Abstract
Background
The diagnosis of reversible cerebral vasoconstriction syndrome (RCVS) is challenging due to its varied clinical manifestations and imaging findings. While it typically presents with a sudden, severe thunderclap headache and multifocal constriction of the cerebral arteries, the wide spectrum of radiological presentations may complicate the diagnosis.
Main Body
This review presents a series of cases that show both typical and atypical presentations of RCVS. Typical cases show the characteristic “string of beads” pattern on angiography, which usually resolves within 3–6 months. However, diagnostic challenges arise when angiography appears normal in the early stages or when imaging artifacts obscure the findings. In addition, the variability in vasoconstriction patterns and the need for a differential diagnosis further complicate the accurate identification. These cases highlight the importance of considering RCVS in patients with recurrent thunderclap headaches, even when the initial imaging is inconclusive. Recognizing these challenges and the variability in presentation, along with the use of high-resolution vessel wall MRI and blood-brain barrier imaging, can improve diagnostic accuracy and improve patient outcomes.
Conclusion
The diagnosis of RCVS requires careful integration of clinical evaluation and advanced imaging techniques, with particular attention to radiological findings that can guide accurate diagnosis and management. Despite challenges, such as normal early stage angiography and imaging variability, maintaining a high suspicion of RCVS is essential, especially in patients with recurrent thunderclap headaches.
Keywords: Reversible cerebral vasoconstriction syndrome, Thunderclap headache, Angiography, Imaging artifacts, Cerebral vasoconstriction, Differential diagnosis, High-resolution vessel wall MRI, Blood-brain barrier imaging
Background
Reversible cerebral vasoconstriction syndrome (RCVS) has been introduced as a clinical entity in 2007 [1]. The initial diagnostic criteria included recurrent thunderclap headaches, multifocal cerebral artery vasoconstriction with reversibility within 12 weeks on angiography, normal cerebrospinal fluid findings, and the exclusion of other causes [1]. Multifocal segmental vasoconstriction of RCVS often accompanies with vasodilation and can be seen with a “string of beads” appearance on angiography, which has traditionally been considered as characteristic for the primary angiitis of the central nervous system (PACNS) [1–3]. Thus, the initial diagnostic criteria seem to emphasize the differentiation from PACNS relying on cerebrospinal fluid analysis and the reversibility of angiographic abnormalities.
However, growing clinical experience has revealed that RCVS may encompass a broader spectrum of clinical and radiological presentations than initially recognized. While the International Classification of Headache Disorders, 3rd edition provides diagnostic criteria specifically for the headaches attributed to RCVS, it does not offer a diagnostic framework for RCVS itself [4]. Given the dynamic nature of vascular changes in RCVS, understanding the full spectrum of its radiological manifestations is essential for accurate diagnosis and differentiation from other conditions.
This paper focuses on the radiological aspects of RCVS, aiming to comprehensively review the diagnostic pitfalls, provide a full spectrum of angiographic findings observed in real-world cases, and make suggestions for the differential diagnosis.
Typical reversible cerebral vasoconstriction syndrome
RCVS typically presents as repetitive sudden and severe thunderclap headaches and reversible multifocal constriction of the cerebral arteries [1, 2, 4, 5]. Angiographic imaging findings characteristic of RCVS is a “string of beads” or “sausage-on-a-string” pattern, which resolved within 3–6 months [4]. These initial cases illustrate the classic presentation of RCVS, emphasizing the critical role of identifying this imaging signature alongside clinical symptoms for an accurate diagnosis.
Case 1
A 37-year-old female at 38 weeks of gestation experienced labor pain and took a shower before heading to the hospital. As soon as the water hit her body, she was struck by a sudden onset, excruciating headache. After childbirth, the patient continued to experience recurrent thunderclap headaches. Her thunderclap headache was triggered by activities such as showering, bending, and urination.
Angiographic imaging revealed segmental stenosis and post-stenotic dilatation in multiple cerebral arteries, presenting a characteristic pattern of alternating constriction and dilation, similar to a “string of beads” or “sausage-on-a-string” pattern (Fig. 1). Based on the clinical presentation and imaging findings, the diagnosis of RCVS was made.
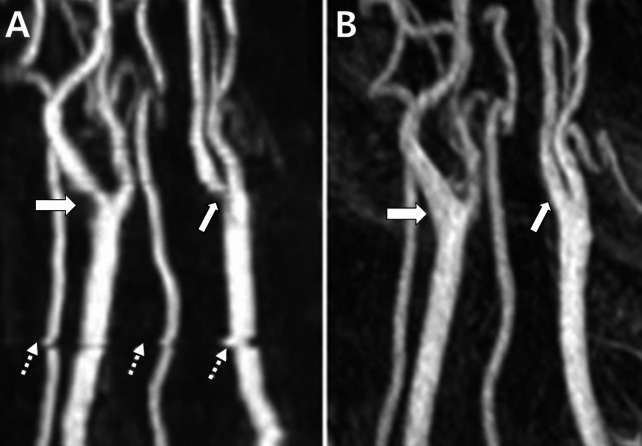
Fig. 1.
Typical steno-dilatation in reversible cerebral vasoconstriction syndrome: case 1. Time-of-flight magnetic resonance angiography (TOF MRA) image taken 17 days after onset in a pregnant woman with RCVS showed segmental stenosis (arrowheads: highlighting only selected regions for clarity) in multiple cerebral arteries and post-stenotic dilatation in the basilar artery (arrow) and other cerebral arteries, presenting the characteristic “string of beads” or “sausage-on-a-string” pattern of RCVS
Case 2
A 56-year-old female came to the clinic after being startled by a fire alarm in her apartment 8 days prior. The following morning, the patient experienced a sudden and severe thunderclap headache while brushing her teeth. The headache recurred multiple times thereafter and was notably triggered by actions such as coughing, bending over, tying her hair, and speaking in tense situations.
The imaging revealed multiple segmental stenoses and dilatations, predominantly in the distal middle and posterior cerebral arteries (Fig. 2). Therefore, the patient was diagnosed with RCVS.
Fig. 2.
Typical steno-dilations in reversible cerebral vasoconstriction syndrome: case 2. Multiple segmental stenoses (arrow heads) and dilations (arrows) in the distal middle cerebral arteries (MCAs) and posterior cerebral arteries (PCAs) in a 56-year-old patient with RCVS experienced a thunderclap headache triggered by brushing teeth, coughing, bending, tying hair, and speaking in tense situations. Only representative regions are marked for clarity
Diagnostic challenges and variability of magnetic resonance angiography
Although the typical cases presented above are straightforward, several scenarios complicate the diagnosis of RCVS. Challenges include normal angiography in the early stages, artifacts and sensitivity issues of angiographic imaging modalities, and variability in the distribution and degree of vasoconstriction. These examples underscore the importance of comprehensive clinical evaluation. Even when imaging is normal or atypical, RCVS should remain a differential consideration, especially when patients present with recurrent thunderclap headaches. In this section, we will further elucidate these diagnostic nuances with case studies.
Normal angiography
Angiographic imaging, including MR, CT, and catheter angiography, can reveal normal findings within the first week of clinical onset [6, 7]. In such cases, patients with recurring thunderclap headaches but with normal angiograms should be considered for RCVS. The ICHD-3 called this condition “probable RCVS,” where a patient shows a typical clinical manifestation of RCVS but normal angiographic findings [4]. This challenge underscores the need for clinical vigilance and a comprehensive evaluation beyond imaging alone, particularly in the early stages of RCVS, when angiography may not reveal characteristic findings.
Case 3
A 31-year-old female experienced a thunderclap headache 1 month before her visit to the clinic. Two weeks before admission, after returning from vigorous exercise, the patient awoke the next morning with another thunderclap headache. The headache was exacerbated by actions such as standing up from a seated position, getting up from lying down, sneezing, and blowing one’s nose. She reported feeling a pulsating sensation in her head that coincided with her heartbeat, accompanied by pain of 8 out of 10 on the numeric rating scale, lasting for approximately 5 min. With the impression of RCVS, brain magnetic resonance imaging (MRI) and MRA were performed, but did not reveal significant findings (Fig. 3). A tentative diagnosis of probable RCVS was made. The patient was treated with nimodipine and did not experience significant headaches thereafter. The diagnosis of probable RCVS was finally made after 3 months of observation.
Fig. 3.
Reversible cerebral vasoconstriction syndrome with normal brain magnetic resonance angiography findings. Brain MRA of a 31-year-old patient with recurrent thunderclap headaches triggered by typical precipitants, which did not show significant abnormalities
Magnetic resonance angiography artifacts and sensitivity issues
MRA and CTA are both effective for evaluating large vessels in RCVS [8]. CTA uses iodinated contrast media to directly visualize vascular structures, providing accurate anatomical representation of vessel morphology [9–11]. However, optimal visualization of distal vessels on CTA depends on precise contrast bolus timing, which can be inconsistent in clinical settings. These factors may lead to variability in the quality of imaging, particularly in the visualization of distal vasculature. Additionally, CTA is often limited by its inability to comprehensively evaluate the vessel wall or brain parenchyma, as well as the risk of nephrotoxicity and radiation exposure associated with the use of iodinated contrast agents.
In contrast, time-of-flight (TOF)-MRA visualizes intracranial arteries through flow-related enhancement rather than contrast media, eliminating the risks associated with nephrotoxicity and radiation exposure [12]. Although it is susceptible to flow-related artifacts and potential signal loss in regions with complex or slow flow patterns, TOF-MRA offers standardized imaging parameters that enhance its reproducibility [8, 13, 14]. TOF-MRA also has the advantage of detecting hyperintensity in the vessel wall, aiding in the diagnosis of conditions such as arterial dissection, and can be further enhanced by combining it with complementary sequences such as Susceptibility-Weighted Imaging, Vessel Wall Imaging or Contrast-Enhanced Fluid-Attenuated Inversion Recovery. These attributes make MRA widely used as a first-line diagnostic modality in clinical practice.
While both modalities are influenced by technical factors during image acquisition, the comprehensive evaluation capabilities and safety profile of TOF-MRA make it the preferred first-line diagnostic modality for RCVS. As noted, there are several factors influencing TOF-MRA acquisition. In this section, we will review potential issues in interpreting TOF-MRA that can lead to misdiagnosis of RCVS.
Stair-step artifact
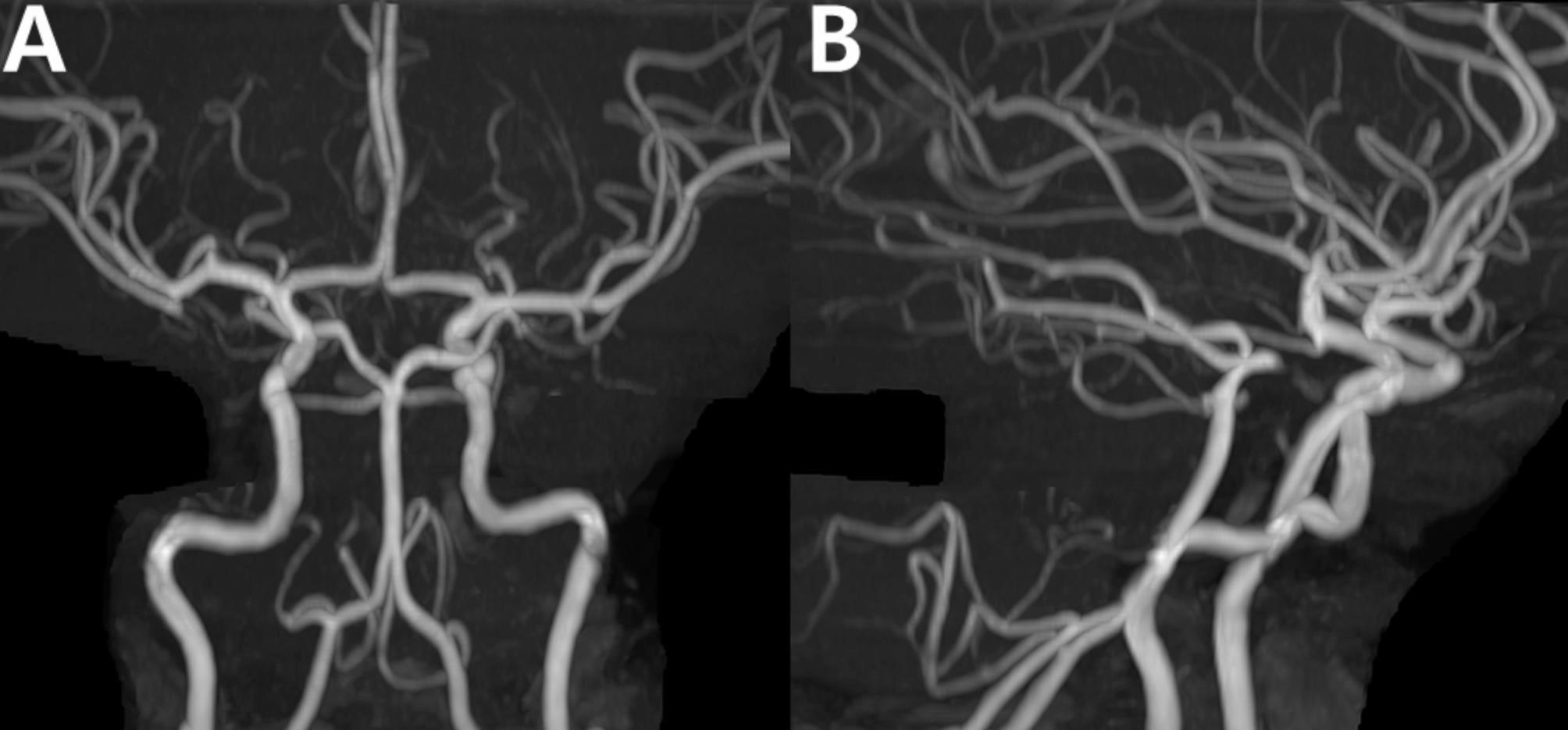
This artifact occurs on 2D TOF MRA where the slices are relatively thick, resulting in a pixelated appearance of obliquely oriented vessels. This can mislead the interpretation of the vessel structure, making it appear as steplike patterns in the image [9, 15]. The vessels appear segmented and pixelated, which can interfere with the accurate diagnosis (Fig. 4).
