Abstract
Chimeric antigen receptor (CAR) T cell therapy has revolutionized the treatment of B cell and plasma cell malignancies, and numerous promising targets against solid tumours are being explored. Despite their initial therapeutic success in hematological cancers, relapse occurs in a significant fraction of patients, highlighting the need for further innovations in advancing CAR T cell therapy. Tumour antigen heterogeneity and acquired tumour resistance leading to antigen escape (antigen loss/downregulation) have emerged as a crucial factor contributing to immune escape and CAR T cell resistance, particularly in the case of solid tumours with only limited success achieved to date. In this review, we discuss mechanisms of tumour relapse in CAR T cell therapy and the promising strategies that are under development to overcome multiple resistance mechanisms, thereby reducing outgrowth of antigen escape variants. Specifically, we emphasize the importance of designing clinical translational strategies to enhance CAR T cell crosstalk with host immune cells, eliciting endogenous antitumour immune responses through antigen/epitope spreading, which offers a genuine solution to the limitations of targeting tumour antigen heterogeneity in solid tumours with monospecific T cell therapies.
Keywords: Chimeric antigen receptor, Tumour heterogeneity, Antigen loss/downregulation, Antigen/epitope spreading
Introduction
Chimeric antigen receptor T (CAR T) cell therapy has exhibited remarkable clinical success, and it has driven a paradigm shift in the treatment of relapsed/refractory (r/r) B cell lymphomas, B cell acute lymphoblastic leukemia and multiple myeloma. At present, CD19 and B cell maturation antigen (BCMA) are the most common targets in CAR T cell therapy, and numerous novel therapeutic targets are being explored for the treatment of solid tumours.
However, despite the promising clinical outcome achieved with CAR T cells, there is still a substantial proportion of patients who ultimately have progressive disease, either initial lack of response, known as primary resistance or disease progression after an initial, transient response, known as second resistance. For instance, the documented complete remission (CR) rate for r/r B-ALL treated with CAR T cells ranged from 70 to 94%. However, around 35–55% of these patients eventually relapsed [1–6]. In the pivotal phase II studies for r/r large B-cell lymphoma, anti-CD19 CAR T achieved the CR rates of 39–58%, and nearly half of the treated patients developed primary resistance to CAR T therapy [7–9]. For r/r multiple myeloma (MM), CAR T cell therapy targeting BCMA, acquired an overall response rates of 70–90% and complete response rates of 30–60% in pivotal trials [10, 11]. However, when examining long-term outcomes, most individuals with multiple myeloma will eventually relapse without any plateau in progression-free survival, as observed when using anti-CD19 CAR T cells for patients with B-cell aggressive lymphomas or leukaemia.
Reasons leading to the failure of CAR T therapies are complex, including tumour heterogeneity, T cell exhaustion, decreased T cell persistence, limited tumour trafficking, the presence of an immunosuppressive tumour microenvironment (TME), metabolic stress, and various other confounding factors [12]. This review focuses specifically on the importance of tumour relapse caused by antigen escape after CAR T therapy and summarizes the potential strategies that are being employed to overcome this serious problem. And most importantly, we will provide an overview of current approaches to elicit epitope/antigen spreading to address this element of CAR T cell therapy.
Antigen escape and its mechanisms
Antigen escape is frequently seen in post-CAR T therapy relapse
Antigen escape refers to the process in which tumour cells evade immune detection and destruction by downregulation or loss of the specific antigens that targeted therapies, such as CAR T therapy, are designed to recognize. This phenomenon allows tumour cells to survive and proliferate, even in the presence of sustained CAR T-mediated cytotoxicity and immune surveillance mechanisms. More and more data indicate that antigen escape is a major mechanism of resistance in patients who undergo CAR T therapy. Indeed, relapse due to antigen escape following CAR T cell treatment has been observed with multiple targets including CD19 [13], BCMA [14], CD22 [15], EGFRvIII [16], IL-13Rα2 [17, 18] and ErbB2 [19]. The rate of disease relapse due to antigen escape varies depending on the type of cancer and the target antigen. In pediatric r/r B-ALL patients treated with tisagenlecleucel, wherein sustained immune pressure was induced by prolonged persistence of the CAR T cells, 94% (15/16) of relapses analyzed were attributed to CD19– relapses, along with one patient CD19 + recurrence [2]. The other study in a real-world setting was reported on patients with r/r B-ALL infused with tisagenlecleucel, among relapses, 59% (30 out of 52) were CD19-positive and 41% (22 out of 52) CD19-negative (three associated with myeloid transformation) [20]. In large B cell lymphoma (LBCL), 10 of 16 LBCL patients with progressive disease after axi-cel treatment had converted from CD19 + pre-therapy to CD19−/low at relapse, exhibiting either complete loss of CD19 or diminished CD19 [21]. It should be pointed out that most previous studies have relied on antigen completely loss as a criterion, which inevitably leads to significant underestimation of incidence of antigen escape, due to a considerable number of patients exhibit low antigen relapse. It is well known that CAR T cells may have a threshold tumour antigen density below which they are unable to kill, so both loss and downregulation of antigen cannot elicit effective antitumour immunity by CAR T cytotoxicity [15]. In practice, antigen escape should include both loss and downregulation of antigen in order to better reflect the incidence of antigen escape. However, there is no uniform thresholds of activation based on antigen levels, which depends on the construction of individual CAR T and different types of targeting antigens [22–24]. It is worth noting that two independent studies evaluated resistance to CD19 CAR T cell therapy in NHL and its association with low levels or loss of CD19 at relapse, using the semiquantitative immunohistochemistry (IHC) to measure CD19 expression at baseline and at disease progression. All patients with paired pre-therapy and post-therapy showed complete loss or significant decrease in CD19 expression at relapse compared to tumour tissue before treatment [21, 25].
In general, the currently available data on anti-BCMA CAR T cells, have demonstrated that BCMA loss after treatment is not a common event, with BCMA expression remaining positive in the majority of patients [26, 27]. However, although some BCMA-positive MM cells still existed in these relapsed patients, the BCMA expression level and the BCMA antigen-binding capacity were strongly reduced. Decreased BCMA expression levels were observed by Cohen et al. in 12 out of 18 patients who received BCMA CAR T [28]. Recently, by integrating genomic and single-cell transcriptome profiling, Da Vià et al. reported for the first time that homozygous BCMA gene deletion led to a complete and irreversible loss of BCMA expression in a r/r MM [29]. Likewise, Samur et al. and Leblay et al. found a similar biallelic BCMA loss (mutation + deletion or deletion + deletion) as a resistance mechanism in other r/r MM patients who relapsed after ide-cel treatment [30]. Functionally, although these mutants retained surface BCMA expression, they escaped the binding and cytotoxicity of anti-BCMA CAR T [31].
Taken together, although the antigen escape after CAR T therapy as the unique cause of tumour relapse is not fully established, these findings underscore the importance of overcoming this kind of drug resistance by developing diversified immune responses to unleash the full potential of the immune system against cancer.
The mechanisms of antigen escape
The mechanisms of antigen escape are also multiple, including the selection of pre-existing antigen-negative clones, the spontaneous antigen loss due to stress [1, 32], acquired mutations [3, 13], splicing site variations [13], T-cell trogocytosis [33] and lineage switching from a lymphoid to a myeloid phenotype [34, 35], failure of surface antigen presentation [36, 37], and epitope masking, among others [38]. For instance, in B-ALL patients with antigen-negative escape after CAR T cell therapy, antigen escape has occurred as a result of CD19 alternative splicing that lack the exon recognized by the CAR or the transmembrane domain, and frameshift mutations leading to truncation or absence of the CD19 transmembrane region [13, 39]. Remarkably, the study demonstrated that anti-CD19 CAR T cell therapy exerted selective pressure on cells, and cells carrying these pre-existing alternatively spliced CD19 isoforms could evolve as a dominant clone, thereby affecting the efficacy of CAR T cell treatment [13]. Single-cell RNA sequencing also confirmed that CD19 − B-ALL cells can exist before CAR T cell therapy and are enriched by selection to be dominant within the tumour [32]. Unfortunately, technologies are not yet available to identify patients at risk for immune escape before therapy.
Strategies to overcome target downregulation
The expression density of the target antigen is an important factor that determines the function of engineered T cells since a minimum threshold of antigen expression is needed for preserved T cell activity [15, 40, 41]. CARs are highly reliant on target antigen density, therefore, CAR T cells lose their functionality when antigen expression drops below a threshold that depends on the type of the target and the CAR binding properties [22, 24, 41]. Given the fact that nearly all targets on solid tumours are heterogeneously expressed and the efficacy of CAR therapies require high antigen expression for significant activity, it has profound implications for the development of therapeutic CAR T for solid tumours.
Generally speaking, there are two strategies to overcome tumour recurrence caused by antigen downregulation in CAR T therapy. One is to increase the expression level of antigens on the target cells, and the other is to enhance the sensitivity of CAR T to recognize low antigen density.
Pharmacologic modulation to induce the expression of targeting antigens
BCMA is a protein expressed on the surface of plasma cells and is cleaved by the plasma cell membrane γ-secretase. γ-Secretase inhibitors abrogate enzymatic function and substantially increase BCMA surface density on plasma cells and reduce its soluble form, thereby improving the activity of anti-BCMA CAR T cells in preclinical models [42]. Recently, Cowan and colleagues combined γ-secretase inhibitors with anti-BCMA CAR T cells in a phase I study to increase BCMA antigen density before CAR T-cell infusion. The potential of this combination strategy provides encouraging preliminary results, with the overall response rate achieving 89% (16 of 18 participants), and the median duration of response achieving 14.4 months [43].
Similarly, bryostatin 1 has been shown to upregulate CD22 protein expressed on the cell surface and increases the efficacy of CD22-targeted CAR T cell therapy in preclinical models, although the mechanism of this CD22 upregulation remains to be elucidated [41].
Epigenetic modulators to induce the expression of targeting antigens
Epigenetic modifications, such as DNA methylation and histone alteration, can silence gene to reduce the expression levels or post-transcriptional modifications upon being targeted by CAR T to avoid immune surveillance [44]. Aberrant hypermethylation occurring in lymphoma patients and their cell lines, which is often located on CpG island within the promoter region of tumour suppressor gene, can lead to the silencing of the gene [45]. In fact, preclinical studies have found promoter hypermethylation in primary CLL cells when challenged with CD19 CAR, and verified an inverse correlation between CD19 promoter DNA methylation and its expression in the relapsed tumours. Importantly, the expression loss was partially reversible by treatment with a DNA methyltransferase (DNMT) inhibitor, azacytidine (AZA), validating the repressive character of methylation [46]. Similarly, rapid restoration of CD20 expression on CD20-negative lymphoma cells after using CD20-targeting rituximab was shown to occur within a few days after treatment with the epigenetic modulators, DNMT inhibitors and histone deacetylase (HDAC) inhibitors, and expression lasted more than 10 weeks in vitro [47].
