Abstract
Gestational exposure to valproic acid (VPA) is a risk factor for autism spectrum disorder (ASD). Rodents exposed to VPA in utero display common features of ASD, including volumetric dysregulation in higher-order cognitive regions like the medial prefrontal cortex (mPFC), the anterior cingulate cortex (ACC), and the hippocampus. Exercise has been shown in elderly populations to boost cognition and to buffer against brain volume losses with age. This study employed an adolescent treadmill exercise intervention to facilitate cognitive flexibility and regional brain volume regulation in rats exposed to VPA during gestation. It was found that exercise improved performance on extra-dimensional shifts of attention on a set-shifting task, which is indicative of improved cognitive flexibility. Exercise decreased frontal cortex volume in females, whereas in males exercise increased the ventral hippocampus. These findings suggest that aerobic exercise may be an effective intervention to counteract the altered development of prefrontal and hippocampal regions often observed in ASD.
Keywords: VPA, ASD, Brain volume, Hippocampus, Anterior cingulate, Cerebellum, Adolescence
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by difficulties with social interactions and restricted or repetitive behaviors. People with ASD also often display impairments in cognitive flexibility, and the underlying developmental brain circuitry that leads to these impairments is not well understood. To identify interventions and treatments for ASD, it is critical to understand how and when neural circuitry is altered in ASD. In ASD, it is known that several developmental brain changes occur in cellular migration, synaptogenesis and cortical organization [1]. This results in an overgrowth of brain volume in childhood and adolescence [1]. Overgrowth in volume or increased cortical thickness has been observed in the frontal cortex [2,3], amygdala [4,5], and hippocampus [4–7]. However, decreases in cerebellar volume have been reported [8–10] and associated with repetitive behaviors [9,11]. These changes in volume across different structures (increases or decreases in volume at different developmental timepoints) have also been associated with symptomology, meaning that identifying developmental mechanisms behind these changes is critical [12,13]. In spite of this, there are very few studies that have examined volumetric changes in animal models of ASD [14,15].
The valproic acid (VPA) model has been used for decades to induce ASD-like phenotypes in rodents [16]. VPA-exposed offspring display impaired sociability and increases in repetitive behaviors [17–19]. The VPA model was developed in part after recognition that children of women on VPA medications, which treat seizures and bipolar disorder, were at increased risk of ASD [20,21]. Thus, the model has both construct and face validity [16]. Mechanisms induced by VPA that may alter development include changes in excitatory and inhibitory balance [22], hyperserotonemia [23], and altered GABAergic regulation in early development [24]. These alterations could contribute to regional changes in volume throughout development as well.
Although core deficits in ASD include changes in communication, social interaction and increased repetitive behaviors, cognitive flexibility deficits frequently co-occur [25]. Around a third of those with ASD have intellectual disability [26] and even in those with normal IQ or above normal IQ, cognitive flexibility is impaired [25]. Changes in cognitive flexibility can interact with and alter repetitive behaviors, perseveration, and rigidity to change across environments, such as preference for sameness and routine [25]. In addition, in those with ASD and Fragile X syndrome, alterations in frontal cortex connectivity and function have been linked to executive function deficits [27–29]. For example, individuals with ASD have impairments on the Wisconsin Card Sorting task [30,31], which is the analog to the test used in this study in rodents. This project used the digging attentional set-shifting task to examine cognitive flexibility in VPA animals. The digging version of the set-shifting task consists of several different, consecutive phases where rats learn to dig for a food reward. After digging correctly, the rules of the task are changed. Phases of the task are: compound discrimination (odor and digging cues used), intra-dimensional (ID) shift (same attention domain; e.g., odor-odor) and extra-dimensional (ED) shifts of attention (different domain of attention; e.g., odor to media). The neural circuits necessary for this task require the anterior cingulate cortex (ACC) for the ID shifts [32,33] and the medial prefrontal cortex (mPFC) for the ED shifts [34].
Changes in brain volume are associated with cognitive decline in elderly populations [35,36]. One intervention in the elderly that has been used to drive brain volume regulation mechanisms is exercise [37], and exercise in elderly and neurologically compromised populations is also associated with cognitive benefits [38]. In those with ASD, exercise has reduced repetitive behaviors in children with ASD [39–41] and has reduced symptoms for those with schizophrenia [42,43]. This approach has not been well characterized for developmental disorders. Accordingly, this study used a treadmill exercise intervention during adolescence, tested cognitive flexibility in an attentional set-shifting task, and then quantified volume for regions of interest (ROI) in control and VPA animals after the intervention (Fig. 1).
Fig. 1.
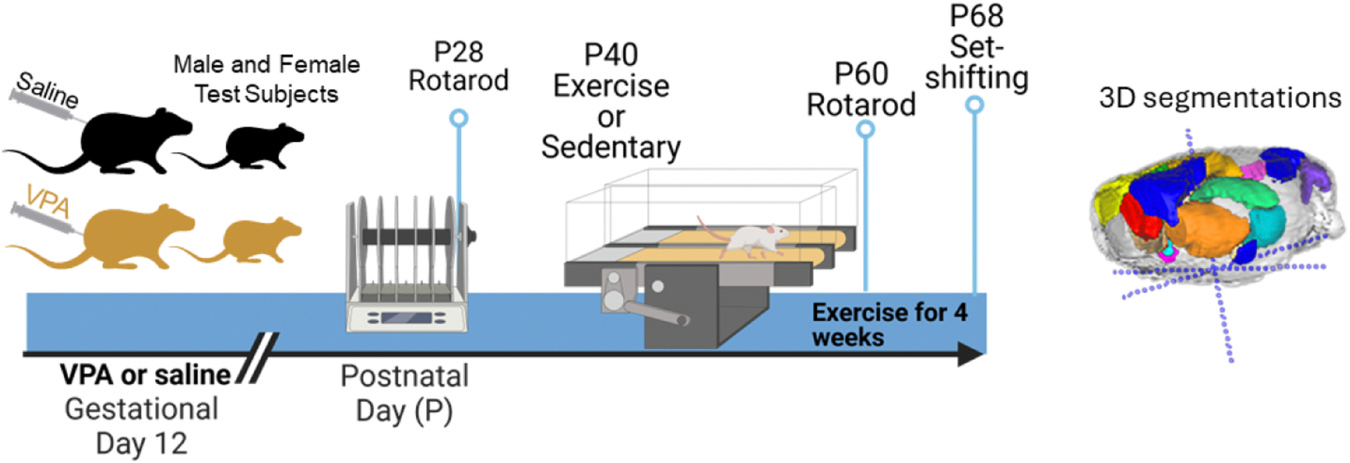
Timeline of behavioral events. (Left panel) Rats were injected at gestational day 12 with saline or VPA. Rats were tested on rotarod at P28 and then began treadmill training at P40 for 4 weeks. Rats were tested on rotarod a second time and performed the attentional set-shifting task. A second rotarod test was conducted at P60. (Right panel) An example brain with regions segmented, red (medial prefrontal cortex), yellow and blue (motor cortex), orange (caudate), light green and aqua (hippocampus), pink, purple and dark blue (cerebellar regions). (Created in part in Biorender.com).
Mechanistically, exercise has also been shown to drive plasticity and to protect against cell loss in the cerebellum [44]. Exercise promotes the release of myokines that bind back to the brain [45] and drives the release of brain derived neurotrophic factor (BDNF) [46]. BDNF signaling is one of the mechanisms thought to drive increased brain volume and cellular regulation [37,47]. Exercise has been demonstrated to boost cognition in humans [38,48] and is associated with increased levels of BDNF [49]. Past results indicate that female VPA rodents are impaired at the extra-dimensional shift of attention [15]. Hence, it was predicted that exercise would primarily impact the ED phase to correct the impairment. It was further hypothesized that an exercise intervention would increase volume within the cerebellum and improve performance on the set-shifting task. To assess this, magnetic resonance imaging (MRI) scans were then collected to measure volumes across brain regions of interest, including the cerebellum, following adolescent exercise and cognitive assessment on the set-shifting task.
2. Material and methods
2.1. Subjects
Pregnant Long-Evans rat dams were shipped from Charles River on gestational day 6 to the research facility. Dams were injected intraperitoneally on gestational day 12 with a single dose of saline or VPA (sodium valproate (Sigma), 250 mg/ml, mixed in saline, 600 mg/kg). Dams were briefly anesthetized on isoflurane gas to administer a less stressful injection. The isoflurane administration (required by the IACUC) was very brief and should not have adversely effected the pups, having lasted only a few minutes, as more prolonged exposure is required to see adverse effects in offspring [50]. All procedures were conducted in accordance with the Kansas State University IACUC guidelines. Rats were given free access to food except for when undergoing preparation for the set-shifting task. Lights were on starting at 7:00 a.m. and turned off 7:00 p.m. Rats were reared with litter mates until weaning and were then pair-housed with a same-sex littermate. Subject numbers by group Females: Control exercise = 13, Control sedentary = 10, VPA exercise 13, VPA sedentary 13; Males: Control exercise = 8, Control sedentary = 11, VPA exercise = 12, VPA sedentary = 13.
2.2. Timeline
Upon weaning on postnatal day (P) 21, one to two male and female pups per litter were randomly assigned to each experimental treatment (exercise or sedentary) [51]. Animals completed a rotarod test both prior to the exercise intervention (P28) and during the last week of exercise. Starting at P40, rats were exercised on the treadmill with air puff delivery to maintain running for 4 weeks. During the fourth week of running, rats were tested on the set-shifting task. After rats completed the task, they were euthanized by pentobarbital overdose and perfused with formaldehyde for head collection. Heads were stored postfixed in 4 % formaldehyde until MRI.
2.3. Rotarod
Before the first week of exercise, and after 4 weeks of exercise, animals performed a rotarod test. Speed was set to 5 rpm and latency to fall of the rod was measured. Rats were removed after a maximum of 180 s (if applicable) to prevent fatigue [52].
2.4. Exercise
Rats were trained on a treadmill and ran 5 days a week for 5 min at 8.3 cm/s, then 5 min at 13.3 cm/s, and then 20 min at 26.6 cm/s. These speeds and times were selected because prior research demonstrated it increased Purkinje cells within the cerebellum [44]. Sedentary rats were placed in the treadmill for 30 min daily, but it was not turned on.
2.5. Attentional set-shifting task and apparatus
2.5.1. Apparatus and stimuli
A Plexiglass box (50 l × 37.5 w × 25 h cm) with a black divider was used as the testing arena. The sliding door allowed access to two flowerpots. All media stimuli were mixed and recycled between rats to allow any odor cues to be disseminated. However, once a pot had been scented with the blotting paper it was only ever scented with the same scent. Crushed Honey Nut Cheerio powder was applied to all digging media to prevent reward odor from serving as a digging cue. Media were shredded manila folders, aspen shavings, foam rubber, felt, burlap ribbon, silk ribbon, plastic beads, plastic jewels, cloth string, wire string, cigarette filters, and shredded cotton pads. Odors were rum, vanilla, lemon, almond, cinnamon, anise, watermelon, grapefruit, strawberry, blueberry, marjoram, and cumin. A pilot study demonstrated that rats could discriminate these odors.
2.5.2. Training
For the set-shifting task, rats were first habituated to Honey Nut Cheerios (General Mills, MN) and a flowerpot in the home cage the night before training began. Rats were then given basic digging training with plain bedding for one to two days. Basic training consisted of shaping digging behavior in the testing arena. Rats were allowed to obtain a small 1/3 piece of cereal on top of the bedding of the flowerpots, after which the cereal was buried progressively deeper until the rat was successfully digging to retrieve it at about 3.5 cm deep. Then one to two days of discrimination training were conducted for animals to learn one media pair (shredded paper vs pine shavings) with unscented pots and one odor pair (tea tree oil vs lavender). Pots themselves were not scented; rather, blotting paper was taped to the inner lip of the ceramic pot and scented with a pipettor (2.5 microliters). The paper was re-scented before every training session. Since prior work has found that adolescent rats were impaired on the compound discriminations compared to adults [53], basic training was extended to ensure that rats could complete the full set-shifting task. After reaching 6 consecutive correct trials on the odor and media pairs, rats performed the full set-shifting task the next day on P68. All rats that completed the set-shifting task were euthanized on P68.
2.5.3. Set-shifting task
Rats were trained in the set-shifting task with seven stages in the following order: compound discrimination (CD), intra-dimensional shift 1–4 (ID), extra-dimensional shift (ED), and a simple discrimination (SD). The SD was included as a quality control measure, and no condition differences were expected. Rats underwent food restriction for around seven days, reaching around 90 % of their body weight. In all sessions, the first four trials were discovery trials; that is, the rat could sample both pots in these trials only, regardless of which one contained the food reward. After these trials, once the rat initiated a dig (defined as moving the medium with nose or paw), the trial was ended, and rats were allowed to finish digging in incorrect pots but not allowed to go back to the correct pot. Trials to criterion was set to six trials as the subjects were past adolescence [54]. Discovery trials were included in the trials to criterion. All stimuli were counterbalanced with a Latin square design and half of the rats were trained with odor to medium shifts, half medium to odor shifts.
