Abstract
Background
Genetic variations in the CYP2C19 gene, which encodes the major enzyme responsible for activating clopidogrel, may influence response to Clopidogrel antiplatelet therapy. This study aimed to assess the prevalence of CYP2C19 variants in Syrian patients with coronary artery disease (CAD) and evaluate the impact of these variants on the clinical efficacy of a doubled maintenance dose of clopidogrel following percutaneous coronary intervention (PCI).
Methods
This study included 50 Syrian CAD patients on dual antiplatelet therapy (DAPT) with a doubled maintenance dose of clopidogrel. CYP2C19 genotypes were determined by PCR, followed by Sanger sequencing. Clinical outcomes, including major acute cardiovascular events (MACE) and bleeding events, were monitored over 18–24 months.
Results
The allele frequencies were 8% for CYP2C19*2, 0% for CYP2C19*3, and 17% for CYP2C19*17. The distribution of our study population by CYP2C19 genotype-predicted metabolizer phenotypes was 56% for normal metabolizers (NMs), 26% for intermediate metabolizers (IMs), 12% for rapid metabolizers (RMs), and 2% for ultra-rapid metabolizers (UMs). No association was found between the CYP2C19*2 allele and recurrent ischemic events or between the CYP2C19*17 allele and bleeding complications in patients treated with a doubled maintenance dose of clopidogrel.
Conclusions
In Syrian patients undergoing PCI, a doubled maintenance dose of clopidogrel (150 mg/day) may help mitigate variability in response due to CYP2C19*2 carrier status, offering potential benefits in optimizing antiplatelet therapy. However, given the study’s limited sample size, these findings should be interpreted with caution, and larger studies are needed to confirm this potential benefit.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-025-04768-8.
Keywords: Clopidogrel, Doubled maintenance dose, CYP2C19, Genotyping, CYP2C19*2, CYP2C19*3, CYP2C19*17, Syria
Background
Coronary artery disease (CAD), also known as ischemic heart disease (IHD), is a widespread health condition and one of the leading causes of death worldwide. This condition is characterized by an imbalance between myocardial oxygen supply and demand, primarily arising due to atherosclerosis of the coronary arteries [1]. Percutaneous coronary intervention (PCI) is a minimally invasive, nonsurgical procedure utilized in the management of IHD, with the aim of relieving the stenosis or obstruction of coronary arteries by restoring blood flow to the affected area, commonly achieved by inflating a balloon or deploying a stent [2]. Following stenting, a dual antiplatelet therapy (DAPT) is administered to patients, comprising aspirin and an oral P2Y12 receptor antagonist (clopidogrel, prasugrel, or ticagrelor), to prevent stent thrombosis and reduce the likelihood of major acute cardiovascular events (MACE) [3].
Clopidogrel, a second-generation thienopyridine, is a prodrug absorbed through the intestines via the P-glycoprotein pump following oral administration. Once absorbed, it undergoes metabolism via two primary pathways. The first pathway involves esterase enzymes, resulting in the hydrolysis of clopidogrel to a non-active carboxylic acid derivative, which accounts for 85% of the parent drug. The second pathway involves two sequential oxidative steps mediated by a group of cytochrome P450 enzymes, mainly CYP2C19. The initial step involves the oxidation of clopidogrel to an intermediate metabolite, 2-oxo-clopidogrel, followed by a second step in which an active thiol metabolite is formed [4]. The resulting metabolite specifically and irreversibly binds to the P2Y12 adenosine diphosphate receptors on platelets, inhibiting platelet aggregation and preventing blood clot formation [5]. Despite the well-established benefits of clopidogrel, a considerable portion of patients exhibit a limited response to its antiplatelet effect, leading to recurrent ischemic events after PCI [6].
The response to clopidogrel has substantial interpatient variability, which has received close attention. Several potential mechanisms have been proposed, likely to be multifactorial, depending on pharmacokinetic, pharmacodynamic, and pharmacogenomic factors. Considering that the CYP2C19 enzyme plays a pivotal role in the hepatic activation of clopidogrel, the examination of CYP2C19 polymorphisms has come to the forefront of clopidogrel pharmacogenetic research [7].
The CYP2C19 gene is located on chromosome 10q23.33 within the CYP2C gene cluster and comprises nine coding exons and eight introns. This gene is highly polymorphic, with the Pharmacogene Variation (PharmVar) Consortium cataloging over 35 different star alleles, each with varying levels of evidence. The majority of these alleles result from specific combinations of single nucleotide polymorphisms (SNPs), while a few arise from copy number variation (CNV), such as the *36 and *37 alleles, which represent complete and partial deletion of the CYP2C19 gene, respectively [9, 10]. The most common non-functional alleles that contribute to poor and intermediate metabolizer phenotypes are CYP2C19*2 (rs4244285; c.681G > A) and CYP2C19*3 (rs4986893; c.636G > A). In contrast, CYP2C19*17 (rs12248560; -806 C > T) is a gain-of-function allele associated with increased gene transcription, resulting in rapid metabolizers (RMs) or ultra-rapid metabolizers (UMs) depending on the copy number of the CYP2C19*17 allele that the individual carries [9].
Considering the absence of studies reporting the prevalence of CYP2C19 polymorphisms in the Syrian population, a highly admixed Mediterranean population, our study aimed to determine the frequencies of the most clinically relevant CYP2C19 alleles among Syrian patients undergoing PCI and assess the impact of these genetic variations on the clinical response to a double maintenance dose of clopidogrel presented by patients’ cardiovascular outcomes.
Methods
Study design and ethics approval
This prospective cohort study was approved by the Scientific Research Bioethics Committee of the Faculty of Pharmacy, Damascus University, in accordance with the World Medical Association Declaration of Helsinki. Informed consent was obtained from all individuals who participated in the study in compliance with ethical standards.
Sample collection
Between June 2020 and January 2021, patient eligibility assessments were conducted at three medical institutions in Damascus, Syria: Martyr Bassel al-Assad Heart Hospital, Damascus Hospital, and Al Fayhaa Hospital. The inclusion criteria were CAD patients aged 18 years or older who underwent PCI and were prescribed DAPT with a double maintenance dose of clopidogrel 150 mg/day (for at least one month after PCI). Patients were excluded if they were prescribed the standard dose of Clopidogrel (75 mg/day), or if they were taking other adenosine diphosphate (ADP) receptor antagonists like Ticagrelor or Prasugrel. Eligible patients participated in face-to-face interviews, during which relevant information (Additional File 1), such as age, gender, body weight, height, smoking status, medical history, and concomitant medication use, were recorded. Furthermore, a peripheral blood sample (5 mL) was collected from each participant using an EDTA tube.
