Abstract
BACKGROUND
Conjoined twins are a rare type of congenital malformation. The point of attachment is the primary factor used to classify conjoined twins; typically, this is front to front, with thoracopagus and omphalopagus twins accounting for about 75% of cases and pygopagus twins only between 6% and 19%.
OBSERVATIONS
This is a case report of 46-day-old female triplets born to a 37-year-old para 4 mother. Triplets A and B were conjoined at the lumbosacrococcygeal area dorsally. Triplet C was not conjoined. The conjoined triplets had a low birth weight; hence, it was decided to start nutritional management and achieve adequate weight gain before surgical separation. While on nutritional management, at 46 days of age, triplet B developed cardiorespiratory failure. Therefore, the multidisciplinary team decided to proceed with emergency neurosurgical separation surgery, with the primary aim of saving triplet A. Triplet B died 7 hours after the surgery due to irreversible cardiorespiratory failure, and triplet A remained in the hospital for 14 days and was discharged with intact neurological function.
LESSONS
Most cases of conjoined twins do not require emergency separation surgery and need nutritional support before the separation procedure. Separation surgery requires a multidisciplinary team meeting, planning ahead of time, and preparation.
Keywords: triplets, conjoined twins, pygopagus, neurosurgical, separation, malformation
ABBREVIATIONS: ASD = atrial septal defect, HC = head circumference, PDA = patent ductus arteriosus
For conjoined twins, several classification schemes have been put forth. As cited in Khan, while Potter and Craig simply categorized conjoined twins based on the most prevalent forms of twinning, Spencer categorized conjoined twins based on the site of union, i.e., ventral (cephalopagus, thoracopagus, omphalopagus, ischiopagus, and parapagus) and dorsal (craniopagus, pygopagus, and rachipagus).1 The lower spine in 100% of pygopagus twins, the lower gastrointestinal tract in 25%, and the genitourinary system (which includes a single bladder and urethra) in 15% of them are shared, while the spinal cords and cauda equinae mostly remain separate. The primary obstacle is separating all three involved systems without sacrificing their anatomy and physiology, in addition to covering significant skin defects.2 The spinal cord’s fused site has been described as having a U, Y, or V form. Because of the hidden anatomical midline, it is more challenging to identify the cleavage plane of twins in a U-shaped fusion, either intraoperatively or with preoperative MRI.3 A surgical separation success rate of conjoined twins as high as 70% has been reported for electively operated cases. The extent of fusion and the presence of associated congenital anomalies are the most important determining factors for survival.2 When nerve roots cross the midline in a fused spinal cord, identifying the midline is challenging. The perineal region, spinal cords, major vessels, and gastrointestinal, urinary, and reproductive systems are shared to varying degrees by pygopagus conjoined twins. They sometimes have a single dural sac and fused spinal cord, and they typically merge at the sacrum. The majority of instances that have been documented had a connected sacrum, either with or without the coccyx or lower lumbar spine involved. Surgical results have indicated that 68% of conjoined twins share the dural sac, whereas 32% have separate dural and spinal structures. To verify nerve distribution and identify the functional midline, urodynamic investigations and intraoperative nerve stimulation have been used in the past. One case study described how a single fused conus was separated in the anatomical midline with an excellent outcome. The optimum time to separate pygopagus conjoined twins is usually after their medical condition has stabilized and they have gained weight.4
Illustrative Case
This is a report of 46-day-old female triplets born to a 37-year-old para 4 mother by cesarean section at a district hospital. Triplets A and B were conjoined dorsally at the lumbosacrococcegeal area (Fig. 1), which was 6 cm in length and had a common spinal canal and dural coverings. Triplet B had multiple vertebral bone segmentation anomalies as well as multiple cardiac, intestinal, and renal anomalies; however, no fusion of vertebral bones between the conjoined triplets was noted. Triplet C was not conjoined.
FIG. 1.
Preoperative photograph of conjoined triplets A and B.