2.6. MRI volumetric measurements
Volumetric measurements were obtained via magnetic resonance imaging (MRI) with a Bruker 600 MHz scanner that had a bottom loading probe measuring 30 × 40 mm. Brains were placed in a flat bottom glass tube and covered with Fomblin, a safe, nontoxic, inert, and MRI-silent perfluoropolyether oil, to increase signal-to-noise ratios and decrease magnetic susceptibility distortions [55]. Brains were secured with a pronged glass stabilizer specially made to ensure sample position consistency. Scans were performed with Rapid Acquisition with Refocused Echoes (RARE) pulse sequences in roughly 1 h and 45 min with one repetition, a repetition time of 2500 ms, echo time of 30.23 ms, and a RARE factor of 16. The pulse sequence used a flip angle of 90°, acquired two averages, with slices acquired in an interlaced pattern. Image resolution was approximately 100 × 100 × 300 μm with matrix sizes, field of view, and number of slices varying according to brain size. Additionally, a pronged glass sample holder was made to ensure consistent orientation and sample position throughout imaging and between samples. The utilization of adaptable parameters for imaging allows for maximal signal-to-noise and contrast-to-noise ratios dependent upon sample size.
Volume of the medial prefrontal cortex (areas A32D and A32V) and the anterior cingulate cortex (ACC) (areas A24a and A24b), defined by the Paxinos and Watson brain atlas (7th edition), were segmented with ITK SNAP software by blind-to-condition researchers. The mPFC, ACC, and PCC (posterior cingulate) were chosen because of their importance to set-shifting performance [32–34]. The amygdala (left, right, central nucleus and basolateral/lateral), dorsal hippocampus, caudate, motor cortex, cerebellum (total), crus I (left/right), and lobule VI were also segmented. Segmentation was screened for consistency through 3-D modeling in ITK-SNAP before evaluation of total volume. Each ROI was outlined based on anatomical landmarks and the volume for each lobule and frontal region was measured. Total brain volume included grey matter, white matter, and ventricles. The active contour segmentation tool was used to fill the entire brain including the cerebellum and brain stem, and any overfilling into the intracranial space was cleaned in coronal slice views. The volume of subregions was normalized to total brain volume (subregion/total brain volume) in mm3.
2.7. Data analysis
Behavioral analysis was run in R statistical computing software and graphs were made with GraphPad Prism software. Trials to criterion data were analyzed by condition and treatment, with litter as a nested factor, and separated by sex. Latency data was log transformed and then analyzed. Volume data were analyzed using R [56]. Volume data for each region of interest were analyzed separately for male and female animals using multilevel gamma regressions [57] with fixed effects of condition, treatment (exercise), and their interaction. These predictors were effect coded so that the estimate of the model’s intercept represented the sample grand mean. The models were nested by litter, with the intercept being allowed to vary as a random effect, thus allowing the detection of volume differences according to condition and litter while controlling for the differences driven by the litter. Sidak-corrected post hoc analyses were conducted following significant interactions. The effect of brain volume on behavior was analyzed separately for male and female animals using multilevel gamma regressions. These models were also nested by litter, which was included as a random effect.
3. Results
3.1. Set-shifting behavioral data
3.1.1. Females
For the CD phase, there were no effects of condition or exercise. For the ID1, ID2, and ID3 phases, there were also no effects. For ID4 phase, there was an interaction between condition and exercise (estimate = .08, SE = .04, t = 1.96, p = .04). Post hoc tests demonstrated that VPA exercised animals completed the phase in fewer trials than VPA sedentary animals. For the ED phase, there was an effect of exercise (estimate = − .18 SE = .04, t = − 4.42, p = .000), where exercised animals, both control and VPA, required fewer trials to reach criterion than sedentary animals (Fig. 2, left). As expected, there were no effects for the SD phase.
Fig. 2.
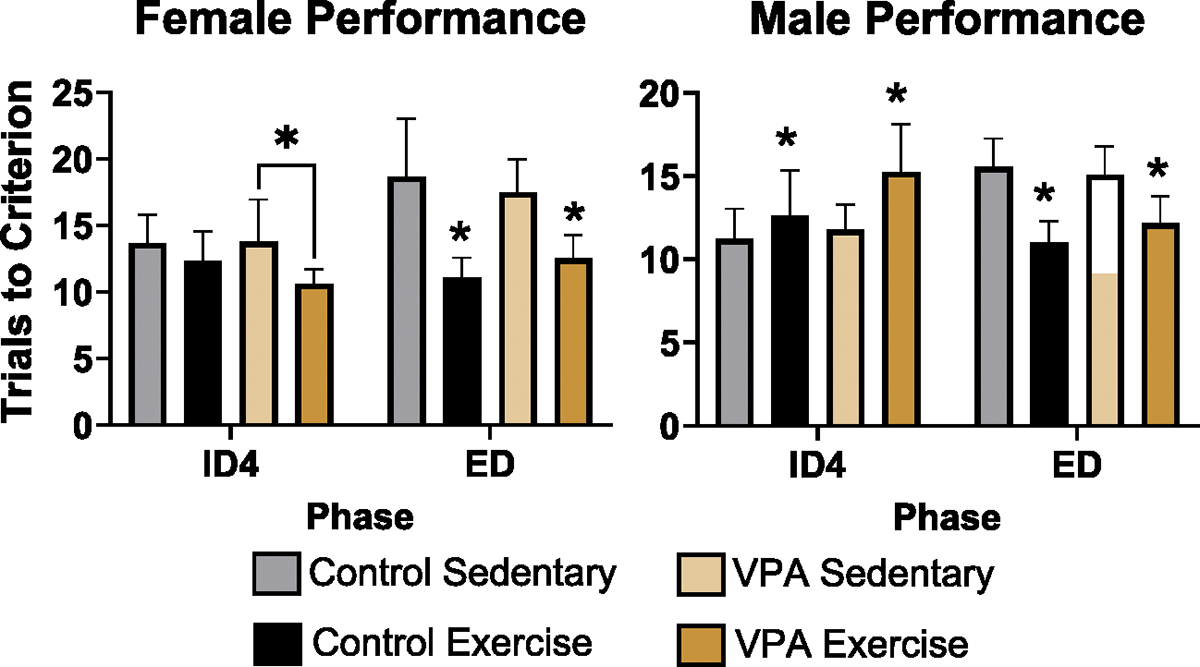
Female (left) and Male (right) set-shifting performance on ID4 and ED phases. Exercise improved VPA female performance on the ID4 phase, fewer trials to criterion is better (p < 0.05). Exercise improved ED performance in control and VPA females (p < 0.05). Exercise impaired control and VPA male performance on ID4 phase (p < 0.05), but improved performance on the ED phase (p < 0.05), with fewer trials to criterion after exercise (dark orange and black bars are lower than grey and light orange bars).
3.1.2. Males
For the CD phase, there were no effects of condition or exercise. For ID1, there was an effect of exercise (estimate = .13, SE = .04, t = 2.89, p = .003), and post hoc tests demonstrated that exercised VPA animals took more trials to complete the phase. For ID2, there was also an effect of exercise in the VPA group (estimate = .13, SE = .04, t = 2.71, p = .006), where exercised VPA animals were impaired. There was an effect of exercise on the ID3 phase (estimate = .16, SE = .04, t = 3.28, p= .001), where exercised animals, regardless of condition, required more trials to reach criterion. The same was found for ID4 (estimate = .12, SE = .05, t = 2.43, p= .01). For the ED phase, there was an effect of treatment (estimate = − .12, SE = .04, t = − 2.71, p = .006) where exercised animals, both control and VPA, required fewer trials to reach criterion than their sedentary counterparts (Fig. 2, right). There were no differences for the SD phase.
3.2. Rotarod performance
Latency data were transformed into log scale for analysis, and mixed analysis with condition, exercise treatment, litter (random effect) and trial (repeated measure) was conducted. There was a main effect of condition (F 1, 87 = 5.24, p < 0.05) and a main effect of trial (F 1, 87 = 39.09, p < 0.05). This demonstrated that control rats took longer to fall off the rod than VPA rats, but that all rats had longer latencies on the second trial compared to the first trial (Fig. 3).
Fig. 3.
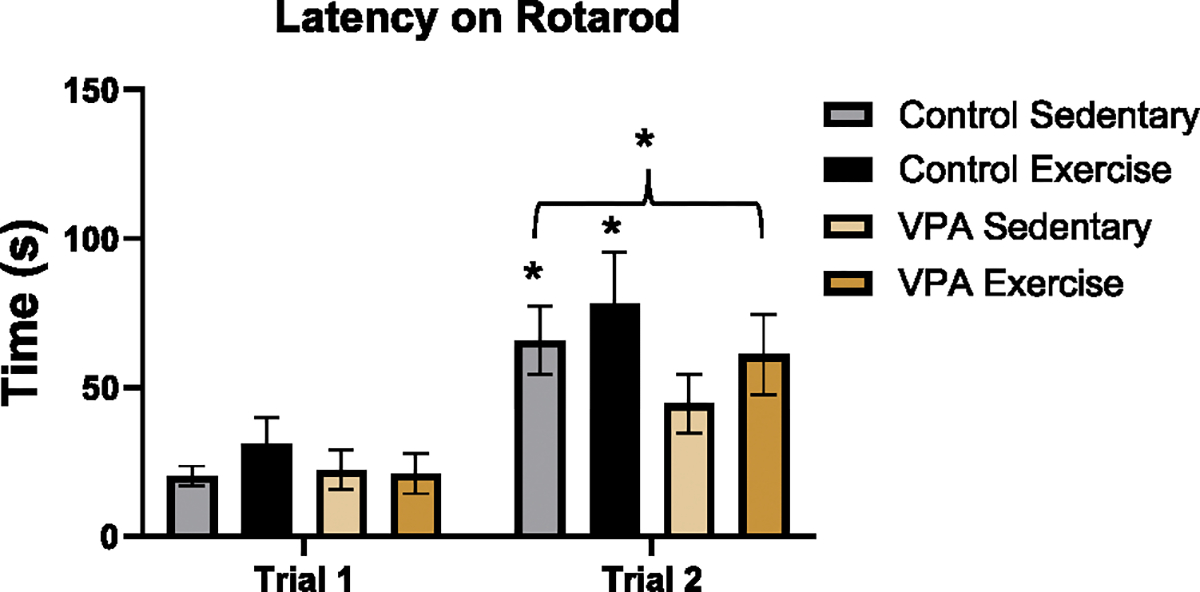
Rotarod performance. All rats stayed on the rotarod longer on trial 2 compared to trial 1 (*p < 0.05), meaning rats did better on the second attempt. There was also an effect of condition (*p < 0.05) where control rats stayed on the rod longer than VPA rats.
3.3. Volume data
Table 1 has raw mm3 volumes for all structures of females, Table 2 has these values for males. Supplemental Tables 1 and 2 have statistical model data.
Table 1.
Female volumes.
| Region | Control |
VPA |
||||||
|---|---|---|---|---|---|---|---|---|
| Exercised |
Sedentary |
Exercised |
Sedentary |
|||||
| Volume (mm3) | SE | Volume (mm3) | SE | Volume (mm3) | SE | Volume (mm3) | SE | |
|
| ||||||||
| mPFC | 17.76 | 1.13 | 16.83 | 1.41 | 18.31 | 0.83 | 17.69 | 1.09 |
| ACC | 19.67 | 0.85 | 20.77 | 0.80 | 19.23 | 0.83 | 19.41 | 0.79 |
| Left motor cortex | 30.10 | 0.51 | 30.28 | 0.52 | 30.10 | 0.52 | 31.62 | 0.64 |
| Right motor cortex | 29.79 | 0.72 | 30.33 | 0.36 | 30.22 | 0.72 | 31.66 | 0.75 |
| Right CE | 1.00 | 0.07 | 0.93 | 0.21 | 0.99 | 0.09 | 1.05 | 0.06 |
| Right BLA | 2.63 | 0.26 | 2.46 | 0.25 | 2.63 | 0.34 | 2.86 | 0.24 |
| Left Ce | 1.00 | 0.07 | 0.85 | 0.06 | 1.02 | 0.08 | 1.05 | 0.08 |
| Left BLA | 2.58 | 0.28 | 2.18 | 0.28 | 2.38 | 0.20 | 2.50 | 0.21 |
| Left Caudate | 32.30 | 1.17 | 32.66 | 0.96 | 34.14 | 1.07 | 32.68 | 1.27 |
| Right Caudate | 33.79 | 0.88 | 33.87 | 0.81 | 35.61 | 1.04 | 33.74 | 1.16 |
| Lobule 6 | 12.39 | 0.73 | 11.43 | 0.89 | 11.99 | 0.68 | 11.89 | 0.46 |
| Left Crus 1 | 17.35 | 0.56 | 16.63 | 0.53 | 15.06 | 0.85 | 16.88 | 0.78 |
| Right Crus 1 | 16.90 | 0.54 | 16.90 | 0.72 | 15.81 | 0.39 | 16.70 | 0.80 |
| Left dorsal hippocampus | 22.92 | 0.65 | 20.06 | 0.99 | 23.08 | 1.11 | 23.37 | 1.20 |
| Right dorsal hippocampus | 22.96 | 0.88 | 20.07 | 1.16 | 22.43 | 1.10 | 22.44 | 1.20 |
| PCC | 26.26 | 1.07 | 24.55 | 1.54 | 24.85 | 1.15 | 25.41 | 1.67 |
| Left ventral hippocampus | 13.42 | 0.98 | 13.38 | 0.73 | 12.91 | 1.04 | 12.75 | 0.81 |
| Right ventral hippocampus | 13.48 | 0.83 | 13.56 | 1.35 | 11.99 | 0.87 | 11.07 | 0.84 |
| Cerebellum | 271.04 | 5.97 | 264.74 | 7.27 | 256.46 | 9.28 | 269.43 | 10.21 |
| Total brain volume | 1854.58 | 27.40 | 1835.22 | 33.86 | 1816.54 | 37.55 | 1826.44 | 52.60 |
Note: ACC = anterior cingulate cortex; BLA = basolateral amygdala; Ce = central nucleus of amygdala; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; VPA = valproic acid.