DNA extraction
Genomic DNA (gDNA) was extracted from whole blood samples using a Blood DNA Preparation Solution Kit (Jena Bioscience®, Jena, Germany) following the manufacturer’s protocol. The quality and purity of the extracted gDNA were assessed using a NanoDrop device (MaestroGen®, Hsinchu City, Taiwan). For analysis, two µL of gDNA were loaded onto the lens of the NanoDrop and measured at a wavelength of 260/280 nm. The NanoDrop lens was meticulously cleaned with distilled water and a cotton swab after each measurement to ensure accuracy and prevent contamination. Subsequent measurements were performed on the remaining samples.
CYP2C19 *2, *3 and *17 genotyping
Genotyping of CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893), and CYP2C19*17 (rs12248560) was performed using polymerase chain reaction (PCR), followed by Sanger sequencing of the amplicons. The PCR reactions were carried out using the Labcycler Basic (011–103) device (SensoQuest GmbH®, Göttingen, Germany), using specific primers and implementing the conditions outlined in Table 1. The targeted locations of the forward and reverse primers for each investigated SNP are illustrated in Fig. 1. a. Each PCR reaction had a final volume of 20 µl and included 50 ng of gDNA, five pmol of 10 pmol/µL of each the forward and reverse primer, and ten µl of 2X Master Mix (Kapa Biosystems®, Wilmington, USA). The resulting PCR products were separated by gel electrophoresis using a (1.5%) agarose gel, stained with ethidium bromide, and visualized under ultraviolet (UV) light (Additional File 2). The observed bands were documented using a digital camera and compared to a 100-bp DNA ladder for identification purposes. Subsequently, 150 PCR amplification products were sent to Macrogen (Seoul, South Korea) for sequencing.
Table 1.
Primers sequences and PCR conditions
| Allele | Primers | PCR Conditions | Product Size | ||||
|---|---|---|---|---|---|---|---|
| Step | TmoC | Time | # of Cycles | ||||
| CYP2C19*2 | F | 5′-ACAACCAGAGCTTGGCATATT-3′ | Initial Denaturation | 95 | 5 min | 203 bp | |
| Denaturation | 95 | 20 s | 40 | ||||
| Primer Annealing | 54.4 | 30 s | |||||
| R | 5′-TGTCCATCGATTCTTGGTGT-3′ | Extension | 72 | 30 s | |||
| Final Extension | 72 | 10 min | |||||
| CYP2C19*3 | F | 5′-ATTGTTTCCAATCATTTAGCTTCAC-3′ | Initial Denaturation | 94 | 5 min | 269 bp | |
| Denaturation | 94 | 1 min | 37 | ||||
| Primer Annealing | 52 | 45 s | |||||
| R | 5′-ACTTCAGGGCTTGGTCAATA-3 | Extension | 72 | 30 s | |||
| Final Extension | 72 | 5 min | |||||
| CYP2C19*17 | F | 5′-GCCCTTAGCACCAAATTCTC-3′ | Initial Denaturation | 95 | 3 min | 473 bp | |
| Denaturation | 94 | 1 min | 35 | ||||
| Primer Annealing | 56.3 | 1 min | |||||
| R | 5′-ATTTAACCCCCTAAAAAAACACG-3′ | Extension | 72 | 1 min | |||
| Final Extension | 72 | 10 min | |||||
Abbreviations: F = forward; R = reverse; Tm = temperature
Fig. 1.
Schematic representation of the CYP2C19 gene location, structure, studied SNPs, and primer positions. (a) Human chromosome 10 with the 10q.23.33 band marked by a red vertical line. (b) A cluster of four cytochrome P450 genes including CYP2C18, CYP2C19, CYP2C9, and CYP2C8. (c) Core sequence variations defining CYP2C19*2, *3 and, *17 alleles. (d) Targeted locations of the forward and reverse primers for each of the studied SNPs. Forward and reverse primers are represented by green and yellow arrows, respectively. The arrow’s tip indicates the 5′➔3’ orientation of the primers. The dotted lines depict the PCR product lengths for each primer set
Sequencing reactions were performed using the MJ Research PTC-225 Peltier Thermal Cycler device and ABI PRISM®BigDyeTM Terminator Cycle Sequencing Kit with AmpliTaq® DAN polymerase (Applied Biosystems®, Waltham, USA). The resulting fluorescently labeled fragments were purified to remove unincorporated terminators using the BigDye® XTerminator™ purification protocol. The purified samples were resuspended in distilled water and subjected to electrophoresis using an ABI 3730xl sequencer (Applied Biosystems®, Waltham, USA). Geneious Prime® software (Biomatters Ltd, Auckland, New Zealand) was used to analyze the sequencing chromatograms and to identify the frequencies of the studied alleles (Additional File 3). Moreover, by using the length of the PCR products, we were able to screen for four additional core SNPs (rs6413438, rs140278421, rs375781227, and rs370803989) that define the *10, *22,*26, and *33 alleles, respectively.
Phenotype prediction
Patient phenotypes were classified according to the guidelines established by the Clinical Pharmacogenetics Implementation Consortium (CPIC) for CYP2C19 Genotype and Clopidogrel Therapy, updated in January 2022. According to these guidelines, individuals who carry two copies of the gain-of-function allele CYP2C19*17 are considered ultrarapid metabolizers (UMs). Those with one copy of the CYP2C19*17 allele and one copy of the normal function allele CYP2C19*1 are classified as rapid metabolizers (RMs). Individuals carrying two copies of the normal function allele CYP2C19*1 are designated as normal metabolizers (NMs). In contrast, individuals with one copy of a non-functional allele (either CYP2C19*2 or CYP2C19*3) along with either a normal function or gain-of-function allele are designated as intermediate metabolizers (IMs). Finally, patients with two copies of a non-functional allele are categorized as poor metabolizers (PMs).
Follow-up
A follow-up period of 18-24 months was conducted for patients, during which they were contacted via phone calls. The purpose of this follow-up was to assess adherence to the prescribed medication regimen and evaluate the occurrence of any subsequent cardiovascular events following the PCI procedure. The assessed cardiovascular events included refractory angina symptoms, MACE, and bleeding events.
Statistical analysis
Data were analyzed using GraphPad Prism® 9.2.0 (283) (GraphPad Software Inc., California, USA) and SPSS® version 26 (IBM Corp, NY, USA). Categorical data were represented as frequency and percentage, while quantitative data that showed a normal distribution were expressed as the mean ± standard deviation. Genotype frequencies were tested for deviations from Hardy–Weinberg equilibrium (HWE) using chi-square analysis. The relationship between genetic and non-genetic variables and clinical responsiveness to clopidogrel was analyzed using the chi-square or Fisher’s exact test. A two-sided P-value of less than 0.05 was considered to indicate statistical significance in all performed tests.
