Abstract
Background
The differentiation characteristics of neutrophils within the gastric cancer (GC) tumor microenvironment (TME) and their interactions with malignant gastric epithelial cells require further investigation. Furthermore, the therapeutic potential of tumor-associated neutrophils (TANs) in immunotherapy remains inadequately explored.
Methods
We integrated two single-cell transcriptome datasets comprising 12 samples, including gastric primary tumors, non-tumor tissues, and metastatic tumors, to profile the epithelial cells and TANs atlas within the TME and examine their interaction modules. In addition, these data were integrated with the bulk transcriptomic including the Cancer Genome Atlas - Stomach Adenocarcinoma (TCGA-STAD) and Asian Cancer Research Group (ACRG) datasets to analyze the expression levels of neutrophil-associated genes across the tumor-associated neutrophil subsets.
Results
We analyzed 3,118 gastric epithelial cells and 2,365 TANs from all samples. Epithelial cells were classified into ten subclusters, while TANs were grouped into five subclusters. In gastric primary tumors, epithelial cell subtypes included primarily MUC16 + and stem-like populations. In metastatic tumors, the epithelial cell subset with high CXCL5 expression was a characteristic subtype. TANs mainly interacted with epithelial cells via the LGALS9-CD45 and CD46-JAG1 pathways. And RGS2 was highly expressed in N4, a tumor-associated neutrophils subcluster characterized by high MMP9 expression, highlighting its potential as an immunotherapy target.
Conclusion
TANs exhibit robust interactions with gastric malignant epithelial cell subsets. Furthermore, RGS2, which is highly expressed in N4, could serve as a promising target for immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-025-03920-0.
Keywords: Gastric cancer, Tumor-associated neutrophils, ScRNAseq, Gastric metastatic tumor, Tumor microenvironment
Introduction
GC ranks fifth globally in both incidence and mortality among all cancers, with over 70% of cases occurring in Asia [1]. Consequently, GC presents a significant global public health challenge, threatening human health and placing a considerable strain on healthcare systems. Despite a gradual decline in GC incidence worldwide [2–4], many patients are diagnosed with distant metastases, including to the lymph nodes, liver, peritoneum, or other organs. Even among patients undergoing curative surgery, the risk of postoperative recurrence or metastasis remains substantial, and overall survival rates are poor [5]. Investigating novel mechanisms driving the development and metastasis of GC remains a key focus of current research efforts in its treatment.
Advances in experimental and clinical research on tumor immunotherapy have enabled the application of immune checkpoint inhibitors (ICBs) in both late-stage and perioperative treatments of advanced GC [6–7]. Patients with Epstein-Barr virus (EBV)-positive and microsatellite instability-high (MSI-H) GC demonstrate greater responsiveness to immunotherapy than those with chromosomal instability (CIN) or genome stability (GS) subtypes [8]. This highlights the importance of further molecular characterization of GC to enhance the precision of immunotherapy and targeted treatments. Recent studies indicate that dual immune therapies for GC improve pathological complete response (pCR) rates [9–10]. However, ICBs such as PD-1/PD-L1 and CTLA-4 primarily targeted T cell-mediated anti-tumor responses [11–12]. Despite their efficacy, patients’ responses to ICBs therapy are highly variable, with many failing to achieve long-term benefits. Thus, identifying additional immune checkpoints, improved treatment strategies and combinatorial therapies remains crucial for advancing cancer therapy. Activation of the Wnt signaling pathway has been identified as a crucial regulatory mechanism in GC development. Several studies have reported upregulation of Wnt-associated proteins, such as Wnt-1, Wnt-2, and Wnt-5a, in GC. In contrast, inhibitors of this pathway — Dickkopf-related protein 1 (DKK-1) and Axin — are often downregulated or lost in GC [13]. Notably, DKK-1 also plays a role in modulating the tumor immune microenvironment and is currently being evaluated in a clinical trial (NCT02013154) as a potential immunotherapeutic target. Another promising immune target is CLDN18, a member of the claudin family, which has been found to be highly expressed in several malignancies, especially gastrointestinal cancers. A chimeric antigen receptor (CAR) T cell therapy specifically targeting CLDN18.2 has demonstrated encouraging therapeutic potential in patients with CLDN18.2-positive advanced gastrointestinal tumors [14].
Neutrophils, as key immune defense cells, have garnered increasing attention in recent years. Within the TME, neutrophils can polarize into anti-tumor (N1) or pro-tumor (N2) subtypes [15]. Activated neutrophils can form neutrophil extracellular traps (NETs), facilitating tumor metastasis [16]. Myeloid-derived suppressor cells (MDSCs), distinct from normal neutrophils, contribute to tumor progression and metastasis within the TME [17–18]. CD300ld, a potential immunotherapy target, is highly expressed on polymorphonuclear-MDSCs (PMN-MDSCs), attracting significant interest [19]. Nevertheless, research on neutrophils and their specific roles in tumor progression remains limited, highlighting the necessity of identifying neutrophil-related targets to advance cancer therapy.
We utilized single-cell RNA sequencing data from primary gastric tumors, adjacent non-tumor tissues, and metastatic tumors to construct a comprehensive atlas of epithelial cells and neutrophils within the tumor microenvironment. This analysis enabled the identification and characterization of distinct subpopulations of epithelial cells and TANs, shedding light on their potential interactions. Our findings underscore the active involvement of specific neutrophil subpopulations in GC progression and reveal promising therapeutic targets for intervention.
Methods
Data retrieval and sources
This study utilized single-cell transcriptome data of primary gastric tumors, non-tumor gastric tissues and metastatic gastric tumors from two distinct cohorts. The GEO database (GSE163558) includes three gastric primary tumor samples (PT1, PT2, PT3) [20], one adjacent non-tumor sample (NT1), and six gastric metastatic tumor samples of GC from the liver (n = 2) (Li1, Li2), peritoneum (n = 1) (P1), ovary (n = 1) (O1) and lymph node metastases (n = 2) (LN1, LN2). Additionally, the scRNA-seq data of GSE184198 dataset were derived from a cohort consisting of one primary tumor tissue (PT4) and one adjacent normal gastric tissue (NT2) from a single GC patient. For further diagnostic value ROC Analysis of key TANs-related genes from specific subclusters, we sourced stomach adenocarcinoma data, along with complete clinical information, from ACRG cohort [21].