Fig. 4.
Stair-step artifacts. (A) Pseudo-stenosis caused by stair-step artifacts in 2D TOF MRA is observed in the right internal carotid artery (ICA) (thick arrow), left ICA (thin arrow), bilateral vertebral arteries, and left common carotid artery (dotted arrows). (B) In the contrast-enhanced MRA of the same patient, no artifacts are seen in the vessels indicated in (A). Figure adapted from McKinney AM: Artifacts of the Craniocervical Arterial System on MRI. In: Atlas of Normal Imaging Variations of the Brain, Skull, and Craniocervical Vasculature. Cham: Springer International Publishing; 2017: 1261–1291, under the terms of the Creative Commons Attribution License [16]
In-plane saturation artifact
TOF MRA achieves a high intravascular signal using the inflow of fresh unsaturated blood, with the signal being most intense when the direction of blood flow is perpendicular to the imaging plane [17]. However, when vessels run within the plane, blood can become saturated, similar to stationary tissues, leading to reduced signals [18] (Fig. 5).
Fig. 5.
In-plane saturation artifact. (A) Pseudo-stenosis in the ICA (thick arrow) and in-plane saturation artifacts in the VA (thin arrow) are observed in a 2D TOF MRA. (B) The contrast-enhanced MRA shows normal findings in the previously observed areas. (C) A pseudo-stenosis that appears as an artificial defect in the right MCA (arrow) is evident on a 3D TOF MRA. (D) However, this defect is not seen in the contrast-enhanced MRA image. Figure adapted from McKinney AM: Artifacts of the Craniocervical Arterial System on MRI. In: Atlas of Normal Imaging Variations of the Brain, Skull, and Craniocervical Vasculature. Cham: Springer International Publishing; 2017: 1261–1291, under the terms of the Creative Commons Attribution License [16]
Venetian blind artifact
In 3D TOF imaging, the signal intensity decreases as a result of repeated radiofrequency pulses as the protons move through the imaged volume. This loss is most noticeable at the slab edges. When the slabs are combined, it creates a “Venetian blind” effect, with low signal intensity at section boundaries [19] (Fig. 6).
Fig. 6.
Venetian blind artifacts in 3D TOF imaging resulting from signal loss due to repeated radiofrequency pulses, particularly at slab edges (arrows). Figure adapted from Sayah A, Mamourian AC: Flow-Related Artifacts in MR Imaging and MR Angiography of the Central Nervous System. Neurographics 2012, 2(4):154–162, under the terms of the Creative Commons Attribution License [19]
Visualization limitations in distal vessels
TOF MRA is effective for imaging large- and medium-sized arteries, but faces challenges with small distal vessels [20]. Loss of signal from repeated radiofrequency pulses reduces the visibility and structural clarity of these vessels [20, 21]. The real-world challenges are exemplified in Case 4.
Case 4
A 58-year-old female stood outside for an extended period on a cold day, 6 days before experiencing a sudden thunderclap headache. Subsequently, the headache recurred several times and was notably triggered by urination and defecation.
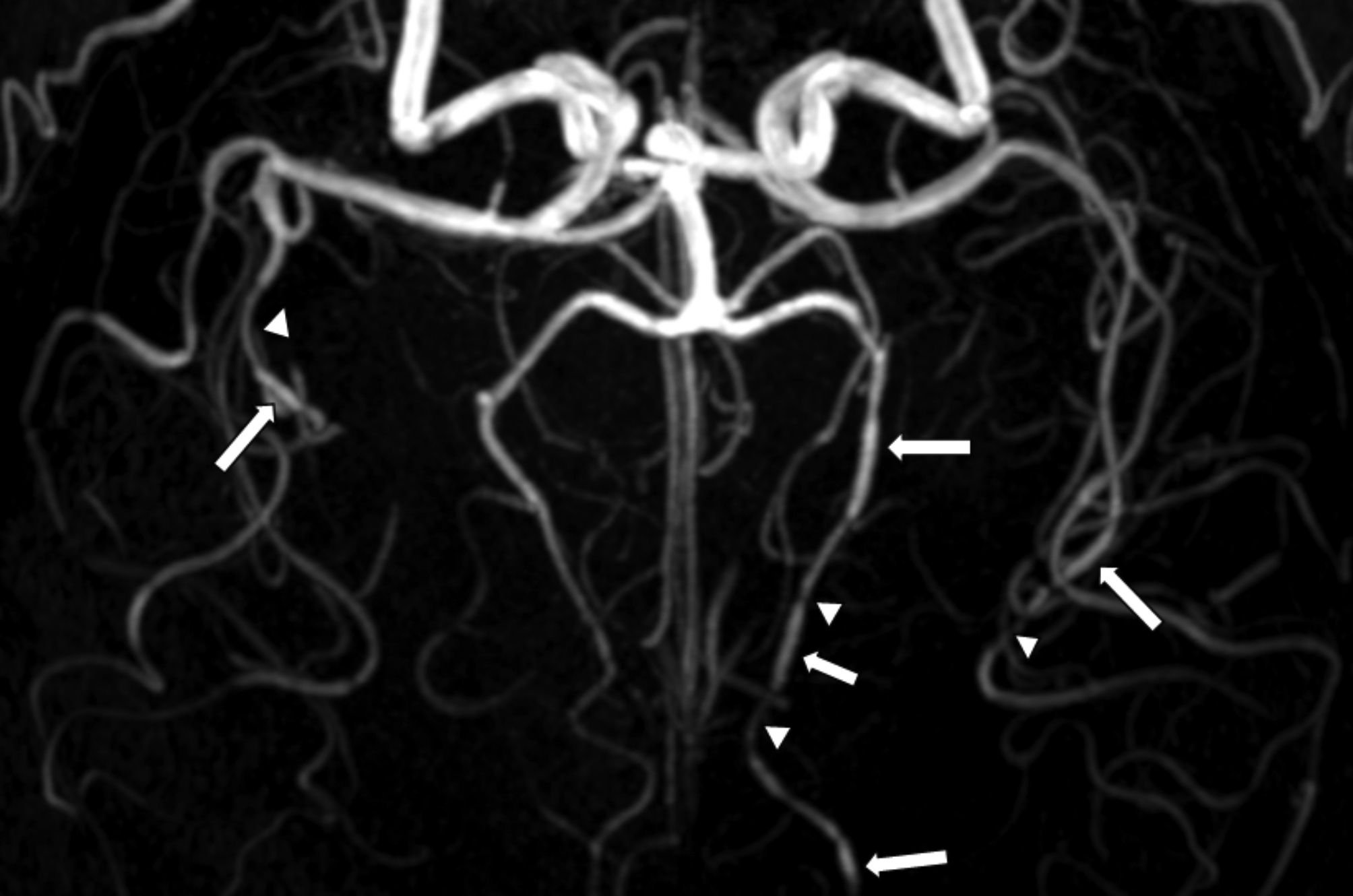
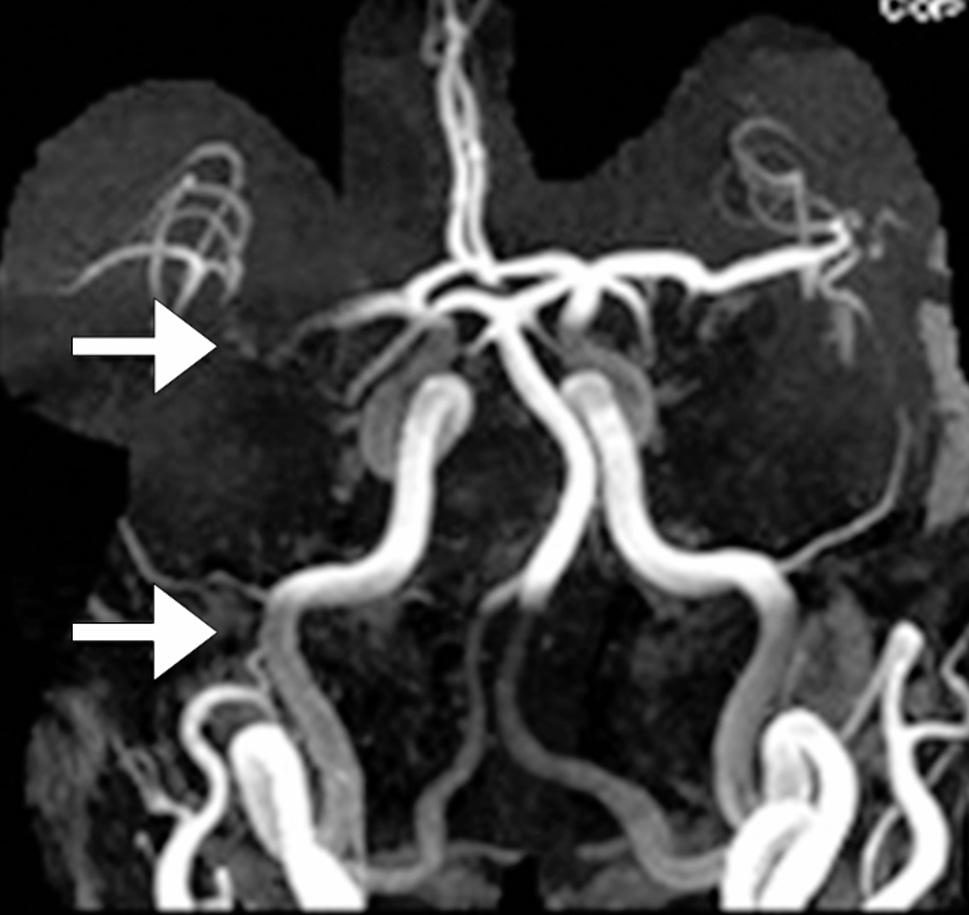
During the MRA examination, 6 days after the onset of the symptoms, it was challenging to distinguish between normal findings and artifacts (Fig. 7A). However, 3 months later, distal vessel dilation became evident, leading to the diagnosis of RCVS (Fig. 7B). This case highlighted the diagnostic limitations of MRA, particularly its reduced sensitivity and specificity for distal vessels, which can obscure early signs of RCVS. This case provides insight into the challenges in diagnosing RCVS, particularly in its early stages, and highlights the importance of follow-up imaging to detect evolving vascular changes indicative of the condition.
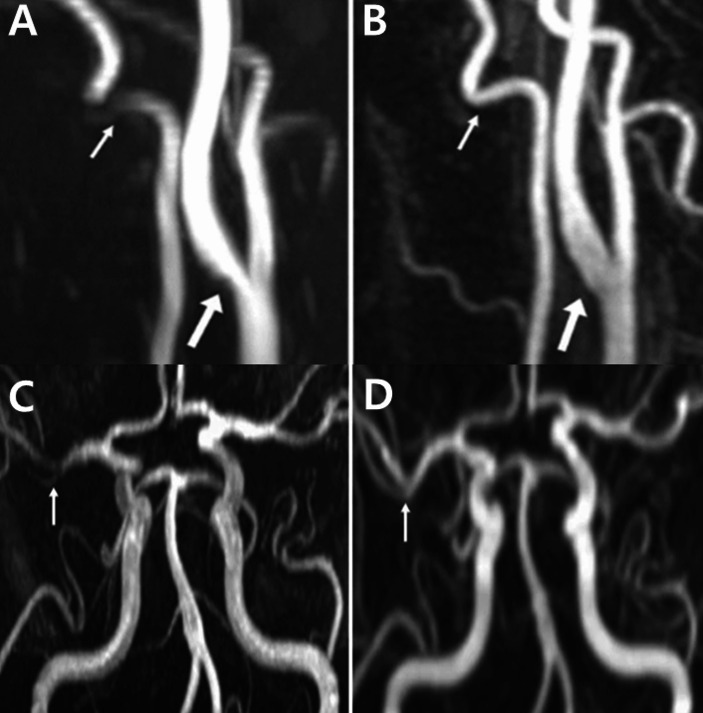
Fig. 7.
Reversible cerebral vasoconstriction syndrome with ambiguous findings: case 4. (A) An initial MRA performed 6 days after onset shows uncertainty between distal artery visualization issues and possible stenosis, particularly in the anterior cerebral arteries (ACAs) (dotted circles) and the MCA (oval). (B) Follow-up MRA 3 months later reveals distal vessel dilation in the same ACAs (dotted circles) and MCA (oval), suggesting RCVS
Diverse spectrum of degree and distribution of Vasoconstriction in Reversible Cerebral Vasoconstriction Syndrome
As discussed above, the typical radiological manifestation of RCVS is the presence of multiple segmental stenoses and dilatations in the cerebral arteries, often described as a “string of beads” or “sausage-on-a-string” appearance and is observed most prominently between 1 and 3 weeks accompanied by recurrent thunderclap headaches [1, 2, 4, 22]. However, RCVS can be captured with different degrees and distributions of vasoconstriction, due to different severities of disease activity or the timing of the imaging.