Results
Patients’ characteristics
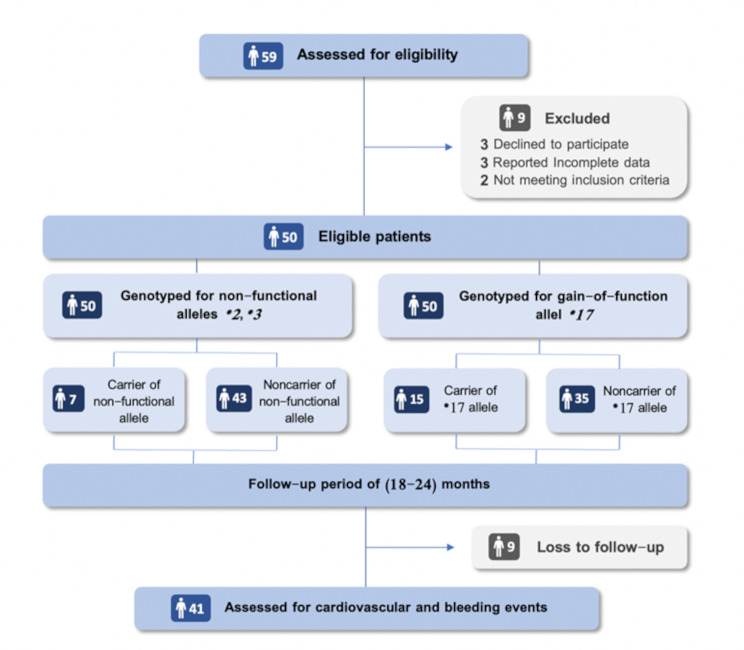
59 CAD patients undergoing PCI were evaluated, and 51 of these subjects met the inclusion criteria, as illustrated in Fig. 2. The general characteristics of the study population in the current research were summarized and categorized in Table 2. The study population comprised 32 males and 18 females. The mean age of the patients was 57.67 ± 9.26 years. The study investigated other clinical features of patients and found that (56%) had hypertension, (28%) had diabetes, and (52%) had a family history of CAD. Furthermore, (12%) of the patients reported a previous PCI, (50%) reported being smokers, and (44%) had a sedentary lifestyle.
Fig. 2.
Flow diagram for study participants
Table 2.
Baseline demographics of study participants
| Mean age, y (SD) | 57.67 (1.35) |
|---|---|
| Gender | |
| Male | 32 (64%) |
| Female | 18 (32%) |
| BMI by class | |
| Normal | 15 (30%) |
| Overweight | 20 (40%) |
| Obese | 15 (30%) |
| Current smoker | 25 (50%) |
| Sedentary lifestyle | 22 (44%) |
| Medical history | |
| Hypertension | 28 (56%) |
| Diabetes | 14 (28%) |
| CAD FH | 26 (52%) |
| Previous PCI | 6 (12%) |
| Comedications | |
| Statin | 50 (100%) |
| ACEIs/ARBs | 26 (52%) |
| Beta blocker | 37 (74%) |
| CCB | 12 (24%) |
| Aspirin | 50 (100%) |
| PPIs | 24 (48%) |
Allelic and genotypic distribution of the CYP2C19 polymorphisms
Table 3 displays the allelic distribution frequencies of the investigated SNPs within the CYP2C19 gene. The frequency of the CYP2C19*2 allele was found to be (8%) with only one individual homozygous for this non-functional allele, resulting in a frequency of (2%) for the (*2/*2) genotype. The allelic frequency of CYP2C19*17 was determined to be (17%), with (4%) of the participants carrying the homozygous (*17/*17) genotype, and (26%) were heterozygous wild-type (*1/*17). The other examined alleles (CYP2C19*3, *10, *22, *26, and *33) were not detected in the study population. The genotype frequencies were not significantly different from those predicted by the Hardy–Weinberg equation (Rx2 = 3.023; P value = 0.082; Table 4). The distribution of patients by CYP2C19 phenotypes was as follows: UMs (4%), RMs (26%), NMs (56%), IMs (12%), and PMs (2%) (Table 4).
Table 3.
Studied CYP2C19 gene polymorphisms
| Allele | rsID number | Nucleis Acid Base Substitution | Frequency |
|---|---|---|---|
| CYP2C19*2 | rs4244285 | 19,154 G > A | 0.08 |
| CYP2C19*3 | rs4986893 | 17,948 G > A | 0 |
| CYP2C19*10 | rs6413438 | 19,153 C > T | 0 |
| CYP2C19*17 | rs12248560 | −806 C > A / C > T | 0.17 |
| CYP2C19*22 | rs140278421 | 17,869 G > A / G > C / G > T | 0 |
| CYP2C19*26 | rs375781227 | 19,239 G > A | 0 |
| CYP2C19*33 | rs370803989 | 17,874 G > A | 0 |
Table 4.
Observed versus expected genotype distribution of CYP2C19
| Phenotype | N (%) | Genotype |
N (%) Observed |
N (%) Expected |
HWE x2 | HWE P value |
|---|---|---|---|---|---|---|
| UMs | 2 (4%) | *17/*17 | 2 (4%) | 1.44 (2.89%) | 0.2131 | 0.64 |
| RMs | 13 (26%) | *1/*17 | 13 (26%) | 12.75 (25.5%) | 0.0049 | 0.94 |
| NMs | 28 (56%) | *1/*1 | 28 (56%) | 28.13 (56.25%) | 0.00055 | 0.98 |
| IMs | 6 (12%) | *1/*2 | 6 (12%) | 6 (12%) | 0 | 1 |
| *1/*3 | 0 (0%) | 0 (0%) | NA | NA | ||
| *17/*2 | 0 (0%) | 1.36 (2.72%) | 1.36 | 0.24 | ||
| *17/*3 | 0 (0%) | 0 (0%) | NA | NA | ||
| PMs | 1 (2%) | *2/*2 | 1 (2%) | 0.32 (0.64%) | 1.445 | 0.22 |
| *2/*3 | 0 (0%) | 0 (0%) | NA | NA | ||
| *3/*3 | 0 (0%) | 0 (0%) | NA | NA |
HWE x2 Hardy–Weinberg equation test (P = 0.05)
NA, not applicable
Follow-up
Out of all the patients initially included in the study, nine individuals could not be followed up. Among the remaining 41 patients for whom data were available, 16 patients (39%) reported experiencing recurrent angina symptoms. Additionally, two individuals (4.88%) experienced MACE, and four patients (9.76%) reported bleeding events.
Refractory angina symptoms and CYP2C19 polymorphism
As represented in Table 5, it was found that two patients (33.33%) from the CYP2C19*2 allele carrier group exhibited symptoms of refractory angina. However, this result did not demonstrate a statistically significant difference when compared with the non-carrier group, in which 14 patients (%38.89) exhibited similar symptoms (P > 0.9999).
Table 5.