Table 2.
Male volumes.
| Region | Control |
VPA |
||||||
|---|---|---|---|---|---|---|---|---|
| Exercised |
Sedentary |
Exercised |
Sedentary |
|||||
| Volume (mm3) | SE | Volume (mm3) | SE | Volume (mm3) | SE | Volume (mm3) | SE | |
|
| ||||||||
| mPFC | 17.69 | 0.92 | 17.95 | 0.78 | 18.17 | 0.43 | 18.02 | 1.01 |
| ACC | 21.41 | 1.06 | 21.52 | 0.79 | 19.80 | 1.05 | 20.07 | 0.92 |
| Left motor cortex | 32.48 | 1.12 | 32.28 | 0.72 | 32.00 | 0.66 | 32.27 | 0.97 |
| Right motor cortex | 32.71 | 0.77 | 32.34 | 0.59 | 31.99 | 0.65 | 31.72 | 0.90 |
| Right CE | 1.17 | 0.15 | 1.17 | 0.11 | 1.09 | 0.07 | 1.04 | 0.05 |
| Right BLA | 3.48 | 0.45 | 2.87 | 0.23 | 3.10 | 0.26 | 2.97 | 0.25 |
| Left Ce | 1.21 | 0.15 | 1.21 | 0.10 | 1.04 | 0.08 | 1.08 | 0.05 |
| Left BLA | 3.20 | 0.44 | 2.69 | 0.21 | 2.40 | 0.23 | 2.55 | 0.18 |
| Left Caudate | 34.40 | 1.10 | 33.85 | 1.18 | 32.86 | 0.81 | 35.10 | 1.26 |
| Right Caudate | 35.59 | 1.13 | 35.65 | 1.29 | 35.62 | 1.08 | 36.27 | 1.13 |
| Lobule 6 | 12.24 | 0.64 | 12.07 | 0.56 | 12.30 | 0.52 | 14.09 | 0.84 |
| Left Crus 1 | 16.68 | 0.63 | 17.11 | 0.95 | 16.23 | 0.51 | 17.40 | 0.68 |
| Right Crus 1 | 17.12 | 0.67 | 17.48 | 0.92 | 16.13 | 0.53 | 17.38 | 0.57 |
| Left dorsal hippocampus | 24.83 | 2.13 | 23.46 | 1.22 | 23.31 | 0.93 | 23.75 | 1.09 |
| Right dorsal hippocampus | 24.90 | 1.91 | 24.44 | 1.05 | 22.43 | 1.18 | 23.45 | 1.29 |
| PCC | 27.47 | 2.05 | 26.13 | 1.05 | 24.44 | 1.05 | 24.45 | 1.08 |
| Left ventral hippocampus | 13.71 | 1.15 | 13.11 | 0.86 | 14.96 | 0.83 | 12.60 | 0.75 |
| Right ventral hippocampus | 14.00 | 1.32 | 13.52 | 1.02 | 10.45 | 0.80 | 12.00 | 0.86 |
| Cerebellum | 292.35 | 8.63 | 297.80 | 9.87 | 288.69 | 8.54 | 296.05 | 7.53 |
| Total brain volume | 1949.61 | 45.40 | 1912.65 | 30.35 | 1914.94 | 33.79 | 1953.47 | 47.75 |
Note: ACC = anterior cingulate cortex; BLA = basolateral amygdala; Ce = central nucleus of amygdala; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; VPA = valproic acid.
3.3.1. Cerebellum
There were no significant effects for male or female rats for total cerebellar volume, lobule VI or crus I on either side.
3.3.2. Frontal cortex and cingulate cortex
There were no differences in mPFC volume in males or females. For female rats, there was an effect of exercise, which decreased ACC volume (estimate = − .03, SE = .01, t = − 2.01, p= .04) (Fig. 4, left). For the motor cortex, there was an effect of exercise in females, with exercise decreasing volume (estimate = − .01, SE = .00, t = − 2.14, p = .03) (Fig. 4, middle). For the PCC, there was an interaction between sex and condition (estimate = .03, SE = .01, t = 2.85, p = .004) and an exercise effect (estimate = .02, SE = .01, t = 1.98, p = .04) (Fig. 4, right). Sidak post hoc tests found that control sedentary females had smaller PCC volumes compared to control exercised females. For males, there were no differences in motor cortex or PCC volume.
Fig. 4.
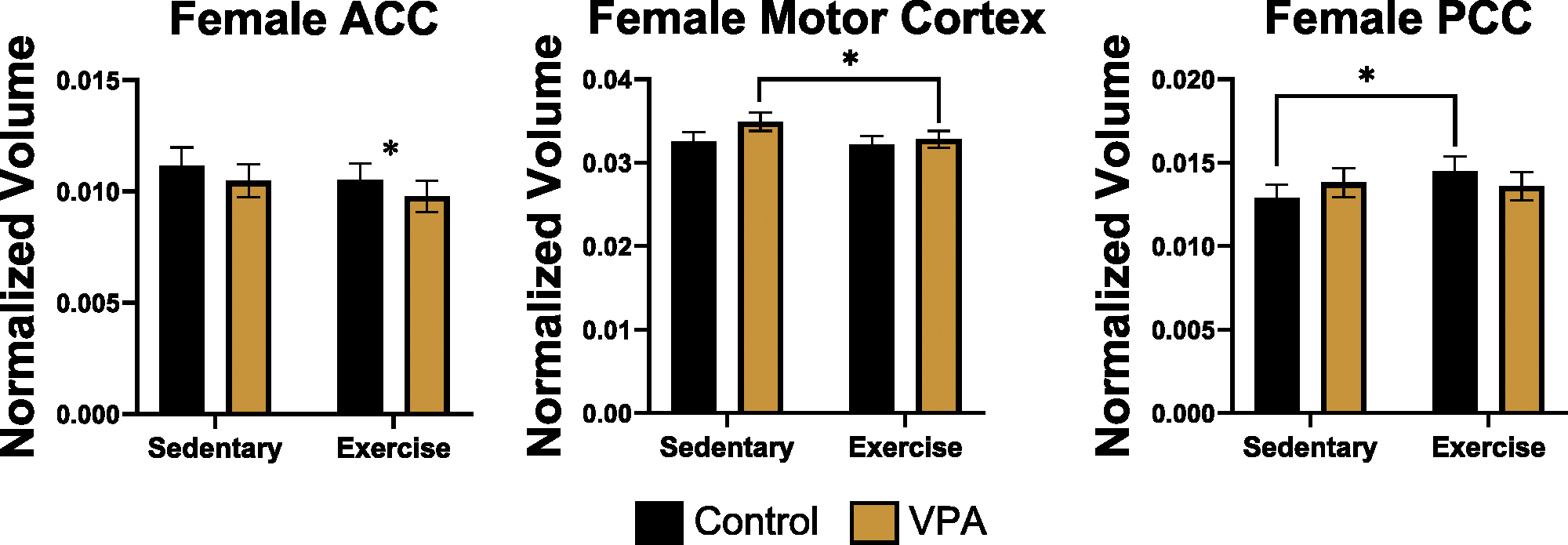
Normalized volume for female ACC (left), motor cortex (middle), and PCC (right). Exercise decreased ACC volume for control and VPA females (p < 0.05), decreased VPA motor cortex volumes (p < 0.05), and increased control female PCC volumes (p < 0.05).
3.3.3. Amygdala, hippocampus and caudate
For the left and right central nuclei of the amygdala, as well as left and right basolateral nucleus, there were no significant differences in males or females. In females, for total left amygdala (central nucleus + basolateral nucleus) there was an interaction (estimate = .05, SE = .004, t = 12.66, p = .000) and effects of condition (estimate = − .03, SE = .004, t = − 8.94, p = .000) and exercise treatment (estimate = .04, SE = .004, t = 10.78, p = .000). Sidak post hoc tests found that exercise increased left amygdala volume in control females (p = .000), and control sedentary females also had smaller left amygdala volumes than exercised VPA (p = .000) or sedentary VPA females (p = .000), (Fig. 5, left). There was a trend for condition in males for the left amygdala (estimate = .10, SE = .06, t = 1.67, p = .09), suggesting that VPA males had smaller left amygdala than control males (Fig. 5, right). For the right amygdala there were no group differences in males or females. There were also no differences for right or left caudate in males or females.
Fig. 5.
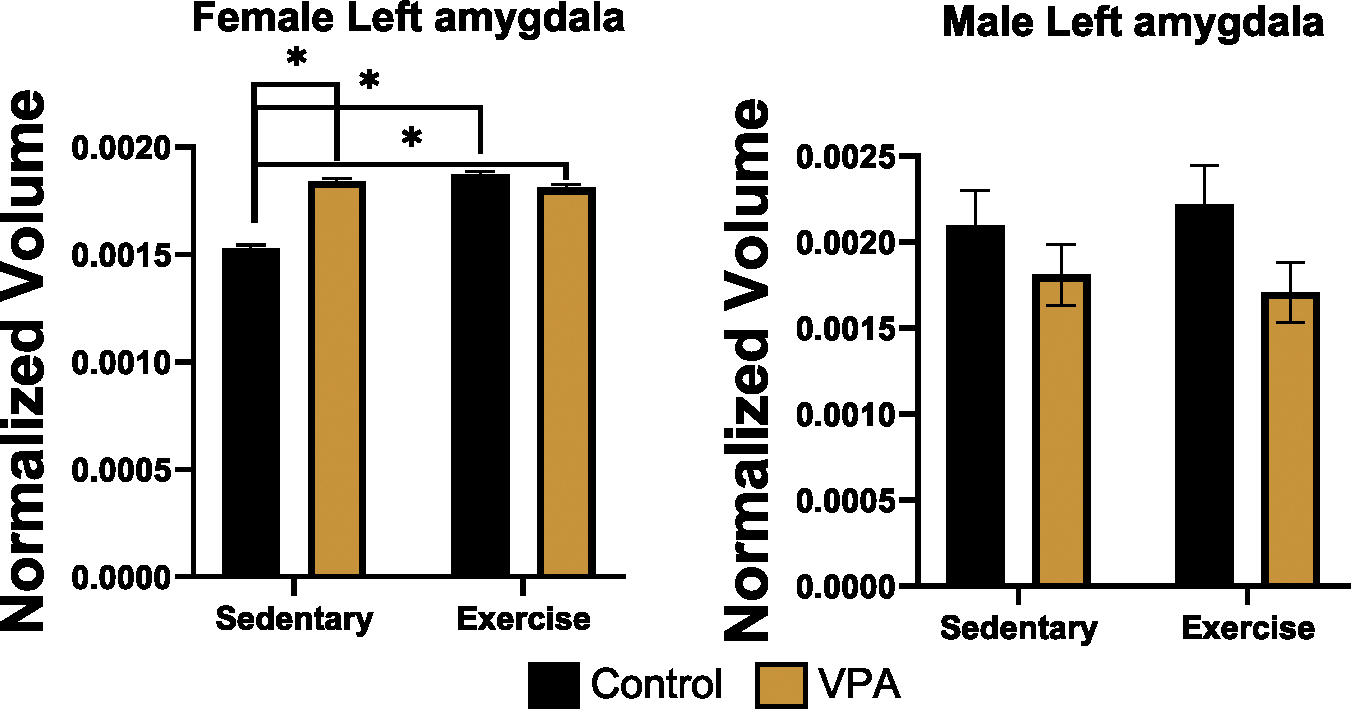
Normalized volume for female (left) and male (right) left amygdala. VPA sedentary females had enlarged left amygdala compared to control sedentary animals (p < 0.05). Exercise increased left amygdala volume in controls only (p < 0.05), whereas there were no changes in VPA animals. VPA males did not show enlargements, suggesting that there may be subtle sex differences in VPA animals within the left amygdala.