Physical Examination Findings of Each Triplet
Triplet A was a 46-day-old female who had an Apgar score between 7 and 8, a birth weight of 1700 g, an anterior fontanel of 2 × 3 cm, and a head circumference (HC) of 37 cm. She was acutely sick and in respiratory distress. Sucking was not sustained, but her grasp was strong.
Triplet B was a 46-day-old female who had an Apgar score between 5 and 7, a birth weight of 1400 g, an anterior fontanel of 2 cm, and an HC of 37 cm. She had multiple congenital malformations, including a rectovaginal fistula with imperforate anus, a hypoplastic left leg with contracture, dextrocardia (L-looped ventricle), situs inverses, a large patent ductus arteriosus (PDA), a small atrial septal defect (ASD), and a right solitary ectopic kidney.
Triplet C, who was not conjoined, was a 46-day-old female who had an Apgar score between 8 and 9 at 5 and 10 minutes, a birth weight of 2440 g, an anterior fontanel of 2 × 3 cm, and an HC 38 cm. She was healthy looking and alert, able to sustain sucking, and had a complete Moro reflex.
For the conjoined triplets (A and B), whole-spine MRI demonstrated a single spinal cord and U-shaped dural covering starting at L2 and descending as a single filum terminale (Fig. 2). CT demonstrated multiple vertebral segmentation anomalies for triplet B, and there was no bone fusion between the triplets. Echocardiography and abdominopelvic ultrasound showed a small ASD, a large PDA, an L-looped ventricle, situs inversus, dextrocardia, and a right solitary ectopic kidney in triplet B.
FIG. 2.
Preoperative T2-weighted MR image demonstrating the U-shaped thecal sac with its contents.
The shared structures and fused body parts in our conjoined triplets were the dura mater, conus medullaris, filum terminale, dorsal lumbosacral coccygeal area muscle, fascia, and skin (Fig. 2). The rectum, anus, and other visceral organs were not shared.
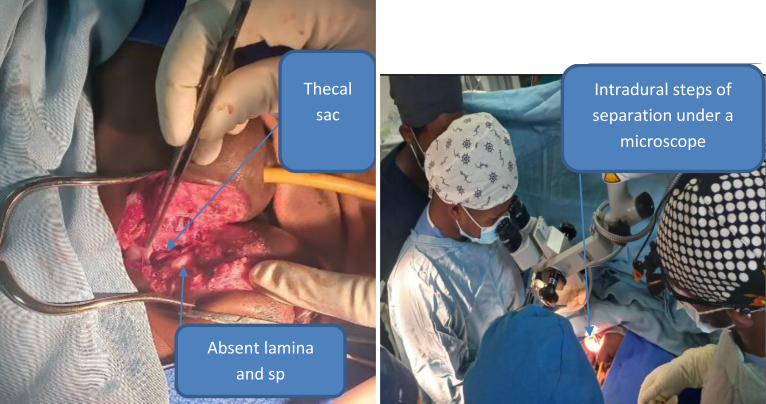
The conjoined triplets had a low birth weight; hence, the multidisciplinary team decided to start nutritional management and achieve adequate weight gain before surgical separation. While on nutritional management, on the 46th day of age, triplet B developed cardiorespiratory failure. The multidisciplinary team decided to intubate triplet B urgently in the neonatal ICU and to proceed with emergency neurosurgical separation surgery on the same day, with the primary aim of saving triplet A. After preparing cross-matched blood, the multidisciplinary team took the patients to the operating room and positioned them laterally facing away from each other with the fused lumbosacrococcygeal area in the middle of the operating table. Triplet A was intubated on the operating table, and all intravenous lines were labeled with colored marks: red for triplet A and blue for triplet B. Then the surgical site was marked, cleaned, and draped. Skin exposure started on the dorsal midline of the fused lumbosacrococcygeal area by the pediatric surgery and plastic and reconstructive surgery teams. After skin exposure (Fig. 3), there was no vertebral bony fusion. In deep dissection, a single U-shaped dura was encountered and opened in the midline vertically. After durotomy, a Y-shaped spinal cord was identified, which means two spinal cords fused at the conus and then descended as a single filum with multiple nerve roots exiting to both sides. At the conus level, there was a cleavage plane with thin bands in between. Separation was performed through the cleavage plane, dissecting the conus along the plane to give each triplet her corresponding conus and roots, with special emphasis on triplet A. After completing spinal cord, conus, filum, and root separation, the dural closure was accomplished by primary repair separately for both triplets. Then closure of the remaining muscle, subcutaneous tissue, and skin was accomplished by a Y-shaped flap for triplet A and a rotational flap for triplet B by the plastic and reconstructive surgery and pediatric surgery teams. We knew that we could not save triplet B as she had multiple congenital malformations that were not compatible with life. Triplet B died 7 hours after the surgery due to nonreversible cardiorespiratory failure.