Distribution of recurrent cardiac ischemic events by CYP2C19*2 polymorphism
| Allele CYP2C19*2 | Refractory angina symptoms | P value | Major adverse cardiac events | P value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Yes | 2 (%33.33) | 4 (%66.67) | >0.9999 | 1 (%16.67) | 5 (%83.33) | 0.2744 |
| No | 14 (%38.89) | 22 (%61.11) | 1 (%2.86) | 34 (%97.14) | ||
Major adverse cardiac events and CYP2C19 polymorphism
Overall, two of the 41 patients who were followed up in the study experienced at least one MACE after PCI. One case was from the CYP2C19*2 allele carrier group and the other was from the non-carrier group. In terms of the distribution of MACE based on CYP2C19*2 status, there was no statistically significant correlation (P = 0.2744).
Recurrent cardiac ischemic events and non-genetic factors
The distribution of refractory angina symptoms and MACE among patients according to various demographic and clinical factors is presented in Table 6. Statistical analyses were conducted to investigate the association between these factors and the clinical response to Clopidogrel, particularly regarding the incidence of recurrent cardiovascular events. The results indicated that the P-value in all tests exceeded 0.05, suggesting no significant association between these factors and cardiovascular events. Specifically, no significant relationship was found between MACE and factors such as gender, body mass index (BMI), hypertension, diabetes mellitus, proton pump inhibitor use, or calcium channel blocker use. These findings highlight the absence of a clear link between these non-genetic factors and major cardiovascular events in the studied population.
Table 6.
Distribution of recurrent cardiac ischemic events by non-genetic factors
| Variables | Refractory angina symptoms | P value | Major adverse cardiac events | P value | |||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| Gender | Male | 4 (14.81%) | 23 (85.19%) | > 0.9999 | 0 (0%) | 27 (100%) | 0.1228 |
| Female | 2 (14.28%) | 12 (85.71%) | 2 (14.28%) | 12 (85.71%) | |||
| BMI | Normal | 1 (7.69%) | 12 (92.31%) | 0.19134 | 0 (0%) | 13 (100%) | 0.1317 |
| Overweight | 1 (7.14%) | 13 (92.86%) | 0 (0%) | 14 (100%) | |||
| Obese | 4 (28.57%) | 10 (71.42%) | 2 (14.28%) | 12 (85.72%) | |||
| Hypertension | Yes | 4 (12.90%) | 27 (87.10%) | 0.6221 | 1 (4.76%) | 20 (95.24%) | > 0.9999 |
| No | 2 (10%) | 18 (90%) | 1 (5%) | 19 (95%) | |||
| Diabetes | Yes | 3 (27.27%) | 8 (72.73%) | 0.3162 | 1 (9.1%) | 10 (90.9%) | 0.4695 |
| No | 3 (10%) | 27 (90%) | 1 (3.33%) | 29 (96.77%) | |||
| Proton Pump Inhibitor Use | Yes | 4 (18.18%) | 18 (81.82%) | 0.6681 | 0 (0%) | 22 (100%) | 0.2085 |
| No | 2 (10.52%) | 17 (89.47%) | 2 (10.53%) | 17 (89.47%) | |||
| Calcium Chanel Blockers Use | Yes | 3 (30%) | 7 (70%) | 0.1435 | 0 (0%) | 10 (100%) | > 0.9999 |
| No | 3 (9.67%) | 28 (90.33%) | 2 (6.45%) | 29 (93.55%) | |||
Bleeding events and CYP2C19 polymorphism
Our results indicated that carriers of the CYP2C19*17 variant experienced a higher incidence of bleeding events (16.67%) compared to non-carriers (6.9%). Despite this observed difference, it is important to note that the statistical analysis found no significant difference between the two groups (P = 0.567; see Table 7). Similarly, there was no statistically significant difference in bleeding events between CYP2C19*2 carriers and non-carriers (P = 0.0952; see Table 7), although a higher proportion of bleeding events was observed among carriers.
Table 7.
Distribution of bleeding events by CYP2C19 polymorphism
| Allele CYP2C19*17 | Bleeding events | P value | Allele CYP2C19*2 | Bleeding events | P value | ||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| Yes | 2 (16.67%) | 10 (83.33%) | 0.567 | Yes | 2 (33.33%) | 4 (66.67%) | 0.0952 |
| No | 2 (6.9%) | 27 (93.1%) | No | 2 (5.71%) | 33 (94.29%) | ||
Discussion
Dual antiplatelet therapy, consisting of aspirin and a P2Y12 inhibitor, is the current recommended treatment strategy for patients with CAD undergoing PCI with stent implantation to reduce the risk of recurrent MACE [27]. Although novel P2Y12 inhibitors, such as prasugrel and ticagrelor are becoming more popular due to their strong antiplatelet effects [28–30], clopidogrel remains the most widely prescribed drug of P2Y12 inhibitors in Syria. This preference is primarily due to its lower cost and reduced risk of bleeding. However, most Syrian physicians tend to duplicate the maintenance dose of clopidogrel for at least one month after stenting to provide additional prevention of recurrent ischemic events, including stent thrombosis. However, despite clopidogrel’s general effectiveness, various studies have shown that a significant number of patients (between 5% and 44%) may not respond adequately to this medication [31]. Clopidogrel response has been attributed to be affected by several factors, which can be categorized as clinical, cellular, and genetic. Polymorphism of CYP2C19 gene is one of the key genetic factors influencing individual responses to clopidogrel [32, 33].
To the best of our knowledge, this is the first study in Syria reporting allelic frequencies of CYP2C19*2, *3, and *17 and assessing their impact on the clinical efficacy and safety of a double maintenance dose of clopidogrel as part of DAPT in CAD patients undergoing PCI. The Syrian population is a highly admixed community with diverse ethnic, cultural, and religious groups, resulting from historical factors such as immigration, political occupations, and trade relations with various countries over the centuries. This diversity has created a unique genetic makeup, making it important to conduct genetic studies specific to this population.
The frequency of CYP2C19*2 allele in our study population (8%) was found to be compatible with that of other populations in the Middle East and North Africa (MENA) region, such as the Jordanian (9.8%) [11], Iraqi (11.7%) [14], Saudi (9.9%) [17], Egyptian (12.6%) [15], and Tunisian (9%) [18]. However, a noteworthy variation was observed when comparing our results to East Asian populations, such as Chinese (24.7%) [25] and Japanese (27.9%) [24]. The CYP2C19*3 allele was absent in our study population, which aligns with the low or diminished frequencies reported in European and Middle Eastern populations. In contrast, this allele has a higher prevalence in Asian populations, ranging from 3.3% in China [25] to 12.8% in Japan [24]. This suggests a notable ethnic difference between Caucasian and Oriental populations, indicating that this genetic variant likely emerged relatively recently after the differentiation of these groups [21]. For the CYP2C9*17 allele, the frequency was found to be (17%), which is close to that reported in European and Middle Eastern populations but higher than those found in other studies on Asians (from 0.3% in Koreans [26] to 1.3% in Japanese [23]). Table 8 summarizes the frequencies of the studied alleles across different countries and populations.