Processing of scRNA-seq data
We processed the scRNA-seq data using R software (version 4.4.1) and the Seurat R package (v5.0.1) [22]. Low-quality cells were filtered out based on the following criteria: fewer than 200 or more than 5500 expressed genes, unique molecular identifers (UMIs) counts exceeding 1000 or ranking in the top 3%, and mitochondrial gene ratios greater than 20%. After quality control, 56,725 single cells were retained. The batch effect was corrected by the function of “Harmony”. The “NormalizeData” and “ScaleData” functions were employed to normalize and scale the transcriptome data, ensuring comparability of gene expression matrices. Principal component analysis (PCA)was conducted using the top 2000 hypervariable genes to determine the principal components (PCs) for further analysis. To address batch effects, we used the “IntegrateLayers” function with the Harmony method to integrate multiple samples. Twenty PCs were selected to calculate intercellular distances, and the clustering resolution was set to 0.2 to identify distinct cell clusters. Finally, UMAP was applied to visualize the high-dimensional gene expression data in a two-dimensional space.
Identification and annotation of cell clusters and subsets
To annotate cell types, we initially examined the expression of canonical marker genes in each cell cluster, referencing findings from previous studies. To further stratification of cell subpopulations, we employed the “FindAllMarkers” function with the parameters logfc.threshold = 0.25 and min.pct = 0.25 to identify differentially expressed genes (DEGs) within each cell subset. DEGs were filtered using criteria of p-value < 0.05 and log2FoldChange (log2FC) > 2. The resulting genes were subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses to elucidate the biological functions of the cell subsets [23–24], and adjusted p.value < 0.01 was used to filter the functional candidates.
Identifying malignant or non-malignant epithelial cell subsets
The “AddModulScore” function was employed to estimate the “Gastric Cancer” score for each epithelial subset to distinguish the malignant from non-malignant cell subsets. This classification was further validated by analyzing chromosomal copy-number variations (CNVs) with the “Infer CNV” R package [25].
Cellular pseudotime analysis
Pseudotime trajectory is a computational method based on single-cell RNA sequencing data employed to analyze the changes in gene expression across different stages of cell development. We utilized the “Monocle 2” R package to assess cellular development and state transitions. Specifically, the “dispersionTable” function was employed to identify the significantly altered genes (mean expression ≥ 0.01) that reflect differentiation state among cell subsets. Concurrently, the “cytoTRACE” R package was used to validate the starting point of cellular differentiation by estimating cellular potency through gene co-expression patterns analysis [26].
Analysis of metabolism characteristics of cell subsets
To investigate active metabolic functions of each cell subset and identify metabolic variations across different cell types, we used the “scMetabolism” R package with the AUCell method [27]. This analysis, based on scRNA-seq data and metabolic pathways curated from the KEGG database., quantified the expression of genes associated with specific metabolic pathways.
Detection of cell‒cell communication
The R package “CellChat” [28] was applied to analyze cell-cell interactions. This analysis aimed to quantify and assess the communication frequency and interaction strength between TANs and epithelial cell subsets based on the “CellChatDB. human” ligand-receptor database. To accomplish this, we isolated and subsetted the expression data corresponding to these two cell types.
Screening of tans marker genes
To assess the discrepancy in neutrophil infiltration between gastric primary tumors and non-tumor tissues, we utilized multiple computational tools, including CIBERSORT, CIBERSORT-ABS, MCPcounter, quanTIseq, TIMER, and xCell. Among these, xCell was selected for its ability to identify infiltration-associated genes effectively.
Identification of differentially expressed genes (DEGs)
DEGs between normal and tumor GC samples were identified using the “limma” package of R (version 3.60.3). Genes with P-value < 0.05 and log2FoldChange (log2FC) > 2 were considered significant. A Venn diagram was used to illustrate the overlapping DEGs shared by both tumor-associated neutrophil (TAN) subgroups and neutrophil infiltration-related genes.
Statistical analysis
Statistical analyses and results visualizations were performed using R software. The Wilcoxon Rank Sum test was applied to identify DEGs between various cell clusters. Pearson’s correlation coefficient was used to measured the linear correlation between two continuous variables. A P-value of less than 0.05 was considered statistically significant.
Results
Identification of cell clusters and analysis of distribution patterns in gastric metastatic tumor
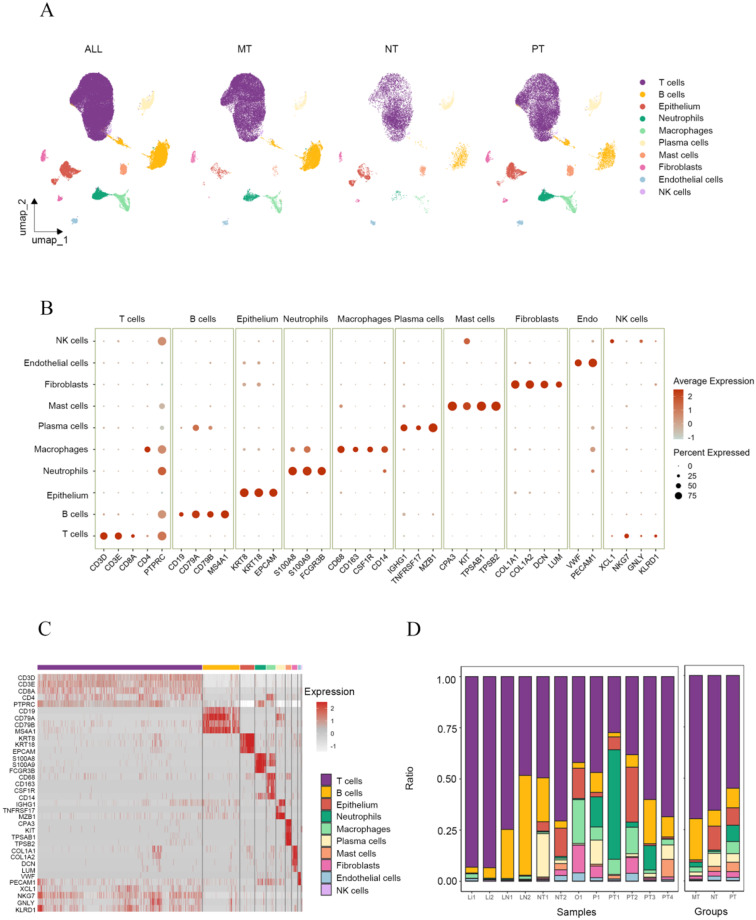
After quality control (Additional file: Fig. S1A-B) and batch effect correction (Additional file: Fig. S1C-D), we analyzed the remaining 56,725 single cells. These cells were divided into 13 clusters (Additional file: Fig. S1E) and classified into 10 cell types (Fig. 1A). Gene markers were used to identify the cell types (Fig. 1B-C). Cell clusters with high expression of KRT8, KRT18 and EPCAM were annotated as epithelial cells. Clusters expressing of COL1A1, COL1A2, DCN and LUM were identified as fibroblasts. While those with high levels of VWF and PECAM1 were identified as endothelial cells. Immune cells were further classified into T cells (CD3D, CD3E, CD8A), neutrophils (S100A8, S100A9, FCGR3B), macrophages (CD68, CD163, CSF1R, CD14), NK cells (XCL1, NKG7, GNLY, KLRD1), B cells (CD19, CD79A, CD79B, MS4A1), plasma cells (IGHG1, TNFRSF17, MZB1), and mast cells (CPA3, TPSAB1, TPSB2) [20, 29].
