Abstract
Background
Migraine has been linked to a heightened risk of cardiovascular disease, and acute treatment drugs, such as triptans, might increase this risk. This study aimed to determine whether the elevated cardiovascular risk is primarily attributable to the underlying migraine condition or the treatment modalities utilized. Additionally, we investigated the effects of managing traditional cardiovascular risk factors and the influence of healthy lifestyle scores on this association.
Methods
This population-based investigation leveraged data from the UK Biobank, encompassing participants recruited between 2006 and 2010, to examine the association between migraine and the long-term risk of atherosclerotic cardiovascular disease and its subtypes. Cox proportional hazard models were employed to conduct this analysis. Furthermore, the study evaluated the relative importance of migraine in predicting atherosclerotic cardiovascular disease by calculating the R² values of the Cox models. Additionally, multiplicative and additive interaction analyses were conducted to examine whether the association between migraine and cardiovascular disease varies based on the degree of risk factor control and lifestyle scores.
Results
Across an average follow-up span of 12.9 years, a total of 24,038 cardiovascular events were documented, comprising 12,451 cases of coronary heart disease, 3,608 cases of ischemic stroke, and 4,493 cases of peripheral artery disease. In a thoroughly adjusted analysis, individuals with migraine demonstrated an increased risk of atherosclerotic cardiovascular disease, with a hazard ratio of 1.12 (95% confidence interval: 1.05 to 1.20) compared to those without migraine, indicating a significant trend (PFDR< 0.001). Among female participants under 45 years of age, migraine emerged as a stronger predictor of ASCVD risk than certain lifestyle factors. Furthermore, a significant interaction between migraine and an unhealthy lifestyle was observed in ASCVD risk, evident at both additive and multiplicative levels (P for additive interaction = 0.016; P for multiplicative interaction = 0.041). However, the interactions between migraine and the degree of traditional risk factor control did not reach statistical significance. Additionally, the acute treatment of migraine, including the use of triptans, was not significantly associated with ASCVD risk.
Conclusion
A significant interaction exists between unhealthy lifestyles and migraine, where their combined effects may contribute to an elevated risk of cardiovascular diseases, potentially amplifying the impact of each factor. Developing effective lifestyle intervention strategies tailored for migraine patients could help improve their long-term cardiovascular health, emphasizing the importance of comprehensive risk management.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-025-02002-6.
Introduction
Atherosclerotic cardiovascular disease (ASCVD), which includes coronary heart disease (CHD), ischemic stroke (IS), and peripheral artery disease (PAD), continues to be a leading cause of global morbidity and mortality [1]. Recent statistics from 2024 indicate that ASCVD accounted for nearly one-third of all deaths in the United States across various causes [2]. Migraine, a chronic and episodic primary headache disorder, is characterized by moderate to severe pain accompanied by symptoms such as nausea, vomiting, photophobia, and phonophobia [3]. Migraine affects approximately 15% of the global population, translating to nearly one billion individuals, and constitutes a significant cause of disability that substantially reduces the quality of life while imposing a considerable burden on society [4]. Migraine presents significant heterogeneity in attack frequency, aura, treatment response, and comorbidities, reflecting its complex genetic and molecular basis. Despite its high prevalence and impact, it remains underrecognized and undertreated, highlighting the need for better education and management strategies [5]. Prior studies have demonstrated that individuals experiencing migraine, particularly young women, exhibit an elevated risk of ischemic stroke and myocardial infarction [6]. The 2021 European Society of Cardiology (ESC) Prevention Guideline acknowledges the significant global impact of migraine, emphasizing the need to consider them in discussions of cardiovascular prevention at the population level [7].
While the majority of the burden of ASCVD is observed in older adults, there is a rising incidence of ASCVD risk among younger populations [8]. Following the establishment of targets for preventable risk factors by the World Health Organization (WHO), there has been a general decline in ASCVD incidence and mortality rates [9]. Nevertheless, this decline has not been observed in instances of early-onset atherosclerotic ASCVD, leading to heightened interest in identifying additional risk factors that may contribute to the onset and progression of ASCVD. Research has suggested a potential link between migraine and an increased risk of ASCVD, but the underlying mechanisms remain unclear. This study aims to determine whether the elevated cardiovascular risk associated with migraine stems primarily from the condition itself or its therapeutic interventions. Additionally, it explores the interplay between migraine, traditional risk factor management, and healthy lifestyle behaviors about ASCVD risk.
Methods
Study design and population
This prospective cohort study analyzed data from the UK Biobank (UKB), comprising over 500,000 individuals aged 37 to 73 years, collected between 2006 and 2010. The North West Multi-centre Research Ethics Committee approved the study, and all participants provided written consent. Only participants with full data at the time of enrollment were included in the analysis. The exclusion criteria were: (i) individuals with pre-existing cardiac conditions, such as rheumatic heart disease, ischemic heart disease, pericardial disease, valvular heart disease, atrial fibrillation, heart failure, stroke, and peripheral artery disease, identified via ICD-10 codes (see Supplementary Data online, Table S1); (ii) individuals with incomplete covariate data; and (iii) individuals diagnosed with or self-reporting migraine during the follow-up period. Individuals meeting at least one of the following criteria were classified as having migraine: (i) a documented clinical diagnosis of migraine, or (ii)self-reported migraine.
Definition of traditional risk factor control
Drawing from the Framingham Heart Study and the ASCVD risk assessment tool [10, 11], participants were stratified based on the management of traditional risk factors, utilizing four principal indicators: glycated hemoglobin (HbA1c), blood pressure (BP), low-density lipoprotein (LDL) cholesterol, and smoking status. Glycemic control was defined by an HbA1c level of less than 48 mmol/mol (6.5%), while lipid control was specified as an LDL cholesterol level below 130 mg/dL. Blood pressure control was determined by maintaining mean systolic and diastolic BP levels below 140/90 mm Hg. Non-current smoking status encompassed both individuals who have never smoked and former smokers. Attaining LDL cholesterol levels below the specified threshold is linked to a substantial decrease in the risk of cardiovascular diseases [12, 13]. For more rigorous evaluations, optimal lipid management is characterized by LDL levels below 100 mg/dL, which are associated with additional reductions in the risk of cardiovascular events [14, 15]. The extent of control over traditional risk factors is categorized into three tiers: low (control of 0–1 risk factors), medium (control of 2–3 risk factors), and high (control of 4 risk factors).
