Abstract
N-acetyl-L-leucine (NALL), a derivative of the branched-chain amino acid leucine, has shown therapeutic potential in neurodegenerative diseases, including in prodromal stages of Parkinson’s disease (PD). However, the mechanism of its protective effects has been largely unknown. Using discovery-based proteomics, we found that treatment with NALL led to upregulation of lysosomal, mitochondrial, and synaptic proteins in PD patient-derived dopaminergic neurons. NALL reduced levels of pathological pS129-alpha-synuclein in dopaminergic neurons from patients harboring GBA1 or LRRK2 mutations. This decrease in pS129-syn was dependent on serine protease HTRA1 that was induced by NALL treatment of dopaminergic neurons. NALL also upregulated expression of wild-type parkin in both GBA1 and LRRK2 mutant neurons, leading to an increase in functional dopamine transporter and synaptic membrane-associated synaptojanin-1, suggesting improved synaptic function. Furthermore, NALL treatment of mutant LRRK2R1441C knock-in mice led to decreased pS129-alpha-synuclein, increased parkin and improved dopamine-dependent motor learning deficits. These findings highlight the therapeutic potential of NALL in PD by its protective effects on α-synuclein pathology and synaptic function in vulnerable dopaminergic neurons.
Introduction
Parkinson’s disease (PD) affects 1–2% of the population and is characterized by resting tremor, rigidity, and bradykinesia. These motor symptoms of PD are primarily due to the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc)1,2 The pathogenesis of PD involves complex interactions between genetic and environmental factors. Among the genetic factors, mutations in the genes encoding leucine-rich repeat kinase 2 (LRRK2), glucocerebrosidase (GBA), and parkin all have been identified as significant contributors that affect mitochondrial, lysosomal, and synaptic pathways3.
A key pathological feature of PD is the age-dependent intracellular aggregation of α-synuclein (α-syn) in spherical cytoplasmic inclusions, termed Lewy bodies, which are also observed in neuronal processes as Lewy neurites4. α-Syn is thought to play a central role in the pathobiology of both familial and sporadic PD. Several posttranslational modifications to α-Syn are known to occur in PD. Among them is phosphorylation of α-Syn at Ser129 (pS129-syn), a modification that plays a critical role in PD pathogenesis5,6. pS129-syn has been reported to enhance α-Syn toxicity both in vivo and in vitro, possibly by increasing the formation of α-Syn aggregates6,7. Several current studies seek to find drugs that lower the accumulation and aggregation of α-Syn in the brains of PD patients8,9 10.
N-acetyl-L-Leucine (NALL) is the L-enantiomer of N-acetyl-DL-leucine (ADLL), a derivative of the branched-chain amino acid leucine. ADLL has been used as a treatment for acute vertigo and vertiginous symptoms (Tanganil®) for many years with an excellent safety profile. More recently, it has been shown that acetyl-leucine is beneficial in patients with lysosomal storage disorders Niemann-Pick Disease Type C and GM2 gangliosidoses11–13. In addition, ADLL has shown promising effects in patients with cerebellar ataxia14–16, restless legs syndrome17, and more recently REM Sleep Behavior Disorder18 that is considered a prodrome of PD. However, the mechanism responsible for these protective effects of NALL is largely unknown.
In this study, we found that NALL promoted the degradation of pS129-syn in human iPSC-derived dopaminergic neurons with GBA1 mutations. Moreover, NALL increased expression of parkin and synaptic vesicle recycling, as well as glycosylated functional dopamine transporter (DAT). Importantly, decreased pS129-syn and increased parkin upon NALL treatment were validated in LRRK2 mutant human dopaminergic neurons and LRRK2R1441C mutant mice. NALL treatment of LRRK2 mutant mice restored their behavior deficits, further pointing to the potential beneficial effects of NALL in PD.
Results
NALL leads to decreased pS129-syn in human dopaminergic neurons with GBA1 mutations
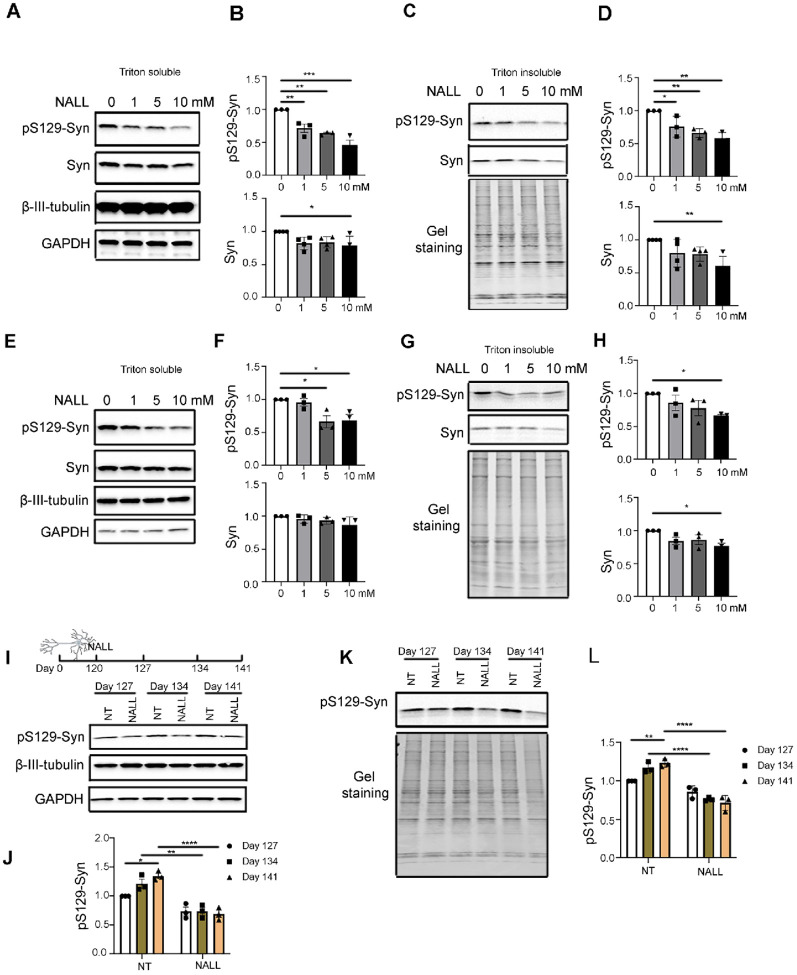
Since NALL exhibited protective effects in lysosomal disorders Niemann-Pick Disease Type C and GM2 gangliosidoses11–13, we hypothesized that NALL might be beneficial to PD patients with lysosomal dysfunction. To test this hypothesis, iPSC-derived dopaminergic neurons with GBA1 mutation (L444P) were treated with several concentrations of NALL on day 120 for 4 weeks. We observed a significant decrease of pS129-syn in a dose-dependent manner in both triton soluble and insoluble fractions (Fig. 1A–D), whereas total α-Syn levels were decreased with 10 mM NALL treatment. This finding was replicated in another GBA1 mutant (N370S) line where we also observed a decrease in pS129 and total syn upon NALL treatment (Fig. 1E–H). We found that phosphorylated a-Syn (pS129-syn) accumulated in both triton soluble and insoluble fractions from mutant GBA1 neurons cultured for 127 to 141 days. Upon treatment with NALL for 14 and 21 days, pS129-syn was reduced in mutant neurons (Fig. 1I–L), further suggesting that NALL treatment prevents the accumulation of a-Syn in GBA1 neurons.
Figure 1. NALL leads to decreased pS129-syn in human dopaminergic neurons with GBA1 mutations.