In the female left dorsal hippocampus, there was a condition by treatment interaction effect (estimate = .03, SE = .01, t = 2.93, p = .01), a condition effect (estimate = − .05, SE = .02, t = − 2.14, p = .03), and an exercise treatment effect (estimate = .02, SE = .01, t = 2.07, p = .03). Sidak post hoc tests found that control sedentary females had smaller volumes than exercised control (p = .02), exercised VPA (p = .03) or sedentary VPA animals (p = .02), (Fig. 6, left). In the right dorsal hippocampus, females had a condition by treatment interaction effect (estimate = .02, SE = .01, t = 2.05, p = .03) and an exercise effect (estimate = .03, SE = .01, t = 2.40, p = .01). Post hoc tests demonstrated that exercise increased volume in control females compared to sedentary females (p = .02), (Fig. 6, right). Males had no differences for left or right dorsal hippocampus. Females had no differences for the left or right ventral hippocampus but, for males, there was a condition by treatment interaction effect for left ventral hippocampus volume (estimate = − .06, SE = .02, t = − 2.67, p = .007). Post hoc tests found that exercised VPA males had larger left ventral hippocampi than VPA sedentary males (p = .000, Fig. 7). There were no significant differences in right ventral hippocampus volume for males.
Fig. 6.
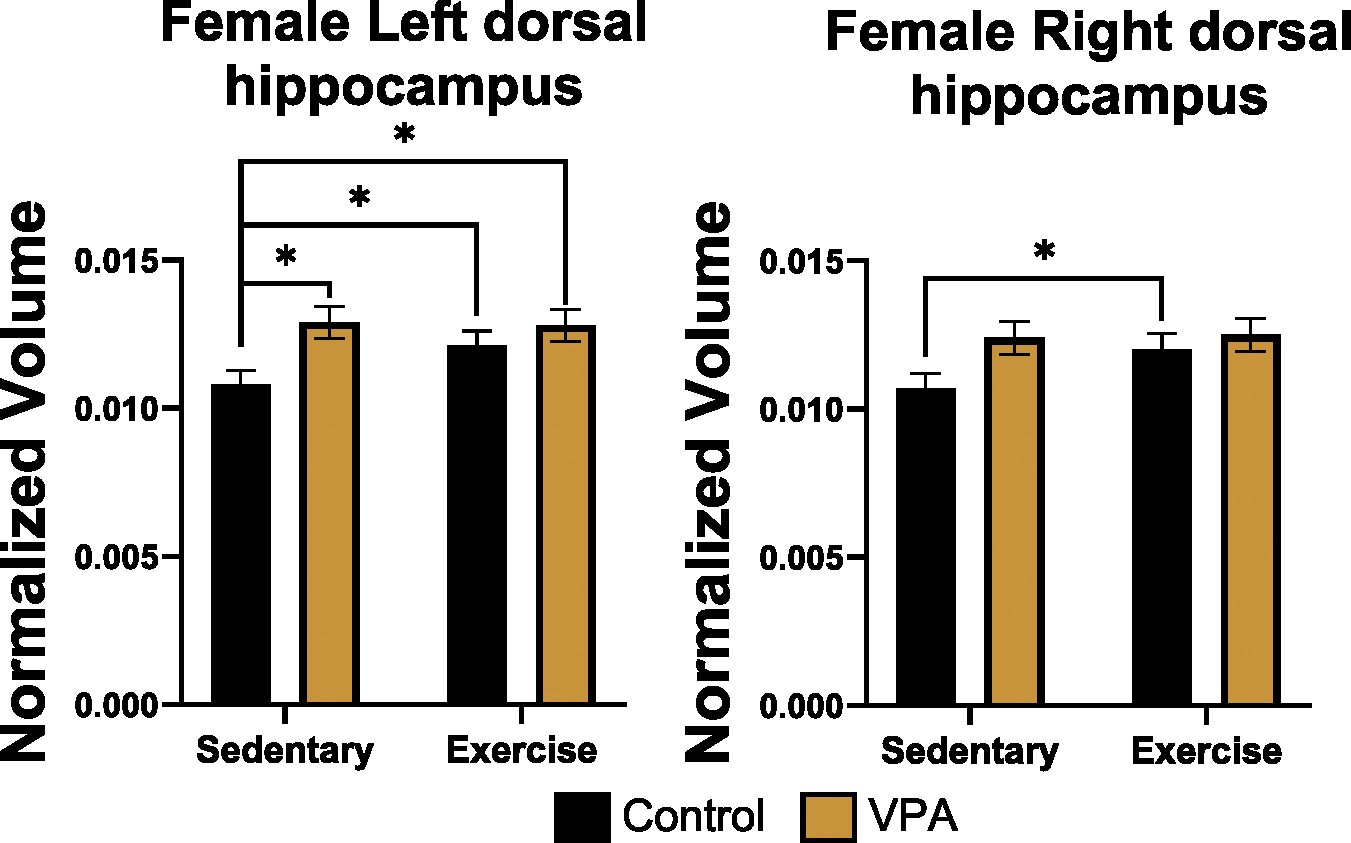
Normalized volume of female left dorsal hippocampus (left) and right dorsal hippocampus (right). Exercise increased left dorsal hippocampal volumes for control females (p < 0.05). Sedentary and VPA females had larger left dorsal hippocampi compared to sedentary controls (p < 0.05). Exercise increased right dorsal hippocampus for control females. After exercise, VPA females had volume similar to control exercised females suggesting exercise was modulating the overgrowth observed in sedentary females.
Fig. 7.
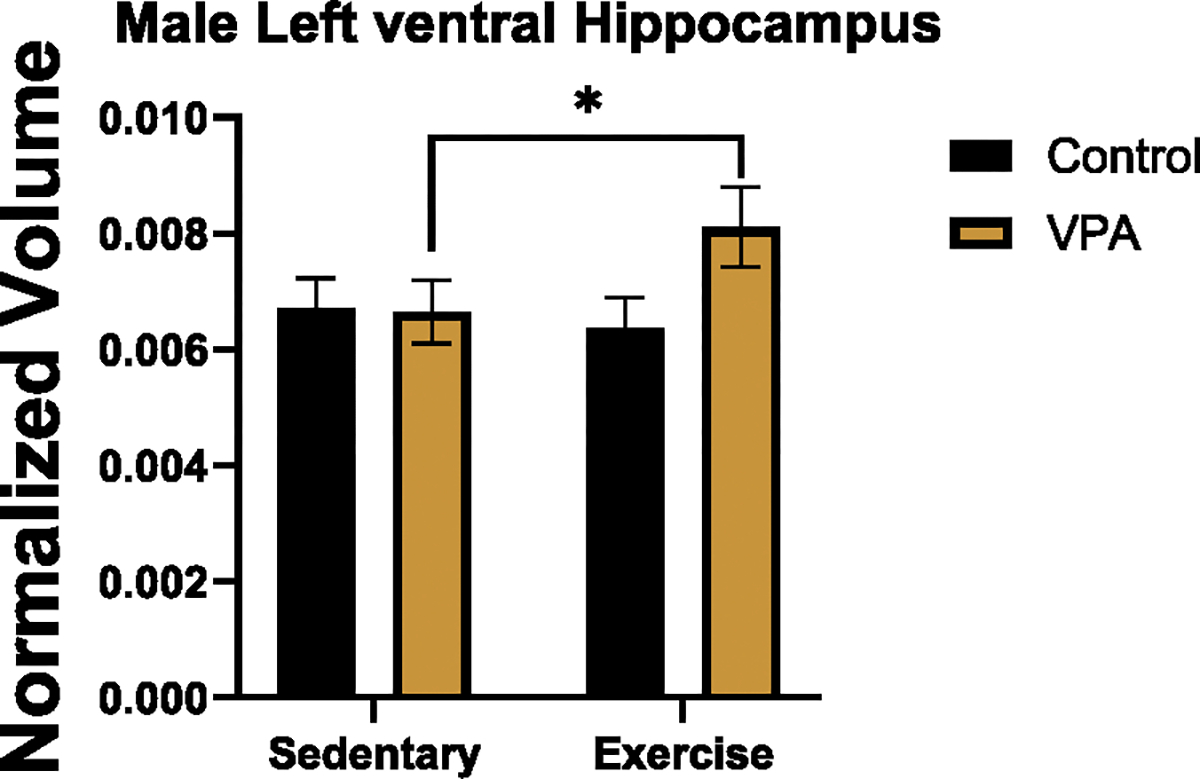
Normalized volume of male left ventral hippocampus. Exercise increased left ventral hippocampus of VPA males (p < 0.05).
3.4. Volume and behavior
3.4.1. Females
To examine the effect of volume on behavior, regression analysis was conducted to assess how volume, condition, and treatment related to different components of the set-shifting task. The phases of the task of interest on the task were the ED and ID4 phases. These are the phases most impacted in ASD [30]. Individuals with ASD make more errors on extra-dimensional phases [30], and in those with ASD there is even greater recruitment of PFC networks during similar task demands [31]. Total trials across the task were also examined as a metric of total performance. On the ED phase, there was an interaction between condition and mPFC volume (estimate = 88.07, SE = 30.14, z = 2.922, p = .003) where controls with smaller volumes performed better and VPA females with larger volumes performed better (Fig. 8). For the left dorsal hippocampus and ED performance, there was an effect of volume (estimate = − 84.34, SE = 39.56, z = − 2.13, p = .03) where, for both control and VPA females, larger volume was associated with improved performance (Fig. 9). For left crus I and ED performance, there was an interaction between volume and condition (estimate = − 35.02, SE = 10.63, z = − 3.29, p = .000) where larger volumes were associated with improved control animal performance, but impaired performance in VPA animals (Fig. 10).
Fig. 8.
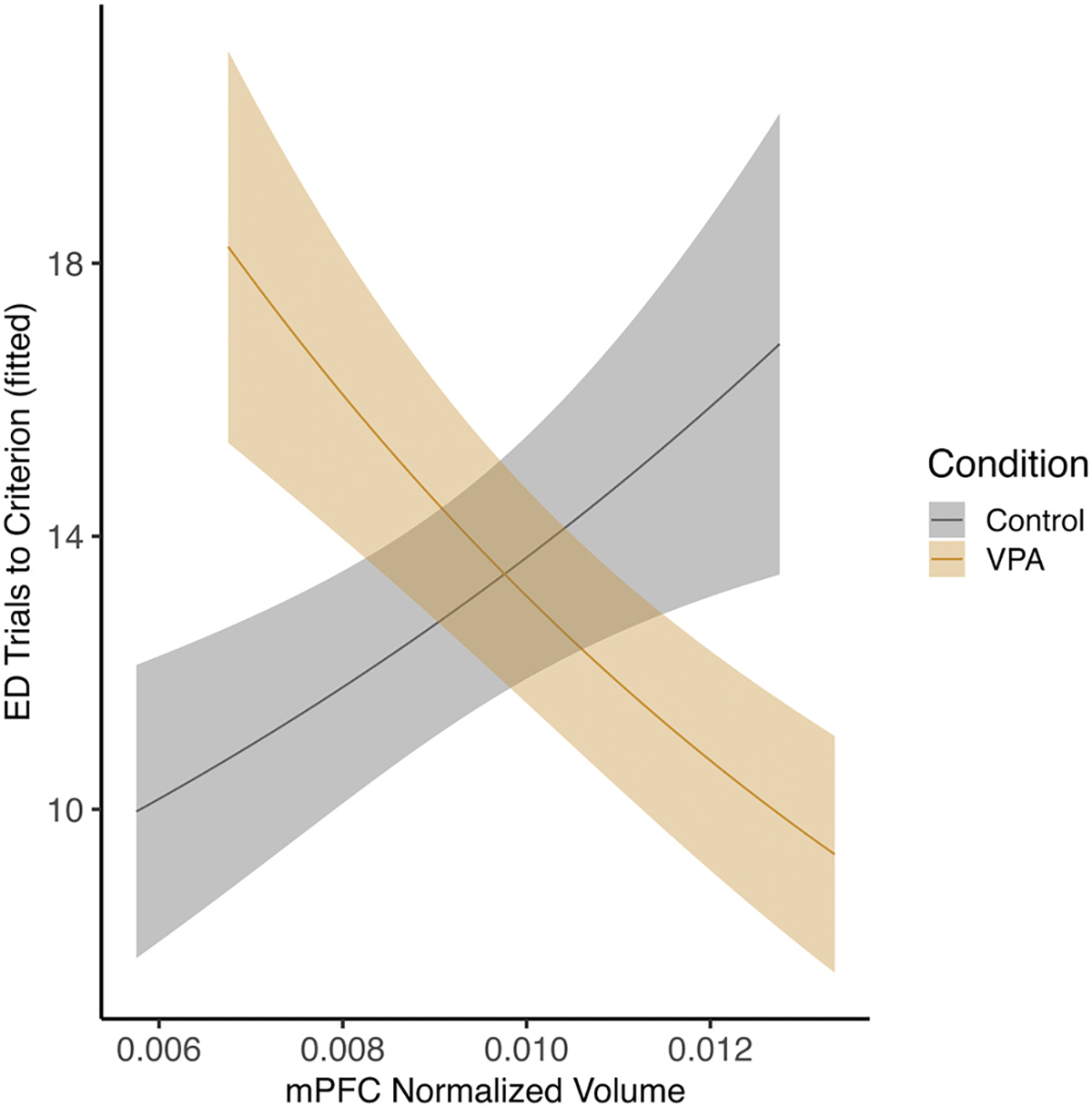
Female mPFC and performance on ED phase. There was an interaction between condition and volume where control females with larger volumes were impaired on the ED phase and VPA females with smaller volumes were impaired (p < 0.05).
