Abstract
Rationale:
Anticipation is a critical antecedent to drug use, in which the prospect of imminent drug availability can potently motivate instrumental actions directed to procure it. Models that capture the behavioral dynamics that precede drug access may allow for the dissociation of key neural mechanisms underlying appetitive or consummatory processes in drug self-administration.
Objectives:
We aimed to isolate measurements attributed to the procurement and consumption of a reward by defining distinct actions for each using a chain-schedule of reinforcement.
Methods:
Male Long-Evans rats were trained to self-administer cocaine or saccharin under a chained schedule of reinforcement (FI-FR) in order to dissociate appetitive (‘seeking’) from consummatory (‘taking’) behaviors. Completion of a fixed-interval (5min) was followed by 5min of continuously reinforced responding (FR1) on another lever.
Results:
The FI-FR chain procedure appears to provide sensitive and dissociable dimensions of cocaine self-administration within a single experimental session. Importantly, we demonstrate that responding during the FI (i.e., seeking) link tracks with the incentive value of anticipated reward access – whereby response rates corresponded to expected reward magnitude, degree of reward-specific satiety, and general motivational state.
Conclusions:
The FI component is a sensitive and reliable index of motivational changes induced by either the extrinsic incentive value of reinforcement (i.e., anticipated dose) or intrinsic motive states (i.e., satiety or deprivation). This procedure provides a valuable tool for interrogating the neural dynamics of drug-seeking and -taking behavior, in isolation.
Keywords: Cocaine Self-Administration, Anticipation, Motivation, Fixed-Interval, Chain Schedule
INTRODUCTION
The anticipation of imminent drug availability can provoke a heightened state of behavioral and physiological arousal in cocaine dependent individuals, which collectively influences the decision to pursue drug as well as the effort exerted toward its procurement. There is considerable evidence that contextual and/or environmental stimuli that set the occasion – wherein drug availability is expected – can potentiate the motivation for drug use (Robinson and Berridge 1993; O’Brien et al. 1998; Weiss et al. 2003). However, few conventional models of drug self-administration are designed to adequately or sensitively dissociate the behavioral, biological, and cognitive process that provoke or invigorate drug-seeking behaviors (in a drug-free state) from those that regulate subsequent drug intake once access has been procured. Efforts to study the neural underpinnings of drug self-administration require procedures that permit the isolation of its appetitive and consummatory aspects. Indeed, the multidimensional nature of substance use disorders has become increasingly evident. Pathophysiological functioning of motivational, reward, stress, memory, and cognitive control processes may independently contribute to the propensity to seek and subsequently consume drug reinforcers (Volkow et al. 2019).
Wise (1987) makes a compelling case that conventional IV self-administration procedures represent a unique form of reinforcement, in which the instrumental action that “earns” a target reward also accomplishes its consumption. Most goal-directed behavior can be separated into a distinct two-phase sequence consisting of 1) an appetitive phase, which is characterized by motor arousal, approach, and goal-directed instrumental actions and 2) a consummatory phase, in which physical interaction with the target object in a stereotyped manner (i.e. chewing and swallowing) serves to achieve the terminal objective, and results in relative state of satiety (Craig 1917; Ikemoto and Panksepp 1996; Berridge 2004). Where food or water ingestion, for example, can be behaviorally, anatomically, and neurochemically distinguished on this basis, such distinctions are obscured in the case of IV self-administration, as a single lever-press conventionally obtains and consumes – via rapid intravenous infusion – the drug. Therefore, simple schedules of drug reinforcement fail to account for the heterogeneity of human drug use, in which the procurement and consumption of a drug require a distinct set of actions that are typically conducted in distinct stimulus environments.
One way to obviate this problem is to separate appetitive and consummatory responding entirely. Drug reinforcement can be effectively maintained under a two-lever heterogenous chained schedule, wherein drug access and drug infusion are conferred by distinct acts (Olmstead et al. 2000; Vanderschuren and Everitt 2004; Veeneman et al. 2012b). For example, completion of a response on one lever (i.e., seeking lever) never produces a primary reward (i.e., drug), but rather triggers access to a separate lever on which responding produces drug delivery (i.e., taking lever. Putative seeking and taking responses under ‘seeking-taking’ chains are coordinated by different biobehavioral mechanisms. Food restriction, for example, produces a larger increase in responses that produce food, compared to those that produced access to the food-taking lever (Balleine and Dickinson 1998; Corbit and Balleine 2003). Similarly, seeking and taking behaviors maintained by cocaine reinforcement can be differentially modulated by dopaminergic drugs (Veeneman et al. 2012b). Therefore, seeking-taking chain schedules provide a temporal window during which to independently assess the neural substrates underlying drug consumption and the factors that motivate its procurement.
In the current study, we implement a chained schedule of reinforcement which provides access to cocaine on a ‘drug-taking lever’ only after completion of a fixed-interval (FI) on a separate ‘drug-seeking lever’. Under FI schedules, the first response made after a specific time interval has elapsed is reinforced (Ferster and Skinner 1957). Although a single response is sufficient to meet the response requirement, FIs reliably produce an accelerating pattern of operant behavior (i.e., lever presses) as predicted reinforcement approaches (Fry et al. 1960; Dougherty and Pickens 1973; Dews 1978; Guilhardi and Church 2004). The tendency to shift from low-rate to high-rate behavior as the interval progresses (i.e., the response scallop) relates to the capacity to predict reward availability in time, while intensity (i.e., rate) of anticipatory responding is thought to bear a closer relationship to the value of expected outcome (Daniels and Sanabria 2017; Covington III et al. 2019). Indeed, a clear dose-response relationship is revealed when unconditioned drug effects are mitigated by prolonged post-infusion timeouts (Balster and Schuster 1973; Corrigall and Coen 1989). Thus, in a drug-free state, responding under a fixed-interval positively correlates with anticipated drug dose. A scalloped pattern of responding emerges in human subjects who self-administer cocaine on an FI under laboratory conditions (Panlilio et al. 2005; Risinger et al. 2005). In addition, accelerating drug-directed responses closely parallel a dramatic rise in real-time measures of subjective craving across the interval (Risinger et al. 2005). Responding under an FI may, therefore, provide a sensitive behavioral index of the motivated state engendered by the hedonic – incentive – value of an expected reward. Nevertheless, it has not yet been evaluated whether a responding under an FI can be maintained by access to doses of cocaine on a separate operandum, such as in a two-lever seeking-taking chain.
In the following studies, we evaluate both cocaine and saccharin self-administration in a seeking-taking procedure. We hypothesized that responses to gain access to rewards (seeking) and responses that delivered rewards (taking) would be sensitive to changes to the value of such primary reinforcers. Therefore, once stable performance was established under the FI-FR chain, this hypothesis was tested over a series of experiments in which the value of expected outcome was modified by either conditioned revaluation or unconditioned manipulation of motivational state.
Initially, we evaluated the propensity for rats to adjust instrumental behavior during the fixed-interval procurement link to correct for changes to outcome contingencies, which is a hallmark feature of a goal-directed action (Colwill and Rescorla 1985; Balleine and Dickinson 1998). By manipulating the unit dose of cocaine available during isolated access to the drug-taking lever, outside the context of the chained schedule, rats learned to associate drug access with higher or lower (i.e., extinction) reinforcing value. If action-outcome contingencies are intact, it is expected that subsequent drug anticipation will be enhanced or suppressed, respectively, in accordance with the perceived incentive value of drug procurement (Olmstead et al. 2001). Given the capacity to effectively anticipate the consequences of an instrumental action, outcome value will prominently impact the animal’s current motivational status.