GD2, a disialoganglioside, is highly expressed by a variety of embryonal cancers, including brain tumours, retinoblastoma, osteosarcoma and Ewing’s sarcoma, but it is barely expressed in normal cells [48], making it a target for CAR T therapy. Kailayangiri et al. established that EZH2 (a histone methyltransferase) inhibitors, GSK126 and tazemetostat, significantly upregulated GD2 surface expression in a majority of GD2 low or negative Ewing’s sarcoma cells, sensitizing the sarcoma cells to killing by GD2-specific CAR T cells [49]. Mechanically, EZH2 inhibitor enhances the expression of two key enzymes of GD2 biosynthesis via reducing H3K27 trimethylation [49]. Coincidentally, further independent clinical observations support the upregulation of genes encoding components of the ganglioside synthesis pathway in H3K27M mutant glioma cells, showing uniform and high expression of GD2 [50, 51].
On top of that, a potential benefit for the administration of epigenetic reprogramming is to maintain the functionality of CAR T cells. Epigenetic remodeling following CAR T infusion can counteract detrimental epigenetic and transcriptional changes that affect T-cell function, exhaustion, and tumour infiltration. In several recent studies, strategies such as using DNMT inhibitor and HDAC inhibitor have enabled CAR T to alleviate exhaustion and improve therapeutic efficacy [52–56].
Chimeric co-stimulatory receptor (CCR) sensitizes CAR T to low antigen expression
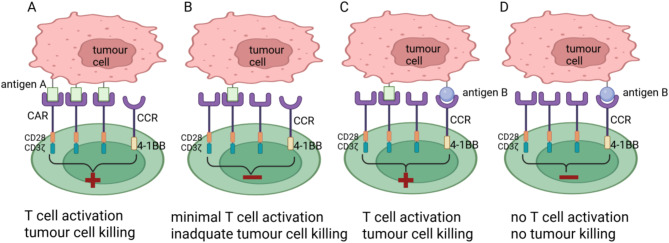
One successful approach involves expressing a fully functional second-generation CAR alongside a chimeric co-stimulatory receptor (CCR) containing CD28 or 4-1BB. With this combinatory approach, this CAR has demonstrated superior antitumour activity against low antigen variants by increasing the overall functional avidity compared to conventional second-generation CAR T cells alone [57, 58]. For instance, Katsarou et al. demonstrated that combining a fully functional 28ζ CAR targeting either BCMA or CD19 with a CCR binding to CD38, an antigen highly expressed in B-cell malignancies, can remarkably improve cytotoxicity of a CAR and better response to lower antigen expression [58]. It is worthy of note that T cells bearing the CD38-CCR alone did not induce cell lysis of CD38 expressing targets [58]. Together, these data support that the combination of a CAR and a CCR allows for multiplexed targeting, as well as co-stimulation, generating potent and durable effectors with enhanced in vivo eradication of antigen-low tumour clones, which were otherwise resistant to treatment with conventional CAR T cells, thus preventing relapses of tumour clones with low target antigen density(Figure 1). Mechanically, the coexpression of a CCR not only allows the engagement of the CCR with a second antigen to strengthen the synaptic tension between the CAR T cell and the tumour cell, but also enables the synergy of CD28 and 4-1BB co-stimulatory signaling as in the process of physiologic T cell activation [59, 60]. Therefore, CAR + CCR T cells improve cytokine and granzyme B production, cell proliferation, as well as impressive persistence and reduced expression of exhaustion related markers in vivo, compared to second-generation CAR T.
Fig. 1.
Combination of a CAR and a CCR sensitizes CAR T to low antigen expression. T cells coexpress a fully functional second-generation CAR specific for antigen A and a CCR specific for antigen B. A, CAR T cells are fully activated if the CAR engages with antigen A expressed at high levels on tumour cells. B, If tumours express antigen A at low levels, they are insufficient to activate second-generation CAR T cells. C, If tumours express antigen A at low levels, full T cell activation requires CCR engagement with antigen B. D, If tumours express antigen B only, they do not activate CAR T cells. Figure created with BioRender (biorender.com)
Optimizing the CAR construction
Refinement of CAR design can tune the threshold for antigen recognition and endow CARs with enhanced capacity to recognize antigen-low targets while retaining a superior capacity for persistence. A promising strategy to overcome this obstacle is the engineering of CAR T with improved co-stimulatory domains, which are crucial for T-cell proliferation, survival, and effector functions. Incorporating potent co-stimulatory domains, such as 4-1BB (CD137) or CD28, contributes to T-cell proliferation, antigen-specific cytokine secretion, and prolonged antitumour activity. For instance, it has been demonstrated that the CD19-CD28ζ CAR T cells confer enhanced efficacy against antigen-low targets, as CAR T cells are less susceptible to trogocytosis-mediated antigen downregulation and contribute to the formation of more stable immunologic synapses than CD19-4-1BBζ CAR T cells [22, 58]. Enhancing signal strength by including additional immunoreceptor tyrosine-based activation motifs (ITAM) of CD3ζ in the CAR enables enhancement of CAR activity against low antigen density cells, whereas ITAM deletions blunt signal and increase the antigen density threshold. Furthermore, the substitution of the CD8 hinge-transmembrane (H/T) region of a 4-1BBζ CAR with a CD28H/T lowers the threshold for CAR reactivity despite identical signaling molecules. Intriguingly, CARs incorporating a CD28-H/T show a more organized and stable synapse that is able to recruit both more CAR–ligand complexes and downstream ZAP70, resulting in superior antitumour activity [22]. To further improve the efficacy of CAR design, GPC2-CAR incorporating CD28TM and c-Jun overexpression enables potent and durable eradication of neuroblastoma with low-density target antigen [61, 62]. Notably, although CARs were designed to mimic T cell receptor (TCR) signaling, TCRs are at least 100-fold more sensitive to antigen [63]. The mechanism underlying this observation is that CAR T cells do not form highly organized immune synapses in contrast to those seen when the T cell receptor engages antigen [64]. To improve the quality of CAR synapse formation, a novel synthetic receptor has been developed by incorporating into the TCR-CD3 complex the same heavy and light chains of the CAR scFv. These receptors, known as HLA-independent TCRs (HIT) receptors, improved the signaling properties of CARs by incorporating native TCR elements, offering new opportunities for the targeting of low-density antigens [65, 66].
Strategies to overcome target loss
Dual-targeted and multi-targeted CAR T cells
The most intuitive strategy to address the risk of antigen loss is to empower T cells with two CARs targeting two different antigens, either by amalgamate two populations of distinct CAR Ts, each engaging a different tumour-associated antigen (pooled CAR T cells), or by using a single CAR T product with two specificities [19, 67, 68]. The latter can be carried out with two types of products: tandem (or bivalent) CARs that link two distinct antigen-binding domains on a single extracellular domain, and bicistronic CARs that are transduced using a single vector encoding two distinct CARs that are expressed in two separate extracellular receptors.
The most extensively investigate combinations, such as CD19/CD20 or CD19/CD22, which are both expressed in malignant B cells, have shown encouraging outcomes in patients with ALL and diffuse large B-cell lymphoma. Initial results from preliminary clinical trials using CD19/CD22 dual-targeted CAR Ts not only preserve bi-functionality against both CD19 and CD22, but also reduced the risk of relapse due to the loss of CD19 expression [21, 69]. Additionally, CD123/CLL-1 tandem CAR Ts demonstrate significantly increased cytotoxicity and cytokine release to AML cells, expressing both CD123 and CLL-1 antigens in comparison to single-target CAR T therapy [70]. Of interest, a tandem HER2/IL13Rα2 CAR demonstrated enhanced potency and antitumour activity in vivo when two CARs were expressed as a single molecule compared with expressing two separate CARs individually on each T cell or co-infusing two populations of cells, each expressing a monospecific receptor [71].
Although most studies have shown muti-targeting CAR could be an alternative way to lower the risk of antigen escape-mediated relapse and enhance therapeutic efficiency, a significant percentage of patients still relapse and die of progressive disease. So far, there is no solid evidence that dual targeting is significantly superior to single target. The possible reasons for the unconvincing advantage of dual targeting may be attributed to the wide intrinsic heterogeneity [72]. In the context of DLBCL, sequential loss of tumour antigens following sequential CAR immunotherapy has been observed [73]. These cases highlight the importance of tumour evolution driven by immune-mediated selective pressure, which results in the loss of antigen expression.
Furthermore, tri-specific CAR T cells, simultaneously targeting three antigens, have been studied in preclinical models of hematological malignancies and glioblastoma, both of which expand tumour antigen coverage and elicit robust immune synapse formation [74, 75]. Nevertheless, with the increase in the size of tri-specific CAR, manufacturing process needs to be optimized, and clinical results are still lacking so far.
The switchable CAR T cell system is another strategy to increase flexibility and expand antigen recognition. The CAR is divided into two distinct components that must interact via intermediary for activation. CAR T cells are directly link to a small intermediary, such as chemicals and peptides. Only when the separate antitumour antibody moiety coupled to this intermediary is present, the CAR T cells are activated. This strategy was designed for multi-antigen targeting with single CAR T cells using multiple adapters simultaneously, conferring CAR T cells with near-infinite antigen [76, 77]. More detail on how engineered T cell therapies may benefit from targeting more than one antigen is discussed in recent reviews [72, 78, 79].
Induce epitope/antigen spreading via cross presentation of endogenous tumour antigens
It has been increasingly recognized that developing diversified immune responses is critical to overcome the limitations of current immunotherapies. Of particular interest are professional antigen-presenting cells (APCs) that, while present in low numbers within tumours and lymphoid organs, play critical roles in initiating and regulating tumour-specific adaptive and innate immunity. APC has important established functions in antitumour response, wherein they can prime antitumour T cell responses through their TCR by presenting antigenic peptides on their cell surface via major histocompatibility complex-I (MHC-I) and MHC-II [80]. Several clinical trials have demonstrated that epitope spreading via T cell–APC crosstalk to elicit effective antitumour immune responses is essential to improving immunotherapy [81, 82].
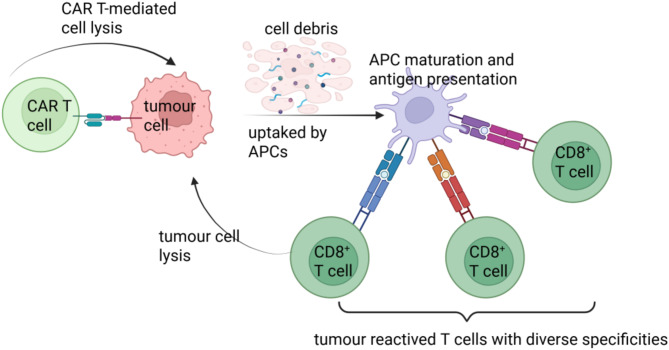
Epitope spreading was initially defined as the diversification of epitope specificity from the initial focused, dominant epitope-specific immune response to subdominant epitopes on the same protein [83, 84]. In this review, epitope spreading, extending this view, is a process in which the specificity of the immune response spreads beyond the initial CAR T targeting epitope. During this process, cancer debris generated by CAR T cytotoxicity is ingested and cross-presented by host APCs. Some mutations in tumour cells may generate neoantigens that are presented to endogenous naive CD8 + T lymphocytes in the context of HLA-I molecules and these neoantigens subsequently activate specific endogenous T-cell responses that were critical to a broader array of tumour antigens(Figure 2). Indeed, a syngeneic mouse model has shown that CAR T cells can induce long-term resistance even if the rechallenge tumour does not show expression of the antigen targeted by CAR T cells. This has demonstrated that by unleashing an endogenous immune response during CAR T therapy could promote antigen spreading to other tumour specific neoantigens, and thus reduce the potential for antigen escape. Recently, several combination strategies are underway to promote the interplay between CAR T cells and the host’s immune system, broadening epitope spreading following CAR T cell therapy [85].
