Abstract
Heme enzymes, with the pentacoordinate heme iron active sites, possess high catalytic activity and selectivity in biosensing applications. However, they are still subject to limited catalytic stability in the complex environment and high cost for broad applications in electrochemical sensing. It is meaningful to develop a novel substitute that has a similar structure to some heme enzymes and mimics their enzyme activities. One emerging strategy is to design the Fe-N-C based single-atomic site catalysts (SASCs). The obtained atomically dispersed Fe-Nx active sites can mimic the active sites of heme enzymes effectively. In this work, we synthesize a SASC (Fe-SASC/NW) by doping single iron atoms in polypyrrole (PPy)-derived carbon nanowire via a zinc-atom-assisted method. The proposed Fe-SASC/NW shows high heme enzyme-like catalytic performance for H2O2 with a specific activity of 42.8 U/mg. An electrochemical sensor based on Fe-SASC/NW is developed for the detection of H2O2. This sensor exhibits a wide detection concentration range from 50 nM to 500 mM and an excellent limit of detection (LOD) of 46.35 nM. Such excellent catalytic activity and electrochemical sensing sensitivity are attributed to the isolated Fe-Nx active sites and their structural similarity with natural metalloproteases.
Keywords: single-atomic site catalysts, heme mimics, hydrogen peroxide, electrochemical sensing
Graphical Abstract

Novel Fe-based single-atomic site catalyst (Fe-SASC/NW) are synthesized by doping single iron atoms in polypyrrole (PPy)-derived carbon nanowires. The atomically dispersed Fe-Nx active sites in the proposed Fe-SASC/NW can mimic the natural metalloproteases active sites and show high heme enzyme-like catalytic performance. The electrochemical sensor based on Fe-SASC/NW exhibits excellent electrochemical sensing sensitivity and potential practical application prospects.
Introduction
Heme-based catalase is a type of the oldest enzymes known, which is commonly used to catalyze and detect hydrogen peroxide (H2O2) with high sensitivity and selectivity.[1, 2] Nevertheless, the limitations of natural enzymes such as high cost, inherent instability, short lifetime, and transducer dependence promote researchers to explore various advanced artificial substitutes.[3–5] Some nanomaterials with peroxidase-like activities, such as Fe3O4, Prussian blue, and some noble- or transition- metal-based nanomaterials, are applied to substitute traditional heme-based catalase enzymes and used in electrochemical sensors.[6–9] However, their catalytic activities are still much inferior to natural peroxidases and do not exhibit structural resemblance to natural enzymes.[10] Besides, it is also hard to control the molecular architecture and calculate the number of active sites in nanomaterials, which significantly hinders their further practical applications.[11, 12]
Single-atomic site catalysts (SASCs), containing exclusively isolated active metal sites, have aroused wide attention due to their specific activity and maximized atomic utilization.[13–21] Among them, Fe-N-C based SASCs have a large amount of Fe-Nx active sites, which can mimic the structure of pentacoordinate heme iron systems in heme enzymes.[22, 23] Due to these unique features, Fe-N-C based SASCs can enhance peroxidase-like catalytic activities and open new avenues to substitute the natural enzymes in the biosensing field.[22, 24–26] For example, our group developed Fe-Nx sites enriched carbon nanotube to replace horseradish peroxidase (HRP) in a commercial ELISA for detecting Alzheimer’s disease in the early stage.[27] Besides, unlike traditional nanomaterials that most of the catalytic activity is caused by surface atoms,[28] the enhanced catalyzing ability of Fe-N-C based SASCs results from almost one hundred percent utilization of iron atoms. Therefore, Fe-N-C based SASCs can be regarded as an ideal candidate to substitute natural enzymes or traditional nanomaterials in immunoassays and biosensing applications, owing to their higher catalytic activity and stability, better simulation of natural metalloenzymes active sites, and easier to optimize and improve catalytic performance.[29, 30]
In this work, we proposed and synthesized a new type of Fe-based SASC with heme enzymes-like active sites for electrocatalytic H2O2 sensing with high sensitivity and selectivity (Scheme 1). The proposed SASC based on Fe-doped polypyrrole (PPy)-derived carbon nanowire (Fe-SASC/NW) was synthesized through a zinc-atom-assisted method.[31] A series of analyses revealed the tortuous and interconnected carbon nanowire possess densely isolated single iron sites, which held a natural heme enzymes-liked Fe-Nx structure. As expected, the Fe-SASC/NW showed excellent peroxidase-like activity and exceptional stability. The electrochemical sensor based on Fe-SASC/NW modified electrode shows excellent sensitivity for H2O2 detection.