Fig. 1.
Overview of the single-cell transcriptome landscape for non-tumor tissues, primary tumors and metastatic tumors in GC. A UMAP plot showing the annotation of all cells from NTs, PTs and MTs into ten major cell types and the distribution of each cell type in NTs, PTs and MTs, highlighting the abundance variations among these groups. B Bubble diagram illustrating the expression levels of canonical gene markers in various cell populations. C Heatmap illustrating the expression levels of canonical gene markers across various cell populations. Color intensity reflects relative gene expression, with higher intensity indicating higher expression levels. D Stacked bar chart displaying the percentage of cell populations in each samples and each groups
To analyze heterogeneity during GC development and metastasis, we compared the composition of different cell types among three groups (Fig. 1A). The proportions of epithelial cells, fibroblasts, endothelial cells, and immune cells varied across non-tumor tissues (NTs), primary tumors (PTs), and metastatic tumors (MTs) (Fig. 1D). Myeloid cells (neutrophils, macrophages and mast cells) were more abundant in PTs than NTs, whereas their proportions were significantly reduced in MTs compared to PTs. The ratio of epithelial cells in NTs, PTs and MTs displayed a gradual decline. However, due to sampling discrepancies, further subdivision of epithelial cell clusters is required to explore their alterations during the development and metastasis of GC. T cells were the most abundant cell type across all samples, comprising approximately 65.59%, 54.89% and 69.74% of the cell population in NTs, PTs and MTs, respectively. Neutrophil infiltration was highest in PTs and lowest in NTs, indicating active recruitment of neutrophils during gastric carcinogenesis. Among the metastatic tissues of GC, peritoneal metastasis exhibited the highest proportion of infiltrating neutrophils. These trends were consistent across all samples (Fig. 1D). Overall, significant heterogeneity was observed among NTs, PTs, and MTs.
Identification of ten epithelial cell subclusters and their heterogeneous in GC carcinogenesis and metastasis
We analyzed 3118 gastric epithelial cells and performed dimensionality reduction and clustering anew, identifying ten subclusters (EPC0-9) at a resolution of 0.2 (Fig. 2A, Additional file: Fig. S2A). Based on gastric epithelial cells — specific marker genes — including PGA4, PGA3, and LIPF for chief cells (also known as zymogenic cells), and PROX1, CHGA, MS4A8, and SCG3 for endocrine cells [29] — EPC1 was identified as chief cells, exhibiting high expression of stomach-specific digestive enzyme and lipases (Additional file: Fig. S2B), consistent with marker gene annotation. EPC4 and EPC7 were identified as endocrine cells. Furthermore, malignant gastric epithelial cell markers, such as CLDN4, CLDN7, and TFF3, were highly expressed in EPC0, EPC2, EPC8 and EPC9 subclusters [20]. Additionally, EPC2 demonstrated elevated expression of marker genes associated with gastric stem/progenitor cells, such as MYC, FABP5, and NME1 [30], characterizing it as a stem-like epithelium (Fig. 2D).
Fig. 2.
Functional characteristics and changes of epithelial subclusters within the TME of GC. A-B UMAP plot and bar chart showing the distribution and proportion of each subcluster across groups. C The CNV profiles of each subcluster in three groups, using chief cells and endocrine cells in NTs as reference cells. D Dot plot showing the canonical marker genes of epithelial subtypes. E UMAP and boxplot illustrating the Gastric cancer score of each epithelial cell subcluster. F GO and KEGG enrichment analyses of MUC16 + epithelium
DEGs analysis of the remaining epithelial cell subclusters revealed distinct functional characteristics. DEGs in the EPC0 subcluster were enriched in immune-related pathways and cell adhesion biological processes, with highly expression of MUC16, a marker associated with cell metastatic capacity. Notably, MUC16 + epithelium displayed the highest EMT scores, and alongside the stem-like epithelium, predominated in the PTs group (Fig. 2F, Additional file: Fig. S2C). EPC3, marked by high CXCL5 expression, emerged as the dominant epithelial cell type in MTs, with DEGs enriched in ribosome biogenesis and cytoplasmic translation, suggesting heightened metabolic activity and roles in proliferation and metastasis. EPC5 showed associations with biological processes related to lymphocyte differentiation and leukocyte cell-cell adhesion, indicating regulatoty roles in immune cell activity within the TME. EPC8 exhibited gene expression patterns linked to phagocytosis and neutrophil extracellular traps (NETs), which are critical for promoting cancer metastasis by evading immune surveillance. Although EPC6 and EPC9 did not show significant biological pathways enrichment, top 10 DEGs analysis revealed that EPC6 displayed significant proliferative activity, marked by high MYCL expression, while EPC9 highly expressed tumor necrosis factor (TNF) (Additional file: Fig. S2B, 2D).
Furthermore, we evaluated the benign versus malignant characteristics of the epithelial cell subclusters. As previously noted, chief cells and endocrine cells expressed canonical genes, which are characteristically enriched in normal gastric epithelium (Fig. 2D). These subclusters were predominantly observed in NTs, sparsely distributed in PTs, and absent in MTs, highlighting their role as nonmalignant epithelial subtypes (Fig. 2B). Using the Gastric Cancer score [31], we determined that the malignancy grades of other subclusters were notably higher than those of the nonmalignant subclusters (Fig. 2E). Subsequently, we conducted inferCNV analysis on individual cell subclusters across different groups, using chief cells and endocrine cells from NTs as reference cells. Copy number variation (CNV) refers to variations in the number of copies of certain DNA sequences in specific regions of the genome. The CNV score can represent the degree of CNV variation. Subclusters in MTs and PTs exhibited chromosomal CNVs, unlike chief cells. Further analysis revealed that EPC6 had the highest CNV score, EPC3, EPC6, and EPC9 displayed elevated scores in the PT group (Fig. 2C, Additional file: Fig. S2E). Notably, EPC6 also showed a relatively high score, possibly attributable to sampling bias.