Table 8.
Comparison of allele frequencies of CYP2C19*2, *3, and *17 reported from different ethnic populations
| Country | *2 allele frequency (%) | *3 allele frequency (%) | *17 allele frequency (%) | Ref. |
|---|---|---|---|---|
| Syria | 8 | 0 | 17 | Current study |
| Lebanon | 13.4 | 0.3 | NE | [1] |
| Jordan | 9.8 | 0 | 28.72 | [2, 3] |
| Palestine | 15.5 | 2.3 | NE | [4] |
| Iraq | 11.7 | 0 | 27.6 | [5] |
| Egypt | 12.6 | 0.25 | 17 | [6] |
| Qatar | 13 | 0.4 | 21 | [7] |
| Saudi Arabia | 9.9 | 0.1 | 25.7 | [8] |
| Tunisia | 9 | 0 | 21.3 | [9] |
| Turkey | 15.27 | 0.43 | 15.85 | [10] |
| Iran | 16.5 | 0.1 | 21.78 | [11] |
| Italy | 11 | 0 | 20.3 | [12] |
| Greek | 13.07 | 0 | 19.61 | [13] |
| Japan | 27.9 | 12.8 | 1.3 | [14, 15] |
| China | 24.7 | 3.3 | 1.2 | [16] |
| Korea | 28 | 11 | 0.3 | [17] |
The present study did not confirm an association between MACE incidence and the carrier status of the non-functional CYP2C19*2 allele, which has been established in many previous studies and meta-analyses [34–39]. Numerous prominent international medicine and regulatory agencies, including the US Food and Drug Administration (FDA), European Medicines Agency (EMA), Health Canada/Santé Canada (HCSC), and Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, have incorporated pharmacogenomics (PGx)-relevant annotations into the clopidogrel drug label under the “Prescribing” section. These annotations indicate that the antiplatelet efficacy of clopidogrel may be impaired or absent in certain patient groups carrying loss-of-function alleles of the CYP2C19 gene [40–43]. Furthermore, extensive literature reviews have been conducted, leading to the development of clinical practice guidelines by professional organizations such as the Clinical Pharmacogenetics Implementation Consortium, the Dutch Pharmacogenetics Working Group (DPWG), and the French National Network of Pharmacogenetics (RNPGx). These guidelines specifically address clopidogrel and CYP2C19 genotyping, recommending various approaches based on the patient’s CYP2C19 phenotype [44–46]. The recommendations provided by the DPWG team suggest considering alternative antiplatelet treatments or doubling the dose of clopidogrel for patients with the Intermediate Metabolizer (IM) phenotype [45]. This may explain the findings of our study. In our study population, all patients who carried the CYP2C19*2 allele and were followed up demonstrated the IM phenotype of the CYP2C19 enzyme, as they carried a single copy of the CYP2C19*2 allele. These patients were prescribed a double maintenance dose of clopidogrel (150 mg/day) for at least one month following (PCI) procedure, as directed by their treating physician. It is possible that by increasing the clopidogrel dosage in individuals with the IM phenotype, sufficient platelet inhibition could have been achieved, potentially reducing the occurrence of recurrent ischemic events.
Our study revealed a noteworthy observation regarding the incidence of bleeding events among the patients we investigated. Despite 30% of the individuals carrying the gain-of-function CYP2C19*17 allele and receiving a double maintenance dose of clopidogrel after stent placement, the occurrence of hemorrhagic events remained relatively low, not exceeding (9.7%). Several potential explanations for these findings include limitations related to the study population size, medication adherence, loss to follow-up, and various clinical and genetic factors.
The CYP2C19*17 allele is well-known to be associated with increased CYP2C19 enzyme activity. However, the clinical significance of this allele remains a topic of ongoing controversy. Although several studies, including a comprehensive meta-analysis, have confirmed that individuals carrying the CYP2C19*17 allele are at an elevated risk of bleeding events when treated with a standard dose of clopidogrel [47–49], conflicting evidence has been reported [50]. In addition to the conflicting evidence, therapeutic recommendations vary among different guidelines issued by professional organizations in the field of pharmacogenetics for patients undergoing (PCI) and carrying the CYP2C19*17 allele [44–46]. Given these controversies and inconsistencies, further research is needed to gain a more comprehensive understanding of the clinical implications of the CYP2C19*17 allele and its impact on treatment outcomes, particularly in patients undergoing PCI.
In addition to the extensively studied CYP2C19, several other genes have been investigated in the literature because of their potential impact on the metabolism and effective concentration of the active metabolites of clopidogrel. These genes included ABCB1 [51], CYP2C9 [52], CYP3A4/5 [53], PON1 [54], and CES1 [55]. Within the ABCB1 gene, a SNP known as C3435T (rs1045642) has been associated with a higher incidence of ischemic cardiac events in patients with acute myocardial infarction treated with clopidogrel. This SNP has been linked to potential sub-therapeutic concentrations of clopidogrel, primarily resulting from decreased intestinal absorption of the drug [51]. The frequency of this genetic variant varies among populations. High frequencies have been observed in nearby Middle Eastern populations, such as Jordanians (58%) [56], Palestinians (46%) [57], and Lebanese (50.8%) [58], making it possible that this SNP could be present in a substantial percentage within our study population.
One of the main limitations of this study was the small number of participants, which may restrict the generalizability of the findings. Additionally, the study did not include laboratory evaluation of Clopidogrel responsiveness through platelet function testing. Such testing could have provided valuable insights into the pharmacodynamic effect of clopidogrel in relation to CYP2C19 polymorphisms and further strengthened the interpretation of our findings. It is crucial to acknowledge that this research represents an initial step towards investigating the genetic profile of the Syrian community in relation to the double maintenance dose of clopidogrel. The utilization of a robust genotyping method (amplicon Sanger sequencing) bolsters the reliability of the genetic data obtained. As such, the findings of this study should be interpreted as modest contributions to the existing literature rather than definitive evidence. Further research with larger sample sizes, more diverse populations, and rigorous study designs is necessary to gain a comprehensive understanding of the genetic factors influencing clopidogrel response within the Syrian community.
Conclusions
Among patients who underwent PCI and were treated with a double maintenance dose of clopidogrel, the carrier status of the CYP2C19*2 allele does not appear to be associated with an increased risk of MACE. This finding could potentially support the increased clopidogrel dosing strategy depending on the CYP2C19 genotype. Nonetheless, given the widespread use of clopidogrel for the treatment of patients undergoing PCI, further evidence regarding the impact of adjusting clopidogrel dosage or utilizing alternative antiplatelet therapy for CYP2C19 non-functional alleles carriers is necessary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our sincere appreciation to Professor Majd N. Aljamali for his invaluable technical guidance. We also acknowledge the Department of Pharmaceutical Biotechnology at the National Commission for Biotechnology for granting us access to their laboratory equipment and facilities.