Scheme 1.
The synthetic process of single-atomic site catalyst based on Fe-doped on polypyrrole (PPy)-derived carbon nanowire (Fe-SASC/NW).
Results and Discussion
The Fe-SASC/NW was synthesized via a zinc-atom-assisted method (Scheme 1).[31] In brief, ammonium peroxydisulfate (APS) was added to cetyltrimethylammonium bromide (CTAB) acid solution to produce white precipitates of (CTA)2S2O8 complex templates. Subsequently, pyrrole, Zn(NO3)2 and Fe(NO3)3 were added dropwise to the above mixture. Pyrrole polymerization occurred on the (CTA)2S2O8 complex templates to form Fe-polypyrrole nanowire (Fe-PPy NW). Transmission electron microscopy (TEM, Figure 1a) images revealed that the Fe-PPy NW showed well-defined nanowire structures with relatively uniform diameter distributions. The Fe-PPy NW was then treated with high-temperature, acid leaching, and a step of NH3 heat treatment to obtain Fe-SASC/NW. It is worth pointing out that the one-dimensional nanowire structure (Figure 1b and Figure S2) was well maintained after the thermal treatment at a high temperature. PPy NW and N-doped/NW were also prepared without adding Zn(NO3)2 and Fe(NO3)3 and were used as control samples (Figure S1 and S2). Raman spectroscopy was conducted to investigate the graphitizing degree. Two peaks belong to D-band and G-band of carbon materials exist in Fe-SASC/NW Raman spectrum (Figure S3), and the intensity ratio of D band to G band (ID/IG) is around 1.14, demonstrating that Fe-SASC/NW not only owns some defects in carbon matrix to load Fe active sites but also satisfies the electrical conductivity required for electrochemical sensing.[32, 33] The high-resolution scanning transmission electron microscopy (STEM) image in Figure 1c indicates that Fe-SASC/NW is constituted of distorted graphitic carbon and no metal nanoparticle is observed. Such structure feature is usually accompanied by an enormous specific surface area and plentiful nanopores, where numerous atomic iron sites are hosted. N2 adsorption-desorption measurement was used to evaluate the specific surface area of Fe-SASC/NW, showing around 639 m2 g−1 calculated from the Brunauer-Emmett-Teller (BET) method. The N2 adsorption/desorption isotherm curve in Figure S4 shows apparent adsorption and hysteresis in the low-pressure area (P/P0= 0–0.1), revealing both micropores and mesopores exist in Fe-SASC/NW.[34] Moreover, specific pore distributions were further analyzed by nonlocal density functional theory (NLDFT) and Barrett-Joyner-Halenda (BJH) in Figure S5. High-angle annular dark-field STEM (HAADF-STEM) was further applied to investigate the atomic-level structure of Fe-SASC/NW. As shown in Figures 1d and 1e, isolated bright spots are detected and marked with red circles, which reveals that abundant dispersed single-atomic Fe sites were anchored on Fe-SASC/NW. Furthermore, energy-dispersive X-ray spectroscopy (EDS) was performed to evaluate the chemical composition and the corresponding dispersity. Figure 1f shows the elemental mapping images, revealing that C, N, and Fe are homogeneously distributed over the entire nanowire.
Figure 1.

TEM images of (a) polypyrrole nanowire and (b) Fe-SASC/NW. (c) STEM image of the Fe-SASC/NW sample. (d-e) Different magnification HAADF-STEM images of Fe-SASC/NW sample. (f) STEM image and EDS C, N, and Fe elemental mapping images of Fe-SASC/NW.