FIG. 3.
Intraoperative photographs showing skin exposure, laminar and spinous process (sp) defects, thecal sac exposure, and intradural conus, nerve root, and filum separation.
Triplet A remained in the hospital for 14 days. At discharge she had full lower extremity strength and was moving spontaneously. At the 1-month follow-up, triplet A had intact neurological function (Fig. 4). Many authors recommend neurophysiological monitoring during intradural procedures and perineal separation stages, but it was not available at our institution.
FIG. 4.
Postoperative photograph of triplet A at the 1-month follow-up.
What Is Unique in Our Case?
A triplet pregnancy with one separate triplet and the remaining two conjoined at the lumbosacrococcygeal area dorsally is an extremely rare occurrence. Separation surgery was accomplished on an emergency basis, unlike most case reports. Separation surgery performed without intraoperative neurophysiological monitoring and stimulation, as it was not available in our institution, may serve as an additional resource for other neurosurgeons in resource-limited settings. Triplet B had multiple congenital heart diseases (large PDA and small ASD) that eventually led the baby to die after the separation, which was not mentioned in other case reports, and she also had a solitary ectopic kidney, which, again, has rarely been reported. This may give insight into how various associated anomalies can occur and affect the patient's overall outcome.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Pygopagus twins in a triplet pregnancy are extremely uncommon; they make up between 10% and 18% of all conjoined twins, or roughly 1 in 1 million live births of this specific kind. Pygopagus twins may share rectums, reproductive organs, urinary tracts, and neural components if they are linked at the sacrum. A single anus is shared by about 25% of them, with one or two rectums, which is similar to our situation. Even though the first pygopagus twin separation went well in 1955, the procedure is still difficult today and requires careful planning, preparation, and teamwork between several multidisciplinary teams. Conjoined twins are typically separated months after birth, when the babies are ready for general anesthesia and major surgery, despite the fact that distinct findings of monochorionic monoamniotic babies and relative fixed positioning of fetal bodies on ultrasound are sufficient to detect conjoined twins as early as 12–16 weeks in utero. A backup plan is necessary if urgent separation is needed, which was missed in our case. It is essential for the separation to assign the various tasks to distinct teams. It is crucial to pay attention to medication dosages and adverse effects because there is a chance of cross-circulation. In pygopagus twins, being guided by muscle stimulation and neuromonitoring modalities during perineal restoration is important because it enhances neurological and functional results, although it is not available in resource-limited settings like our institution. To reduce the danger of contamination and meningitis, neural structure separation should be carried out before anorectal separation.5
Observations
By definition, pygopagus conjoined twins are twins joined at the buttocks and base of the spine, and they might share gastrointestinal genitourinary systems, spine, and spinal cord. The first pygopagus conjoined twins lived 34 years joined at the hips and buttocks, sharing a vagina and anus. When one of them died, the other refused to be separated and died 6 hours later.6 Conjoined twins have an unclear etiology; however, it is generally agreed that the condition results from a uniovular pregnancy in which the embryonic disc fails to fully separate at about day 15 or 17. Of the pygopagus twins, roughly 50% have anomalies unrelated to the usual fused organs (Table 1), and spinal malformations are very common, which were confirmed to present in multiple parts of the body in our case. In the literature, congenital vertical talus deformity and congenital talipes equinovarus have been described.6 Thirty-four sets of pygopagus twins have been reported in the literature. Further investigation of the spectrum of anorectal anomalies revealed that 13 sets of rectums were entirely nonfused and 13 sets were fused in a Y configuration. Female conjoined twins made up 70% of the cases (Table 1). Eighty percent of the pygopagus twins that were stillborn were boys. Eighty-six percent of the twins that were delivered alive were female (Table 1). Every known live-born male pygopagus twin (100%) had a separated rectum. Just 41% of the female pygopagus twins had nonfused rectums,6 like ours. Conjoined twins with fusions at the head or cranium (craniopagus or cephalopagus) and unions at the chest (thoracopagus) are typically difficult to separate and have a low survival probability. The actual occurrence of conjoined twins in Ethiopia is unknown, yet a few publications exist that describe the cases. In one journal, a case of conjoined twins, ischiopagus dicephalus tetrabrachius bipus (meaning conjoined twins fused at the pelvis with the vertebral axis at 180°), born alive, was documented.7
TABLE 1.