Definition of healthy lifestyle scores
Four adjustable and recognized lifestyle factors were identified to evaluate healthy lifestyle scores.: alcohol consumption, diet, sleep patterns, and physical activity. Alcohol intake was quantified by aggregating the consumption of some alcoholic drinks, including wine (red, white, and fortified), champagne, beer, cider, spirits, and other alcoholic beverages. Established guidelines recommend a daily alcohol consumption limit of 0–14 g for women and 0–28 g for men in the UKB [16]. Diet quality was assessed within the framework of the healthy lifestyle factor score developed by Bradbury et al. [17], which is predicated on the consumption of commonly consumed food groups that correspond with recommendations for cardiometabolic health. These dietary targets included: fruits (≥ 3 servings/day), vegetables (≥ 3 servings/day), fish (≥ 2 servings/week), processed meats (≤ 1 serving/week), unprocessed red meats (≤ 1.5 servings/week), whole grains (≥ 3 servings/day), and refined grains (≤ 1.5 servings/day). Regular exercise is defined as at least 150 min of moderate activity or 75 min of vigorous activity weekly, or engaging in moderate activity five days a week, as per American Heart Association guidelines. Healthy sleep was evaluated by examining five sleep-related factors. Each factor deemed low-risk was awarded 1 point, whereas all other conditions were assigned 0 points. The cumulative score could reach a maximum of 5, with a score of 4 or higher indicating a healthy sleep pattern. Each lifestyle factor was scored with 1 point for a healthy level and 0 points for an unhealthy level [18]. Participants were sorted into three groups according to their total lifestyle score, which ranged from 0 to 4: unhealthy (0–1 points), intermediate (2–3 points), and healthy (4 points).
Acute treatment of migraine
The study investigated four categories of acute-phase pharmacological treatments for migraine, namely triptans, nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and dihydroergotamine. Detailed information can be found in the Supplementary Material online, Tables S2.
Outcomes
The principal endpoints of this study encompassed the occurrence of atherosclerotic cardiovascular disease and its three major components: coronary heart disease, ischemic stroke, and peripheral artery disease. These outcomes were identified utilizing the International Classification of Diseases, 10th Revision (ICD-10) codes: I20–I25 for coronary heart disease, I63 for ischemic stroke, and I70–I72, in addition to I73.9 for peripheral artery disease. The follow-up period was determined from the baseline to the earliest event of an outcome diagnosis, mortality, or the censoring date, which was October 31, 2022.
Covariates
A touch-screen questionnaire was used to collect self-reported data, which included age, gender, race, income, education, comorbidities, and family history of heart disease or stroke among first-degree relatives (including father, mother, and siblings). Medication information was obtained from both self-reported data and a verbal interview conducted by a nurse. Body mass index (BMI) was determined by dividing weight in kilograms by the height in meters squared. Total household income before tax was categorized into four categories: low = “£18,000-£51,999,” middle = “£52,000-£100,000,” high = “>£100,000,” and unknown. Comorbidities included hypertension, diabetes, and dyslipidemia. Hypertension was identified as a systolic blood pressure (SBP) of more than 140 mmHg or a diastolic blood pressure (DBP) of more than 90 mmHg, and hospital inpatient record or self-reported use of antihypertensive medications. Diabetes diagnosis relied on self-reported, hospital records, anti-diabetic treatment (such as oral medication or insulin), or a HbA1c level of ≥ 6.5%. Dyslipidemia is described as having any of the following conditions: total cholesterol ≥ 5.2 mmol/L, low-density lipoprotein (LDL) cholesterol ≥ 3.4 mmol/L, high-density lipoprotein (HDL) cholesterol < 1.0 mmol/L, or triglyceride levels ≥ 1.7 mmol/L.
Statistical analysis
Participant characteristics were summarized with medians and IQRs for continuous variables, and frequencies and percentages for categorical ones. The Mann-Whitney U test assessed differences in continuous variables between groups, while the chi-squared test (χ2) was used for categorical variables. Cox proportional hazard models were employed to assess the association between migraine and the risk of atherosclerotic cardiovascular disease, utilizing follow-up years as the baseline time reference. Schoenfeld residuals were employed to test the proportional hazards assumption. While this assumption was generally met (P > 0.05 for most models), minor violations were observed in some cases. Therefore, HRs were interpreted as weighted averages over the follow-up period [19]. The multivariable models were adjusted for a range of covariates, including age, gender, race, income, education, family history of coronary heart disease (CHD) or stroke, body mass index (BMI), and comorbidities. Additionally, lifestyle factors and traditional risk factors were incorporated into the models both individually and collectively. To enhance the reliability of our results, we conducted sensitivity analyses. We used multiple imputations with chained equations to handle missing data in exposure variables and covariates, assessing the impact of these gaps. We applied Fine and Gray’s model to consider mortality as a competing risk. Additionally, we excluded participants with follow-up periods under 2 and 5 years. To reduce possible confounding factors, propensity score matching (PSM) was used to form a matched cohort, where the main analysis was repeated. (refer to online supplementary material, Supplementary Methods). These analyses were further conducted in subgroups stratified by factors such as age, sex, ethnicity, education, income level, obesity, healthy lifestyle, comorbidities, and medication usage to explore potential effect modifiers.
To determine the importance of migraine in predicting ASCVD, we compared its significance against other risk factors across various age and gender groups using the R² value from Cox models. We also examined how migraine’s link to ASCVD varies with risk factor control and lifestyle scores through multiplicative and additive interaction analyses. Multiplicative interactions were assessed by adding interaction terms to the original Cox models. For additive interaction evaluation, variables were coded by designating the lowest risk stratum (no migraine with four controlled risk factors, or no migraine with a lifestyle score of 4) as the reference and treating it as a continuous variable. Based on previous research [20], the Relative Excess Risk due to Interaction (RERI) was computed as a measure of additive interaction. We also evaluated the attributable proportion (AP) due to additive interaction between migraine and a low degree of risk factor control or poor lifestyle. The AP quantifies the fraction of incident cases that can be ascribed to the synergistic effect of both exposures when they occur simultaneously. To determine the potential influence of acute-phase migraine treatments on ASCVD, Cox proportional hazards regression models were applied. Migraine patients who did not use these medications were designated as the reference group, enabling the evaluation of ASCVD risk associated with medication use.