Additional studies examine whether fixed-interval responding correlates with shifts in motivational state prior to reward access. Pre-session exposure to the terminal reinforcer, either cocaine or saccharin, was used to induce a transient state of satiety that was expected to suppress motivation to procure and consume the outcome (Corbit and Balleine 2003). Furthermore, in a logical extension of these studies, we examined patterns of drug-seeking exhibited in anticipation of drug access after a period of forced-abstinence. It is well-established that a vulnerability to reinstate drug-taking is heightened in the midst of cues and contexts previously associated with drug use (O’Brien et al. 1998; Preston and Epstein 2011). Moreover, the capacity for drug-stimuli to elicit cravings may increase progressively during early abstinence (Gawin 1986; Bedi et al. 2011; Wang et al. 2013; Parvaz et al. 2016); a phenomenon that has been reliably reproduced in animal models of cue-evoked drug-seeking (Grimm et al. 2001; Madangopal et al. 2019). It was expected that forced-abstinence from established drug-taking would augment efforts directed towards drug procurement when renewed access is imminent. However, there is less clarity around the relationship between drug-seeking and subsequent drug consumption once abstinence is broken.
Finally, an exploratory factor analysis (EFA) was conducted to evaluate where fixed-interval responding and cocaine intake, our putative measures of motivational arousal and consumption, lie amongst other indices of drug self-administration within a dimensional space. It is important to identify commonalities between the proposed procedure and more conventional measures of motivation and reinforcement, which possess a degree of established predictive, external validity (Deroche-Gamonet et al. 2004; Venniro et al. 2020).
METHODS
Subjects
Experimental Animals.
All experiments were conducted using Male Long-Evans rats (Charles River Laboratories, Wilmington, MA) Rats weighed 275–300g upon arrival and were individually housed within a temperature-controlled vivarium on a reverse light/dark cycle (lights on: 20:00–8:00), with ad libitum access to food and water. All rats were allowed to acclimate for one week prior to experiments. All procedures were approved by the Tufts University Institutional Animal Care and Use Committee, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011)
Surgical Procedures
Intravenous Catheter Surgery.
Rats were anesthetized with ketamine (100mg/kg, i.p.) and xylazine (6mg/kg, i.p.) and implanted with an indwelling catheter (Silastic® silicon tubing, ID 0.63mm, OD 1.17 mm) into the right jugular vein, using sterile surgical procedures. Tubing was then affixed to a plastic pedestal mounted within an adjustable harness (SAI Infusion Technologies). Animals were allowed to recover for five days in their home-cage prior to self-administration training. Upon recovery, rats were transported to IV self-administration chambers, dimensionally identical to the standard home-cage, for the duration of experiments. Each morning, catheters were flushed with 0.20mL saline and 0.20mL of heparinized saline (20 IU/mL) and, overnight, saline was delivered in 0.17ml pulses every 30 minutes to maintain patency.
Heterogeneous Chain Schedule for Self-Administration
Cocaine Self-Administration
In order to dissociate the anticipatory and consummatory features of cocaine “use”, rats were trained to self-administer cocaine intravenously under a chained schedule of reinforcement (FI-FR). Under these contingencies, completion of a fixed-interval on one lever (designated ‘drug-seeking lever’) was followed by a period of continuous reinforcement on another lever (designated ‘drug-taking lever’; see Figure 1). Thereby, the procurement and consumption of cocaine is engendered by separate, quantifiable actions.
Fig. 1. FI-FR chain procedure.

A) Schematic timeline of events during a single trial of FI5m-CRf(5m)-TO20m heterogeneous response chain. Arrows indicate theoretical lever-presses during the fixed-interval (FI) or continuous reinforcement (CRf) phase. B) Frequency of responding lawfully accelerates across the fixed-interval (left). Representative raster plot shows responses (black circles) for 8 rats during a single trial. The first response emitted after the interval elapsed (red circle) produced protraction of the drug-taking lever. Pattern of responding summarized as cumulative response record according to group means per 10s bin (solid trace, ±SEM shaded region). C) Cocaine-taking is typically front-loaded during the continuous reinforcement component. Event record depicts taking-lever responses (tick marks) across the 5min interval (top). Mean cocaine intake (mg/kg) is visualized in 15s bins across the access period (bottom; blue trace with filled AUC, ±SEM shading). D) Representative behavioral event record for a FI-FR test session, comprising 4 trials. Estimated brain [cocaine] is plotted on the right y-axis (dashed grey trace) to demonstrate spiking patterns of cocaine levels which approach zero prior to subsequent trial onset. Cumulative response output during each FI component is plotted on the left y-axis (red trace). Tick marks denote drug infusion events (blue trace). Unless otherwise noted, the first trial was used for all analyses.
The terminal schedule [FI5m -CRf(5min)-TO20m] was designed to approximate drug access conditions maintained under intermittent access (IntA) procedures for cocaine self-administration: 5 min periods of unrestricted drug-access on and FR1 schedule (i.e., continuous reinforcement; CRf) separated by 20 min drug-free timeouts (Zimmer et al. 2012; Calipari et al. 2014; Kawa et al. 2016). Importantly, this schedule of access allows for sufficient drug metabolism to occur between trials, thus mitigating the influence of cocaine reinforcement on subsequent behavior (Zimmer et al. 2012). Accordingly, FI responding during the ‘drug-seeking’ sequence of the chained schedule should occur in the relative absence of on-board cocaine. Indeed, it has been shown that enforcement of long timeout periods between intervals (i.e. 15min) produces a lawful relationship between cocaine injection dose (i.e. reward-magnitude) and response rates (Balster and Schuster 1973; Dougherty and Pickens 1973).
Saccharin Self-Administration
A separate group of rats was trained to self-administer saccharin (0.1% dissolved in H2O; 0.1ml/reinforcer) under a similar FI-FR response chain. Whereby responding on a ‘seeking-lever’ was maintained under a 5-minute FI that was reinforced with a period of unlimited access to saccharin (10min). Saccharin reward was produced by a nose-poke entry into a ‘taking-port”, which delivered 0.10mL of fluid into a dipper within the port. Due to inherent differences between how natural/food rewards are consumed in comparison to intravenous cocaine, procedural parameters were adjusted into order to capture individual variability in the consummatory component of the response chain. First, the 0.1% concentration was chosen because it lies on the ascending limb of a concentration-consumption function (Scalfani et al., 2010; confirmed with pilot testing), as it was presumed that a lower concentration would be more sensitive to bi-directional changes in consummatory behavior under experimental conditions. Similarly, the consumption period was lengthened to 10-minutes in order to increase sensitivity of the measure. Training sessions were composed of 4 trials, while testing was conducted during a single trial.
Analysis of FI-FR Behavior.
Since the principal interest of the present studies is in the anticipatory arousal that precedes drug-taking, emphasis is given behavior observed during the first trial. Although test sessions were composed of 4 discrete seeking-taking trials, trials 2–4 were omitted from analysis unless otherwise noted.
Total responses during the FI component to the response chain was used as the primary dependent measure of motivated arousal. Although there is no clear consensus on primary measures of FI behavior, it is important to consider that response patterns can vary on two critical axes – the temporal allocation and magnitude/intensity of responding. Since responses emitted before the interval has elapsed produce no consequence, measures of response distribution (i.e., index of curvature) have been used to establish the degree of temporal discrimination (Guilhardi and Church 2004). Consequently, response rates are difficult to interpret without accounting for the temporal distribution of responding of responding across an interval (Fry et al. 1960; Dougherty and Pickens 1973; Daniels and Sanabria 2017). Factors that might facilitate more precise timing on the part of the animal, such as extended training or longer intervals will reliably produce rightward right in response allocation (Fry et al. 1960). However, post-acquisition changes to microstructure of responding during a fixed-interval trial may also indicate a variety of behavioral disruptions that are unrelated to motivational state, such as general disruption of timing processes, loss of stimulus control, or nonspecific motoric effects induced by a particular manipulation. Only when patterns are consistent across conditions, might comparison of response rates be appropriate for assessment of motivational status.