Case 5
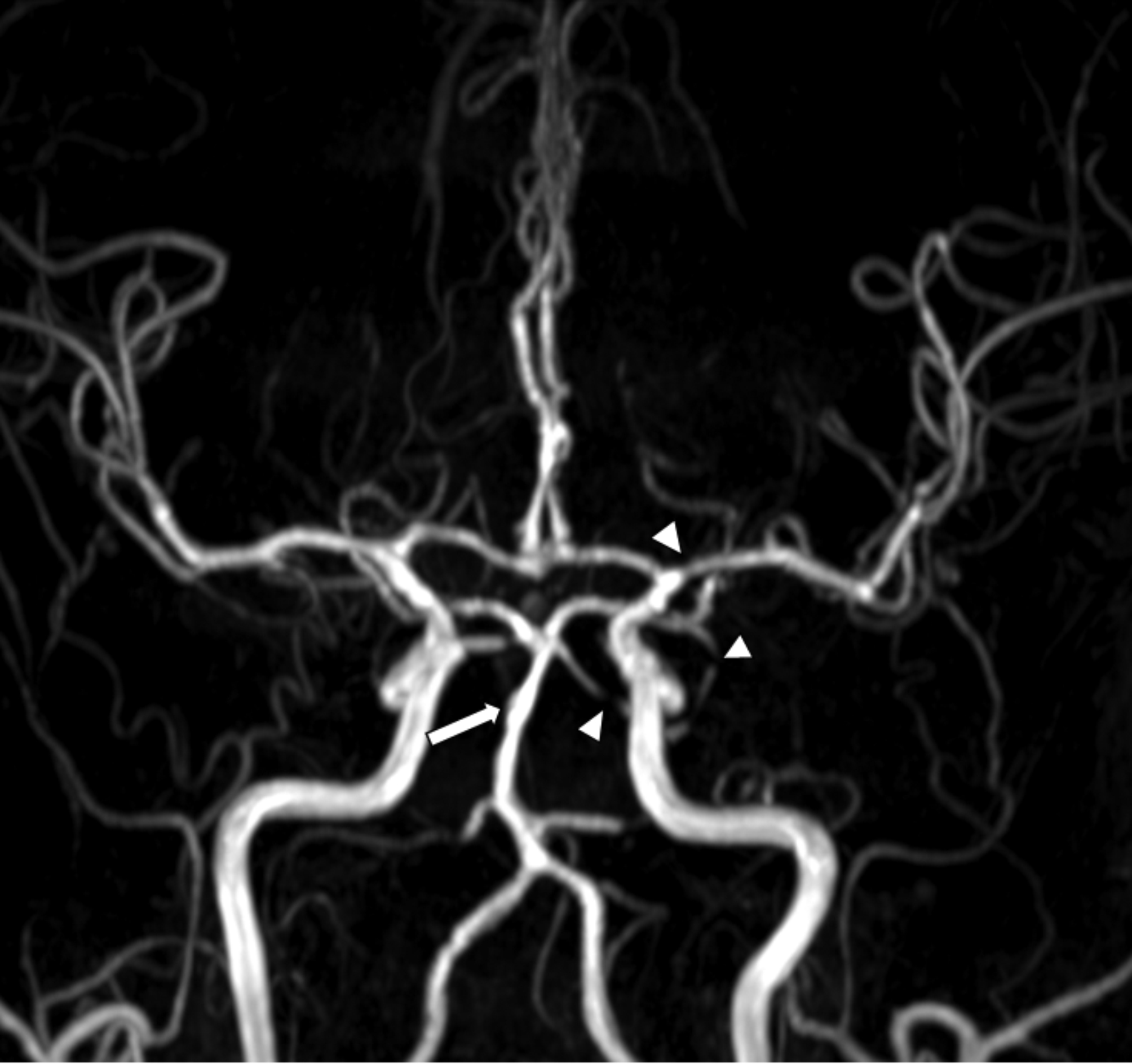
A 67-year-old female experienced a sudden thunderclap headache while bending over to pick up a fallen object 10 days prior, which resolved spontaneously. MRA revealed a single focal stenosis of the left PCA (Fig. 8A). Since the vasoconstriction involved only a single segment, the diagnosis of RCVS could not be confirmed, and the differential diagnoses included atherosclerosis and dissection. Two days later, the patient returned to the hospital with sudden diplopia, right-hand weakness and transient aphasia, each lasting about 10 min. These neurological symptoms occurred alongside recurrent thunderclap headaches. Follow-up imaging revealed normalization of focal segmental stenosis in the left PCA (Fig. 8B). The diagnosis of RCVS was supported by the rapid reversibility of stenosis, which helped exclude other diagnoses. In this case, a new focal dilation was observed in the right ACA and the left MCA (Fig. 8D), which was not apparent in the initial MRA (Fig. 8C). This case illustrates that RCVS can manifest itself as a single-segment vasoconstriction or dilatation.
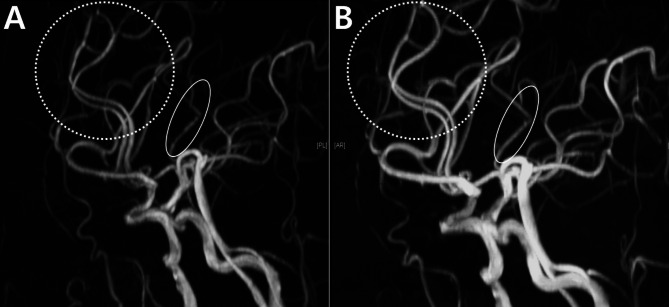
Fig. 8.
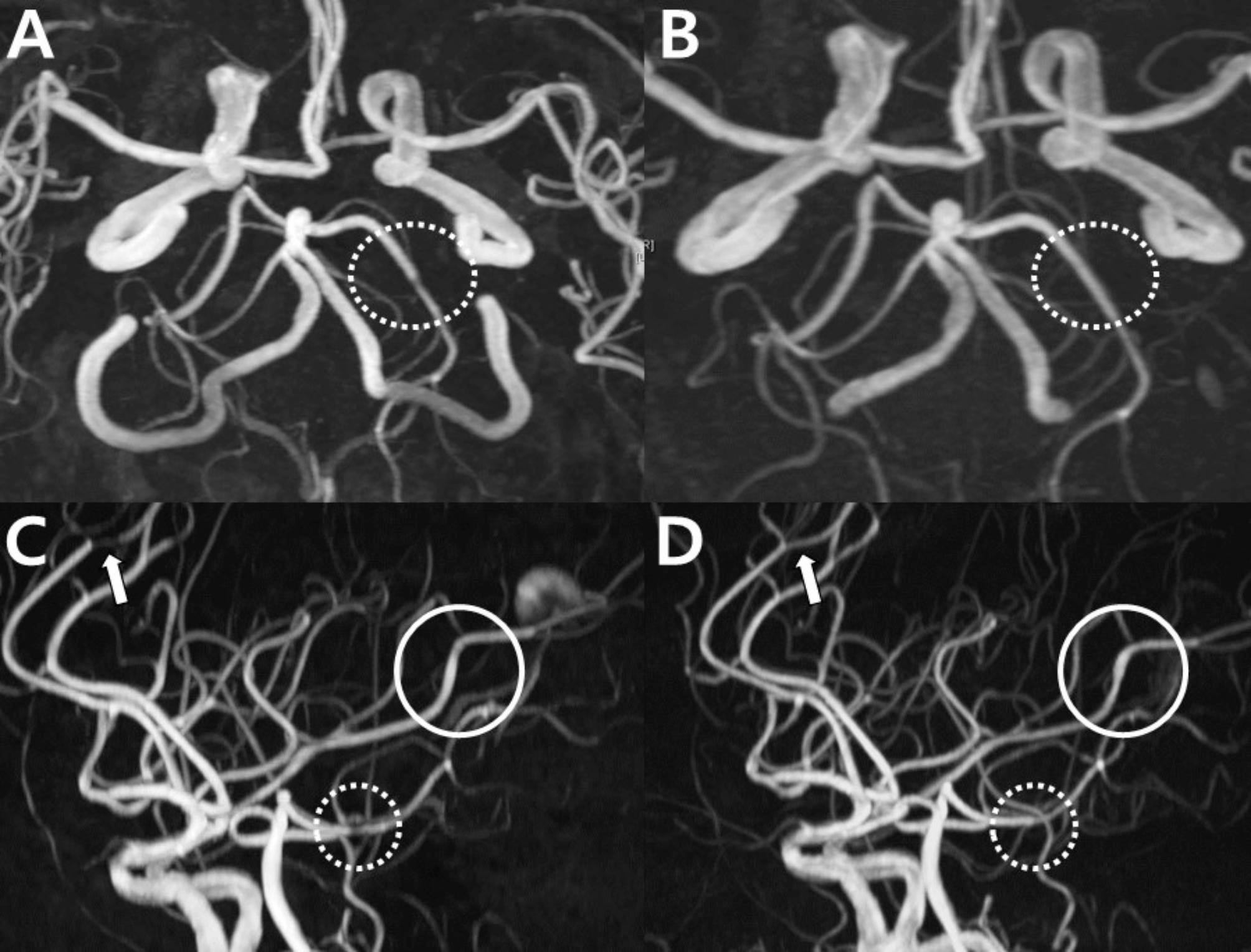
Rapid reversibility of stenosis and new focal dilation in reversible cerebral vasoconstriction syndrome: Case 5. (A) Initial MRA with focal stenosis in the left PCA (dotted circle). (B) Follow-up MRA in 2 days showing normalized stenosis (dotted circle). (C) Initial MRA suspicious for stenosis vs. artifact in the right anterior (arrow) and left PCAs (dotted circle). (D) The follow-up MRA shows normalization of the right anterior (arrow) and left PCAs (dotted circle) and a focal dilation in the left MCA (circle) newly developed
Case 6
A 30-year-old female, 7 days postpartum, experienced her first headache, which gradually began with only moderate intensity. Her MRA revealed multifocal narrowing of the bilateral M1, A1, and A2 segments (Fig. 9A). Although she did not have a typical thunderclap headache, the diagnosis of RCVS was made once her follow-up imaging showed normalization of the vasoconstrictions. Interestingly, the basilar artery, which initially appeared normal, showed an increase in diameter in the follow-up imaging (Fig. 9B), suggesting the possibility that she initially had a “diffuse narrowing” in the basilar artery (Fig. 9A). This observation raises the possibility that cases deemed “angiographically normal” could harbor such a diffuse narrowing, which warrants a more cautious evaluation. The diffuse narrowing of the vessel can initially be overlooked. Follow-up imaging can help, even in patients with normal-looking MRA.
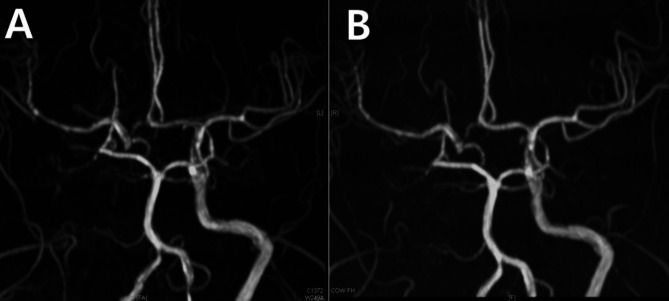
Fig. 9.
Diffuse narrowing of the basilar artery as a reversible cerebral vasoconstriction syndrome phenomenon: case 6. (A) Multifocal narrowing of the bilateral M1, A1, and A2 segments is observed in the initial time-of-flight MR angiography of a 30-year-old patient with postpartum RCVS. In this imaging, the basilar artery (arrows) appeared normal. (B) The follow-up imaging obtained 3 months later showed normalization of the overall vessels. The diameter of the basilar artery increased compared to the initial imaging (arrows), indicating that there is a diffuse narrowing in the basilar artery, which can be missed if assessed cross-sectionally
Reversible cerebral vasoconstriction syndrome-mimicking clinical conditions
Intracranial arterial atherosclerosis
Case 7
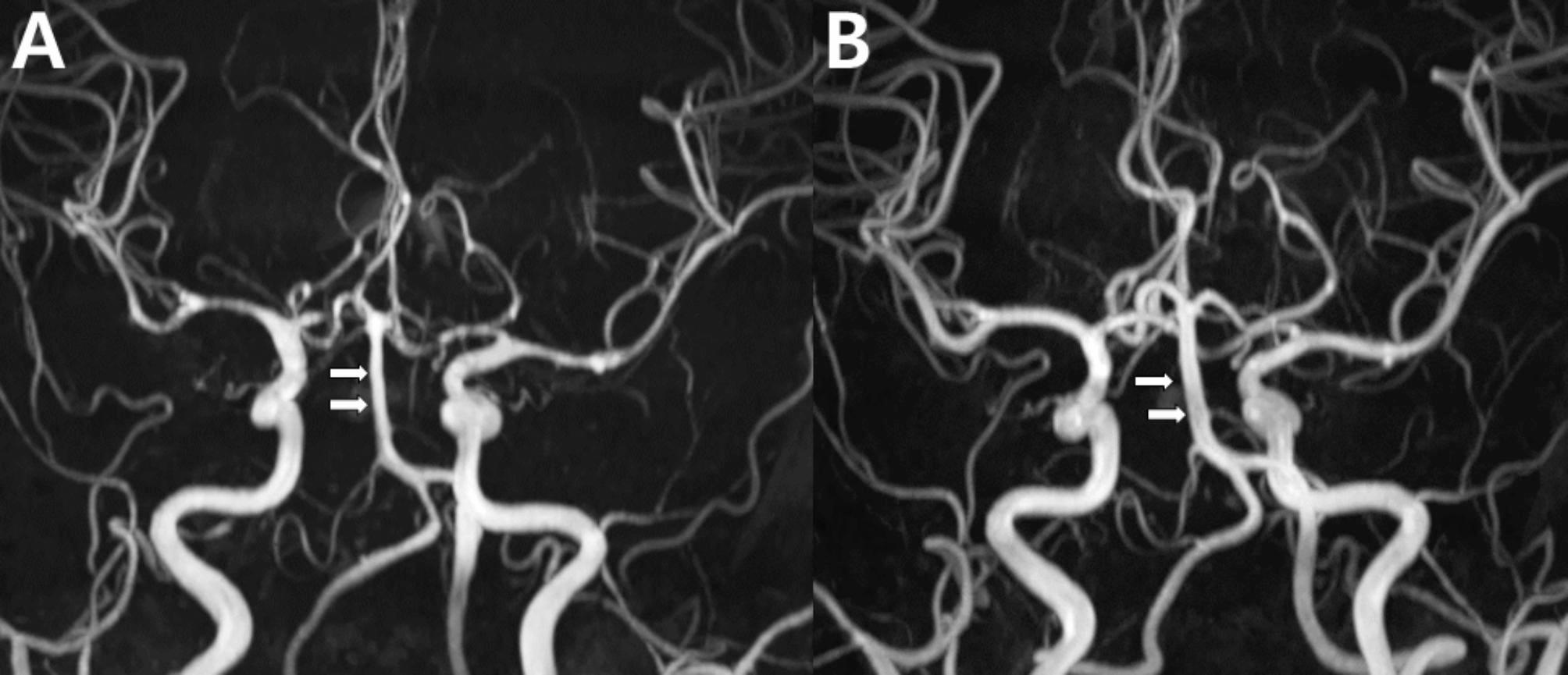
A 51-year-old female visited our headache clinic 7 days after experiencing a thunderclap headache while swimming. The headache resolved and did not recur after stopping swimming. MRA revealed multifocal stenosis and dilatation of the ACA [23], MCA, and distal ICA (Fig. 10A). Vasospasm improved after 6 months, except for a lesion in the right distal ICA (Fig. 10B). High-resolution vessel wall MRI revealed an atherosclerotic plaque in the right distal ICA (Fig. 10C and D). Therefore, the right distal ICA was determined to have atherosclerosis probably prior to the onset of RCVS, while the vasoconstrictions in the other vessels were confirmed as RCVS.