Abbreviations
- CAD
Coronary artery disease
- CNV
Copy number variation
- CPIC
The Clinical Pharmacogenetics Implementation Consortium
- DAPT
Dual antiplatelet therapy
- DPWG
Dutch Pharmacogenetics Working Group
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- gDNA
Genomic DNA
- HCSC
Health Canada/Santé Canada
- HWE
Hardy–Weinberg equilibrium
- IHD
Ischemic heart disease
- IMs
Intermediate metabolizers
- MACE
Major acute cardiovascular events
- MENA
Middle East and North Africa
- NMs
Normal metabolizers
- PCI
Percutaneous coronary intervention
- PGx
Pharmacogenomics
- PharmVar
Pharmacogene Variation
- PMDA
Pharmaceuticals and Medical Devices Agency
- PMs
Poor metabolizers
- RMs
Rapid metabolizers
- RNPGx
French National Network of Pharmacogenetics
- SNPs
Single nucleotide polymorphisms
- UMs
Ultra-rapid metabolizers
- UV
Ultraviolet
Author contributions
LAY contributed to the conception and design of the study, data interpretation, and revisions of the manuscript. NHS was responsible for assessing eligibility, collecting data, conducting laboratory work, performing statistical analyses, interpreting the data, and drafting the manuscript.
Funding
This research was partially supported by special funding from Damascus University and a grant awarded by The Ministry of Higher Education and Scientific Research-Fund to support scientific research and technological development.
Data availability
The datasets generated and/or analysed during the current study are available in the ClinVar repository, with the accession numbers SCV005438651 to SCV005438653.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Scientific Research Ethics Committee at the Faculty of Pharmacy, Damascus University in accordance with the Declaration of Helsinki (1964). All patients provided written informed consent.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kovell LC, Aurigemma GP. Coronary Artery Disease. Diastology: Clinical Approach to Heart Failure with Preserved Ejection Fraction [Internet]. 2023 Feb 9 [cited 2023 May 8];308–21. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564304/
- 2.Ahmad M, Mehta P, Reddivari AKR, Mungee S. Percutaneous Coronary Intervention. StatPearls [Internet]. 2022 Sep 30 [cited 2023 May 5]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK556123/
- 3.Giantini A, Timan IS, Dharma R, Sukmawan R, Setiabudy R, Alwi I et al. The role of clopidogrel resistance-related genetic and epigenetic factors in major adverse cardiovascular events among patients with acute coronary syndrome after percutaneous coronary intervention. Front Cardiovasc Med [Internet]. 2022 Feb 8 [cited 2023 May 5];9. Available from: /pmc/articles/PMC9944402/. [DOI] [PMC free article] [PubMed]
- 4.Jiang XL, Samant S, Lesko LJ, Schmidt S. Clinical Pharmacokinetics and Pharmacodynamics of Clopidogrel. Clin Pharmacokinet [Internet]. 2015 Jan 24 [cited 2023 May 5];54(2):147. Available from: /pmc/articles/PMC5677184/ [DOI] [PMC free article] [PubMed]
- 5.Oldenburg PJ, Clopidogrel. Reference Module in Biomedical Sciences [Internet]. 2018 Jan 1 [cited 2023 May 5]; Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128012383981297
- 6.Angiolillo DJ, Galli M, Collet JP, Kastrati A, O’Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):E1371–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown SA, Pereira N. Pharmacogenomic Impact of CYP2C19 Variation on Clopidogrel Therapy in Precision Cardiovascular Medicine. J Pers Med [Internet]. 2018 Mar 1 [cited 2023 May 17];8(1). Available from: /pmc/articles/PMC5872082/ [DOI] [PMC free article] [PubMed]
- 8.Botton MR, Whirl-Carrillo M, Del Tredici AL, Sangkuhl K, Cavallari LH, Agúndez JAG et al. PharmVar GeneFocus: CYP2C19. Clin Pharmacol Ther [Internet]. 2021 Feb 1 [cited 2023 May 6];109(2):352. Available from: /pmc/articles/PMC7769975/ [DOI] [PMC free article] [PubMed]
- 9.Scott SA, Sangkuhl K, Shuldiner AR, Hulot JS, Thorn CF, Altman RB, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genomics. 2012;22(2):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jureidini ID, Chamseddine N, Keleshian S, Naoufal R, Zahed L, Hakime N. Prevalence of CYP2C19 polymorphisms in the Lebanese population. Mol Biol Rep. 2011;38(8):5449–52. [DOI] [PubMed] [Google Scholar]
- 11.Rjoub M, Saleh A, Hakooz N, Imraish A, Jarrar Y, Zihlif M. Allelic frequency of PON1 Q192R, CYP2C19*2 and CYP2C19*17 among Jordanian patients taking clopidogrel. Trop J Pharm Res. 2018;17(11):2275–80. [Google Scholar]
- 12.Yousef AM, Bulatova NR, Newman W, Hakooz N, Ismail S, Qusa H, et al. Allele and genotype frequencies of the polymorphic cytochrome P450 genes (CYP1A1, CYP3A4, CYP3A5, CYP2C9 and CYP2C19) in the Jordanian population. Mol Biol Rep. 2012;39(10):9423–33. [DOI] [PubMed] [Google Scholar]
- 13.Ayesh BM, Al-Astal IR, Yassin MM. The clinical effects of CYP2C19 *2 allele frequency on Palestinian patients receiving clopidogrel after percutaneous coronary intervention. Int J Clin Pharm. 2019;41(1):96–103. [DOI] [PubMed] [Google Scholar]
- 14.Mohammad AM, Al-Allawi NAS. CYP2C19 genotype is an independent predictor of adverse cardiovascular outcome in Iraqi patients on clopidogrel after percutaneous coronary intervention. J Cardiovasc Pharmacol. 2018;71(6):347–51. [DOI] [PubMed] [Google Scholar]
- 15.Khalil B, Shahin M, Solayman M, Langaee T, Schaalan M, Gong Y, et al. Genetic and nongenetic factors affecting clopidogrel response in the Egyptian population. Clin Transl Sci. 2016;9(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali ZO, Bader L, Mohammed S, Arafa S, Arabi A, Cavallari L et al. Effect of CYP2C19 genetic variants on bleeding and major adverse cardiovascular events in a cohort of Arab patients undergoing percutaneous coronary intervention and stent implantation. Pharmacogenet Genomics [Internet]. 2022 Jul 1 [cited 2023 Jan 15];32(5):183–91. Available from: https://journals.lww.com/jpharmacogenetics/Fulltext/2022/07000/Effect_of_CYP2C19_genetic_variants_on_bleeding_and.2.aspx [DOI] [PubMed]
