Abstract
The Drosophila compound eye is a precisely patterned tissue that has revealed molecular mechanisms and biological processes that drive morphogenesis. It is a simple structure of repeating unit eyes, termed ommatidia, that is used to characterize genetic interactions and gene functions. Mutations that affect eye architecture can be easily detected and analyzed; hence, this system is frequently used in under-resourced institutions. Further phenotypic analysis often includes a Scanning Electron Microscope (SEM) to generate high-magnification images suitable for quantitative analysis. However, SEMs are expensive and require costly reagents; sample preparation spans days; and, often, they need full-time staff for sample preparation and instrument maintenance. This limits their utility at under-resourced institutions or during budgetary austerity. In entomology, the use of high-resolution digital imaging technology is a common practice for the identification and characterization of species. This paper describes a method that combines strategies and allows for high-resolution digital imaging of adult Drosophila structures and quantitative analysis using the open software ImageJ. The workflow is a rapid and student-friendly alternative that remedies the limitations of underfunded and under-resourced research facilities with a cost-effective and rapid approach to quantitative phenotypic analysis.
Introduction
Drosophila melanogaster is a powerful genetic model organism that has been used for decades to elucidate molecular signaling pathways and cellular behaviors. Many of the evolutionarily conserved signaling pathways that are essential for multicellular development were first identified and their mechanism of action defined in Drosophila. About 65-75% of all human disease-associated genes have orthologs in Drosophila1 , 2 . The adult Drosophila eye is an important model that has allowed for unbiased genetic screens that facilitated the discovery of important conserved genes implicated in human diseases, including cancer3 , 4 , neurodegeneration5 , and metabolic disorders6 .
The Drosophila eye is composed of ~800 unit eyes, termed ommatidia, that are precisely arrayed in a hexagonal pattern across the surface of the adult eye7 . Each ommatidium is composed of eight photoreceptor neurons that occupy a distinct location within an asymmetrical trapezoid. These are supported by four non-neural cone cells and two primary pigment cells that secrete lens and pseudo-cone to focus light onto the light-sensing rhabdomeres of the photoreceptor neurons. Neighboring ommatidia are separated by a single row of interommatidial lattice cells, comprised of secondary pigment cells, tertiary pigment cells, and mechanosensory bristle complexes8 , 9 , 10 .
Perturbations in eye development are visible in adult eyes as increased or decreased eye size, abnormal abundance or structure of lenses or bristles, or as a “rough eye” where the normally invariant hexagonal patterning is disrupted such that a row of ommatidia can no longer be followed across the surface of the eye. These phenotypes can be scored at the gross tissue level using dissecting microscopes. Detailed analysis of phenotypes traditionally includes scanning electron microscopy followed by quantitative image analysis11 . However, scanning electron microscopy requires expensive instrumentation, costly reagents, sample preparation that spans days, and often, full-time staff to maintain.
This paper presents a workflow that is relatively inexpensive, has a short sample preparation time, can easily be set up on a 3-foot lab bench, does not require hazardous materials, and could be a long-lived addition to Drosophila research labs (Figure 1). Point mounting is an entomological technique used to air dry and preserve small, soft-bodied insects, such as Drosophila12 . This method relies on combining microscope objectives with high-resolution DSLR cameras for effective magnifications of 10x to 1,000x. The limited depth of field inherent to macrophotography is overcome by focus stacking: stitching together a series of images with the focal plane moving through the specimen of interest13 . This method yields high-resolution images suitable for the quantification of phenotypes and could easily be adapted for other structures of interest, such as the wing, leg, thorax, and abdomen. The image analysis workflow uses the free image analysis program FIJI (NIH ImageJ). This methodology makes sample preparation, high-resolution imaging, and analysis accessible for undergraduate students and scientists at under-resourced institutions.
Figure 1: Workflow for imaging adult Drosophila structures.