Multiple testing correction was applied using the Benjamini-Hochberg False Discovery Rate (FDR) correction. Using R version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria), the statistical analysis was carried out. Two-tailed P-values were used, and those below 0.05 were considered statistically significant.
Results
Participant baseline characteristics
The study included a total of 265,794 participants, consisting of 11,743 individuals diagnosed with migraine and 254,051 individuals without migraine at baseline. As presented in Table 1, the median age at recruitment was 56 years (interquartile range [IQR], 49–62 years), with females representing 55.4% of the cohort. Compared to participants without migraine, those with migraine were generally younger, predominantly female, and exhibited higher levels of educational attainment, alongside a slightly lower BMI. Regarding lifestyle factors, individuals with migraine typically exhibited moderate alcohol consumption and adherence to healthier dietary practices. Nonetheless, they generally engaged in lower physical activity levels and were less likely to maintain healthy sleep patterns. Participants with migraine had lower rates of hypertension, diabetes, and dyslipidemia. Concerning traditional risk factors, these individuals demonstrated a reduced prevalence of current smoking and better control of HbA1c and blood pressure, although their LDL cholesterol levels were marginally elevated.
Table 1.
Baseline characteristic of participants in the biobank
| Characteristics | People without migraine | People with migraine | P value | |
|---|---|---|---|---|
| No.of participants (%) | 254,051 | 11,743 | ||
| Age (years) | 55.73 ± 8.08 | 54.67 ± 7.75 | < 0.001 | |
| Female sex, n (%) | 138,193 (54.4) | 9077 (77.3) | < 0.001 | |
| White ethnicity, n (%) | 243,187 (95.7) | 11,413 (97.2) | < 0.001 | |
| College or university, n (%) | 90,398 (35.6) | 4574 (39.0) | < 0.001 | |
| Income level (%) | Low | 158,306 (62.3) | 7265 (61.9) | 0.012 |
| Middle | 53,562 (21.1) | 2459 (20.9) | ||
| High | 14,916 (5.9) | 652 (5.6) | ||
| Unknown | 27,267 (10.7) | 1367 (11.6) | ||
| Body mass index (Kg/m2) | 27.12 ± 4.58 | 26.84 ± 4.81 | < 0.001 | |
| Family history, n (%) | ||||
| Heart disease | 105,477 (41.5) | 5358 (45.6) | < 0.001 | |
| Stroke | 65,059 (25.6) | 3168 (27.0) | 0.001 | |
| smoking status (%) | Never | 141,848 (55.8) | 7151 (60.9) | < 0.001 |
| Previous | 87,142 (34.3) | 3692 (31.4) | ||
| Current | 25,061 (9.9) | 900 (7.7) | ||
| Moderate Alcohol drinking, n (%) | 144,227 (56.8) | 7125 (60.7) | < 0.001 | |
| Regularly physical activity, n (%) | 192,982 (76.0) | 8619 (73.4) | < 0.001 | |
| Healthy diet, n (%) | 143,376 (56.4) | 7082 (60.3) | < 0.001 | |
| Healthy sleep pattern, n (%) | 84,734 (33.4) | 3696 (31.5) | < 0.001 | |
| Comorbidities | ||||
| Hypertension (yes, %) | 136,960 (53.9) | 5884 (50.1) | < 0.001 | |
| Diabetes (yes, %) | 11,704 (4.6) | 292 (2.5) | < 0.001 | |
| Dyslipidemia (yes, %) | 125,117 (49.2) | 5642 (48.0) | 0.011 | |
| Glycated hemoglobin | 5.41 ± 0.56 | 5.35 ± 0.44 | < 0.001 | |
| LDL-cholesterol | ||||
| Millimoles per liter | 3.61 ± 0.84 | 3.64 ± 0.84 | < 0.001 | |
| Millimoles per deciliter | 139.64 ± 32.58 | 140.91 ± 32.48 | < 0.001 | |
| Blood pressure, mm Hg | ||||
| Systolic | 139.32 ± 19.54 | 135.39 ± 18.82 | < 0.001 | |
| Diastolic | 82.31 ± 10.62 | 81.17 ± 10.54 | < 0.001 | |
| Aspirin intake, percent | 23,752 (9.3) | 1308 (11.1) | < 0.001 | |
| Statin intake, percent | 32,048 (12.6) | 1121 (9.5) | < 0.001 | |
| Beta-Blockers & ACEI & ARB intake, percent | 29,598 (11.7) | 1801 (15.3) | < 0.001 | |
| Number of risk-factor control | 2.45 ± 0.89 | 2.58 ± 0.88 | < 0.001 | |
| Healthy lifestyle score | 2.23 ± 1.01 | 2.26 ± 1.00 | < 0.001 | |
Data are present as mean ± SD or n (%)
The body mass index is the weight in kilograms divided by the square of the height in meters; To convert values for cholesterol to milligrams per deciliter, divide by 0.02586; to convert values for glycated hemoglobin to percent, divide by 10.929 plus 2.15. LDL, low-density lipoprotein. ACEI, Angiotensin-Converting Enzyme Inhibitor. ARB, Angiotensin II Receptor Blocker
Long-term relative risk of atherosclerotic cardiovascular disease is higher in people with migraine
During an average follow-up period of 12.9 years, 24,038 new ASCVD events were recorded, including 12,451 CHD cases, 3,608 strokes, and 4,493 PAD cases. In a multivariable-adjusted model, participants with migraine had a hazard ratio of 1.12 (95% CI: 1.05, 1.20) for total ASCVD compared to those without migraine (PFDR < 0.001). These findings persisted even after adjusting for lifestyle factors and traditional risk factor control in the model. The association between migraine and ASCVD risk persisted when all covariates were included, with HRs (95% CI) of 1.14 (1.07–1.22) (PFDR < 0.001) (Table 2). However, migraine was not significantly associated with stroke in any of the models. Results were consistent even after considering the competing risk of death, using multiple imputations for missing data, and excluding participants with follow-ups under 2 or 5 years (see Supplementary material online, Tables S8). Furthermore, the findings from the propensity score matching were congruent with the primary results of this study. (see Supplementary material online, Tables S10 and S11).
Table 2.