Fig. 9.
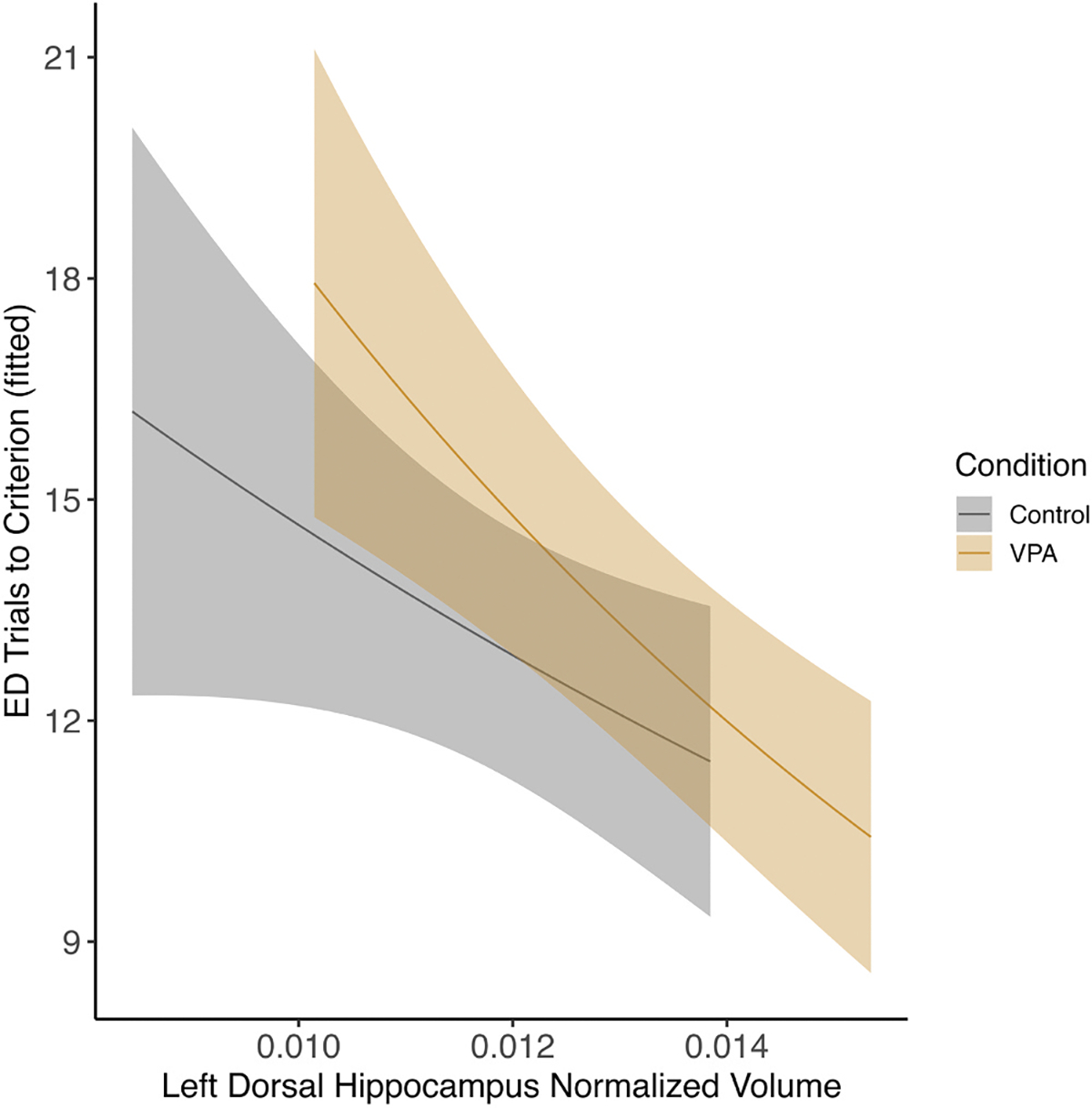
Female left dorsal hippocampus and ED phase There was an effect of left dorsal hippocampus volume on ED performance where control and VPA females with larger volumes performed better (p < 0.05).
Fig. 10.
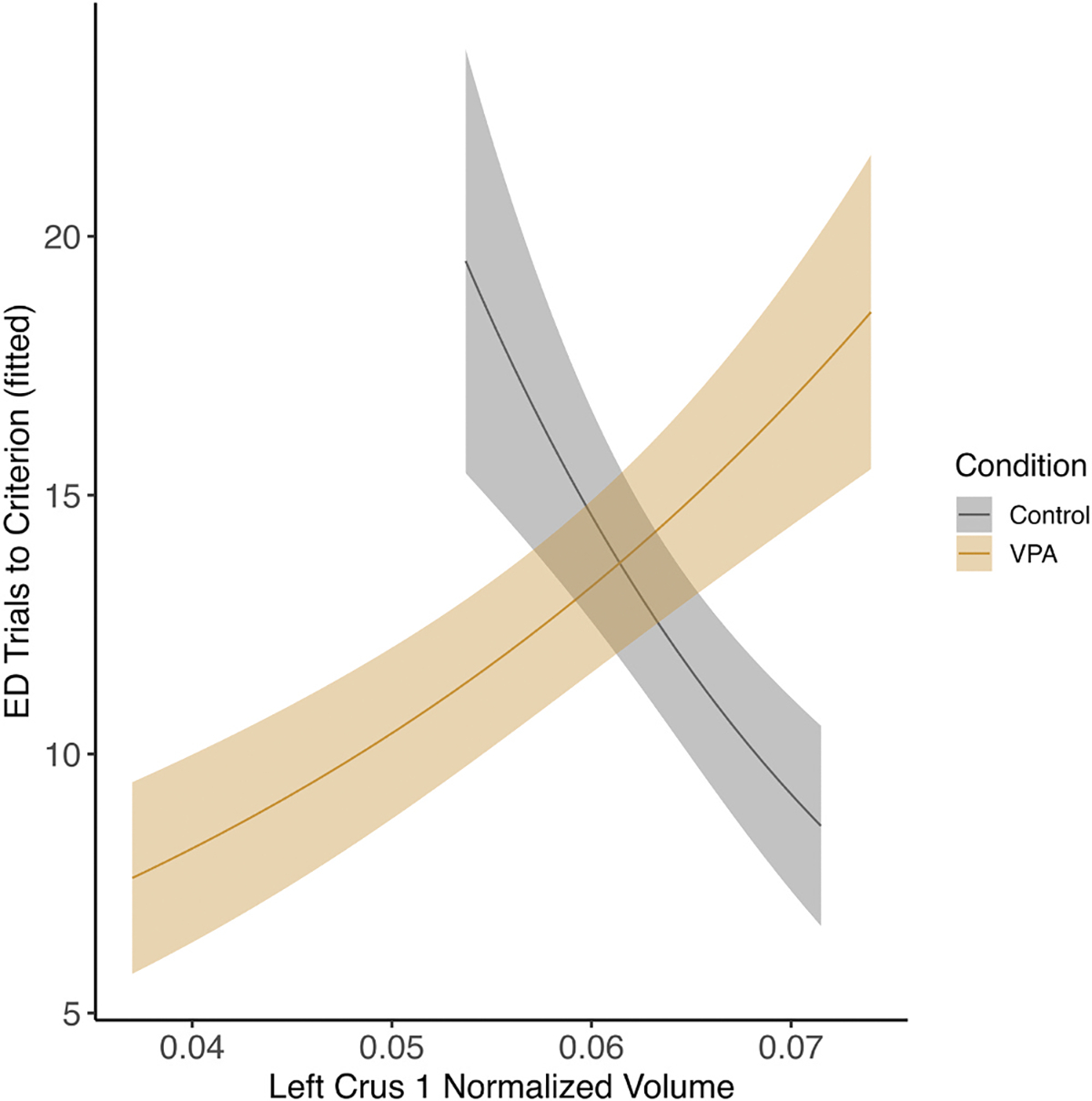
Female left crus I and ED phase. There was an interaction between condition and left crus I volume on ED performance where control females with larger volumes performed worse and VPA females with larger volumes performed better (p < 0.05).
3.4.2. Males
In males, for left crus I there was an effect of volume on total trials across the task (estimate = − 14.54, SE = 4.74, z = − 3.06, p = .002). Both control and VPA males with larger volumes performed better overall (Fig. 11). For the right dorsal hippocampus, there was an interaction between condition and volume (estimate = 35.28, SE = 16.18, z = 2.18, p = .02) where control males with smaller volumes performed better, but VPA males with larger volumes performed better across the task on total trials, (Fig. 12).
Fig. 11.
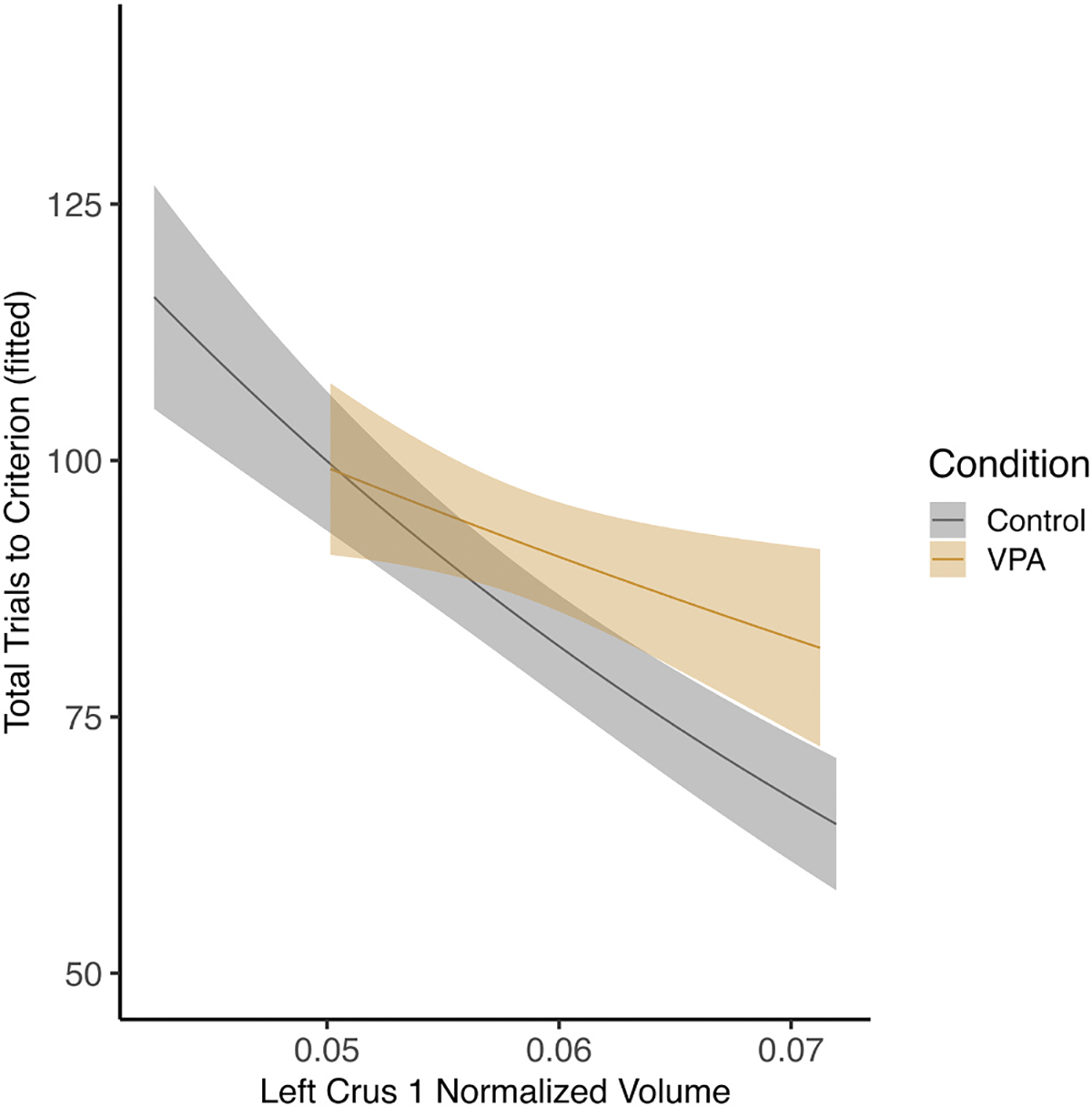
Male left crus I and total trials across the task. There was an effect of left crus I volume on total trials where, for both control and VPA males, larger left crus I was associated with better overall task performance (p < 0.05).
Fig. 12.
