Abstract
The seasonal influenza vaccine contains strains of viruses from distinct subtypes that are grown independently and then combined. However, most individuals exhibit a more robust response to one of these strains and thus are vulnerable to infection by others. By studying a monozygotic twin cohort, we found that although prior exposure is a factor, host genetics are a stronger driver of subtype-bias to influenza viral strains. We found that covalent coupling of heterologous hemagglutinin (HA) from different viral strains could largely eliminate subtype bias in an animal model and in a human tonsil organoid system. We proposed that coupling of heterologous antigens improves antibody responses across influenza strains by broadening T cell help, and we found that using this approach substantially improved the antibody response to avian influenza HA.
Introduction
Influenza virus infection causes an estimated 290,000 to 650,000 deaths and millions of hospitalizations worldwide each year (1). Despite the large number of known influenza subtypes, human infections are primarily restricted to influenza A (H1N1 and H3N2 subtypes) and B viruses (Victoria and Yamagata lineages) (2). Each influenza subtype includes several viral strains. A seasonal vaccine is formulated each year with the strains of each type that are predicted to be most prevalent in circulation. A mismatch between the circulating and vaccine strain(s) can be devastating (3), but even with a precise match, the vaccine affords limited efficacy (4). Overall influenza vaccine effectiveness over the past decade has varied from 19% [95% confidence interval (CI), 10 to 27] to 60% [95% CI, 53 to 66] (5). This has led to calls for the development of a more effective vaccine (5).
A large fraction of vaccinated individuals elicit a higher response to one strain in the vaccine formulation, and a major challenge is to understand the factors contributing to this. An explanation for this subtype-bias was proposed by Thomas Francis – which he termed “original antigenic sin” (OAS) – to describe how the first influenza infection a person is exposed to could skew their immune system towards that particular strain (6). OAS refers to the preferential induction of antibodies with higher affinity to the priming immunogen resulting from prior exposures as opposed to the boosting immunogens present in the seasonal vaccine formulation. Thus, memory from past exposures can divert and limit the response to influenza strains in circulation (7, 8). By contrast, other studies have suggested that memory from past exposures can cross-react and boost the response to seasonal vaccination (9–11). This sequential recruitment of memory B cells upon exposure to drifted antigens is essential for the development of cross-reactive antibodies (2, 12). However, such antibodies are typically rare and constitute a limited fraction of the seasonal vaccine response necessitating annual changes in the formulation (2, 12). Pre-existing antibodies in circulation from past exposures can also bind vaccine antigens and limit the response by blocking access to vaccine-specific B cells. Although OAS may influence part of the response, the magnitude of its impact on the response to drifted immunogens in humans is not clear (13).
Previous studies have also investigated the relationship between a vaccine response and polymorphism of genes with immunological functions (14). Of these, the cell surface major histocompatibility complex (MHC) molecules encoded by the polygenic and highly polymorphic human leukocyte antigen (HLA) gene locus are an important regulator of adaptive immunity (14). Vaccine antigens are processed into peptide fragments by B cells and presented on MHC molecules, which in turn are recognized by helper T cells. There is wide variation in the peptides presented by distinct MHC molecules because of variations in their peptide-binding grooves. The relative contributions of previous heterologous exposures and host genetics are poorly understood.
Results
Seasonal influenza vaccination induces a heterogeneous response
A major component of vaccine mediated protection is the generation of neutralizing antibodies against the viral surface glycoprotein, hemagglutinin (HA), blocking viral entry into cells (2, 4). This is commonly measured by using the hemagglutination inhibition (HAI) assay (15). The seasonal influenza vaccine includes multiple strains selected from the H1, H3 and B subtypes [B(vi) and B(ya) indicate Victoria and Yamagata lineages]. To determine the variation in response to vaccination, we measured the HAI response in a cohort of healthy, vaccinated individuals. Baseline HAI titer, age, and sex only partly explain the post-vaccination HAI because the subtype-specific post-vaccination HAI titer varied widely across individuals despite having identical baseline HAI titer (Figs. S1 and S2). We visualized this heterogeneity in the response across individuals using uniform manifold approximation and projection (UMAP) used for dimensionality reduction with baseline and post-vaccination HAI titers determined for each vaccine strain as input (Fig. 1A). A large fraction (65.9%) of vaccinated individuals could be assigned to subtype-specific bias groups (Fig. 1A). The vaccine induced response in the bias groups, both in terms of the post-vaccination HAI titer and fold-change, was higher to an individual subtype in the formulation (Fig. 1B). We did not observe any age or sex association with subtype-bias (Figs. S3A–B). Individuals in the high responder group displayed high post-vaccination HAI titers and fold-change against all influenza subtypes in the vaccine formulation (Fig. S4A). We also measured full-length HA-specific antibody responses with ELISA to evaluate differences in total antigen-specific titers because HAI assay mainly measures antibodies targeting the receptor binding site (13). Consistent with HAI measurements, we observed skewing in the HA-specific antibody titers in individuals assigned to subtype-bias groups (Fig. S5).
Figure 1: Host genetics contributes to influenza subtype-bias after vaccination.