Association between migraine and ASCVD in the UK biobank
| People without migraine | People with migraine | P value | P FDR | |
|---|---|---|---|---|
| ASCVD | ||||
| No. of cases/total | 23,095/254,051 | 943/11,743 | ||
| Multivariable adjusteda | 1(Reference) | 1.12(1.05,1.20) | <0.001 | <0.001 |
| Multivariable adjusteda+ lifestyle factors | 1(Reference) | 1.12(1.05,1.20) | <0.001 | <0.001 |
| Multivariable adjusteda+ traditional factor control | 1(Reference) | 1.13(1.05,1.20) | <0.001 | <0.001 |
| Model included all covariates | 1(Reference) | 1.12(1.05,1.20) | <0.001 | <0.001 |
| Coronary heart disease | ||||
| No. of cases/total | 11,743/254,051 | 708/11,743 | ||
| Multivariable adjusteda | 1(Reference) | 1.09(1.01,1.18) | 0.021 | 0.023 |
| Multivariable adjusteda+ lifestyle factors | 1(Reference) | 1.09(1.01,1.17) | 0.029 | 0.030 |
| Multivariable adjusteda+ traditional factor control | 1(Reference) | 1.09(1.02,1.18) | 0.019 | 0.020 |
| Model included all covariates | 1(Reference) | 1.09(1.01,1.18) | 0.026 | 0.029 |
| Ischaemic stroke | ||||
| No. of cases/total | 3466/254,051 | 142/11,743 | ||
| Multivariable adjusteda | 1(Reference) | 1.13(0.95,1.34) | 0.162 | 0.199 |
| Multivariable adjusteda+ lifestyle factors | 1(Reference) | 1.13(0.96,1.34) | 0.151 | 0.188 |
| Multivariable adjusteda+ traditional factor control | 1(Reference) | 1.14(0.96,1.34) | 0.141 | 0.188 |
| Model included all covariates | 1(Reference) | 1.14(0.96,1.35) | 0.133 | 0.177 |
| Peripheral artery disease | ||||
| No. of cases/total | 4314/254,051 | 179/11,743 | ||
| Multivariable adjusteda | 1(Reference) | 1.23(1.05,1.42) | 0.008 | 0.011 |
| Multivariable adjusteda+ lifestyle factors | 1(Reference) | 1.23(1.06,1.43) | 0.007 | 0.009 |
| Multivariable adjusteda+ traditional factor control | 1(Reference) | 1.24(1.07,1.44) | 0.005 | 0.008 |
| Model included all covariates | 1(Reference) | 1.25(1.07,1.45) | 0.005 | 0.006 |
Adjusteda for sex, age, race, income level, education, BMI, family history of cardiovascular disease, comorbidities, and lifestyle factors, including physical activity, diet, sleep pattern, and drinking; traditional risk factor control including glycated hemoglobin control, blood pressure control, low-density-lipoprotein cholesterol control, and no current smoking; HR, hazard ratio; CI, confidence interval
Gender and age-specific atherosclerotic cardiovascular disease risks associated with migraine
Among female participants with migraine, the hazard ratios for overall ASCVD were 1.19 (95% CI: 1.09–1.29, P < 0.001), for CHD 1.15 (95% CI: 1.04–1.26, PFDR = 0.005), and IS 1.28 (95% CI: 1.04–1.57, PFDR = 0.035), with no significant associations for PAD. Women under 45 had a notably higher ASCVD risk (HR 1.6, 95% CI: 1.16–2.2, PFDR = 0.014), while those aged 45–55 showed no significant results. Women aged 55–65 had a moderate risk increase (HR 1.15, 95% CI: 1.02–1.29, PFDR = 0.027), and those over 65 had an HR of 1.27 (95% CI: 1.05–1.54, PFDR = 0.028). No significant associations were found between migraine and ASCVD risk in men across all ages (Table 3). Subtype-specific analyses showed that women under 45 with migraine had a significantly higher risk of CHD (HR: 1.68, 95% CI: 1.17–2.40, PFDR = 0.037). No significant associations were found in other demographic groups. (see online supplementary material, Tables S3–S7).
Table 3.
Multivariable-adjusted HRs (95% CIs) of migraine for ASCVD by gender and age group
| ASCVD | No. of events/total | HR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Women | People without migraine | People with migraine |
Model 1 | Model 2 | Model 3 | Model 4 |
| <45 | 318/19,744 | 45/1404 | 1.66 (1.21, 2.28) | 1.62 (1.18, 2.23) | 1.64 (1.2, 2.26) | 1.6 (1.16, 2.2) |
| P value | 0.001 | 0.003 | 0.002 | 0.004 | ||
| P FDR | 0.004 | 0.009 | 0.007 | 0.014 | ||
| 45–55 | 1570/44,301 | 136/3399 | 1.1 (0.92, 1.31) | 1.09 (0.91, 1.3) | 1.1 (0.92, 1.31) | 1.09 (0.92, 1.3) |
| P value | 0.294 | 0.337 | 0.284 | 0.326 | ||
| P FDR | 0.340 | 0.373 | 0.360 | 0.416 | ||
| 55–65 | 4448/58,336 | 312/3571 | 1.14 (1.02, 1.28) | 1.14 (1.02, 1.28) | 1.15 (1.02, 1.29) | 1.15 (1.02, 1.29) |
| P value | 0.022 | 0.025 | 0.018 | 0.020 | ||
| P FDR | 0.027 | 0.031 | 0.024 | 0.027 | ||
| >65 | 2118/15,812 | 114/703 | 1.28 (1.06, 1.54) | 1.28 (1.06, 1.54) | 1.27 (1.05, 1.54) | 1.27 (1.05, 1.54) |