Fig. 10.
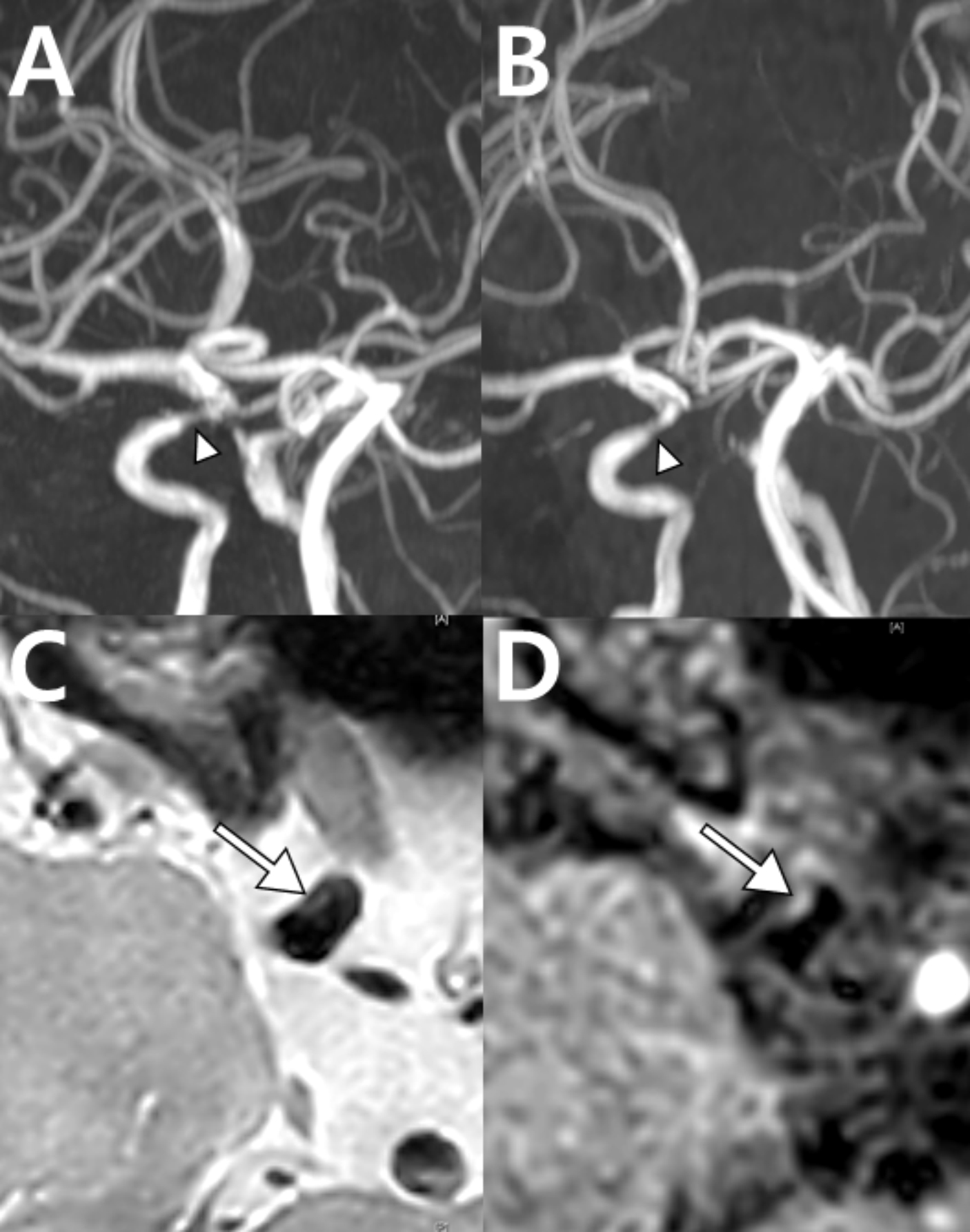
Mimickers of reversible cerebral vasoconstriction syndrome: focal intracranial atherosclerosis in a patient with reversible cerebral vasoconstriction syndrome (case 7). (A) Multifocal stenosis is observed in the ACA, MCA, and most prominently in the right distal ICA (arrowhead) in the initial time-of-flight MR angiography. (B) Six months later, overall vasospasm improved, except for the right distal ICA (arrowhead). (C, D) High-resolution vessel wall MRI (C, T2 weighted image; D, T1 post-contrast image) show an atherosclerotic plaque in the right distal ICA (arrow)
Case 8
A 74-year-old female came to the emergency room with a thunderclap headache while defecating. Her headache recurred three times, when bending or defecating. Right ICA occlusion with multifocal stenosis was observed in the distal M1, M2, M3, A3, and V4 segments (Fig. 11A). Although clinical symptoms were consistent with RCVS and some minor narrowing appeared to have normalized, most of the arterial narrowing persisted for more than 1 year, indicating that these vasoconstrictions were of atherosclerotic origin (Fig. 11B). The patient was finally diagnosed with asymptomatic atherosclerosis and probable (angiogram-negative) RCVS (Fig. 11).
Fig. 11.
Mimickers of reversible cerebral vasoconstriction syndrome: asymptomatic atherosclerosis (case 8). (A) MR angiography shows right ICA occlusion with multifocal stenosis in the distal M1, M2, M3, A3, and V4 segments in a 74-year-old patient with clinically suspected RCVS. (B) There is no significant change 1 year after onset. The diagnosis of asymptomatic intracranial atherosclerosis and probable RCVS (angiogram-negative) is made
Intracranial atherosclerotic stenosis (ICAS) is an important differential diagnosis of RCVS due to its high prevalence. Asymptomatic ICAS is common in the general population (6–13%), with an even higher prevalence (9–65%) in hospital-based studies from Asian countries [24–26]. ICAS is one of the most common causes of stroke worldwide and is associated with substantial morbidity and mortality [25]. Differentiating between ICAS and RCVS is essential because they require different treatment strategies. Although the distinct feature of RCVS is its reversibility, serial imaging is required to document reversibility; consequently, a definitive diagnosis is delayed. High-resolution vessel wall MRI (HR-vwMRI) can prompt differential diagnosis between atherosclerosis and RCVS. The HR-vwMRI characteristics of ICAS include the presence of eccentric atheromas with various signal intensities, positive remodeling of the affected segment, and eccentric plaque enhancement [27]. Additionally, these plaques may show juxtaluminal T2 hyperintensity of the fibrous cap, a lipid-rich necrotic core, and thickening or remodeling of the vessel wall [27, 28]. In contrast, HR-vwMRI of RCVS can only show mild enhancement without positive remodeling or eccentric atheroma [28–31].
Another differential point is that RCVS rarely involves the ICA [32, 33], which is represented in the RCVS2 scoring system (see below for further discussion). Two scoring systems are used for the differential diagnosis of RCVS: RCVS2 and RCVS-TCH. The RCVS2 score is particularly helpful in distinguishing RCVS in patients with intracranial vasculopathies, while the RCVS-TCH score is designed to identify RCVS in patients with thunderclap headaches. The acronym RCVS2 denotes the variables: recurrent or single thunderclap headache (+ 5 points), carotid (intracranial) artery involvement (-2 points), vasoconstrictive trigger (+ 3 points), female sex (+ 1 point), and subarachnoid hemorrhage (+ 1 point) [32, 33]. An RCVS2 score, primarily based on CTA findings, of ≥ 5 is 90% sensitive and 99% specific to identify RCVS from other intracranial vasculopathies, while a score of ≤ 2 is 85% sensitive and 100% specific to rule out the condition [33, 34]. The RCVS-TCH score, which includes both patients evaluated with CTA and MRA, which includes recurrent thunderclap headache (+ 2 points), female sex (+ 3 points), blood pressure surge (+ 4 points), and triggering factors (multiple + 3 points, single + 2 points), suggests RCVS in patients with thunderclap headache at a cut-off point of ≥ 7, with a sensitivity of 80% and specificity of 97% [35]. The RCVS-TCH score has shown better performance compared to the RCVS2 score in identifying RCVS among patients with thunderclap headaches [35].
Intracranial arterial dissection
Case 9
A 50-year-old female came to the emergency room after experiencing her first thunderclap headache while performing push-ups. MRArevealed a single dilation of the left A2 segment (Fig. 12A). Initially, RCVS was suspected; however, a high-resolution vessel wall MRIrevealed a dissecting flap, leading to a diagnosis of ACA dissection (Fig. 12). This case highlights the importance of consideringintracranial arterial dissection, especially in Asian populations, where it is relatively more common.
Fig. 12.
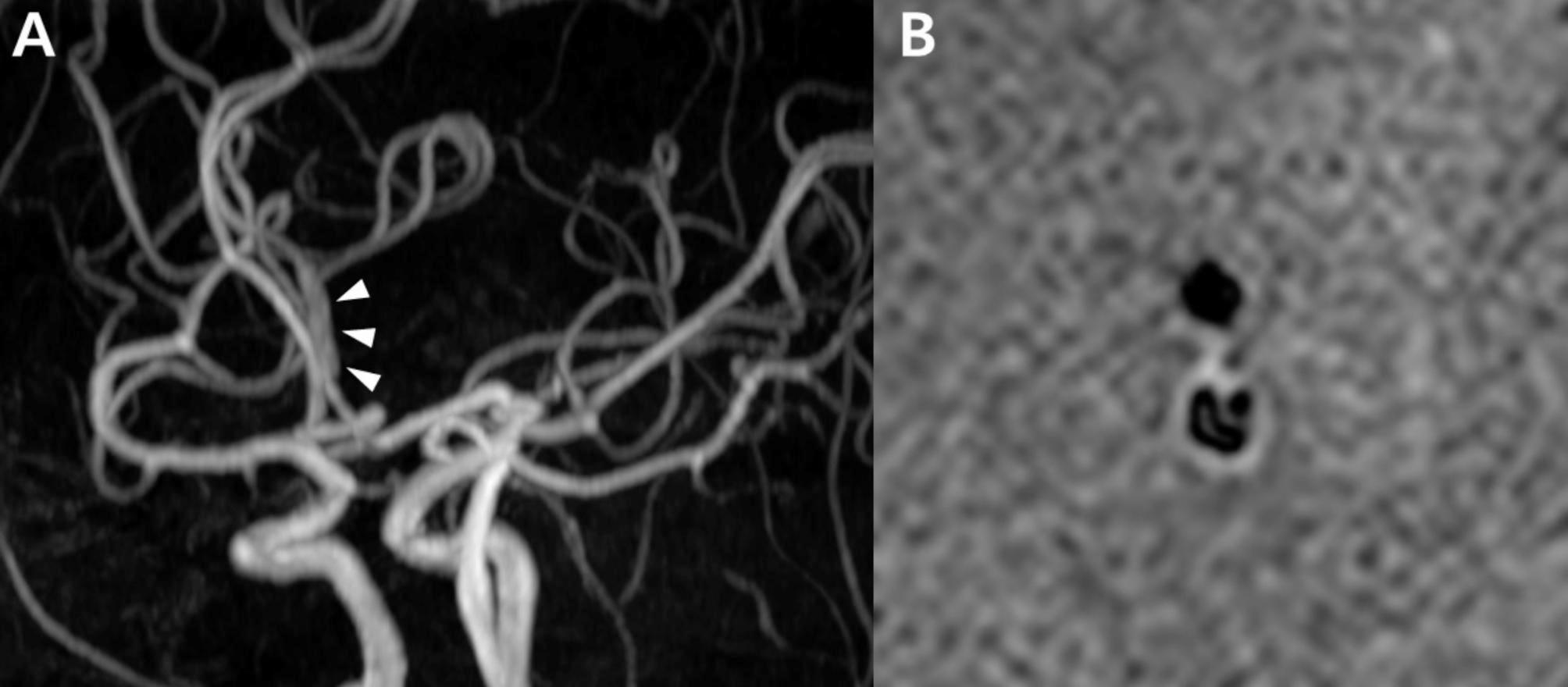
Mimickers of reversible cerebral vasoconstriction syndrome: Intracranial arterial dissection (case 9). The MRA shows a single dilation (arrowhead) in the left A2 segment in a patient with thunderclap headache (Case 9), who is ultimately diagnosed with dissection by (B) high-resolution vessel wall MRI revealing a dissecting flap
Case 10
A 47-year-old female experienced a thunderclap headache while performing push-ups 8 days before presentation. She initially visited another hospital 2 days after the onset, where cerebrospinal fluid (CSF) analysis was performed to rule out subarachnoid hemorrhage (SAH) but showed normal findings. MRA revealed no significant abnormalities (Fig. 13A), and she was diagnosed with stress-induced headache and discharged without further intervention. However, she experienced another thunderclap headache on day 8 after onset and visited our headache clinic. Imaging on day 8 revealed a single flame-shaped occlusion in the left ACA, leading to considerations of an arterial dissection (Fig. 13B). However, when comparing the MRI/MRA results of the initial hospital visit, normal findings were observed on day 2 after the onset (Fig. 13A). This dynamic change suggested RCVS rather than dissection.
Fig. 13.