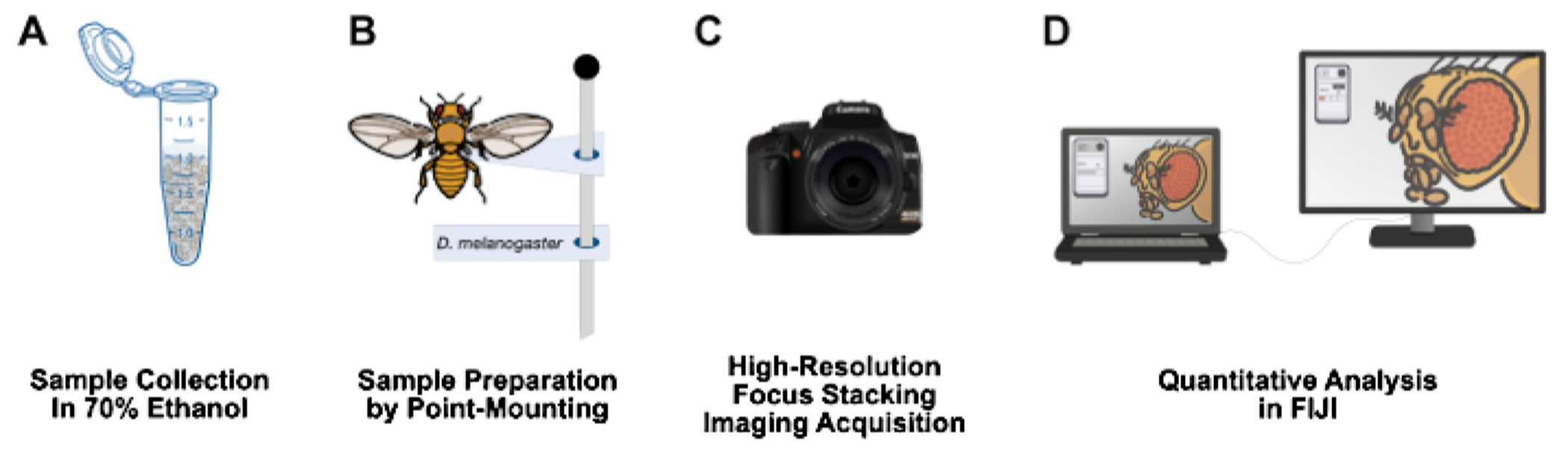
(A) Collect and fix adult Drosophila in 70% ethanol. (B) Prepare samples for imaging by point mounting and affixing to pins. (C) Acquire high-resolution images through focus stacking and integration. (D) Quantify images using FIJI.
Protocol
1. Adult Drosophila collection and fixation
Set up Drosophila crosses or select strains and place them in vials containing fly food. Incubate the vials at the desired temperature (usually 25 °C) until the flies have developed and adults eclose (~10-14 days at 25 °C).
Anesthetize the flies with CO2 and place them on a CO2 pad.
Sort the flies with a feather and select the individuals with the desired phenotype (e.g., straight wings). Make a feather fly sorter by trimming a goose feather to fit in the tapered end of a 1 mL serological pipette.
-
Prepare a 1.7 mL microcentrifuge tube with 1 mL of 70% ethanol. Place the selected flies in the microcentrifuge tube and put on ice. Store the microcentrifuge tubes at 4 °C overnight (Figure 2A).
NOTE: Do not preserve flies in ethanol for more than 24 h. Long-term storage of flies in 70% ethanol will result in loss of eye and body pigment.
Figure 2: Sample preparation.
