Abstract
Phaeohyphomycosis caused by Phialophora americana is relatively rare in clinical practice. Deficiency in the human caspase recruitment domain-containing protein 9 (CARD9) is associated with infections caused by Phialophora americana. In this case, the patient has had a decade-long history of recurrent tinea corporis and recently presented with an invasive, deep subcutaneous infection in the right axilla caused by Phialophora americana. Metagenomic next-generation sequencing (mNGS) confirmed that the pathogen infecting the patient was Phialophora americana. Whole exome sequencing (WES) revealed that the patient had compound heterozygous CARD9 gene mutations, with a c.952-1G > A mutation in intron 6 and a c.184 + 5G > T mutation in intron 2. The expression of the CARD9 protein and the levels of cytokines, including IL-17 and IFN-γ, were observed to be decreased in the patient. After an ineffective treatment with amphotericin B, voriconazole was administered for antifungal therapy and yielded satisfactory results. Following discharge, the patient continued oral voriconazole for ongoing antifungal treatment. One month after discharge, the patient returned to the hospital for a follow-up examination, during which it was observed that the symptoms had been successfully resolved. The novel compound heterozygous mutations may lead to CARD9 deficiency, which in turn results in susceptibility to Phialophora americana infection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-10973-9.
Keywords: CARD9, Dematiaceous fungi, Phaeohyphomycosis, Phialophora americana, Compound heterozygous mutation
Introduction
The dematiaceous fungi are characterized by the dark pigmentation in the walls of their hyphae and/or spores, and they are widespread in nature [1]. They can cause a wide range of subcutaneous and invasive infections in humans, including phaeohyphomycosis and chromoblastomycosis [2]. Phialophora americana is a dematiaceous fungus belonging to the Phialophora species, which can cause phaeohyphomycosis in animals such as cats and dogs [3, 4]. However, reports of infections caused by Phialophora americana are rare in the clinical field [5, 6]. The occurrence of Phialophora americana infection has been documented in both patients with primary and secondary immunodeficiency [5, 6]. Therefore, investigating the clinical and genetic features of Phialophora americana infections is of significant importance for the diagnosis and treatment of phaeohyphomycosis.
The caspase recruitment domain-containing protein 9 (CARD9) is a member of the CARD family, playing a crucial role in modulating innate immunity and exhibiting high expression in myeloid cells such as monocytes, neutrophils, macrophages, and dendritic cells [7]. Fungi are recognized by the innate immune system through pattern recognition receptors (PRRs), such as C-type lectin receptors (CLRs) [8]. CARD9 acts as a crucial downstream signaling molecule of CLRs, initiating signaling cascades against fungal invasion through ligand-receptor binding [9]. CARD9 deficiency is an autosomal recessive primary immunodeficiency that leads to increased susceptibility to fungal infections, primarily manifesting as invasive infections of the central nervous system, oral mucosa, and skin [10]. At present, approximately 101 cases of CARD9 gene defects accompanied by fungal infections have been reported, involving over 40 mutations [11–20]. Chen et al. found that patients hold with a homozygous frameshift CARD9 mutation were prone to infection by Phialophora americana and Phialophora expanda [6, 11]. Additionally, both patients exhibited impaired production of pro-inflammatory cytokines and decreased responses associated with Th17 and Th22 upon stimulation specific to the fungus [6, 11]. Although a previous study has reported an association between a CARD9 gene mutation and Phialophora americana infection, clinical manifestations and prognoses still vary among different mutation sites. Therefore, the discovery of new mutations and the detailed observation of clinical symptoms continue to contribute to a better understanding of Phialophora americana infection related to CARD9 deficiency.
In this report, we present a case of a patient with phaeohyphomycosis who harbored novel compound heterozygous mutations in CARD9, allowing us to gain new insights into the clinical and genetic characteristics of Phialophora americana infection.
Case description
A 35-year-old male patient presented with an unexplained mass in his right axilla and a hyperpigmented complexion one and a half years ago. Initially, the patient had no symptoms of pain or itching in the affected area and was seen at a community hospital. Laboratory tests failed to identify the underlying cause but ruled out tuberculosis infection. The patient’s condition subsequently deteriorated due to ineffective treatment, manifesting as progressive swelling and pain in the affected area for approximately one month before he sought treatment at our hematology clinic. He was initially diagnosed with a significantly enlarged right axillary lymph node of unknown origin. During the consultation, it was discovered that the patient had a long-standing history of recurrent tinea corporis spanning over 10 years and had exhibited irregular usage of voriconazole, resulting in suboptimal management of the condition. A dermatologist was subsequently consulted, and based on the patient’s general clinical features, a deep fungal infection was suspected. The patient was ultimately referred to our dermatology department for further evaluation and treatment.