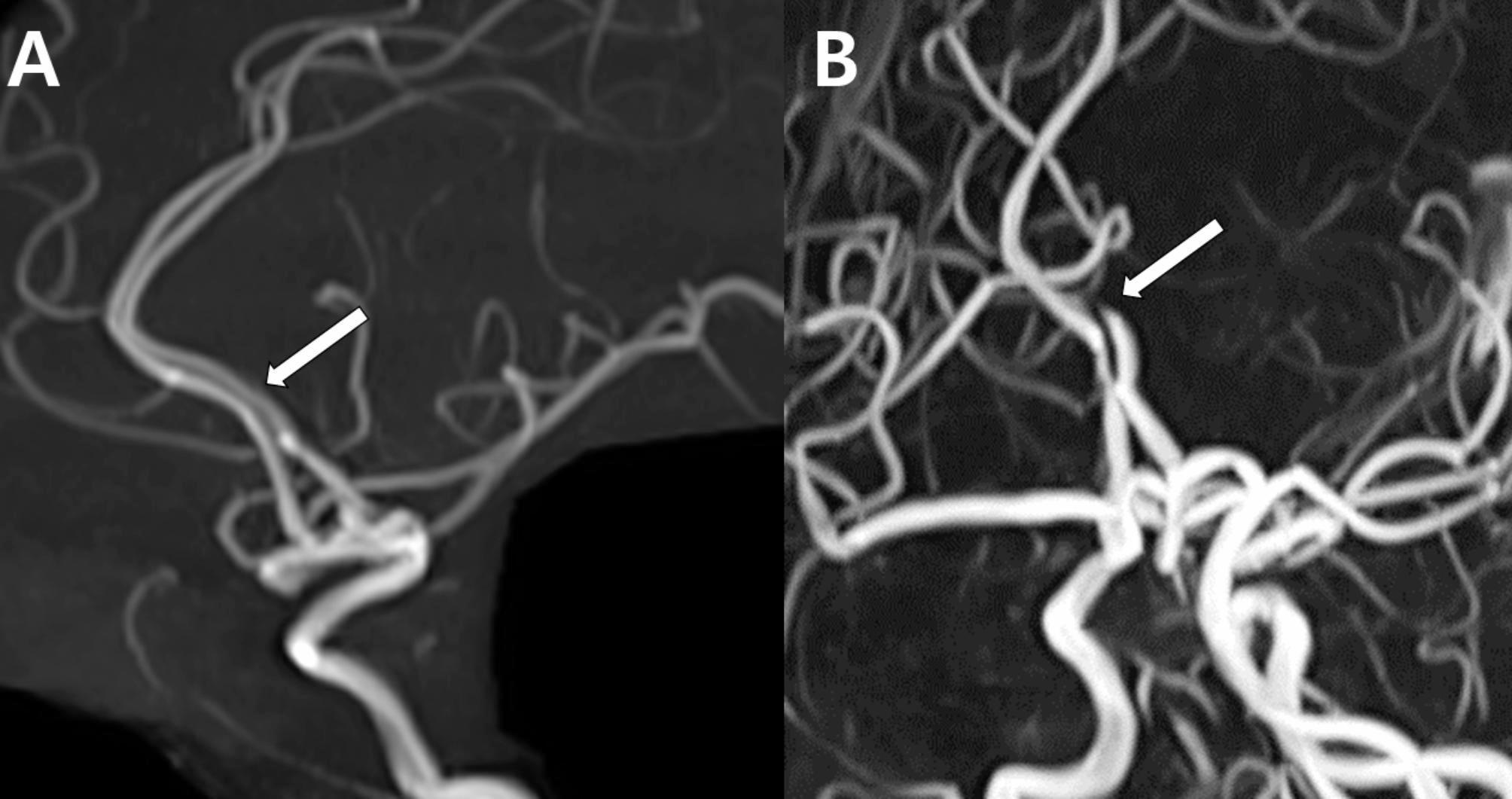
Reversible cerebral vasoconstriction syndrome resembling intracranial arterial dissection (case 10). (A) MRA taken 2 days after thunderclap headache showed normal findings in the left ACA (arrow). (B) However, a single flame-shaped occlusion in the left ACA (arrow) was observed 8 days after onset, suggesting RCVS rather than arterial dissection due to its dynamic imaging characteristics
Intracranial dissection and RCVS are important considerations in the differential diagnosis of patients with thunderclap headache. Intracranial dissection typically involves a single artery in approximately 70−80% of cases, while RCVS usually affects large-to medium-sized cerebral arteries in various vascular regions, leading to multisegmental constriction [1, 33, 36]. Intracranial dissection should be considered when there is arterial occlusion and recanalization with aneurysmal dilation, fusiform or irregular aneurysmal dilation at a non-branching site, or long irregular stenosis with double lumen, intramural hematoma, intimal flap, rapid changes, or focal stenosis [23, 37]. In East Asian studies, where most cases were reported due to recruitment from neurosurgery or neurointervention departments, intracranial artery dissection comprised 67–78% of cervicocephalic artery dissections [38, 39]. In South Korea, intracranial dissection accounts for approximately 1 in 4 cases of idiopathic MCA stenosis in young adults [40]. Furthermore, intracranial dissection was the most common cause of isolated ACA infarction in a study conducted in Japan [41]. Therefore, HR-vwMRI can aid in the differential diagnosis of intracranial arterial dissection and RCVS. Intracranial arterial dissection typically presents with eccentric luminal narrowing, intimal flaps, and intramural hematomas [42]. Among these findings [43, 44], intimal flaps (42−91.4% of cases) and intramural hematomas (23.1−61% of cases) are pathognomic for arterial dissection [44–46]. It should be noted that arterial dissection can trigger RCVS, and several reports document the coexistence of RCVS and dissection, with one study finding that 12% of RCVS cases and 7% of cervical artery dissection cases had both conditions [47–50].
Moyamoya disease
Case 11
A 30-year-old female experienced a recurrent transient ischemic attack on the second postpartum day. These episodes involved temporary right arm paralysis lasting 10–30 min. In particular, she did not experience any thunderclap headache, but a mild headache (NRS, 3−4) following the ischemic attacks.
The initial findings on the first day revealed isolated left distal ICA occlusion, prompting considerations of atherosclerosis, moyamoya disease (MMD), or dissection (Fig. 14A). Subsequent imaging performed 8 days after onset demonstrated rapid progressive right distal ICA stenosis along with new left distal multifocal stenosis and a string-of-beads pattern (Fig. 14B). These findings initially led us to consider a possibility of RCVS superimposed on chronic left distal ICA occlusion. However, the findings 1 year after onset showed no reversal of the bilateral distal ICA steno-occlusion or the vasoconstriction in the left distal arteries (Fig. 14C). These cumulative observations led to a final diagnosis of unilateral MMD, which was confirmed by high-resolution vessel wall MRI (Fig. 14D).
Fig. 14.
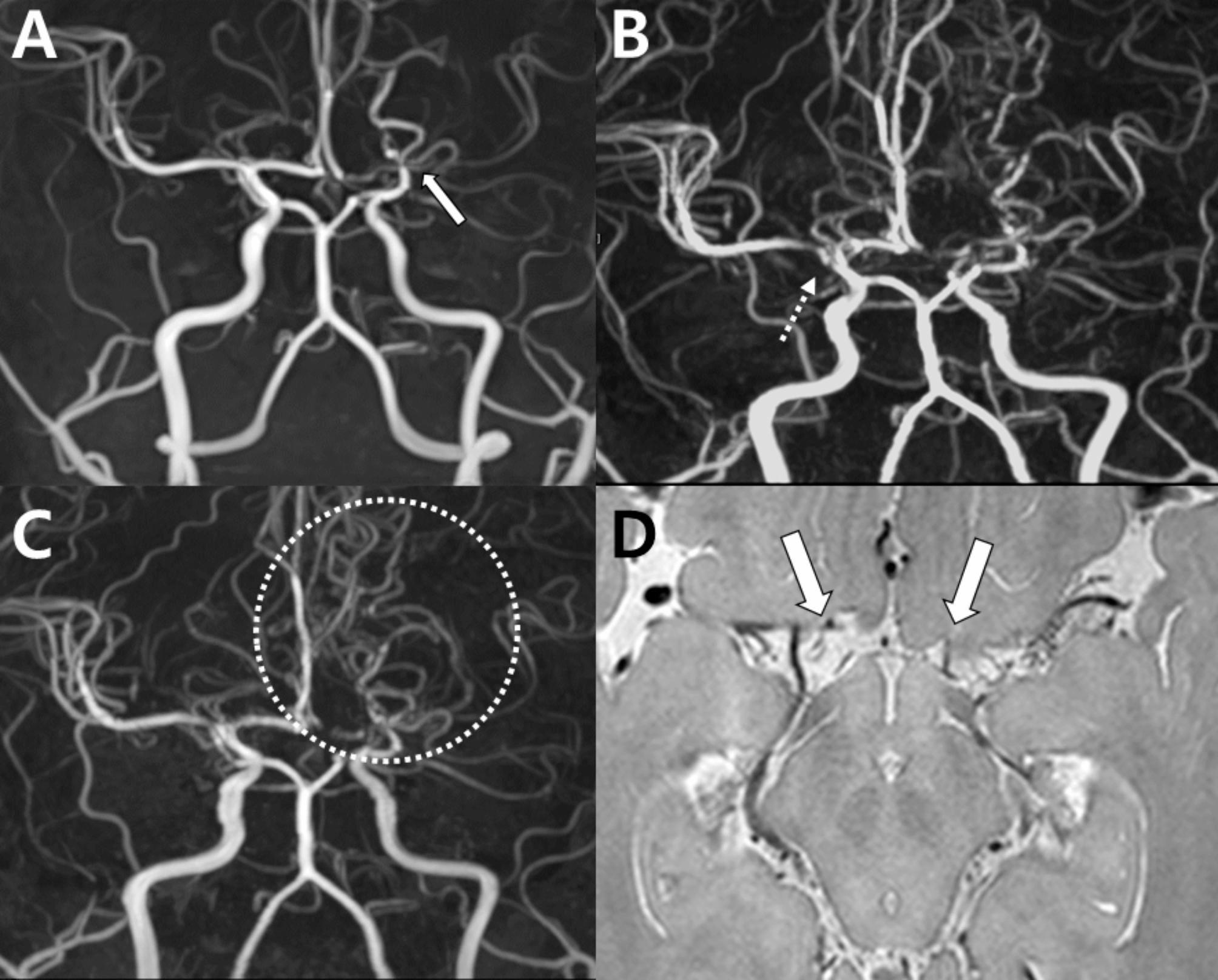
Mimickers of reversible cerebral vasoconstriction syndrome: unilateral moyamoya disease with rapid progression to bilateral disease (case 11). (A-C) Serial MRA angiographic imaging in a 30-year-old postpartum patient with recurrent transient ischemic attacks, right arm paralysis, and mild headache. (A) Initial imaging on the first day of onset shows isolated left distal ICA occlusion (arrow). (B) Subsequent imaging at 8 days after onset revealed rapid progression to right distal ICA stenosis (dotted arrow) along with left distal multifocal stenosis and a string-of-beads pattern. (C) At 1 year, no reversal of the bilateral distal ICA steno-occlusion or the vasoconstriction in the left distal arteries with formation of basal collateral vessels (dotted circle) were observed. (D) High-resolution vessel wall MRI showed significant obliteration of the ICAs (arrows), suggesting moyamoya disease. These cumulative observations led to the final diagnosis of moyamoya disease
MMD is prevalent in Northeast Asian populations, particularly in Japan, Korea, and China [51]. A known genetic susceptibility factor is a polymorphism in the ring finger protein 213 [52]. Previously considered primarily a pediatric disease, with 48% of cases occurring in individuals under the age of 10 years, MMD now shows an increasing prevalence in adults [53]. Recent studies have indicated that the peak age of onset is 45−49 years, making it essential to include MMD in the differential diagnosis of adults [53]. Diagnostic challenges arise in adult-onset cases because characteristic basal collaterals decrease with age, making diagnosis more difficult.
This case highlights the complexity of distinguishing between RCVS and MMD. A typical MMD involves the terminal segments of the ICAs, leading to compensatory basal collaterals that resemble a “hazy puff of smoke” and progressively affect the proximal ACA and MCAs [54–56]. On high-resolution MRI wall imaging, MMD is characterized by negative remodeling (i.e., reduced outer diameter of the affected vessels) and concentric gadolinium enhancement of the active lesion [57, 58].
In Case 11, the differential diagnosis was challenging due to unilateral involvement at baseline imaging and rapid progression in the second imaging. Although bilateral presentation is more common, unilateral moyamoya vasculopathy is observed in up to 18% of patients with MMD, and recent studies have shown contralateral progression in up to 78.6% of unilateral cases [59–62]. Unilateral MMD is more prevalent in adults, and collateral vessel development is less pronounced than that in younger patients [62–65].
In a study from the Stanford University Medical Center, seven out of 18 patients (38.9%) showed angiographic progression to bilateral disease within an average of 12.7 months, with most cases requiring surgical intervention and primarily affecting adults [61]. Koruda et al. reported 15 patients with worsening unilateral MMD, and the earliest interval for detecting worsening was 1 month [66]. A surprisingly rapid progression (8 days) in Case 11, along with the postpartum setting, raised a strong suspicion of RCVS or delayed vasospasm associated with PRES rather than MMD. However, prolonged observation and high-resolution MRI suggested a diagnosis of MMD.
Vasospasm as a complication of subarachnoid hemorrhage
Case 12
A 23-year-old female presented with episodes of excruciating pain, peaking at a Numeric Rating Scale of 10/10 within 1 min while walking, occurring 20 days prior to her visit. She experienced two similar episodes of nausea, vomiting, and pain radiating from the head, neck, and back. Despite receiving analgesic treatment, the patient continued to have a persistent mild headache that progressively worsened and was unresponsive to pain medications, prompting her to seek medical attention.
An initial CT scan was performed to rule out SAH, but the result was negative. Given the unusual localization of severe stenosis in both distal ICAs and M1 segments of the ACAs on MRA (Fig. 15A) and the presence of a small amount of hemorrhage along the Sylvian fissure (Fig. 15B), SAH with associated vasospasm was considered a potential differential diagnosis over RCVS.
Fig. 15.