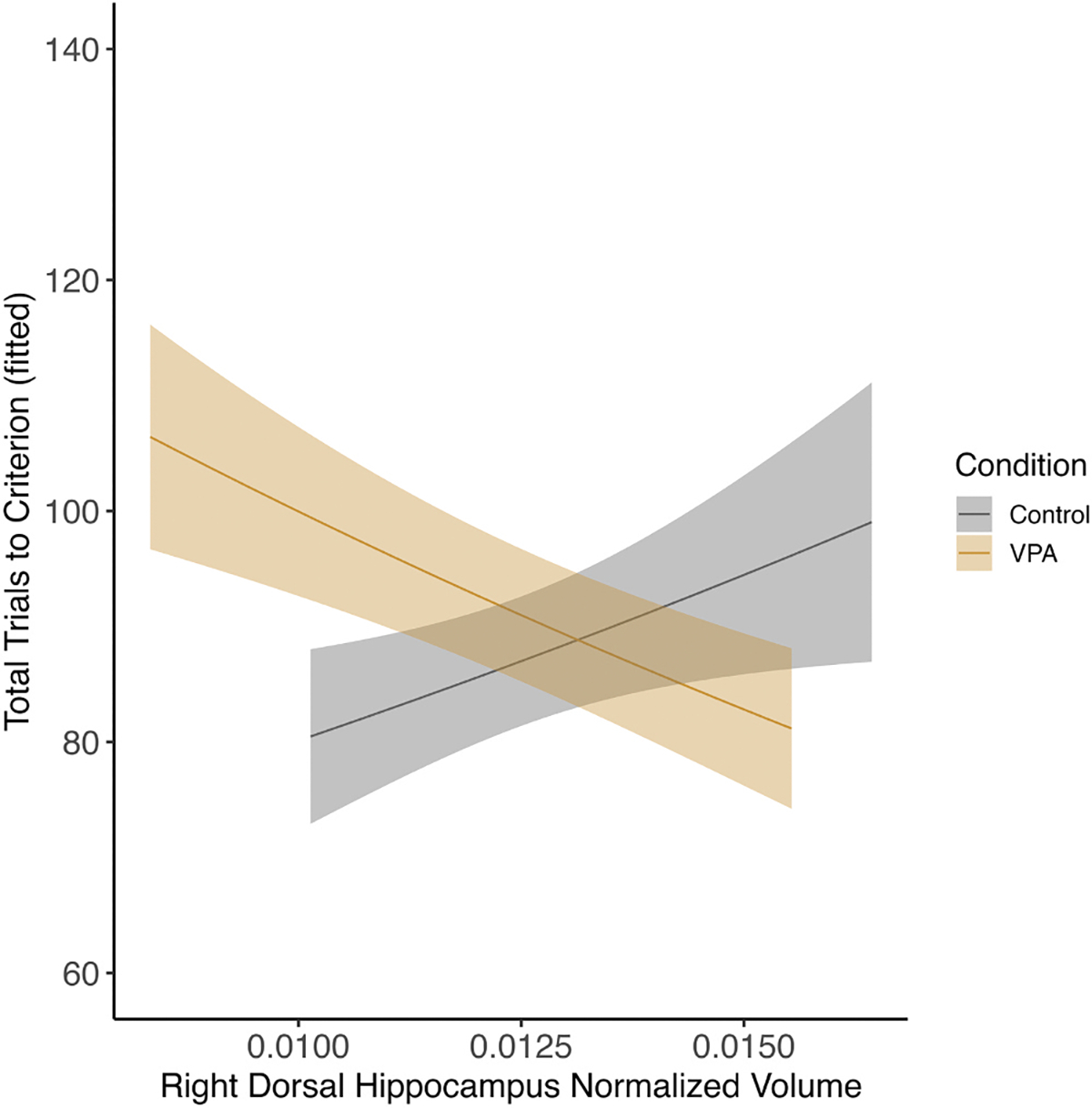
Male right dorsal hippocampus and total trials across the task. There was interaction between right dorsal hippocampus and condition for total trials. Control males with smaller volumes performed better across the task, whereas VPA males with larger volumes performed better across the task (p < 0.05).
4. Discussion
4.1. Set-shifting performance
This study used an aerobic exercise intervention in adolescence to drive brain plasticity in a rodent model of ASD. Both control and VPA exposed animals benefited cognitively from exercise, including better performance on the ED phase of the task. This suggests that exercise assists with modulation of the circuits that regulate set-shifting performance. Exercised females displayed more cognitive benefits than males in that they had improvements for both the ID4 and ED phases, whereas for males exercise impaired performance across the ID phases (ID1-ID4), and the benefit was not observed until the ED phase. This may in part be due to differences in male and female VPA animals. Female VPA animals have been found to be more impaired at set-shifting than VPA males [15,58,59] and therefore exercise may drive more benefits. The difference may also be accounted for by the fact that rats were exercised during adolescence, and stressors (in this case forced exercise) applied during adolescence can impact cognitive performance in males but not in females [60,61]. Stressed adolescent males demonstrate different behaviors from females such as increased head shakes in other tasks [62], and it is possible these types of differences impacted early learning during the ID phases in exercised males. However, a cognitive benefit did emerge during the later stages of the task. Individuals with ASD struggle more with modifying behavior flexibly when rules change and the ED phase is indicative of this requirement [30]. It appears that exercise may be most beneficial during this portion of the task.
4.2. VPA exposure sex differences and cognitive performance
Prior studies have verified the VPA phenotype using behavioral outcomes [17,63–65], and brain volume [14,59,66], an endophenotypic marker [67,68]. This study was focused on measuring cognitive outcomes in exercised and sedentary VPA exposed animals. On the CD phase of the task neither sedentary or exercised, male or female VPA animals were impaired on the task. This is consistent with prior findings [58] and suggests that the cognitive differences observed later in VPA animals is not related to basic learning impairments. In humans with ASD, there are differences in cognitive performance in similar tasks with ED components [30,69–72] and females with ASD frequently have lower cognitive ability [73,74] suggesting they may benefit more from exercise [75]. Prior work in VPA females also found greater impairment in VPA females compared to VPA males [15,58]. This study suggests exercise improved performance for these types of demands in VPA exposed animals and this aligns with human data suggesting exercise improves executive functioning in those with ASD [75]. Other domains of ASD-like behaviors were not assessed due to COVID-19 restrictions in the research facility at the time of data collection. Whether exercise could reduce anxiety and repetitive behavior, or improve social interactions remains to be explored. However, studies in humans suggests exercise may provide benefits in those domains as well [39,40].
4.3. Regional volume changes impacted by exercise
This study employed an adolescent exercise intervention to assess whether aerobic exercise modulated volume changes in young adult VPA animals. Exercise increased dorsal hippocampus volume in control females, whereas exercise maintained already increased volumes in VPA females. Therefore, after exercise control and VPA females have similar volumes. This is evidence of a modulatory benefit of exercise in VPA females. In males, VPA animals had increased ventral hippocampal volumes, suggesting that there may be slight differences in hippocampal responses to exercise by sex. To date, most of the studies that have examined volumetric changes have been in elderly populations where loss of volume has already occurred. For this reason, it is not surprising that exercise did not drive large increases in volume across the brain, as young animals have not yet had the significant grey matter reductions that the elderly do. However, exercise may still be exerting pro-cognitive impacts via changes at the molecular level via an irisin-BDNF axis [76,77]. Irisin is a molecule that is released from skeletal muscle during exercise, and it activates receptors in the brain that help modulate brain derived neurotrophic factor (BDNF) expression [77,78]. BDNF has been shown to improve synaptic maintenance and is upregulated by exercise in VPA animals (under review). The maintenance of hippocampal volume by exercise in VPA females could be arising due to molecular changes related to the BDNF pathway.
4.3.1. Frontal cortex and cingulate
A past study found overgrowth of frontal cortices and the ACC in VPA females during adolescence [15]. This is consistent with findings of excessive frontal gray matter in humans with ASD [2] where females with ASD have larger frontal gyrification compared to males with ASD [79]. In the current study, females were exercised from P40 into young adulthood, where control and VPA sedentary females did not have differences in frontal or ACC volumes. This suggests that frontal areas may have begun to prune, and that exercise may assist this effort, which is supported by the findings that exercise further decreased frontal volumes in both control and VPA females. The motor cortex was another frontal region that was modulated by exercise. The motor cortex decreased in volume for exercised females, and this was primarily driven by changes in the VPA group. Typically, exercise is studied as an intervention to increase brain volume. However correct pruning and refinement of networks is also an important component of brain function, particularly during development. Exercise can modulate microglial functions, which are critical for proper pruning and the preservation of memory function [80]. It is possible that exercise is assisting with some of these molecular and cellular processes related to pruning and synaptic maintenance in frontal regions and improving cognitive function as a result. Prior research has also found altered metabolic rates in frontal regions of rodents [81,82]. These metabolic changes could be another mechanism that impacts cognition in ways undetectable by MRI. Indeed, metabolic dysregulation in these frontal regions has been associated with changes in cognition in humans with ASD [83–85].
4.3.2. PCC and amygdala
In the PCC, control females had increased volumes after exercise, and VPA sedentary females already had slightly higher volumes than sedentary controls. This suggests that exercise is modulating function by increasing volume in control females and maintaining already overgrown volume in VPA animals, rather than further increasing them, perhaps through molecular regulation. Control sedentary females had the smallest left amygdala volumes compared to exercised control and VPA (both exercised and sedentary) females. Additionally, VPA sedentary females had enlarged left amygdala compared to sedentary control females. Taken together, this suggests exercise increased left amygdala volume in control animals but did not further increase the already enlarged structure in VPA females.
4.4. Volume and behavior relationships
4.4.1. VPA Female behavior and volume differences
Regression analysis examined if brain volume was predictive of cognitive performance on the set-shifting task. The mPFC is a critical structure for accurate ED performance [86]. Prior research found that adolescent VPA females with enlarged frontal cortices had impaired cognitive performance on the set-shifting task, specifically on the ED phase [15]. In the current study, and at a later age when overgrowth of the frontal cortex appears to have stopped, decreased mPFC volumes were associated with improved performance on the ED phase of the task for control females, but not for VPA females. Across frontal regions, exercise decreased volumes in female rats, which may suggest that pruning, regulation of microglia, or network refinement is assisting the function of this region. Smaller volumes were not beneficial to VPA females, though, and this could be because of neural compensation or extra reliance on the structure. In ASD, some regions may overgrow or over activate to compensate for dysfunctions in others [7,87]. Past research also found that VPA females with smaller left crus I volumes were impaired on set-shifting [59]. In this study, VPA females with enlarged left crus I volumes performed worse on the ED phase of the task. This same relationship was not present in control females, which suggests that cerebellar pathology may be contributing to worse outcomes in this task for VPA females. Crus I is a region that functionally connected to the frontal cortex through the thalamus [88,89], and in humans this region can affect cognitive flexibility [90].
4.4.2. VPA Male behavior and volume differences
For males, left and right crus I had a similar relationship where larger volumes were associated with improved performance across the entire set-shifting task. This is a novel finding that further implicates cerebellar function in cognitive flexibility. Furthermore, the sex differences in the association between crus I volume and performance on the set-shifting task suggest that cerebellar alterations in ASD may be sex-specific. Disentangling these sex effects may reconcile conflicting findings of hypoplasia and hyperplasia of cerebellar regions in ASD [91]. In the hippocampus, VPA males that had larger right dorsal and ventral hippocampi performed better during the ED phase of the task. This in part may be explained by the exercise-mediated increase in ventral volume. In the dorsal hippocampus, there was a split where larger volumes improved VPA performance, but impaired control performance. It has been hypothesized that individuals with ASD rely more on hippocampus as a compensatory mechanism for posterior medial network dysfunction [92], which may explain the divergent findings between VPA and control males. In a past study, VPA males with enlarged ACC volumes had impaired performance on the ID phase of the set-shifting task [15]. Here, this finding was replicated; VPA males with larger ACCs were worse on the ID4 phase. Future studies could examine different molecular markers of plasticity such as changes in microglia or BDNF signaling to better understand what is contributing to cognitive and volumetric changes across developmental time.
4.5. VPA regional volumes across development parallel those found in ASD
Regional volume changes in the development of ASD are extraordinarily complex and are both age-, sex-, and region-specific [87,93]. Region-specific volume changes in ASD and the VPA model follow similar developmental trajectories. For instance, during adolescence, individuals with ASD have enlarged frontal cortices [94,95] and hippocampi [5,92]. Prior findings show that VPA animals also have enlarged frontal cortices [15] and hippocampi during adolescence [66] and that these increases in volumes are associated with impairments in cognition [15]. These volumetric findings are replicated here where sedentary VPA females still have enlarged dorsal hippocampi compared to control sedentary females. A novel finding from this study is that in sedentary VPA females the left amygdala is enlarged compared to sedentary control females. This is not the case in males, where sedentary VPA and controls were not different. In human males with ASD, there is excessive oligodendrocyte proliferation in the amygdala at the same approximate age measured in the current study [96], which could be contributing to the observed enlargement. This aligns with human studies in young adult males, where amygdala volumes are not different between those with ASD and controls [4]. The results found here support these findings for males, and further research is warranted to understand if the same trajectory is followed in females with ASD.