Previous case reports with conjoined pygopagus twins
| Authors & Year | Shared Structures | Age at Op | Elective or Emergency Separation | Neurological Outcome After Separation | Alive or Dead After Separation | Unique Scenario in the Case | Complications | Sex | Type of Pregnancy | Associated Anomalies |
|---|---|---|---|---|---|---|---|---|---|---|
| Cloutier et al., 197912 | Anus, rectum, vertebral bone, large vein, common dura mater | 4 mos | Elective | Excellent | Alive | Colostomy done | Minimal serous discharge | Female | Twin | Meningocele, dermal sinus |
| Mahmoud et al., 202313 | Sacrum, dura mater, nerve roots | 1 yr 7 mos | Elective | Excellent | Alive | Colostomy done for rectovaginal fistula for both | None | Female | Twin | Leg shortening or discrepancy in 1 twin |
| Cromeens et al., 201714 | Dura mater, spinal cords, common anal sphincter | 11 mos | Elective | Excellent | Alive | Colostomy for imperforate anus | None | Female | Twin | Lipomyelomeningocele |
| Toyama et al., 202215 | 1 anus, 1 penis, 1 scrotum, spinal cord, sacrum, rectum | 5 mos | Elective | Excellent | Alive | Colostomy | None | Male | Twin | None |
| Chou et al., 201116 | Anus, rectum, dura, spinal cord | 5 mos | Elective | Excellent | Alive | Colostomy | None | Female | Twin | Epidermoid cyst |
| Can et al., 202011 | Sacrum, dura mater, spinal cord, perineal fistula, rectovaginal fistula | 13 mos | Elective | Excellent | Alive | Diverting colostomy | CSF leak | Female | Twin | Congenital hip dysplasia, synarthrosis |
| Awasthi et al., 20159 | Lumbar & sacrum, low-lying conus, spinal cord, dura | 1 mo 2 wks | Elective | Excellent | Alive | None | None | Female | Twin | Spina bifida |
Conjoined twin diagnosis and workup is possible prenatally in facilities where pregnant mothers undergo obstetric ultrasound examinations as early as week 12 of pregnancy. The diagnosis is necessary because the care of conjoined twins should be relocated while they are in utero to a facility that can manage their future care.6 Among investigation modalities, spine MRI should be used to look for the number of spinal cords, a single or separate dural covering, the end of the filum, filum thickness, associated cysts, and anomalies, to determine anorectal malformation. Spine CT should be used to look for bone fusion sites and bony anomalies. Echocardiography should be used to look for congenital heart disease, and abdominopelvic ultrasound to look for gastrointestinal, genitourinary, and other abdominopelvic organ anomalies. Other invasive examinations like anoscopy, cystourethroscopy, and vaginoscopy may be needed when there is a strong suspicion of anorectal or rectovaginal fistula. A thorough radiographic assessment of the urinary, reproductive, and gastrointestinal systems is necessary for the surgical management of pygopagus twins, with special emphasis on the anatomy of the spinal cord.