Therefore, comparison between trials may only be valid when measures of timing – demonstrating task fidelity – are sufficiently intact. In the current studies, a previously characterized quantitative measure of FI responding known as Index of Curvature (IoC) was used as the primary means of assessing consistency of response patterns across testing conditions (Fry et al. 1960; Narayanan et al. 2012). Application of the equation shown in Figure 2 provides an index of the extent to which a cumulative response curve deflects from a linear response pattern. This is achieved by quantifying area-under-the-curve (AUC) differences between an observed response trace and a hypothetical linear pattern. As such, a delayed acceleration in responding will produce a positive index. This pattern of late-accelerating responding is commonly referred to as a response “scallop”, and demonstrates schedule-controlled behavior (Ferster and Skinner 1957; Dews 1978). Conversely, premature responding early in the interval will produce negative curvature indices. In addition to IoC, quarter-life values - defined as the time into a fixed-interval at which a quarter of the responses were made (Herrnstein and Morse 1957) – were recorded as a secondary measure of timing.
Fig. 2. Schedule control (timing) is demonstrated by the development of a “scalloped” pattern of responding over the fixed-interval.
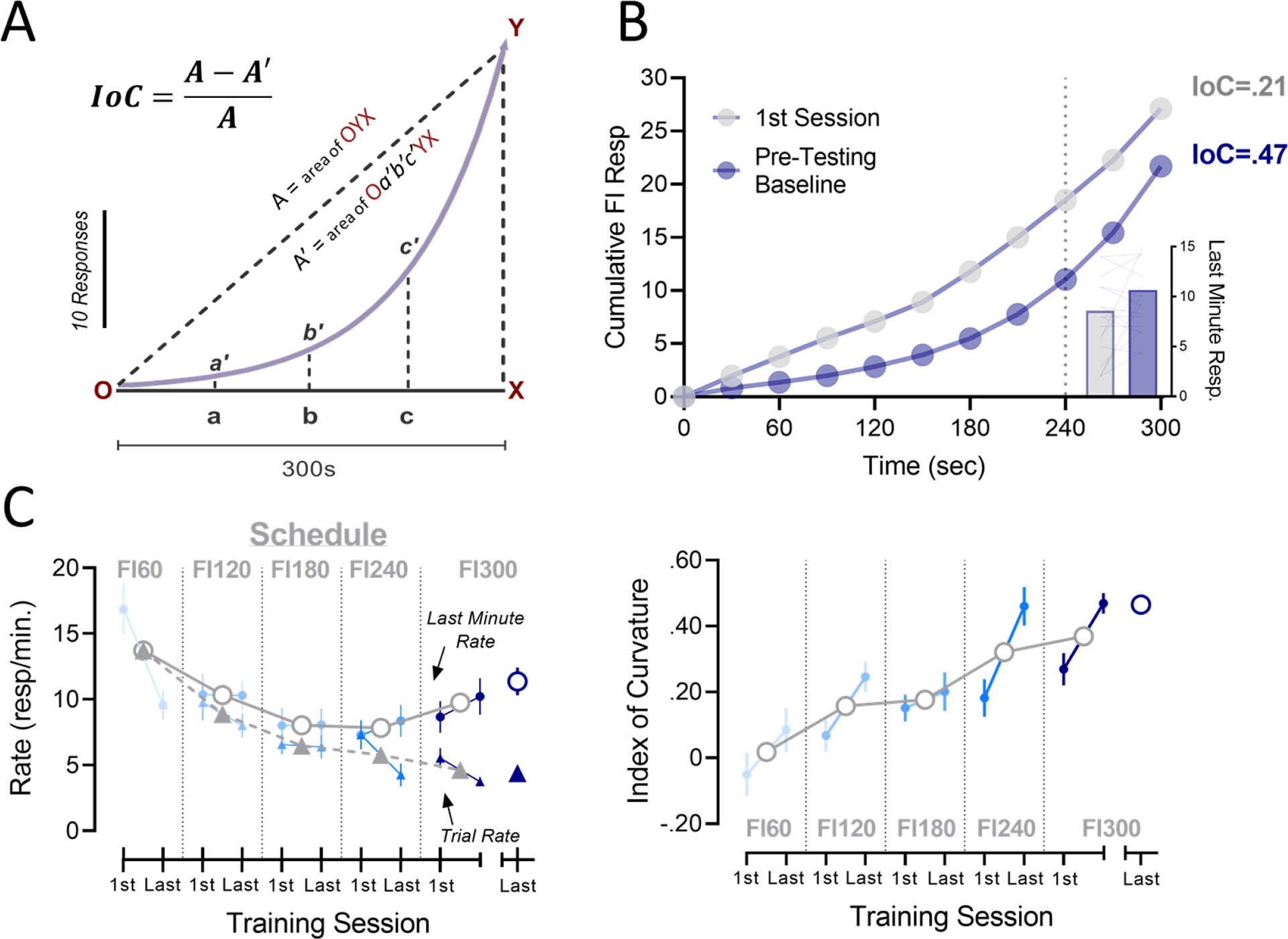
A) Hypothetical cumulative response record illustrating how Index of Curvature is calculated. B) Pattern of responding across an FI exhibits a higher IoC at the completion of training (blue circles) when compared to a more linear pattern early in training (grey circles). The accelerating response pattern is characterized by a high density of lever-presses during the final minute of the FI (inset bars). C) Trial response rates decline over the course of training (left; triangles with dotted trace) as function of reduced responding early in the interval. Mean lever-pressing during the last minute of the FI (left; circles with solid trace) does not differ between the first and last training session. Mean index of curvature (Right) increases across training sequence. All training data is shown as group means across training at each schedule (grey symbols), along with final baseline values (right x-axis). Values from the first and last training session demonstrate changes withing training block.
Group-level effects of treatment conditions on both cocaine- or saccharin-intake and fixed-interval responses for each drug test were analyzed using one-way ANOVAs, and all significant results were always followed by post hoc analysis using Dunnett’s test for comparisons against vehicle controls. For the purposes of analysis, only intervals in which rats emitted at least four operant responses were included, as this is the minimum output necessary to conduct analyses on response patterning (i.e. index of curvature).
Conditioned Manipulation of Outcome Value: Outcome Expectancy
Experiment 1: Outcome Revaluation Training
After an initial baseline test (Pre-revaluation BL), rats were given 5 sessions to self-administer either 0, 0.32 or 1.0mg/kg cocaine on the drug-taking lever. Groups were matched to produce similar mean fixed-interval response rates prior to training. Three-hour training sessions were capped at a maximum of 15mg/kg cumulative intake to maintain consistency across drug-taking groups, and to preclude potential intake-dependent effects on incentive motivation (i.e. Ahmed and Koob 1998)). On day seven, rats were tested for responding on the FI-FR chain under standard training conditions. FI responses from the first trial were compared to pre-revaluation baseline.