(A) Western blot showing levels of pS129-syn and total a-Syn (Syn) upon different concentrations of NALL in Triton-soluble faction of GBA1 L444P mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as loading controls. (B) Quantification of the fold change of decreased pS129-syn (top) and total α-Syn (Syn)(bottom) upon NALL treatment in A. Protein levels normalized toβ-III-Tubulin (n = 3 or 4 independent experiments). (C) Western blot of pS129-syn and total a-Syn (Syn) upon different concentrations of NALL in Triton-insoluble faction of GBA1 L444P mutant dopaminergic neurons. Gel staining was used as a loading control. (D) Quantification of the fold change of decreased pS129-syn (top) and total a-Syn (Syn)(bottom) upon NALL treatment in B. Protein level was normalized with β-III-Tubulin (n = 3 or 4 independent experiments). (E) Western blot of pS129-syn and total a-Syn (Syn) upon different concentrations of NALL treatment in Triton-soluble faction of GBA1 N370S mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as loading controls. (F) Quantification of the fold change of pS129-syn (top) and total α-Syn (Syn)(bottom) upon NALL treatment in E. The protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (G) Western blot of pS129-syn and total a-Syn (Syn) upon NALL treatment in Triton-insoluble faction of GBA1 N370S mutant dopaminergic neurons. Gel staining was used as a loading control. (H) Quantification of the fold change of pS129-syn (top) and total a-Syn (Syn)(bottom) upon NALL treatment in G. Protein level was normalized with total protein (n = 3 independent experiments). (I) (Top) Schematic illustration of the NALL treatment on dopaminergic neurons. GBA1 L444P mutant neurons were grown for 120 days and treated with or without 10 mM of NALL for another 7, 14, or 21 days. Cells were collected on days 127, 134, or 141. (Bottom) Western blot showing the protein level of pS129-syn with (NALL) or without (NT) NALL treatment in Triton-soluble faction. β-III-Tubulin and GAPDH were used as loading controls. (J) Quantification of the fold change of decreased pS129-syn in I. The protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (K) Western blot of pS129-syn with (NALL) or without (NT) NALL treatment in Triton-insoluble faction of GBA1 L444P mutant dopaminergic neurons. Gel staining was used as a loading control. (L) Quantification of the fold change of pS129-syn in K. Protein level was normalized with total protein (n = 3 independent experiments). All data are represented as mean ± SEM, *p<.05, **p<.01 and ***p<.005 and ****p<.0001.
Decreased pS129-syn upon NALL treatment is mediated by HTRA1
To better understand the underlying mechanisms of NALL treatment, we performed discovery-based proteomic analysis in iPS-derived dopaminergic neurons. This analysis revealed 896 proteins with significantly elevated levels and 283 proteins with decreased levels (Table S1). The proteins that were increased by at least 1.5-fold are involved in mitochondrial, lysosomal, and synaptic pathways whereas proteins that were decreased are involved in cytoskeletal function (Fig. 2A, Fig S1, Table S1, and S2). One of the top candidates was HTRA1 (Fig. 2B), an ATP-independent PDZ serine protease that cleaves various proteins such as fibronectin and amyloid precursor protein. It has been reported that HTRA1 can disaggregate a-Syn amyloid fibrils in vitro and prevent the accumulation of Lewy Body-like inclusions comprised of hyperphosphorylated a-Syn in mice19. Therefore, we hypothesized that increased HTRA1 might explain the effects of NALL on pS129-syn. We observed significantly elevated fold change of HTRA1 levels upon NALL treatment compared to the non-treated group (Fig. 2C and D), consistent with the proteomic data (Fig. 2B). Consistent with a previous finding that HTRA1 prominently partitions to the insoluble fraction when incubated without a-Syn, and soluble fraction in the presence of a-Syn in vitro19, we found a significant decrease of HTRA1 in the Triton soluble fraction but an increase in the Triton insoluble fraction with NALL treatment (Fig. 2E–H). Next, to test if HTRA1 indeed mediated NALL-induced lowering of pS129-syn, two different shRNA lentiviral constructs were used to knockdown HTRA1 (KD-1 and KD-2) in both non-treated (NT) and treated (NALL) conditions (Fig. 2I and J top panel). While HTRA1 was significantly higher and pS129-syn lower in the scramble control group, there was no significant decrease of pS129-syn upon NALL treatment when HTRA1 was knocked down (Fig. 2I and J bottom). These results suggested that NALL-mediated decrease in pS129-syn in mutant GBA1 neurons was at least in part mediated by HTRA1.
Figure 2. The effect of NALL on pS129-syn was mediated through increasing HTRA1.
(A) Heatmap summarizing protein levels significantly altered (adj. *p*-value < 0.05) by NALL treatments in GBA1 L444P mutant neurons. Each condition includes three biological replicates (BR). Red and blue reflect increased and decreased, respectively. (N =3 replicates). (B) Volcano plot summarizing protein level alterations induced by 10 mM NALL treatment compared to non-treated (NT) samples (n = 3). Proteins significantly altered (ratio NALL/NT > 1.5 and adjusted *p*-value < 0.05) are highlighted in blue, red, or green. The green dot indicates HTRA1. The dashed line represents an adjusted *p*-value threshold of 0.05 (one-way ANOVA, Benjamini–Hochberg correction). (C) Western blot of HTRA1 and pS129-syn upon different concentrations of NALL treatment in total lysates of GBA1 L444P mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as loading controls. (D) Quantification of the fold change of increased HTRA1 upon NALL treatment in C. The protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (E) Western blot of HTRA1 upon NALL treatment in Triton-soluble faction of GBA1 L444P mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as a loading control. (F) Quantification of the fold change of decreased HTRA1 upon NALL treatment in E. The protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (G) Western blot of HTRA1 upon NALL treatment in Triton-insoluble faction of GBA1 L444P mutant dopaminergic neurons. Gel staining was used as a loading control. (H) Quantification of the fold change of increased HTRA1 upon NALL treatment in G. The protein level was normalized with total protein (n = 3 independent experiments). (I) Western blot of HTRA1 and pS129-syn with (NALL) or without (NT) NALL treatment upon HTRA1 knockdown (KD-1 and KD-2) in GBA1 L444P mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as a loading control. (J) Quantification of the fold change of HTRA1 (top) and pS129-syn (bottom) with or without NALL treatment in I. The protein level was normalized with β-III-Tubulin (n = 3 independent experiments). All data are represented as mean ± SEM, ns: not significant, *p<.05, **p<.01, ***p<.005 and ****p<.0001.