| P value | 0.011 | 0.011 | 0.012 | 0.012 | ||
| P FDR | 0.021 | 0.022 | 0.025 | 0.028 | ||
| Men | ||||||
| <45 | 759/17,316 | 24/405 | 1.3 (0.86, 1.95) | 1.31 (0.87, 1.97) | 1.29 (0.86, 1.94) | 1.3 (0.86, 1.95) |
| P value | 0.212 | 0.198 | 0.220 | 0.210 | ||
| P FDR | 0.265 | 0.250 | 0.298 | 0.283 | ||
| 45–55 | 3047/35,519 | 84/890 | 1.09 (0.88, 1.36) | 1.1 (0.88, 1.36) | 1.11 (0.9, 1.38) | 1.11 (0.89, 1.38) |
| P value | 0.414 | 0.4 | 0.336 | 0.339 | ||
| P FDR | 0.414 | 0.445 | 0.375 | 0.410 | ||
| 55–65 | 7520/48,442 | 173/1099 | 1.02 (0.88, 1.18) | 1.02 (0.87, 1.18) | 1.02 (0.88, 1.19) | 1.02 (0.88, 1.19) |
| P value | 0.82 | 0.829 | 0.755 | 0.781 | ||
| P FDR | 0.82 | 0.829 | 0.755 | 0.781 | ||
| >65 | 3315/14,581 | 55/272 | 0.89 (0.68, 1.16) | 0.89 (0.68, 1.16) | 0.9 (0.69, 1.17) | 0.9 (0.69, 1.17) |
| P value | 0.401 | 0.397 | 0.435 | 0.428 | ||
| P FDR | 0.401 | 0.419 | 0.473 | 0.492 | ||
Adjusteda for sex, age, race, income level, education, BMI, family history of cardiovascular disease, comorbidities, and lifestyle factors, including physical activity, diet, sleep pattern, and drinking; traditional risk factor control including glycated hemoglobin control, blood pressure control, low-density-lipoprotein cholesterol control, and no current smoking; HR, hazard ratio; CI, confidence interval
The relative importance of migraine in predicting atherosclerotic cardiovascular disease
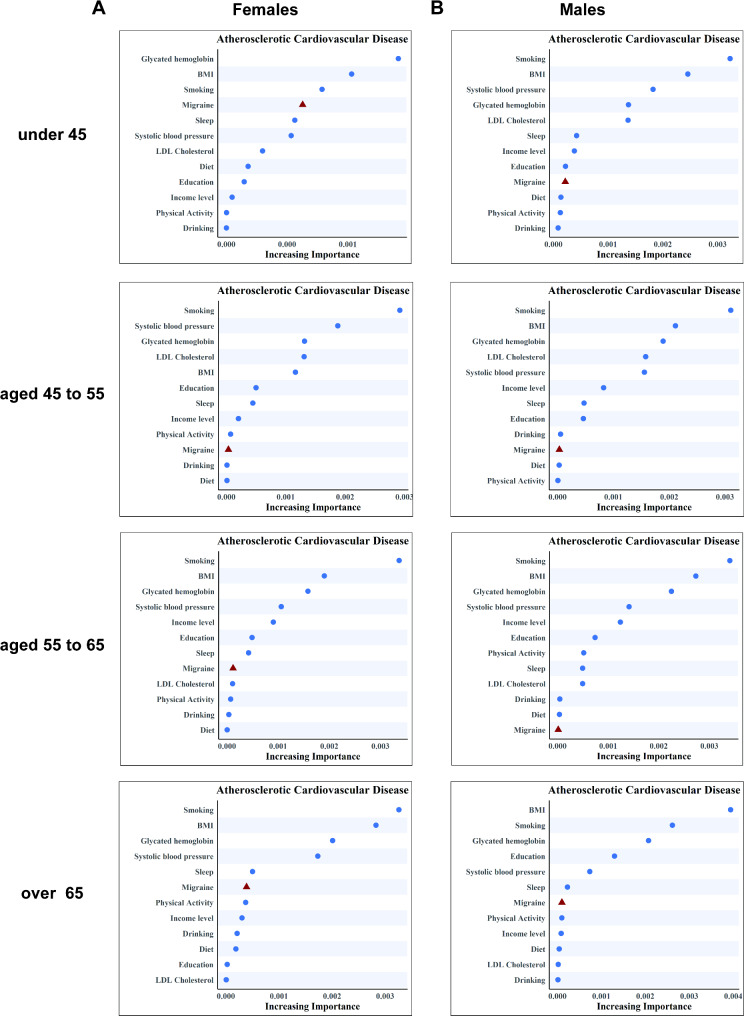
We evaluated the relative significance of migraine as a predictor of ASCVD among participants of the UK Biobank, comparing it to other established risk factors (Fig. 1). Our findings indicate that, within the selected variables, migraine was the fourth most significant predictor of ASCVD risk in women under the age of 45. HbA1c, BMI, and smoking surpassed its predictive capability, yet it was comparable to sleep patterns and systolic blood pressure. Importantly, migraine demonstrated a higher predictive value than LDL cholesterol and lifestyle factors such as diet, physical activity, and alcohol consumption. However, the predictive value of migraine for ASCVD was relatively much lower in the total population and other subgroups (see Supplementary material online, Fig. 2).
Fig. 1.
The relative importance of risk factors for predicting atherosclerotic cardiovascular disease
A. The relative importance of migraine as a predictor of atherosclerotic cardiovascular disease in females, stratified by age groups (under 45, 45–55, 55–65, and over 65 years)
B. The relative importance of migraine as a predictor of atherosclerotic cardiovascular disease in males, stratified by age groups (under 45, 45–55, 55–65, and over 65 years)
BMI, body mass index; LDL, low-density lipoprotein
Joint association of migraine and risk factor control with atherosclerotic cardiovascular disease
We evaluated the combined impact of migraine and risk factor control on ASCVD risk (see Supplementary material online, Table S12). Compared to individuals without migraine and high-risk factor control (4 factors), those without migraine but minimal control (0–1 factors) had an HR of 1.58 (95% CI: 1.48–1.69). For individuals with migraine and similarly low levels of risk factor control, the HR was 1.57 (95% CI: 1.33–1.85). No significant multiplicative (P = 0.062) or additive (P = 0.123) interactions between migraine and risk factor control levels affected ASCVD risk. A sensitivity analysis with a stricter LDL cholesterol cut-off (< 100 mg/dL) yielded similar results (see Supplementary material online, Table S13). To further investigate the influence of individual risk factors, we performed additional analyses employing restricted cubic splines to assess the associations between HbA1c, LDL-C, and SBP with various cardiovascular outcomes in patients with migraine. Supplementary Fig. 3 illustrates that HbA1c levels exceeding 35 mmol/mol were correlated with an elevated risk across all outcomes, indicating that migraine patients may benefit from more stringent glycemic control than the standard target of < 48 mmol/mol. Conversely, LDL-C and SBP demonstrated linear relationships with ASCVD risk, which were consistent with established control thresholds (LDL-C < 130 mg/dL and SBP < 140 mmHg). Some associations lacked statistical significance, likely due to the small subgroup size.