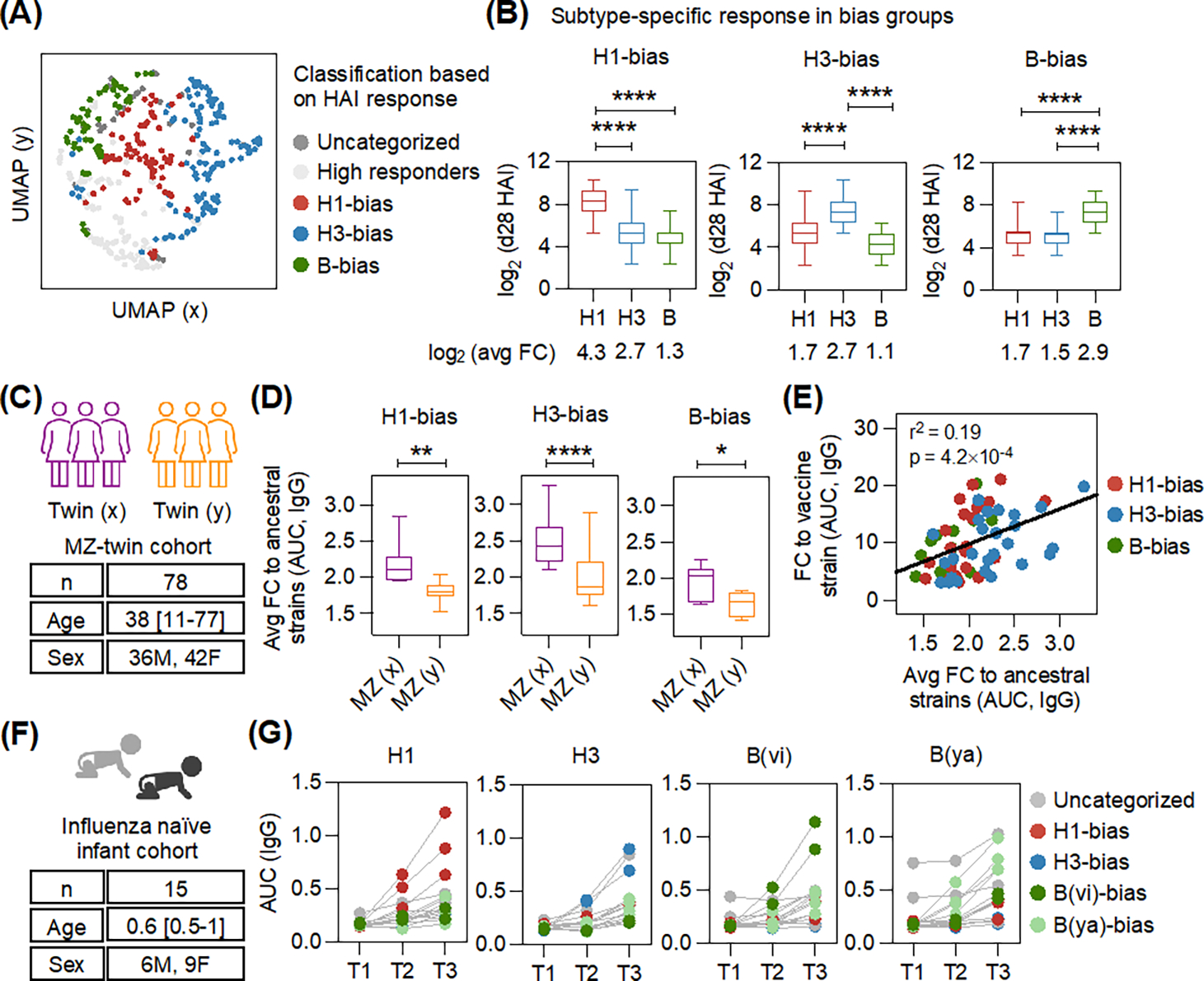
(A) The uniform manifold approximation and projection (UMAP) shows heterogeneity in the immune response measured using hemagglutination inhibition (HAI) in a cohort of vaccinated individuals (n = 402). A large fraction (65.9%) clustered into subtype-specific bias groups. (B) Box plots display the day 28 (d28) post-vaccination HAI titers for each group. The mean fold-change (FC) is also indicated. Adjusted p-values were calculated using one-way ANOVA with Dunnett’s test for pairwise comparisons. (C) Characteristics of the monozygotic (MZ)-twin cohort (n = 78, 39 pairs). The age (mean, range) and sex distribution are indicated. Twins were assigned to groups (x) or (y). (D) Box plots show the average antibody response in twins to ancestral influenza strains in each subtype-bias group. The fold-change (FC), day 28 (d28)/day 0 (d0), in response to vaccination was measured by ELISA against full-length HAs (Fig. S7). P-values were calculated using two-tailed paired t-test. (E) Linear regression between subtype-specific responses (FC, d28/d0) against vaccine and ancestral strains determined by ELISA for twins with subtype-bias after vaccination. (F) Characteristics of the influenza naïve infant cohort. The age (mean, range) and sex distribution are indicated. (G) Response to full-length HAs in infants was determined by ELISA, with serum samples collected before influenza antigen exposure (T1) and after vaccination (T2 and T3). Each datapoint shows total binding for each individual subject, colored by subtype-bias observed after vaccination. *p < 0.05, **p < 0.01, ****p < 0.0001.
Host genetics contributes to response heterogeneity
Previous studies have suggested that both past exposures and host genetics can influence the response to vaccination (9, 14, 16–18). To compare the relative contributions of these influences, we measured influenza vaccine response in a monozygotic twin cohort (Fig. 1C). We observed that 77% of the twin-pairs had the same subtype-bias (Fig. S6). To assess the contribution of past heterologous exposures on subtype-bias, we measured anti-HA antibody titers at baseline and after vaccination against a panel of 50 ancestral influenza strains representing a sequence divergence of >40 years (Fig. S7A–C). The fold-change in antibody titer to ancestral strains after vaccination estimates the contribution of past exposures to the variation in vaccine strain specific antibody responses. The remaining unexplained variation is likely attributable to host genetics. We observed that the fold-change in antibody titer to ancestral strains after vaccination between monozygotic twins with the same subtype-bias was different (Figs. 1D and S7A–C). This finding suggested that twins could develop the same subtype preference after vaccination despite dissimilar memory responses from past exposures. Furthermore, the response to ancestral strains after vaccination could only partly explain the antibody levels elicited against the seasonal vaccine strains (Fig. 1E), suggesting that the impact of heterologous past exposures on subtype-bias observed after vaccination was a factor, but that shared genetics may be a larger one.
To explore this further, we analyzed the response in a cohort of infants who had never been infected with influenza on the basis of weekly nasal swabs and low pre-vaccination anti-HA antibody titers (Fig. S8), with the primary exposure to influenza antigens likely resulting from the seasonal vaccine (Fig. 1F). In this naïve infant cohort, 73% of the children had a skewed subtype-specific anti-HA antibody response after vaccination (Fig. 1G). This finding indicated that prior influenza exposure was not essential for the development of subtype-bias and that genetics, or other microbial exposures (19, 20) are likely the main drivers of this phenomenon.