NALL increases functional dopamine transporter and synaptic function through Parkin
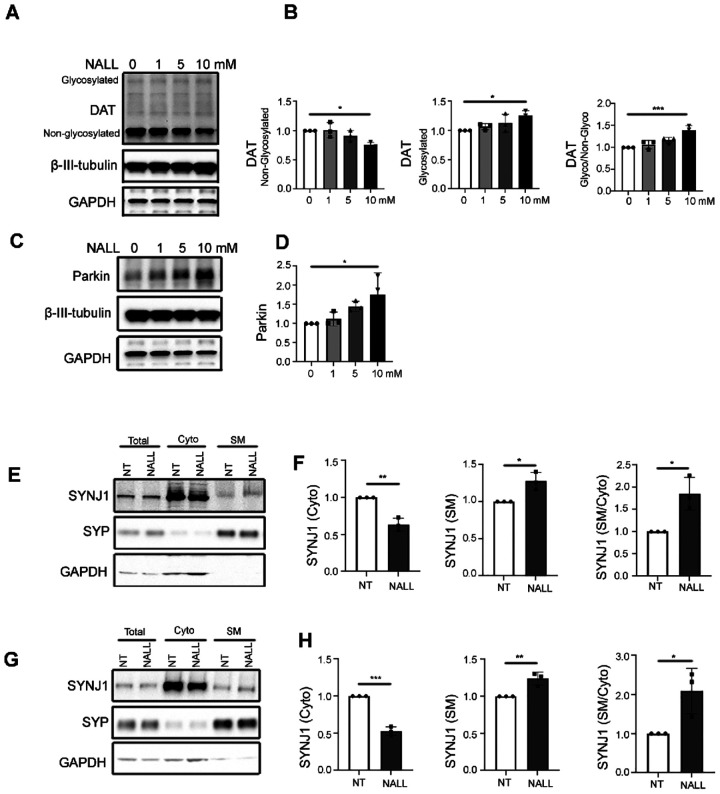
Recent findings suggest that ADLL improved the DAT-SPECT binding ratios in patients with REM sleep behavior disorder18. Consistent with this finding, we found that non-glycosylated DAT was decreased (Fig. 3A and B left), whereas glycosylated DAT increased in a dose-dependent manner upon NALL treatment (Fig. 3A and B middle). As the glycosylated DAT is cell surface expressed DAT20,21, the increased ratio of glycosylated versus non-glycosylated DAT (Fig. 3B right) suggested an increase in functional DAT. It has been reported that parkin enhances cell surface expression of DAT by ubiquitinating and degrading non-glycosylated misfolded DAT, which affects the oligomerization and surface expression of native DAT molecules21. To test whether the effects of NALL on DAT might be mediated by parkin, we examined the parkin levels in GBA1 mutant dopaminergic neurons. Interestingly, we found a dose-dependent increase in parkin upon treatment with NALL (Fig. 3C and D), suggesting that increased parkin might account for the increased cell surface or functional DAT. Our previous data in dopaminergic neurons showed that parkin facilitates synaptic vesicle recycling through ubiquitination-mediated membrane recruitment of synaptojanin-1 (SYNJ1), which promotes clathrin uncoating during synaptic vesicle recycling pathway22. Interestingly, we observed a significant decrease in cytosolic SYNJ1 but an increase of synaptic membrane-associated SYNJ1 upon NALL treatment in both GBA1 L444P (Fig. 3E and F) and N370S (Fig. 3G and H) mutant neurons, suggesting increased synaptic vesicle endocytosis pathway upon NALL treatment. These results suggest that NALL-mediated increase in parkin protein promotes efficient synaptic vesicle recycling in mutant GBA1 neurons.
Figure 3. NALL increases functional dopamine transporter and synaptic function through Parkin.
(A) Western blot of glycosylated and non-glycosylated DAT upon different concentrations of NALL treatment in GBA1 L444P mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as a loading control. (B) Quantification of the fold change of non-glycosylated DAT (left), glycosylated DAT (middle), and the ratio of glycosylated versus non-glycosylated DAT (right) upon NALL treatment in A. Protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (C) Western blot of parkin upon different concentrations of NALL treatment in GBA1 L444P mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as a loading control. (D) Quantification of the fold change of parkin upon NALL treatment in C. Protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (E) SYNJ1 is decreased in the cytosolic fraction (Cyto) and increased in the synaptic membrane fraction (SM) upon NALL treatment compared to non-treated (NT) dopaminergic neurons carrying GAB L444P mutation. Fractions were obtained from original total cell homogenate (Total). Synaptophysin (SYP) was used as a synaptic marker. GAPDH was used as a cytosolic marker. (F) Quantification of the fold change of cytosolic SYNJ1, normalized with GAPDH (left), synaptic membrane fraction of SYNJ1, normalized with SYP (middle), and the ratio of synaptic membrane (SM) versus cytosolic (Cyto) SYNJ1 (right) upon NALL treatment in E (n = 3 independent experiments). (G) SYNJ1 is decreased in the cytosolic fraction (Cyto) and increased in the synaptic membrane fraction (SM) upon NALL treatment compared to non-treated (NT) dopaminergic neurons carrying GAB N370S mutation. Fractions were obtained from original total cell homogenate (Total). Synaptophysin (SYP) was used as a synaptic marker. GAPDH was used as a cytosolic marker. (H) Quantification of the fold change of cytosolic SYNJ1, normalized with GAPDH (left), synaptic membrane fraction of SYNJ1, normalized with SYP (middle), and the ratio of synaptic membrane (SM) versus cytosolic (Cyto) SYNJ1 (right) upon NALL treatment in G (n = 3 independent experiments). All data are represented as mean ± SEM, *p<.05, **p<.01 and ***p<.005.
NALL leads to decreased pS129-syn and increased parkin in mutant human and mouse LRRK2 dopaminergic neurons
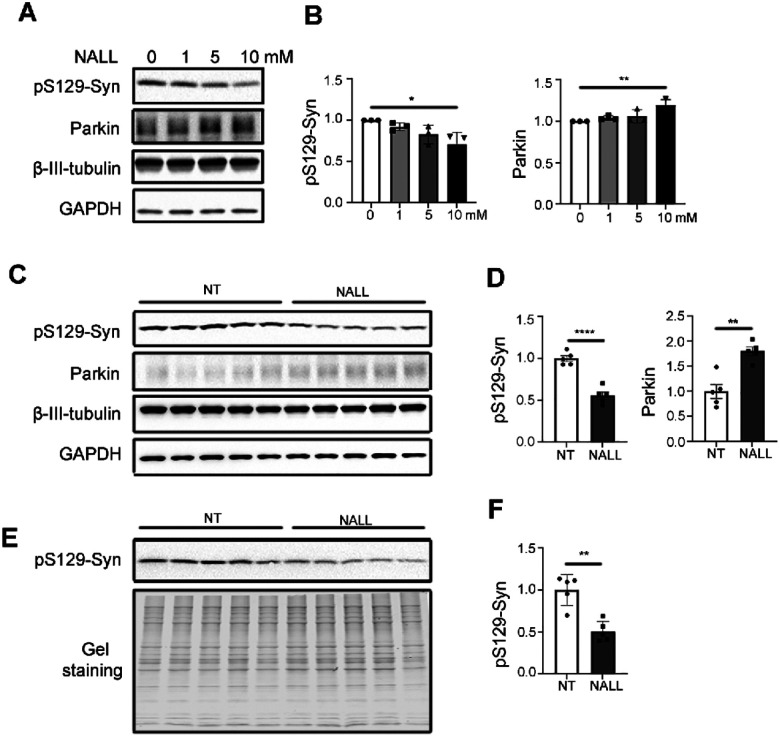
Our data so far suggested that NALL could decrease pathogenic pS129-syn level through HTRA1 and improve synaptic function through parkin in mutant GBA1 dopaminergic neurons. To understand if NALL may be beneficial in other forms of PD, we examined NALL in patient-derived dopaminergic neurons carrying LRRK2 R1441C mutation. Like the findings in mutant GBA1 neurons, we observed decreased pS129-syn and increased parkin in mutant LRRK2 neurons upon NALL treatment for 2 weeks (Fig. 4A and B). In addition, we found a significant decrease of pS129-syn in both Triton-soluble (Fig. 4C and D) and Triton-insoluble fraction (Fig. 4E and F) in LRRK2R1441C mice23 treated with NALL. Furthermore, we also observed a significant increase of parkin protein in LRRK2 mice upon NALL treatment (Fig. 4C and D), suggesting that NALL might be beneficial in LRRK2-linked PD.
Figure 4. NALL leads to decreased pS129-syn but increased parkin in LRRK2 mutant dopaminergic neurons in both human and mice.
(A) Western blot of pS129-syn and parkin upon different concentrations of NALL treatment in LRRK2 R1441C mutant dopaminergic neurons. β-III-Tubulin and GAPDH were used as a loading control. (B) Quantification of the fold change of decreased pS129-syn (left) and parkin (right) upon NALL treatment in A. Protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (C) Western blot of pS129-syn and parkin with (NALL) or without (NT) NALL treatment in Triton-soluble faction of LRRK2R1441C mice substantial nigra. β-III-Tubulin and GAPDH were used as a loading control. (D) Quantification of the fold change of decreased pS129-syn (left) and increased parkin upon NALL treatment in C. Protein level was normalized with β-III-Tubulin (n = 3 independent experiments). (E) Western blot of pS129-syn with (NALL) or without (NT) NALL treatment in Triton-insoluble faction of LRRK2R1441C mice substantial nigra. Gel staining was used as a loading control. (F) Quantification of the fold change of decreased pS129-syn upon NALL treatment in E. Protein level was normalized with total protein (n = 3 independent experiments). All data are represented as mean ± SEM, *p<.05, **p<.01, ***p<.005 and ****p<.0001.