Trajectory inference of epithelial cells in GC scRNA-seq
Cancer development involves a process of dedifferentiation [32–33]. To investigate variations in epithelial cell differentiation states during carcinogenesis and metastasis, we extracted epithelial expression data from GC scRNA-seq. Our results revealed that MUC16 + epithelium and stem-like epithelium were the final cell subtypes to emerge during cancer cell dedifferentiation. These cells also exhibited the highest differentiation potential score (DPS), consistent with their stem-cell characteristics observed in stem-like epithelium. In contrast, chief cells and endocrine cells exhibited relatively intermediate level of differentiation. Interestingly, EPC3 and EPC6, the dominant epithelial cell types in MTs (liver and lymph node metastases), had the lowest DPS, suggesting a reduced differentiation capacity in these malignant subclusters. These findings indicate that epithelial cell subclusters in metastatic tumors seem to be the higher level of differentiation, while in the gastric primary tumor, epithelium is characterized by lowest level of differentiation (Fig. 2A, D). The variations in gene expression during epithelial cell differentiation are illustrated in the heatmap (Fig. 3B).
Fig. 3.
Differentiation and metabolic characteristics of epithelial subclusters within the TME of GC. A Pseudotime analysis diagram displaying the differentiation trajectory of each epithelial subset. B Significantly expressed pseudotime-related genes during epithelial cell differentiation. Colors indicate relative expression levels, with red representing high expression and blue representing low expression. C Active levels of metabolism-related pathways in each epithelial cell subcluster based on scRNA-seq data. D Box plot showing the differentiation score for each subcluster. A score closer to 1 indicates a lower differentiation status, while a score closer to 0 indicates a higher differentiation status
Metabolic difference in epithelial cell subsets
Cancer cells reprogram the metabolic pathways to enhance their proliferation, invasion, and adaptability [34]. To investigate metabolic alterations during gastric tumorigenesis and metastasis, we evaluated the metabolic pathway differences across epithelial cell subsets. Our findings revealed that MUC16 + epithelium and stem-like epithelium, the predominant cell subsets in gastric primary tumors, share similar metabolic profiles. In contrast, other cancer cell subsets also demonstrated distinct metabolic characteristics (Fig. 3C).
TANs diversity in gastric tumorigenesis and metastasis from singlecell perspective
To investigate the potential role of TANs in priming response mechanisms during gastric tumorigenesis and metastasis, we extracted a total of 2,365 TANs from all samples. These neutrophils were clustered into five subclusters (N0-N4) (Fig. 4A). N0 and N2 were the dominant subclusters in PTs, while N1 was identified as a key subcluster in MTs, particularly in peritoneal metastasis, where it represented the highest proportion among metastasis samples (peritoneum: 14.7%; liver: 0.5%; lymph node: 0.3%; ovary: 0.6%). The N3 subcluster was consistently detected across all groups (Fig. 4A and D).
Fig. 4.
Heterogeneity of TANs within the TME of GC and gastric metastases. A UMAP plot showing the distribution of each TAN subcluster across groups. B Violin plot displaying genes specifically expressed in TANs. C Top 10 DEGs in each TANs subcluster. D Stacked bar chart showing the proportion of each TAN subcluster across groups. E Pseudotime analysis illustrating the differentiation trajectory of each TAN subcluster. F Significantly expressed pseudotime-related genes during TANs differentiation. Colors indicate relative expression levels, with red representing high expression and blue representing low expression. G GO enrichment analyses of N0 cluster. H Box plot showing the differentiation score for each subcluster
We analyzed the top 10 specifically expressed genes in each TAN subcluster and performed GO enrichment analysis on the DEGs of each subcluster (Fig. 4G). The results indicated that the N0 subcluster was enriched in pathways related to immune activation, suggesting N0 is in a highly activated state. N0 also showed high expression of the SOCS3 gene (Fig. 4B), which encodes a suppressor involved in negative feedback regulation of cytokine signaling. And SOCS3 plays a critical role in inhibiting the JAK2/STAT signaling pathway, suppressing immune cell functions and contributing to tumor immune evasion [35]. Additionally, N0 expressed high levels of PROK2 and TUBA1A (Fig. 4B), both of which promote tumor angiogenesis and create an adaptive environment for cancer cell metastasis. These findings indicate that N0 exhibits characteristics of pro-tumor TANs, a type of polarized TANs. The N2 subcluster, another cluster type primarily distributed in PTs, showed high expression of PLAU and VEGFA (Fig. 4B), both closely associated with tumor cell proliferation and angiogenesis [36]. GO enrichment analysis further revealed that N2 was enriched in pro-tumor pathways. The N1 subcluster, a predominant type in MTs, specifically expressed interferon-related genes (ISG15, RSAD2, IFIT1, IFI44L, and HERC5) (Fig. 4C). This subcluster was involved in tumor immunity, aligning with the characteristics of immunomodulatory TANs. A study in vitro found that neutrophils effectively recruited to the tumor site in samples with favorable responses to immunotherapy showed upregulation of interferon-stimulated genes [37]. Finally, the N4 subcluster expressed high levels of MMP9 (Fig. 4B), a marker of pro-tumor TANs, although N4 cells were present in the lowest numbers. As a result, N1 was identified as a typical anti-tumor cell type in gastric metastases, while N4 was the pro-tumor type. N0 and N2 were the predominant pro-tumor types in gastric primary tumors, and N3 was present in all three groups (Fig. 4D). The N3 subcluster expressed both canonical markers of neutrophils and T/NK cells, along with cytotoxic granule-associated genes such as GZMA and GZMB, suggesting the presence of a potential hybrid immune cell population or a transitional cell state about this cluster.
Next, we performed pseudotime trajectory analysis for the subclusters (Fig. 4E). Although both N0 and N2 exhibited pro-tumor properties, their developmental trajectories diverged in different directions. Interestingly, the differentiation trajectory of N1 did not align with N2. During the process of maturation, the expression of CD2, RPL11, RPL5, RPS27, and RPS8 gradually decreased (Fig. 4H, F). The heatmap illustrated the dynamic changes in differentially expressed genes associated with key developmental transitions.
Analysis of cell‒cell interactions between epithelial cells and tans
Given that TANs can either promote or inhibit tumor growth depending on the molecular subtype, this section explores the specific interaction patterns between different TAN subtypes and GC epithelial cell subtypes based on the human ligand-receptor interaction database (CellChatDB.human). The results revealed strong interactions between various TAN and epithelial cell subclusters, with particularly strong interactions observed between the N0 cluster and the MUC16 + epithelium and stem-like epithelium that primarily distributed in the PTs (Fig. 5A).
Fig. 5.