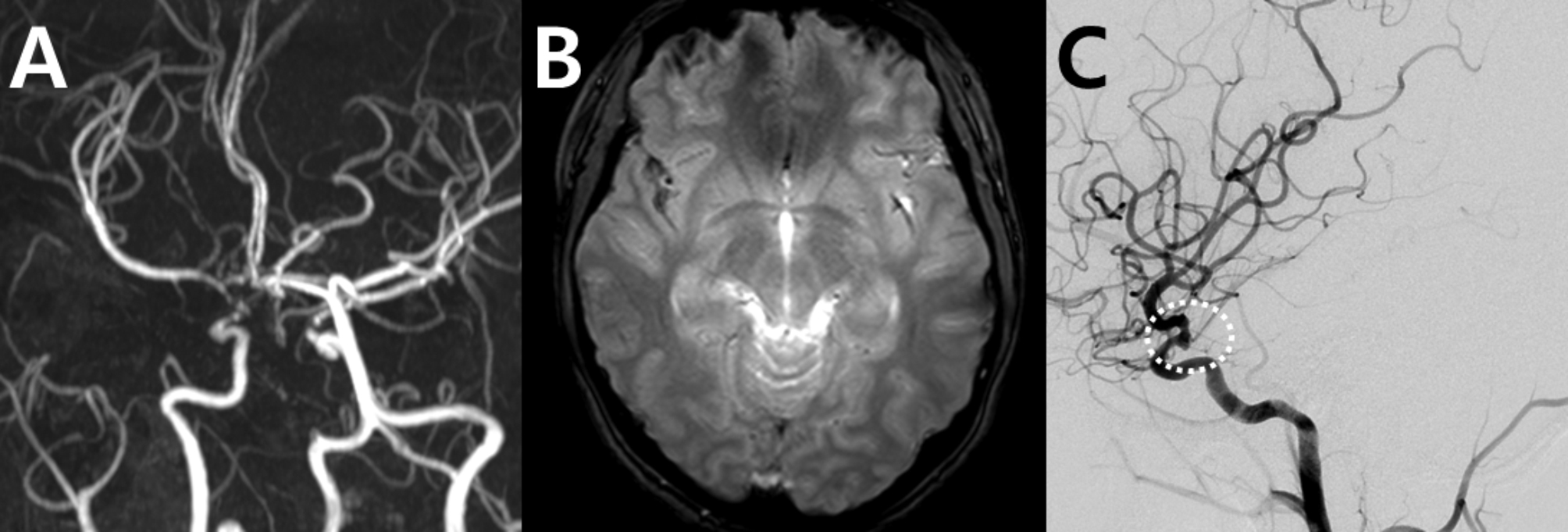
Mimickers of reversible cerebral vasoconstriction syndrome: aneurysmal subarachnoid hemorrhage and vasospasm (case 12). A 23-year-old female who had severe headache episodes was initially misdiagnosed with RCVS. (A) Magnetic resonance angiography (MRA) shows severe stenosis in the right distal ICA and M1 segment of the ACAs. (B) A small amount of subarachnoid hemorrhage is observed along the right Sylvian fissure in the gradient-echo image. (C) Catheter angiography reveals a ruptured aneurysm over the right distal ICA aneurysm (dotted circles), requiring emergency coil embolization
To further clarify the diagnosis, CSF analysis was performed, revealing xanthochromia, consistent with the presence of red blood cells, indicative of SAH. This finding led to follow-up catheter angiography, which confirmed a ruptured aneurysm in the right distal ICA (Fig. 15C), requiring emergency coil embolization. This case underscores the importance of a thorough, stepwise diagnostic approach to differentiate SAH from RCVS, especially when atypical radiological features are present.
The differential diagnosis of vasospasm after aneurysmal SAH and RCVS with SAH can be challenging because patients can present with severe headaches, brain hemorrhage, and potential neurologic deficits. However, each type has distinct characteristics. In aneurysmal SAH, hemorrhage typically occurs in the basal cisterns or Sylvian fissure, while it is commonly found in cerebral convexity in RCVS [67, 68]. Vasoconstriction in SAH is located mainly on the bleeding site, often unilateral, and correlated with the amount of hemorrhage [69]. In contrast, RCVS involves diffuse vasoconstriction, usually bilateral, with severity disproportionate to the amount of hemorrhage (i.e., vasoconstriction is more severe and widespread, while the amount of SAH is often small) [70]. Regarding timing, SAH-related vasoconstriction peaks between 4 and 14 days after onset, while vasoconstriction in RCVS is already pronounced at onset in cases complicated by SAH [71]. Neurological deficits are more pronounced in SAH, often worsening with vasospasm, while they are transient in the majority of patients with RCVS [72]. Approximately 70% of patients with SAH experience headache, with a thunderclap pattern as the primary symptom in 50% of cases [73–76]. Headache in SAH can persist and commonly accompany neck rigidity, while the headache associated with RCVS typically presents as a thunderclap at onset, is often self-limiting, and is triggered by unique provocating factors such as urination, showering, and bending. Both diseases can be triggered by physical exertion, sexual activity, intense emotions, and Valsalva maneuvers [77–79].
Primary angiitis of the Central Nervous System
RCVS and primary angiitis of the central nervous system (PACNS) are often considered differential diagnoses of cerebral arteriopathies [80]. This topic will be further explored and debated separately in this special collection.
Several clinical and imaging findings can differentiate between these two conditions. Clinically, RCVS often begins with a thunderclap headache and a recurrent thunderclap headache [81]. In contrast, PACNS rarely presents with a thunderclap headache; instead, it manifests itself as a gradual-onset, dull, and progressive headache [82]. Neurological deficits in RCVS are often transient, while in PACNS, they are common and persistent, frequently accompanied by cognitive and emotional impairments associated with encephalopathy, often including hemiparesis or aphasia [83, 84].
RCVS involves multiple arteries with a severe and relatively symmetric vasoconstriction and a “sausage-on-a-string” appearance [4]. PACNS may show normal angiographic findings or less severe and symmetric vasoconstriction [85]. Infarcts in the PACNS are located in multiple territories, including the deep gray matter and brainstem, and brain MRI findings are almost always abnormal [80]. In RCVS, infarcts are less common and typically small or wedge-shaped, affecting hemispheric border zones rather than deep structures; brain MRI can be normal in uncomplicated cases [80]. Hemorrhage patterns also differ; PACNS more commonly involves parenchymal hemorrhage, whereas RCVS more commonly involves convexity SAH [81]. PRES is very rare in PACNS, but not uncommon in RCVS [86]. High-resolution vessel wall MRI shows strong, concentric enhancements of the affected arterial walls in the PACNS, while RCVS shows a normal or mild enhancement, concentrically or eccentrically [87, 88].
In summary, RCVS is characterized by a thunderclap headache, transient neurological deficits, and a monophasic course, while PACNS presents with persistent symptoms, infarctions, and more severe brain parenchymal lesions. Imaging plays a vital role in diagnosis, and high-resolution vessel wall MRI and clinical history can be helpful for differentiation.
Imaging the blood brain barrier breakdown for the differential diagnosis of reversible cerebral vasoconstriction syndrome
As discussed, imaging findings of vasoconstriction alone may be unsuccessful in diagnosing RCVS because vasoconstriction in RCVS can have a broader spectrum than its typical multifocal vasoconstriction-vasodilation pattern, and differential diagnosis with a variety of vasculopathies can be challenging. HR-vwMRI can help in the differential diagnosis as an ancillary test for selected cases of proximal arterial involvement to differentiate RCVS from conditions such as dissection, ICAS, MMD, and PACNS. Its use is limited in evaluating distal arteries due to their thin walls, which are smaller than the voxel resolution of HR-vwMRI, and the resulting low signal-to-noise ratio [89]. Our group showed that the pathophysiology of RCVS includes BBB breakdown, which can be visualized using contrast-enhanced fluid-attenuated inversion recovery (FLAIR) MRI by detecting BBB disruption through hyperintense CSF when gadolinium leaks into CSF and parenchyma, shortening T1 and reducing signal suppression [90–93]. CE-FLAIR imaging is also considered an ancillary test, but its routine use in clinical practice is strongly recommended as it provides valuable information about small arteriolar and capillary involvement which cannot be visualized in current angiographic imaging. It further assists in differentiating RCVS from conditions such as ICAS, MMD, and dissection. Imaging findings in RCVS typically peak at 1–2 weeks but can still be detected up to 1 month after symptom onset, offering an extended diagnostic window [94].
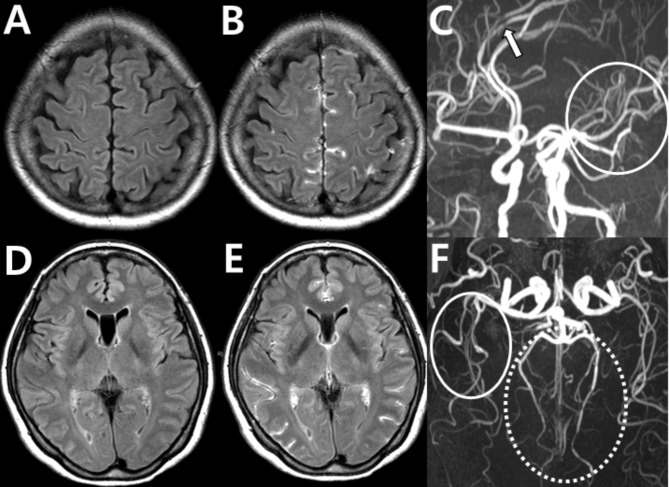
In our study, BBB breakdown was documented in 69% of patients with definite RCVS and 25% of those with probable RCVS but not in those with other secondary causes of thunderclap headache, underscoring its diagnostic value [93]. Subsequently, this finding was reproduced in several studies [94–96]. Chen et al. (2021) elaborated on this idea and successfully demonstrated increased BBB permeability using dynamic contrast-enhanced MRI in patients with RCVS [95, 96]. BBB breakdown in RCVS appears earlier than vasoconstriction, with a peak prevalence in the first and second weeks, while vasoconstriction peaks in the third week [94]. This explains the different timings of neurological complications associated with RCVS: hemorrhagic lesions and PRES develop earlier (1–2 weeks after onset), while ischemic stroke appears later (2–3 weeks after onset) [94, 97, 98]. Therefore, BBB breakdown can serve as a useful marker for the early diagnosis and prediction of neurological complications in RCVS [93]. However, caution is necessary when interpreting BBB breakdown, as BBB disruption can also occur in conditions other than RCVS, such as SAH, posterior reversible encephalopathy syndrome, and cerebral infarction [22, 93, 99], and sulcal hyperintensity in CE-FLAIR can also be caused by leptomeningeal enhancement from infectious meningitis or leptomeningeal carcinomatosis. Therefore, a complete picture of the clinicoradiological presentation and time course should be considered when diagnosing RCVS (Fig. 16).
Fig. 16.
Blood-brain barrier disruption in reversible cerebral vasoconstriction syndrome. BBB breakdown is observed using contrast-enhanced FLAIR imaging in a 56-year-old female with RCVS (case 2). (A, D) FLAIR images without contrast. (B, E) The same sections with contrast-enhanced FLAIR MRI show BBB disruption through hyperintense CSF. (C, F) MRA shows typical multifocal steno-dilatation in the ACA (arrow), left MCA (solid circles), and distal PCAs (dotted circles)
Summary and Suggested Diagnostic Flow
As shown in our real-world cases, RCVS diagnosis poses significant challenges due to its varied clinical presentation and limitations in imaging modalities. The diagnosis is often complicated by the fact that angiography can appear normal in the early stages of RCVS and the interpretation of MRA can be difficult due to potential artifacts or focal or diffuse lesions. Differential diagnoses such as atherosclerosis, arterial dissection, MMD, vasospasm after SAH, and PACNS require a thorough evaluation (Table 1). RCVS should be considered because imaging findings can vary beyond the radiological peak, and even when imaging findings are normal or atypical, it should still be included in the differential diagnosis.
Table 1.
Differential diagnoses of reversible cerebral vasoconstriction syndrome
| Reversible Cerebral Vasoconstriction Syndrome | Intracranial Atherosclerosis | Intracranial Arterial Dissection | Moyamoya Disease | Subarachnoid Hemorrhage | Primary Angiitis of the Central Nervous System | |
|---|---|---|---|---|---|---|
| Clinical Symptoms | Recurrent, sudden-onset thunderclap headaches triggered by sexual activity, exertion, Valsalva maneuvers | Often asymptomatic until significant blockage | Variable symptoms; may present with headache or focal neurologic deficits | Transient ischemic attack, stroke, headache during exertion, stress, fever | Persistent thunderclap headache, neck stiffness or focal neurologic deficits | Gradual-onset, dull headache with persistent neurological deficits, cognitive changes |
| MRI findings | ||||||
| Angiographic findings | “String of beads” appearance with reversible vasoconstriction | Less severe, less symmetric; segmental dilatation rare | Typically affects a single artery |
“Puff of smoke” collateral pattern; progressive stenosis of internal carotid artery terminal segments |
Vasoconstriction near the bleeding site, often unilateral and peak between 4–14 days after onset | Normal or less severe, symmetric vasoconstriction |
| Parenchymal findings | Hemorrhage usually in convexity; blood brain barrier breakdown shown in contrast-enhanced fluid-attenuated inversion recovery MRI | May show ischemic changes in advanced stages | May include ischemia or hematoma in advanced stages | May include chronic ischemia, infarctions, encephalomalacia, or hemorrhage | Hemorrhage in basal cisterns or Sylvian fissure | Multiple infarcts in deep gray matter or brainstem |
| HR-vwMRI findings | Mild or no enhancement | Eccentric atheromas, positive remodeling, plaque enhancement | Eccentric narrowing, intimal flaps, intramural hematoma | Negative remodeling, concentric enhancement | Useful in identifying rupture site of aneurysmal subarachnoid hemorrhage | Concentric enhancement |
| Differential Point | Diffuse, bilateral vasoconstriction with reversibility which rarely involves internal carotid artery | HR-vwMRI findings | Number of vessels involved and HR-vwMRI findings | Angiographic and HR-vwMRI findings | Hemorrhage site and vasoconstriction timing | Parenchymal and HR-vwMRI findings |
**Abbreviations: MRI, magnetic resonance imaging; HR-vwMRI, high-resolution vessel wall magnetic resonance imaging;
As shown in these cases, relying solely on MRA has intrinsic limitations. A thorough clinical evaluation, and follow-up imaging, with additional consideration of high-resolution vessel imaging can help with the diagnosis. Further to these, BBB breakdown is another important diagnostic and pathophysiologic marker of RCVS, which can be documented by using a delayed contrast-enhanced FLAIR protocol after gadolinium injection [91, 92, 95, 99, 100]. A real-world study found that 69% of patients with definite RCVS, one-fourth of patients with probable RCVS, and one-eighth of patients with primary thunderclap headache had a BBB breakdown [93]. Using BBB breakdown as a complementary finding, the diagnostic rate of RCVS increased from 33 to 41% in patients with normal angiography [93]. When BBB breakdown and clinical manifestations were considered together, RCVS was diagnosed in 61% of patients with thunderclap headache compared to 40% based only on angiographic findings [93]. This technique can help in the diagnosis of RCVS in angiogram-negative patients.