NALL improves dopamine-dependent motor learning in LRRK2 mice
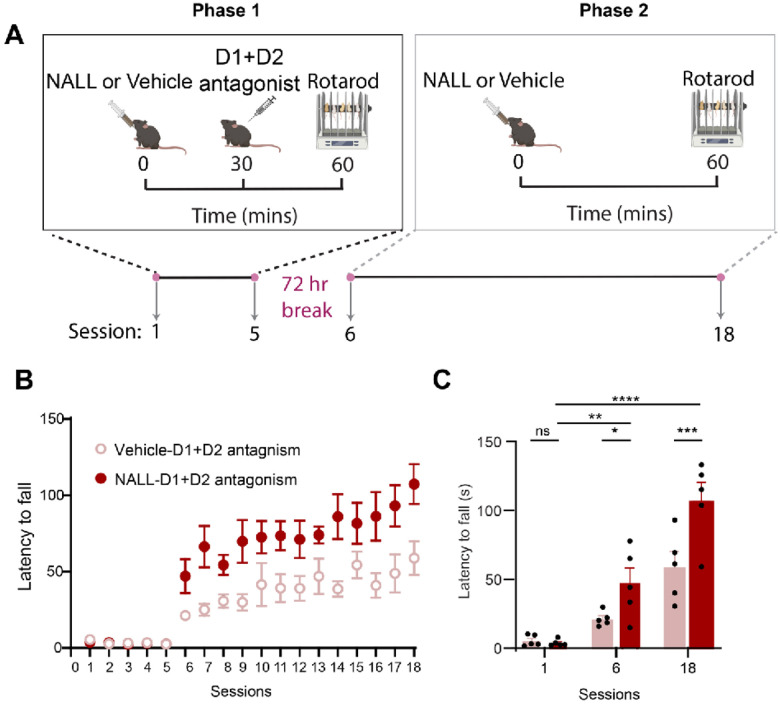
Since NALL treatment exhibited similar effects on a-Syn and parkin in LRRK2 patient derived neurons and mice, we examined whether NALL could improve the behavioral alterations in mutant LRRK2R1441C knock-in mice. Earlier studies showed that the loss of dopamine antagonism during the learning of a motor skill can hinder future motor performance even after the blockade has ended24,25. Considering the significance of dopamine’s role in motor learning and the decrease in dopamine release in the dorsal striatum of LRRK2R1441C mice23, we explored how dopamine antagonism affects striatal motor learning in these mice, using a two-stage accelerating rotarod paradigm, during which we applied D1 and D2 receptor antagonism in the first phase23. After a 72-hour break, the performance of the mice was assessed in the accelerating rotarod by measuring their latency to fall. We showed that wild-type LRRK2wt but not mutant LRRK2R1441C mice gradually improved their performance during the antagonism-free phase, indicating that the R1441C mutation disrupts dopamine-mediated motor learning in the striatum23.
To test potential beneficial effects of NALL on motor performance of mutant LRRK2R1441C mice, NALL was administered to LRRK2R1441C mice throughout the task (Fig. 5). The treated LRRK2R1441C mice showed a marked improvement compared to vehicle-treated mice across the second phase of the Rotarod protocol (treatment x session interaction < p value = .011, 2-way ANOVA). Next, we compared the performance of mutant LRRK2R1441C mice across selective task sessions. As expected, the dopamine antagonist impacted the performance at the first stage of the task; no difference was found (session 1) across treatments. However, at both sessions 6 and 18 of phase II, NALL improved LRRK2 mice performance compared to vehicle-treated controls (p value = .04 for session 6 and p value = .0004 for session 18; Sidak’s multiple comparison tests after two-way ANOVA). These data suggest that NALL improves dopamine-dependent motor learning of mutant LRRK2R1441C mice.
Figure 5. NALL improved dopamine-dependent motor learning impairments in LRRK2r1441C mice.
A. Schematic of the rotarod training paradigm. Mice were evaluated over a total of 18 daily sessions, with each session consisting of five trials. LRRK2 R1441C mice were administered either NALL or a vehicle control 30 minutes before receiving either saline or a cocktail of dopamine D1 receptor antagonists (SCH23390) and D2 receptor antagonists (eticlopride), both at a dosage of 1 mg/kg. Thirty minutes after the cocktail administration, the mice were trained on an accelerated rotarod for five consecutive days. After a 72-hour break, the mice were returned to the rotarod for an additional 13 days, with either NALL or vehicle administered 60 minutes before the accelerated rotarod test. B Improved performance of LRRK2 R1441C mice received NALL ( treatment x session interaction < p=0.011) C. Summarized data of the average latency in the session 1, session 6 and session 18 in the B. Data are represented as mean±SEM. Asterisks in C show statistical significance for Sidak’s multiple comparison tests after two-way ANOVA; *p < .05, **p< .01, ***p < .001, ****p < .0001. n=5 mice/treatment.
Discussion
Our patient-derived dopaminergic neuron data demonstrated that NALL decreased pS129-syn in GBA1 and LRRK2 mutant neurons. In GBA1 L444P neurons, we also observed a decrease in total α-Syn after 4 weeks of NALL treatment. To elucidate the mechanism of NALL-mediated effects on α-Syn, we employed discovery-based proteomics that identified an increase in HTRA1 protein in human dopaminergic neurons treated with NALL. It has been previously reported that HTRA1 protects against α-Syn aggregation and hyperphosphorylation19. In line with this finding, we found that the decrease of pS129-syn in NALL treated neurons was dependent on the expression of HTRA1, suggesting that targeting HTRA1 activation could be a novel strategy for counteracting α-Syn pathology. Since prior studies with purified HTRA1 protein demonstrated similar effects with other aggregation-prone proteins such as FUS, TDP-4319 and fibrillar tau26,27, it would be of interest to examine whether NALL treatment exhibited protective effects in models of amyotrophic lateral sclerosis, frontotemporal dementia, and Alzheimer’s Disease.
A recent study found that acetyl-leucine reversed the loss of striatal dopamine-transporter binding in the nigrostriatal system of patients with prodromal PD18. However, the mechanism of these effects has been unexplored. Our results demonstrate that NALL enhanced synaptic function by increasing parkin levels and stabilizing functional DAT in patient-derived GBA1 and LRRK2 dopaminergic neurons. This aligns with previous studies indicating that parkin facilitates DAT surface expression by ubiquitinating and degrading misfolded, non-glycosylated DAT21. Emerging evidence suggests that parkin plays a crucial role in synaptic function, particularly in the regulation of synaptic vesicle recycling22,28. Neuronal activity-dependent activation of parkin in human dopaminergic neurons leads to ubiquitination of SYNJ1, facilitating its interaction with endophilin A1 and promoting synaptic vesicle recycling22. In agreement with these findings, NALL-mediated increase in parkin expression enhanced the localization of SYNJ1 at synaptic membrane, suggesting that NALL promotes efficient synaptic endocytosis and dopamine neurotransmission. This notion is supported by our findings in LRRK2R1441C knock-in mice that exhibit impaired dopamine-dependent motor learning23, indicating striatal dopamine signaling dysfunction. Treatment of the LRRK2 R1441C mice with NALL led to upregulation of parkin in nigral dopaminergic neurons and improvements in their motor learning performance. Given the known effects of parkin22, these findings suggest that NALL-mediated parkin upregulation may help restore synaptic vesicle dynamics29,30 and DAT function in LRRK2 mutant neurons31,32.