Subtype-specific CD4+ T cell help correlates with antibody subtype-bias
MHC-II molecules on B cells present antigen-derived peptides to T follicular helper cells (Tfh), a subset of CD4+ T cells that provide help to B cells and stimulate antibody production (21). Because the peptide binding sites of these MHC-II molecules can be very different, variations in the number of peptides presented by a given MHC-II molecule could be a major factor in the T cell help received by B cells and the subsequent antibody response (22). Thus, we analyzed the number of HA-derived peptides from H1, H3, and B subtypes predicted to be high-affinity binders to 27 HLA class-II alleles (23), using the immune epitope database (IEDB) (24). Overall, we found that MHC-II molecules exhibited a wide, subtype-specific variation in the number of HA peptides predicted to bind with high-affinity (Fig. 2A). Thus, these differences could be a driver of subtype-bias in the antibody response.
Figure 2: Differences in HA peptide presentation by host MHC-II molecules leads to bias in CD4+ T cell help after vaccination.
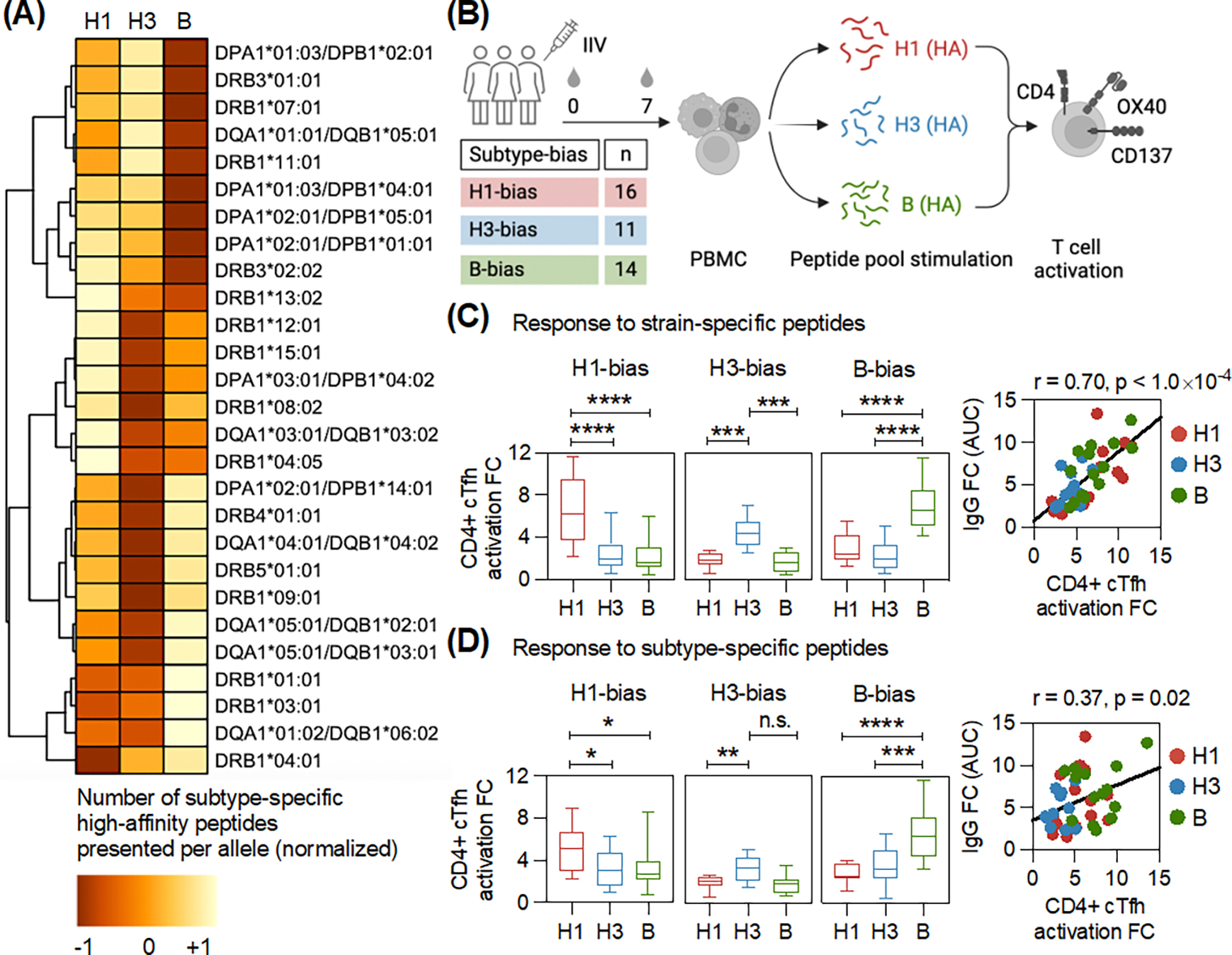
(A) The affinity of HA peptides from strains of distinct influenza subtypes (H1, H3 and B) to 27 HLA class-II alleles was predicted using the immune epitope database (IEDB). The heatmap displays the average number of high-affinity peptides predicted for each influenza subtype per HLA-II allele. Normalization was applied to visualize the data across alleles, which show wide variation in the number of predicted peptides. Using the standard settings of the pheatmap package in R, each row was normalized to have a mean of zero and a standard deviation of one. Values closer to +1 indicate a higher number of predicted peptides. (B) PBMCs collected before (day 0, d0) and after (day 7, d7) vaccination from individuals with antibody subtype-bias were stimulated separately with strain- and subtype-specific HA peptide pools to measure CD4+ T cell activation (gating in Fig. S9). PBMCs were also stimulated with DMSO. Box plots show the fold-change (d7/d0) in CD4+ cTfh activation after stimulation with (C) Strain-specific, and (D) Subtype-specific HA peptides for individuals with distinct subtype-bias after vaccination. Adjusted p-values were calculated using one-way ANOVA with Dunnett’s test for pairwise comparisons. The scatter plot shows the antibody binding titer (d28/d0) and the corresponding CD4+ cTfh activation (d7/d0) against the preferred vaccine strain in response to vaccination in individuals with subtype-bias. Each dot shows the data from one individual. Pearson correlation was calculated. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and not significant (n.s.).
