Abstract
Background and Purpose
Pediatric neurointerventional radiology is an evolving subspecialty with growing indications and technological advancement such as miniaturization of devices and decreased radiation dose. The ability to perform these procedures is continuously balanced with necessity given the inherently higher risks of radiation and cerebrovascular injury in infants. The purpose of this study is to review our institution's neurointerventional experience in infants less than one year of age to elucidate trends in this patient population.
Methods
We retrospectively identified 132 patients from a neurointerventional database spanning 25 years (1997–2022) who underwent 226 procedures. Treatment type, indication, and location as well as patient demographics were extracted from the medical record.
Results
Neurointerventional procedures were performed as early as day of life 0 in a patient with an arteriovenous shunting malformation. Average age of intervention in the first year of life is 5.9 months. Thirty-eight of 226 procedures were completed in neonates. Intra-arterial chemotherapy (IAC) for the treatment of retinoblastoma comprised 36% of neurointerventional procedures completed in infants less than one year of age followed by low flow vascular malformations (21.2%), vein of Galen malformations (11.5%), and dural arteriovenous fistulas (AVF) (9.3%). Less frequent indications include non-Galenic pial AVF (4.4%) and tumor embolization (3.0%). The total number of interventions has increased secondary to the onset of retinoblastoma treatment in 2010 at our institution.
Conclusion
The introduction of IAC for the treatment of retinoblastoma in the last decade is the primary driver for the increased trend in neurointerventional procedures completed in infants from 1997 to 2022.
Keywords: Pediatric, history, angiography, brain, congenital
Introduction
Interventional neuroradiology is a primarily adult-focused practice with a minority of neurointerventionalists completing more than 100 pediatric endovascular procedures per year. 1 Pediatric interventional neuroradiology is an evolving subspecialty with growing indications and technological advances such as miniaturization of devices and updated angiography suites with improved imaging at lower radiation doses. The ability to perform these procedures is continuously balanced with their necessity given the inherently higher risks of radiation,2,3 contrast, and cerebrovascular injury in infants less than one year of age.
Endovascular treatment of arteriovenous shunting lesions including vein of Galen malformations (VOGM), arteriovenous malformations (AVM), and arteriovenous fistulas (AVF) has been well established in pediatric patients including those of a very young age particularly when intracranial hemorrhage or high-output heart failure complicate those diagnoses.4–6 More recently, intra-arterial chemotherapy (IAC) for the treatment of retinoblastoma (RB) has been used in this age group.7–9 The purpose of this study is to provide a descriptive review of our institution's neurointerventional experience in infants less than one year of age, to describe trends in the procedures and indications, as well as to provide historical context. Specific attention is paid to the subgroup of youngest patients—neonates within the first month of life—as they are the most sensitive to radiation, anesthetics, and contrast media. The most common indications for procedures performed by the pediatric neurointerventional service are then reviewed.
Methods
A retrospective review was conducted of pediatric patients less than one year of age who underwent a procedure for diagnosis and/or treatment by the interventional neuroradiology division at our single institution between the years of 1997 and 2022. Procedure type and indication as well as patient demographics were extracted from the medical records under an institutional review board approved protocol. Procedures included cerebral angiography, embolization, intra-arterial chemoinfusion, sclerotherapy of low flow vascular malformations (LFVM), and peripheral angiography and/or embolization. Patient data was analyzed according to year of treatment as well as with respect to indication for angiography or sclerotherapy in the first year of life.