The LRRK2R1441C knock in mice23,33,34 serve as a valuable in vivo model for studying the early stages of PD as they present dopamine-related phenotypes but not overt dopaminergic cell loss. Changes in the dopamine-dependent striatal motor learning restored with NALL likely reflect dysfunctions in dopamine-associated circuits present in asymptomatic LRRK2 mutation carriers35,36. A recent work focusing on a progressive PD model provides additional evidence linking dopamine-dependent motor learning deficits with early (synaptic) dopamine neuron dysfunctions during PD progression37. This study found that decreased dopamine release from dopaminergic axons results in striatal motor learning deficits in the absence of PD motor impairments, which are only observed upon a significant loss of dopamine neurons. These findings further support the potential neuroprotective effects of NALL that have been reported in the clinical setting18. In addition, we found a reduction of pS129-syn in human dopaminergic neurons carrying LRRK2 mutation and in LRRK2 mutant mice, further supporting the role of NALL in enhancing α-Syn clearance mechanisms.
Our proteomic results indicate that NALL treatment of human dopaminergic neurons primarily increased levels of mitochondrial, synaptic and lysosomal proteins. While there could be many reasons underlying this observation, it is also possible that NALL-mediated elevation of parkin contributed to these proteomic changes in treated neurons. It has been well-established that parkin plays an important role in the mitochondrial quality control pathway38 39–41, as well as in lysosomal function by regulating direct contacts and transfer of amino acids between lysosomes and mitochondria42. It is also possible that high levels of intracellular leucine affect mTOR function43 and autophagy44,45 that in turn impact mitochondrial and lysosomal function. Further mechanistic studies will be required to address these questions.
Together, our findings support NALL as a promising therapeutic candidate for PD, by targeting α-synuclein clearance, synaptic dysfunction, and dopaminergic deficits.
Methods
Ethics statement
All research complied with regulatory committees for research safety (Safety Protocol ID: BIO20230025). All mouse experiments were performed in compliance with Northwestern University Animal Care and Use Committee guidelines.
Cells and animals
Human iPSC GBA1 L444P line was a gift from Thomas Gasser46; GBA1 N370S line and LRRK2 R1441C line were from the Northwestern University Biorepository. 3 month old C57BL/6 (Jax 000664, RRID:IMSR_JAX:000664) and homozygous LRRK2 R1441C knock-in mice ( RRID:IMSR_JAX:009346) were group-housed on a standard 12/12 hr light/dark cycle with standard feeding. Littermates were randomly assigned to the experimental procedures. Both males and females were used.
iPSC culture and neuronal differentiation:
Human iPS cells were cultured and maintained as described previously47. Directed differentiation toward dopaminergic neurons was conducted as described previously47–50. Briefly, undifferentiated iPS cells were plated onto Cultrex-coated (BD Bioscience) 6-well plates in mTeSR media (Stemcell technologies). Differentiation was started by adding knock-out serum replacement medium (Invitrogen) containing Noggin (R&D Systems) and SB431542 (Tocris Bioscience). To help control neuralization variability, cells were passaged by chunking manually (en bloc: size of 1–2mm) and plated onto 10 cm dishes precoated with poly-D-lysine (Sigma) and laminin (Roche) on day 13. Differentiation into dopaminergic neurons was conducted by adding Neuralization growth factors. All the experiments were done between day 80 to day 150. For N-Acetyl-l-Leucine (NALL) (Sigma, 441511) treatment, NALL was dissolved in DMSO at 1M for stock and diluted into cell culture media to get final concentrations as indicated in the figures. Neurons were treated with different concentrations of NALL twice a week for indicated days.
Mouse motor learning
NALL was dissolved in ethanol to prepare 50 mg/ml solution, which was then diluted in water to get 10 mg/ml. 0.25 ml of NALL solution (10 mg/ml) was orally administered in mice at 100 mg/kg/day dose (2.5 mg NALL/25g mouse)51. Motor learning was assessed using an accelerating rotarod with 8–9 weeks old LRRK2 R1441C mice. The rotarod apparatus (Panlab) is equipped with a mouse rod (3 cm diameter) and set to 4–40 rpm acceleration over 300 s. The task consisted of eighteen daily sessions (five trials per session; intertrial-interval = 15 s, max trial duration = 300 s) divided into two phases*2. In the first phase—session 1–5— mice were administered either NALL or vehicles via oral gavage 30 min before an intraperitoneal (i.p.) injection of a cocktail of D1 receptor (SCH23390, Sigma D054) and D2 receptor (eticlopride, Sigma E101). Each antagonist was administered a dose of 1 mg/kg for 30 minutes. After a 72-hour break, the mice were tested over thirteen sessions without dopamine antagonists. Instead, they received either NALL or vehicle via oral gavage 60 minutes before the rotarod test.
HTRA1 lentivirus
Lentiviral shRNA constructs (MISSION pLKO.1-puro) for non-target control and HTRA1 were obtained from Millipore Sigma. For lentiviral packaging, HEK293FT cells were transfected with lentiviral expression constructs together with psPAX2 (Addgene, 12260) and pLP3 (Invitrogen) using X-tremeGENE HP DNA Transfection Reagent (Roche). A media change was performed the day after transfection. 48 h after transfection, virus-containing supernatant was collected and cleared by centrifuge at 500 x g for 10 minutes. Virus particles were concentrated using Lenti-X Concentrator (Takara Bio, 631232) following manufacturer’s instructions. The concentration of virus particles was determined by ELISA using RETRO-TEK HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix, 0801111).
Western blot analysis
Cells were scraped in cold PBS and centrifuged at 300 x g for 5 min. Pellets were resuspended in Triton buffer (containing 1% Triton X-100) with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher) and incubated on ice for 30 min followed by homogenized for 2 minutes. Lysates were cleared by centrifugation at 100,000 × g for 30 minutes. Protein concentration of the supernatants, as Triton soluble fraction, was measured using the Bicinchoninic Acid assay (Sigma). Pellets were resolved in 2% SDS buffer, followed by sonication and centrifugation at 150,000 × g for 30 minutes. The supernatant was Triton-insoluble fraction. Both Triton-soluble and insoluble fractions were denatured by heating in 4X Laemmli sample buffer (BioRad). Equal amounts of protein were loaded in 4–20% Tris-glycine gels. Proteins were transferred onto Nitrocellulose membrane using the Transblot Turbo transfer system (BioRad). Membranes were blocked with 5% milk in TBS-T (50 mM tris, pH 7.4, 150 mM NaCl, 0.1% Tween20) and incubated with primary antibody overnight at 4°C. After washing, membranes were incubated with anti-rabbit or anti-mouse secondary antibody for 1 h at room temperature. Chemiluminescence was assessed using pico or femto chemiluminescence substrates (Thermo Fisher). ChemiDoc XRS + imaging station with a 16-bit CCD camera was used for imaging. Quantification was done using ImageJ software (NIH). Antibodies used for immunoblotting include anti-pS129-syn antibody (Cell Signaling), anti-SYP antibody (Millipore), anti-a-syn antibody (Santa Cruz), anti-β-III-Tubulin antibody (BioLegend), anti-synaptojanin-1 (SYNJ1) antibody (LsBio), anti-parkin antibody (Santa Cruz), anti-HTRA1 (R& D Systems), anti-DAT antibody (Santa Cruz), and anti-GAPDH antibody (Millipore).
Synaptic membrane enrichment assay
Cells grown in 6 well plates were washed with cold PBS and scrapped in Syn-PER™ Synaptic Protein Extraction Reagent (Syn-PER) (Thermo Fisher Scientific) followed by centrifuging at 1200 xg for 10 min at 4°C. Supernatants were collected as homogenates (Total) and further centrifuged at 15,000 xg for 20 min at 4°C. The supernatants from the second spin contained cytosolic proteins (Cyto). The pellets were re-solubilized in Syn-PER and centrifuge at 70,000 × g for 45 min at 4°C. The supernatants from this spin were added into the Cyto fraction, and the pellets were washed with Syn-PER buffer twice and finally resuspended in Syn-PER as the synaptic membrane fraction (SM) which contained SV membrane and synaptic plasma membrane. Equal amounts of protein from each fraction were loaded and detected by western blot using indicated antibodies.