Because these data are dependent on the accuracy of the prediction algorithms, we measured the fold-change in circulating CD4+ Tfh (cTfh) cell activation in response to vaccination in individuals with HA-specific antibody subtype-bias. The peripheral blood mononuclear cells (PBMCs) from these donors were stimulated with HA peptides from strains in the seasonal vaccine formulation (Figs. 2B and S9). The peptides from each antigen (H1, H3, and B HAs) in the formulation were divided into two independent pools: strain-specific and subtype-specific. The subtype-specific pool consisted of HA peptides that are conserved within the given subtype. By contrast, the strain-specific pool included HA peptides that are largely exclusive to the strain in the formulation. The subtype-specific peptide pools enabled us to estimate the effect of memory generated from heterologous influenza exposures on T cell activation and its correlation with antibody responses after vaccination, relative to strain-specific responses. We observed biases in the activation of CD4+ cTfh cells that matched the antibody bias with both strain-specific and subtype-specific peptide pools (Figs. 2C–D). However, the magnitude of difference in CD4+ cTfh activation between influenza subtypes was higher with strain-specific peptides in comparison with the subtype-specific peptides. Furthermore, the T cell response to subtype-specific peptides had a lower correlation with the antibody response in comparison with the strain-specific peptides. This suggested that antibody subtype-specificity in the bias groups may be more affected by the strain-specific CD4+ T cell responses. Individuals with high antibody responses against all vaccine subtypes did not exhibit subtype-specific differences in the CD4+ cTfh cell activation (Figs. S4B–C).
B cells can internalize heterologous antigens after covalent coupling
In current vaccine formulations, antigens from multiple influenza strains are separate, although heterogeneous complexes with varying numbers of HA may occur (25, 26). We hypothesized that B cells expressing cell-surface B cell receptors (BCRs) that are subtype-specific would bind to and primarily internalize their cognate HA in the current vaccines. Consequently, these B cells would present peptides from only one antigen, which could limit CD4+ T cell responses owing to a bias in MHC-II peptide presentation (Fig. S10A). However, covalently coupling heterologous HA antigens from distinct influenza subtypes might allow B cells to internalize the entire complex and recruit help from a much broader array of T cells to induce an antibody response to multiple strains after vaccination (Fig. S10B).
We investigated whether HA from distinct subtypes interact to form aggregates in Fluzone, the inactivated influenza vaccine (IIV) formulation used here. In Fluzone, HA is present as a mixture of isolated trimers and complexes containing 5 to 12 HA trimers (26). We performed sequential binding using a panel of monoclonal antibodies (mAbs) that bind either to the head or stem domains of HA from distinct subtypes. We also used mAbs against non-HA proteins to test whether other viral proteins interact with HA. With sequential binding, if intermolecular interactions were prevalent, we would expect similar signals for subtype-specific mAbs binding to IIV, whether measured directly or after capturing IIV using a mAb specific to another subtype. Non-competing antibody pairs binding the same antigen (head versus stem) were used to optimize the assay. The signal from direct binding of CR8033 (α-B HA head mAb) to IIV was comparable to the signal from sequential binding, where IIV was captured by 5A7 (an α-B HA stem mAb) (Fig. S11A). Similar results were observed for CR8020 (α-H3 HA stem mAb) binding to IIV (Fig. S11B). However, we did not detect any robust interaction between HA of distinct subtypes (Figs. S11A–B). Furthermore, the signal of antibody binding to non-HA proteins was much lower in comparison with HA (Figs. S11A–B). This is consistent with non-HA proteins being present at much lower amounts in inactivated vaccines. We also did not detect any interaction between HA and non-HA viral proteins. These data indicate that heterotypic aggregates in the vaccine is limited. It is unlikely that the composition of these aggregates containing 5 to 12 HA trimers are composed evenly of HA from all influenza subtypes.
To generate a defined coupled heterologous antigen with similar amounts of distinct influenza subtypes we used a sortase ligation system (27). Recently, nanoparticle (I53_dn5, mi3) and virus-like particle (AP205) platforms have been used to display HA antigens from multiple strains (28, 29). In this study, we developed an alternate platform to couple HA antigens. For the scaffold, we modified the enzyme lumazine synthase (LS), a self-assembling 60-mer (18.3-kDa monomer) to carry a pentaglycine tag at the N-terminus of each subunit (G5LS). The HA antigens were modified with an LPETG motif at the C-terminus to facilitate the sortase reaction (Fig. S12A). HAs from different subtypes were purified separately and iteratively conjugated to the functional scaffold (Fig. S12B). The product of each sortase reaction was purified by size-exclusion chromatography before subsequent conjugation cycles (Fig. S12C). The coupled heterologous HA antigen displayed four copies of each influenza subtype. The conjugation of HA antigens to the functional scaffold was also confirmed by negative-stain electron microscopy (Fig. S12D). The binding of this coupled heterologous HA antigen against a panel of influenza-specific mAbs (30, 31) suggested that orthogonal conjugation did not affect their native conformation (Fig. S12E).
We next monitored the internalization of the coupled heterologous antigen in engineered B cells. The presentation of peptides on MHC-II in B cells begins with endocytosis of the antigen. We generated a B cell line that expressed a cell surface B cell receptor (BCR) that binds H1 HA, but not H3 or B HA. We found that a H1 HA-specific B cell could internalize H3 and B HA after covalent coupling (Figs. S13A–C).
Coupled heterologous antigen can limit antibody subtype-bias
We tested the primacy of MHC class-II preferences in modulating subtype-bias and the potential of a coupled heterologous antigen to limit this bias in mice. As C57BL/6 mice has one MHC class-II (I-Ab), we analyzed HA proteins from different influenza strains to identify those that are predicted to have many or few high-affinity peptides binding to I-Ab. We then immunized mice with a mixture of three different HAs, each predicted to have a different number of high-affinity peptides. The immunized mice showed a clear antibody subtype-bias consistent with the predicted CD4+ T cell help. Combining HA from different strains onto one vaccine platform to form a coupled heterologous antigen limited this subtype-bias (Figs. 3A–B).
Figure 3: Covalent coupling of heterologous HA antigens can largely eliminate subtype-bias.