Fig. 2.
CAR T elicits epitope/antigen spreading to immunity against tumour antigen heterogeneity. CAR T cells can recognize their cognate antigen on the surface of tumour cells and further cytolytic activity against cells expressing the target antigen. New antigens derived from dead tumour cells are engulfed by APCs such as macrophages or DCs, processed, and cross-presented to naive antigen-specific T cells, leading to the priming and expansion of polyclonal T cell responses with diverse specificities to initiate tumour cytotoxicity. Figure created with BioRender (biorender.com)
Combining CAR T cell therapy with immune checkpoint inhibitors (ICIs)
In addition to targeting tumours, CAR T cells also need to overcome the direct T cell inhibitory signals present in TME. While multiple inhibitory signals can be present in the TME, the best characterized pathway involves PD-1. PD-1 is an immune-checkpoint receptor expressed on activated T cells and, when bound by PD-L1, which can be expressed by tumour cells as well as other cell types, induces T cells to adopt an exhausted, ineffective phenotype. Recent studies have demonstrated that proper T cell priming is critical for the clinical efficacy of PD-1 blockade and that PD-1 blockade can unleash the inhibition of immune response and restore their ability to attack cancer cells. A recent study has revealed that PD-1 blockade leads to the expansion of novel tumour reactive T cell clones instead of pre-existing tumour-infiltrating T lymphocytes in patients with advanced skin cancer [86]. Similarly, in patients with advanced melanoma, non-small cell lung cancer or bladder cancer who received a personalized neoantigen vaccine concurrently with anti-PD-1 antibody, T cell responses against 33 of 330 nonvaccine neoepitopes across 25 patients were detected, indicating epitope spreading in a larger cohort of patients [87]. In a separate study in patients with melanoma, anti-PD-1 therapy broaden the spectrum of vaccine-induced neoantigen-specific T cell responses, as well as additional nonvaccine antigen-directed responses [88]. Of note, epitope spreading was associated with prolonged progression-free survival [87].
Given that treatment with anti-PD-1 antibody alone, the T cell response may undergo epitope spreading after initial priming by the original tumour antigens [89], it holds promise in combining CAR T cell immunotherapy with immune checkpoint inhibition as a means of both enhancing CAR T cells as well as endogenous T cell accumulation and activation, overall resulting in improved tumour control. Underpinning this strategy is the concept that in cancers where endogenous tumour-reactive T cells are sparse, infusing a bolus of tumour antigen-specific T cells would provide effectors that can respond more vigorously in vivo following the reduced inhibition and increased metabolic fitness afforded by checkpoint blockade. Numerous studies are underway to evaluate the combination of PD-1 pathway blockade and CAR T. Considerable evidence has demonstrated increased efficacy of CAR T cell therapy by epitope spreading with coadministration of PD-1 blockade in ALL [90] and diffuse large B cell lymphoma [91]. Furthermore, CAR T cells engineered to secrete PD-1 blocking single-chain variable fragments have been developed and investigated in clinical trials for the treatment of EGFR-positive solid malignancies [92, 93]. Alternatively, another approach involves using CRISPR/Cas9 to knock out genes responsible for immune checkpoint proteins or inhibitory receptors on CAR Ts, such as PD-1 and TGFBR2. By removing these inhibitory signals, CAR Ts can maintain their activity and continue targeting tumour cells effectively while antigen expression is reduced [94, 95]. Furthermore, CRISPR–Cas9 allows the insertion of CAR cassette into the PD-1 locus successfully by a two-in-one approach [25, 96]. Thus, the long-term persistence of functional CAR T cells, in conjunction with expansion and diversification of neoantigen-specific T cell clones, through immune-checkpoint blockade, may be very suitable to improve antitumour efficacy.
Combining CAR T cell therapy with cytokines
Cytokines can shape both T cells and the surrounding tumour microenvironment. They can be administered systemically or produced by the CAR T cells themselves, known as T cells redirected for universal cytokine-mediated killing (TRUCKs) or armoured CARs. Especially in the context of solid tumours, where T cell survival and trafficking are of immense importance, cytokines play an important role. Cytokines can enhance CAR T cell persistence by promoting survival and growth, and they also play a crucial role in enabling effective migration to tumours. CAR T cells may be engineered to produce several distinct cytokines that boost their persistence or favorably modulate the TME. In this section, we mainly discuss the importance of cytokines in enhancing the crosstalk between CAR T cells and host immune cells, and triggering nearby T cells to eliminate antigen-negative cancer cells at the target site.
Several different cytokines that are naturally not secreted by T cells have been introduced into CAR T cells, such as interleukin (IL)-12, which is canonically secreted by licensed dendritic cells (DCs). In the context of IL-12 armoured CAR T cell therapy, it was shown to improve the antitumour response by affecting ‘‘bystander’’ cells presented in the tumour microenvironment. IL-12-secreting CARs can induce accumulation of activated macrophages, as demonstrated in an immune competent mouse model, resulting in the ability to target antigen-negative tumour cells [97, 98].
IL-18 exerts several beneficial effects on both CAR T cells and the TME. IL-18 secretion by CAR T cells can also improve antitumour function with enhanced proliferation, IFN-γ production and a reduction in markers of exhaustion compared to IL-12 secreting CAR T cells [99, 100]. Furthermore, IL-18 secretion from CAR T cells engaged the local immune response in immunocompetent tumour-bearing mice by inducing an increase in M1- polarized macrophages (M1) and NK cells and a decrease in T regulatory cells (Tregs), suppressive DCs and M2-polarized macrophages (M2) within the TME [99, 101].
IL-36γ armoured CARs have also been shown to mediate enhanced antitumour activity by engaging endogenous immune responses. IL-36γ CAR T cells increase IL-6 production by DCs and IFN-γ and tumour necrosis factor (TNF)-α release by endogenous T cells, which could then eradicate a secondary challenge with antigen-negative tumour cells [102].
The coexpression of IL-7 with either CCL19 or CCL21 can enhance CAR T cell persistence and antitumour function [103, 104]. Previous studies have established that IL-7 supports the survival of naive T cells and selectively expands tumour-redirected cytotoxic T lymphocytes. In lymphoid organs IL-7 and CCL19 produced by T zone reticular cells are crucial for the formation and maintenance of T cell regions [105]. A recent study showed that engineered CAR T cells coexpress both IL-7 and CCL19 can enhance the antitumour potential of CAR T cells in coordination with activation of tumour-reactive recipient T cells and their memory formation. IL-7 and CCL19 (7 × 19) secretion promotes T cell and dendritic cell (DC) recruitment and tumour infiltration in an immunocompetent mouse model. Remarkably, mice treated with 7 × 19 CAR T cells could eradicate both antigen positive and antigen-negative tumour rechallenge, indicating the development of a robust memory response and epitope spreading [104, 106].
Tumour antigen spreading mediated by vaccine-boosted CAR T cells
The main rationale behind developing a vaccine approach combined with CAR T cells in cancer immunotherapy is the addition of vaccine can potentially expand and boost antitumour efficacy of CAR T cells, making it possible to achieve complete remission. One group used an RNA vaccine strategy combined with claudin 6 (CLDN6)-targeting CAR T cells [107]. They administered liposomal CLDN6-encoding RNA (CLDN6-LPX), which designed for body-wide delivery of the CLDN6 antigen into lymphoid compartments, stimulates adoptively transferred CLDN6 CAR T cells. In addition, it is likely that the same APCs concurrently process and present CLDN6 on MHC molecules, which may result in priming and activation of endogenous CLDN6-specific T cells. Collectively, presentation of the natively folded target on resident APCs promotes cognate and selective expansion of CAR T cells. In preclinical study, improved engraftment of CAR T cells and regression of large tumours in difficult-to-treat mouse models was achieved at subtherapeutic CAR T cell doses [107] (Figure 3). Building on these initial promising results, follow-up work has shed a positive light in patients administered these agents in a Phase I clinical trial, 7 of 21 treated patients with tumours that express CLDN6 achieved an objective response (ORR 33%), including one complete response. Particularly, patients with germ cell tumours treated at the higher dose level exhibited the highest response rate (ORR 57% (4 of 7)) [108].
Fig. 3.
Vaccine-boosted CAR T crosstalk with host immunity to reject tumours with antigen heterogeneity. DCs within lymph nodes internalize the vaccine and efficiently display it as a surface ligand for CAR T cells. CAR T cells interact with DCs via vaccine can enhance their metabolic profile, expansion, cytotoxicity, and increase IFNγ expression. IFNγ secreted by vaccine-boosted CAR T cells can promote macrophage and DC activation, in turn resulting in augmented tumour antigen uptake, increased intratumoural IL-12 production, priming of endogenous T cells, and elimination of antigenically heterogenous tumours. Adapted from Alizadeh and colleagues [109]. Figure created with BioRender (biorender.com)
Another novel strategy for vaccination involved administering a CAR T ligand with amphiphilic properties, called amph-ligand that, upon administration, efficiently trafficked to draining lymph nodes and decorated the surfaces of APCs with CAR T ligands, thereby priming CAR Ts in the native lymph node microenvironment. CAR T cells encountering ligand-decorated DCs in the lymph node receive stimulation through the CAR in tandem with native co-stimulatory receptor signals and cytokine stimulation from the ligand-APCs, leading to CAR T massive cell expansion and enhanced functionality in multiple immunocompetent mouse tumour models [110]. Interestingly, stimulation of CAR T cells by these APCs in lymph nodes enabled a proportion of complete responses even when the initial tumour was 50% CAR antigen negative. They further delineated how vaccine boosted CAR T cells can eradicate antigenically heterogenous tumours and induce antigen spreading [111]. Mechanistically, they demonstrated that vaccine-boosted CAR T cells exhibited an improved metabolic profile and polyfunctionality, which, in turn, amplified CAR T cell IFNγ production. Enhanced IFNγ secretion by vaccine-boosted CAR T cells promote recruitment of macrophage and DC expressing IL-12 to the tumour microenvironment and resulted in augmented solid tumour antigen uptake by DCs as well as priming of endogenous T cells to eliminate antigenically heterogenous solid tumours [111](Figure 3). In short, vaccine boosting CAR T cell promote crosstalk between CAR T cells and endogenous immunity to elicit and sustain antigen spreading, thereby effectively treating tumours with antigen heterogeneity.