Joint association of migraine and lifestyle factor scores with atherosclerotic cardiovascular disease
Our analysis evaluated how migraine and lifestyle factors together affect ASCVD risk (Table 4). Compared to the reference cohort, comprising individuals without migraine who adhered to a healthy lifestyle (4 points), the HR for individuals without migraine but engaging in an unhealthy lifestyle (0–1 points) was 1.27 (95% [CI]: 1.20–1.34). This HR increased to 1.51 (95% CI: 1.32–1.72) for individuals with migraine who also followed an unhealthy lifestyle (0–1 points). A significant additive interaction between migraine and lifestyle scores on ASCVD risk was observed (Table 5). The relative excess risk due to interaction (RERI) was 0.36 (95% CI: 0.07–0.66), and the attributable proportion (AP) of the additive interaction was 24.02% (95% CI: 5.93–42.11%), with a P-value of 0.016. We also discovered a notable multiplicative interaction between migraine and lifestyle scores on ASCVD risk (P = 0.041). Subgroup analysis among individuals younger than 45 years (Supplementary Table S14–S15) yielded consistent trends. Compared to the reference group, individuals without migraine but with an unhealthy lifestyle (0–1 points) exhibited an HR of 1.76 (95% CI: 1.26–2.46), while those with both migraine and an unhealthy lifestyle demonstrated an HR of 2.24 (95% CI: 1.40–3.58). In the additive interaction model, the RERI was 1.44 (95% CI: -0.22–3.11) and the AP was 53.04% (95% CI: 8.45–98.52%), with a P-value of 0.022. However, the 95% CI for RERI included zero (-0.22 to 3.11), necessitating cautious interpretation. No significant multiplicative interaction was detected (P = 0.977).
Table 4.
Joint association of migraine and healthy lifestyle scores with the risk of ASCVD
| Migraine Status | Healthy lifestyle scores | |||||
|---|---|---|---|---|---|---|
| Healthy (4 points) | intermediate (2–3 points) | poor (0–1 points) | ||||
| Cases/total | HR (95% CI) | Cases/total | HR (95% CI) | Cases/total | HR (95% CI) | |
| No | 1744/25,328 | 1 (reference) | 14,850/168,223 | 1.12(1.07,1.18) | 6501/60,500 | 1.27(1.20,1.34) |
| Yes | 57/1191 | 0.88(0.67,1.14) | 630/7923 | 1.27(1.16,1.39) | 256/2629 | 1.51(1.32,1.72) |
| P for multiplicative interaction = 0.041 | ||||||
| P for additive interaction = 0.016 | ||||||
Four lifestyle factors including physical activity, diet, sleep pattern, and drinking; (range from 0 to 4), models are adjusted for age, sex, race, income level, educational attainment, family history of CHD or stroke, BMI, comorbidities, and traditional risk factor control
Table 5.
Attributing effects to the interaction between migraine and poor healthy lifestyle scores on ASCVD risk
| ASCVD | |||
|---|---|---|---|
| Multiplicative interaction | Estimate(95%CI) | P value | |
| 1.356(1.013,1.816) | 0.041 | ||
| Additive interaction | Relative excess risk due to interaction | Attributable proportion, % | P value |
| 0.36(0.07,0.66) | 24.02 (5.93,42.11) | 0.016 |
Models are adjusted for age, sex, race, income level, educational attainment, family history of CHD or stroke, BMI, comorbidities, and traditional risk factor control
Acute treatment of migraine shows no significant association with the risk of atherosclerotic cardiovascular disease
We examined the influence of several acute-phase medications on the risk of ASCVD, including triptans, ergotamines, NSAIDs, and acetaminophen. By comparing the cardiovascular risk among migraine patients who used these medications with those who did not, our analysis found no statistically significant association between the use of any of these medications and the overall risk of ASCVD or its subtypes. Thus, our study fails to offer adequate proof of the notable influence of these acute-phase medications on cardiovascular risk. (see Supplementary material online, Table S16).
Discussion
This decade-long study found that women with migraine had a higher risk of atherosclerotic cardiovascular disease, coronary artery disease, and ischemic stroke, but not peripheral artery disease. Conversely, men exhibited a 35% increased risk of PAD. The study further confirmed that migraine itself, rather than its treatments, is responsible for the increased cardiovascular risk. Moreover, the presence of both migraine and a low lifestyle score substantially amplified the risk of ASCVD, indicating significant additive and multiplicative interactions.
Numerous population-based studies [6, 21, 22] and meta-analyses [21, 23] have found a strong link between migraine and cardiovascular events, especially in women. A 2006 Women’s Health Study reported that women with migraine and aura had a higher risk of major coronary heart disease events with an HR of 2.08 (95% CI: 1.30–3.31) [24]. Subsequent investigations by the same research group identified analogous patterns in men, reporting an HR of 1.42 (95% CI: 1.15–1.77) [25], and another study showed that the risk of MI associated with migraine, regardless of aura, was consistent across genders (HR: 2.2, 95% CI: 1.7 to 2.8) [26]. Research on women indicates that migraine with aura significantly increases the risk of ischemic stroke, while migraine without aura does not [24, 27, 28]. A 2018 Danish study [6] identified a positive correlation between migraine and IS, with an HR of 2.26 (95% CI: 2.11 to 2.41). However, it reported no significant link with PAD. These findings support our observation that female migraine patients have a higher stroke risk. However, unlike the Danish study, our research shows that migraine is linked to an increased risk of PAD, with an HR of 1.25 (95% CI: 1.14–1.37) in the general population and 1.35 (95% CI: 1.22–1.49) in men. Limited research on age-related cardiovascular risks of migraine shows mixed results. Our study identifies an association between migraine and coronary artery disease, ischemic stroke, and peripheral artery disease, influenced by gender and age. Women under 45 with migraine face higher ASCVD risk, while men show no significant risk. We evaluated the significance of migraine compared to traditional ASCVD risk factors. Our findings suggest that, in women under the age of 45, migraine exhibits a stronger association with ASCVD risk than lifestyle factors such as diet, physical activity, and alcohol consumption.
Some biological mechanisms have been put forward to clarify the connection between migraine and increased cardiovascular event risk. These mechanisms encompass endothelial dysfunction [29], paradoxical embolism resulting from patent foramen ovale [30], genetic susceptibility [31, 32], and the utilization of NSAIDs or other analgesics. Additionally, cortical spreading depression associated with migraine aura may contribute to the occurrence of strokes and other vascular complications [33].
