016;27:305–311. doi: 10.4103/1319-2442.178547. [DOI] [PubMed] [Google Scholar]
- 25.Boils C.L., Nasr S.H., Walker P.D., Couser W.G., Larsen C.P. Update on endocarditis-associated glomerulonephritis. Kidney Int. 2015;87:1241–1249. doi: 10.1038/ki.2014.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmood S.B., Aziz M., Malepati D., et al. Evaluating sex differences in the characteristics and outcomes of lupus nephritis: A systematic review and meta-analysis. Glomerular Dis. 2024;4:19–32. doi: 10.1159/000535981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cattran D.C., Reich H.N., Beanlands H.J., et al. The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant. 2008;23:2247–2253. doi: 10.1093/ndt/gfm919. [DOI] [PubMed] [Google Scholar]
- 28.Tampe D., Korsten P., Ströbel P., Hakroush S., Tampe B. Comprehensive analysis of sex differences at disease manifestation in ANCA-associated glomerulonephritis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.736638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gool I.C., Kers J., Bakker J.A., et al. Antineutrophil cytoplasmic antibodies in infective endocarditis: A case report and systematic review of the literature. Clin Rheumatol. 2022;41:2949–2960. doi: 10.1007/s10067-022-06240-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraaij T., Kamerling S.W.A., van Dam L.S., et al. Excessive neutrophil extracellular trap formation in ANCA-associated vasculitis is independent of ANCA. Kidney Int. 2018;94:139–149. doi: 10.1016/j.kint.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Sangaletti S., Tripodo C., Chiodoni C., et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120:3007–3018. doi: 10.1182/blood-2012-03-416156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Forest plot of tested variables associated with end stage kidney disease in all-comers with endocarditis associated glomerulonephritis. Table S1. Details of patients with Bartonella-endocarditis associated glomerulonephritis.