Combining CAR T cell therapy with radiotherapy
The benefits of abscopal effect, the effect whereby radiotherapy at one site may lead to regression of distant metastatic cancer that has not been irradiated, is connected to mechanisms priming the immune system to target both local and distant tumour sites [112]. Mechanically, radiotherapy was originally used because of its ability to induce DNA damage, resulting in cell death via mitotic catastrophe, apoptosis, necrosis, and autophagy [113, 114]. Antigens from damaged immunogenic tumour cells can be taken up by APCs, which travel to the lymph node to cross-prime naïve T cells [115, 116]. This usually takes place in tumour-draining lymph nodes, where the T cells are primed, proliferate, and develop into T effector cells. Subsequently, activated T cells travel to both irradiated tumours and distant metastases, and eliciting an endogenous tumour‐specific cellular immune responses [112]. Moreover, radiation has been shown to upregulate the expression of MHC I molecules and tumour-associated antigens (TAAs) in a dose-dependent manner in a variety of different tumour types, rendering them better presentation of tumour antigens and more susceptible to immune cell attacks [117–119]. Importantly, irradiation can stimulate the secretion of various chemokines, such as CXCL10 and CXCL16, which can attract effector T cells into the tumour microenvironment [120]. Evidence is emerging that radiation can modulate all of the steps in driving T‐cell‐mediated immune responses, and has opened a new era in which radiation is being tested as an adjuvant to immunotherapy.
In accordance with this, radiation is confirmed working synergistically with CAR T cell therapy by increasing the release of tumour-specific antigens, thus improving tumour recognition by immune cells as well as increasing the sensitivity of tumour to the cytotoxic effects by CAR T cells. In addition, tumour cells exposed to low-dose radiation can be eliminated through TRAIL-mediated death by locally activated CAR T cells. In a model of pancreatic adenocarcinoma, with heterogeneously expressing sialyl Lewis-A (sLeA), it proved that not only sLeA + but also sLeA– tumour cells exposed to low-dose radiation become susceptible to CAR therapy, reducing antigen negative tumour relapse. They find that sLeA targeted CAR T cells produce TRAIL upon activated by engaging sLeA + tumour cells, and activated CAR T exerts a significant apoptotic effect on sLeA- tumour cells previously exposed to low-dose radiation in a TRAIL dependent manner irrespective of immunogenicity. This approach enhances the prospects for successfully applying CAR therapy to heterogeneous solid tumours [121]. Local delivery of radiation followed by CAR T cell infusion demonstrated superior attenuation of orthotopic glioblastoma tumour and pancreatic ductal adenocarcinoma growth compared to radiation or CAR T cell treatment alone. Moreover, the combination therapy induced an abscopal effect in a dual-flank tumour model [122–124]. Therefore, local or systemic radiation is integral to many tumours’ standard of care and can be easily implemented as a CAR T conditioning regimen [125]. In a phase II trial focusing on CAR T therapy for r/r DLBCL, patients who received radiotherapy before their CAR T cell treatment achieved a 100% response rate [126].
Combining CAR T cell therapy with oncolytic virotherapy (OVT)
OVT has its unique advantages and prospects, because oncolytic viruses (OVs) selectively replicate in and kill cancer cells either due to natural tumour tropism or genetic manipulation of the wild-type virus [127]. OVs can mediate antitumour effects through a variety of mechanisms, including direct tumour cell destruction and activation of the antitumour immune response through the release of TAAs, intracellular damage associated molecular patterns (DAMPs), viral pathogen-associated molecular patters (PAMPs), as well as release of stimulatory cytokines and chemokines to reverse the immunosuppressive TME [128]. This activation promotes the recruitment and infiltration of immune effector cells into the TME, turning immunologically “cold” tumours into “hot” tumours [129, 130]. Importantly, the destruction of tumour cells by OVs results in a debris field consisting of PAMPs and DAMPs to be taken up and cross-presented by APCs, contributing to an additional downstream recruitment and infiltration of additional TAA-specific CTLs, effectively eliciting bystander killing of uninfected tumour cells in TME.
However, to date, OVs monotherapies have exhibited only mild antitumour effects in patients. Recently, preclinical studies have demonstrated the promising potential of combining CAR T therapy with oncolytic viruses. This synergistic approach has resulted in enhanced tumour infiltration, persistence, and antitumour efficacy [131–134].
Park et al. engineered an oncolytic virus to deliver truncated CD19 to tumours, enabling targeting by CD19-CAR T cells, thereby passively sensitizing them to CD19 CAR T cells and triggering local immunity characterized by both endogenous and adoptively transferred T cells [135]. Evgin et al. further innovated this synergistic approach and developed the dual-specific CAR T that not only recognizes CAR specific antigens, but also has natural TCR reactivity against oncolytic vesicular stomatitis virus (VSV). These CAR T cells could be stimulated through their native TCR upon reactivity against viral or virally encoded VSV antigens. In a tumour model with EGFRvIII expression in 10% of tumour cells, it showed that the treatment promote the activation and proliferation of endogenous T cells, as well as epitope spreading to protect against CAR antigen-negative tumours [136]. Moreover, these viruses can be armed with several immunostimulatory molecules, such as chemokine ligand 5 (CCL5) and IL-12, to further tip the balance in favor of therapeutic efficacy [132, 137].
Boost DC at the tumour site by Flt3L or CD40
Whilst the above strategies emphasize the importance of engaging APCs in engendering host antitumour immunity, none of these approaches have investigated the potential for directly activating both migratory and resident DC, as a way to activate and recruit endogenous polyclonal T cells able to recognize non-CAR target antigens, such as neoantigens.
FMS-like tyrosine kinase 3 ligand (FLT3L), a DC growth factor, is associated with DC proliferation [138]. Binding of FLT3L to its receptor FLT3 induces DC expansion in circulation, thereby promoting their immune function and tumour-killing behavior. Beavis et al. designed a CAR T that engineered to secrete Flt3L, effectively driving the DC proliferation of intratumoural DCs in mice bearing colon adenocarcinoma and breast cancer. Once activated, the intratumoural DCs expand and stimulate endogenous tumour-infiltrating T cells. The activated polyclonal endogenous T cells are able to recognize a broad range of tumour-associated antigens to more effectively target heterogeneous tumours. Overall, this result suggests that increasing the number of endogenous DCs and CD8 + T cells is a promising strategy to overcome the challenge of antigen negative tumour escape following CAR T therapy [139].
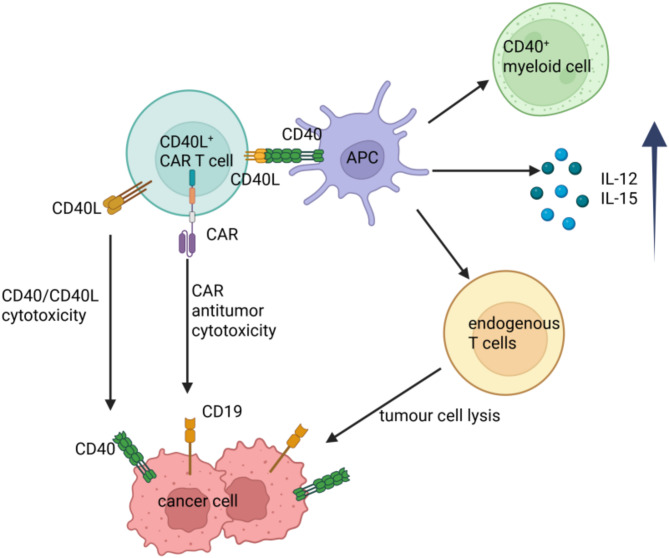
CD40, a member of the TNF receptor superfamily, is expressed on a variety of APCs, including DCs, and is essential for their activation and proliferation [140]. Human CAR T cells modified to constitutively express CD40L, an immune-stimulatory molecule CD40 ligand, are capable of activate neighboring CD40-expressing DCs in vivo, and enhanced recruitment of immune effectors, and mobilized endogenous tumour recognizing T cells, inducing an endogenous antitumour response. Accordingly, CD19-CD40L CAR T cell treatment protected mice from antigen-negative tumour outgrowth in both xenograft leukemia and lymphoma models. Additionally, CD40L + CAR T cells can directly lyse CD40 + tumour cells in a CAR-independent fashion, regardless of target antigen expression, through CD40/CD40L-mediated cytotoxicity on CD40-expressing tumour cells [141]. (Fig. 4)
Fig. 4.
CD40L-armoured CD19 CAR T cells enhance antitumour function by eliciting an endogenous antitumour response. CD40L + CAR T cells kill antigen-negative tumour cells through direct CD40/CD40L-mediated cytotoxicity. Furthermore, CD40L + CAR T cells license APCs prime non-CAR T cells to recognize tumour cells and produce cytokines to recruit and activate myeloid cells with enhanced antigen-presenting features, resulting in endogenous immune responses. Adapted from Kuhn and colleagues [141]. Figure created with BioRender (biorender.com)
Combining CAR T cell therapy with CAR-macrophages (CAR-M)
APC cells are essential for exerting the effect of epitope spreading, although originally thought to be an exclusive characteristic of DCs, recently it is now firmly established that various types of tissue-resident macrophages are able to cross-present via similar cellular pathways as DCs. Macrophages are also abundant within the TME and could be particularly well suited to trafficking to and surviving within it. Therefore, CAR-M is capable of better migrating to the tumour mass, directly phagocytose cancer cells, and naturally act as professional APCs, and further play a role in boosting the host antitumour immune.
One major concern in macrophage-based therapies is the high plasticity of macrophages. In response to external stimuli like cytokines, macrophages differentiate into antitumour proinflammatory M1 or immunosuppressive M2 phenotypes. M1 macrophages are equipped with antitumourigenic properties that can efficiently eliminate tumour cells through phagocytosis and cytotoxicity, while M2 macrophages on the other hand help to promote tumour angiogenesis, immunosuppression, growth, and metastasis [142]. Although M1 macrophages have demonstrated antitumour abilities, the majority of tumour-associated macrophages (TAMs) exhibit behavior characteristic of M2 macrophages, which promote tumour progression. Thus, reprogramming M2 TAMs into M1 TAMs may be an effective and promising strategy for macrophage-based immunotherapy. Remarkably, Klichinsky et al. utilized an adenoviral vector (Ad5f35) to transfer anti-HER2 CAR into human macrophages. Importantly, the authors observed that, regardless of CAR expression, adenovirally transduced macrophages induced a pro-inflammatory M1 phenotype in the infected macrophages, as well as induced a proinflammatory milieu, skewing the bystander myeloid cells towards a M1 phenotype, with upregulation of interferon-induced and antigen-presenting machinery genes [143].