The study revealed that individuals with migraine exhibit a heightened risk of cardiovascular disease compared to those without the condition. However, among participants exhibiting poor control of risk factors, the risk of atherosclerotic cardiovascular disease was comparable between the two groups, with hazard ratios of 1.57 for migraine sufferers and 1.58 for non-sufferers. No significant interaction was identified between migraine and poor risk factor control. Even in the context of inadequate risk factor management, migraine did not substantially elevate ASCVD risk beyond the established risk factors, underscoring the predominant influence of traditional risk factors. Notably, our analysis demonstrated significant additive and multiplicative interactions between migraine and an unhealthy lifestyle, with their concurrent presence markedly increasing cardiovascular disease risk beyond the individual contributions of each factor. Specifically, the co-occurrence of migraine and a low healthy lifestyle score was associated with a 36% increase in ASCVD cases. Additive interactions provide crucial insights into the disproportionate impact of combined risk factors on specific subgroups, such as individuals with migraine. In contrast to multiplicative interactions, which concentrate on relative risks, additive interactions highlight the absolute increase in disease incidence. This focus renders them particularly pertinent for the development of targeted public health interventions aimed at addressing the distinct needs of high-risk populations [34]. In individuals under 45 years old, although the AP was significant, the RERI confidence interval included zero, limiting conclusions on additive interaction. Further research is needed to elucidate their combined impact on ASCVD risk.
In addition to lifestyle modifications, managing migraine encompasses both acute and preventive treatment strategies, dietary interventions, physiotherapy, and rehabilitation treatments [35, 36]. Acute treatment is designed to provide rapid and effective relief of symptoms during an attack and applies to all patients, whereas preventive treatment is tailored according to the frequency and severity of migraine episodes. Currently, a variety of acute treatments for migraine are available. Prior research has demonstrated that triptans and ergot alkaloids exhibit vasoactive properties; thus, they are not recommended for patients with a history of heart attack, stroke, or numerous vascular risk factors. Furthermore, NSAIDs have been associated with nephrotoxicity and cardiotoxicity. Therefore, we assessed the link between using triptans, NSAIDs, acetaminophen, and dihydroergotamine and the risk of atherosclerotic cardiovascular disease [37]. Our findings indicate that there is insufficient evidence to suggest that these medications elevate the risk of ASCVD. Hence, more randomized controlled trials are needed to evaluate the link between acute migraine treatments and ASCVD risk. This study explores the interactions among migraine, traditional cardiovascular risk factors, and lifestyle patterns with atherosclerotic cardiovascular disease risk. Utilizing extensive datasets and conducting long-term follow-up, the research identifies a significant additive and multiplicative effect of migraine and unhealthy lifestyle choices on ASCVD risk. In contrast, no significant interaction is observed with traditional cardiovascular risk factors. The study also investigates the link between acute migraine medications and ASCVD risk, providing new insights for improving ASCVD prevention in migraine patients. Still, there are a few limitations that should be mentioned. First, using self-reported migraine data might lead to errors in classification. Second, the lack of information regarding migraine aura precludes further stratification based on aura status. Third, the study fails to consider potential temporal variations in migraine frequency and the impact these fluctuations may have on the association between migraine and the incidence of cardiovascular diseases. Fourth, the self-reported medication information in the UK Biobank may involve certain inaccuracies. Additionally, as follow-up data on time-varying covariates were unavailable, our analysis relied only on baseline measurements, limiting our ability to capture changes in factors like lifestyle or migraine status.
Conclusion
Our research indicates that migraine elevates the long-term risk of developing atherosclerotic cardiovascular disease irrespective of acute treatment interventions. Notably, among females under the age of 45, migraine is identified as a more substantial predictor of ASCVD compared to several lifestyle risk factors. Furthermore, the combination of migraine and low lifestyle scores exhibits both greater than additive and multiplicative effects on the risk of cardiovascular disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our profound gratitude to all UK Biobank participants for their invaluable data contributions and to the committed staff engaged in executing the study.
Author contributions
Y. Huang, W. Yan, and Y. F. Jia contributed equally to this work and are joint first authors. Y. Huang, W. Yan, Y. F. Jia, Z.M. Xiao, and Y. J. Zhou designed the study. Y. Huang, W. Yan, Y. F. Jia, Q.F. Xie, Y. X. Lei and Z. P. Chen conducted the statistical analysis. Y. Huang, W. Yan, and Y. F. Jia drafted the manuscript.Y. J. Zhou and Z.M. Xiao revised it. All authors thoroughly reviewed and approved the final manuscript.
Funding
Funding for this study was provided by the National Natural Science Foundation of China (82471577,81971055), the Interdisciplinary Innovative Talents Foundation of Renmin Hospital at Wuhan University (JCRCYG-2022-006), Clinical Cooperation Project of serious diseases (National Administration of Traditional Chinese Medicine), Research on Degree and Graduate Education Teaching Reform at Wuhan University, Teaching Research Project of Wuhan University Medical Science Center (2024YB08).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was conducted using UK Biobank resources under application number 143136.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Huang, Wen Yan and Yifan Jia contributed equally to this work.
Contributor Information
Yanjie Zhou, Email: yanjiezhou@whu.edu.cn.
Zheman Xiao, Email: zmxiao@whu.edu.cn.