X-ray absorption spectroscopy analysis was performed to reveal the chemical state and coordination environment of the Fe center, as shown in Figure 2a. The X-ray absorption near-edge structure (XANES) spectra show that the absorption edge position of Fe-SASC/NW is located between FeO and Fe2O3, indicating that the average valence state of Fe atoms is between FeII and FeIII. The corresponding Fourier transforms (FT) obtained from the extended X-ray absorption fine structure (EXAFS) shows that the main peak of Fe-SASC/NW only exhibits a prominent peak at 1.48 Å (Figure 2b), which is attributed to Fe-N scattering, suggesting that Fe exists in Fe-SASC/NW as single-atomic form. The X-ray photoelectron spectroscopy (XPS) analysis was further carried out to evaluate the chemical composition and doping configurations quantitatively (Figure S6). C (90.82 at.%), N (7.18 at.%), O (1.10 at.%), and Fe (0.66 at.%) elements are detected in Fe-SASC/NW (Figure 2c), in concordance with EDS result and again confirms that N and Fe are successfully doped into carbon matrix. The inductively coupled plasma mass spectrometry (ICP-MS) was further used to quantify the Fe content in Fe-SASC/NW, which is 2.05 wt %. As shown in Figure 2d, the C 1s XPS spectra of Fe-SASC/NW is deconvoluted into five peaks, which belong to C-sp2 (~284.6 eV), C-sp3 (~285.3 eV), C-N (~286.0 eV), C-O (~287.3 eV), and C=O (~289.3 eV), respectively.[35, 36] Compared to other reported Fe-N-C materials, Fe-SASC/NW shows a relatively low ratio of C-sp2, which possibly due to its distorted graphitic carbon, inducing more defects to support single-atomic Fe sites.[37] The high-resolution N 1s are shown in Figure 2e. The peaks around 398.0 eV, 400.9 eV, 401.9 eV, and 404.1 eV can be assigned to pyridinic, pyrrolic, graphitic, and oxidized N, respectively, suggesting that nitrogen was indeed doped into the carbon matrix.[38, 39] A spectral valley at 399.5 eV between two dominating pyridinic peak and pyrrolic peak should be attributed to Fe-Nx species based on binding energy.[40] The percentage of Fe-Nx configuration is 15.2% (inset of Figure 2c). Figure 2f shows the Fe 2p spectrum, and according to previous reports, the peaks at 709.1 eV, 712.1 eV, 723.0 eV, and 724.9 eV are corresponding to the 2p3/2 orbitals of Fe2+ and Fe3+ as well as 2p1/2 band of Fe2+ and Fe3+, respectively.[41, 42] This demonstrates Fe atoms exist in FeII and FeIII forms, which is well in line with the result of XANES.
Figure 2.
(a) Fe K-edge XANES spectrum of Fe-SASC/NW and reference samples of FePc, Fe foil, FeO and Fe2O3. (b) FT k3-weighted EXAFS spectrum of Fe-SASC/NW, FePc, Fe foil, FeO and Fe2O3. (c) C, N, O, and Fe contents in Fe-SASC/NW recorded by XPS measurements. Inset: the percentage of N configurations. High-resolution (d) C 1s, (e) N 1s and (f) Fe 2p spectra of Fe-SASC/NW. Inset in (d): the percentage of C-sp2 configuration.