A thorough initial multidisciplinary planning session and insertion of a tissue-expanding balloon beneath the joining skin bridge ahead of time, to aid in skin closure during the separation process, were mentioned in the literature, but we did not use this technique in our case as there were separate rectums and separation was performed on an emergency basis. Cross-matched blood should be prepared and the patients intubated on one operating table with two machines. Each intravenous line should be labeled and the cable color-coded. The lateral position suffices for most pygopagus twins, with an underneath buttock roll or cushion to elevate the sacrum and perineum. Both twins should be catheterized, and an electrode needle should be inserted into all limbs and buttocks for spinal cord or root monitoring, but it is not available in most developing countries. After preparing the skin, we make a vertical skin incision and expose the deeper layers, layer by layer, and then the dura by removing the vertebral bone posterior elements with laminectomies. Sometimes there is a posterior element bone defect, in which case we reach directly into the dura and open the dura vertically under an operating microscope. We explore and divide the spinal cords and roots, giving special attention to the twin with relatively better neurological and general health, as in our case. Identifying the midline of the cord and identifying which root belongs to which twin is challenging, and there are also crossing roots and adjoining bands. These cases need frequent neuromonitoring. Some authors recommend just separating the midline in two halves in a fused conus with no plane of separation for an excellent outcome. A tethering bone dividing the spinal cords may be encountered; it should be excised. Sometimes the aberrant roots or bands need to be cut for separation. After completing the cord and root separation, a watertight dural closure is crucial, either primarily, as in our case, or with a graft, to avoid cerebrospinal fluid leaks and meningitis. The perineal stage is next and is undertaken by the pediatric surgery team to separate the rectum, anus, and midline crossing vessels. Then the reconstruction is carried out by the plastic and reconstructive surgery team. After completing the above steps to separate the twins, the remaining attachments and skin may need to be divided, and repositioning may be needed for muscle, fascia, and skin closure and reconstruction. In patients with rectovaginal fistulas and an imperforate anus, a diversion colostomy is necessary before the separation procedure.8
Emergency surgery carries a much greater mortality rate than elective surgery. Eighty-two percent of the separation operations in the literature, from 1 week to 19 months after birth, were done electively (Table 1). One of the twins died during or after surgery in 3 of the 5 urgent procedures (60%). On the other hand, in 17 pairs of 20 cases involving elective procedures, both twins lived (85%).4 When one child has a serious illness, however, emergency separation is the only way to save one or both of them.4 The distribution of organs between the twins, the order of separation, the meticulous aseptic surgical techniques, the reconstruction of divided organs and structures with tension-free wound closure, and the mode of treatment (nonoperative, emergency, or elective procedure as needed) are all important factors that contribute to a successful outcome.9 Of the pygopagus twins with conjoined cords that survived surgery, 37.5% had CSF leakage. This particular issue is especially concerning when it comes to those who have already had intestinal surgery, as this carries a higher risk of sepsis. One remarkable instance consisted of a tethered cord in conjoined spinal cords. Without separation, ambulation would be exceedingly challenging and most likely further hampered by the neurological effects of tethering.10 While surgical separation is a significant achievement, the long-term care and ongoing monitoring of these individuals are just as crucial to detecting and treating late urological and orthopedic complications.11 Pygopagus conjoined twinning is associated with different types of malformations, making separation challenging, and during separation surgery use of neuromonitoring is recommended.
Limitations
This case report had a short-term follow-up.
Lessons
Separation surgery requires a multidisciplinary team involving pediatric neurosurgeons, pediatric surgeons, plastic and reconstructive surgeons, and anesthesiologists. The overall outcome is determined by the associated malformations, particularly the presence of cardiac defects, and multiple malformations on one neonate require early separation surgery.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: all authors. Acquisition of data: Ali, Abebe, Bayable. Analysis and interpretation of data: Ali, Abebe, Bayable. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: Ali, Abebe, Bayable. Approved the final version of the manuscript on behalf of all authors: Ali. Statistical analysis: Bayable. Administrative/technical/material support: Ali, Haile, Bayable. Study supervision: Ali, Haile, Bayable.