4.6. Limitations
There are limitations to this study. Due to practical constraints, it was not possible to take MRI images longitudinally within-subject. This would have been beneficial in examining developmental trajectory changes between brain regions impacted by VPA exposure and sex. Future studies could also combine magnetic resonance spectroscopy and diffusion tensor imaging to learn more about molecular changes and fiber pathways over time. Also, as discussed above, the forced nature of the exercise could be viewed as a stressor, and future studies could employ running wheels to determine if voluntary exercise alleviates the observed deficits in males on ID4 performance.
4.7. Conclusion
This study used an adolescent aerobic exercise intervention in VPA-exposed animals and found that exercise enhanced cognitive flexibility as measured by a set-shifting task. This change in cognitive flexibility was coupled with volumetric alterations in key regions for set-shifting performance like the ACC in female rats and the hippocampus in both sexes. Outside of these regions and the amygdala, this study did not find robust exercise effects modulating volume across the brain. However, volume was associated with performance on the set-shifting task for other regions like the mPFC in females and cerebellar crus I in both sexes. This study also replicates past MRI findings in the VPA model in adolescence, including enlarged hippocampi. At the developmental timepoint of this study, though, some structures like the ACC are no longer enlarged as they are in adolescence [15]. This suggests that pruning of these structures has likely started. Future studies should measure volume in these structures at later ages where it is predicted that a decrease in volume would be accelerated. These findings highlight the unique developmental trajectories across brain structures that occur in VPA animals, and these are mirrored in humans with ASD [2–6]. Additionally, sedentary VPA males trended toward enlarged lobule VI regions compared to control males, replicating previous findings [59].
The exercise intervention used in this study was not vigorous or especially taxing, and the animals walked at low speeds. This suggests that, even in young animals, exercise is successful at driving cognitive function. Thus, exercise may serve as an excellent intervention for children with ASD, especially in combination with sensory stimulation therapies, as it is inexpensive and generally accessible. Thus, exercise may improve cognitive function in ASD, similarly to how it does in aging, in part by regulating the development of brain volume.
Supplementary Material
Acknowledgements
This work was funded by a Developmental Research Project Award to B. Plakke (K-INBRE P20 GM103418), a University small research grant from KSU to B. Plakke, and supported by (CNAP Center NIGMS-GM113109 NIH). We would like to thank Anvesha Sharda for her contributions as well.
Footnotes
CRediT authorship contribution statement
Ivina Mali: Methodology. Cole King: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Bhavana Sivayokan: Writing – review & editing, Writing – original draft, Formal analysis. Bethany Plakke: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Stefan Bossmann: Resources, Methodology, Funding acquisition. Ellie Warnes: Investigation. Hunter Strating: Investigation. Macy Payne: Writing – original draft, Methodology.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbr.2024.115136.
Data availability
Data will be made available on request.
References
- [1].Courchesne E, Pramparo T, Gazestani VH, Lombardo MV, Pierce K, Lewis NE, The ASD living biology: from cell proliferation to clinical phenotype, Mol. Psychiatry 24 (2019) 88–107, 10.1038/s41380-018-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, Calderoni S, Daly E, Deruelle C, Di Martino A, Dinstein I, Duran FLS, Durston S, Ecker C, Fair D, Fedor J, Fitzgerald J, Freitag CM, Gallagher L, Gori I, Haar S, Hoekstra L, Jahanshad N, Jalbrzikowski M, Janssen J, Lerch J, Luna B, Martinho MM, McGrath J, Muratori F, Murphy CM, Murphy DGM, O’Hearn K, Oranje B, Parellada M, Retico A, Rosa P, Rubia K, Shook D, Taylor M, Thompson PM, Tosetti M, Wallace GL, Zhou F, Buitelaar JK, Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group, Am. J. Psychiatry 175 (2018) 359–369, 10.1176/appi.ajp.2017.17010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Braden BB, Riecken C, Thinning faster? Age-related cortical thickness differences in adults with autism spectrum disorder, Res. Autism Spectr. Disord. 64 (2019) 31–38, 10.1016/j.rasd.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG, The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages, J. Neurosci. Off. J. Soc. Neurosci. 24 (2004) 6392–6401, 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Groen W, Teluij M, Buitelaar J, Tendolkar I, Amygdala and hippocampus enlargement during adolescence in autism, J. Am. Acad. Child Adolesc. Psychiatry 49 (2010) 552–560, 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- [6].Xu M-X, Ju X-D, Abnormal brain structure is associated with social and communication deficits in children with autism spectrum disorder: a voxel-based morphometry analysis, Brain Sci. 13 (2023), 10.3390/brainsci13050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Banker SM, Gu X, Schiller D, Foss-Feig JH, Hippocampal contributions to social and cognitive deficits in autism spectrum disorder, Trends Neurosci. 44 (2021) 793–807, 10.1016/j.tins.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mosconi MW, Wang Z, Schmitt LM, Tsai P, Sweeney JA, The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders, Front. Neurosci. 9 (2015) 296, 10.3389/fnins.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ, Cerebellar gray matter and lobular volumes correlate with core autism symptoms, NeuroImage Clin. 7 (2015) 631–639, 10.1016/j.nicl.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pierce K, Courchesne E, Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism, Biol. Psychiatry 49 (2001) 655–665 ([pii]). [DOI] [PubMed] [Google Scholar]
- [11].D’Mello AM, Stoodley CJ, Cerebro-cerebellar circuits in autism spectrum disorder, Front. Neurosci. 9 (2015), 10.3389/fnins.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tunç B, Yankowitz LD, Parker D, Alappatt JA, Pandey J, Schultz RT, Verma R, Deviation from normative brain development is associated with symptom severity in autism spectrum disorder, Mol. Autism 10 (2019) 46, 10.1186/s13229-019-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bieneck V, Bletsch A, Mann C, Schäfer T, Seelemeyer H, Herøy N, Zimmermann J, Pretzsch CM, Hattingen E, Ecker C, Longitudinal changes in cortical thickness in adolescents with autism spectrum disorder and their association with restricted and repetitive behaviors, Genes 12 (2021), 10.3390/genes12122024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spisák T, Román V, Papp E, Kedves R, Sághy K, Csölle CK, Varga A, Gajári D, Nyitrai G, Spisák Z, Kincses ZT, Lévay G, Lendvai B, Czurkó A, Purkinje cell number-correlated cerebrocerebellar circuit anomaly in the valproate model of autism, Sci. Rep. 9 (1) (2019) 15, 10.1038/s41598-019-45667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mali I, Payne M, King C, Maze TR, Davison T, Challans B, Bossmann SH, Plakke B, Adolescent female valproic acid rats have impaired extra-dimensional shifts of attention and enlarged anterior cingulate cortices, Brain Res. 1800 (2023) 148199, 10.1016/j.brainres.2022.148199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mabunga DF, Gonzales EL, Kim JW, Kim KC, Shin CY, Exploring the validity of valproic acid animal model of autism, Exp. Neurobiol. 24 (2015) 285–301, 10.5607/en.2015.24.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schneider T, Ziolkowska B, Gieryk A, Tyminska A, Przewlocki R, Prenatal exposure to valproic acid disturbs the enkephalinergic system functioning, basal hedonic tone, and emotional responses in an animal model of autism, Psychopharmacology 193 (2007) 547–556, 10.1007/s00213-007-0795-y. [DOI] [PubMed] [Google Scholar]
- [18].Rinaldi T, Silberberg G, Markram H, Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid, Cereb. Cortex 18 (2008) 763–770, 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- [19].Kim KC, Kim P, Go HS, Choi CS, Yang SI, Cheong JH, Shin CY, Ko KH, The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats, Toxicol. Lett. 201 (2011) 137–143, 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- [20].Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M, Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism, Jama 309 (2013) 1696–1704, 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, Dowling NF, Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2014, Morb. Mortal. Wkly. Report. Surveill. Summ. (Wash. D. C. 2002) 67 (2018) 1–24, 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee E, Lee J, Kim E, Excitation/inhibition imbalance in animal models of autism spectrum disorders, Biol. Psychiatry 81 (2017) 838–848 ([pii]). [DOI] [PubMed] [Google Scholar]
- [23].Tanaka M, Sato A, Kasai S, Hagino Y, Kotajima-Murakami H, Kashii H, Takamatsu Y, Nishito Y, Inagaki M, Mizuguchi M, Hall FS, Uhl GR, Murphy D, Sora I, Ikeda K, Brain hyperserotonemia causes autism-relevant social deficits in mice, Mol. Autism 9 (2018) 60, 10.1186/s13229-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y, Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring, Science 343 (2014) 675–679, 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- [25].Leung RC, Zakzanis KK, Brief report: cognitive flexibility in autism spectrum disorders: a quantitative review, J. Autism Dev. Disord. 44 (2014) 2628–2645, 10.1007/s10803-014-2136-4. [DOI] [PubMed] [Google Scholar]
- [26].Shenouda J, Barrett E, Davidow AL, Sidwell K, Lescott C, Halperin W, Silenzio VMB, Zahorodny W, Prevalence and disparities in the detection of autism without intellectual disability, Pediatrics 151 (2023), 10.1542/peds.2022-056594. [DOI] [PubMed] [Google Scholar]
- [27].Schmitt LM, Li J, Liu R, Horn PS, Sweeney JA, Erickson CA, Pedapati EV, Altered frontal connectivity as a mechanism for executive function deficits in fragile X syndrome, Mol. Autism 13 (2022) 47, 10.1186/s13229-022-00527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Petanjek Z, Sedmak D, Džaja D, Hladnik A, Rašin MR, Jovanov-Milosevic N, The protracted maturation of associative layer IIIC pyramidal neurons in the human prefrontal cortex during childhood: a major role in cognitive development and selective alteration in autism, Front. Psychiatry 10 (2019) 122, 10.3389/fpsyt.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Uddin LQ, Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder, Biol. Psychiatry 89 (2021) 172–183, 10.1016/j.biopsych.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy LE, Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the Intradimensional/extradimensional shift test correlate with repetitive behaviors, Autism 13 (2009) 523–539, 10.1177/1362361309335716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yerys BE, Antezana L, Weinblatt R, Jankowski KF, Strang J, Vaidya CJ, Schultz RT, Gaillard WD, Kenworthy L, neural correlates of set-shifting in children with autism, Autism Res. 8 (2015) 386–398, 10.1002/aur.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A, Double dissociation of attentional resources: prefrontal versus cingulate cortices, J. Neurosci. 27 (2007) 12123–12132 ([pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bubb EJ, Aggleton JP, Mara SMO, Nelson AJD, ORIGINAL ARTICLE chemogenetics reveal an anterior cingulate–thalamic pathway for attending to task-relevant information, Cereb. Cortex (2020) 1–18, 10.1093/cercor/bhaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Birrell JM, Brown VJ, Medial frontal cortex mediates perceptual attentional set shifting in the rat, J. Neurosci. 20 (2000) 4320–4325 ([pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF, Exercise, brain, and cognition across the life span, J. Appl. Physiol. 111 (2011) 1505–1513, 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, van Praag H, Exercise and hippocampal memory systems, Trends Cogn. Sci. 23 (2019) 318–333, 10.1016/j.tics.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF, Aerobic exercise training increases brain volume in aging humans, J. Gerontol. - Ser. A Biol. Sci. Med. Sci. 61 (2006) 1166–1170, 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- [38].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF, Exercise training increases size of hippocampus and improves memory, Proc. Natl. Acad. Sci. USA 108 (2011) 3017–3022, 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ferreira JP, Ghiarone T, Júnior CRC, Furtado GE, Carvalho HM, Rodrigues AM, Toscano CVA, Effects of physical exercise on the stereotyped behavior of children with autism spectrum disorders, Med 55 (2019) 1–18, 10.3390/medicina55100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zarafshan H, Salmanian M, Aghamohammadi S, Mohammadi MR, Mostafavi SA, Zarafshan H, Salmanian M, Aghamohammadi S, Mohammadi MR, Mostafavi S-A, Effectiveness of non-pharmacological interventions on stereotyped and repetitive behaviors of pre-school children with autism: a systematic review, Basic Clin. Neurosci. 8 (2017) 95–104, 10.18869/nirp.bcn.8.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schmitz Olin S, McFadden BA, Golem DL, Pellegrino JK, Walker AJ, Sanders DJ, Arent SM, The effects of exercise dose on stereotypical behavior in children with autism, Med. Sci. Sports Exerc. 49 (2017) 983–990, 10.1249/MSS.0000000000001197. [DOI] [PubMed] [Google Scholar]
- [42].Firth J, Stubbs B, Rosenbaum S, Vancampfort D, Malchow B, Schuch F, Elliott R, Nuechterlein KH, Yung AR, Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis, Schizophr. Bull. 43 (2017) 546–556, 10.1093/schbul/sbw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Falkai P, Malchow B, Schmitt A, Aerobic exercise and its effects on cognition in schizophrenia, Curr. Opin. Psychiatry 30 (2017) 171–175, 10.1097/YCO.0000000000000326. [DOI] [PubMed] [Google Scholar]
- [44].Cho HS, Kim TW, Ji ES, Park HS, Shin MS, Baek SS, Treadmill exercise ameliorates motor dysfunction through inhibition of Purkinje cell loss in cerebellum of valproic acid-induced autistic rats, J. Exerc. Rehabil. 12 (2016) 293–299, 10.12965/jer.1632696.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barbalho SM, Prado Neto EV, De Alvares Goulart R, Bechara MD, Baisi Chagas EF, Audi M, Guissoni Campos LM, Landgraf Guiger E, Buchaim RL, Buchaim DV, Cressoni Araujo A, Myokines: a descriptive review, J. Sports Med. Phys. Fit. 60 (2020) 1583–1590, 10.23736/S0022-4707.20.10884-3. [DOI] [PubMed] [Google Scholar]
- [46].Walsh JJ, Tschakovsky ME, Exercise and circulating bdnf: mechanisms of release and implications for the design of exercise interventions, Appl. Physiol. Nutr. Metab. 43 (2018) 1095–1104, 10.1139/apnm-2018-0192. [DOI] [PubMed] [Google Scholar]
- [47].Erickson KI, Leckie RL, Weinstein AM, Physical activity, fitness, and gray matter volume, Neurobiol. Aging 35 (2014) S20–S28, 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu-Ambrose T, Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans, Front. Neuroendocrinol. 46 (2017) 71–85, 10.1016/j.yfrne.2017.04.002. [DOI] [PubMed] [Google Scholar]
- [49].de M Coelho FG, Gobbi S, Andreatto CAA, Corazza DI, Pedroso RV, Santos-Galduróz RF, Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly, Arch. Gerontol. Geriatr. 56 (2013) 10–15, 10.1016/j.archger.2012.06.003. [DOI] [PubMed] [Google Scholar]
- [50].Andropoulos DB, Effect of Anesthesia on the Developing Brain: Infant and Fetus, vol. 77030, 2018, pp. 1–11. ⟨ 10.1159/000475928⟩. [DOI] [PubMed] [Google Scholar]
- [51].Lazic SE, Essioux L, Improving basic and translational science by accounting for litter-to-litter variation in animal models, BMC Neurosci. 14 (2013), 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Seo T-B, Cho H-S, Shin M-S, Kim C-J, Ji E-S, Baek S-S, Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats, J. Exerc. Rehabil. 9 (2013) 220–229, 10.12965/jer.130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Newman LA, Mcgaughy J, Adolescent rats show cognitive rigidity in a test of attentional set shifting, Dev. Psychobiol. 53 (2011) 391–401, 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cain RE, Wasserman MC, Waterhouse BD, McGaughy JA, Atomoxetine facilitates attentional set shifting in adolescent rats, Dev. Cogn. Neurosci. 1 (2011) 552–560, 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li R, Liu X, Sidabras JW, Paulson ES, Jesmanowicz A, Nencka AS, Hudetz AG, Hyde JS, Restoring susceptibility induced MRI signal loss in rat brain at 9.4 T: a step towards whole brain functional connectivity imaging, PLoS One 10 (2015), 10.1371/journal.pone.0119450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].R Core Team, R: A Language and Environment for Statistical Computing, 2023. ⟨https://www.r-project.org/⟩.