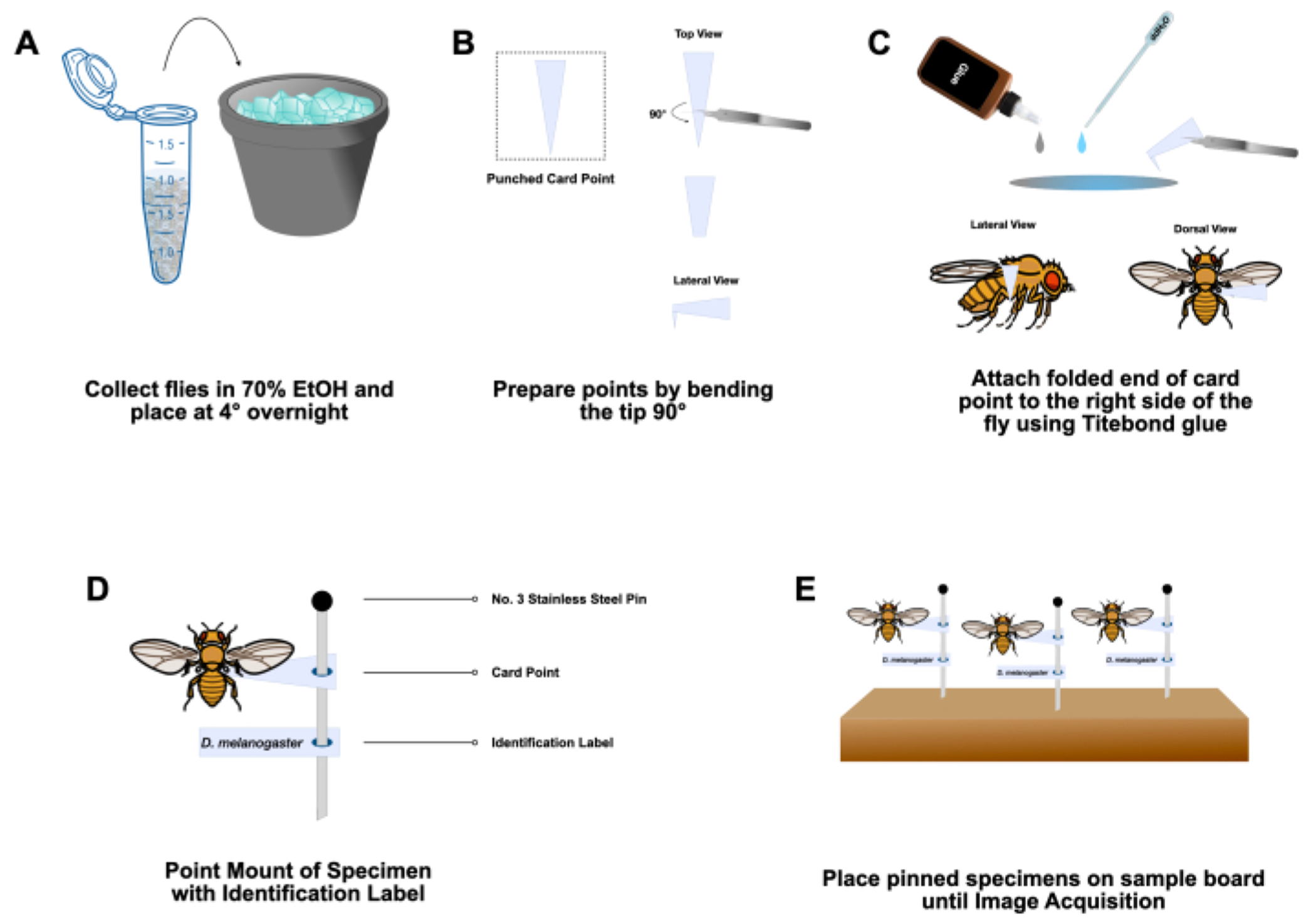
(A) Adult Drosophila are sorted based on phenotypic markers and collected into labeled microcentrifuge tubes containing 70% ethanol on ice. Flies are stored at 4° C overnight. (B) Paper card points are prepared by bending the narrow end 90° from the rest of the card using a pair of #5 forceps. (C) Flies are recovered from tubes and briefly allowed to air dry. Hide glue is applied to the small, folded end of the prepared card point and glued to the adult fly at abdominal segments 2-3. (D) Specimens are mounted, with an identification label, onto a #3 stainless steel insect pin. (E) Pinned specimens are stored on a sample board until ready for image acquisition.
2. Sample preparation by point-mounting
NOTE: Drosophila are soft-bodied insects that become brittle and collapse when air-dried; therefore, this protocol requires samples to be imaged the same day as they are mounted. Work in small sets of ~5 flies at a time to prevent sample loss. Increase the number of samples in a set based on efficiency. Specimens that require more time before imaging can be dehydrated through an increasing concentration series of hexamethyldisilazane (HMDS)14 .
Cut small triangular points (7.1 mm x 2.7 mm) from archival 65 lb cardstock using a specialized point punch. Prepare points by bending the tip (narrowest 25%) to a 90° angle with Dumont #5 fine-tip forceps (Figure 2B).
-
Using Dumont #5 fine-tip forceps, remove the flies from the microcentrifuge tubes (step 1.4). Gently blot the flies with lint-free lab tissue to remove excess ethanol. Position each fly on its left side on an index card under a dissecting microscope.