Results
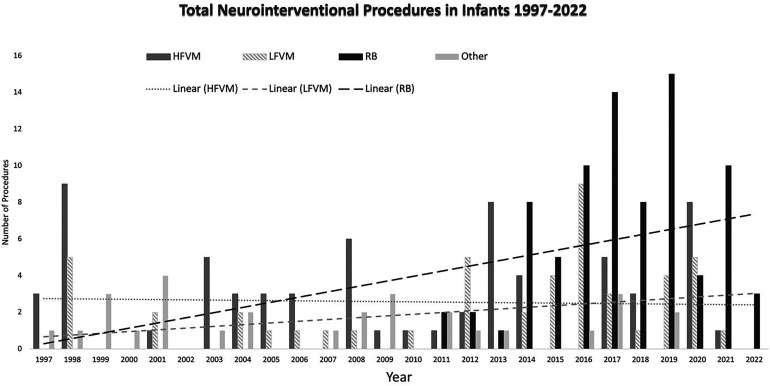
In a 25-year period (January 1997 to April 2022), 132 patients less than one year of age underwent 226 neurointerventional procedures. The total number of procedures is shown in Figure 1, as divided into three main indications: RB, high flow vascular malformations (HFVM) including VOGM, AVM, dAVF, and pAVF lesions, LFVM, RB and “other” including brain aneurysms and diagnostic angiograms. Neurointerventional procedures were performed as early as day of life 0, specifically in a patient with an AVF causing heart failure and hydrops fetalis. The average age of the patient undergoing a neurointerventional procedure in the first year of life was 5.9 months, or 177 days. Patient age ranged from 0 days to 361 days in this cohort. Twenty-one neonates (age < 30 days) accounted for 38 of 226 (16.8%) procedures. Fifty-eight of 226 (25.6%) procedures were completed in the first 90 days of life.
Figure 1.
Total number of neurointerventional procedures performed per indication subtype from 1997 through 2022 with a linear-fit regression model for each indication subtype.
The most common procedure performed in the first year of life over a 25-year period was IAC for the treatment of RB which comprised 82 of 226 total procedures (36%) in 36 patients. Since its initiation at our institution in 2010, IAC for RB has accounted for half (51%) of the total number of 160 total procedures between 2010 and 2022. Other relatively common neurointerventional procedures in patients less than one year of age were sclerotherapy for LFVM accounting for 48 of 226 procedures (21.2%) in 30 patients, embolization of VOGM accounting for 26 of 226 procedures (11.5%) in 15 patients, and embolization of intracranial dural AVF accounting for 21 of 226 procedures (9.3%) in 7 patients. Less frequent indications included non-Galenic pial AVF in 10 of 226 procedures (4.4%), brain AVM in 8 of 226 procedures (3.5%), tumor evaluation and/or embolization in 7 of 226 procedures (3.0%), and brain aneurysm in 4 of 226 procedures (1.8%). Thirteen diagnostic cerebral angiograms were normal. In the most recent decade, the total number of interventions has increased secondary to the onset of RB treatment in 2010 at our institution.
Neonates (age < 30 days)
Thirty-eight of 266 procedures (16.8%) were completed in 21 neonates who had an average age of 8.1 days (0–26 days). The most common indication for neuroangiography in the first month of life was for the evaluation and treatment of VOGM accounting for 14 of 38 procedures (36.8%) in eight patients followed closely by the evaluation and treatment of dAVF which comprised 12 of 38 procedures (31.5%) in four patients. Additional procedure indications included: five lymphatic malformations (13.1%), two negative diagnostic angiograms in patients with subarachnoid hemorrhage and intraparenchymal hematoma, two dural venous sinus thromboses, one holohemispheric brain AVM, one non-Galenic pial AVF, one chest wall hemangioendothelioma causing high-output heart failure, and one desmoplastic infantile ganglioglioma for pre-operative vascular mapping and embolization. Figure 2 demonstrates sporadic occurrence of procedures completed in neonates in the 25-year span without demonstrable trend from 1997 to 2022, likely related to the rarity of the diagnoses and dependence on clinical severity.
Figure 2.
Total number of neurointerventional procedures performed in neonates (<30 days of age) from 1997 through 2022 with a linear-fit regression model.
High flow vascular malformations
Vein of Galen malformations
A subset of patients well known to neuroangiographers in the first year of life are those with VOGMs, an example shown in Figure 3. In our study, 28 of 226 procedures (12.3%) in 16 patients underwent neuroangiography and accounted for the most common indication of neuroangiography in the neonatal period. The average age was 95.2 days (range 1–361 days). VOGMs were divided into the following angioarchitectural subtypes: six mixed, five choroidal, four mural, and one unknown.