In addition to the aforementioned benefits, CAR-M are able to remodel the extracellular matrix (ECM) by secreting a wide range of matrix metalloproteinases (MMPs) that are engaged in ECM and basement membrane degradation, which, in turn, increased intratumoural infiltration of tumour infiltrating cells, such as CAR-M, cytotoxic T cells (CTL), antitumoural neutrophils, and NK cells, leading to wide communication between macrophages and other immune components, promoting antigen spread and endogenous antitumour immunity [144, 145]. Distinctively, in recent preclinical studies, adopt CAR-M or CAR-iMAC, demonstrated that macrophages were able to cross-present tumour-derived antigens after phagocytosis, suggesting that CAR-Ms lead to epitope spreading [143, 146]. Collectively, CAR-Ms leverage the innate properties of macrophages with the specificity and potency of CAR technology, engaging the entire immune system aimed at eliminating tumour cells through multiple mechanisms.
Conclusion
CAR T cell therapy has emerged as one of the most rapidly advancing modalities in immunotherapy, and clinical success observed in hematological malignancies struggle to be extended to solid tumours, which issues of tumour heterogeneity and immune escape may be even more prevalent. Levels of antigen expression in tumour cells either decreased or were eliminated under selective pressure by CAR T cells, ultimately leading to tumour relapse. So, insufficient reactivity against cells with low antigen density has emerged as an important cause of CAR T-cell resistance.
With immune escape and regrowth of antigen negative tumour cells now being a well-documented mechanism of resistance to CAR T therapy, we suggest that curative strategies should aim at activating the endogenous antitumour response to reach durable response and allow for the eradication of antigen escape variants. Currently, multiple efforts are underway to enable CAR T cells to boost host immunity, therefore triggering antigen spreading and effectively preventing antigen-negative relapse. These approaches include armoured CARs to recruit endogenous T cells via the secretion of activating cytokines, optimization of CAR signaling to improve clinical efficacy, the checkpoint inhibitor to reinvigorate both CAR T cells and endogenous tumour-reactive T cells and activating DC to orchestrate a sustained endogenous antitumour response by mobilizing innate and adaptive members of the immune system(Table 1).
Table 1.
A summarized table about the aforementioned strategies
| Antigen escape | Strategies to overcome antigen escape | |
|---|---|---|
| target downregulation | 1. induce the expression of targeted antigen | |
| pharmacologic modulation | ||
| epigenetic modulators | ||
| 2. sensitize CAR T | ||
| chimeric co-stimulatory receptor (CCR) | ||
| optimization of the CAR design | ||
| target loss | 1. dual-targeted and multi-targeted CAR T cells | |
| pooled CAR T cells, tandem/bivalent CARs, bicistronic CARs | ||
| tri-specific CAR T cell | ||
| switchable CAR T cell | ||
| 2. induce epitope/antigen spreading via cross presentation of endogenous tumour antigens | ||
|
(1) engage APC in engendering host antitumour immunity |
immune checkpoint inhibitors (ICI) | |
| cytokines | ||
| vaccine-boosted CAR T cells | ||
| radiotherapy | ||
| oncolytic virotherapy | ||
| (2) directly activate DC | boost DC at the tumour site by Flt3L or CD40 | |
| (3) activate macrophage | CAR-macrophages | |
In summary, this review suggest that CAR T cells can serve not only as direct antitumour effector cells, but also as cellular vectors to deliver immune-regulatory molecules to the tumour microenvironment. The ability to stimulate broad, durable, and adaptable immune responses toward multiple tumour antigens by recruiting and activating the endogenous T cell offers a practicable solution to the limitations of targeting tumour antigen heterogeneity in solid tumours with monospecific CAR T cell therapies.
Acknowledgements
Supported by grants from Beijing Natural Science Foundation (grant number: 7252005 to FZ).
Abbreviations
- CAR
Chimeric antigen receptor
- r/r
Relapsed/refractory
- BCMA
B cell maturation antigen
- CR
Complete remission
- MM
Multiple myeloma
- TME
Tumour microenvironment
- LBCL
Large B cell lymphoma
- IHC
Immunohistochemistry
- DNMT
DNA methyltransferase
- AZA
Azacytidine
- HDAC
Histone deacetylase
- CCR
Chimeric co-stimulatory receptor
- ITAM
Immunoreceptor tyrosine-based activation motifs
- H/T
Hinge-transmembrane
- TCR
T cell receptor
- APCs
Antigen-presenting cells
- MHC-I
Major histocompatibility complex-I
- ICIs
Immune checkpoint inhibitors
- TRUCKs
T cells redirected for universal cytokine-mediated killing
- IL
Interleukin
- DCs
Dendritic cells
- M1
M1- polarized macrophages
- M2
M2-polarized macrophages
- TNF
Tumour necrosis factor
- CLDN6
Claudin 6
- CLDN6-LPX
CLDN6-encoding RNA
- ORR
Objective response rate
- TAAs
Tumour-associated antigens
- sLeA
Sialyl Lewis-A
- OVT
Oncolytic virotherapy
- DAMPs
Damage associated molecular patterns
- PAMPs
Pathogen-associated molecular patters
- VSV
Vesicular stomatitis virus
- CCL5
Chemokine ligand 5
- FLT3L
FMS-like tyrosine kinase 3 ligand
- CAR-M
CAR-macrophages
- TAMs
Tumour-associated macrophages
- ECM
Extracellular matrix
- MMPs
Matrix metalloproteinases
- CTL
Cytotoxic T cells
Author contributions
YL wrote the paper; FZ edited the paper; YL made the fgures; FZ made final edits. All authors read and approved the final manuscript.
Funding
This work was supported by Beijing Natural Science Foundation (grant number: 7252005 to FZ).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA. Grupp, tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, Levine JE, Qayed M, Grupp SA, Boyer M, De Moerloose B, Nemecek ER, Bittencourt H, Hiramatsu H, Buechner J, Davies SM, Verneris MR, Nguyen K, Brogdon JL, Bitter H, Morrissey M, Pierog P, Pantano S, Engelman JA, Winckler W. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–6. [DOI] [PubMed] [Google Scholar]
- 4.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-Term Follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene Ciloleucel CAR T-Cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G. Maziarz, tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 9.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, Sehgal A, Solomon SR, Ghosh N, Albertson TM, Garcia J, Kostic A, Mallaney M, Ogasawara K, Newhall K, Kim Y, Li D, Siddiqi T. Lisocabtagene Maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. [DOI] [PubMed] [Google Scholar]
- 10.Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M, Lesokhin A, Deol A, Munshi NC, O’Donnell E, Avigan D, Singh I, Zudaire E, Yeh TM, Allred AJ, Olyslager Y, Banerjee A, Jackson CC, Goldberg JD, Schecter JM, Deraedt W, Zhuang SH, Infante J, Geng D, Wu X, Carrasco-Alfonso MJ, Akram M, Hossain F, Rizvi S, Fan F, Lin Y, Martin T, Jagannath S. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–24. [DOI] [PubMed] [Google Scholar]
- 11.Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, Raje N, Lin Y, Siegel D, Oriol A, Moreau P, Yakoub-Agha I, Delforge M, Cavo M, Einsele H, Goldschmidt H, Weisel K, Rambaldi A, Reece D, Petrocca F, Massaro M, Connarn JN, Kaiser S, Patel P, Huang L, Campbell TB, Hege K. San-Miguel, Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16. [DOI] [PubMed] [Google Scholar]
- 12.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, Martinez NM, Harrington CT, Chung EY, Perazzelli J, Hofmann TJ, Maude SL, Raman P, Barrera A, Gill S, Lacey SF, Melenhorst JJ, Allman D, Jacoby E, Fry T, Mackall C, Barash Y, Lynch KW, Maris JM, Grupp SA. Thomas-Tikhonenko, convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, Kanakry JA, Ali SA, Mikkilineni L, Feldman SA, Stroncek DF, Hansen BG, Lawrence J, Patel R, Hakim F, Gress RE, Kochenderfer JN. T cells genetically modified to express an Anti-B-Cell maturation antigen chimeric antigen receptor cause remissions of Poor-Prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL. CD22-targeted CAR T cells induce remission in B-ALL that is Naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, Isaacs R, Mohan S, Plesa G, Lacey SF, Navenot JM, Zheng Z, Levine BL, Okada H, June CH, Brogdon JL, Maus MV. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9(399) (2017). [DOI] [PMC free article] [PubMed]
- 17.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of glioblastoma after chimeric antigen receptor T-Cell therapy. N Engl J Med. 2016;375(26):2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu H, Dotti G, Balyasnikova IV, Gottschalk S. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol Res. 2017;5(7):571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, Byrd TT, Krebs S, Wu MF, Liu H, Heslop HE, Gottschalk S, Yvon E, Ahmed N. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz LM, Eaton A, Baggott C, Rossoff J, Prabhu S, Keating AK, Krupski C, Pacenta H, Philips CL, Talano JA, Moskop A, Baumeister SHC, Myers GD, Karras NA, Brown PA, Qayed M, Hermiston M, Satwani P, Wilcox R, Rabik CA, Fabrizio VA, Chinnabhandar V, Kunicki M, Mavroukakis S, Egeler E, Li Y, Mackall CL, Curran KJ, Verneris MR, Laetsch TW, Stefanski H. Outcomes after nonresponse and relapse Post-Tisagenlecleucel in children, adolescents, and young adults with B-Cell acute lymphoblastic leukemia. J Clin Oncol. 2023;41(2):354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, Frank MJ, Shiraz P, Sahaf B, Craig J, Iglesias M, Younes S, Natkunam Y, Ozawa MG, Yang E, Tamaresis J, Chinnasamy H, Ehlinger Z, Reynolds W, Lynn R, Rotiroti MC, Gkitsas N, Arai S, Johnston L, Lowsky R, Majzner RG, Meyer E, Negrin RS, Rezvani AR, Sidana S, Shizuru J, Weng WK, Mullins C, Jacob A, Kirsch I, Bazzano M, Zhou J, Mackay S, Bornheimer SJ, Schultz L, Ramakrishna S, Davis KL, Kong KA, Shah NN, Qin H, Fry T, Feldman S, Mackall CL, Miklos DB. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majzner RG, Rietberg SP, Sotillo E, Dong R, Vachharajani VT, Labanieh L, Myklebust JH, Kadapakkam M, Weber EW, Tousley AM, Richards RM, Heitzeneder S, Nguyen SM, Wiebking V, Theruvath J, Lynn RC, Xu P, Dunn AR, Vale RD. Mackall, tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 2020;10(5):702–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fioretti S, Matson CA, Rosenberg KM, Singh NJ. Host B cells escape CAR-T immunotherapy by reversible downregulation of CD19. Cancer Immunol Immunother. 2023;72(1):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, Sakemura R, Goto T, Hanajiri R, Imahashi N, Shimada K, Tomita A, Kiyoi H, Nishida T, Naoe T, Murata M. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 Ζ chimeric antigen receptor-modified effector CD8 + T cells. J Immunol. 2015;194(3):911–20. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, Zhang L, Wei G, Tian Y, Zhao K, Chen A, Tan B, Cui J, Li D, Li Y, Qi Y, Wang D, Wu Y, Li D, Du B, Liu M, Huang H. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609(7926):369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34(4):985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Rasche L, Kortüm KM, Mersi J, Einsele H. BCMA loss in the epoch of novel immunotherapy for multiple myeloma: from biology to clinical practice. Haematologica. 2023;108(4):958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K, Nelson A, Plesa G, Chen F, Davis MM, Hwang WT, Young RM, Brogdon JL, Isaacs R, Pruteanu-Malinici I, Siegel DL, Levine BL, June CH, Milone MC. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Da MC, Vià O, Dietrich M, Truger P, Arampatzi J, Duell A, Heidemeier X, Zhou S, Danhof S, Kraus M, Chatterjee M, Meggendorfer S, Twardziok ME, Goebeler MS, Topp M, Hudecek S, Prommersberger K, Hege S, Kaiser V, Fuhr N, Weinhold A, Rosenwald F, Erhard C, Haferlach H, Einsele KM, Kortüm AE, Saliba L, Rasche. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat Med. 2021;27(4):616–9. [DOI] [PubMed] [Google Scholar]