- 17.Tayeb HT, Bakheet DH, Zaza K, Wakil SM, Dzimiri N. Genotyping of CYP2C19 polymorphisms and its clinical validation in the ethnic Arab population. J Pharm Pharmacol. 2015;67(7):972–9. [DOI] [PubMed] [Google Scholar]
- 18.Abdelhedi R, Bouayed A, Alfadhli N, Abid S, Rebai L, Kharrat A. Characterization of drug-metabolizing enzymes CYP2C9, CYP2C19 polymorphisms in Tunisian, Kuwaiti and Bahraini populations. 94, J Genet. 2015. [DOI] [PubMed]
- 19.Saydam F, Değirmenci İ, Birdane A, Özdemir M, Ulus T, Özbayer C, et al. The CYP2C19*2 and CYP2C19*17 polymorphisms play a vital role in clopidogrel responsiveness after percutaneous coronary intervention: A pharmacogenomics study. Basic Clin Pharmacol Toxicol. 2017;121(1):29–36. [DOI] [PubMed] [Google Scholar]
- 20.Mahdieh N, Rabbani A, Firouzi A, Zahedmehr A, Hoseinimoghaddam M, Saedi S, et al. Clopidogrel pharmacogenetics in Iranian patients undergoing percutaneous coronary intervention. Cardiovasc Toxicol. 2018;18(5):482–91. [DOI] [PubMed] [Google Scholar]
- 21.Scordo MG, Caputi AP, D’Arrigo C, Fava G, Spina E. Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol Res. 2004;50(2):195–200. [DOI] [PubMed] [Google Scholar]
- 22.Ragia G, Arvanitidis KI, Tavridou A, Manolopoulos VG. Need for reassessment of reported CYP2C19 allele frequencies in various populations in view of CYP2C19*17 discovery: the case of Greece. Pharmacogenomics. 2009;10(1):43–9. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto K, Uno T, Yamazaki H, Tateishi T. Limited frequency of the CYP2C19*17 allele and its minor role in a Japanese population. Br J Clin Pharmacol. 2008;65(3):437–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakata T, Miyahara M, Nakatani K, Wada H, Tanigawa T, Komada F, et al. Relationship between CYP2C19 loss-of-function polymorphism and platelet reactivities with clopidogrel treatment in Japanese patients undergoing coronary stent implantation. Circ J. 2013;77(6):1436–44. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Qin S, Xie J, Tang J, Yang L, Shen W et al. Genetic polymorphism analysis of CYP2C19 in Chinese Han populations from different geographic areas of mainland China. Pharmacogenomics [Internet]. 2008 [cited 2023 May 20];9(6):691–702. Available from: https://pubmed.ncbi.nlm.nih.gov/18518848/ [DOI] [PubMed]
- 26.Ramsjö M, Aklillu E, Bohman L, Ingelman-Sundberg M, Roh HK, Bertilsson L. CYP2C19 activity comparison between Swedes and Koreans: effect of genotype, sex, oral contraceptive use, and smoking. European Journal of Clinical Pharmacology 2010 66:9 [Internet]. 2010 May 25 [cited 2022 Aug 27];66(9):871–7. Available from: https://link.springer.com/article/10.1007/s00228-010-0835-0 [DOI] [PubMed]
- 27.Lawton J, Tamis-Holland J, Bangalore S. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. J Am Coll Cardiol [Internet]. 2022 Jan [cited 2023 Jun 4];79(2):e21–129. Available from: https://www.jacc.org/doi/10.1016/j.jacc.2021.09.006 [DOI] [PubMed]
- 28.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med [Internet]. 2009 Sep 10 [cited 2023 Jun 4];361(11):1045–57. Available from: https://pubmed.ncbi.nlm.nih.gov/19717846/ [DOI] [PubMed]
- 29.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med [Internet]. 2007 Nov 15 [cited 2023 Jun 4];357(20):2001–15. Available from: https://pubmed.ncbi.nlm.nih.gov/17982182/ [DOI] [PubMed]
- 30.Navarese EP, Verdoia M, Schaffer A, Surian P, Kozinski M, Castriota F et al. Ischaemic and bleeding complications with new, compared to standard, ADP-antagonist regimens in acute coronary syndromes: a meta-analysis of randomized trials. QJM [Internet]. 2011 Jul [cited 2023 Jun 4];104(7):561–9. Available from: https://pubmed.ncbi.nlm.nih.gov/21572108/ [DOI] [PubMed]
- 31.Ray S. Clopidogrel resistance: The way forward. Indian Heart J [Internet]. 2014 Sep 1 [cited 2023 May 27];66(5):530. Available from: /pmc/articles/PMC4223168/ [DOI] [PMC free article] [PubMed]
- 32.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol [Internet]. 2007 Apr 10 [cited 2023 Jun 4];49(14):1505–16. Available from: https://pubmed.ncbi.nlm.nih.gov/17418288/ [DOI] [PubMed]
- 33.Camilleri E, Jacquin L, Paganelli F, Bonello L. Personalized antiplatelet therapy: review of the latest clinical evidence. Curr Cardiol Rep [Internet]. 2011 Aug [cited 2023 Jun 4];13(4):296–302. Available from: https://pubmed.ncbi.nlm.nih.gov/21594586/ [DOI] [PubMed]
- 34.Xi Z, Fang F, Wang J, AlHelal J, Zhou Y, Liu W. CYP2C19 genotype and adverse cardiovascular outcomes after stent implantation in clopidogrel-treated Asian populations: A systematic review and meta-analysis. Platelets [Internet]. 2019 Feb 17 [cited 2023 Jun 4];30(2):229–40. Available from: https://pubmed.ncbi.nlm.nih.gov/29257922/ [DOI] [PubMed]
- 35.Alkattan AN, Radwan NM, Mahmoud NE, Alfaleh AF, Alfaifi AH, Alabdulkareem KI. The efficacy of clopidogrel in preventing recurrent cardiovascular events among Arab population carrying different CYP2C19 mutations: systematic review and meta-analysis. Egyptian Journal of Medical Human Genetics [Internet]. 2022 Dec 1 [cited 2023 Jun 4];23(1):1–10. Available from: https://jmhg.springeropen.com/articles/10.1186/s43042-022-00313-w
- 36.Mao L, Jian C, Changzhi L, Dan H, Suihua H, Wenyi T, et al. Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel-treated patients: A meta-analysis based on 23,035 subjects. Arch Cardiovasc Dis. 2013;106(10):517–27. [DOI] [PubMed] [Google Scholar]