Cell-cell interactions between epithelial cells and TANs. A The number and strength of communications between in epithelial and TAN subsets. B Heatmap showing the relative strength of specific incoming and outgoing signaling pathways between epithelial and TAN subsets. Color intensity reflects relative gene expression, with higher intensity indicating higher expression levels. C Hierarchical clustering illustrating distinct signaling pathways in the TME of gastric primary tumors. D Dot plot displaying detailed ligand-receptor communication probabilities targeting specific cell subsets. E Clustering analysis of communication patterns of secreting cells and target cells
While the incoming and outgoing signaling patterns between TANs and epithelial cells were generally similar (Fig. 5E). MUC16 + epithelium, stem-like epithelium, EPC8 and EPC9 demonstrated stronger outgoing signaling within the PT group. Conversely in TANs, the N2 subcluster exhibited relatively highest outgoing signal intensity. Remarkable ligand-receptor signaling pathways from epithelial cells to TANs included AGRN, NOTCH, Glutamate and GALECTIN. While TANs signaled to epithelial cells through the IL1, ADGRE and ICAM pathways (Fig. 5B). Moreover in metastatic tumors (MTs), EPC3 and EPC6 displayed relatively weak outgoing and incoming signaling activity, indicating limited interactions between TANs and epithelial cells within the metastatic group. MUC16 + epithelium and stem-like epithelium displayed strong interactions with N0 and N2. The LGALS9/CD45 signaling pathway is highly specific to interactions between the two type of epithelium subclusters with the N0 and N2. Conversely, the CD46/JAG1 signaling pathway acts as a key receptor-ligand axis targeted to the MUC16 + epithelium and stem-like epithelium clusters, with JAG1 in promoting tumor progression, epithelial-mesenchymal plasticity, and cancer stem cell (CSC) properties [38] (Fig. 5C and D). And the interaction between CD46 and JAG1 can regulate the TH1 immunity response has been investigated by previous report [39]. But its role in GC progression remains to be experimentally validated.
Validation and ROC analysis of key genes in tans subsets
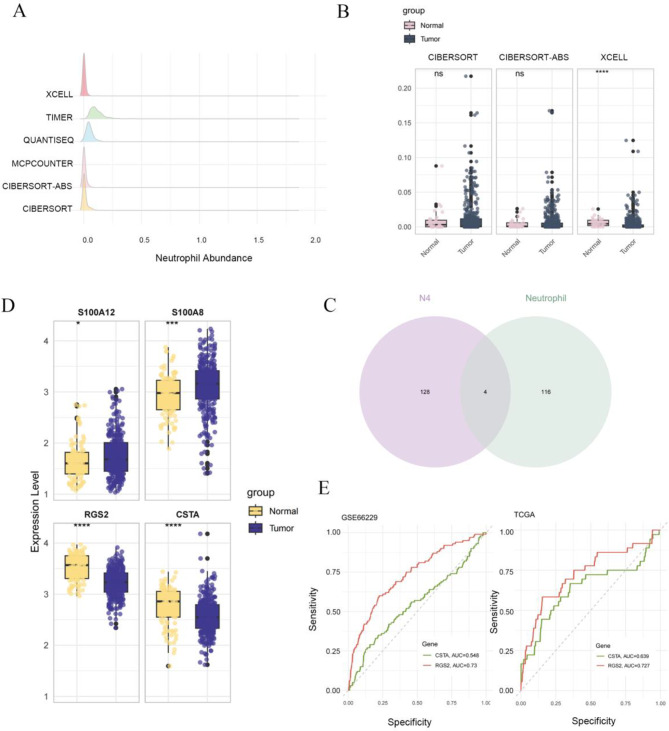
To investigate the role of TAN subclusters in tumorigenesis and their potential as targets for immunotherapy, we performed an in-depth analysis using six immune cell composition algorithms—CIBERSORT, CIBERSORT-ABS, MCPCOUNTER, QUANTISEQ, TIMER, and XCELL—to estimate neutrophil abundance within the TME based on gene expression data from the TCGA-STAD database. The results indicated relatively high neutrophil abundance as assessed by the XCELL, CIBERSORT-ABS, and CIBERSORT algorithms (Fig. 6A). We then categorized the TCGA-STAD transcriptomic data into normal and tumor groups to evaluate differences in neutrophil abundance between these groups using the three algorithms. The XCELL algorithm revealed a statistically significant difference in neutrophil abundance between tumor and normal samples (Fig. 6B).
Fig. 6.
Identification of gene markers for TAN subsets. A Evaluation of neutrophil abundance in TCGA-STAD data using six algorithms. B Box plot displaying the differential expression of TAN abundance cross three algorithms. C Venn diagram illustrating overlap down-regulated genes in gastric tumors from TCGA and DEGs in the N4 subset. D Expression levelS of four selected genes in GSE66229 dataset. E ROC curves calculating the diagnostic potential of CSTA and RGS2 in two datasets
Depending on neutrophil scores from the XCELL algorithm, we identified four downregulated genes from N4 subcluster associated with neutrophil infiltration that were differentially expressed between the tumor and normal groups (Fig. 6C). Among these genes, the expression patterns of RGS2 and CSTA in the GSE66229 dataset were consistent with those in TCGA-STAD (Fig. 6D). We further examined the diagnostic potential of these two genes in GC: in TCGA data, RGS2 had an area under the curve (AUC) of 0.727 and CSTA had an AUC of 0.639; in GSE66229, RGS2 achieved an AUC of 0.73, while CSTA had an AUC of 0.548. Notably, RGS2 expression within the N4 TAN subcluster exhibited a stronger classification effect (Fig. 6E), while in gastric caner patients, the inhibition of RGS2 expression seems significantly associated with poorer overall survival and neutrophil infiltration correlation. These findings suggest that the N4 subcluster may represent a suppressive tumor TAN phenotype. Targeting the RGS2 pathways in N4-associated could provide a novel therapeutic approach in GC. Furthermore, RGS2 holds potential as a biomarker for neutrophil-driven tumor biology.
Discussion
Given the high incidence and mortality rates of GC, exploring novel therapeutic approaches and improving patient outcomes remain central research themes. With immunotherapy gaining prominence in cancer treatment, the limitations of widely used ICBs drugs in achieving sustained disease remission underscore the urgent need for additional immunotherapeutic targets. In neutrophil-related immunotherapy research, blocking the IL-8–CXCR1/2 and C5a/C5aR pathways, which are critical for tumor-mediated neutrophil recruitment, emerges as a promising cancer treatment strategy. Furthermore, approaches aimed at restoring the antitumor immune functions of neutrophils, such as inhibiting CD47, STAT3, and TGF-β, offer significant therapeutic potential [40–43].