The physical examination revealed dark red pigmentation on the medial aspect of the patient’s right axilla (Fig. 1A). Palpation revealed a firm mass measuring approximately 8 × 4 cm in size in the patient’s right axilla, accompanied by tenderness (Fig. 1A). Additionally, palpated lymph nodes in the right supraclavicular and submental areas of the patient, with sizes ranging from approximately 1 × 1 cm. These lymph nodes were firm in consistency, without tenderness or adhesion. The patient tested positive for the [1, 3]-β-D glucan (G test), with a quantitative measurement of [1, 3]-β-D glucan exceeding 600 pg/ml. However, the galactomannan (GM) test for this patient was negative. These findings collectively suggest the presence of a deep-seated fungal infection. The biopsy pathological result of the right axillary mass puncture tissue indicated the presence of a significant amount of necrotic tissue (Fig. 1B). The GMS staining of the puncture tissue showed a positive response, indicating the presence of a fungal infection (Fig. 1B). Subsequent metagenomic next-generation sequencing (mNGS) of the puncture tissue revealed four pathogens: Phialophora americana, Staphylococcus aureus, Staphylococcus capitis, and Epstein-Barr virus (EBV). Fungal cultures of the puncture tissue were conducted using potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) media. After 14 days, grayish-black colonies were observed on PDA at 28 °C (Fig. 1C) and on SDA at 35 °C (Fig. 1D). Microscopic examination revealed that the hyphae were septate and branched, with phialides being lateral or terminal. They appeared flask-shaped or elongated to cylindrical. Spherical to ellipsoidal conidia were observed on the hyphae (Fig. 1E). The patient was diagnosed with phaeohyphomycosis, which resulted from an infection caused by Phialophora americana.
Fig. 1.
Clinical manifestations and laboratory findings of the patient. (A) The skin on the inner side of the patient’s right axilla presented a dark red color, and there was a firm mass approximately 8 × 4 cm in size located at that site. (B) The biopsy pathological result of the right axillary mass puncture tissue of this patient indicated the presence of a significant amount of necrotic tissue. The GMS staining of the puncture tissue showed a positive response, indicating the presence of fungal infection. (C, D) After a period of 14 days, grayish-black colonies were observed in both environments at temperatures of 28 °C on PDA medium and 35 °C on SDA medium. (E) Microscopic examination revealed that the hyphae were septate and branched, and the phialides were lateral or terminal. They appeared flask-shaped or elongated to cylindrical. Spherical to ellipsoidal conidia could be observed on the hyphae
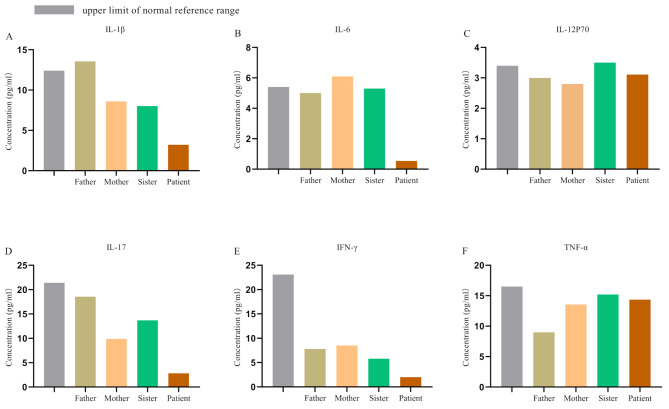
Phialophora americana is an opportunistic pathogen. The patient has no history of diabetes, cancer, HIV infection, or use of immunosuppressive drugs. Additionally, there was no evidence of a hereditary predisposition to chronic fungal infections in his immediate family. We suspected that the patient might be suffering from a primary immunodeficiency caused by a genetic mutation. Cytokine assays revealed that the levels of IL-1β, IL-6, IL-17, and IFN-γ in the patient were lower compared to those of the patient’s family members (Fig. 2). We stimulated the peripheral blood mononuclear cells (PBMCs) of both the patient and his family members using lipopolysaccharide (LPS) to evaluate cytokine expression profiles. Under the stimulation of LPS, the expression levels of cytokines such as IL-1β, IL-6, IL-17, IFN-γ and TNF-α in PBMCs of the patient were significantly lower than those of the controls (Fig. 3).