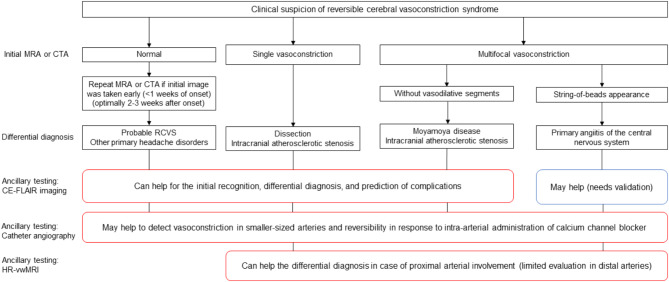
Figure 17 is a diagnostic flow chart with a focus on radiologic evaluation to guide differential diagnoses and imaging options for RCVS. MRA and CTA are both viable options for initial imaging; however, MRA is recommended as the initial imaging modality due to its standardized imaging parameters and superior safety profile, as it is non-invasive and avoids the risks of nephrotoxicity and radiation exposure. Repeat scans are advised 2–3 weeks after onset if the initial findings are normal and the scan was performed early (< 1 week after onset). Single lesions may indicate dissection or ICAS, while multifocal vasoconstriction without vasodilative segments suggests MMD or ICAS. A “string-of-beads” pattern raises suspicion for PACNS alongside RCVS. CE-FLAIR helps differentiate RCVS from dissection, ICAS, or MMD, although its utility in PACNS requires further validation. Catheter angiography, although invasive, may be useful across all stages, detecting vasoconstriction in smaller vessels and assessing reversibility with intra-arterial calcium channel blockers. HRMR is particularly effective for evaluating proximal arterial involvement in dissection, ICAS, MMD, and PACNS, though its application to distal arteries remains limited.
Fig. 17.
Diagnostic flow chart for radiologic evaluation and differential diagnosis in suspected reversible cerebral vasoconstriction syndrome. **Abbreviations: MRA, Magnetic Resonance Angiography; CTA, Computed Tomography Angiography; RCVS, Reversible Cerebral Vasoconstriction Syndrome; CE-FLAIR, Contrast-Enhanced Fluid-Attenuated Inversion Recovery; HR-vwMRI, High-Resolution Vessel Wall Magnetic Resonance Imaging;
This flow chart summarizes imaging approaches and differential diagnoses for suspected RCVS. It provides guidance on the use of specific imaging modalities, including contrast-enhanced fluid-attenuated inversion recovery, catheter angiography, and high-resolution vessel wall magnetic resonance imaging. Red-outlined boxes in the flow chart indicate ancillary tests, which are not mandatory but may provide additional diagnostic information in specific clinical scenarios.
In conclusion, the diagnosis of RCVS requires a comprehensive approach that integrates clinical and imaging findings. Awareness of diagnostic challenges and differential diagnoses, combined with advances such as high-resolution vessel wall MRI and BBB breakdown assessment, can improve diagnostic accuracy and improve patient outcomes.
Acknowledgements
None.
Abbreviations
- RCVS
Reversible cerebral vasoconstriction syndrome
- MRA
Magnetic resonance angiography
- TOF
Time-of-flight
- ICA
Internal carotid artery
- MCA
Middle cerebral artery
- ACA
Anterior cerebral artery
- PCA
Posterior cerebral artery
- ICHD-3
International Classification of Headache Disorders, 3rd edition
- BBB
Blood-brain barrier
- HR-vwMRI
High-resolution vessel wall MRI
- SAH
Subarachnoid hemorrhage
- CE-FLAIR
Contrast-enhanced fluid-attenuated inversion recovery
- PRES
Posterior reversible encephalopathy syndrome
- ICAS
Intracranial atherosclerotic stenosis
- MMD
Moyamoya disease
- CSF
Cerebrospinal fluid
- PACNS
Primary angiitis of the central nervous system
Author contributions
SAK and MJL were responsible for conceptualization, data collection, analysis, and the preparation of figures and cases, as well as literature research, writing, and manuscript review and editing. EYK and SJW contributed through manuscript review and editing. All authors have reviewed the final version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT)(RS-2024-00357394) and the Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project number: RS-2023-00229484).
Data availability
The data used in this study consisted of patient cases and were subject to privacy protection under Institutional Review Board (IRB) regulations. Due to ethical restrictions, raw data cannot be publicly available or shared on request. Data and materials supporting this study’s conclusions are included in this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB (2007) Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med 146(1):34–44 [DOI] [PubMed] [Google Scholar]
- 2.Ducros A (2012) Reversible cerebral vasoconstriction syndrome. Lancet Neurol 11(10):906–917 [DOI] [PubMed] [Google Scholar]
- 3.Cho SH, Kim BK, Lee MJ (2022) Clinical characteristics of reversible cerebral vasoconstriction syndrome: a large korean multicenter study. Headache Pain Res 23(2):27–32.
- 4.Headache Classification Committee of the International Headache Society (IHS) (2018) The International classification of Headache disorders, 3rd edition. Cephalalgia 38(1):1–211 [DOI] [PubMed]
- 5.Song TJ, Lee KH, Li H, Kim JY, Chang K, Kim SH, Han KH, Kim BY, Kronbichler A, Ducros A et al (2021) Reversible cerebral vasoconstriction syndrome: a comprehensive systematic review. Eur Rev Med Pharmacol Sci 25(9):3519–3529 [DOI] [PubMed] [Google Scholar]
- 6.Chen SP, Wang SJ (2014) Hyperintense vessels: an early MRI marker of reversible cerebral vasoconstriction syndrome? Cephalalgia 34(13):1038–1039 [DOI] [PubMed]
- 7.Ducros A (2013) L37. Reversible cerebral vasoconstriction syndrome: distinction from CNS vasculitis. Presse medicale (Paris, France: 1983) 42(4 Pt 2):602–604 [DOI] [PubMed]
- 8.Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D (2015) Reversible cerebral vasoconstriction syndrome, part 2: Diagnostic Work-Up, Imaging evaluation, and Differential diagnosis. AJNR Am J Neuroradiol 36(9):1580–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman RR, Koktzoglou I (2019) Noncontrast MR angiography: an update. J Magn Reson Imaging: JMRI 49(2):355–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz RB, Tice HM, Hooten SM, Hsu L, Stieg PEJR (1994) Evaluation of cerebral aneurysms with helical CT: correlation with conventional angiography and MR angiography. 192(3):717–722 [DOI] [PubMed]
- 11.Harrison MJ, Johnson BA, Gardner GM, Welling BG (1997) Preliminary results on the management of unruptured intracranial aneurysms with magnetic resonance angiography and computed tomographic angiography. Neurosurgery 40(5):947–955 discussion 955– 947 [DOI] [PubMed] [Google Scholar]
- 12.Katzberg RW, Haller C Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Supplement 2006(100):S3–7 [DOI] [PubMed]
- 13.Chen SP, Fuh JL, Wang SJ, Chang FC, Lirng JF, Fang YC, Shia BC, Wu JC (2010) Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol 67(5):648–656 [DOI] [PubMed] [Google Scholar]
- 14.Griffith B, Kelley BP, Patel SC, Marin H (2018) Cerebrovascular Imaging (CT, MRI, CTA, MRA). In: Extracranial Carotid and Vertebral Artery Disease: Contemporary Management. Edited by Hans SS. Cham: Springer International Publishing;: 85–111
- 15.Okanovic M, Hillig B, Breuer F, Jakob P, Blaimer M (2018) Time-of-flight MR-angiography with a helical trajectory and slice-super-resolution reconstruction. Magn Reson Med 80(5):1812–1823 [DOI] [PubMed] [Google Scholar]
- 16.McKinney AM (2017) Artifacts of the craniocervical arterial system on MRI. Atlas of Normal Imaging variations of the Brain, Skull, and Craniocervical Vasculature. Springer International Publishing, Cham, pp 1261–1291 [Google Scholar]
- 17.Nishimura DG (1990) Time-of-flight MR angiography. Magn Reson Med 14(2):194–201 [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki M, Lee VS (2008) Nonenhanced MR Angiography. Radiology 248(1):20–43 [DOI] [PubMed] [Google Scholar]
- 19.Sayah A, Mamourian AC (2012) Flow-related artifacts in MR Imaging and MR Angiography of the Central Nervous System. Neurographics 2(4):154–162 [Google Scholar]
- 20.Heverhagen JT, Bourekas E, Sammet S, Knopp MV, Schmalbrock P (2008) Time-of-flight magnetic resonance angiography at 7 Tesla. Invest Radiol 43(8):568–573 [DOI] [PubMed] [Google Scholar]
- 21.Harteveld AA, De Cocker LJ, Dieleman N, van der Kolk AG, Zwanenburg JJ, Robe PA, Luijten PR, Hendrikse J (2015) High-resolution postcontrast time-of-flight MR angiography of intracranial perforators at 7.0 Tesla. PLoS ONE 10(3):e0121051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG (2007) The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 130(Pt 12):3091–3101 [DOI] [PubMed] [Google Scholar]
- 23.Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, Goeggel-Simonetti B, Engelter ST, Pezzini A, Bijlenga P et al (2015) Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 14(6):640–654 [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez J, Turan TN, Hoh BL, Chimowitz MI (2022) Intracranial atherosclerotic stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 21(4):355–368 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, Wang Y, Zou X, Leung TW, Cai Y et al (2014) Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (CICAS) study. Stroke 45(3):663–669 [DOI] [PubMed] [Google Scholar]
- 26.Kim YD, Choi HY, Cho HJ, Cha MJ, Nam CM, Han SW, Nam HS, Heo JH (2010) Increasing frequency and burden of cerebral artery atherosclerosis in Korean stroke patients. Yonsei Med J 51(3):318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Havenon A, Mossa-Basha M, Shah L, Kim SE, Park M, Parker D, McNally JS (2017) High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology 59(12):1193–1202 [DOI] [PubMed] [Google Scholar]
- 28.Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, Tirschwell DT, Hatsukami T, Anzai Y, Yuan C (2015) Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke 46(6):1567–1573 [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Lee DH, Kwon JY, Kang DW, Suh DC, Kim JS, Kwon SU (2013) High resolution MRI difference between moyamoya disease and intracranial atherosclerosis. Eur J Neurol 20(9):1311–1318 [DOI] [PubMed] [Google Scholar]
- 30.Yuan C, Kerwin WS, Ferguson MS, Polissar N, Zhang S, Cai J, Hatsukami TS (2002) Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging: JMRI 15(1):62–67 [DOI] [PubMed] [Google Scholar]
- 31.Chen CY, Chen SP, Fuh JL, Lirng JF, Chang FC, Wang YF, Wang SJ (2018) Vascular wall imaging in reversible cerebral vasoconstriction syndrome - a 3-T contrast-enhanced MRI study. J Headache Pain 19(1):74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha EA, Topcuoglu MA, Silva GS, Singhal AB (2019) RCVS(2) score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology 92(7):e639–e647 [DOI] [PubMed] [Google Scholar]
- 33.Shimoyama T, Uchino K, Hajj-Ali RA (2020) Reversible cerebral vasoconstriction syndrome: an update of recent research. Curr Treat Options Rheumatol 6(1):55–70 [Google Scholar]
- 34.Kumar N, Kumar S, Rocha E, Lioutas VA (2023) Vasoconstriction and long-term headache in reversible cerebral vasoconstriction syndrome. J Neurol 270(3):1647–1653 [DOI] [PubMed] [Google Scholar]
- 35.Cho S, Lee MJ, Gil YE, Chung CS (2021) RCVS-TCH score can predict reversible cerebral vasoconstriction syndrome in patients with thunderclap headache. Sci Rep 11(1):7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Néel A, Guillon B, Auffray-Calvier E, Hello M, Hamidou M (2012) [Reversible cerebral vasoconstriction syndrome]. La Revue De Med Interne 33(10):586–592 [DOI] [PubMed] [Google Scholar]
- 37.Hassan AE, Zacharatos H, Mohammad YM, Tariq N, Vazquez G, Rodriguez GJ, Suri MF, Qureshi AI (2013) Comparison of single versus multiple spontaneous extra- and/or intracranial arterial dissection. J Stroke Cerebrovasc Diseases: Official J Natl Stroke Association 22(1):42–48 [DOI] [PubMed] [Google Scholar]
- 38.Kwak JH, Choi JW, Park HJ, Chae EY, Park ES, Lee DH, Suh DC (2011) Cerebral artery dissection: spectrum of clinical presentations related to angiographic findings. Neurointervention 6(2):78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YC, Chen YF, Wang YH, Tu YK, Jeng JS, Liu HM (2009) Cervicocranial arterial dissection: experience of 73 patients in a single center. Surg Neurol 72(Suppl 2):S20–27 discussion S27 [DOI] [PubMed] [Google Scholar]
- 40.Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, Kang DW, Kim JS (2015) Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke 46(3):697–703 [DOI] [PubMed] [Google Scholar]
- 41.Sato S, Toyoda K, Matsuoka H, Okatsu H, Kasuya J, Takada T, Shimode A, Uehara T, Naritomi H, Minematsu K (2010) Isolated anterior cerebral artery territory infarction: dissection as an etiological mechanism. Cerebrovasc Dis 29(2):170–177 [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Shan Y, Guo R, Zheng T, Zhang X, Liu Z, Liu K (2022) Three-Dimensional High-Resolution magnetic resonance imaging for the Assessment of cervical artery dissection. Front Aging Neurosci 14:785661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arai D, Satow T, Komuro T, Kobayashi A, Nagata H, Miyamoto S (2016) Evaluation of the arterial wall in Vertebrobasilar Artery Dissection using high-resolution magnetic resonance Vessel Wall Imaging. J Stroke Cerebrovasc Diseases: Official J Natl Stroke Association 25(6):1444–1450 [DOI] [PubMed] [Google Scholar]