Previous studies have reported that neutrophils infiltrating the TME differentiate into two distinct subtypes: N1, with antitumor properties, and N2, which promotes tumor progression [13]. However, emerging evidence suggests that neutrophils transition through three differentiation states. Immature and mature neutrophils undergo reprogramming upon infiltrating the TME, transitioning into a terminal differentiation state (T3) characterized by high dcTRAIL-R1 expression [44]. Further findings reveal that TANs exhibit remarkable plasticity, and remodeling of the immune environment by TANs in the TME represents a key aspect of their role in tumor regulation. Including antigen-presenting functions initiated by leucine accumulation, which activates T cells and positively influences immune dynamics [45]. While the level of neutrophil infiltration in the TME has been correlated with patient prognosis, outcomes vary significantly. For instance, sustained neutrophil infiltration often promotes tumorigenesis and correlates with poor patient outcomes [46–47], whereas in colorectal cancer, high neutrophil infiltration is linked to improved overall survival due to enhanced interactions with CD8 + T cells [48]. These discrepancies may reflect anatomical and histological differences across cancer types. Recent studies have revealed that neutrophil ferroptosis within the TME is a critical driver of immune escape primarily by suppressing the activity of anti-tumor CD8 + T cells and thereby facilitating tumor progression. This process is regulated by a subset of CD4 + Tcells known as Fer-CD4 + T cells. Notably, inhibiting neutrophil ferroptosis has been shown to elicit anti-tumor immune responses [49]. In addition, neutrophil-derived NETs can promote tumor proliferation by further suppressing T cell function [50]. A study on pancreatic ductal adenocarcinoma found that TANs upregulate the expression of Nectin2 on their cell membrane, leading to CD8 + T cell exhaustion via direct cell-cell interaction [51]. Moreover, TANs can enhance macrophage activation and polarization towards a pro-tumor phenotype by abundantly secreting cytokines such as TGF-βwithin the TME [52]. Collectively, these findings underscore the pivotal role of TANs in reshaping the immunosuppressive TME and highlight their potential as promising targets for cancer immunotherapy. TANs also exert direct regulatory effects on tumor cells. TANs may also directly contribute to carcinogenesis and metastasis through the release of cytotoxic substances, such as nitric oxide (NO) which induce DNA damage [53–54]. Conversely, neutrophils expressing high levels of MET may inhibit tumor growth and metastasis via NO release. Our study seeks to elucidate whether TANs interact directly with gastric epithelial cells to influence tumorigenesis and metastasis. Additionally, we aim to identify TAN subclusters and key genes involved in GC progression, providing a theoretical foundation for future research.
Regulator of G-protein signaling 2 (RGS2), a member of the RGS family, is known to terminate G-protein coupled receptor (GPCR) signaling [55]. Its function has been reported in various cancers. RGS2 highly expressed in breast cancer [56] and is associated with the prognosis and cancer metastasis in prostate cancer [57]. Interestingly, the role of RGS2 appears to vary among different cancers. For instance, in high-grade serous ovarian cancer, reduced RGS2 expression correlates with adverse clinical outcomes [58]. Mechanistically, RGS2 can directly inhibit adenylyl cyclase activity and modulate mitogen-activated protein kinase pathways [59–60]. Additionally, RGS2 cab form a complex with Toll-like receptor 4 (TLR4), thereby preventing Gαq-mediated induction of interferon-gamma (IFN-γ) [61]. Given these diverse functional roles, its inhibition may enhance the efficacy of immune checkpoint inhibitors by promoting a more favorable immune landscape. This could improve immune cell activity to suppress the tumor cells. In our study, the opposite expression patterns of RGS2 in GC samples and the N4 subcluster suggest its context-dependent and potentially multifunctional roles in different cell types. Investigating its specific function in tumor-associated neutrophils (TANs) will be an important focus of our future research.We need further studies to explore this potential combination therapy and its clinical applications.
In this study, we identified epithelial cell subsets and their functional characteristics from datasets, shedding light on the heterogeneity of epithelial cells during GC carcinogenesis and metastasis. MUC16 + epithelium and stem-like epithelium represent the least differentiated epithelial subsets in primary gastric tumors. Previous research has shown that MUC16 promotes tumor cell proliferation via the JAK2/STAT3 pathway and enhances cell migration and invasion [62–64]. We observed that MUC16 + epithelium displayed elevated epithelial-to-mesenchymal transition (EMT) scores, indicating significant metastatic potential. Stem-like epithelium exhibited stem cell-like properties and high proliferative capacity. Importantly, our findings suggested that chief cells share differentiation trajectories with MUC16 + epithelium and stem-like epithelium, supporting the hypothesis that chief cells may serve as a potential origin of malignant epithelial cells [65]. In metastases, EPC3 and EPC6 were identified as dominant epithelial subsets, with EPC3 being almost absent in normal tissues. Both subsets, characterized by high differentiation levels, were considered primary drivers of tumor metastasis. Within the TME, infiltrating TANs exhibited diverse pro- and anti-tumor properties. For instance, N0 supported immune evasion and tumor angiogenesis, while N2, marked by PLAU and VEGFA co-expression, similarly facilitate angiogenesis [66]. In contrast, the N4 elevated MMP9 expression, signified pro-tumor activity and was predominantly found in liver metastasis samples. The N1 subcluster, with high interferon-stimulated gene (ISG) expression, was enriched in metastatic tissues, particularly peritoneal metastases [67–68]. MUC16 + epithelium and stem-like epithelium strongly interacted with N0, predominantly through the LGALS9/CD45 signaling pathway. In contrast, the CD46/JAG1 receptor-ligand axis played a pivotal role in interactions between epithelial subsets and TANs.
We identified DEGs in each TAN subset associated with neutrophil infiltration, and these genes also showed statistically significant differences between tumor and normal gastric tissues through analyzing data from the TCGA-STAD and ACRG databases. Notably, RGS2, a member of the regulator of G protein signaling (RGS) family [69], was highly expressed specifically in the N4 TAN subset. Although generally downregulated in gastric tumors, RGS2 was markedly upregulated within N4 TANs, suggesting potential as a diagnostic marker for GC. Its unique expression profile positions RGS2 as a promising therapeutic target, capable of modulating N4 TAN functionality during transitional differentiation states and influencing tumor progression.