NOTE: Remove the flies from the tube by holding onto an anatomical structure that is not the area of interest-when imaging the head, we hold samples by the wing or leg. Do not hold samples by the abdomen, as that structure is used to glue the fly onto the card point.
-
Prepare the hide glue, adjusting its consistency to the desired viscosity. Mix 1-2 drops of hide glue with 1-2 drops of deionized (DI) water, and mix with a transfer pipet on an index card. Pick up a prepared card point at the broad end with forceps, and place a small amount of the diluted glue on the bent tip of the point by dabbing it into the glue-water mix (Figure 2C).
NOTE: The glue should be spreadable but not runny.
Apply the bent tip of the point to the anterior side of the right abdomen around abdominal segments 2-3 (Figure 2C). Before the glue dries, make slight adjustments to the fly such that the anterior-posterior axis of the fly is perpendicular to the bent tip of the point.
Insert a No. 3 mounting pin into the wide end of the card-point (Figure 2D) and secure it to an insect pinning block (Figure 2E). Label each pin or row of pins with the corresponding genotype.
3. High-resolution focus stacking imaging acquisition
- Acquire high-resolution photographs of fly eyes with an assembled and customized focus stacking imaging system assembled and customized.
- Capture photographs with a DSLR Camera Body with a 70-200 mm telephoto lens connected to a 20x Apo Microscope Objective via a 77 mm lens adapter.
- Ensure that the specimen is illuminated with a flash through a diffuser (Figure 3).
- Control the Z positioning using a Stackshot Controller and Macro Rail.
- Connect the camera, flash, and motorized stage to a heavy-duty anodized aluminum tripod.
-
Position each point-mounted sample on a universal stage gimbal with the head oriented so that the eye is facing toward the lens. Make head position adjustments by gently moving the head with the forceps.
CAUTION: Large and fast adjustments can result in accidental decapitation.
-
With the camera tethered to a laptop computer, adjust the acquisition settings in the software. Photograph specimens at 20x magnification with these settings: Flash Power 1/32, Shutter Speed 1/200, Aperture F2.8, and ISO 400. Ensure that any Autofocus and Image Stabilization features are turned off.
NOTE: These settings balance optimal flash illumination, shutter speed, and depth of field. They would need to be adjusted for other magnifications and/or lens combinations.
Set the location for saving the resulting image stack (10-50 images) to the desired file folder. Ensure enough storage capacity for the images (~8.5 MB per image).
- Adjust the focus stack settings on the Stackshot control unit in Auto Distance mode.
- Set the step size to 5 μm and calculate the number of steps by setting the start and stop positions of the focus stack.
- View the specimen in the LiveView Mode and with the camera in Auto Shoot Mode to identify the start and stop positions.
- Move the rail so that the closest part of the specimen is in focus (set start position), then move to where the furthest feature of interest is in focus (set end position).
-
Return the camera to the Manual Shoot Mode and start the image acquisition from the Stackshot control unit.
NOTE: Image acquisition time depends on the size of the specimen. The greater the depth of field necessary for large specimens, the more slices are included in the image stack, which will extend the overall acquisition time.
Open files in the referenced focus stacking software. Generate a stacked image by clicking Stack | Align & Stack All (PMax).
-
Save the final image to the computer’s hard drive as a .tif file by clicking File | Save Output Image.
NOTE: Depending on the resolution of the stacked image file and the number of specimens imaged, large external hard drives (1 TB) may be necessary for image backup. In this protocol, stacked images are approximately 100 MB each before being compressed.
Figure 3: Image acquisition.

(A) Imaging apparatus with parts labeled as follows: a) DSLR Camera Body; b) telephoto lens; c) 20x Apo Microscope Objective and Adapter; d) Flash; e) Lens and Dome Diffusers; f) Stackshot Controller, Macro Rail, and Rotary Stage; g) Universal Stage Gimbal; h) Tripod. (B) Imaging apparatus with light diffuser in place. (C) Close-up of mounted specimen in position for imaging.
4. FIJI analysis workflow to calculate adult eye area
For image analysis, obtain the FIJI software15 from the ImageJ.net website.
Choose images for analysis where the eye is centered and aligned with adequate lighting and minimal peripheral blurring, indicating proper alignment with the camera.
- Calibrate the image scale.