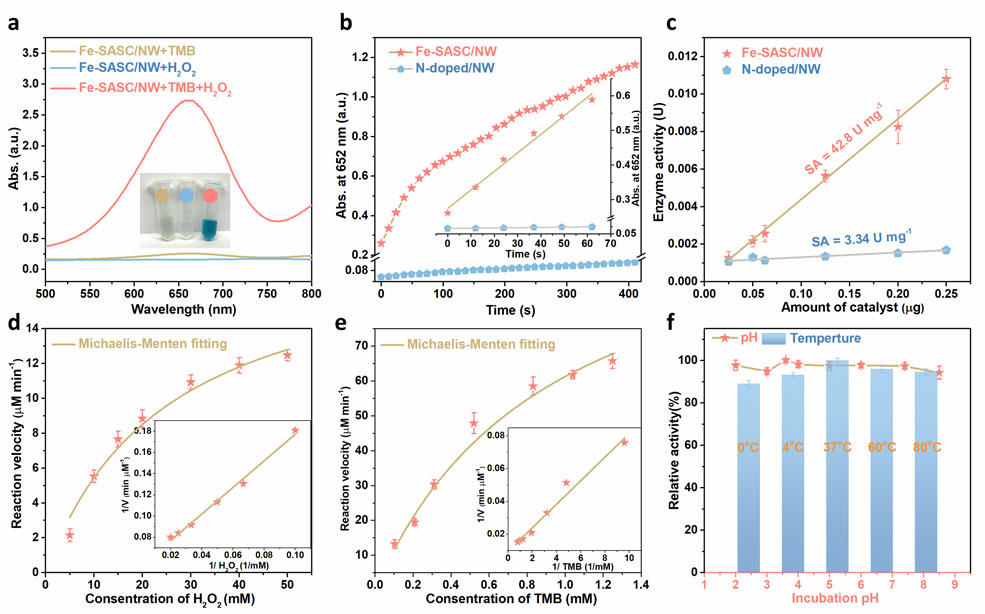
The peroxidase-mimicking properties of the Fe-SASC/NW were verified through typically chromogenic reactions with H2O2, where 3,3′,5,5′-tetramethylbenzidine (TMB), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 1,2-diaminobenzene (OPD) were used as substrates and strong color change was observed (Figure 3a and Figure S7). The absorbance spectrum shows that substrates are oxidized by the Fe-SASC/NW in the presence of H2O2. As a comparison, TMB was also used to trigger chromogenic reactions of N-doped/NW (Figure S8), and no such color change was observed and the absorbance value was trivial. In Figure 3b, the absorbance at 652 nm increases with reaction time and shows a linear relation in the first minute. In addition, we quantitatively analyzed the specific activities of Fe-SASC/NW by measuring the colorimetric signal intensity (Figure 3c). As expected, the specific activity of Fe-SASC/NW reaches 42.8 U/mg, much higher than that of other reported works, including densely isolated Fe-N-C SAzymes (25.33 U/mg),[43] boron-doped Fe-N-C single-atom nanozymes (15.41 U/mg),[44] and heterogeneous single iron atom catalyst (6.75 U/mg).[45] Such results further prove that the synthesized Fe-SASC/NW possesses unprecedented peroxidase-like properties. Natural HRP was analyzed by the same process and the result showed it exhibits a specific activity of 258.9 U mg−1, which is in accordance with the manufacture’s value (≥ 250 U mg−1). Even though the specific activity of the Fe-SASC/NW cannot pass the natural HRP, it is nearly comparable. Michaelis-Menten kinetics were analyzed to probe the catalytic property of Fe-SASC/NW towards H2O2 and TMB (Figure 3e), respectively. Meanwhile, Michaelis-Menten kinetics of HRP were also calculated as a comparison (Figure S9). The detailed kinetics parameters are listed in Table S1. Thereinto, the Km of the Fe-SASC/NW towards H2O2 is at the same level as that of HRP. Using TMB as a substrate, Fe-SASC/NW has a smaller Km value than that of natural HRP, indicating a better affinity ability toward TMB. Besides, the calculated maximum rate (Vmax) of Fe-SASC/NW (19.31 μM/min) almost catches that of natural HRP, demonstrating its potential to substitute natural HRP. The Fe-SASC/NW can also provide comparable Kcat and Kcat/Km with natural HRP, which once again illustrates the impressive catalytic activity and efficiency. In Figure 3f, Fe-SASC/NW maintains excellent stability under pH and temperature variation, indicating that the Fe-SASC/NW retains outstanding robustness in harsh environments.
Figure 3.
(a) Absorption curves of the Fe-SASC/NW in the solution of TMB, H2O2, and TMB +H2O2. Insert: photographs of the color changes (blue). (b) Absorbance-time curves of TMB chromogenic reaction catalyzed by Fe-SASC/NW and N-doped/NW. The corresponds magnified initial linear portion. (c) Specific activities of Fe-SASCs/NW and N-doped/NW. (d-e) Steady-state kinetics curves of Fe-SASC/NW toward H2O2 and TMB, respectively. Inset: double-reciprocal plots for determining the kinetic constants for H2O2 and TMB substrate, respectively. (f) Robustness of Fe-SASC/NW against the harsh environment of temperature and pH, respectively.