Label-free Quantitative Proteomics:
Sample preparation:
iPSC-derived midbrain dopaminergic neurons treated with 0, 5, or 10 mM of NALL twice a week for 4 weeks. Cells were harvested for label-free quantitative proteomic analysis. Briefly, neurons were harvested in 1x PBS and pelleted at 3000 × g for 5 min at 4°C. RIPA buffer contains complete protease inhibitor cocktail was added to the pellet at a volume of 1 mL of lysis buffer per 250uL cell pellet volume. Samples were homogenized using a tip sonicator 3 × 30 sec with rests of 1 min in between. Lysates were centrifuged at 10,000 × g for 10 min at 4°C. Supernatant was collected for protein quantification using the BCA assay. Protein pellets were resuspended in 8 M urea (Thermo Fisher Scientific, Cat# 29700) prepared in 100 mM ammonium bicarbonate solution (Fluka, Cat# 09830). Dithiothreitol (DTT, DOT Scientific Inc, Cat# DSD11000) was applied to a final concentration of 5 mM. After incubation at RT for 20 mins, iodoacetamide (IAA, Sigma-Aldrich, Cat# I1149) was added to a final concentration of 15 mM and incubated for 20 mins at RT in the dark. Excess IAA was quenched with DTT for 15 mins. Samples were diluted with 100 mM ammonium bicarbonate solution, and digested for three hrs with Lys-C protease (1:100, Thermo Fisher Scientific, Cat# 90307_3668048707) at 37°C. Trypsin (1:100, Promega, Cat# V5280) was then added for overnight incubation at 37°C with intensive agitation (1000 rpm). The next day, reaction was quenched by adding 1% trifluoroacetic acid (TFA, Fisher Scientific, Cat# O4902–100). The samples were desalted using Peptide Desalting Spin Columns (Thermo Fisher Scientific, Cat# 89852). All samples were vacuum centrifuged to dry.
Tandem Mass Tag (TMT) labeling:
Our protocol was based on previously reported methods52–54. C18 column-desalted peptides were resuspended with 100 mM HEPES pH 8.5 and the concentrations were measured by micro BCA kit (Fisher Scientific, Cat# PI23235). For each sample, 100 μg of peptide labeled with TMT reagent (0.4 mg, dissolved in 40 μl anhydrous acetonitrile, Thermo Fisher Scientific, Cat# A44520) and made at a final concentration of 30% (v/v) acetonitrile (ACN). Following incubation at RT for 2 hrs with agitation, hydroxylamine (to a final concentration of 0.3% (v/v)) was added to quench the reaction for 15 min. TMT-tagged samples were mixed at a 1:1:1:1:1:1:1:1:1 ratio. Combined sample was vacuum centrifuged to dryness, resuspended, and subjected to Peptide Desalting Spin Columns (Thermo Fisher Scientific, Cat# 89852).
Peptide fractionation
We used a high pH reverse-phase peptide fractionation kit (Thermo Fisher Scientific, Cat# 84868) to get eight fractions (5.0%, 10.0%, 12.5%, 15.0%, 17.5%, 20.0%, 22.5%, 25.0% and 50% of ACN in 0.1% triethylamine solution). The high pH peptide fractions were directly loaded into the autosampler for MS analysis without further desalting.
Tandem Mass spectrometry.
Two micrograms of each fraction or sample were auto-sampler loaded with a Thermo Vanquish Neo UHPLC system onto a PepMap Neo Trap Cartridge (Thermo Fisher Scientific, 174500; diameter, 300 μm; length, 5 mm; particle size, 5 μm; pore size, 100 Å; stationary phase, C18) coupled to a nanoViper analytical column (Thermo Fisher Scientific, 164570; diameter, 0.075 mm; length, 500 mm; particle size, 3 μm; pore size, 100 Å; stationary phase, C18) with a stainless-steel emitter tip assembled on the Nanospray Flex Ion Source with a spray voltage of 2,000 V. An Orbitrap Ascend (Thermo Fisher Scientific) was used to acquire all the MS spectral data. Buffer A contained 99.9% H2O and 0.1% formic acid, and buffer B contained 80.0% acetonitrile, 19.9% H2O with 0.1% formic acid. For each fraction, the chromatographic run was for 4 hours in total with the following profile: 0–7% for 7, 10% for 6, 25% for 160, 33% for 40, 50% for 7, 95% for 5 and again 95% for 15 mins receptively.
We used a multiNotch MS3-based TMT method to analyze all the TMT samples55–57. The scan sequence began with an MS1 spectrum (Orbitrap analysis, resolution 120,000, 400–1400 Th, AGC target 2×105, maximum injection time 200 ms). MS2 analysis, ‘Top speed’ (2 s), Collision-induced dissociation (CID, quadrupole ion trap analysis, AGC 4×103, NCE 35, maximum injection time 150 ms). MS3 analysis, top ten precursors, fragmented by HCD prior to Orbitrap analysis (NCE 55, max AGC 5×104, maximum injection time 250 ms, isolation specificity 0.5 Th, resolution 60,000).
MS data analysis and quantification:
Protein identification/quantification and analysis were performed with Integrated Proteomics Pipeline - IP2 (Bruker, Madison, WI. http://www.integratedproteomics.com) using ProLuCID58,59, DTASelect260,61, Census and Quantitative Analysis (For TMT-MS experiments). Spectrum raw files were extracted into MS1, MS2 and MS3 (For TMT experiments) files using RawConverter (http://fields.scripps.edu/downloads.php). The tandem mass spectra were searched against UniProt human (downloaded on 01–01-2014) protein databases62 and matched to sequences using the ProLuCID/SEQUEST algorithm (ProLuCID version 3.1) with 5 ppm peptide mass tolerance for precursor ions and 600 ppm for fragment ions. The search space included all fully and half-tryptic peptide candidates within the mass tolerance window with no-miscleavage constraint, assembled, and filtered with DTASelect2 through IP2. To estimate peptide probabilities and false-discovery rates (FDR) accurately, we used a target/decoy database containing the reversed sequences of all the proteins appended to the target database62. Each protein identified was required to have a minimum of one peptide of minimal length of six amino acid residues; however, this peptide had to be an excellent match with an FDR < 1% and at least one excellent peptide match. After the peptide/spectrum matches were filtered, we estimated that the peptide FDRs were ≤ 1% for each sample analysis. Resulting protein lists include subset proteins to allow for consideration of all possible protein forms implicated by at least two given peptides identified from the complex protein mixtures. Then, we used Census and Quantitative Analysis in IP2 for protein quantification of TMT-MS experiments and protein quantification was determined by summing all TMT report ion counts. For TMT experiments: static modification: 57.02146C for carbamidomethylation, 304.2071 for 16-plex TMT tagging; differential modifications: 304.2071 for N-terminal 16-plex TMT tagging, 42.0106 for N-terminal Acetylation. Resulting protein lists include subset proteins to allow for consideration of all possible protein isoforms implicated by at least two given peptides identified from the complex protein mixtures. Each possible protein isoforms was quantified individually63.
Spyder (MIT, Python 3.7, libraries, ‘pandas’, ‘numpy’, ‘scipy’, ‘statsmodels’ and ‘bioinfokit’) was used for data statistical analyses. RStudio (version, 2024.09.1 Build 394, packages, ‘tidyverse’, ‘pheatmap’) was used for data virtualization. The Database for Annotation, Visualization and Integrated Discovery (DAVID) was used for protein functional annotation analysis
Statistical analysis
Statistical calculations were performed using GraphPad Prism 9 software. Datasets with two samples were analyzed using a two-tailed t-test. Datasets with more than two samples were analyzed using one-way ANOVA followed by Tukey’s post-hoc test.
Acknowledgments:
We thank Robert William Coukos for assisting with the analysis of the proteomic data.
Funding:
This work was supported by National Institutes of Health grants R37 NS096241 and R35 NS122257 (to D.K.) and S10 OD032464 (to J.S.)
Funding Statement
This work was supported by National Institutes of Health grants R37 NS096241 and R35 NS122257 (to D.K.) and S10 OD032464 (to J.S.)