Experiment 2: Outcome Devaluation
Using a modified protocol adapted from Olmstead et al. (2001), drug-taking was devalued by replacing cocaine reinforcement with saline. Rats were given daily 2-hr extinction sessions in which the drug-taking lever was inserted and SD+ (LED) light illuminated at the onset of each session. Each lever press produced an infusion of saline and illumination of the drug-associated stimulus light (CS+). Extinction session continued until response rates were reduced to >15% of baseline (i.e., first extinction session). Total saline infusions obtained during the first session were also used as an index of ‘resistance to extinction’ in subsequent factor analyses. The following day, drug-seeking behavior was reassessed by presenting animals with the ‘drug-seeking’ context for 5 min. During this test, the ‘drug-seeking’ lever was inserted and the green stimulus light illuminated, and responses were recorded but had no consequence. As the ‘drug-seeking’ test should be indistinguishable from the FI-link of the chained schedule, response rates were compared to a single FI-FR trial conducted immediately prior to the outcome devaluation protocol (Pre-Devaluation Test). To determine whether basal fixed-interval performance predicted sensitivity to outcome devaluation, an index of devaluation was obtained for each rat (FI responses during Pre-Devaluation Test / (FI responses during Pre-Devaluation Test + FI responses during Post-Devalued Test)), such that indices approaching 1 indicate increasing sensitivity to outcome devaluation (ie. goal-directed behavior; (Chevée et al. 2023).
Unconditioned Manipulation of Motivational State: Reward Satiety or Restriction
Experiment 3: Cocaine Pretreatment Tests
Non-Contingent Administration.
On test days, rats (n=8) received a non-contingent infusion of cocaine (0, 0.18, 0.56 or 1.8mg/kg; IV) 5min prior to the start of cocaine self-administration. This dose range was based on previous intake values. The peak dose tested (1.8mg/kg) closely approximated the mean drug consumption during a drug-taking component and was therefore hypothesized to produce satiation-like effects. All doses were administered in a counterbalanced manner and separated by at least two days. Sessions were composed of four trials each. The first trial was used to evaluate the acute effects of non-contingent cocaine on the anticipation (FI responding) and consumption of additional drug. Subsequent trials (2–4) were used as a within-session comparison, under the assumption that the direct pharmacological effects of cocaine pretreatment would be diminished.
To determine whether the effects of cocaine pretreatment were reinforcer-specific, a similar procedure was conducted in rats (n=11) trained to self-administer saccharin under the FI-FR chain. On test days, rats received cocaine (0, 5.6, 10 or 17.8mg/kg) via IP injection 30min prior to saccharin self-administration. Doses were administered in a counterbalanced manner and separated by at least two days.
Contingent Administration.
To account for potential differences in the manner of drug administration (i.e., Response-dependent vs response-independent; Markou et al. 1999), we examined the effects of self-administered cocaine on subsequent performance on an FI-FR seeking-taking chain. At the onset of each test session, rats (n=8) were given access to the drug-taking lever and allowed to self-administer cocaine (0.32 mg/kg/infusion) for a maximum of 1, 2 or 4 infusions (0.32, 0.64, 1.28 mg/kg). Although rats were permitted a maximum of 5 min to consume the target dose for each condition, all subjects achieved intended consumption on each test day. Upon completion of the final infusion, a 5min timeout period was enforced prior to the onset of self-administration under the FI-FR seeking-taking chain.
Experiment 4: Pre-feeding and Fluid Restriction Tests.
To manipulate the intrinsic motivational value of saccharin reward, rats (n=9) were exposed to either 8-hrs of fluid restriction or given 1-hr unrestricted access to saccharin (0.1%) prior to FI-FR self-administration. Testing blocks consisted of a baseline session (Test BL), which served as a control comparison for the Value Test conducted the following day. Tests were separated by three training sessions and conditions were presented in a counterbalanced manner across animals.
Experiment 5: Reinstatement Tests
Yohimbine-Induced Reinstatement.
One day after post-devaluation testing, rats were administered a single dose (1.5mg/kg i.p) of yohimbine 30 minutes prior to the reinstatement session. Reinstatement tests were composed of a single fixed-interval component (5min) that was followed by 2hr ‘-taking’ period under extinction conditions (see extinction training). Access to the drug-taking lever was not contingent on seeking-lever responses during testing, and the FI component was terminated after 5min, regardless of responding. Lever-presses on the taking-lever produced saline infusion and elicited the presentation of the drug-conditioned cue light.
Although all rats were tested, only animals that demonstrated a significant reduction in responding after outcome devaluation (<70% pre-devaluation responding) were considered for analysis. Two subjects were omitted.
Cocaine-Induced Reinstatement.
A separate group of rats (n=16) was administered cocaine (5 or 10mg/kg i.p) 30 min prior to an identical reinstatement session following the drug-lever extinction protocol. Drug taking response was extinguished to >20% responding between test sessions
Experiment 6: Forced Abstinence Tests
Four groups of rats were used to assess the effects of withdrawal from either cocaine (n=30) or saccharin (n=16) on both the anticipation and consumption of rewards, according to previously described procedures (Holly et al. 2016). Enforced abstinence periods of 1, 7 or 15 days were examined. The effects of 1-day were examined in all subjects, and rats were subsequently tested after an abstinence period of either 7 or 15 days.
Cocaine Abstinence.
After three consecutive sessions with >25% variability in responding, rats were transferred from the self-administration room to a separate vivarium for a period of forced abstinence (ABST), during which no self-administration sessions were conducted. In deviation from the protocol described by Holly et al. (2016), rats were housed in a “shoebox cage” with pine-shaving bedding throughout the drug-free period in order to establish a visually and tactilely distinct environment from the drug-taking context. Self-administration chambers and outer enclosures were not cleaned or used in the interim, to retain any potential contextual odor cues. Rats were weighed and catheters were flushed with heparinized saline (20 IU/mL) daily.
After 7 or 15 days, rats were returned to their previous cocaine-self-administration chamber and FI-FR self-administration test was initiated immediately. The Post-ABST test session was composed of four trials and did not otherwise differ from standard self-administration conditions. Since catheter patency was not evaluated during ABST (to prevent extraneous stress), rats were tested for propofol immediately after the Post-ABST test. If catheters failed, drug-intake data was omitted from analysis, but fixed-interval responding was retained.
Exploratory Factor Analysis of Behavioral Dimensions
An exploratory factor analysis (EFA) was conducted to evaluate where fixed-interval responding and cocaine intake, our putative measures of motivational arousal and consumption, lie amongst other indices of drug self-administration within a dimensional space. This analysis contains behavioral data collected upon conclusion of previous studies (N = 24). All measures were collected at the conclusion of their primary study. Seven measure were analyzed: 1) FI (Anticipatory Arousal): the mean number of FI responses collected over nine FI-FR baseline sessions spread throughout the experimental timeline; 2) FR (Acute Consumption): the mean cocaine intake (mg/kg/first trial) during the FI-FR baseline tests; 3) Post-ABST (‘Incubation’): The net change in seeking-lever responses after a 15-day drug-free period, compared to an average of the last three sessions pre-ABST; 4) Resistance to Extinction: Total taking-lever responses during the first session of extinction/devaluation training; 5) Yohimbine Reinstatement: Total seeking-lever responses produced by yohimbine treatment after devaluation procedure; 6) Progressive Ratio: mean drug-taking lever responses emitted over three progressive ratio sessions; 7) Binge: Total cocaine consumption (mg/kg) during a 16-hr period of unlimited cocaine access. See Supplementary Information (SI) for further detail on behavioral metrics.
Modeling Brain-Cocaine Concentration
Running estimates of cocaine levels in the brain (Cbrain; μM) were produced according the well- characterized pharmacokinetic model below (Pan et al. 1991). See Supplementary Information (SI) for more detail.
Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse (Research Triangle Park, NC), and prepared in sterile 0.9% saline solution for intravenous administration. Yohimbine was obtained from Sigma-Aldrich (Saint Louis, MO, USA) and dissolved in sterile distilled water.
Data Analysis
Basic statistical analyses were conducted using Prism version 7.0 (Graphpad Software Inc.). Total responses during the fixed-interval and total cocaine (mg/kg) or saccharin (mL) intake during the continuous reinforcement phase (FR) were the primary dependent measures of cocaine anticipation and consumption, respectively. FI responding or intake before and after fluid restriction, pre-feeding or devaluation training were compared via paired t-test. One-way ANOVA was used to assess the effects of the cocaine pretreatment or revaluation training, and two-way ANOVAs were used to evaluate the time- and reinforcer-dependent effects of enforced abstinence on either measure of self-administration. All significant effects (p<.05) here were followed by post hoc analyses with Dunnett’s tests for multiple comparisons.