Figure 3.
Reprinted with permission (Figure 29 Griscom NT. Radiologic History Exhibit: History of Pediatric Radiology in the United States and Canada: Images and Trends. Radiographics 1995; 15: 1399–1422). Lateral cerebral angiogram from 1947 in an infant with direct carotid artery access as shown by the retractors and needle position over the neck.
Dural arteriovenous fistulas
Seven patients underwent 21 neuroangiography procedures for the evaluation and treatment of dAVF. The average age in this cohort was 70.9 days (range 3–342).
Non-Galenic pial arteriovenous fistulas
Five patients underwent 10 neuroangiography procedures for the evaluation and treatment of pAVF. The average age in this cohort was 118.9 days (range 1–258).
Arteriovenous malformations
Five patients underwent eight neuroangiography procedures for the evaluation and treatment of nidus-type brain AVM. One of the patients presented with AVM rupture, two patients presented with hydrocephalus, one patient with seizure and mild CHF in the setting of Wyburn-Mason syndrome, one patient with developmental delay, and one patient with severe congestive heart failure leading to hydrops fetalis. Average age at time of procedure was 130.5 days (range 0–273).
Low flow vascular malformations
At our institution, LFVM regardless of anatomic location referred through the multidisciplinary Birthmarks and Vascular Anomalies Clinic are treated by the neurointerventional radiology section via percutaneous sclerotherapy. In infancy, these lesions are typically treated because of airway compromise. For example, 21 of 48 procedures (43.8%) were completed on patients with lymphatic malformations in the neck and/or floor of mouth. The next most common location for percutaneous sclerotherapy treatment in the first year of life was the orbit, completed in 7 of 48 procedures (14.5%). The average age was 153.4 days (range 2–333 days).
Retinoblastoma
The advent of IAC for the treatment of RB accounts for the largest practice shift in procedures completed on patients less than one year of age, an example shown in Figure 4. Although we initially did not offer IAC under age 6 months, preferring instead to begin with systemic chemotherapy in these small infants, we have more recently treated patients as young as 3 months old with IAC. Since 2011, 82 procedures were completed on 36 patients in the first year of life. Average age was 261.5 days (range 98–358 days).
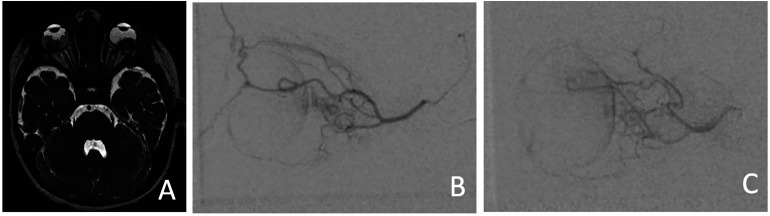
Figure 4.
Neonate, day of life 0 (A-E) through 41 (F), with a vein of Galen malformation. (A) Coronal ultrasound image with color Doppler through the brain demonstrating a large vascular pouch in the region of the median prosencephalic vein of Markowski. (B) Multiplanar reconstruction of the time-of-flight MRA, T2-weighted MRI in (C) coronal and (D) sagittal planes confirm enlarged flow voids corresponding to choroidal and thalamoperforator arterial supply to a markedly enlarged vein of Galen and torcula. (E) Pre-embolization and (F) post-embolization lateral angiograms of a left vertebral artery injection confirms a mixed-type VOGM with high flow arteriovenous shunting, which persisted after four staged embolization procedures including transarterial coiling and glue as well as transvenous coiling.
Discussion
Our study evaluated the evolution of indications for neurointerventional procedures in infants over 25 years. The rise of interventional neuro-oncology in the form of IAC infusions for RB accounted for a significant increase in the number of procedures in the most recent decade. Other common indications such as congenital high-flow AV fistulas remained relatively stable throughout the study period.