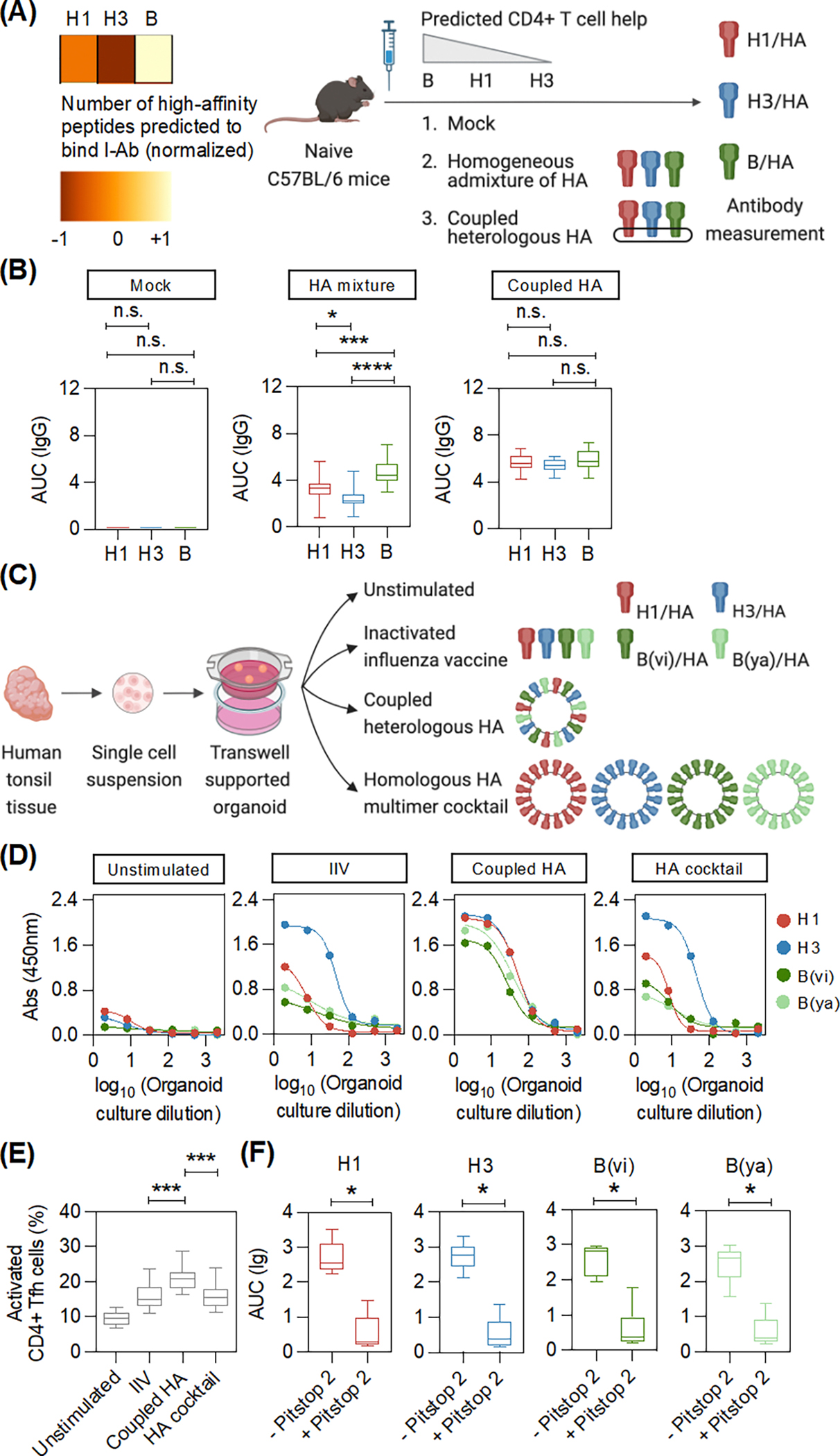
(A) The number of peptides from each influenza subtype (H1, H3, and B) that can bind to MHC-II I-Ab with high-affinity was predicted using the immune epitope database (IEDB). Data was normalized for representation in a heatmap. Naïve, male C57BL/6 mice (5–6 weeks old, n = 15) were immunized with the indicated formulations. Serum was collected at day 14 after immunization to determine the anti-HA antibody titers by ELISA. The data was collected from two independent experiments. (B) Box plots show the anti-HA binding against full-length HAs in the indicated groups. Adjusted p-values were calculated using one-way ANOVA with Tukey’s test for pairwise comparisons. (C) Transwell supported organoids were generated using surgically resected human tonsil tissue. Organoids made from each donor were stimulated separately with the indicated formulations. (D) ELISA binding curves of the tonsil organoid culture supernatant with full-length HAs after the indicated stimulation for a representative donor. Each dot shows the absorbance signal (binding) at the indicated organoid culture dilution. (E) Box plots shows the activation of CD4+ Tfh cells (CD38+) after stimulation of the tonsil organoids with the indicated formulations (n = 10) (gating in Fig. S19). Adjusted p-values were calculated using one-way ANOVA with Dunnett’s test for pairwise comparisons. (F) The antibody response in tonsil organoids after coupled heterologous antigen stimulation, with or without Pitstop 2, an inhibitor of receptor-mediated endocytosis, was measured by ELISA (n = 6). Box plots display antibody binding to full-length HA. P-values were calculated using two-tailed Wilcoxon test. *p < 0.05, ***p < 0.001, ****p < 0.0001, and not significant (n.s.).
To test whether subtype-bias could be overcome in humans, we took advantage of human tonsil organoids that could recapitulate many aspects of an influenza vaccine response (32, 33). Here, we evaluated the antibody response to a coupled heterologous antigen versus the same platform displaying HA from multiple subtypes individually (HA cocktail) (Fig. 3C). IIV and HA cocktail stimulation of organoid cultures from different donors showed subtype-bias (Figs. 3D and S14). By contrast, cultures from the same donors stimulated with the coupled heterologous HA antigen resulted in generally high antibody responses against multiple subtypes (Figs. 3D and S14). Antibody response to the head-domain was higher in comparison with the stem-domain (34–36) (Figs. S15 and S16). However, the stem-domain response was higher after coupled heterologous antigen stimulation in comparison with IIV (Fig. S17). Our data suggested that orthogonal display of protein arrays increased both strain-specific and cross-reactive antibody responses in comparison with IIV (Fig. S18).