Exploratory factor analyses were conducted in R ver. 4.1.1, and unrotated loading plots were produced using SigmaPlot 13.0 (Systat Inc.). Three factors were initially identified on the basis of containing eigenvalues >1.0. Subsequent parallel analysis confirmed the extraction of the first three factors.
RESULTS
Self-Administration under an FI-FR Chain
An example of response patterns during each component of the chained sequence is depicted in Figure 1. At behavioral stability, the pattern of responding on the drug seeking-lever accelerates across the fixed-interval as drug availability becomes imminent, resulting in a characteristic “scallop” (Figure 1B). Due to the high rate of responding towards the end of the interval, there was typically a short latency (0–20sec) between elapse of the fixed-interval and response initiating the continuous reinforcement phase. Consumption of cocaine via the taking-lever was characterized by a high density of intake during the first minute of drug access (Figure 1C), which is typical of drug-taking maintained by intermittent periods of limited availability (Zimmer et al. 2012; Kawa et al. 2016; Allain et al. 2018). Few infusions were obtained during minutes 2–5. Importantly, FI responding did not significantly correlate with consumption of either cocaine (R2 = .0192) or saccharin (R2 = .0173) during the consummatory phase (Figure S2), supporting the assumption that ‘seeking’ and ‘taking’ behaviors are dimensionally distinct.
The overarching trial structure supported brief peaks in estimated brain cocaine concentration which decreased to near-zero prior to subsequent drug-seeking intervals (Figure 1D), thus it is presumed that individual trails were conducted with minimal influence of unconditioned drug effects on behavior. Accordingly, both seeking-lever and taking-lever responding remained relatively consistent between trials.
Acquisition of an FI-FR Response Chain
An average of 44.1 (±6.05) sessions were required for rats to acquire stable performance prior to testing. Training data corresponding to fixed-interval responding and cocaine intake from a representative group (n=20) are displayed in Figure 2 and Supplemental Figure S1, respectively. A stable mean index of curvature (IoC) developed gradually over time, indicating increased temporal discrimination with training. Although IoC values significantly increased between the first and last training session under a 5min FI (Student’s t-test: t(19) = 6.882, p<0.001), improvement was asymptotic, and there was no further change in response distribution over the 10 pre-test baseline sessions (Session 1 vs. Session 10 IoC; t(19) = 0.202, n.s). Despite the extended training protocol, levels of cocaine consumption remained consistent throughout training, and there was no evidence of escalating intake. Mean consumption during the first trial did not significantly differ between the first and last training sessions (t(19) = 0.472, n.s).
Conditioned Manipulation of an Expected Outcome Value
Experiment 1: Effects of revaluation training on fixed-interval performance
Figure 3 illustrates the experimental protocol for outcome revaluation, in which rats were trained to associate taking-lever responses with an outcome of either higher (1.0mg/kg/inf.), equivalent (0.32mg/kg/inf.), or lower (0mg/kg/inf.) reinforcing value than initial training conditions. After 5 sessions with access to 1.0mg/kg cocaine on the drug taking-lever, rats responded at a significantly higher rate upon reintroduction of the seeking-lever contingency (Student’s t-test: t(5)=6.169, p<0.01). Conversely, rats that self-administered saline (0mg/kg/inf.) displayed markedly suppressed responding during the fixed-interval (t(5)=7.039, p<.0001). Self-administration of the training dose (0.32.mg/kg/inf.) had no consequences on subsequent anticipatory responding, relative to pretraining (t(5)=1.577, n.s). A one-way ANOVA confirmed a main effect of revaluation training dose (F(2, 19) = 27.03, p<0.0001), and showed that both the high-dose (Dunnett’s Test: q=4.118, p<0.05) and low-dose (q=5.615, p<0.01) training resulted in significant deviations from the equivalent-dose control condition. Importantly, the augmented FI response pattern produced by revaluation training was structurally identical to pre-training behavior. Mean index of curvature did not differ between pre- and post-training tests when compared alongside baseline response patterns (F(2, 10) = 0.09347, n.s). Moreover, when transformed to a proportion of maximal responding, mean cumulative response curves for each group overlap entirely (Supplemental Figure S3).
Fig. 3. Effects of outcome revaluation training on fixed-interval responding.
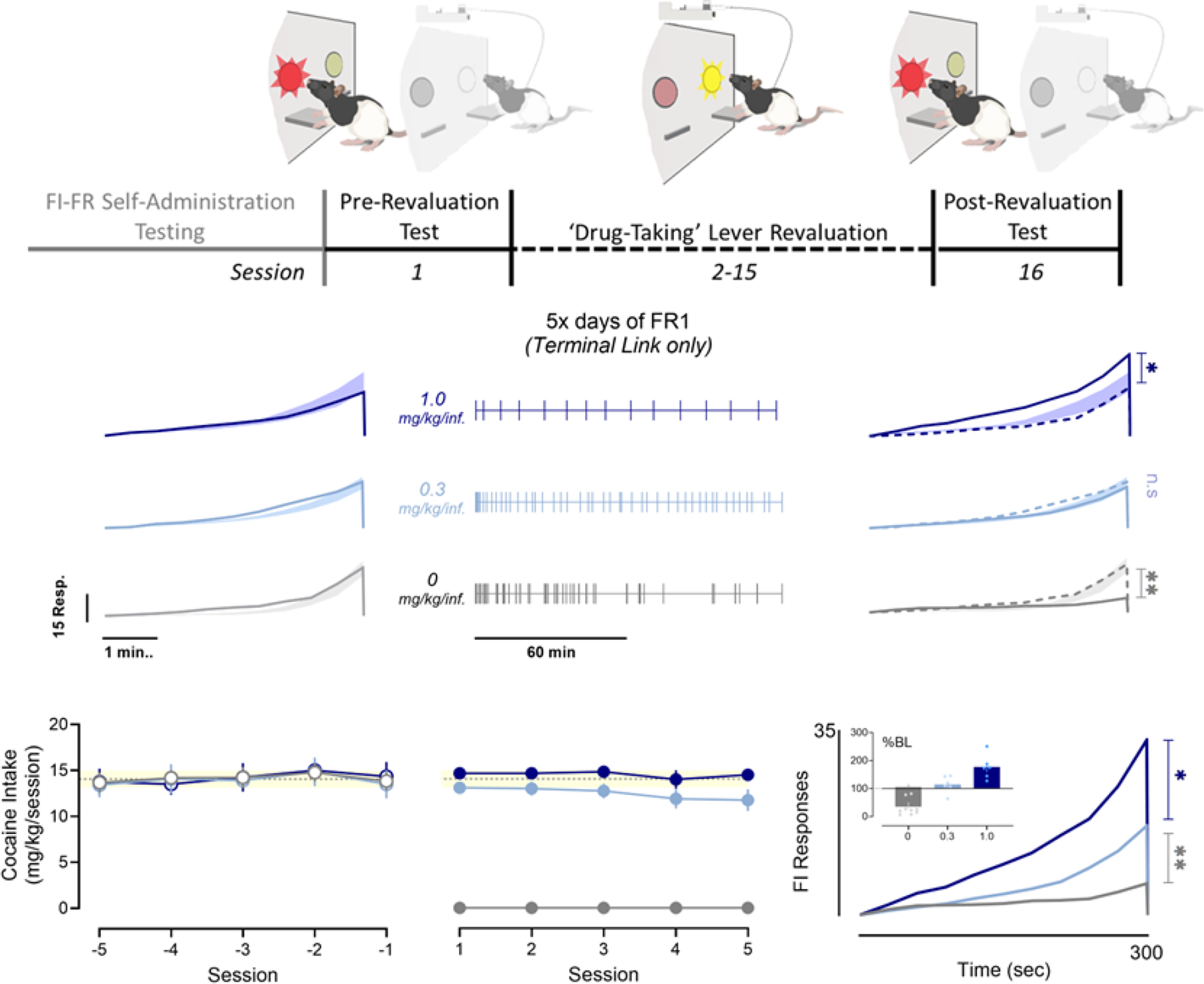
The event record depicts a representative 5-day period of self-administration on various doses of cocaine, when only the drug-taking lever was available. Tick marks indicate infusions, session means (± SEM) shown below. Mean fixed-interval response records for trials immediately before (Left) or after training (Right) for each group are shown as a solid trace, along with pre-revaluation test values (dotted trace). The shaded region that joins accompanies each FI trace represents the ± SEM of each group’s pre-testing baseline. Post-training response records are overlaid for between-group comparison (Bottom Right). Inset figure depicts total FI response during the post-test as percent pre-test.