Abramson et al. 10 evaluated the feasibility of intra-arterial infusion of melphalan into the ophthalmic arteries in nine children in 2008. The clinical use of melphalan evolved from the study of chemosensitivity of RB cells against various therapeutic agents, of which the mustard agent, melphalan proved most tumoricidal. 11 Since 2008, the use of melphalan and additional agents, such as topotecan,12,13 have been increasingly used for the treatment of both unilateral and bilateral RB, especially later stage disease (Group D-E), to reduce the rate of enucleation.7,14
Our study indicates there is a temporally sporadic presentation of infants under the age of one month who present for neuroangiographic evaluation and treatment. Over a 25-year period, there is a slight increase in use of percutaneous sclerotherapy for the treatment of LFVM, primarily lymphatic malformations, further described at UCSF by Caton et al. 15 Additionally, there is a linear increase in the treatment of RB in the past decade. The use of catheter neuroangiography for HFVM in the context of intended endovascular treatment has remained constant at our institution, an experience most recently described by Hetts et al.5,16
Despite an increase in the number of cerebrovascular cases evaluated at UCSF over the last 25 years, many of the youngest patients do not come to the angiography suite. Unless there is an indication for diagnosis to guide emergent or urgent treatment or with the intention to perform concomitant endovascular therapy, catheter angiography may be delayed until an infant is at least 6–12 months old in effort to reduce procedural risks. The procedural and periprocedural risk of neuroangiography are compounded in children, particularly those less than one year of age. Increased attention to the constraints of neuroangiography within the pediatric patient population includes the use of contrast media, particularly within neonates and infants, anesthesia time, and devices optimized for children. For example, femoral access site complications remain one of the highest risks of cerebral angiography in this patient population despite advances in technique using smaller sheath sizes 17 or slender sheaths intended for radial artery access in adults.18,19
Multiple studies have investigated the radiation risk to children incurred from neuroangiography, specifically. Children are particularly sensitive to the dose and detrimental effects of ionizing radiation. Orbach et al. demonstrated the <1 year old subgroup had the highest mean brain-absorbed dose following neuroangiographic procdeures. 3 In a study evaluating 50 children who underwent cerebral embolization, one of the factors that affects higher radiation dose is younger patient age, presumably from the thin calvarium in younger patients. 20 Beyond the affect of age on radiation dose, younger patients with more rapidly dividing cells are also more susceptible to the effects of ionizing radiation. As such, it is imperative to maintain the principle of ALARA “as low as reasonably achievable” in the pediatric neuroangiography suite. 21
Our study is limited by a single institution experience and the availability of data in PACS and our electronic medical record, beginning in 1996. Pediatric neuroangiography is remarkably variable through the United States and the world due to relatively low incidence of pediatric neurovascular disease, variability in operator experience, and the small subset of patients at higher risk for procedures necessitating radiation. Neuroangiography in infants is highly variable throughout the United States and the world due to relatively low incidence of pediatric neurovascular disease, variability in operator experience, and higher risks of performing invasive procedures in the very young as compared to older patients.
Brief history of pediatric cerebral angiography
Egaz Moniz (1874–1955) is typically considered the father of neuroangiography as he performed the first cerebral angiograms in a dog in 1926 and human patients in 1927 by direct injection in the internal carotid artery.22,23 His work included one pediatric patient who was 11 years old in 1928. 24 His life and contributions of the field of neurointerventional radiology are well described in the literature. 25
There is a relative paucity of literature describing the advent of pediatric neuroangiography. Pediatric angiocardiography preceded documented cerebral angiography in the late 1930s. 26 Probably the first reported cerebral angiogram in the American literature was performed at Boston Children's Hospital in 1947 showing diminutive contrast reaching the intracranial circulation, as reproduced in Figure 5. 27 This may have been secondary to under-injection as posited by the author, or related to inadequate contrast opacification, as contrast was limited in supply in the years following World War II.
Figure 5.
(A) Axial T2-weighted MRI showing bilateral lobulated masses within the posterior orbits consistent with retinoblastoma. (B) Right and (C) left lateral ophthalmic arteriograms with choroidal blushes immediately prior to intra-arterial chemotherapy infusion.