References
- 1.Mj JB, Rm C K, et al (2020) Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J 41. 10.1093/eurheartj/ehz962 [DOI] [PMC free article] [PubMed]
- 2.Ss M, Aw A, Zi A et al (2024) 2024 heart disease and stroke statistics: A report of US and global data from the American heart association. Circulation 149. 10.1161/CIR.0000000000001209 [DOI] [PMC free article] [PubMed]
- 3.Headache Classification Committee of the International Headache Society (IHS) (2023) The International Classification of Headache Disorders, 3rd edition - PubMed. https://pubmed.ncbi.nlm.nih.gov/29368949/. Accessed 11 Aug [DOI] [PubMed]
- 4.Vos T, Flaxman AD, Naghavi M et al (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet Lond Engl 380:2163–2196. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raggi A, Leonardi M, Arruda M et al (2024) Hallmarks of primary headache: part 1 - migraine. J Headache Pain 25:189. 10.1186/s10194-024-01889-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelborg K, Szépligeti SK, Holland-Bill L et al (2018) Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 360:k96. 10.1136/bmj.k96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(2021) ESC Guidelines on cardiovascular disease prevention in clinical practice| European Heart Journal| Oxford Academic. https://academic.oup.com/eurheartj/article/42/34/3227/6358713?login=false. Accessed 27 Sep 2024
- 8.Rs V, Rj S, van den Er H (2022) Temporal trends in incidence of premature cardiovascular disease over the past 7 decades: the Framingham heart study. J Am Heart Assoc 11. 10.1161/JAHA.122.026497 [DOI] [PMC free article] [PubMed]
- 9.Khan MA, Hashim MJ, Mustafa H et al (2020) Global epidemiology of ischemic heart disease: results from the global burden of disease study. Cureus 12:e9349. 10.7759/cureus.9349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson PW, D’Agostino RB, Levy D et al (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847. 10.1161/01.cir.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 11.Goff DC, Lloyd-Jones DM, Bennett G et al (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/american heart association task force on practice guidelines. Circulation 129:S49–73. 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 12.Peng K, Li X, Wang Z et al (2022) Association of low-density lipoprotein cholesterol levels with the risk of mortality and cardiovascular events: A meta-analysis of cohort studies with 1,232,694 participants. Med (Baltim) 101:e32003. 10.1097/MD.0000000000032003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullah SM, Defina LF, Leonard D et al (2018) Long-Term association of low-Density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-Year risk of atherosclerotic cardiovascular disease. Circulation 138:2315–2325. 10.1161/CIRCULATIONAHA.118.034273 [DOI] [PubMed] [Google Scholar]
- 14.Navarese EP, Andreotti F, Raggi P et al (2019) Baseline low-density lipoprotein cholesterol to predict the extent of cardiovascular benefit from lipid-lowering therapies: a review. Eur Heart J Cardiovasc Pharmacother 5:47–54. 10.1093/ehjcvp/pvy038 [DOI] [PubMed] [Google Scholar]
- 15.Mach F, Baigent C, Catapano AL et al (2020) 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41:111–188. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 16.He P, Zhang Y, Ye Z et al (2023) A healthy lifestyle, life’s essential 8 scores and new-onset severe NAFLD: A prospective analysis in UK biobank. Metabolism 146:155643. 10.1016/j.metabol.2023.155643 [DOI] [PubMed] [Google Scholar]
- 17.Lourida I, Hannon E, Littlejohns TJ et al (2019) Association of lifestyle and genetic risk with incidence of dementia. JAMA 322:430–437. 10.1001/jama.2019.9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan M, Sun D, Zhou T et al (2020) Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J 41:1182–1189. 10.1093/eurheartj/ehz849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stensrud MJ, Hernán MA (2020) Why test for proportional hazards? JAMA 323:1401–1402. 10.1001/jama.2020.1267 [DOI] [PMC free article] [PubMed]
- 20.Ma H, Zhou T, Li X et al (2022) Early-life educational attainment, APOE Ε4 alleles, and incident dementia risk in late life. GeroScience 44:1479–1488. 10.1007/s11357-022-00545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schürks M, Rist PM, Bigal ME et al (2009) Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 339:b3914. 10.1136/bmj.b3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gudmundsson LS, Scher AI, Aspelund T et al (2010) Migraine with aura and risk of cardiovascular and all cause mortality in men and women: prospective cohort study. BMJ 341:c3966. 10.1136/bmj.c3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmoud AN, Mentias A, Elgendy AY et al (2018) Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 8:e020498. 10.1136/bmjopen-2017-020498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurth T, Gaziano JM, Cook NR et al (2006) Migraine and risk of cardiovascular disease in women. JAMA 296:283–291. 10.1001/jama.296.3.283 [DOI] [PubMed] [Google Scholar]
- 25.Kurth T, Gaziano JM, Cook NR et al (2007) Migraine and risk of cardiovascular disease in men. Arch Intern Med 167:795–801. 10.1001/archinte.167.8.795 [DOI] [PubMed] [Google Scholar]
- 26.Bigal ME, Kurth T, Santanello N et al (2010) Migraine and cardiovascular disease. Neurology 74:628–635. 10.1212/WNL.0b013e3181d0cc8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma TK, Cs S K, et al (2005) Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 64. 10.1212/01.WNL.0000154528.21485.3A [DOI] [PubMed]
- 28.Kurth T, Schürks M, Logroscino G et al (2008) Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ 337:a636. 10.1136/bmj.a636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tietjen GE, Khubchandani J, Herial N et al (2018) Migraine and vascular disease biomarkers: A population-based case-control study. Cephalalgia Int J Headache 38:511–518. 10.1177/0333102417698936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwerzmann M, Nedeltchev K, Lagger F et al (2005) Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology 65:1415–1418. 10.1212/01.wnl.0000179800.73706.20 [DOI] [PubMed] [Google Scholar]
- 31.Eikermann-Haerter K, Lee JH, Yuzawa I et al (2012) Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation 125:335–345. 10.1161/CIRCULATIONAHA.111.045096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winsvold BS, Bettella F, Witoelar A et al (2017) Shared genetic risk between migraine and coronary artery disease: A genome-wide analysis of common variants. PLoS ONE 12:e0185663. 10.1371/journal.pone.0185663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreier JP (2011) The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 17:439–447. 10.1038/nm.2333 [DOI] [PubMed] [Google Scholar]
- 34.VanderWeele TJ, Robins JM (2007) The identification of synergism in the sufficient-component-cause framework. Epidemiol Camb Mass 18:329–339. 10.1097/01.ede.0000260218.66432.88 [DOI] [PubMed] [Google Scholar]
- 35.Jibril AT, Shab-Bidar S, Djafarian K et al (2022) Effect of major dietary interventions on migraine: a systematic review of randomized control trials. SN Compr Clin Med 4:185. 10.1007/s42399-022-01270-6 [Google Scholar]
- 36.Onan D, Arıkan H, Can İ et al (2024) Comparative insights into physiotherapy expectations among chronic migraine patients with and without OnabotulinumtoxinA treatment: A Case–Control study. SN Compr Clin Med 6:125. 10.1007/s42399-024-01759-2 [Google Scholar]
- 37.Buse DC, Reed ML, Fanning KM et al (2017) Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: results from the American migraine prevalence and prevention (AMPP) study. Headache 57:31–44. 10.1111/head.12962 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.