Footnotes
Competing interests: D.K. is the Founder and Scientific Advisory Board Chair of Vanqua Bio; serves on the scientific advisory boards of The Silverstein Foundation, AcureX, and is a Venture Partner at OrbiMed. Other authors declare no other competing financial interests.
Additional Declarations: There is NO Competing Interest.
Supplementary Files
Contributor Information
Pingping Song, Northwestern University Feinberg School of Medicine.
Rossella Franchini, Department of Neuroscience, Biomedicine and Movement Sciences, University of Verona.
Chuyu Chen, Department of Pharmacology, Feinberg School of Medicine, Northwestern University.
Bryan Duong, Northwestern University Feinberg School of Medicine.
Yi-Zhi Wang, Northwestern University.
Jeffrey Savas, Department of Neurology, Feinberg School of Medicine, Northwestern University, Chicago, IL.
Loukia Parisiadou, Northwestern University.
Dimitri Krainc, Department of Neurology Northwestern University Feinberg School of Medicine.
Data availability
The raw mass spectrometry data presented in this study was deposited in Mass Spectrometry Interactive Virtual Environment (MassIVE) and ProteomeXchange upon acceptance.
References
- 1.Klein C, Krainc D (2012) Movement disorders in 2011: Translating new research findings into clinical practice. Nat reviews Neurol 8:65–66. 10.1038/nrneurol.2011.212 [DOI] [PubMed] [Google Scholar]
- 2.Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. 10.1038/nrn.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D (2019) Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson’s Disease. Trends Neurosci 42:140–149. 10.1016/j.tins.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Periquet M, Wang X, Negro A, McLean PJ, Hyman BT, Feany MB (2009) Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest 119:3257–3265. 10.1172/JCI39088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. 10.1038/ncb748 [DOI] [PubMed] [Google Scholar]
- 7.Smith WW, Margolis RL, Li X, Troncoso JC, Lee MK, Dawson VL, Dawson TM, Iwatsubo T, Ross CA (2005) Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci 25:5544–5552. 10.1523/JNEUR0SCI.0482-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers JT, Mikkilineni S, Cantuti-Castelvetri I, Smith DH, Huang X, Bandyopadhyay S, Cahill CM, Maccecchini ML, Lahiri DK, Greig NH (2011) The alpha-synuclein 5’untranslated region targeted translation blockers: anti-alpha synuclein efficacy of cardiac glycosides and Posiphen. J Neural Transm (Vienna) 118:493–507. 10.1007/s00702-010-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen S, West AB (2009) The therapeutic potential of LRRK2 and alpha-synuclein in Parkinson’s disease. Antioxid Redox Signal 11:2167–2187. 10.1089/ars.2009.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, Eklund AC, Ney PA, Ng J, McGoldrick M, Mollenhauer B et al. (2008) GATA transcription factors directly regulate the Parkinson’s disease-linked gene alpha-synuclein. Proc Natl Acad Sci U S A 105:10907–10912. 10.1073/pnas.0802437105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bremova-Ertl T, Ramaswami U, Brands M, Foltan T, Gautschi M, Gissen P, Gowing F, Hahn A, Jones S, Kay R et al. (2024) Trial of N-Acetyl-l-Leucine in Niemann-Pick Disease Type C. N Engl J Med 390:421–431. 10.1056/NEJMoa2310151 [DOI] [PubMed] [Google Scholar]
- 12.Bremova-Ertl T, Claassen J, Foltan T, Gascon-Bayarri J, Gissen P, Hahn A, Hassan A, Hennig A, Jones SA, Kolnikova M et al. (2022) Efficacy and safety of N-acetyl-L-leucine in Niemann-Pick disease type C. J Neurol 269:1651–1662. 10.1007/s00415-021-10717-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaya E, Smith DA, Smith C, Morris L, Bremova-Ertl T, Cortina-Borja M, Fineran P, Morten KJ, Poulton J, Boland B et al. (2021) Acetyl-leucine slows disease progression in lysosomal storage disorders. Brain Commun 3:fcaa148. 10.1093/braincomms/fcaa148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strupp M, Teufel J, Habs M, Feuerecker R, Muth C, van de Warrenburg BP, Klopstock T, Feil K (2013) Effects of acetyl-DL-leucine in patients with cerebellar ataxia: a case series. J Neurol 260:2556–2561. 10.1007/s00415-013-7016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schniepp R, Strupp M, Wuehr M, Jahn K, Dieterich M, Brandt T, Feil K (2016) Acetyl-DL-leucine improves gait variability in patients with cerebellar ataxia-a case series. Cerebellum Ataxias 3:8. 10.1186/s40673-016-0046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martakis K, Giorgi M, Spanou M, Neubauer BA, Dinopoulos A, Hahn A (2025) N-Acetyl-leucine in progressive CACNA1A ataxia: A case series. Eur J Paediatr Neurol 54:64–67. 10.1016/j.ejpn.2024.12.006 [DOI] [PubMed] [Google Scholar]
- 17.Fields T, Schoser B, Oertel W, Strupp M (2021) Acetyl-DL-leucine improves restless legs syndrome: a case report. J Neurol 268:2595–2596. 10.1007/s00415-021-10625-3 [DOI] [PubMed] [Google Scholar]
- 18.Oertel WH, Janzen A, Henrich MT, Geibl FF, Sittig E, Meles SK, Carli G, Leenders K, Booij J, Surmeier DJ et al. (2024) Acetyl-DL-leucine in two individuals with REM sleep behavior disorder improves symptoms, reverses loss of striatal dopamine-transporter binding and stabilizes pathological metabolic brain pattern-case reports. Nat Commun 15:7619. 10.1038/s41467-024-51502-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Puri A, Bell B, Fritsche J, Palacios HH, Balch M, Sprunger ML, Howard MK, Ryan JJ, Haines JN et al. (2024) HTRA1 disaggregates alpha-synuclein amyloid fibrils and converts them into non-toxic and seeding incompetent species. Nat Commun 15:2436. 10.1038/s41467-024-46538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME (2004) The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem 279:21012–21020. 10.1074/jbc.M311972200 [DOI] [PubMed] [Google Scholar]
- 21.Jiang H, Jiang Q, Feng J (2004) Parkin increases dopamine uptake by enhancing the cell surface expression of dopamine transporter. J Biol Chem 279:54380–54386. 10.1074/jbc.M409282200 [DOI] [PubMed] [Google Scholar]
- 22.Song P, Peng W, Sauve V, Fakih R, Xie Z, Ysselstein D, Krainc T, Wong YC, Mencacci NE, Savas JN et al. (2023) Parkinson’s disease-linked parkin mutation disrupts recycling of synaptic vesicles in human dopaminergic neurons. Neuron 111:3775–3788e3777. 10.1016/j.neuron.2023.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xenias HS, Chen C, Kang S, Cherian S, Situ X, Shanmugasundaram B, Liu G, Scesa G, Chan CS, Parisiadou L (2022) R1441C and G2019S LRRK2 knockin mice have distinct striatal molecular, physiological, and behavioral alterations. Commun Biol 5. 10.1038/s42003-022-04136-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beeler JA, Frank MJ, McDaid J, Alexander E, Turkson S, Bernardez Sarria MS, McGehee DS, Zhuang X (2012) A role for dopamine-mediated learning in the pathophysiology and treatment of Parkinson’s disease. Cell Rep 2:1747–1761. 10.1016/j.celrep.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang X, Mazzoni P, Kang UJ (2013) The role of neuroplasticity in dopaminergic therapy for Parkinson disease. Nat Rev Neurol 9:248–256. 10.1038/nrneurol.2013.57 [DOI] [PubMed] [Google Scholar]
- 26.Poepsel S, Sprengel A, Sacca B, Kaschani F, Kaiser M, Gatsogiannis C, Raunser S, Clausen T, Ehrmann M (2015) Determinants of amyloid fibril degradation by the PDZ protease HTRA1. Nat Chem Biol 11:862–869. 10.1038/nchembio.1931 [DOI] [PubMed] [Google Scholar]