- Download the scale bar image for 20x magnification that correlates to 500 μm. Alternately, at the time of image acquisition, photograph a ruler using the same settings. Open the image in FIJI Software.
- Calculate pixels per micron. Use this to convert pixel measurements into micrometer measurements.
Open the stacked image file in FIJI (Figure 4C).
Select Magnifying Glass from the toolbar to enlarge the area of focus. Try to fill the screen with the eye and immediate surrounding head cuticle (Figure 4D).
Select Freehand Select Tool from the toolbar. Outline the retinal area as closely as possible, following the contours of the outermost row of ommatidia (Figure 4E). To remove part of the selection, hold down the option button and select the pixels to remove. To add to the selection, hold down the option and shift buttons and select the pixels to add.
To calculate the area, select Analyze | Measure from the top menu (Figure 4F). A new window will appear with area, mean, minimum, and maximum parameters. Copy and paste these data into a spreadsheet for documentation and conversion from pixels to micrometer measurements.
Perform appropriate statistical analyses.
Figure 4: Image analysis in FIJI.

(A) Scale the original image. Download the calibration image and measure the length of the 500 μm bar. (B) Adjust the scaling using the Set Scale function. (C) Open the stacked image. (D) Magnify the image so that the eye is centered and nearly full screen. (E) Use the Freehand Select tool to outline the eye at the border between the outermost row of ommatidia and the surrounding cuticle. (F) Measure the area within the selected region is calculated by clicking Analyze | Measure | Area.
Representative Results
The Drosophila eye is an excellent model system for studying tissue patterning, growth control, and cell death.
We recently published a study investigating how intracellular pH (pHi) influences tissue growth. First, we established a genetic system where overexpression of the sodium-proton exchanger DNhe2 (the ortholog of mammalian NHE1) in the developing eye causes patterning defects and increased proliferation16 . Increased proliferation with higher pHi is observed across species from yeasts to mammals17 . However, we also observed that with increased pHi, the adult eye appeared smaller in size compared to controls.
Quantitative analysis of adult eye area was done to determine how elevated DNhe2 expression decreases tissue size in the Drosophila eye. We first acquired low-resolution images using an ocular insert microscope (Figure 5A), but these were of insufficient quality to perform quantitative measurements. Specifically, the edges of the eye are not in focus, thus preventing an accurate measurement of the eye area.
Figure 5: Rescue of decreased retinal size associated with increased intracellular pH with decreased autophagy.

(A) Low-resolution images of adult fly eyes taken with an ocular insert microscope. (B) High-resolution images of adult fly eyes used for eye size quantification for the following genotypes: w1118; GMRGAL4; GMR>DNhe2; GMRGAL4; Atg13; GMR>DNhe2; Atg13. (C) Mean measured adult eye sizes were: w1118 (125.83 μm2, N = 7); GMRGAL4 (142.49 μm2, N = 9), and GMR>DNhe2 (74.28 μm2, N = 6), GMRGAL4; Atg13 (146.4 μm2, N = 15); GMR>DNhe2; Atg13 (129.9 μm2, N = 19) and indicated by showing median values with interquartile ranges. Statistical significance was determined by unpaired t-tests with Welch’s correction. The data in B and C were previously published18 .
We measured the area of each eye in high-resolution images of adult Drosophila eyes (Figure 5B). GMR>DNhe2-expressing flies had significantly smaller eyes (mean 74.28 μm2) than the genetic background control w1118 flies (mean 125.83 μm2 ) or the driver control GMRGAL4 heterozygous flies (mean 142.49 μm2 ), which is a 41% and 48% reduction in area, respectively (Figure 5C). This decrease in eye size, in spite of increased proliferation, could be attributed to elevated levels of cell death.
Known autophagy genes, including Atg1, which induces phagophore formation, were used in genetic interaction studies (Figure 5). Effects on adult eye size were quantified using high-resolution photography and area analysis (Figure 5B). Eye size was restored (129.9 μm2 vs 74.28 μm2) in GMR>DNhe2 flies heterozygous for Atg13 compared to GMR>DNhe2 alone and was similar to controls (Figure 5C). Combined with molecular marker analysis, our results support increased autophagic cell death at higher pHi.