- 30.Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai YT, Prabhala R, Alonso A, Sperling AS, Campbell T, Petrocca F, Hege K, Kaiser S, Loiseau HA, Anderson KC, Munshi NC. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun. 2021;12(1):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Ahn S, Maity R, Leblay N, Ziccheddu B, Truger M, Chojnacka M, Cirrincione A, Durante M, Tilmont R, Barakat E, Poorebrahim M, Sinha S, McIntyre J, A MYC, Wilson H, Kyman S, Krishnan A, Landgren O, Walter W, Meggendorfer M, Haferlach C, Haferlach T, Einsele H, Kortüm MK, Knop S, Alberge JB, Rosenwald A, Keats JJ, Rasche L, Maura F, Neri P, Bahlis NJ. Mechanisms of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in multiple myeloma. Nat Med. 2023;29(9):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabilloud T, Potier D, Pankaew S, Nozais M, Loosveld M, Payet-Bornet D. Single-cell profiling identifies pre-existing CD19-negative subclones in a B-ALL patient with CD19-negative relapse after CAR-T therapy. Nat Commun. 2021;12(1):865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, Eyquem J, Zhao Z, Whitlock BM, Miele MM, Li Z, Cunanan KM, Huse M, Hendrickson RC, Wang X, Rivière I, Sadelain M. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamble AJ, Myers RM, Taraseviciute A, John S, Yates B, Steinberg SM, Sheppard J, Kovach AE, Wood B, Borowitz MJ, Stetler-Stevenson M, Yuan CM, Pillai V, Foley T, Chung P, Chen L, Lee DW, Annesley C, DiNofia A, Grupp SA, Verneris MR, Gore L, Laetsch TW, Bhojwani D, Brown PA, Pulsipher MA, Rheingold SR, Gardner RA, Shah NN. Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Adv. 2023;7(4):575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moskop A, Pommert L, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, Stefanski HE, Talano JA, Margossian SP, Verneris MR, Myers GD, Karras NA, Brown PA, Qayed M, Hermiston ML, Satwani P, Krupski C, Keating AK, Wilcox R, Rabik CA, Fabrizio VA, Chinnabhandar V, Goksenin AY, Curran KJ, Mackall CL, Laetsch TW, Guest EM, Breese EH, Schultz LM. Real-world use of tisagenlecleucel in infant acute lymphoblastic leukemia. Blood Adv. 2022;6(14):4251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P, Bumm T, Böttcher S, Bargou RC, Binder M. Resistance to anti-CD19/CD3 bite in acute lymphoblastic leukemia May be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129(1):100–4. [DOI] [PubMed] [Google Scholar]
- 37.Heard A, Landmann JH, Hansen AR, Papadopolou A, Hsu YS, Selli ME, Warrington JM, Lattin J, Chang J, Ha H, Haug-Kroeper M, Doray B, Gill S, Ruella M, Hayer KE, Weitzman MD, Green AM, Fluhrer R, Singh N. Antigen glycosylation regulates efficacy of CAR T cells targeting CD19. Nat Commun. 2022;13(1):3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, Klichinsky M, Shestova O, Patel PR, Kulikovskaya I, Nazimuddin F, Bhoj VG, Orlando EJ, Fry TJ, Bitter H, Maude SL, Levine BL, Nobles CL, Bushman FD, Young RM, Scholler J, Gill SI, June CH, Grupp SA, Lacey SF, Melenhorst JJ. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer J, Paret C, El Malki K, Alt F, Wingerter A, Neu MA, Kron B, Russo A, Lehmann N, Roth L, Fehr EM, Attig S, Hohberger A, Kindler T, Faber J. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, Lopomo P, Vigny M, Fry TJ, Orentas RJ, Mackall CL. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol Ther. 2017;25(9):2189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramakrishna S, Highfill SL, Walsh Z, Nguyen SM, Lei H, Shern JF, Qin H, Kraft IL, Stetler-Stevenson M, Yuan CM, Hwang JD, Feng Y, Zhu Z, Dimitrov D, Shah NN, Fry TJ. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin Cancer Res. 2019;25(17):5329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, Hudecek M, Comstock ML, Rajan A, Patel BKR, Voutsinas JM, Wu Q, Liu L, Cowan AJ, Wood BL, Green DJ. Riddell, γ-Secretase Inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19):1585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowan AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby EN 3rd, Coffey DG, Tuazon SA, Wood B, Gooley T, Wu VQ, Voutsinas J, Song X, Shadman M, Gauthier J, Chapuis AG, Milano F, Maloney DG, Riddell SR. Green, γ-Secretase inhibitor in combination with BCMA chimeric antigen receptor T-cell immunotherapy for individuals with relapsed or refractory multiple myeloma: a phase 1, first-in-human trial. Lancet Oncol. 2023;24(7):811–22. [DOI] [PMC free article] [PubMed]
- 44.Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19(11):776–800. [DOI] [PubMed] [Google Scholar]
- 45.Esteller M. Epigenetic gene Silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16(Spec 1):R50–9. [DOI] [PubMed] [Google Scholar]
- 46.Ledererova A, Dostalova L, Kozlova V, Peschelova H, Ladungova A, Culen M, Loja T, Verner J, Pospisilova S, Smida M, Mancikova V. Hypermethylation of CD19 promoter enables antigen-negative escape to CART-19 in vivo and in vitro. J Immunother Cancer 9(8) (2021). [DOI] [PMC free article] [PubMed]
- 47.Hiraga J, Tomita A, Suzuki N, Takagi Y, Narita M, Kagami Y. Partial restoration of CD20 protein expression and rituximab sensitivity after treatment with Azacitidine in CD20-negative transformed diffuse large B cell lymphoma after using rituximab. Ann Hematol. 2018;97(11):2253–5. [DOI] [PubMed] [Google Scholar]
- 48.Sait S, Modak S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev Anticancer Ther. 2017;17(10):889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kailayangiri S, Altvater B, Lesch S, Balbach S, Göttlich C, Kühnemundt J, Mikesch JH, Schelhaas S, Jamitzky S, Meltzer J, Farwick N, Greune L, Fluegge M, Kerl K, Lode HN, Siebert N, Müller I, Walles H, Hartmann W, Rossig C. EZH2 Inhibition in ewing sarcoma upregulates G(D2) expression for targeting with Gene-Modified T cells. Mol Ther. 2019;27(5):933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, Labanieh L, Hulleman E, Woo PJ, Rietberg SP, Vogel H, Monje M, Mackall CL. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018;24(5):572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monje M, Mahdi J, Majzner R, Yeom KW, Schultz LM, Richards RM, Barsan V, Song KW, Kamens J, Baggott C, Kunicki M, Rietberg SP, Lim AS, Reschke A, Mavroukakis S, Egeler E, Moon J, Patel S, Chinnasamy H, Erickson C, Jacobs A, Duh AK, Tunuguntla R, Klysz DD, Fowler C, Green S, Beebe B, Carr C, Fujimoto M, Brown AK, Petersen AG, McIntyre C, Siddiqui A, Lepori-Bui N, Villar K, Pham K, Bove R, Musa E, Reynolds WD, Kuo A, Prabhu S, Rasmussen L, Cornell TT, Partap S, Fisher PG, Campen CJ, Grant G, Prolo L, Ye X, Sahaf B, Davis KL, Feldman SA, Ramakrishna S, Mackall C. Intravenous and intracranial GD2-CAR T cells for H3K27M(+) diffuse midline gliomas. Nature. 2025;637(8046):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Tong C, Dai H, Wu Z, Han X, Guo Y, Chen D, Wei J, Ti D, Liu Z, Mei Q, Li X, Dong L, Nie J, Zhang Y, Han W. Low-dose decitabine priming endows CAR T cells with enhanced and persistent antitumour potential via epigenetic reprogramming. Nat Commun. 2021;12(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.López-Cobo S, Fuentealba JR, Gueguen P, Bonté PE, Tsalkitzi K, Chacón I, Glauzy S, Bohineust A, Biquand A, Silva L, Gouveia Z, Goudot C, Perez F, Saitakis M, Amigorena S. SUV39H1 ablation enhances Long-term CAR T function in solid tumors. Cancer Discov. 2024;14(1):120–41. [DOI] [PubMed] [Google Scholar]
- 54.Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, Nguyen P, Houke H, Bell M, Haydar D, Brown C, Boi SK, Alli S, Crawford JC, Riberdy JM, Park JJ, Zhou S, Velasquez MP, DeRenzo C, Lazzarotto CR, Tsai SQ, Vogel P, Pruett-Miller SM, Langfitt DM, Gottschalk S, Youngblood B, Krenciute G. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021;13(620):eabh0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jain N, Zhao Z, Koche RP, Antelope C, Gozlan Y, Montalbano A, Brocks D, Lopez M, Dobrin A, Shi Y, Gunset G, Giavridis T, Sadelain M. Disruption of SUV39H1-Mediated H3K9 methylation sustains CAR T-cell function. Cancer Discov. 2024;14(1):142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porazzi P, Nason S, Yang Z, Carturan A, Ghilardi G, Guruprasad P, Patel RP, Tan M, Padmanabhan AA, Lemoine J, Fardella E, Zhang Y, Pajarillo R, Chen L, Ugwuanyi O, Markowitz K, Delman D, Angelos MG, Shestova O, Isshiki Y, Blanchard T, Béguelin W, Melnick AM, Linette GP, Beatty GL, Carreno BM, Cohen IJ, Paruzzo L, Schuster SJ, Ruella M. EZH1/EZH2 Inhibition enhances adoptive T cell immunotherapy against multiple cancer models. Cancer Cell. 2025;43(3):537–e5517. [DOI] [PubMed] [Google Scholar]
- 57.Hirabayashi K, Du H, Xu Y, Shou P, Zhou X, Fucá G, Landoni E, Sun C, Chen Y, Savoldo B, Dotti G. Dual targeting CAR-T cells with optimal costimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat Cancer. 2021;2(9):904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katsarou A, Sjöstrand M, Naik J, Mansilla-Soto J, Kefala D, Kladis G, Nianias A, Ruiter R, Poels R, Sarkar I, Patankar YR, Merino E, Reijmers RM, Frerichs KA, Yuan H, de Bruijn J, Stroopinsky D, Avigan D, van de Donk N, Zweegman S, Mutis T, Sadelain M, Groen RWJ, Themeli M. Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence, Sci Transl Med. 2021;13(623):eabh1962. [DOI] [PMC free article] [PubMed]
- 59.Melero I, Bach N, Hellström KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28(3):1116–21. [DOI] [PubMed] [Google Scholar]