Fig. 2.
Cytokine levels in the patient and his family members. The levels of cytokines in the peripheral blood serum were measured in the clinical laboratory using a commercially available 12-plex cytokine detection kit (RAISECARE) and analyzed using the BDFACSCanto™ flow cytometer. Results revealed that the levels of IL-1β, IL-6, IL-17, and IFN-γ in the patient were lower compared to those of the patient’s family members
Fig. 3.
Cytokine expressions in LPS stimulated PBMCs. Peripheral blood mononuclear cells (PBMCs) were obtained from the patient and his family members. After stimulating PBMCs with LPS, the expression levels of cytokines were detected using a commercial ELISA kit. Results revealed that PBMCs from the patient expressed low levels of IL-1β, IL-6, IL-17, IFN-γ, and TNF-α after stimulation
At present, approximately 101 cases of CARD9 gene defects accompanied by fungal infections have been reported, involving over 40 mutations [20]. Fungus pathogen and genetic and clinical features of patients with CARD9 mutations were summarized in Supplementary Table 1. We performed whole-exome sequencing (WES) analysis on his family, and Sanger sequencing was used to confirm the WES results. Bioinformatics analysis revealed that the patient had three potential pathogenic gene mutations: LRP5, NLRP1, and CARD9. However, only CARD9 is associated with fungal infections. Finally, we propose that the compound heterozygous CARD9 mutations, characterized by a c.952-1G > A mutation in intron 6 and a c.184 + 5G > T mutation in intron 2, are associated with fungal infections (Fig. 4). Among them, two reports have indicated that c.184 + 5G > T is associated with fungal infection, and c.952-1G > A is a novel mutation [14, 21]. The expression of the CARD9 protein in the PBMCs of the patient and his parents was detected by Western blot (WB). The results revealed that, compared to that of his parents, the expression level of the CARD9 protein in the patient was significantly reduced (Fig. 4D). The results indicate that the compound heterozygous mutations of CARD9 are indeed deleterious.
Fig. 4.
Pedigree of CARD9 mutation, genetic sequencing and protein expression. (A) The patient was the proband in the family. (B, C) The patient had a compound heterozygous CARD9 gene mutation, which included a c.952-1G > A variation in intron 6 and a c.184 + 5G > T variation in intron 2. (D) Compared to his parents, the patient exhibited a significant decrease in CARD9 protein expression
Treatment
During hospitalization, the patient initially received a daily dosage of 200 mg of amphotericin B cholesterol sulfate complex for 8 days. Throughout this period, the patient exhibited symptoms indicative of impaired renal function, including elevated levels of creatinine and a reduced glomerular filtration rate. The patient also presented with additional adverse reactions, such as decreased appetite and low mood. After consulting with experts in nephrology and pharmacy, we adjusted the patient’s treatment plan. The new treatment plan involved administering voriconazole at a dose of 400 mg once daily for 6 consecutive days, along with cefuroxime for the treatment of bacterial infection. After completing one treatment course, the mass in the patient’s right axilla and the lymph nodes were significantly reduced. After undergoing an additional course of treatment, the patient’s condition stabilized, and he was discharged from the hospital. Following discharge, the patient continued to take 400 mg of voriconazole daily while also taking Jinshuibao capsules to enhance kidney function and glutathione tablets to protect the liver. One month after discharge, the patient returned to the hospital for a follow-up examination, during which it was observed that the patient’s symptoms had resolved.
At present, reports on phaeohyphomycosis caused by Phialophora americana infection are relatively rare, and the sharing of relevant treatment experiences is also scarce. We reviewed the literature on phaeohyphomycosis in patients with CARD9 gene mutations that has been published, summarized the general clinical features, pathogenic species, treatment regimens, and prognosis of the patients, with the aim of providing references for subsequent treatments. (Table 1).
Table 1.