We also observed a higher frequency of activated CD4+ Tfh cells after stimulation with the coupled heterologous antigen in comparison with IIV (Figs. 3E and S19). This finding suggested that the coupled heterologous antigen augments CD4+ T cell help. The epitope-specific contribution of CD4+ T cell responses at a single-peptide resolution after coupled heterologous antigen stimulation warrants further investigation. Scaffold-specific CD4+ T cell responses could also be detected, which may contribute to the overall response as previously reported (37). However, the T cell response to HA was higher than the response to the scaffold (Fig. S20). Inhibiting endocytosis in the cultures with Pitstop 2 (38) decreased the antibody response, indicating that antigen uptake is required (Fig. 3F).
“Borrowing” T cell help boosts the avian influenza response
Highly pathogenic avian influenza with its very high mortality rate represents a major pandemic threat if it becomes transmissible through air. Thus, we tested whether we could augment the response to an avian influenza HA by providing additional T cell help (Fig. 4A). Whereas tonsil organoids stimulated with H5N1 HA alone induced very weak antibody responses, a coupled heterologous antigen with an optimal ratio of H5N1 HA and seasonal HA that maximized cross-subtype T cell help induced higher antibody responses (Figs. 4B–C). However, consistent with the results described previously, the help from seasonal HA was not as effective when H5N1 and seasonal HAs were not covalently linked. We also analyzed the frequency of antigenic-specific B cells using H5 HA specific and H1 HA specific spheromer probes (Figs. 4D and S21), a highly sensitive multimer reagent (39). We observed a higher frequency of both H5 HA+ (strain-specific) and cross-reactive (H5 and H1 HA+) non-naïve B cells after stimulation with the coupled heterologous H5 and seasonal HA antigen in comparison to stimulation with H5 HA alone. The frequency of strain-specific B cells was ~five-fold higher than cross-reactive B cells after coupled heterologous antigen stimulation. This result suggested that coupling antigens with dissimilar sequences resulted in higher antibody titers, primarily because of better strain-specific responses, and to a much lesser extent, cross-reactive responses.
Figure 4: Antibody responses to avian influenza HA can be boosted by borrowing CD4+ T cell help.
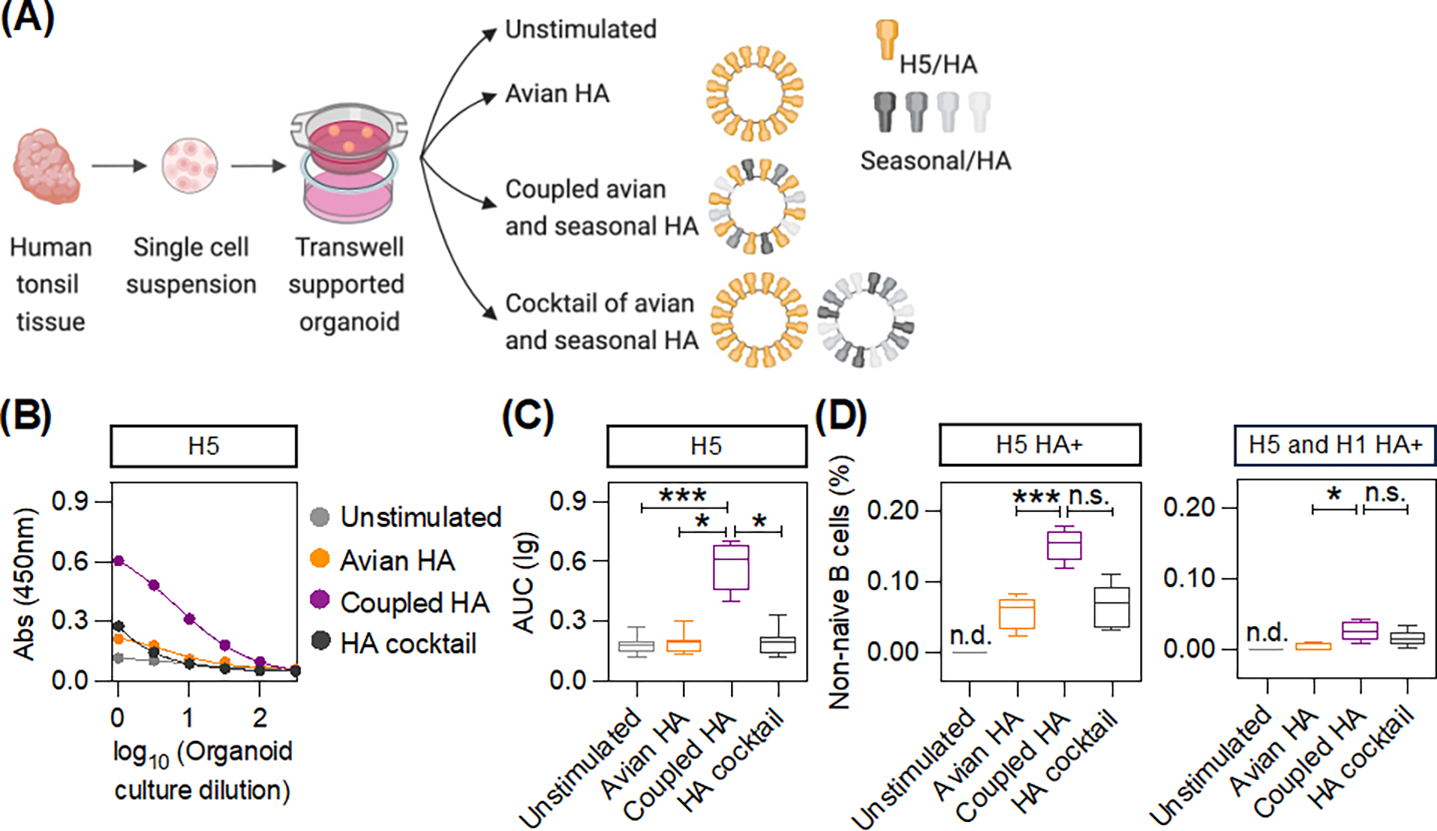
(A) Transwell supported organoids were generated using surgically resected human tonsil tissue. Organoids made from each donor were stimulated separately with the indicated formulations. (B) ELISA binding curves of the tonsil organoid culture supernatant with H5 HA after the indicated stimulations for a representative donor. (C) Box plots show the binding to H5 HA after the indicated stimulations across all donors (n = 8). Adjusted p-values were calculated using one-way ANOVA with Dunn’s test for multiple comparisons. (D) The frequency of H5 HA+ and H1 HA+ specific non-naïve B cells in the tonsil organoids was determined using spheromer probes (gating in Fig. S21). Box plots show the frequency of strain-specific (H5 HA+) and cross-reactive (H5 and H1 HA+) B cells after the indicated stimulations (n = 5). Not determined (n.d.). Adjusted p-values were determined using one-way ANOVA with Dunn’s test for multiple comparisons. *p < 0.05, ***p < 0.001, and not significant (n.s.).