- 27.Tennstaedt A, Popsel S, Truebestein L, Hauske P, Brockmann A, Schmidt N, Irle I, Sacca B, Niemeyer CM, Brandt R et al. (2012) Human high temperature requirement serine protease A1 (HTRA1) degrades tau protein aggregates. J Biol Chem 287:20931–20941. 10.1074/jbc.M111.316232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K, Yan Z, Feng J (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 3, 668. 10.1038/ncomms1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen M, Krainc D (2018) LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proc Natl Acad Sci U S A 115:5576–5581. 10.1073/pnas.1717590115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan PY, Li X, Wang J, Powell J, Wang Q, Zhang Y, Chen Z, Wicinski B, Hof P, Ryan TA, Yue Z (2017) Parkinson’s Disease-Associated LRRK2 Hyperactive Kinase Mutant Disrupts Synaptic Vesicle Trafficking in Ventral Midbrain Neurons. J Neurosci 37:11366–11376. 10.1523/JNEUR0SCI.0964-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longo F, Mercatelli D, Novello S, Arcuri L, Brugnoli A, Vincenzi F, Russo I, Berti G, Mabrouk OS, Kennedy RT et al. (2017) Age-dependent dopamine transporter dysfunction and Serine129 phospho-alpha-synuclein overload in G2019S LRRK2 mice. Acta Neuropathol Commun 5. 10.1186/s40478-017-0426-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volta M, Beccano-Kelly DA, Paschall SA, Cataldi S, Maclsaac SE, Kuhlmann N, Kadgien CA, Tatarnikov I, Fox J, Khinda J et al. (2017) Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. Elife 6. 10.7554/eLife.28377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C, Soto G, Dumrongprechachan V, Bannon N, Kang S, Kozorovitskiy Y, Parisiadou L (2020) Pathway-specific dysregulation of striatal excitatory synapses by LRRK2 mutations. Elife 9. 10.7554/eLife.58997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J (2009) R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A 106:14622–14627. 10.1073/pnas.0906334106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandhagopal R, Mak E, Schulzer M, McKenzie J, McCormick S, Sossi V, Ruth TJ, Strongosky A, Farrer MJ, Wszolek ZK, Stoessl AJ (2008) Progression of dopaminergic dysfunction in a LRRK2 kindred: a multitracer PET study. Neurology 71:1790–1795. 10.1212/01.wnl.0000335973.66333.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simuni T, Uribe L, Cho HR, Caspell-Garcia C, Coffey CS, Siderowf A, Trojanowski JQ, Shaw LM, Seibyl J, Singleton A et al. (2020) Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson’s Progression Markers Initiative (PPMI): a cross-sectional study. Lancet Neurol 19:71–80. 10.1016/S1474-4422(19)30319-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornejo-Olivas M, Wu L, Noyce A (2022) Disruption of Mitochondrial Complex I Induces Progressive Parkinsonism. Mov Disord 37:478. 10.1002/mds.28961 [DOI] [PubMed] [Google Scholar]
- 38.Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Song P, Du L, Tian W, Yue W, Liu M, Li D, Wang B, Zhu Y, Cao C et al. (2011) Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem 286:11649–11658. 10.1074/jbc.M110.144238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM (2011) PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell 144:689–702. 10.1016/j.cell.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147:893–906. 10.1016/j.cell.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng W, Schroder LF, Song P, Wong YC, Krainc D (2023) Parkin regulates amino acid homeostasis at mitochondria-lysosome (M/L) contact sites in Parkinson’s disease. Sci Adv 9:eadh3347. 10.1126/sciadv.adh3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH (2007) Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 56:1647–1654. 10.2337/db07-0123 [DOI] [PubMed] [Google Scholar]
- 44.Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721. 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto A, Cremona ML, Rothman JE (2006) Autophagy-mediated clearance of huntingtin aggregates triggered by the insulin-signaling pathway. J Cell Biol 172:719–731. 10.1083/jcb.200510065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK et al. (2014) iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5:4028. 10.1038/ncomms5028 [DOI] [PubMed] [Google Scholar]
- 47.Song P, Trajkovic K, Tsunemi T, Krainc D (2016) Parkin Modulates Endosomal Organization and Function of the Endo-Lysosomal Pathway. J Neurosci 36:2425–2437. 10.1523/JNEUROSCI.2569-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A et al. (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480:547–551. 10.1038/nature10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burbulla LF, Song PP, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C et al. (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357:1255-. 10.1126/science.aam9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song P, Krainc D (2024) Protocol to investigate Parkinson’s patient-derived dopaminergic neurons by live-cell microscopy and oxidized dopamine assays. STAR Protoc 5:102889. 10.1016/j.xpro.2024.102889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegdekar N, Lipinski MM, Sarkar C (2021) N-Acetyl-L-leucine improves functional recovery and attenuates cortical cell death and neuroinflammation after traumatic brain injury in mice. Sci Rep 11:9249. 10.1038/s41598-021-88693-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YZ, Castillon CCM, Gebis KK, Bartom ET, d’Azzo A, Contractor A, Savas JN (2024) Notch receptor-ligand binding facilitates extracellular vesicle-mediated neuron-to-neuron communication. Cell Rep 43:113680. 10.1016/j.celrep.2024.113680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang YZ, Perez-Rosello T, Smukowski SN, Surmeier DJ, Savas JN (2024) Neuron type-specific proteomics reveals distinct Shank3 proteoforms in iSPNs and dSPNs lead to striatal synaptopathy in Shank3B(−/−) mice. Mol Psychiatry 29:2372–2388. 10.1038/s41380-024-02493-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weekes MP, Tomasec P, Huttlin EL, Fielding CA, Nusinow D, Stanton RJ, Wang ECY, Aicheler R, Murrell I, Wilkinson GWG et al. (2014) Quantitative temporal viromics: an approach to investigate host-pathogen interaction. Cell 157:1460–1472. 10.1016/j.cell.2014.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weekes MP, Tomasec P, Huttlin EL, Fielding CA, Nusinow D, Stanton RJ, Wang EC, Aicheler R, Murrell I, Wilkinson GW et al. (2014) Quantitative temporal viromics: an approach to investigate host-pathogen interaction. Cell 157:1460–1472. 10.1016/j.cell.2014.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McAlister GC, Nusinow DP, Jedrychowski MP, Wuhr M, Huttlin EL, Erickson BK, Rad R, Haas W, Gygi SP (2014) MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem 86:7150–7158. 10.1021/ac502040v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ting L, Rad R, Gygi SP, Haas W (2011) MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 8:937–940. 10.1038/nmeth.1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eng JK, McCormack AL, Yates JR (1994) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5:976–989 10.1016/1044-0305(94)80016-2 [DOI] [PubMed] [Google Scholar]
- 59.Xu T, Park SK, Venable JD, Wohlschlegel JA, Diedrich JK, Cociorva D, Lu B, Liao L, Hewel J, Han X et al. (2015) ProLuCID: An improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J Proteom 129:16–24. 10.1016/j.jprot.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cociorva D, D L.T., and, Yates JR (2007) Validation of tandem mass spectrometry database search results using DTASelect. Curr Protoc Bioinformatics Chap. 13, Unit 13 14. 10.1002/0471250953.bi1304s16 [DOI] [PubMed] [Google Scholar]
- 61.Tabb DL, McDonald WH, Yates JR 3rd (2002) DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.UniProt C (2015) UniProt: a hub for protein information. Nucleic Acids Res 43:D204–212. 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raj B, Blencowe BJ (2015) Alternative Splicing in the Mammalian Nervous System: Recent Insights into Mechanisms and Functional Roles. Neuron 87:14–27. 10.1016/j.neuron.2015.05.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw mass spectrometry data presented in this study was deposited in Mass Spectrometry Interactive Virtual Environment (MassIVE) and ProteomeXchange upon acceptance.