Our conclusions are strengthened by the sample preparation, high-resolution imaging, and the analysis described here. These methods allowed for a precise and quantitative description of the size of adult Drosophila eyes with various genotypes. We are continuing to use this approach to assess other genetic interactions that will elucidate mechanisms of pH-dependent growth control.
Discussion
Here we describe a method for sample preparation, high-resolution imaging, and analysis of adult Drosophila structures. The Drosophila eye is a genetically tractable model system that has yielded critical insights into molecular mechanisms underlying diseases including cancer19 , neurodegeneration20 and metabolic diseases21 . In particular, cancer patient “avatars” are generated where transgenic Drosophila carrying oncogenic mutations are generated and can be used for high-throughput drug screening22 . Although we focus on the adult eye, this protocol can be adapted to analyze other structures of interest such as wing, leg, thorax, and abdomen.
Fine motor skills are required at several critical steps of this protocol. Sample preparation of the adult Drosophila by point-mounting requires patience and practice. We advise beginners to practice point-mounting before preparing rare or precious samples. There are several critical steps that will require the practice of fine motor skills and careful attention.
The first critical steps in the protocol are the preparation of the card points and the application of the glue to adhere the card point to the abdomen (protocol step 2.4-2.5). It is important to apply just the right amount of glue because target anatomical structures (i.e., the head) may be covered by glue if too much is applied or it is not the right consistency. The sample must also be glued in an appropriate position, with the anterior-posterior axis perpendicular to the mounting pin. The second critical step is positioning the head for high-resolution imaging (protocol step 3.2). It is crucial that the head is oriented with the eye facing the camera lens. To adjust the head, small adjustments are made using forceps. Gentle and incremental movements are crucial as accidental decapitation may occur with large or forceful adjustments. An additional step where fine motor skills are required is when using the Freehand Select Tool from the FIJI toolbar (protocol step 4.6). Here, it is crucial to trace as closely to the outer row of ommatidia of the eye as possible. This precision is key for rigorous and reproducible area measurements of the eye (Figure 4E).
Imaging methods such as scanning electron microscopy (SEM) remain the primary approach to visualize and analyze external structures. However, SEM protocols require the user to handle hazardous chemicals and operate multiple instruments, including critical point dryers, sputter coating apparatuses, and the SEM itself. Sample preparation takes several days and requires intensive training and skill. A significant challenge academic institutions face is the financial investment and commitment to maintaining the instrument and, in our experience, personnel with expertise in maintaining and operating this equipment are generally not supported at an institutional level. Our protocol mitigates cost and eliminates hazardous chemical exposure at the cost of lower-resolution imaging compared to SEM.
The system described in this study was a preassembled package, but its modular nature permits lowering the cost by substituting individual components with equipment fabricated in-house (e.g., sample stages, flash diffusers), alternative brands or models, or equipment that might already be present in the lab. While connecting microscope objectives to macro cameras is increasing in popularity among hobbyists and professional photographers, some trial and error may be required for different combinations of objectives and lenses. To replicate the system described here, an infinity-corrected microscope objective must be connected to a tube lens (telephoto lens here) with an appropriately threaded adapter. Another consideration when exchanging components is to ensure that the working distance of the lens is compatible with the dimensions of the camera mount, specimen mount, and macrofocusing rail. Finally, the focus stacking software selected here is commercial, but many low-cost or free post-processing focus stacking solutions exist, including ImageJ plugins.
While this protocol requires practice to master sample handling techniques, it is designed to acquire and analyze high-resolution images for detailed phenotypic analysis. The procedures presented here constitute a rapid, low-cost alternative to traditional imaging methodologies. By using freeware-based digital imaging techniques for quantitative analysis, our methodology is accessible and will not require costly software purchases or updates. The imaging setup requires some cost and effort but once established, should be a long-lived addition to Drosophila labs. This method yields high-resolution images suitable for the quantification of phenotypes and could easily be adapted for other structures of interest, such as the wing, leg, thorax, and abdomen. This methodology makes sample preparation, high-resolution imaging, and analysis accessible for undergraduate students and scientists at under-resourced institutions.