Experiment 2 Effects of outcome devaluation on fixed-interval performance
Both extended operant training and prolonged cocaine exposure have been associated with the emergence of habit-like behavior (Balleine and Dickinson 1998; Nelson and Killcross 2006; Zapata et al. 2010). In particular, behavior that is initially driven by action-outcome contingencies (i.e. goal-directed) may shift towards a stimulus–response process. Whereby behavior (i.e. drug-seeking) becomes insensitive to changes in outcome, and can be maintained in the face of adverse consequences or diminished reward magnitude – often considered a hallmark feature of addiction (Schoenbaum and Setlow 2005; Hogarth et al. 2019; Epstein 2020; Lüscher et al. 2020). Therefore, upon conclusion of self-administration studies, all rats were evaluated on an outcome devaluation procedure wherein the ‘drug-taking’ link of the response chain was devalued by extinction in order to determine whether responding during the ‘drug-seeking’ link reflects goal-directed or habit-driven behavior (Figure 4).
Fig. 4. The fixed-interval link of an FI-FR response chain is sensitive to outcome devaluation.
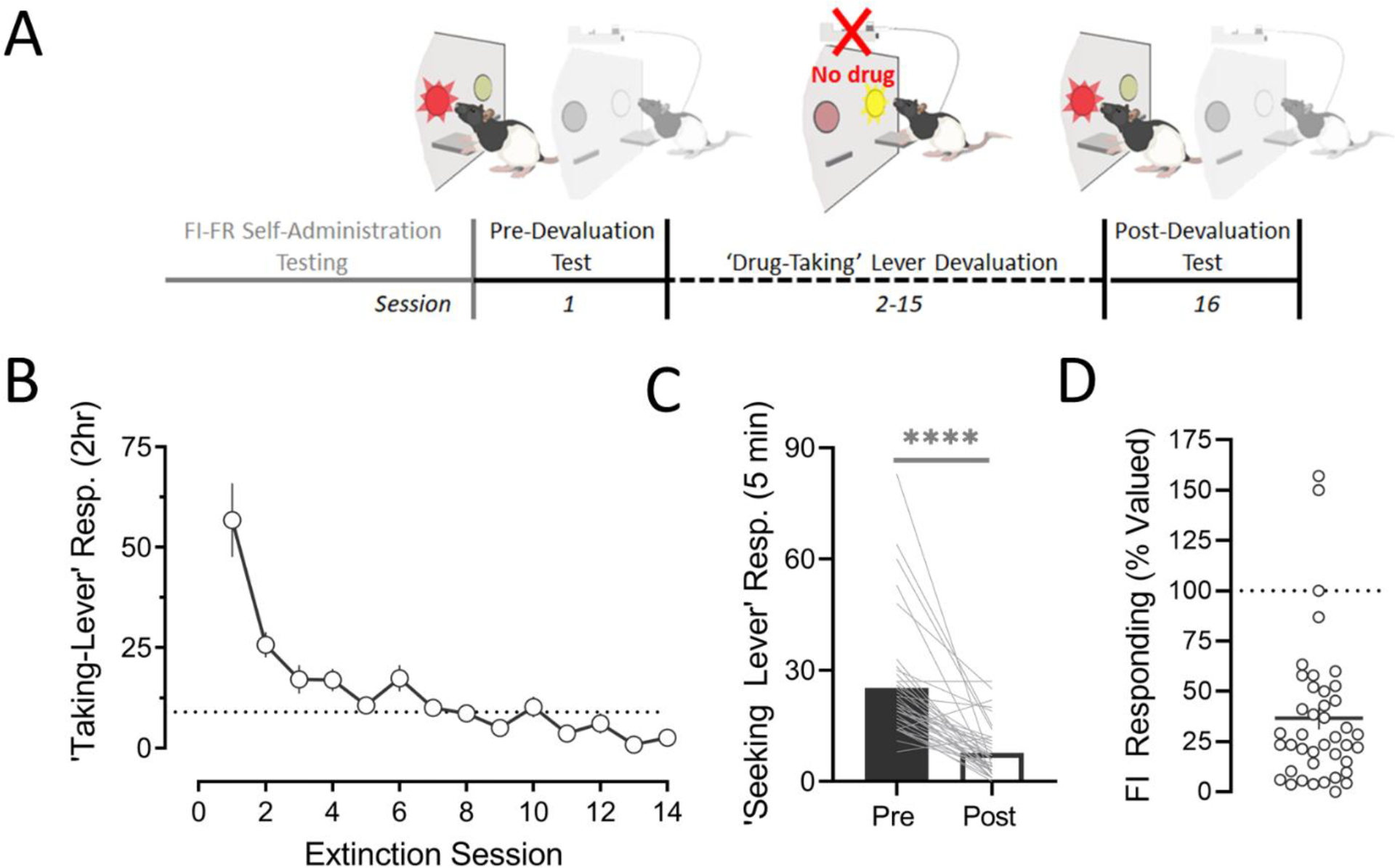
A) Timeline of devaluation procedure. B) Mean responses (± SEM) on drug-taking lever across extinction training. C) Seeking-lever responses during tests conducted before and after extinction training. D) Effects of devaluation training on ‘drug-seeking’ is summarized as a percentage of initial response levels (i.e. pre-devaluation test). *p<.05, ,**p < .01,***p < 0.001.
A paired t-test shows that 14 extinction sessions was sufficient to markedly suppress responses on the drug-taking lever (t(41) = 11.82, p < .0001). Upon reintroduction of the ‘drug-seeking’ contingency, 38 of the 42 rats showed a reduction in responding (<70% of pre-extinction baseline). Overall, mean responses during the FI link of the schedule were significantly reduced (t(41) = 7.128, p < .0001). Because FI performance remained contingent on the value of the terminal outcome (cocaine reinforcement), the putative ‘drug-seeking’ behavior can be considered goal-directed.
Unconditioned Manipulations of an Expected Outcome Value
Experiment 3: Effects of cocaine pretreatment on appetitive and consummatory phases of FI-FR chain
Figure 5 depicts the pattern of responding across the FI and continuous reinforcement phases of the FI-FR chain from representative animal following cocaine pretreatment. Behavioral output is shown alongside a running estimate of brain cocaine concentration in order to demonstrate the relationship between on-board drug and responses directed towards procurement (i.e., FI) and consumption (i.e. FR) of additional cocaine. Of note, 1) fixed-interval responding is low when on-board drug levels are highest and 2) drug infusions during the FR are spaced to achieve similar levels of cocaine by the end of the access period, regardless of drug levels at the onset of the consumption interval.