To further understand the role of Fe-Nx sites in determining the catalytic efficiency, the influence of thiocyanate ions (SCN-) on the peroxidase-like activity was explored since SCN- can form a stable chelate complex with metal-centered catalytic sites.[46–48] A schematic mechanism is illustrated in Figure 4a. As shown in the UV-Vis results in Figure 4b, the catalytic activity of Fe-SASC/NW decreases significantly after adding KSCN, indicating that the atomically dispersed Fe-Nx sites are major active centers responsible for the peroxidase-like activity. Generally, it is well-established that reactive oxygen species (ROS) have been regarded as active intermediates.[49] Herein, sodium azide (NaN3) is selected as the scavenger of •OH/1O2; β-carotene serves as a scavenger for 1O2; and isopropanol acts as the scavenger for •OH.[50, 51] As shown in Figure 4c and S10, the absorbance value of ox-TMB decreases dramatically with the addition of NaN3 in Fe-SASC/NW/H2O2/TMB solution. This result indicates that the participation of •OH/1O2 is related to the oxidation coloration reaction. Experimental results related to β-carotene verify that the amount of 1O2 (Figure S11) and isopropanol (Figure 4d) is very low, which strongly confirms the existence of •OH.[52] At the same time, terephthalic acid (TA) is also selected as a fluorescent probe to confirm the existence of •OH. This experiment is based on the principle that the highly fluorescent 2-hydroxyterephthalic acid is produced in the reaction system after capturing the •OH. (Figure 4e). The results show that the fluorescent intensity is enhanced by increasing TA concentration, indicating that •OH is the active intermediate involved in the reaction.
Figure 4.
(a) Schematic illustration of the mechanism of the influence after adding SCN-. Red ball: Fe, gray ball: C, blue ball: N, white ball: H, pink ball: O, yellow ball: S. (b) Absorption change of Fe-SASC/NW/TMB/H2O2 solution upon the addition of KSCN with various concentrations. (c) The percent inhibition and absorbance of Fe-SASC/NW/TMB/H2O2 solution after adding the ·OH/1O2 scavenger NaN3. (d) The absorbance of Fe-SASC/NW/TMB/H2O2 solution after adding isopropanol. (e) The formation of •OH using different concentrations of TA as a fluorescent probe.
After validation of the peroxidase-like property and mechanism, the Fe-SASC/NW is feasible for fabricating a high-performance electrode owing to its high activity for H2O2 catalysis. Herein, an electrochemical sensor based on Fe-SASC/NW was developed for detecting H2O2. A certain volume of Fe-SASC/NW was coated onto the work electrode’s surface with the loading of 0.1 mg/cm2. Then, the electrochemical measurements were performed using a standard three-electrode cell by CHI1030C electrochemical workstation. Cyclic voltammetry (CV) was used to evaluate the catalytic performance of Fe-SASC/NW in hydrogen peroxide. The CV curve of Fe-SASC/NW shows a significantly enhanced response in the presence of 0.1 mM H2O2 (Figure 5a). Moreover, the electrocatalytic performance of Fe-SASC/NW for H2O2 is also proved by linear sweep voltammetry (LSV) results (Figure S12). The linear current response of Fe-SASC/NW-modified electrode is presented in Figure 5b, which ranges from 50 nM to 500 mM with a lower detection limit of 46.35 nM. Most importantly, Fe-SASC/NW-modified electrode exhibits a high sensitivity while compared with the relevant electrochemical H2O2 sensors in Table S2. The Fe-SASC/NW-modified electrode also exhibits specific selectivity to H2O2 while adding a set of redox or interference molecules (Figure 5c and 5d). Excellent reproducibility is displayed in Figure 5e. The relative response still keeps almost the same as original values after six repeated measurements, indicating good repeatability of Fe-SASC/NW-modified electrode.
Figure 5.