- 37.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K et al. Reduced-Function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A Meta-Analysis. 2010;304(16):1821–30. [DOI] [PMC free article] [PubMed]
- 38.Kambhampati NT, Ahamed H, David KKV, Hakeem S, chandra S, Pillai G et al. Cytochrome P450 2C19 Polymorphisms and Its Association With Major Adverse Cardiac Events in Post-coronary Intervention Patients on Clopidogrel in the Tertiary Care Center. Cureus [Internet]. 2023 Feb 7 [cited 2023 Jun 4];15(2). Available from: https://www.cureus.com/articles/133507-cytochrome-p450-2c19-polymorphisms-and-its-association-with-major-adverse-cardiac-events-in-post-coronary-intervention-patients-on-clopidogrel-in-the-tertiary-care-center [DOI] [PMC free article] [PubMed]
- 39.Rothenbacher D, Hoffmann MM, Breitling LP, Rajman I, Koenig W, Brenner H. Cytochrome P450 2C19*2 polymorphism in patients with stable coronary heart disease and risk for secondary cardiovascular disease events: Results of a long-term follow-up study in routine clinical care. BMC Cardiovasc Disord [Internet]. 2013 Aug 27 [cited 2023 Jun 4];13(1):1–11. Available from: https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/1471-2261-13-61 [DOI] [PMC free article] [PubMed]
- 40.Annotation of FDA Label for clopidogrel and CYP2C19 [Internet]. [cited 2023 Jun 4]. Available from: https://www.pharmgkb.org/labelAnnotation/PA166104777
- 41.Annotation of EMA Label for clopidogrel and CYP2C19 [Internet]. [cited 2023 Jun 4]. Available from: https://www.pharmgkb.org/labelAnnotation/PA166104841
- 42.Annotation of HCSC Label for clopidogrel and CYP2C19 [Internet]. [cited 2023 Jun 4]. Available from: https://www.pharmgkb.org/labelAnnotation/PA166127653
- 43.Annotation of PMDA Label for clopidogrel and CYP2C19 [Internet]. [cited 2023 Jun 4]. Available from: https://www.pharmgkb.org/labelAnnotation/PA166123535
- 44.Lee CR, Luzum JA, Sangkuhl K, Gammal RS, Sabatine MS, Stein CM, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112(5):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annotation of DPWG Guideline for clopidogrel and CYP2C19 [Internet]. [cited 2023 Jun 4]. Available from: https://www.pharmgkb.org/guidelineAnnotation/PA166104956
- 46.Annotation of RNPGx. Guideline for clopidogrel and CYP2C19 [Internet]. [cited 2023 Jun 4]. Available from: https://www.pharmgkb.org/guidelineAnnotation/PA166202501
- 47.Li Y, Tang HL, Hu YF, Xie HG. The gain-of-function variant allele CYP2C19*17: A double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost. 2012;10(2):199–206. [DOI] [PubMed] [Google Scholar]
- 48.Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation [Internet]. 2010 Feb [cited 2023 Jun 4];121(4):512–8. Available from: https://pubmed.ncbi.nlm.nih.gov/20083681/ [DOI] [PubMed]
- 49.Ali ZO, Bader L, Mohammed S, Arafa S, Arabi A, Cavallari L et al. Effect of CYP2C19 genetic variants on bleeding and major adverse cardiovascular events in a cohort of Arab patients undergoing percutaneous coronary intervention and stent implantation. Pharmacogenet Genomics [Internet]. 2022 Jul 1 [cited 2023 Jun 4];32(5):183–91. Available from: https://pubmed.ncbi.nlm.nih.gov/35389962/ [DOI] [PubMed]
- 50.Yiu D, So F, Baudhuin L, Farkouh M, Goodman S, Mathew V, ISCHEMIC AND BLEEDING OUTCOMES OF CARRIERS OF CYP2C19*17 TREATED WITH CLOPIDOGREL: INSIGHTS FROM THE TAILOR-PCI STUDY. J Am Coll Cardiol [Internet]. 2021 May [cited 2022 Sep 20];77(18):1068. Available from: https://www.jacc.org/doi/10.1016/S0735-1097(21)02427-X
- 51.Su J, Xu J, Li X, Zhang H, Hu J, Fang R et al. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis. PLoS One [Internet]. 2012 Oct 9 [cited 2023 Jun 4];7(10). Available from: https://pubmed.ncbi.nlm.nih.gov/23056288/ [DOI] [PMC free article] [PubMed]
- 52.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost [Internet]. 2007 Dec [cited 2023 Jun 4];5(12):2429–36. Available from: https://pubmed.ncbi.nlm.nih.gov/17900275/ [DOI] [PubMed]
- 53.Suh JW, Koo BK, Zhang SY, Park KW, Cho JY, Jang IJ et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ [Internet]. 2006 Jun 6 [cited 2023 Jun 4];174(12):1715–22. Available from: https://pubmed.ncbi.nlm.nih.gov/16754899/ [DOI] [PMC free article] [PubMed]
- 54.Bouman HJ, Schömig E, Van Werkum JW, Velder J, Hackeng CM, HirschhÄuser C et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med [Internet]. 2011 Jan [cited 2023 Jun 4];17(1):110–6. Available from: https://pubmed.ncbi.nlm.nih.gov/21170047/ [DOI] [PubMed]
- 55.Lewis JP, Horenstein RB, Ryan K, O’Connell JR, Gibson Q, Mitchell BD et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics [Internet]. 2013 [cited 2023 Jun 4];23(1):1–8. Available from: https://pubmed.ncbi.nlm.nih.gov/23111421/ [DOI] [PMC free article] [PubMed]
- 56.Khabour OF, Alzoubi KH, Al-Azzam SI, Mhaidat NM. Frequency of MDR1 single nucleotide polymorphisms in a Jordanian population, including a novel variant. Genetics and Molecular Research [Internet]. 2013 Mar 13 [cited 2022 Sep 20];12(1):801–8. Available from: https://www.geneticsmr.com/articles/2174 [DOI] [PubMed]
- 57.Nassar S, Amro O, Abu-Rmaileh H, Alshaer I, Korachi M, Ayesh S. ABCB1 C3435T and CYP2C19*2 polymorphisms in a Palestinian and Turkish population: A Pharmacogenetic perspective to clopidogrel. Meta Gene. 2014;2(1):314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milane A, Khazen G, Olaywan L, Zarzour F, Mohty R, Sarkis A et al. Frequency of ABCB1 C3435T and CYP3A5*3 Genetic Polymorphisms in the Lebanese Population. Exp Clin Transplant [Internet]. 2021 May 1 [cited 2022 Sep 20];19(5):434–8. Available from: https://pubmed.ncbi.nlm.nih.gov/34053421/ [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the ClinVar repository, with the accession numbers SCV005438651 to SCV005438653.