Fig. 5. Unconditioned manipulation of outcome value via cocaine pretreatment alters anticipation and consumption of cocaine under the FI-FR response chain.
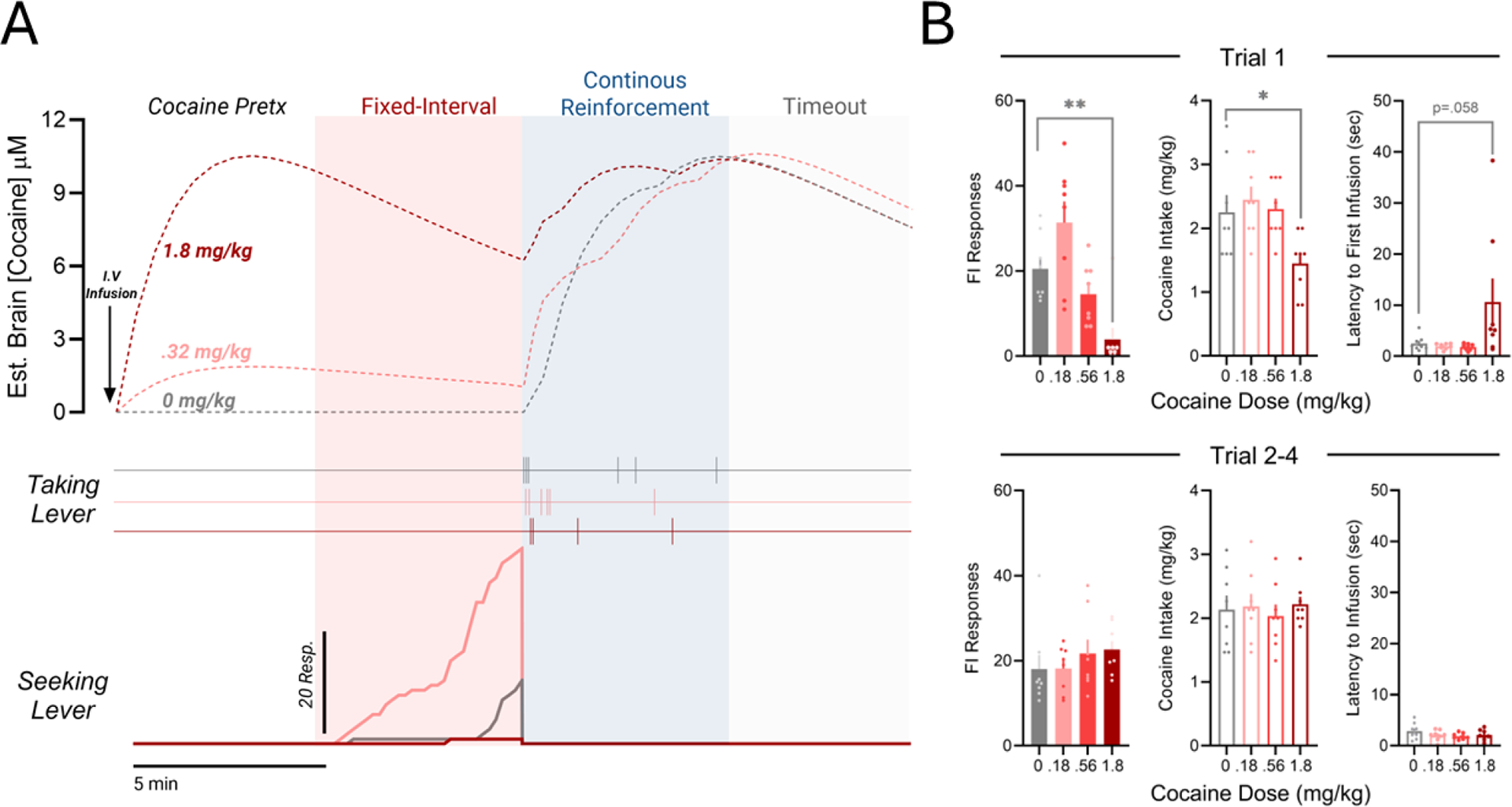
A) Representative response records for behavior during the first trial after cocaine infusion (bottom). Corresponding estimates for momentary brain cocaine levels (top; dotted lines). B) Mean (± SEM) FI responses, cocaine intake and latency to first self-administered infusion following a non-contingent cocaine priming infusion (top). No behavioral compensation for drug pre-loading is evident in subsequent trials. Mean (± SEM) FI responses and cocaine intake averaged across the last 3 trials of test session, which were not preceding by a cocaine prime (bottom). *p<.05, ,**p < .01,***p < 0.001
Anticipatory Phase (Fixed-Interval)
A repeated measures ANOVA verifies that, at the group level, non-contingent cocaine administration (IV) produced dose-dependent effects on the fixed-interval component of the seeking-taking chain, and these were restricted to the first trial (Figure 5B; F(3, 21) = 10.25, p<0.001). Compared to vehicle, the lowest dose tested (0.18mg/kg) produced a modest – but nonsignificant – increase in total responding during the FI (Dunnett’s Test: q=2.142, p=0.086), while responding was suppressed by the highest dose (1.8mg/kg; q=3.275; p<0.01). A similar biphasic dose-effect function on fixed-interval performance was observed after contingent pre-session cocaine consumption (data not shown; F(3, 21) = 3.91, p<0.05). Importantly, analysis of the mean index of curvature values for each condition indicated that response patterning was not significantly affected by non-contingent cocaine pretreatment (F(4, 28) = 2.127, n.s), and thus sufficient schedule-control of behavior was intact.
Consummatory Phase (Cocaine Intake)
Mean intake during the drug-taking phase of the chain was also affected by pre-session cocaine infusion (F(3, 21) = 6.358, p<.05). Compared to vehicle, consumption was significantly reduced after non-contingent pretreatment with 1.8mg/kg cocaine (q=3.171, p<0.05).
Trials 2–4.
The behavioral effects of cocaine pretreatment were absent in subsequent trials (Figure 5B). Repeated-measures ANOVAs suggest that neither mean FI responses (F(3, 21) = 1.241, n.s) nor mean cocaine intake (F(4, 28) = 0.4204, n.s) over trials 2–4 were impacted by non-contingent drug infusion.
Effects of Cocaine Pretreatment on FI-FR for Saccharin.
Repeated-measures ANOVAs revealed a main effect of IP cocaine administration on fixed-interval responding for saccharin access (F(2, 20) = 3.476, p<0.05) Although the 5.6mg/kg dose produced a modest increase in responding, no treatment condition met statistical significance upon post hoc comparison to the vehicle control (Supplemental Figure S5). Similarly, there were no main effects of cocaine injection on saccharin intake during the seeking-taking sequence (F(2, 20) = 0.047, n.s). The highest dose (18mg/kg) produced a marked behavioral disruption, as indicated by a significant reduction in IoC (1=2.815, p<0.05). Consequently, this dose was omitted from analysis.
Experiment 4: Effects of saccharin pre-feeding (satiety) and fluid-restriction (thirst) on FI-FR performance for saccharin reward
Figure 6 shows the effects of induced satiety or thirst on the anticipation and consumption of saccharin under an FI-FR chain. A state of satiety induced by 1-hour of unlimited access to saccharin (0.1%) resulted in a significant reduction in responding during the fixed-interval component (Student’s t-test: t(9) =3.514, p<0.01), compared to the previous session. Subsequent saccharin consumption was also markedly suppressed after pre-feeding (t(9)=4.905, p<0.001). On the other hand, 4-hours of fluid restriction prior to self-administration increased both mean response output during the FI (t(9)=2.929, p<0.05) and saccharin intake (t(9)=5.321, p<0.001).