As with any new procedure, increased reporting of technical feasibility, safety, and utility in pediatric angiography occurred from the early 1950s and onward. For example, in 1952, Dr Picaza 28 published his series of 50 angiograms obtained in children for brain tumors most commonly. Drs Poser and Taveras 24 from Columbia University College of Physicians and Surgeons reported their experience in 1955 in a total of 131 cerebral angiograms completed in 90 children under 12 years old, of whom 11 were under one year of age. In their series, the 63 cases were obtained for intracranial space-occupying lesions most commonly neoplasm or vascular malformation. In 1963, the Seldinger technique was described in children for catheterization of vessels in the cardiac, peripheral, and craniocervical vasculature, 29 although percutaneous intravascular access had been in clinical use in older children for nearly a decade. In that same year, a report of normal pediatric neurovascular anatomy, specifically how the angiographic appearance of the carotid siphon and proximal anterior cerebral artery may differ from adults, was published. 30 Shortly thereafter in 1966 at UCLA, percutaneous intravascular access in children as young as two years old was further described without significant complications. 31 Transumbilical access for catheter angiography in newborn infants was described the next year at the same institution as a safe method to perform cerebral angiography from a right carotid artery injection in one infant for hypotonia, tremor, and overriding sutures, or from a proximal aortic injection for in two infants for lethargy secondary to acute subdural hemorrhage as well as lethargy and macrocephaly with absence of the bilateral pericallosal arteries. 32
In the 1960s and later, the evolution of reporting pediatric angiography focused also on the pathologic conditions for which angiography was obtained and most useful in children. For example at UCSF in 1968, Drs Newton and Gooding 33 described two years of experience with percutaneous transfemoral arterial access for cerebral angiography in children, in which 20 of 76 studied children were less than two years of age. Femoral artery access via surgical cutdown was obtained in neonates. No major complications were reported. The most common indication for positive angiographic studies was neoplasm (10/76 studies or 13%) as compared to our current study in which neoplasm accounted for only 3% of patients. This is not surprising given the advent and widespread adoption of cross-sectional imaging techniques including computed tomography (CT) and magnetic resonance imaging (MRI).
Prior to CT and MRI, diagnostic tools for intracranial hemorrhage and masses were lacking despite the obvious clinical signs. CT was introduced in early 1970s, and by 1980, Dr Hounsfield 34 reported its global use in over 1000 hospitals. The introduction of CT shortly followed by MRI shifted the focus of diagnostic angiography from that of space-occupying lesions to the diagnosis and treatment of vascular pathologies. The evolution of endovascular therapy began to improve upon traditional surgical methods for vascular pathologies in the brain and spine, including in the neonatal and infant age groups.35,36 Multiple reports describing the treatment of VOGMs using detachable balloons and coils via the transarterial and/or percutaneous transtorcular approaches were published, with particularly robust experiences by Drs Lasjunas, Berenstein, ter Brugge and Hieshima and colleagues.6,37,38 Then, in 1990, Dowd et al. 39 at UCSF described the treatment of VOGMs via a transfemoral, transvenous approach. The UCSF experience from the late 1990s through present is described in our study.