This study provides a comprehensive evaluation of neutrophil diversity within the TME and their interactions with malignant epithelial cells in GC. These findings deepen our understanding of TAN functions in cancer progression and identify potential therapeutic targets for immunotherapy. However, Our study is based primarily on publicly available datasets and computational predictions. The imbalance in sample size between tumor and normal tissues, particularly in the TCGA-STAD dataset, may affect the statistical power of differential expression analyses. And the findings need further validations involving in vitro and in vivo assays — such as co-culture systems, Co-Immunoprecipitation (Co-IP), CRISPR and animal models — will be essential to confirm the exact biological roles of RGS2 and the CD46/JAG1-mediated interactions in TAN-driven GC progression and immune modulation. And although we employed robust quality control measures and integrated multiple datasets to minimize batch effects, these factors may still influence the interpretation of our results.
Conclusion
In conclusion, we found that TANs exhibit strong interactions with gastric malignant epithelial cell subsets and promote carcinogenesis through the CD46/JAG1 signaling pathway. Additionally, RGS2, which is highly expressed in N4 (MMP9 + neutrophils), could serve as a promising immunotherapy target. However, these findings require further experimental validation.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank the editor and reviewers for their constructive and valuable comments, which have significantly improved the quality and clarity of our manuscript. We also gratefully acknowledge the availability of publicly accessible datasets, which were essential for the completion of this study.
Abbreviations
- TME
Tumor Microenvironment
- TANs
Tumor-associated Neutrophils
- TCGA-STAD
The Cancer Genome Atlas - Stomach Adenocarcinoma
- ACRG
Asian Cancer Research Group
- GC
Gastric Cancer
- LGALS9
Galectin-9
- CD45
PTPRC, Protein Tyrosine Phosphatase Receptor Type C
- CD46
Membrane Cofactor Protein
- JAG1
Jagged Canonical Notch Ligand 1
- RGS2
Regulator Of G Protein Signaling 2
- ICBs
Immune Checkpoint Inhibitors
- ROC
Receiver Operating Characteristic
- PCA
Principal Component Analysis
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- CNVs
Copy-number Variations
Author contributions
Tongtong Qi contributed to the original concepts, design, data analysis and visualization as well as write the original manuscript. Sijiang Zhou collected and materials sources. Zhu Yu performed language polishing and formal review. Yong Li Data assisted to the data analysis. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No.82060430), The National Natural Science Foundation of Guangxi (No.2023GXNSFBA026164), Guangxi Key Research and Development Project (No.AB24010149), Guangxi Clinical Research Center for Enhanced Recovery after Surgery, Guangxi Science and Technology Base and Talent Project (No.AD19245196), Guangxi key Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer (No.YYZS2020003).
Data availability
In this study, publicly available datasets were analyzed. Additional materials and further inquiries should be directed to the corresponding authors.
Declarations
Ethics approval and consent to participate
This study was based entirely on publicly available datasets. No experiments involving human participants or animals were conducted by the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ward ZJ, Gaba Q, Atun R. Cancer incidence and survival for 11 cancers in the commonwealth: a simulation-based modelling study. Lancet Oncol. 2024;25(9):1127–34. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Wang Y, Luan F, et al. Chinese and global burdens of gastric cancer from 1990 to 2019. Cancer Med. 2021;10(10):3461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzsimmons D, Osmond C, George S, et al. Trends in stomach and pancreatic cancer incidence and mortality in England and Wales, 1951–2000. Br J Surg. 2007;94(9):1162–71. [DOI] [PubMed] [Google Scholar]
- 4.Johnston FM, Beckman M. Updates on management of gastric Cancer. Curr Oncol Rep. 2019;21(8):67. [DOI] [PubMed] [Google Scholar]
- 5.llemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang YK, Terashima M, Kim YW, et al. Adjuvant nivolumab plus chemotherapy versus placebo plus chemotherapy for stage III gastric or gastro-oesophageal junction cancer after gastrectomy with D2 or more extensive lymph-node dissection (ATTRACTION-5): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2024;9(8):705–17. [DOI] [PubMed] [Google Scholar]
- 7.Shitara K, Rha SY, Wyrwicz LS, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25(2):212–24. [DOI] [PubMed] [Google Scholar]
- 8.Sohn BH, Hwang JE, Jang HJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin Cancer Res. 2017;23(15):4441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.André T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite Instability-High gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2023;41:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietrantonio F, Raimondi A, Lonardi S, et al. A multicentre, single-arm, multi-cohort, phase II trial of Tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023;INFINITY:41: 358–358. [Google Scholar]
- 11.André P, Denis C, Soulas C, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes Anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voissière A, Gomez-Roca C, Chabaud S, et al. The CSF-1R inhibitor pexidartinib affects FLT3-dependent DC differentiation and May antagonize durvalumab effect in patients with advanced cancers. Sci Transl Med. 2024;16(731):eadd1834. [DOI] [PubMed] [Google Scholar]
- 13.Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med. 2015;5(2):84–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi C, Liu C, Gong J, et al. Claudin18.2-specific CAR T cells in Gastrointestinal cancers: phase 1 trial final results. Nat Med. 2024;30(8):2224–34. [DOI] [PubMed] [Google Scholar]
- 15.Ohms M, Möller S, Laskay T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front Immunol. 2020;11:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrilovich DI. Myeloid-Derived suppressor cells. Cancer Immunol Res. 2017;5(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-Derived suppressor cells as a therapeutic target for Cancer. Cells. 2020;9(3):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Zheng X, Zhang J, et al. CD300ld on neutrophils is required for tumour-driven immune suppression. Nature. 2023;621(7980):830–9. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Yu D, Yang P, Guo R, et al. Revealing the transcriptional heterogeneity of organ-specific metastasis in human gastric cancer using single-cell RNA sequencing. Clin Transl Med. 2022;12(2):e730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ooi CH, Ivanova T, Wu J, Lee M, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5(10):e1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao Y, Stuart T, Kowalski MH, et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat Biotechnol. 2024;42(2):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Gene Ontology Consortium. Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Res. 2017;45(D1):D331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M, Goto S, Hattori M, et al. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006;34:D354–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kou W, Zhao N, Zhao L, et al. Single-cell characterization revealed hypoxia-induced metabolic reprogramming of gastric cancer. Heliyon. 2022;8(11):e11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng S, Li Z, Gao R, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184(3):792–809. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Yang S, Ma J, et al. Spatiotemporal immune landscape of colorectal Cancer liver metastasis at Single-Cell level. Cancer Discov. 2022;12(1):134–53. [DOI] [PubMed] [Google Scholar]