Acknowledgments
The authors would like to thank members of the Grillo-Hill pHly lab for discussions and support. We thank Tim Andriese, Randy Kirschner, Kitty (Ngoc-Huong) Nguyen, Marco Parent, Jonny Shaloub, and Librado Veliz for excellent technical support. This work was supported by NIH SC3GM132049 and 1R16GM153640 awards (BKGH), a CSU Biotechnology Faculty-Student Research Award (LM and BKGH), and start-up funds from the College of Science and the Department of Biological Sciences at San José State University (FJL). Special mention goes to Bernd Becker for their resourcefulness and assistance during this process. We thank the BioIcons (https://bioicons.com/) community for providing high-quality icons for our figures and especially to Serviere for the pipet icon, and DBCLS for the Drosophila, forceps, and desktop electron microscope icons used in Figure 1 and Figure 2, which are licensed under CC-BY 4.0 Unported. We also thank the SciDraw (https://scidraw.io/) community for providing high-quality icons for our figures, especially Diogo Losch De Oliveira (doi.org/10.5281/zenodo.3925953), which are licensed under Creative Commons 4.0 license (CC-BY).
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/67485.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Rubin GM et al. Comparative genomics of the eukaryotes. Science. 287 (5461), 2204–2215 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ugur B, Chen K, Bellen HJ Drosophila tools and assays for the study of human diseases. Dis Model Mech. 9 (3), 235–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgson JA, Parvy J-P, Yu Y, Vidal M, Cordero JB Drosophila larval models of invasive tumorigenesis for in vivo studies on tumour/peripheral host tssue interactions during cancer cachexia. Int J Mol Sci. 22 (15), 8317 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam Wong KK, Verheyen EM Metabolic reprogramming in cancer: mechanistic insights from Drosophila. Dis Model Mech. 14 (7), dmm048934 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonini NM A perspective on Drosophila genetics and its insight into human neurodegenerative disease. Front Mol Biosci. 9, e1060796 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond-Barbosa D, Tennessen JM Reclaiming Warburg: using developmental biology to gain insight into human metabolic diseases. Development. 147 (11), dev189340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ready DF, Hanson TE, Benzer S Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 53 (2), 217–240 (1976). [DOI] [PubMed] [Google Scholar]
- 8.Johnson RI Hexagonal patterning of the Drosophila eye. Dev Biol. 478, 173–182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weasner BP, Kumar JP The early history of the eye-antennal disc of Drosophila melanogaster. Genetics. 221 (1), iyac041 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichaud F, Casares F Shaping an optical dome: The size and shape of the insect compound eye. Semin Cell Dev Biol. 130, 37–44 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Oster II, Crang RE Scanning electron microscopy of Drosophila mutant and wild type eyes. Trans Am Microsc Soc. 91 (4), 600–602 (1972). [PubMed] [Google Scholar]
- 12.Gibb TJ, Oseto C Insect collection and identification: Techniques for the field and laboratory. Academic Press; (2019). [Google Scholar]
- 13.Mertens J, Roie MV, Merckx J, Dekoninck W The use of low cost compact cameras with focus stacking functionality in entomological digitization projects. ZooKeys. 712, 141–154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown BV A further chemical alternative to critical-point-drying for preparing small (or large) flies. Fly Times. 11, 10 (1993). [Google Scholar]
- 15.Schindelin J. et al. Fiji: an open-source platform for biological-image analysis. Nat Method. 9 (7), 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grillo-Hill BK, Choi C, Jimenez-Vidal M, Barber DL Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. eLife. 4, e03270 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White KA, Grillo-Hill BK, Barber DL Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci. 130 (4), 663–669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peralta J. et al. Drosophila Nhe2 overexpression induces autophagic cell death. Mol Biol Cell. 35 (7), br13 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munnik C, Xaba MP, Malindisa ST, Russell BL, Sooklal SA Drosophila melanogaster: A platform for anticancer drug discovery and personalized therapies. Front Genet. 13, 949241 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitta Y, Sugie A Studies of neurodegenerative diseases using Drosophila and the development of novel approaches for their analysis. Fly. 16 (1), 275–298 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pletcher RC et al. A genetic screen using the Drosophila melanogaster TRiP RNAi collection to identify metabolic enzymes required for eye development. G3: Genes|Genomes|Genetics. 9 (7), 2061–2070 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangi E. et al. A personalized platform identifies trametinib plus zoledronate for a patient with KRAS-mutant metastatic colorectal cancer. Sci Adv. 5 (5), eaav6528 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