Treatment and prognosis of phaeohyphomycosis in patients with CARD9 gene defect
| No | Sex | Age | Year | Pathogenic fungi | CARD9 mutations | Treatment plan | Therapeutic outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 35 | 2025 | Phialophora americana | c.952-1G > A + c.184 + 5G > T | AMB | No response | This study |
| VOR | improve | |||||||
| 2 | W | 6 | 2024 | Exophiala dermatitidis | c.820dupG | VOR, FLC, AMB | died | Ma et al.,2024 |
| 3 | M | 29 | 2023 | Phialophora verrucosa | c.1118G > C + c.819-820insG | FLC, ITZ, VOR, POS, AMB, TBF | improve | Zhang et al.,2023 |
| 4 | M | 6 | 2021 | Alternaria species | c.1526G > A + c. 586 A > G | AMB, VOR | improve | Lai et al.,2021 |
| 5 | W | 26 | 2021 | Exserohilum rosatratum | c.1108 C > T | FLC, ITZ | improve | Kalantri et al.,2021 |
| AMB, ITZ | No response | |||||||
| 6 | W | 56 | 2020 | Exophiala spinifera/ Aspergillus nomius | Q289* | AMB, TER, ITZ, VOR, POS | died | Perez et al.,2020 |
| 7 | W | 35 | 2019 | Pallidocercospora crystallina | c.1118G > C | ITZ, TER, ITZ | mild improvement | Guo et al.,2019 |
| surgery, ITZ, TER | improve | |||||||
| 8 | M | 28 | 2019 | Phialophora americana | c.819-820insG, | ITZ, VOR, POS | improve | Huang et al.,2019 |
| 9 | M | 23 | 2019 | Exophiala dermatitidis | c.759dup | AMB, VOR | died | Wang et al.,2019 |
| 10 | F | 18 | 2018 | Exophiala spinifera | c.68 C > A + c.819-820insG | TER, ITZ | No response | Wang et al.,2018 |
| 11 | F | 53 | 2018 | Ochroconis musae | c.819e820insG | ITZ, TER, AMB | mild improvement | Wang et al.,2018 |
| 12 | F | 38 | 2018 | Corynespora cassiicola | c.191e192insTGCT + c.819e820insG | AMB, ITZ, TER | mild improvement | Wang et al.,2018 |
| 13 | W | 12 | 2018 | Corynespora cassiicola | c.865 C > T + c.23_29del | AMB | mild improvement | Franco et al.,2018 |
| 14 | W | 37 | 2016 | Corynespora cassiicola | c.191-192InsTGCT | AMB | mild improvement | Yan et al.,2016 |
| 15 | M | 21 | 2014 | Phialophora verrucosa | c.191-192insTGCT, c.472 C > T | ITZ, AMB | mild improvement | Wang et al.,2014 |
| 16 | M | 17 | 2014 | Phialophora verrucosa | c.819-820insG | AMB, ITZ | mild improvement | Wang et al.,2014 |
| 17 | F | 43 | 2014 | Phialophora verrucosa | c.819- 820insG | surgery, ITZ | improve | Wang et al.,2014 |
| 18 | M | 64 | 2014 | Phialophora verrucosa | c.819- 820insG | ITZ, TER | mild improvement | Wang et al.,2014 |
Abbreviations: Voriconazole, VOR; Amphotericin B, AMB; flucytosine, FLC; itraconazole, ITZ; Terbinafine, TER; posaconazole, POS
Discussion
The present study reported a case analysis of a Chinese patient who harbored novel compound heterozygous CARD9 gene mutations, characterized by a c.952-1G > A mutation in intron 6 and a c.184 + 5G > T mutation in intron 2. The patient has had a decade-long history of recurrent tinea corporis and recently presented with an invasive, deep subcutaneous infection of the right axilla caused by Phialophora americana.