- 60.Rudolf D, Silberzahn T, Walter S, Maurer D, Engelhard J, Wernet D, Bühring HJ, Jung G, Kwon BS, Rammensee HG, Stevanović S. Potent costimulation of human CD8 T cells by anti-4-1BB and anti-CD28 on synthetic artificial antigen presenting cells. Cancer Immunol Immunother. 2008;57(2):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, Anbunathan H, Lattin J, Jones R, Tieu V, Nagaraja S, Granja J, de Bourcy CFA, Majzner R, Satpathy AT, Quake SR, Monje M, Chang HY. Mackall, c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576(7786):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heitzeneder S, Bosse KR, Zhu Z, Zhelev D, Majzner RG, Radosevich MT, Dhingra S, Sotillo E, Buongervino S, Pascual-Pasto G, Garrigan E, Xu P, Huang J, Salzer B, Delaidelli A, Raman S, Cui H, Martinez B, Bornheimer SJ, Sahaf B, Alag A, Fetahu IS, Hasselblatt M, Parker KR, Anbunathan H, Hwang J, Huang M, Sakamoto K, Lacayo NJ, Klysz DD, Theruvath J, Vilches-Moure JG, Satpathy AT, Chang HY, Lehner M, Taschner-Mandl S, Julien JP, Sorensen PH, Dimitrov DS, Maris JM, Mackall CL. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell. 2022;40(1):53–e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salter AI, Rajan A, Kennedy JJ, Ivey RG, Shelby SA, Leung I, Templeton ML, Muhunthan V, Voillet V, Sommermeyer D, Whiteaker JR, Gottardo R, Veatch SL, Paulovich AG, Riddell SR. Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function. Sci Signal 14(697) (2021). [DOI] [PMC free article] [PubMed]
- 64.Correction for et al. Davenport Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity, Proc Natl Acad Sci U S A. 2019;116(22):11075–11076. [DOI] [PMC free article] [PubMed]
- 65.Mansilla-Soto J, Eyquem J, Haubner S, Hamieh M, Feucht J, Paillon N, Zucchetti AE, Li Z, Sjöstrand M, Lindenbergh PL, Saetersmoen M, Dobrin A, Maurin M, Iyer A, Garcia Angus A, Miele MM, Zhao Z, Giavridis T, van der Stegen SJC, Tamzalit F, Rivière I, Huse M, Hendrickson RC, Hivroz C, Sadelain M. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat Med. 2022;28(2):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang D, Li Y, Rui W, Sun K, Zhou Z, Lv X, Yu L, Chen J, Zhou J, Liu V, Wang J, Lan X, Fu YX, Zhao X, Lin X. TCR-mimicking STAR conveys superior sensitivity over CAR in targeting tumors with low-density neoantigens. Cell Rep. 2024;43(11):114949. [DOI] [PubMed] [Google Scholar]
- 67.Fernández de Larrea C, Staehr M, Lopez AV, Ng KY, Chen Y, Godfrey WD, Purdon TJ, Ponomarev V, Wendel HG, Brentjens RJ, Smith EL. Defining an optimal Dual-Targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA Escape-Driven relapse in multiple myeloma. Blood Cancer Discov. 2020;1(2):146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider D, Xiong Y, Wu D, Nӧlle V, Schmitz S, Haso W, Kaiser A, Dropulic B, Orentas RJ. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 2017;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei G, Zhang Y, Zhao H, Wang Y, Liu Y, Liang B, Wang X, Xu H, Cui J, Wu W, Zhao K, Nagler A, Chang AH, Hu Y, Huang H. CD19/CD22 Dual-Targeted CAR T-cell therapy for relapsed/refractory aggressive B-cell lymphoma: A safety and efficacy study. Cancer Immunol Res. 2021;9(9):1061–70. [DOI] [PubMed] [Google Scholar]
- 70.Wang XY, Bian MR, Lin GQ, Yu L, Zhang YM, Wu DP. Tandem bispecific CD123/CLL-1 CAR-T cells exhibit specific cytolytic effector functions against human acute myeloid leukaemia. Eur J Haematol. 2024;112(1):83–93. [DOI] [PubMed] [Google Scholar]
- 71.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, Brawley VS, Byrd TT, Krebs S, Gottschalk S, Wels WS, Baker ML, Dotti G, Mamonkin M, Brenner MK, Orange JS, Ahmed N. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brillembourg H, Martínez-Cibrián N, Bachiller M, Alserawan L, Ortiz-Maldonado V, Guedan S, Delgado J. The role of chimeric antigen receptor T cells targeting more than one antigen in the treatment of B-cell malignancies. Br J Haematol. 2024;204(5):1649–59. [DOI] [PubMed] [Google Scholar]
- 73.Shalabi H, Kraft IL, Wang HW, Yuan CM, Yates B, Delbrook C, Zimbelman JD, Giller R, Stetler-Stevenson M, Jaffe ES, Lee DW, Shern JF, Fry TJ, Shah NN. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103(5):e215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, Joseph SK, Hegde M, Ahmed N. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20(4):506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider D, Xiong Y, Wu D, Hu P, Alabanza L, Steimle B, Mahmud H, Anthony-Gonda K, Krueger W, Zhu Z, Dimitrov DS, Orentas RJ, Dropulić B. Trispecific CD19-CD20-CD22-targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci Transl Med 13(586) (2021). [DOI] [PubMed]
- 76.Lohmueller JJ, Ham JD, Kvorjak M, Finn OJ. mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. Oncoimmunology. 2017;7(1):e1368604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173(6):1426–e143811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao J, Lin Q, Song Y, Liu D. Universal cars, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schlegel LS, Werbrouck C, Boettcher M, Schlegel P. Universal CAR 2.0 to overcome current limitations in CAR therapy. Front Immunol. 2024;15:1383894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. [DOI] [PubMed] [Google Scholar]
- 81.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, Carrum G, Ramos C, Fayad L, Shpall EJ, Pro B, Liu H, Wu MF, Lee D, Sheehan AM, Zu Y, Gee AP, Brenner MK, Heslop HE, Rooney CM. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chapuis AG, Roberts IM, Thompson JA, Margolin KA, Bhatia S, Lee SM, Sloan HL, Lai IP, Farrar EA, Wagener F, Shibuya KC, Cao J, Wolchok JD, Greenberg PD, Yee C. T-Cell therapy using Interleukin-21-Primed cytotoxic T-Cell lymphocytes combined with cytotoxic T-Cell lymphocyte Antigen-4 Blockade results in Long-Term cell persistence and durable tumor regression. J Clin Oncol. 2016;34(31):3787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14(5):203–8. [DOI] [PubMed] [Google Scholar]
- 84.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358(6382):155–7. [DOI] [PubMed] [Google Scholar]
- 85.Sampson JH, Choi BD, Sanchez-Perez L, Suryadevara CM, Snyder DJ, Flores CT, Schmittling RJ, Nair SK, Reap EA, Norberg PK, Herndon JE 2nd, Kuan CT, Morgan RA, Rosenberg SA, Johnson LA. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014;20(4):972– 84. [DOI] [PMC free article] [PubMed]
- 86.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, Gupta RK, Curtis C, Bucktrout SL, Davis MM, Chang ALS, Chang HY. Clonal replacement of tumor-specific T cells following PD-1 Blockade. Nat Med. 2019;25(8):1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD, Lin JJ, Friedlander T, Bushway ME, Balogh KN, Sciuto TE, Kohler V, Turnbull SJ, Besada R, Curran RR, Trapp B, Scherer J, Poran A, Harjanto D, Barthelme D, Ting YS, Dong JZ, Ware Y, Huang Y, Huang Z, Wanamaker A, Cleary LD, Moles MA, Manson K, Greshock J, Khondker ZS, Fritsch E, Rooney MS, DeMario M, Gaynor RB, Srinivasan L. A phase Ib trial of personalized neoantigen therapy plus Anti-PD-1 in patients with advanced melanoma, Non-small cell lung cancer, or bladder Cancer. Cell. 2020;183(2):347–e36224. [DOI] [PubMed] [Google Scholar]
- 88.Hu Z, Leet DE, Allesøe RL, Oliveira G, Li S, Luoma AM, Liu J, Forman J, Huang T, Iorgulescu JB, Holden R, Sarkizova S, Gohil SH, Redd RA, Sun J, Elagina L, Giobbie-Hurder A, Zhang W, Peter L, Ciantra Z, Rodig S, Olive O, Shetty K, Pyrdol J, Uduman M, Lee PC, Bachireddy P, Buchbinder EI, Yoon CH, Neuberg D, Pentelute BL, Hacohen N, Livak KJ, Shukla SA, Olsen LR, Barouch DH, Wucherpfennig KW, Fritsch EF, Keskin DB, Wu CJ, Ott PA. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat Med. 2021;27(3):515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lo JA, Kawakubo M, Juneja VR, Su MY, Erlich TH, LaFleur MW, Kemeny LV, Rashid M, Malehmir M, Rabi SA, Raghavan R, Allouche J, Kasumova G, Frederick DT, Pauken KE, Weng QY, da Pereira M, Xu AAJ, van der Sande W, Silkworth E, Roider EP, Browne DJ, Lieb B, Wang LA, Garraway CJ, Wu KT, Flaherty CE, Brinckerhoff DW, Mullins DJ, Adams N, Hacohen MP, Hoang GM, Boland GJ, Freeman AH, Sharpe D, Manstein DE, Fisher. Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint Blockade responses. Sci Transl Med 13(581) (2021). [DOI] [PMC free article] [PubMed]
- 90.Li AM, Hucks GE, Dinofia AM, Seif AE, Maude SL. Checkpoint inhibitors augment CD19-Directed chimeric antigen receptor (CAR) T cell therapy in relapsed B-Cell acute lymphoblastic leukemia. Blood. 2018;132(Suppl_1):556–556. [Google Scholar]
- 91.Chong EA, Alanio C, Svoboda J, Nasta SD, Landsburg DJ, Lacey SF, Ruella M, Bhattacharyya S, Wherry EJ, Schuster SJ. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood. 2022;139(7):1026–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint Blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ. PD-1 Blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer. 2022;21(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Razeghian E, Nasution MKM, Rahman HS, Gardanova ZR, Abdelbasset WK, Aravindhan S, Bokov DO, Suksatan W, Nakhaei P, Shariatzadeh S, Marofi F, Yazdanifar M, Shamlou S, Motavalli R, Khiavi FM. A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies. Stem Cell Res Ther. 2021;12(1):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019;68(3):365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697–706. [DOI] [PubMed] [Google Scholar]
- 98.Kueberuwa G, Kalaitsidou M, Cheadle E, Hawkins RE, Gilham DE. CD19 CAR T cells expressing IL-12 eradicate lymphoma in fully lymphoreplete mice through induction of host immunity. Mol Ther Oncolytics. 2018;8:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Avanzi MP, Yeku O, Li X, Wijewarnasuriya DP, van Leeuwen DG, Cheung K, Park H, Purdon TJ, Daniyan AF, Spitzer MH, Brentjens RJ. Engineered Tumor-Targeted T cells mediate enhanced Anti-Tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep. 2018;23(7):2130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J, Zhao Y, June CH. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20(13):3025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chmielewski M, Abken H, Cells Releasing CART. IL-18 convert to T-Bet(high) FoxO1(low) effectors that exhibit augmented activity against advanced solid tumors. Cell Rep. 2017;21(11):3205–19. [DOI] [PubMed] [Google Scholar]
- 102.Li X, Daniyan AF, Lopez AV, Purdon TJ, Brentjens RJ. Cytokine IL-36γ improves CAR T-cell functionality and induces endogenous antitumor response. Leukemia. 2021;35(2):506–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo H, Su J, Sun R, Sun Y, Wang Y, Dong Y, Shi B, Jiang H, Li Z. Coexpression of IL7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion. Clin Cancer Res. 2020;26(20):5494–505. [DOI] [PubMed] [Google Scholar]
- 104.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36(4):346–51. [DOI] [PubMed] [Google Scholar]
- 105.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169(1):424–33. [DOI] [PubMed] [Google Scholar]
- 106.Adachi SY, Tamada K. K., CAR-T cells expressing both IL-7 and CCL19 induce epitope spreading via cross presentation of endogenous tumor antigens. J Immunother Cancer, 2023.