(a) CV responses of Fe-SASC/NW, N-doped/NW, and bare glassy carbon electrode (GCE) in the presence of 0.1 mM H2O2 in 0.01 M PBS (pH=7.4), respectively. (b) The linear calibration response of Fe-SASC/NW or N-doped/NW modified electrode for detecting H2O2. (c) The selectivity of the Fe-SASC/NW. (d) Current density changes induced by different species at their respective concentrations as shown in Figure 5C. (e) The repeatability of the Fe-SASC/NW. (f) The detected current density changes and the calculated concentration of H2O2 of the contact lenses disinfectant.
A commercial contact lenses disinfectant was used as a real sample to validate the sensor based on the Fe-SASC/NW-modified electrode. Contact lenses cleaning solutions should maintain suitable H2O2 concentrations that guarantee the sterilization effect yet are safe to the eyes. Therefore, accurate detection of H2O2 is of great significance to control the product quality. Here, we used Fe-SASC/NW-modified electrodes to detect diluted disinfecting solutions with different concentrations (Figure 5f). According to the calibration curve of H2O2 detection in Figure 5b, the original concentration of H2O2 in disinfecting solution is calculated to be 0.875 ± 0.029 M, which approximately matched the biomedical disinfectant standards (3% concentration, around 0.88 M). The results indicate that the Fe-SASC/NW-modified electrode is suitable for quality control of the contact lenses disinfectant.
Conclusion
In summary, we have successfully synthesized a Fe-based single-atomic site catalyst (Fe-SASC/NW) with heme enzyme-like active sites (the atomically dispersed Fe-Nx center sites) via a zinc-atom-assisted method. As expected, Fe-SASC/NW owns excellent peroxidase-like activity and exceptional stability. Accordingly, the Fe-SASC/NW-modified electrode exhibits excellent sensitivity and specificity for H2O2 sensing. The single Fe atom active sites and •OH/1O2 active intermediates are also fully investigated by various scavengers. Results show that this SASCs-based sensor has a wide linear current response ranging from 50 nM to 500 mM with a LOD of 46.35 nM toward H2O2. As an exploratory application, detecting H2O2 in a commercial disinfectant was performed, which further proved the practical sensing application prospects of the single-atomic site catalyst. Since the Fe-SASC/NW has significant advantages in biocatalytic activity, stability, and selectivity, it has a great potential in substituting natural enzymes for various biomedical applications, such as intercellular imaging, electrochemical sensors, immunoassays and DNA assays, and wearable biochemical sensors.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences of the US National Institutes of Health under Award Number 1R43ES031885-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also would like to thank the WSU Franceschi Microscopy & Imaging Center for TEM measurements. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Authors would like to acknowledge the helpful discussions with Dr. Xiangheng Niu and Dr. Yang Zhou.
References