Conclusion
The evolution of pediatric neuroangiography over the past century has evolved from diagnosis of space-occupying lesions to the treatment of complex vascular malformations and embolization of tumors. In the past decade, the advent of IAC for the treatment of RB has increased the involvement of neurointerventional techniques in pediatric oncology.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Amanda Baker https://orcid.org/0000-0001-9973-5792
Michael Travis Caton https://orcid.org/0000-0003-1581-7702
Kazim H Narsinh https://orcid.org/0000-0002-2019-5461
Steven W Hetts https://orcid.org/0000-0001-5885-7259
References
- 1.Chaudhary N, Elijovich L, Martinez M, et al. Pediatric diagnostic cerebral angiography: practice recommendations from the SNIS pediatric committee. J Neurointerv Surg 2021; 13: 762–766. [DOI] [PubMed] [Google Scholar]
- 2.Chen K, Paramasivam S, Berenstein A. Pediatric neurointervention: collimation on radiation exposure-associated lifetime excess tumor risk. J Neurointerv Surg 2017; 9: 895–898. [DOI] [PubMed] [Google Scholar]
- 3.Orbach DB, Stamoulis C, Strauss KJ, et al. Neurointerventions in children: radiation exposure and its import. AJNR Am J Neuroradiol 2014; 35: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hetts SW, Cooke DL, Nelson J, et al. Influence of patient age on angioarchitecture of brain arteriovenous malformations. AJNR Am J Neuroradiol 2014; 35: 1376–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetts SW, Moftakhar P, Maluste N, et al. Pediatric intracranial dural arteriovenous fistulas: age-related differences in clinical features, angioarchitecture, and treatment outcomes. J Neurosurg Pediatr 2016; 18: 602–610. [DOI] [PubMed] [Google Scholar]
- 6.terBrugge KG. Neurointerventional procedures in the pediatric age group. Childs Nerv Syst 1999; 15: 751–754. [DOI] [PubMed] [Google Scholar]
- 7.Manjandavida FP, Stathopoulos C, Zhang Jet al. et al. Intra-arterial chemotherapy in retinoblastoma - A paradigm change. Indian J Ophthalmol 2019; 67: 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pekacka A. The role of intraarterial chemotherapy in the management of retinoblastoma. J Ophthalmol 2020; 2020: 3638410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyse E, Handa JT, Friedman ADet al. et al. A review of the literature for intra-arterial chemotherapy used to treat retinoblastoma. Pediatr Radiol 2016; 46: 1223–1233. [DOI] [PubMed] [Google Scholar]
- 10.Abramson DH, Dunkel IJ, Brodie SEet al. et al. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology 2008; 115: 1398–1404, 1404 e1391. [DOI] [PubMed] [Google Scholar]
- 11.Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured human retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res 1987; 78: 858–868. [PubMed] [Google Scholar]
- 12.Kaufmann SH, Peereboom D, Buckwalter CA, et al. Cytotoxic effects of topotecan combined with various anticancer agents in human cancer cell lines. J Natl Cancer Inst 1996; 88: 734–741. [DOI] [PubMed] [Google Scholar]
- 13.Schaiquevich P, Buitrago E, Ceciliano A, et al. Pharmacokinetic analysis of topotecan after superselective ophthalmic artery infusion and periocular administration in a porcine model. Retina 2012; 32: 387–395. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Yamane T, Mohri Met al. et al. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology 2011; 118: 2081–2087. [DOI] [PubMed] [Google Scholar]
- 15.Caton MT, D M, Baker Aet al. Percutaneous Sclerotherapy For Head And Neck Lymphatic Malformations In Neonates And Infants (≤ 12 Months): Indications, Technique, Safety, And Outcome. In. Society of Neurointerventional Surgery 19th Annual Meeting2022. [DOI] [PubMed]
- 16.Hetts SW, Keenan K, Fullerton HJ, et al. Pediatric intracranial nongalenic pial arteriovenous fistulas: clinical features, angioarchitecture, and outcomes. AJNR Am J Neuroradiol 2012; 33: 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross BA, Orbach DB. Addressing challenges in 4 F and 5 F arterial access for neurointerventional procedures in infants and young children. J Neurointerv Surg 2014; 6: 308–313. [DOI] [PubMed] [Google Scholar]