- 107.Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, Klein O, Kuna K, Ouchan Y, Wöll S, Christ E, Weber D, Suchan M, Bukur T, Birtel M, Jahndel V, Mroz K, Hobohm K, Kranz L, Diken M, Kühlcke K, Türeci Ö, Sahin U. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367(6476):446–53. [DOI] [PubMed] [Google Scholar]
- 108.Mackensen A, Haanen J, Koenecke C, Alsdorf W, Wagner-Drouet E, Borchmann P, Heudobler D, Ferstl B, Klobuch S, Bokemeyer C, Desuki A, Lüke F, Kutsch N, Müller F, Smit E, Hillemanns P, Karagiannis P, Wiegert E, He Y, Ho T, Kang-Fortner Q, Schlitter AM, Schulz-Eying C, Finlayson A, Flemmig C, Kühlcke K, Preußner L, Rengstl B, Türeci Ö, Şahin U. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat Med. 2023;29(11):2844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alizadeh D, Brown CE. CAR T cells ignite antitumor immunity. Trends Immunol. 2023;44(10):748–50. [DOI] [PubMed] [Google Scholar]
- 110.Ma L, Dichwalkar T, Chang JYH, Cossette B, Garafola D, Zhang AQ, Fichter M, Wang C, Liang S, Silva M, Kumari S, Mehta NK, Abraham W, Thai N, Li N, Wittrup KD, Irvine DJ. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365(6449):162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma L, Hostetler A, Morgan DM, Maiorino L, Sulkaj I, Whittaker CA, Neeser A, Pires IS, Yousefpour P, Gregory J, Qureshi K, Dye J, Abraham W, Suh H, Li N, Love JC, Irvine DJ. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell. 2023;186(15):3148–e316520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palumbo S, Comincini S. Autophagy and ionizing radiation in tumors: the survive or not survive dilemma. J Cell Physiol. 2013;228(1):1–8. [DOI] [PubMed] [Google Scholar]
- 114.Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys. 1995;33(4):781–96. [DOI] [PubMed] [Google Scholar]
- 115.Hlavata Z, Solinas C, De Silva P, Porcu M, Saba L, Willard-Gallo K, Scartozzi M. The abscopal effect in the era of Cancer immunotherapy: a spontaneous synergism boosting Anti-tumor immunity?? Target Oncol. 2018;13(2):113–23. [DOI] [PubMed] [Google Scholar]
- 116.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent cytosolic DNA sensing promotes Radiation-Induced type I Interferon-Dependent antitumor immunity in Immunogenic tumors. Immunity. 2014;41(5):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holtermann A, Gislon M, Angele M, Subklewe M, von Bergwelt-Baildon M, Lauber K, Kobold S. Prospects of synergy: local interventions and CAR T cell therapy in solid tumors. BioDrugs. 2024;38(5):611–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hellström KE, Hellström I, Kant JA, Tamerius JD. Regression and Inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978;148(3):799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, Demaria S. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, Sadelain M. Low-Dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26(11):2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amit U, Uslu U, Verginadis II, Kim MM, Motlagh SAO, Diffenderfer ES, Assenmacher CA, Bicher S, Atoche SJ, Ben-Josef E, Young RM, June CH, Koumenis C. Proton radiation boosts the efficacy of mesothelin-targeting chimeric antigen receptor T cell therapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2024;121(31):e2403002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murty S, Haile ST, Beinat C, Aalipour A, Alam IS, Murty T, Shaffer TM, Patel CB, Graves EE, Mackall CL, Gambhir SS. Intravital imaging reveals synergistic effect of CAR T-cells and radiation therapy in a preclinical immunocompetent glioblastoma model. Oncoimmunology. 2020;9(1):1757360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ni H, Reitman ZJ, Zou W, Akhtar MN, Paul R, Huang M, Zhang D, Zheng H, Zhang R, Ma R, Ngo G, Zhang L, Diffenderfer ES, Motlagh SAO, Kim MM, Minn AJ, Dorsey JF, Foster JB, Metz J, Koumenis C, Kirsch DG, Gong Y, Fan Y. FLASH radiation reprograms lipid metabolism and macrophage immunity and sensitizes Medulloblastoma to CAR-T cell therapy. Nat Cancer (2025). [DOI] [PMC free article] [PubMed]
- 125.Lai JZ, Zhu YY, Ruan M, Chen L, Zhang QY. Local irradiation sensitized tumors to adoptive T cell therapy via enhancing the Cross-Priming, homing, and cytotoxicity of Antigen-Specific CD8 T cells. Front Immunol. 2019;10:2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qu C, Ping N, Kang L, Liu H, Qin S, Wu Q, Chen X, Zhou M, Xia F, Ye A, Kong D, Li C, Yu L, Wu D, Jin Z. Radiation priming chimeric antigen receptor T-Cell therapy in relapsed/refractory diffuse large B-Cell lymphoma with high tumor burden. J Immunother. 2020;43(1):32–7. [DOI] [PubMed] [Google Scholar]
- 127.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498–513. [DOI] [PubMed] [Google Scholar]
- 129.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu Y, Feng J, Wan R, Huang W, Cell Therapy CART. Remedies of current challenges in design, injection, infiltration and working. Drug Des Devel Ther. 2023;17:1783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao X, Liu J, Sun R, Zhang J, Cao X, Zhang Y, Zhao M. Alliance between titans: combination strategies of CAR-T cell therapy and oncolytic virus for the treatment of hematological malignancies. Ann Hematol. 2024;103(8):2569–89. [DOI] [PubMed] [Google Scholar]
- 132.Fang L, Tian W, Zhang C, Wang X, Li W, Zhang Q, Zhang Y, Zheng J. Oncolytic adenovirus-mediated expression of CCL5 and IL12 facilitates CA9-targeting CAR-T therapy against renal cell carcinoma. Pharmacol Res. 2023;189:106701. [DOI] [PubMed] [Google Scholar]
- 133.Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V, Bouchier-Hayes L, Savoldo B, Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74(18):5195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang G, Zhang Z, Zhong K, Wang Z, Yang N, Tang X, Li H, Lu Q, Wu Z, Yuan B, Zheng M, Cheng P, Tong A, Zhou L. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31(1):134–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park AK, Fong Y, Kim SI, Yang J, Murad JP, Lu J, Jeang B, Chang WC, Chen NG, Thomas SH, Forman SJ, Priceman SJ. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci Transl Med 12(559) (2020). [DOI] [PMC free article] [PubMed]
- 136.Evgin L, Kottke T, Tonne J, Thompson J, Huff AL, van Vloten J, Moore M, Michael J, Driscoll C, Pulido J, Swanson E, Kennedy R, Coffey M, Loghmani H, Sanchez-Perez L, Olivier G, Harrington K, Pandha H, Melcher A, Diaz RM, Vile RG. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci Transl Med. 2022;14(640):eabn2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Porter CE, Rosewell Shaw A, Jung Y, Yip T, Castro PD, Sandulache VC, Sikora A, Gottschalk S, Ittman MM, Brenner MK, Suzuki M. Oncolytic adenovirus armed with bite, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol Ther. 2020;28(5):1251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208(8):1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lai J, Mardiana S, House IG, Sek K, Henderson MA, Giuffrida L, Chen AXY, Todd KL, Petley EV, Chan JD, Carrington EM, Lew AM, Solomon BJ, Trapani JA, Kedzierska K, Evrard M, Vervoort SJ, Waithman J, Darcy PK, Beavis PA. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat Immunol. 2020;21(8):914–26. [DOI] [PubMed] [Google Scholar]
- 140.Richards DM, Sefrin JP, Gieffers C, Hill O, Merz C. Concepts for agonistic targeting of CD40 in immuno-oncology. Hum Vaccin Immunother. 2020;16(2):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuhn NF, Purdon TJ, van Leeuwen DG, Lopez AV, Curran KJ, Daniyan AF, Brentjens RJ. CD40 Ligand-Modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell. 2019;35(3):473–e4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huo Y, Zhang H, Sa L, Zheng W, He Y, Lyu H, Sun M, Zhang L, Shan L, Yang A, Wang T. M1 polarization enhances the antitumor activity of chimeric antigen receptor macrophages in solid tumors. J Transl Med. 2023;21(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, Cummins KD, Shen F, Shan X, Veliz K, Blouch K, Yashiro-Ohtani Y, Kenderian SS, Kim MY, O’Connor RS, Wallace SR, Kozlowski MS, Marchione DM, Shestov M, Garcia BA, June CH, Gill S. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, Zheng W, Lu Y, Zhang W, Bei Y, Shen P. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121(10):837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cassetta L, Kitamura T. Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology. 2018;155(3):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lei A, Yu H, Lu S, Lu H, Ding X, Tan T, Zhang H, Zhu M, Tian L, Wang X, Su S, Xue D, Zhang S, Zhao W, Chen Y, Xie W, Zhang L, Zhu Y, Zhao J, Jiang W, Church G, Chan FK, Gao Z, Zhang J. A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat Immunol. 2024;25(1):102–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.