- 28.Jin S, Guerrero-Juarez CF, Zhang L, et al. Inference and analysis of cell-cell communication using cellchat. Nat Commun. 2021;12(1):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Hu S, Min M, et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. 2021;70(3):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong J, Wu X, Zhou X, et al. Spatially resolved expression landscape and gene-regulatory network of human gastric corpus epithelium. Protein Cell. 2023;14(6):433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Y, Lin Y, Gan L, et al. Potential crosstalk between SPP1 + TAMs and CD8 + exhausted T cells promotes an immunosuppressive environment in gastric metastatic cancer. J Transl Med. 2024;22(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta PB, Pastushenko I, Skibinski A, et al. Phenotypic plasticity: driver of Cancer initiation, progression, and therapy resistance. Cell Stem Cell. 2019;24(1):65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Stanger BZ. How tumor cell dedifferentiation drives immune evasion and resistance to immunotherapy. Cancer Res. 2020;80(19):4037–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73(2):377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue C, Yao Q, Gu X, et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. 2023;8(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath L, Puschmann C, Scheiber A, et al. Beyond binary: bridging neutrophil diversity to new therapeutic approaches in NSCLC. Trends Cancer. 2024;10(5):457–74. [DOI] [PubMed] [Google Scholar]
- 37.Gungabeesoon J, Gort-Freitas NA, Kiss M, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. 2023;186(7):1448–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bocci F, Gearhart-Serna L, Boareto M, et al. Toward Understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc Natl Acad Sci USA. 2019;116(1):148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Friec G, Sheppard D, Whiteman P, et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol. 2012;13(12):1213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daver N, Vyas P, Chao M, et al. A phase 3, randomized, open-label study evaluating the safety and efcacy of Magrolimab in combination with Azacitidine in previously untreated patients with TP53-mutant acute myeloid leukemia. Blood. 2021;138:3426. [Google Scholar]
- 41.Mehta A, Harb W, Xu C, et al. Lemzoparlimab, a diferentiated anti-CD47 antibody in combination with rituximab in relapsed and refractory non-Hodgkin’s lymphoma: initial clinical results. Blood. 2021;138:3542. [Google Scholar]
- 42.Shah MA, Yoshino T, Tebbutt NC, et al. Napabucasin plus FOLFIRI in patients with previously treated metastatic colorectal cancer: results from the open-label, randomized phase III CanStem303C study. Clin Colorectal Cancer. 2023;22:100–10. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki T, Gunderson AJ, Gilchrist M, et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a single-arm, phase 2 trial. Lancet Oncol. 2022;23:1189–200. [DOI] [PubMed] [Google Scholar]
- 44.Ng MSF, Kwok I, Tan L, et al. Deterministic reprogramming of neutrophils within tumors. Sci Jan. 2024;383(6679):eadf6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Ma J, Yang X, et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell. 2024;187(6):1422–39. [DOI] [PubMed] [Google Scholar]
- 46.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carnevale S, Di Ceglie I, Grieco G, et al. Neutrophil diversity in infammation and cancer. Front Immunol. 2023;14:1180810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Governa V, Trella E, Mele V, et al. The interplay between neutrophils and CD8 + T cells improves survival in human colorectal Cancer. Clin Cancer Res. 2017;23(14):3847–58. [DOI] [PubMed] [Google Scholar]
- 49.Zeng W, Zhang R, Huang P, et al. Ferroptotic neutrophils induce immunosuppression and chemoresistance in breast Cancer. Cancer Res. 2025;85(3):477–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaltenmeier C, Yazdani HO, Morder K, et al. Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Front Immunol. 2021;12:785222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo H, Ikenaga N, Nakata K, et al. Tumor-associated neutrophils upregulate Nectin2 expression, creating the immunosuppressive microenvironment in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2024;43(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang F, Wang H, Wang X, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canli Ö, Nicolas AM, Gupta J, et al. Myeloid Cell-Derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32:869–83. [DOI] [PubMed] [Google Scholar]
- 54.Butin-Israeli V, Bui TM, Wiesolek HL, et al. Neutrophil-induced genomic instability impedes resolution of infammation and wound healing. J Clin Invest. 2019;129:712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho J, Min HY, Lee H et al. RGS2-mediated translational control mediates cancer cell dormancy and tumor relapse. J Clin Invest. 2023;133(10), e171901. [DOI] [PMC free article] [PubMed]
- 56.Smalley MJ, Iravani M, Leao M, et al. Regulator of G-protein signalling 2 mRNA is differentially expressed in mammary epithelial subpopulations and over-expressed in the majority of breast cancers. Breast Cancer Res. 2007;9(6):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linder A, Hagberg Thulin M, Damber JE, Welén K. Analysis of regulator of G-protein signalling 2 (RGS2) expression and function during prostate cancer progression. Sci Rep. 2018;8(1):17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ihlow J, Monjé N, Hoffmann I, Bischoff P, et al. Low expression of RGS2 promotes poor prognosis in High-Grade serous ovarian Cancer. Cancers (Basel). 2022;14(19):4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinnarajah S, Dessauer CW, Srikumar D, et al. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature. 2001;409(6823):1051–5. [DOI] [PubMed] [Google Scholar]
- 60.Nunn C, Zou MX, Sobiesiak AJ, Roy AA, et al. RGS2 inhibits beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Cell Signal. 2010;22(8):1231–9. [DOI] [PubMed] [Google Scholar]
- 61.Joshi JC, Joshi B, Zhang C et al. RGS2 is an innate immune checkpoint for TLR4 and Gαq-mediated IFNγ generation and lung injury. bioRxiv., 2023.09.22.559016.
- 62.Shen H, Guo M, Wang L, et al. MUC16 facilitates cervical cancer progression via JAK2/STAT3 phosphorylation-mediated cyclooxygenase-2 expression. Genes Genomics. 2020;42(2):127–33. [DOI] [PubMed] [Google Scholar]
- 63.Lakshmanan I, Salfity S, Seshacharyulu P, et al. MUC16 regulates TSPYL5 for lung Cancer cell growth and chemoresistance by suppressing p53. Clin Cancer Res. 2017;23(14):3906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival- a review of the epidemiological literature. J Ovarian Res. 2009;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leushacke M, Tan SH, Wong A, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol. 2017;19(7):774–86. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Liu Y, Dai Y, et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated Glycolysis in pancreatic tumour microenvironment. Gut. 2023;72(5):958–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wauters E, Van Mol P, Garg AD, et al. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of Bronchoalveolar lavages. Cell Res. 2021;31(3):272–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Combes AJ, Courau T, Kuhn NF, et al. Global absence and targeting of protective immune States in severe COVID-19. Nature. 2021;591(7848):124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kehrl JH, Sinnarajah S. RGS2: a multifunctional regulator of G-protein signaling. Int J Biochem Cell Biol. 2002;34(5):432–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In this study, publicly available datasets were analyzed. Additional materials and further inquiries should be directed to the corresponding authors.