- 1.Whitehead TP, Thorpe GH, Carter TJ, Groucutt C, Kricka LJ, Nature 1983, 305, 158. [Google Scholar]
- 2.Iaropolov A, Malovik V, Varfolomeev S, Berezin I, Doklady Akademii Nauk SSSR 1979, 249, 1399. [Google Scholar]
- 3.Huang Y, Ren J, Qu X, Chem. Rev. 2019, 119, 4357. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, Liu C, Yu Y, Yin M, Sun J, Huang J, Chen N, Wang H, Fan C, Song H, Adv. Mater. 2020, 2003708. [DOI] [PubMed]
- 5.Ding S, Zhang N, Lyu Z, Zhu W, Chang Y-C, Hu X, Du D, Lin Y, Mater. Today 2020. 10.1016/j.mattod.2020.11.015 [DOI]
- 6.Jiang B, Duan D, Gao L, Zhou M, Fan K, Tang Y, Xi J, Bi Y, Tong Z, Gao GF, Nat. Protoc. 2018, 13, 1506. [DOI] [PubMed] [Google Scholar]
- 7.Karyakin AA, Karyakina EE, Gorton L, Anal. Chem. 2000, 72, 1720. [DOI] [PubMed] [Google Scholar]
- 8.Das P, Das M, Chinnadayyala SR, Singha IM, Goswami P, Biosens. Bioelectron. 2016, 79, 386. [DOI] [PubMed] [Google Scholar]
- 9.Niu X, Li X, Lyu Z, Pan J, Ding S, Ruan X, Zhu W, Du D, Lin Y, Chem. Commun. 2020, 56, 11338. [DOI] [PubMed] [Google Scholar]
- 10.Scott S, Zhao H, Dey A, Gunnoe TB, ACS Catal. 2020, 10, 14315. [Google Scholar]
- 11.Gumpelmayer M, Nguyen M, Molnár G, Bousseksou A, Meunier B, Robert A, Angew. Chem. 2018, 130, 14974. [DOI] [PubMed] [Google Scholar]
- 12.Gooding JJ, ACS Sensors 2019, 4, 2213. [DOI] [PubMed] [Google Scholar]
- 13.Jasinski R, Nature 1964, 201, 1212. [Google Scholar]
- 14.Fu Q, Saltsburg H, Flytzani-Stephanopoulos M, Science 2003, 301, 935. [DOI] [PubMed] [Google Scholar]
- 15.Lefèvre M, Proietti E, Jaouen F, Dodelet J-P, Science 2009, 324, 71. [DOI] [PubMed] [Google Scholar]
- 16.Qiao B, Wang A, Yang X, Allard LF, Jiang Z, Cui Y, Liu J, Li J, Zhang T, Nat. Chem. 2011, 3, 634. [DOI] [PubMed] [Google Scholar]
- 17.Zhu C, Fu S, Shi Q, Du D, Lin Y, Angew. Chem. Int. Edit. 2017, 56, 13944. [DOI] [PubMed] [Google Scholar]
- 18.Ji S, Chen Y, Wang X, Zhang Z, Wang D, Li Y, Chem. Rev. 2020, 120, 11900–11955. [DOI] [PubMed] [Google Scholar]
- 19.Xu C, Zhi X, Vasileff A, Wang D, Jin B, Jiao Y, Zheng Y, Qiao S-Z, Small Struct. 2021, 2, 2000058. [Google Scholar]
- 20.Yang J, Li W, Wang D, Li Y, Small Struct. 2021, 2, 2000051. [Google Scholar]
- 21.Ding S, Lyu Z, Zhong H, Liu D, Sarnello E, Fang L, Xu M, Engelhard MH, Tian H, Li T, Pan X, Beckman SP, Feng S, Du D, Li J-C, Shao M, Lin Y, Small 2020, 17, 2004454. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Chen J, Gan L, Wang J, Dong S, Sci. Adv. 2019, 5, eaav5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W, Huang L, Wang E, Dong S, Chem. Sci. 2020, 11, 9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Wang H, Wang W, Gao L, Li S, Pan X, Wang H, Yang H, Meng X, Wu Q, Angew. Chem. 2019, 131, 4965. [DOI] [PubMed] [Google Scholar]
- 25.Jiao L, Yan H, Wu Y, Gu W, Zhu C, Du D, Lin Y, Angew. Chem. 2020, 132, 2585. [DOI] [PubMed] [Google Scholar]
- 26.Jiao L, Xu W, Wu Y, Yan H, Gu W, Du D, Lin Y, Zhu C, Chem. Soc. Rev. 2021, 50, 750. [DOI] [PubMed] [Google Scholar]
- 27.Lyu Z, Ding S, Zhang N, Zhou Y, Cheng N, Wang M, Xu M, Feng Z, Niu X, Cheng Y, Zhang C, Du D, Lin Y, Research 2020, 2020, 4724505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei H, Wang E, Chem. Soc. Rev. 2013, 42, 6060. [DOI] [PubMed] [Google Scholar]