Phialophora americana is a dematiaceous fungus that belongs to the Phialophora species. Currently, there is a scarcity of literature on Phialophora americana infections. Phialophora americana can cause phaeohyphomycosis in animals such as cats and dogs [3, 4], and it rarely affects humans, including patients with CARD9 deficiency [6]. CARD9-/- mice are susceptible to infection with various fungal species, including Candida albicans, Aspergillus fumigatus, and Phialophora americana [22–24]. The scaffold protein CARD9 facilitates the transmission of signals from CLRs to mitogen-activated protein kinase (MAPK) and transcription factor nuclear factor-κB (NF-κB), thereby activating downstream signaling molecules and promoting the production of inflammatory cytokines [25]. CLRs, including Dectin-1, Dectin-2, and Dectin-3, play significant roles in recognizing fungi and initiating antifungal immune responses in humans [26]. Dectin-1 is the most representative CARD9- dependent pathway, which can activate inflammasomes and induce the production of proinflammatory cytokines and chemokines, such as IL-6, TNF-α, GM-CSF, and IFN-γ under the regulation of CARD9 [27]. Furthermore, the Dectin-1 signaling pathway exerts regulatory control over macrophage polarization, Th1 and Th17 differentiation, as well as IL-17 production in response to fungal infections [28, 29]. The heterodimers formed by Dectin-2 and Dectin-3 exhibit enhanced recognition of fungal α-mannans compared to pure homodimers [30]. Patients with CARD9 deficiency are prone to fungal infections, possibly due to dysregulation of the CARD9-CLRs cascade signaling pathway.
Genetic mutations in CARD9 can cause functional deficiency. CARD9 mutations are autosomal recessive, with homozygous mutations being the most pathogenic, and compound heterozygous mutations have also been reported. In 2019, Chen et al.. reported the first case of a male patient infected with Phialophora americana carrying a homozygous CARD9 mutation [6]. They subsequently identified the same mutation of the CARD9 gene in another female patient who was infected with Phialophora expanda [11]. Both patients had antifungal immunodeficiency, which was characterized by impaired production of inflammatory cytokines and reduced Th17 and Th22 responses. In this study, we discovered that the patient harbored novel compound heterozygous mutations in CARD9 and expressed low levels of CARD9 protein. Compound heterozygous mutations in the CARD9 gene may lead to a defect in the expression of the CARD9 protein. Similar to the previous cases, this patient also exhibited compromised immune function, characterized by reduced IL-17 levels and other pro-inflammatory cytokines. We speculated that the compound heterozygous mutation also leads to CARD9 deficiency, which subsequently affects antifungal immunity and renders patients susceptible to Phialophora americana. However, further investigation is needed to understand the mechanisms underlying the effects of CARD9 deficiency on antifungal immunity.
In previous reported cases, phaeohyphomycosis has exhibited the characteristics of persistent chronicity and recurrent infection [6, 11]. The two patients reported by Chen et al.. both exhibited severe facial skin lesions, whereas the patients in this study presented with milder skin lesions primarily localized in the axilla. This is also a specific manifestation of the diverse clinical presentations of phaeohyphomycosis. Therefore, timely and accurate diagnosis of deep fungal infections is crucial. Additionally, it is advisable to consider the following points. Firstly, we should pay attention to whether the patient has recurrent fungal infections, such as tinea corporis and chronic mucocutaneous candidiasis, which are potential indicators of antifungal immunodeficiency. Secondly, clinical laboratory examinations, such as G and GM tests, cytokine and immune cell analyses, etc., can facilitate the diagnosis of deep fungal infections. Thirdly, pathological biopsy of the punctured tissue is indispensable for diagnosing phaeohyphomycosis. Fourthly, pathogenic fungi can be accurately identified by employing fungal culture and utilizing advanced detection techniques such as mNGS sequencing and mass spectrometry. Lastly, pedigree analysis using WES sequencing can be employed to identify the genetic characteristics of the patient and discover genetic markers associated with phaeohyphomycosis, facilitating risk screening and analysis of the pathogenesis.
The treatment of infections caused by dematiaceous fungi is challenging and varies among different individuals. In 2014, ESCMID and ECMM provided treatment guidelines for phaeohyphomycosis [31]. Oral itraconazole is considered the preferred drug due to its extensive clinical experience [31]. Voriconazole may potentially have superiority in the treatment of central nervous system infections, while amphotericin B could be considered as an alternative therapeutic approach in certain circumstances [31]. In this study, due to a lack of experience, we initially chose amphotericin B for the treatment of patients. However, the results were unsatisfactory and accompanied by adverse reactions. Subsequently, we switched to voriconazole and achieved satisfactory outcomes. In the study by Chen et al., Phialophora americana infection was effectively treated with itraconazole [6]. The patient indicated that despite having previously received voriconazole, the fungal infection was poorly controlled due to non-standard medication usage. The patient expressed satisfaction with the current medication plan and affirmed his commitment to strictly following the doctor’s instructions. However, this study failed to monitor azole levels during the treatment, which is a limitation of this study. Collectively, it is recommended to use voriconazole and itraconazole as the preferred drugs for treating Phialophora americana infection.