Discussion
The development of an effective influenza vaccine remains challenging. A major issue with the current vaccines is that most individuals respond better to the strain that they are biased for, and thus, may have little protection against infection by other strains. Here, we have shown that coupling heterologous HA antigens can limit this subtype-bias in both mice and human tonsil organoids, most likely by increasing T cell help. We have also shown that T cell help can be “borrowed” from the favored seasonal strain to boost antibody response to an avian strain in tonsil organoids.
Repeated exposure to drifted influenza strains can result in the generation of cross-reactive memory B cells that may contribute to vaccine response (9, 17), but the weak association between the response to ancestral and vaccine strains after vaccination seen in our twin cohort suggests that broadly cross-reactive memory B cells are engaged at a relatively low level. Even though the ancestral panel may be inexact in capturing the typically unknown exposure histories, our data suggests that heterologous exposures have a limited impact on subtype-bias. This may explain the rarity of potent cross-reactive antibodies after seasonal vaccination (2, 12). Observation of subtype-bias after vaccination in an influenza naïve infant cohort further suggests that prior exposure is not essential and supports our thesis that host genetics may be a major driver of subtype-bias. The low levels of anti-HA antibodies in most infants before vaccination suggests that maternal antibodies may have limited impact on subtype-bias.
We also analyzed the role of HLA class-II alleles, an important genetic regulator of adaptive immunity and identified wide subtype-specific variation in the number of high-affinity HA peptides predicted to bind MHC-II. Previous studies have evaluated population-wide association of HLA alleles with influenza vaccine responses (14, 40). Based on our analysis, we suggest that future studies consider how the complete array of HLA class-II alleles within an individual may modulate T cell response, since humans express multiple MHC-II molecules that may have very different peptide binding characteristics.
We have shown that covalent coupling of heterologous HA antigens can limit antibody subtype-bias by enhancing the recruitment of T cell help from strain-specific B cells through the presentation of a broader array of HA peptides. The cell surface BCR enhances a B cells capacity to present antigen to T cells (41). Here, we have demonstrated that a B cell can internalize the ~40nm wide coupled heterologous antigen that we developed through BCR-mediated antigen recognition, which has an upper size limit of < 200nm (42). Our data support earlier studies suggesting that B and T cell epitopes do not need to be on the same protein (43, 44). However, intermolecular help can be limited if the antigen internalized by the B cell lacks both B- and T-cell epitopes, as is seen with large viruses (45).
Antigen multimerization has been shown to increase antibody responses (28, 29, 46), to some extent by stimulating cross-reactive B cells that recognize epitopes conserved across the co-displayed antigens (47). However, the fraction of cross-reactive antibodies in a polyclonal response is unclear. A recent study showed that co-display of SARS-CoV-2 receptor-binding domains (78% to 93% sequence similarity) elicited marginally higher (~1.5 to 2-fold) cross-reactive antibodies in mice, but most neutralizing antibodies in the polyclonal serum recognized only one of the two components (46). In human tonsil organoids, although we observed that coupling of heterologous HA antigens could promote cross-reactive HA stem-specific responses, the response to the head-domain (typically strain-specific) was much greater. Therefore, we speculate that in the context of antigens that co-display proteins with low sequence similarity, recruitment of more CD4+ T cell help is a salient outcome because broadly cross-reactive antibodies are typically rare (2, 12).
For many decades, the OAS hypothesis has influenced the explanation of subtype-bias. However, it suggests no practical way for influenza vaccines to be altered to overcome this problem. By contrast, our study shows that coupling heterologous antigens may broaden T cell help and improve vaccine efficacy. This strategy to augment T cell help is readily applicable to vaccines for other pathogens for which multi-strain coverage is needed. In addition, incorporating immunogenic peptides, such as tetanus toxoid, into the scaffolding or the displayed antigen would likely further enhance the CD4+ T cell response. We also note that immune organoids, such as the tonsil cell cultures used here, provide a rapid way to test vaccine constructs and hypotheses.
Supplementary Material
Acknowledgements:
We are immensely thankful to all the volunteers for their participation in this study. We thank the staff at the Stanford-LPCH Vaccine program for enrolling volunteers and sample collection. We thank the mothers and infants for volunteering their time and efforts to participate in the Influenza IMPRINT Cohort Study. We thank the staff at the Human Immune Monitoring Center (HIMC) for help with sample processing and immunoassays. We thank Janine Bodea Sung and Yael Rosenberg-Hasson for assisting with sample procurement and data curation for this study. Data generated by HIMC was supervised by Dr. Holden T. Maecker. We thank Drs. Barney S. Graham and Jeffrey Boyington for the CMV/R-based plasmid. We thank Dr. Elizabeth D. Mellins for providing access to Octet QK. We appreciate the critical feedback from Drs. Bali Pulendran and Sai Charan Koduru. We thank members of Drs. Mark Davis and Yueh-Hsiu Chien laboratories for helpful discussions. Flow cytometry analysis was done on instruments in the Stanford Shared FACS Facility. Illustrations were made using www.BioRender.com.
Funding:
This work was supported by NIH grants 5U19AI090019 and 5U19AI057229, and the Howard Hughes Medical Institute to MMD. Additional support was provided by the Bill and Melinda Gates Foundation (OPP1113682, Center for Human Systems Immunology) and Good Ventures funding from Open Philanthropy to MMD. This work was supported in part by the High Impact Technology (HIT) Fund of Stanford University to MMD and VM. VM also received support from ITI-YIA. The Influenza IMPRINT Cohort is supported by an NIH Cooperative Agreement 5U01AI144673 and Good Ventures funding from Open Philanthropy to MAS. The study was also supported by the Collaborative Influenza Vaccine Innovation Centers (CIVICs) contract 75N93019C00051 and U01AI144616 to TTW. Stanford Shared FACS Facility procured instruments using NIH S10 Shared Instrument Grant 1S10OD026831–01.