- 29.Cheng N, Li JC, Liu D, Lin Y, Du D, Small 2019, 15, 1901485. [DOI] [PubMed] [Google Scholar]
- 30.Niu X, Shi Q, Zhu W, Liu D, Tian H, Fu S, Cheng N, Li S, Smith JN, Du D, Biosens. Bioelectron. 2019, 142, 111495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J-C, Xiao F, Zhong H, Li T, Xu M, Ma L, Cheng M, Liu D, Feng S, Shi Q, ACS Catal. 2019, 9, 5929. [Google Scholar]
- 32.Wang J, Ciucci F, Small 2017, 13, 1604103. [DOI] [PubMed] [Google Scholar]
- 33.Tan H, Li Y, Kim J, Takei T, Wang Z, Xu X, Wang J, Bando Y, Kang YM, Tang J, Adv. Sci. 2018, 5, 1800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia W, Tang J, Li J, Zhang S, Wu KCW, He J, Yamauchi Y, Angew. Chem. Int. Edit. 2019, 58, 13354. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Wei Z, Gou X, Acs Catal. 2015, 5, 4133. [Google Scholar]
- 36.Yi S, Qin X, Liang C, Li J, Rajagopalan R, Zhang Z, Song J, Tang Y, Cheng F, Wang H, Appl. Catal. B: Environ. 2020, 264, 118537. [Google Scholar]
- 37.Tao L, Wang Q, Dou S, Ma Z, Huo J, Wang S, Dai L, Chem. Commun. 2016, 52, 2764. [DOI] [PubMed] [Google Scholar]
- 38.Shin D, Jeong B, Mun BS, Jeon H, Shin H-J, Baik J, Lee J, J. Phys. Chem. C 2013, 117, 11619. [Google Scholar]
- 39.Ganesan S, Leonard N, Barton SC, Phys. Chem. Chem. Phys. 2014, 16, 4576. [DOI] [PubMed] [Google Scholar]
- 40.Leonard N, Ju W, Sinev I, Steinberg J, Luo F, Varela AS, Cuenya BR, Strasser P, Chem. Sci. 2018, 9, 5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin L, Zhu Q, Xu A-W, J. Am. Chem. Soc. 2014, 136, 11027. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Li J-C, Shi Q, Feng S, Lyu Z, Ding S, Hao L, Zhang Q, Wang C, Xu M, ACS Appl. Mater. Inter. 2019, 11, 39820. [DOI] [PubMed] [Google Scholar]
- 43.Jiao L, Wu J, Zhong H, Zhang Y, Xu W, Wu Y, Chen Y, Yan H, Zhang Q, Gu W, Gu L, Beckman SP, Huang L, Zhu C, ACS Catal. 2020, 10, 6422. [Google Scholar]
- 44.Jiao L, Xu W, Zhang Y, Wu Y, Gu W, Ge X, Chen B, Zhu C, Guo S, Nano Today 2020, 35, 100971. [Google Scholar]
- 45.Zhao C, Xiong C, Liu X, Qiao M, Li Z, Yuan T, Wang J, Qu Y, Wang X, Zhou F, Chem. Commun. 2019, 55, 2285. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Zhou Z-Y, Lai Y-J, You Y, Liu J-G, Wu X-L, Terefe E, Chen C, Song L, Rauf M, J. Am. Chem. Soc. 2014, 136, 10882. [DOI] [PubMed] [Google Scholar]
- 47.Liu W, Zhang L, Liu X, Liu X, Yang X, Miao S, Wang W, Wang A, Zhang T, J. Am. Chem. Soc. 2017, 139, 10790. [DOI] [PubMed] [Google Scholar]
- 48.Pedone D, Moglianetti M, Lettieri M, Marrazza G, Pompa PP, Anal.l Chem. 2020, 92, 8660. [DOI] [PubMed] [Google Scholar]
- 49.Huo M, Wang L, Wang Y, Chen Y, Shi J, ACS nano 2019, 13, 2643. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Jiang S, Shao W, Zhang X, Chen S, Sun X, Zhang Q, Luo Y, Xie Y, J. Am. Chem. Soc. 2018, 140, 3474. [DOI] [PubMed] [Google Scholar]
- 51.Zhan Y, Zeng Y, Li L, Guo L, Luo F, Qiu B, Huang Y, Lin Z, Anal.l Chem. 2019, 92, 1236. [DOI] [PubMed] [Google Scholar]
- 52.Mu Q, Sun Y, Guo A, Xu X, Qin B, Cai A, J. Hazard. Mater. 2020, 402, 123939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.