In conclusion, we presented a case study of a patient with phaeohyphomycosis who harbored novel compound heterozygous mutations in CARD9. This research elaborates on the clinical characteristics and diagnostic and therapeutic processes of the patient, aiming to provide insights for diagnosing and treating patients with occult deep fungal infections. Clinicians should remain vigilant about the possibility of primary immunodeficiency in patients with fungal infections without an obvious cause and promptly conduct genetic risk factor screening.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patient and the family members for their participation in this study.
Abbreviations
- AMB
Amphotericin B
- FLC
Flucytosine
- ITZ
Itraconazole
- mNGS
Metagenomic next-generation sequencing
- PBMCs
Peripheral blood mononuclear cells
- POS
Posaconazole
- TER
Terbinafine
- VOR
Voriconazole
- WES
Whole exome sequencing. EBV, Epstein-Barr virus
Author contributions
JW and YX: designing and drafting the study, analyzing the data, and writing manuscript. FML: laboratory testing and data analysis. XDL: collecting data. JG and NND: designing the study, revising and finalizing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the China Postdoctoral Science Foundation (2022M710852), the National Natural Science Foundation of China (82102420), and the Natural Science Foundation of Shandong Provincial (ZR2024QH034).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the ethics committee of Shandong Provincial Hospital, affiliated to Shandong First Medical University. The patient provided written informed consent for their participation in the study and for the publication of potentially identifiable images or data included in this article.
Consent for publication
All authors have consented to publication. The patient and other participants provided written informed consent for their personal or clinical details along with any identifying images to be published in this study.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Wu and Yang Xiang contributed equally to this work.
References
- 1.He Y, Zheng HL, Mei H, Lv GX, Liu WD, Li XF. Phaeohyphomycosis in China. Front Cell Infect Microbiol. 2022;12:895329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23(4):884–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrás P, Messina F, Abrantes R, Iachini R, Minatel L, Santiso G. First report of phaeohyphomycosis caused by Phialophora americana in a domestic cat from Argentina. JFMS Open Rep. 2022;8(1):20551169221077611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martini F, Seehusen F, Krudewig C, Beckmann KM, Favrot C, Fischer NM, et al. Phaeohyphomycosis caused by Phialophora americana in a dog. Vet Dermatol. 2022;33(5):446–9. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed SA, Bonifaz A, González GM, Moreno LF, Menezes da Silva N, Vicente VA et al. Chromoblastomycosis caused by Phialophora-proven cases from Mexico. J Fungi (Basel). 2021;7(2). [DOI] [PMC free article] [PubMed]
- 6.Huang C, Zhang Y, Song Y, Wan Z, Wang X, Li R. Phaeohyphomycosis caused by Phialophora americana with CARD9 mutation and 20-year literature review in China. Mycoses. 2019;62(10):908–19. [DOI] [PubMed] [Google Scholar]
- 7.Vaezi A, Fakhim H, Abtahian Z, Khodavaisy S, Geramishoar M, Alizadeh A, et al. Frequency and geographic distribution of CARD9 mutations in patients with severe fungal infections. Front Microbiol. 2018;9:2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond RA, Lionakis MS. Mechanistic insights into the role of C-Type lectin receptor/CARD9 signaling in human antifungal immunity. Front Cell Infect Microbiol. 2016;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu A, Hu Z, Zou H, Zhang J, Zhang D, Wang H, et al. CARD9 in host immunity to fungal, bacterial, viral, and parasitic infections: an update. Front Microbiol. 2022;13:1021837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goel S, Kuehn HS, Chinen J, Niemela J, Stoddard J, Yamanaka D, et al. CARD9 expression pattern, gene dosage, and immunodeficiency phenotype revisited. J Clin Immunol. 2022;42(2):336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Deng W, Zhang Y, Zhang K, Ma Y, Song Y, et al. CARD9 deficiency predisposing chromoblastomycosis: a case report and comparative transcriptome study. Front Immunol. 