Footnotes
Competing interests: VM and MMD are inventors on a patent application (PCT/US2024/020935) submitted by Stanford Office of Technology Licensing that covers the strategy to broaden T cell help by heterologous antigen coupling described in this work. MMD is a co-founder, stockholder and paid consultant to NextVivo Inc. which has licensed aspects of the organoid technology used here for commercial purposes. LEW and MMD are co-inventors on a patent (US18/094,851) assigned to Stanford University describing the immune organoid methodology. The other authors declare that they have no competing interests.
Data and materials availability:
All data needed to evaluate the conclusions in the paper are present in the paper or the supplementary materials. The reagents required for heterologous antigen coupling are available from Mark M. Davis under a material transfer agreement with Stanford University School of Medicine.
References and notes
- 1.Iuliano AD et al. , Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391, 1285–1300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rathore U, Kesavardhana S, Mallajosyula VV, Varadarajan R, Immunogen design for HIV-1 and influenza. Biochim Biophys Acta 1844, 1891–1906 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Carrat F, Flahault A, Influenza vaccine: the challenge of antigenic drift. Vaccine 25, 6852–6862 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Schotsaert M, Garcia-Sastre A, Inactivated influenza virus vaccines: the future of TIV and QIV. Curr Opin Virol 23, 102–106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erbelding EJ et al. , A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 218, 347–354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis T, On the Doctrine of Original Antigenic Sin. Proceedings of the American Philosophical Society 104, 572–578 (1960). [Google Scholar]
- 7.Fazekas de St G, Webster RG, Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med 124, 331–345 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Skountzou I, Compans R, Jacob J, Original antigenic sin responses to influenza viruses. J Immunol 183, 3294–3301 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews SF et al. , Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 7, 316ra192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonville JM et al. , Antibody landscapes after influenza virus infection or vaccination. Science 346, 996–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO, Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 354, 722–726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellebedy AH, Ahmed R, Re-engaging cross-reactive memory B cells: the influenza puzzle. Front Immunol 3, 53 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yewdell JW, Santos JJS, Original Antigenic Sin: How Original? How Sinful? Cold Spring Harb Perspect Med 11, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poland GA, Ovsyannikova IG, Jacobson RM, Immunogenetics of seasonal influenza vaccine response. Vaccine 26 Suppl 4, D35–40 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E, Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines (Basel) 2, 707–734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguirre-Gamboa R et al. , Differential Effects of Environmental and Genetic Factors on T and B Cell Immune Traits. Cell Rep 17, 2474–2487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auladell M et al. , Influenza virus infection history shapes antibody responses to influenza vaccination. Nat Med 28, 363–372 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Cobey S, Hensley SE, Immune history and influenza virus susceptibility. Curr Opin Virol 22, 105–111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brodin P et al. , Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagan T et al. , Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 178, 1313–1328 e1313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crotty S, A brief history of T cell help to B cells. Nat Rev Immunol 15, 185–189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber CA et al. , T cell epitope: friend or foe? Immunogenicity of biologics in context. Adv Drug Deliv Rev 61, 965–976 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenbaum J et al. , Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics 63, 325–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreatta M et al. , Accurate pan-specific prediction of peptide-MHC class II binding affinity with improved binding core identification. Immunogenetics 67, 641–650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koroleva M et al. , Heterologous viral protein interactions within licensed seasonal influenza virus vaccines. NPJ Vaccines 5, 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers ML et al. , Commercial influenza vaccines vary in HA-complex structure and in induction of cross-reactive HA antibodies. Nat Commun 14, 1763 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witte MD et al. , Site-specific protein modification using immobilized sortase in batch and continuous-flow systems. Nat Protoc 10, 508–516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyoglu-Barnum S et al. , Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 592, 623–628 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen AA et al. , Construction, characterization, and immunization of nanoparticles that display a diverse array of influenza HA trimers. PLoS One 16, e0247963 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corti D et al. , Tackling influenza with broadly neutralizing antibodies. Curr Opin Virol 24, 60–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Y et al. , Mapping of a Novel H3-Specific Broadly Neutralizing Monoclonal Antibody Targeting the Hemagglutinin Globular Head Isolated from an Elite Influenza Virus-Immunized Donor Exhibiting Serological Breadth. J Virol 94, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kastenschmidt JM et al. , Influenza vaccine format mediates distinct cellular and antibody responses in human immune organoids. Immunity 56, 1910–1926 e1917 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagar LE et al. , Modeling human adaptive immune responses with tonsil organoids. Nat Med 27, 125–135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallajosyula VV et al. , Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A 111, E2514–2523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallajosyula VVA, Swaroop S, Varadarajan R, Influenza Hemagglutinin Head Domain Mimicry by Rational Design. Protein J 39, 434–448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton TC et al. , Protective efficacy of influenza group 2 hemagglutinin stem-fragment immunogen vaccines. NPJ Vaccines 2, 35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson SA et al. , CD4 T cell epitope abundance in ferritin core potentiates responses to hemagglutinin nanoparticle vaccines. NPJ Vaccines 7, 124 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rennick JJ, Johnston APR, Parton RG, Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol 16, 266–276 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Mallajosyula V et al. , CD8(+) T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Sci Immunol 6, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelder CM et al. , Associations between human leukocyte antigens and nonresponsiveness to influenza vaccine. J Infect Dis 185, 114–117 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Lanzavecchia A, Antigen-specific interaction between T and B cells. Nature 314, 537–539 (1985). [DOI] [PubMed] [Google Scholar]
- 42.Roche PA, Furuta K, The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 15, 203–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell SM, Liew FY, T cells primed by influenza virion internal components can cooperate in the antibody response to haemagglutinin. Nature 280, 147–148 (1979). [DOI] [PubMed] [Google Scholar]
- 44.Scherle PA, Gerhard W, Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med 164, 1114–1128 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sette A et al. , Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity 28, 847–858 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zang T et al. , Heteromultimeric sarbecovirus receptor binding domain immunogens primarily generate variant-specific neutralizing antibodies. Proc Natl Acad Sci U S A 120, e2317367120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanekiyo M et al. , Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol 20, 362–372 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the supplementary materials. The reagents required for heterologous antigen coupling are available from Mark M. Davis under a material transfer agreement with Stanford University School of Medicine.