- 18.Miao TL, Figueroa EL, Bajunaid Ket al. et al. Use of a radial artery ‘slender’ sheath for facilitating transfemoral arterial access for neuroendovascular embolization in a very young infant. Interv Neuroradiol 2019; 25: 353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha W, Dmytriw AA, Bickford Set al. et al. Use of radial access sheaths for transfemoral neuroendovascular procedures in children. Neuroradiology 2021; 63: 633–635. [DOI] [PubMed] [Google Scholar]
- 20.Thierry-Chef I, Simon SL, Miller DL. Radiation dose and cancer risk among pediatric patients undergoing interventional neuroradiology procedures. Pediatr Radiol 2006; 36: 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swoboda NA, Armstrong DG, Smith Jet al. et al. Pediatric patient surface doses in neuroangiography. Pediatr Radiol 2005; 35: 859–866. [DOI] [PubMed] [Google Scholar]
- 22.Morris P. Practical Neuroangiography. Third ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 23.Doby T. Cerebral angiography and Egas Moniz. AJR Am J Roentgenol 1992; 159: 364. [DOI] [PubMed] [Google Scholar]
- 24.Poser CM, Taveras JM. Clinical aspects of cerebral angiography in children. Pediatrics 1955; 16: 73–80. [PubMed] [Google Scholar]
- 25.Artico M, Spoletini M, Fumagalli L, et al. Egas Moniz: 90 years (1927–2017) from cerebral angiography. Front Neuroanat 2017; 11: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caffey J. The first sixty years of pediatric roentgenology in the United States, 1896 to 1956. Am J Roentgenol Radium Ther Nucl Med 1956; 76: 437–454. [PubMed] [Google Scholar]
- 27.Griscom NT. History of pediatric radiology in the United States and Canada: images and trends. Radiographics 1995; 15: 1399–1422. [DOI] [PubMed] [Google Scholar]
- 28.Picaza JA. Cerebral angiography in children; an anatomoclinical evaluation. J Neurosurg 1952; 9: 235–244. [DOI] [PubMed] [Google Scholar]
- 29.Lurie PR, Armer RM, Klatte EC. Percutaneous guide wire catheterization–diagnosis and therapy. Am J Dis Child 1963; 106: 189–196. [PubMed] [Google Scholar]
- 30.Siqueira EB, Amador LV. Normal angiographic configuration of carotid Siphon in the pediatric patient. J Neurosurg 1964; 21: 216–218. [DOI] [PubMed] [Google Scholar]
- 31.Desilets DT, Ruttenberg HD, Hoffman RB. Percutaneous catheterization in children. Radiology 1966; 87: 119–122. [DOI] [PubMed] [Google Scholar]
- 32.Emmanouilides GC, Hoy RC. Transumbilical aortography and selective arteriography in newborn infants. Pediatrics 1967; 39: 337–343. [PubMed] [Google Scholar]
- 33.Newton TH, Gooding CA. Catheter techniques in pediatric cerebral angiography. Am J Roentgenol Radium Ther Nucl Med 1968; 104: 63–65. [DOI] [PubMed] [Google Scholar]
- 34.Hounsfield GN. Computed medical imaging. Science 1980; 210: 22–28. [DOI] [PubMed] [Google Scholar]
- 35.Cronqvist S, Granholm L, Lundstrom NR. Hydrocephalus and congestive heart failure caused by intracranial arteriovenous malformations in infants. J Neurosurg 1972; 36: 249–254. [DOI] [PubMed] [Google Scholar]
- 36.Pellegrino PA, Milanesi O, Saia OSet al. et al. Congestive heart failure secondary to cerebral arterio-venous fistula. Childs Nerv Syst 1987; 3: 141–144. [DOI] [PubMed] [Google Scholar]
- 37.Lasjaunias P, Hui F, Zerah M, et al. Cerebral arteriovenous malformations in children. Management of 179 consecutive cases and review of the literature. Childs Nerv Syst 1995; 11: 66–79; discussion 79. [DOI] [PubMed] [Google Scholar]
- 38.Ciricillo SF, Edwards MS, Schmidt KG, et al. Interventional neuroradiological management of vein of Galen malformations in the neonate. Neurosurgery 1990; 27: 22–27; discussion 27–28. [DOI] [PubMed] [Google Scholar]
- 39.Dowd CF, Halbach VV, Barnwell SLet al. et al. Transfemoral venous embolization of vein of Galen malformations. AJNR Am J Neuroradiol 1990; 11: 643–648. [PMC free article] [PubMed] [Google Scholar]