2022;13:984093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan H, Yang Z, Wu Y, Lu X, Li T, Lu X, et al. Human inborn errors of immunity underlying talaromyces marneffei infections: a multicenter, retrospective cohort study. Front Immunol. 2025;16:1492000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang H, Duan X, Li T, Hu L, Guo J. Disseminated combined talaromyces marneffei and enterococcus faecium bloodstream infection presenting as gastrointestinal perforation in a patient with CARD9 gene mutation. Infect Drug Resist. 2024;17:4783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou LH, Qiu WJ, Que CX, Cheng JH, Zhu RS, Huang JT, et al. A novel inherited CARD9 deficiency in an otherwise healthy woman with CNS candidiasis. Clin Immunol. 2024;265:110293. [DOI] [PubMed] [Google Scholar]
- 15.Ma N, Zhao Y, Tang M, Xia H, Li D, Lu G. Concurrent infection of exophiala dermatitidis and angiostrongylus cantonensis in central nervous system of a child with inherited CARD9 deficiency: a case report and literature review. J Mycol Med. 2024;34(1):101455. [DOI] [PubMed] [Google Scholar]
- 16.Tomomasa D, Lee BH, Hirata Y, Inoue Y, Majima H, Imanaka Y, et al. Inherited CARD9 deficiency due to a founder effect in East Asia. J Clin Immunol. 2024;44(5):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Lu H, Li J, Duan J, Wang Z, Yang J, et al. Heterozygous CARD9 mutation favors the development of allergic bronchopulmonary aspergillosis. Chin Med J (Engl). 2023;136(16):1949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng R, Meng X, Li R, Wang A, Song Y. Asymptomatic Candida glabrata urinary tract infection in an immunocompetent young female: a case report. Med (Baltim). 2023;102(20):e33798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallahi M, Mahdaviani SA, Shafiei M, Ghadimi S, Rezaei N, Klein C, et al. CARD9 deficiency with allergic bronchopulmonary aspergillosis (ABPA)-like presentation: a case report. Oxf Med Case Rep. 2023;2023(10):omad103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dantas MDS, Cintra MEC, Lucini F, Venturini J, de Souza GHA, Rossato L. CARD9 mutations in patients with fungal infections: an update from the last 5 years. Mycoses. 2024;67(3):e13712. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Ding H, Chen Z, Zeng X, Sun J, Chen H, et al. CARD9 deficiency in a Chinese man with cutaneous mucormycosis, recurrent deep dermatophytosis and a review of the literature. Mycopathologia. 2020;185(6):1041–50. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Huang C, Song Y, Ma Y, Wan Z, Zhu X, et al. Primary cutaneous aspergillosis in a patient with CARD9 deficiency and aspergillus susceptibility of Card9 knockout mice. J Clin Immunol. 2021;41(2):427–40. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Zhang R, Wu W, Song Y, Wan Z, Han W, et al. Impaired specific antifungal immunity in CARD9-deficient patients with phaeohyphomycosis. J Invest Dermatol. 2018;138(3):607–17. [DOI] [PubMed] [Google Scholar]
- 24.Zajta E, Csonka K, Tóth A, Tiszlavicz L, Németh T, Orosz A, et al. Signaling through Syk or CARD9 mediates species-specific anti-candida protection in bone marrow chimeric mice. mBio. 2021;12(4):e0160821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong X, Chen B, Yang L, Yang Z. Molecular and physiological roles of the adaptor protein CARD9 in immunity. Cell Death Dis. 2018;9(2):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiokawa M, Yamasaki S, Saijo S. C-type lectin receptors in anti-fungal immunity. Curr Opin Microbiol. 2017;40:123–30. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Zhang D, Hou Y, Shen S, Wang T. The adaptor protein CARD9, from fungal immunity to tumorigenesis. Am J Cancer Res. 2020;10(8):2203–25. [PMC free article] [PubMed] [Google Scholar]
- 28.Drummond RA, Desai JV, Hsu AP, Oikonomou V, Vinh DC, Acklin JA et al. Human Dectin-1 deficiency impairs macrophage-mediated defense against phaeohyphomycosis. J Clin Invest. 2022;132(22). [DOI] [PMC free article] [PubMed]
- 29.Carvalho A, Giovannini G, De Luca A, D’Angelo C, Casagrande A, Iannitti RG, et al. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol Immunol. 2012;9(3):276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, et al. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39(2):324–34. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect. 2014;20(Suppl 3):47–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.