- [57].Bates D, Mächler M, Bolker BM, Walker SC, Fitting linear mixed-effects models using lme4, J. Stat. Softw. 67 (2015), 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- [58].McKinnell ZE, Maze T, Ramos A, Challans B, Plakke B, Valproic acid treated female Long-Evans rats are impaired on attentional set-shifting, Behav. Brain Res. 397 (2021) 112966, 10.1016/j.bbr.2020.112966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Payne M, Mali I, McKinnell ZE, Vangsness L, Shrestha TB, Bossmann SH, Plakke B, Increased volumes of lobule VI in a valproic acid model of autism are associated with worse set-shifting performance in male Long-Evan rats, Brain Res. 1765 (2021) 147495, 10.1016/j.brainres.2021.147495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Klinger K, Gomes FV, Rincón-Cortés M, Grace AA, Female rats are resistant to the long-lasting neurobehavioral changes induced by adolescent stress exposure, Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 29 (2019) 1127–1137, 10.1016/j.euroneuro.2019.07.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Horovitz O, Tsoory MM, Yovell Y, Richter-Levin G, A rat model of pre-puberty (juvenile) stress-induced predisposition to stress-related disorders: sex similarities and sex differences in effects and symptoms, World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 15 (2014) 36–48, 10.3109/15622975.2012.745604. [DOI] [PubMed] [Google Scholar]
- [62].Harris EP, McGovern AJ, Melo TG, Barron A, Nolan YM, O’Leary OF, Juvenile stress exerts sex-independent effects on anxiety, antidepressant-like behaviours and dopaminergic innervation of the prelimbic cortex in adulthood and does not alter hippocampal neurogenesis, Behav. Brain Res. 421 (2022) 113725, 10.1016/j.bbr.2021.113725. [DOI] [PubMed] [Google Scholar]
- [63].Melancia F, Schiavi S, Servadio M, Cartocci V, Campolongo P, Palmery M, Pallottini V, Trezza V, Sex-specific autistic endophenotypes induced by prenatal exposure to valproic acid involve anandamide signalling, Br. J. Pharm. 175 (2018) 3699–3712, 10.1111/bph.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schneider T, Turczak J, Przewlocki R, Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism, Neuropsychopharmacology 31 (2006) 36–47 ([pii]). [DOI] [PubMed] [Google Scholar]
- [65].Markram K, Rinaldi T, La Mendola D, Sandi C, Markrams H, Abnormal fear conditioning and amygdala processing in an animal model of autism, Neuropsychopharmacology 33 (2008) 901–913 ([pii]). [DOI] [PubMed] [Google Scholar]
- [66].King C, Mali I, Strating H, Fangman E, Neyhard J, Payne M, Bossmann SH, Plakke B, Region-specific brain volume changes emerge in adolescence in the valproic acid model of autism and parallel human findings, Dev. Neurosci. (2024), 10.1159/000538932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sacco R, Gabriele S, Persico AM, Head circumference and brain size in autism spectrum disorder: a systematic review and meta-analysis, Psychiatry Res. 234 (2015) 239–251, 10.1016/j.pscychresns.2015.08.016. [DOI] [PubMed] [Google Scholar]
- [68].Segovia F, Holt R, Spencer M, Górriz JM, Ramírez J, Puntonet CG, Phillips C, Chura L, Baron-Cohen S, Suckling J, Identifying endophenotypes of autism: a multivariate approach, Front. Comput. Neurosci. 8 (2014) 60, 10.3389/fncom.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yasuda Y, Hashimoto R, Ohi K, Yamamori H, Fujimoto M, Umeda-Yano S, Fujino H, Takeda M, Cognitive inflexibility in Japanese adolescents and adults with autism spectrum disorders, World J. Psychiatry 4 (2014) 42–48, 10.5498/wjp.v4.i2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kiep M, Spek AA, Executive functioning in men and women with an autism spectrum disorder, Autism Res. 10 (2017) 940–948, 10.1002/aur.1721. [DOI] [PubMed] [Google Scholar]
- [71].Wang Z, Jing J, Igarashi K, Fan L, Yang S, Li Y, Jin Y, Executive function predicts the visuospatial working memory in autism spectrum disorder and attention-deficit/hyperactivity disorder, Autism Res. Off. J. Int. Soc. Autism Res. 11 (2018) 1148–1156, 10.1002/aur.1967. [DOI] [PubMed] [Google Scholar]
- [72].Westwood H, Stahl D, Mandy W, Tchanturia K, The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: a systematic review and meta-analysis, Psychol. Med. 46 (2016) 1809–1827, 10.1017/S0033291716000581. [DOI] [PubMed] [Google Scholar]
- [73].Lai MC, Lombardo MV, Pasco G, Ruigrok ANV, Wheelwright SJ, Sadek SA, Chakrabarti B, Baron-Cohen S, A behavioral comparison of male and female adults with high functioning autism spectrum conditions, PLoS One 6 (2011), 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen C, Van Horn JD, Developmental neurogenetics and multimodal neuroimaging of sex differences in autism, Brain Imaging Behav. 11 (2017) 38–61, 10.1007/s11682-015-9504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liang X, Li R, Wong SHS, Sum RKW, Wang P, Yang B, Sit CHP, The effects of exercise interventions on executive functions in children and adolescents with autism spectrum disorder: a systematic review and meta-analysis, Sports Med. 52 (2022) 75–88, 10.1007/s40279-021-01545-3. [DOI] [PubMed] [Google Scholar]
- [76].Pedersen BK, Febbraio MA, Muscles, exercise and obesity: skeletal muscle as a secretory organ, Nat. Rev. Endocrinol. 8 (2012) 457–465, 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- [77].Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM, Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway, Cell Metab. 18 (2013) 649–659, 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Islam MR, Valaris S, Young MF, Haley EB, Luo R, Bond SF, Mazuera S, Kitchen RR, Caldarone BJ, Bettio LEB, Christie BR, Schmider AB, Soberman RJ, Besnard A, Jedrychowski MP, Kim H, Tu H, Kim E, Choi SH, Tanzi RE, Spiegelman BM, Wrann CD, Exercise hormone irisin is a critical regulator of cognitive function, Nat. Metab. 3 (2021) 1058–1070, 10.1038/s42255-021-00438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schaer M, Kochalka J, Padmanabhan A, Supekar K, Menon V, Sex differences in cortical volume and gyrification in autism, Mol. Autism 6 (2015), 10.1186/s13229-015-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tuan L-H, Tsao C-Y, Lee LJ-H, Lee L-J, Voluntary exercise ameliorates synaptic pruning deficits in sleep-deprived adolescent mice, Brain Behav. Immun. 93 (2021) 96–110, 10.1016/j.bbi.2020.12.017. [DOI] [PubMed] [Google Scholar]
- [81].Sumiyoshi A, Nonaka H, Kawashima R, Sexual differentiation of the adolescent rat brain: a longitudinal voxel-based morphometry study, Neurosci. Lett. 642 (2017) 168–173, 10.1016/j.neulet.2016.12.023. [DOI] [PubMed] [Google Scholar]
- [82].Choi H, Choi Y, Kim KW, Kang H, Hwang DW, Kim EE, Chung J, Lee DS, Maturation of metabolic connectivity of the adolescent rat brain, 2015, pp. 1–12. ⟨ 10.7554/eLife.11571⟩. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Agam Y, Joseph RM, Barton JJ, Manoach DS, Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders, Neuroimage 52 (2010) 336–348, 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Leiguarda R, Sigman M, State-dependent changes of connectivity patterns and functional brain network topology in autism spectrum disorder, Neuropsychologia 50 (2012) 3653–3662, 10.1016/j.neuropsychologia.2012.09.047. [DOI] [PubMed] [Google Scholar]
- [85].Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG, Neural correlates of executive function in autistic spectrum disorders, Biol. Psychiatry 59 (2006) 7–17 ([pii]). [DOI] [PubMed] [Google Scholar]
- [86].Bissonette GB, Powell EM, Roesch MR, Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex, Behav. Brain Res. 250 (2013) 91–102, 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Prigge MBD, Lange N, Bigler ED, King JB, Dean DC 3rd, Adluru N, Alexander AL, Lainhart JE, Zielinski BA, A 16-year study of longitudinal volumetric brain development in males with autism, Neuroimage 236 (2021) 118067, 10.1016/j.neuroimage.2021.118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kelly RM, Strick PL, Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate, J. Neurosci. 23 (2003) 8432–8445 ([pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bostan AC, Dum RP, Strick PL, Cerebellar networks with the cerebral cortex and basal ganglia, Trends Cogn. Sci. 17 (2013) 241–254, 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].D’Angelo E, Casali S, Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition, Front. Neural Circuits 6 (2013) (https://doi.org/116). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schreibman L, Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging, AJR Am. J. Roentgenol. 162 (1994) 123–131, 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- [92].Hogeveen J, Krug MK, Geddert RM, Ragland JD, Solomon M, Compensatory hippocampal recruitment supports preserved episodic memory in autism spectrum disorder, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5 (2020) 97–109, 10.1016/j.bpsc.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shan X, Uddin LQ, Xiao J, He C, Ling Z, Li L, Huang X, Chen H, Duan X, Mapping the heterogeneous brain structural phenotype of autism spectrum disorder using the normative model, Biol. Psychiatry 91 (2022) 967–976, 10.1016/j.biopsych.2022.01.011. [DOI] [PubMed] [Google Scholar]
- [94].DeRamus TP, Kana RK, Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders, NeuroImage Clin. 7 (2015) 525–536, 10.1016/j.nicl.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zielinski BA, Prigge MBD, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N, Alexander AL, Bigler ED, Lainhart JE, Longitudinal changes in cortical thickness in autism and typical development, Brain 137 (2014) 1799–1812, 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Morgan JT, Barger N, Amaral DG, Schumann CM, Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder, PLoS One 9 (2014) e110356, 10.1371/journal.pone.0110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