Correspondence
Endris Hussen Ali: Ethiopian Police Hospital, Addis Ababa, Ethiopia. idrisdoc2007@gmail.com.
References
- 1.Khan YA.. Ischiopagus tripus conjoined twins. APSP J Case Rep. 2011;2(1):5. [PMC free article] [PubMed] [Google Scholar]
- 2.Jain P Kundal AK Sharma R Khilnani P Kumar P Kumar P.. Surgical separation of pygopagus twins: a case report. J Pediatr Surg Case Rep. 2014;2(3):119-122. [Google Scholar]
- 3.Yokota C, Kagawa N, Bamba Y.Successful neurosurgical separation of conjoined spinal cords in pygopagus twins: illustrative cases. J Neurosurg Case Lessons. 2021;1(9):CASE218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirokazu T Takayuki I Yoshinori H Kazunari K Akio A Keiji K.. Separation surgery of pygopagus asymmetrical conjoined twins sharing U-shaped spinal cord: case report and literature review. Childs Nerv Syst. 2013;29(4):699-706. [DOI] [PubMed] [Google Scholar]
- 5.Sabeh-Ayoun K Al Tahan H Akel S Zaghal A.. Successful separation of pygopagus conjoined twins: a case report. J Pediatr Surg Case Rep. 2023;99. [Google Scholar]
- 6.Matta H, Jacobsz A, Auchincloss J.Successful separation of pygopagus conjoined twins. J Pediatr Surg. 2006;41(3):586-588. [DOI] [PubMed] [Google Scholar]
- 7.Alene TD Abebe MS.. A case of ischiopagus dicephalus conjoined twins with tetrabrachius bipus from Dessie, Ethiopia. Int Med Case Rep J. 2022;15:425-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hockley AD, Gornall P, Walsh R.Management of pyopagus conjoined twins. Childs Nerv Syst. 2004;20(8-9):635-639. [DOI] [PubMed] [Google Scholar]
- 9.Awasthi R Iyengar R Rege S Jain N.. Surgical management of pygopagus conjoined twins with spinal bifida. Eur Spine J. 2015;24(suppl 4):S560-S563. [DOI] [PubMed] [Google Scholar]
- 10.Fieggen G Millar A Rode H Ngiloi P Andronikou S Peter J.. Spinal cord involvement in pygopagus conjoined twins: case report and review of the literature. Childs Nerv Syst. 2003;19(3):183-187. [DOI] [PubMed] [Google Scholar]
- 11.Can DDT, Lepard JR, Tri TT.The growth of pediatric neurosurgery in southern Vietnam and the first separation of pygopagus twins: case report. J Neurosurg Pediatr. 2020;25(4):445-451. [DOI] [PubMed] [Google Scholar]
- 12.Cloutier R, Levasseur L, Copty M, Roy JP. The surgical separation of pygopagous twins. Journal of Pediatric Surger. 1979;14(5):554-556. [Google Scholar]
- 13.Mahmoud MA Elshawady SB Korkar GH Hussein SM Elserry TH Mahmoud MWS.. Separation of conjoined spinal cords in symmetrical pygopagus twins using intraoperative neuromonitoring: pearls and pitfalls. Childs Nerv Syst. 2023;39(7):1949-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cromeens BP, McKinney JL, Leonard JR.Pygopagus conjoined twins: a neurophysiologic intraoperative monitoring schema. J Clin Neurophysiol. 2017;34(2):e5-e8. [DOI] [PubMed] [Google Scholar]
- 15.Toyama C, Tazuke Y, Yokota C.Successful separation of male pygopagus with anal canal and urethral reconstruction: a case report. Surg Case Rep. Springer; 2022;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou YC, Peng HC, Chu CH.Successful separation of the conjoined thecal sac with an epidermal cyst in pygopagus twins. J Pediatr Surg. 2011;46(9):e25-e27. [DOI] [PubMed] [Google Scholar]