- 44.Tritanon O, Mataeng S, Apirakkan M, Panyaping T (2023) Utility of high-resolution magnetic resonance vessel wall imaging in differentiating between atherosclerotic plaques, vasculitis, and arterial dissection. Neuroradiology 65(3):441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Lou X, Li Y, Sui B, Sun S, Li C, Jiang P, Siddiqui A, Yang X (2014) Imaging investigation of intracranial arterial dissecting aneurysms by using 3 T high-resolution MRI and DSA: from the interventional neuroradiologists’ view. Acta Neurochir 156(3):515–525 [DOI] [PubMed] [Google Scholar]
- 46.Han M, Rim NJ, Lee JS, Kim SY, Choi JW (2014) Feasibility of high-resolution MR imaging for the diagnosis of intracranial vertebrobasilar artery dissection. Eur Radiol 24(12):3017–3024 [DOI] [PubMed] [Google Scholar]
- 47.Bayer-Karpinska A, Patzig M, Adamczyk C, Dimitriadis K, Wollenweber FA, Dichgans M, Jahn K, Opherk C (2013) Reversible cerebral vasoconstriction syndrome with concurrent bilateral carotid artery dissection. Cephalalgia 33(7):491–495 [DOI] [PubMed] [Google Scholar]
- 48.Dakay K, Kaur G, Gulko E, Santarelli J, Bowers C, Mayer SA, Gandhi CD, Al-Mufti F (2020) Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection. J Stroke Cerebrovasc Diseases: Official J Natl Stroke Association 29(9):105011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon M, Kim T (2022) Extracranial reversible cerebral vasoconstriction syndrome associated with vertebral artery dissection: a case report. Brain Circulation 8(4):222–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mawet J, Boukobza M, Franc J, Sarov M, Arnold M, Bousser MG, Ducros A (2013) Reversible cerebral vasoconstriction syndrome and cervical artery dissection in 20 patients. Neurology 81(9):821–824 [DOI] [PubMed] [Google Scholar]
- 51.Liao X, Deng J, Dai W, Zhang T, Yan J (2017) Rare variants of RNF213 and moyamoya/non-moyamoya intracranial artery stenosis/occlusion disease risk: a meta-analysis and systematic review. Environ Health Prev Med 22(1):75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bang OY, Chung JW, Kim DH, Won HH, Yeon JY, Ki CS, Shin HJ, Kim JS, Hong SC, Kim DK et al (2020) Moyamoya Disease and spectrums of RNF213 Vasculopathy. Translational Stroke Res 11(4):580–589 [DOI] [PubMed] [Google Scholar]
- 53.Baba T, Houkin K, Kuroda S (2008) Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry 79(8):900–904 [DOI] [PubMed] [Google Scholar]
- 54.Ihara M, Yamamoto Y, Hattori Y, Liu W, Kobayashi H, Ishiyama H, Yoshimoto T, Miyawaki S, Clausen T, Bang OY et al (2022) Moyamoya disease: diagnosis and interventions. Lancet Neurol 21(8):747–758 [DOI] [PubMed] [Google Scholar]
- 55.Suzuki J, Takaku A (1969) Cerebrovascular moyamoya disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20(3):288–299 [DOI] [PubMed] [Google Scholar]
- 56.Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR (2009) Moyamoya disease: a summary. NeuroSurg Focus 26(4):E11 [DOI] [PubMed] [Google Scholar]
- 57.Yu LB, Zhang Q, Shi ZY, Wang MQ, Zhang D (2015) High-resolution magnetic resonance imaging of Moyamoya Disease. Chin Med J 128(23):3231–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan M, Liu ZQ, Wang ZQ, Li B, Xu LJ, Xiao XL (2015) High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci Lett 584:77–82 [DOI] [PubMed] [Google Scholar]
- 59.Strunk D, Diehl RR, Veltkamp R, Meuth SG, Kraemer M (2023) Progression of initially unilateral Moyamoya angiopathy in caucasian europeans. J Neurol 270(9):4415–4422 [DOI] [PubMed] [Google Scholar]
- 60.Church EW, Bell-Stephens TE, Bigder MG, Gummidipundi S, Han SS, Steinberg GK (2020) Clinical course of unilateral Moyamoya Disease. Neurosurgery 87(6):1262–1268 [DOI] [PubMed] [Google Scholar]
- 61.Kelly ME, Bell-Stephens TE, Marks MP, Do HM, Steinberg GK (2006) Progression of unilateral moyamoya disease: a clinical series. Cerebrovasc Dis 22(2–3):109–115 [DOI] [PubMed] [Google Scholar]
- 62.Hayashi K, Horie N, Izumo T, Nagata I (2014) A nationwide survey on unilateral moyamoya disease in Japan. Clin Neurol Neurosurg 124:1–5 [DOI] [PubMed] [Google Scholar]
- 63.Hayashi K, Suyama K, Nagata I (2010) Clinical features of unilateral moyamoya disease. Neurologia medico-chirurgica 50(5):378–385 [DOI] [PubMed] [Google Scholar]
- 64.Matsushima T, Inoue T, Natori Y, Fujii K, Fukui M, Hasuo K, Kuwabara Y (1994) Children with unilateral occlusion or stenosis of the ICA associated with surrounding moyamoya vessels–unilateral moyamoya disease. Acta Neurochir 131(3–4):196–202 [DOI] [PubMed] [Google Scholar]
- 65.Smith ER, Scott RM (2008) Progression of disease in unilateral moyamoya syndrome. NeuroSurg Focus 24(2):E17 [DOI] [PubMed] [Google Scholar]
- 66.Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y (2005) Incidence and clinical features of disease progression in adult moyamoya disease. Stroke 36(10):2148–2153 [DOI] [PubMed] [Google Scholar]
- 67.Connolly ES Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43(6):1711–1737 [DOI] [PubMed] [Google Scholar]
- 68.Cuvinciuc V, Viguier A, Calviere L, Raposo N, Larrue V, Cognard C, Bonneville F (2010) Isolated acute nontraumatic cortical subarachnoid hemorrhage. AJNR Am J Neuroradiol 31(8):1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vergouwen MD, Algra A, Rinkel GJ (2012) Endothelin receptor antagonists for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke 43(10):2671–2676 [DOI] [PubMed] [Google Scholar]
- 70.Muehlschlegel S, Kursun O, Topcuoglu MA, Fok J, Singhal AB (2013) Differentiating reversible cerebral vasoconstriction syndrome with subarachnoid hemorrhage from other causes of subarachnoid hemorrhage. JAMA Neurol 70(10):1254–1260 [DOI] [PubMed] [Google Scholar]
- 71.Ansari SA, Rath TJ, Gandhi D (2011) Reversible cerebral vasoconstriction syndromes presenting with subarachnoid hemorrhage: a case series. J Neurointerventional Surg 3(3):272–278 [DOI] [PubMed] [Google Scholar]
- 72.Nussbaum ES, Mikoff N, Paranjape GS (2021) Cognitive deficits among patients surviving aneurysmal subarachnoid hemorrhage. A contemporary systematic review. Br J Neurosurg 35(4):384–401 [DOI] [PubMed] [Google Scholar]
- 73.Ferrante E, Tassorelli C, Rossi P, Lisotto C, Nappi G (2011) Focus on the management of thunderclap headache: from nosography to treatment. J Headache Pain 12(2):251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ducros A, Bousser MG (2013) Thunderclap headache. BMJ (Clinical Res ed) 346:e8557 [DOI] [PubMed] [Google Scholar]
- 75.Schwedt TJ (2015) Thunderclap Headache. Continuum (Minneapolis Minn) 21(4 Headache):1058–1071 [DOI] [PubMed] [Google Scholar]
- 76.Roberts T, Horner DE, Chu K, Than M, Kelly AM, Klim S, Kinnear F, Keijzers G, Karamercan MA, Wijeratne T et al (2022) Thunderclap headache syndrome presenting to the emergency department: an international multicentre observational cohort study. Emerg Med J 39(11):803–809 [DOI] [PubMed] [Google Scholar]
- 77.Ling YH, Wang YF, Lirng JF, Fuh JL, Wang SJ, Chen SP (2021) Post-reversible cerebral vasoconstriction syndrome headache. J Headache Pain 22(1):14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ducros A, Wolff V (2016) The typical thunderclap headache of reversible cerebral vasoconstriction syndrome and its various triggers. Headache 56(4):657–673 [DOI] [PubMed] [Google Scholar]
- 79.Song JT, Lee JY (2017) Exercise headache associated with reversible cerebral vasoconstriction syndrome. Headache Pain Res 18(2):39–41
- 80.Singhal AB, Topcuoglu MA, Fok JW, Kursun O, Nogueira RG, Frosch MP, Caviness VS Jr. (2016) Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 79(6):882–894 [DOI] [PubMed] [Google Scholar]
- 81.Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, Calabrese LH (2011) Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol 68(8):1005–1012 [DOI] [PubMed] [Google Scholar]
- 82.Biscetti L, De Vanna G, Cresta E, Corbelli I, Gaetani L, Cupini L, Calabresi P, Sarchielli P (2021) Headache and immunological/autoimmune disorders: a comprehensive review of available epidemiological evidence with insights on potential underlying mechanisms. J Neuroinflamm 18(1):259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kraemer M, Berlit P (2021) Primary central nervous system vasculitis - an update on diagnosis, differential diagnosis and treatment. J Neurol Sci 424:117422 [DOI] [PubMed] [Google Scholar]
- 84.Godasi R, Pang G, Chauhan S, Bollu PC (2024) Primary Central Nervous System Vasculitis. In: StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Graeme Pang declares no relevant financial relationships with ineligible companies. Disclosure: Shaylika Chauhan declares no relevant financial relationships with ineligible companies. Disclosure: Pradeep Bollu declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © StatPearls Publishing LLC.; 2024
- 85.Beuker C, Schmidt A, Strunk D, Sporns PB, Wiendl H, Meuth SG, Minnerup J (2018) Primary angiitis of the central nervous system: diagnosis and treatment. Ther Adv Neurol Disord 11:1756286418785071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singhal AB (2021) Posterior reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome as syndromes of Cerebrovascular Dysregulation. Continuum (Minneapolis Minn) 27(5):1301–1320 [DOI] [PubMed] [Google Scholar]
- 87.Sundaram S, Kumar PN, Sharma DP, Kesavadas C, Sreedharan SE, Prasad BA, Sylaja PN (2021) High-resolution Vessel Wall Imaging in primary angiitis of Central Nervous System. Ann Indian Acad Neurol 24(4):524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mossa-Basha M, Shibata DK, Hallam DK, de Havenon A, Hippe DS, Becker KJ, Tirschwell DL, Hatsukami T, Balu N, Yuan C (2017) Added Value of Vessel Wall magnetic resonance imaging for differentiation of Nonocclusive Intracranial vasculopathies. Stroke 48(11):3026–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, Johnson MH, Daemen MJ, Vossough A, Edjlali M et al (2017) Intracranial Vessel Wall MRI: principles and Expert Consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 38(2):218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ivens S, Gabriel S, Greenberg G, Friedman A, Shelef I (2010) Blood-brain barrier breakdown as a novel mechanism underlying cerebral hyperperfusion syndrome. J Neurol 257(4):615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Muñoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B (2009) Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol 65(2):194–202 [DOI] [PubMed] [Google Scholar]
- 92.Merino JG, Latour LL, Tso A, Lee KY, Kang DW, Davis LA, Lazar RM, Horvath KA, Corso PJ, Warach S (2013) Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol 34(3):518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee MJ, Cha J, Choi HA, Woo SY, Kim S, Wang SJ, Chung CS (2017) Blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome: implications for pathophysiology and diagnosis. Ann Neurol 81(3):454–466 [DOI] [PubMed] [Google Scholar]
- 94.Cho S, Ling YH, Lee MJ, Chen SP, Fuh JL, Lirng JF, Cha J, Wang YF, Wang SJ, Chung CS (2020) Temporal Profile of blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome. Stroke 51(5):1451–1457 [DOI] [PubMed] [Google Scholar]
- 95.Wu CH, Lirng JF, Wu HM, Ling YH, Wang YF, Fuh JL, Lin CJ, Ling K, Wang SJ, Chen SP (2021) Blood-brain barrier permeability in patients with reversible cerebral vasoconstriction syndrome assessed with dynamic contrast-enhanced MRI. Neurology 97(18):e1847–e1859 [DOI] [PubMed] [Google Scholar]
- 96.Ling YH, Chi NF, Pan LH, Wang YF, Wu CH, Lirng JF, Fuh JL, Wang SJ, Chen SP (2023) Association between impaired dynamic cerebral autoregulation and BBB disruption in reversible cerebral vasoconstriction syndrome. J Headache Pain 24(1):170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Topcuoglu MA, Singhal AB (2016) Hemorrhagic reversible cerebral vasoconstriction syndrome: features and mechanisms. Stroke 47(7):1742–1747 [DOI] [PubMed] [Google Scholar]
- 98.Chen SP, Wang SJ (2022) Pathophysiology of reversible cerebral vasoconstriction syndrome. J Biomed Sci 29(1):72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Y, Rosenberg GA (2011) Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42(11):3323–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee EK, Lee EJ, Kim S, Lee YS (2016) Importance of Contrast-Enhanced Fluid-Attenuated Inversion Recovery Magnetic Resonance Imaging in various intracranial pathologic conditions. Korean J Radiol 17(1):127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study consisted of patient cases and were subject to privacy protection under Institutional Review Board (IRB) regulations. Due to ethical restrictions, raw data cannot be publicly available or shared on request. Data and materials supporting this study’s conclusions are included in this article.