Fig. 6. Unconditioned manipulation of outcome value alters performance for saccharin reward under FI-FR response chain.
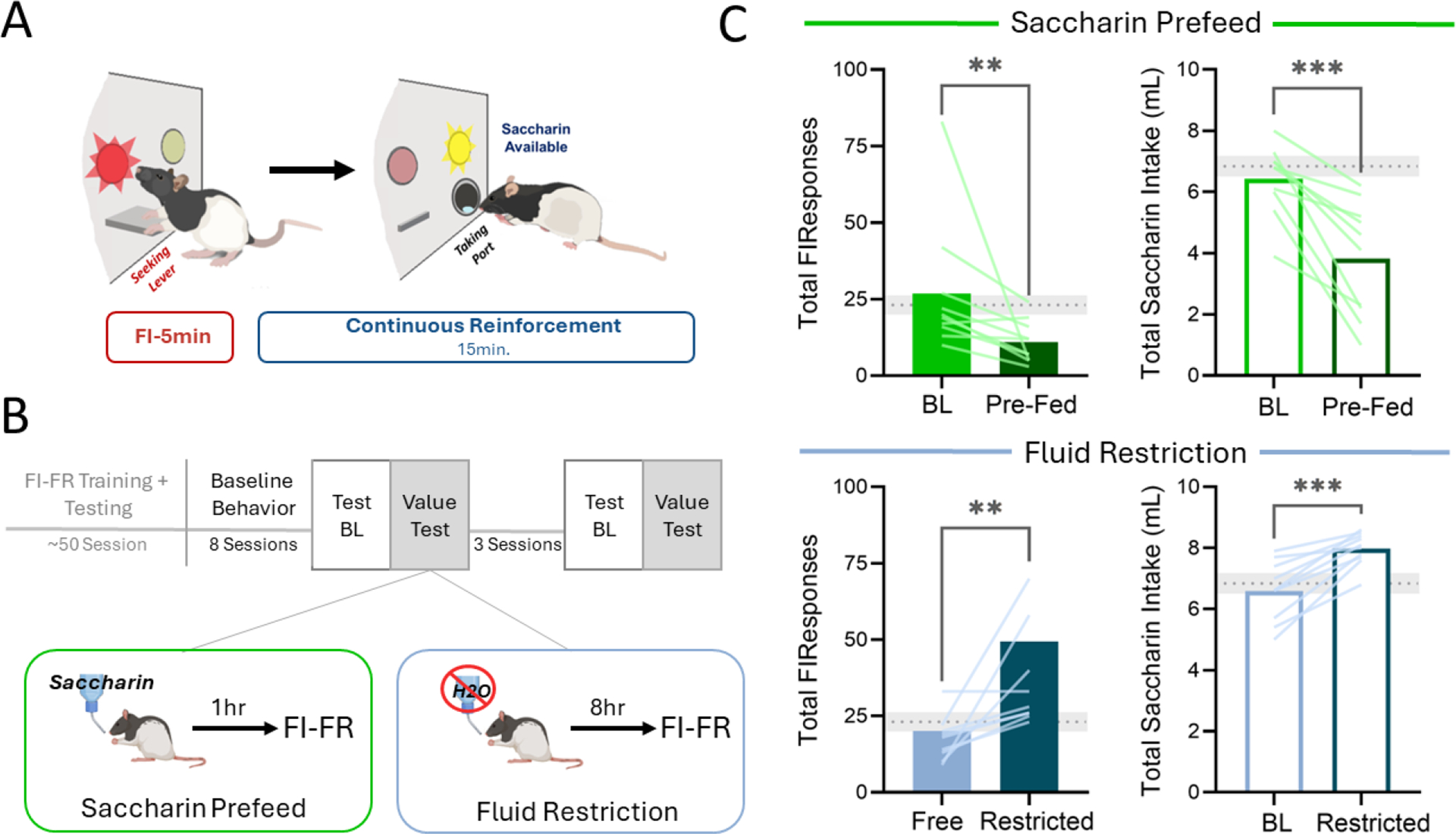
A) Cartoon diagram of FI-FR procedure with 0.1% saccharin solution as the primary reinforcer and B) schematic for outcome value testing. C) Pre-session access to saccharin resulted in both reduced FI responding and saccharin intake (top). Fluid restriction prior to testing enhanced both responding for saccharin access and subsequent saccharin intake (bottom). Grey dotted lines indicate mean (± SEM; shaded) FI responses and saccharin intake, respectively, from the 8 FI-FR sessions immediately prior to testing. *p<.05, ,**p < .01,***p < 0.001
Experiment 5: Acute cocaine- or yohimbine-induced reinstatement
As in Figure 4, isolated extinction of the taking-lever response resulted in significantly reduced responding on the drug-seeking lever when re-introduced during a post-devaluation probe prior to reinstatement testing (Figure 7). A priming injection of cocaine failed to elicit responding on the seeking-lever at either a 5 or 10mg/kg dose (Figure 7B), and FI response rates remained comparable to post-devaluation levels (F(3, 43) = 1.651). However, cocaine dose-dependently increased responding on the taking-lever, despite the absence of cocaine reinforcement (Main effect of cocaine pretreatment: F(3, 43) = 22.71, p<0.0001; Bonferroni comparisons of cocaine vs post-devaluation p<0.001; cocaine vs saline: p<.01).
Fig. 7. Acute cocaine and yohimbine differentially reinstate features of cocaine-seeking after outcome devaluation.

A) Timeline for cocaine- or yohimbine induced-reinstatement experiments B) Mean (± SEM) ‘seeking-lever’ (left) and ‘taking-lever’ responses following cocaine priming injection or C) yohimbine. Inset figures depict the mean cumulative response record for fixed-interval responses. Asterisks (*) denote significant deviation from vehicle treatment condition *p<0.05; **p<0.01, ***p<0.001. Significant comparisons against post-devaluation test values are indicated by (#) #p<0.05; ##p<0.01, ###p<0.001.
Yohimbine administration restored responding on the seeking-lever to pre-devaluation levels (Figure 7C; Main effect of yohimbine F(2, 30) = 11.91,p<0.001; Bonferroni comparisons of yohimbine vs post-devaluation, p<.01; yohimbine vs saline, p<0.001). However, the temporal structure of responding was significantly disrupted by yohimbine, as indicated by a significantly reduced index of curvature (Main effect of yohimbine F(2, 30) = 3.879; p<.05). Like the cocaine prime, yohimbine also provoked a robust increase in responding on the taking-lever for presentation of the drug conditioned cue (Main effect of yohimbine F(2, 30) = 22.41, p<0.001; Bonferroni comparisons of yohimbine vs post-devaluation p<.0001; yohimbine vs saline, p<0.001).
Experiment 6: Effects of forced abstinence on FI-FR performance
Figure 8A depicts mean FI responding for access to either cocaine or saccharin after 0, 1, 7, or 15 days removed from self-administration. A simple two-way ANOVA was used to assess the effects of ABST (0, 1, 7 or 15 days) and Reinforcer (cocaine or saccharin) on FI responding. Analysis revealed a main effect of ABST (F(3, 92) = 5.637, p<0.05) and an interaction between ABST duration and Reinforcer (F(3, 92) = 4.896, p<0.01), reflecting a time-dependent – incubation-like – enhancement of responding for cocaine access that was absent in rats self-administering saccharin. Indeed, post hoc comparisons show that anticipatory responding for cocaine was significantly elevated after either a 7-day (q=3.824, p<0.001) or 15-day drug-free abstinence period (q=5.201, p<0.0001) compared to baseline (0-days). Forced abstinence had no significant effects on responding for access to saccharin at any duration.
Fig. 8. Effects of drug-free (abstinence) periods on anticipation and consumption of cocaine under the FI-FR response chain.
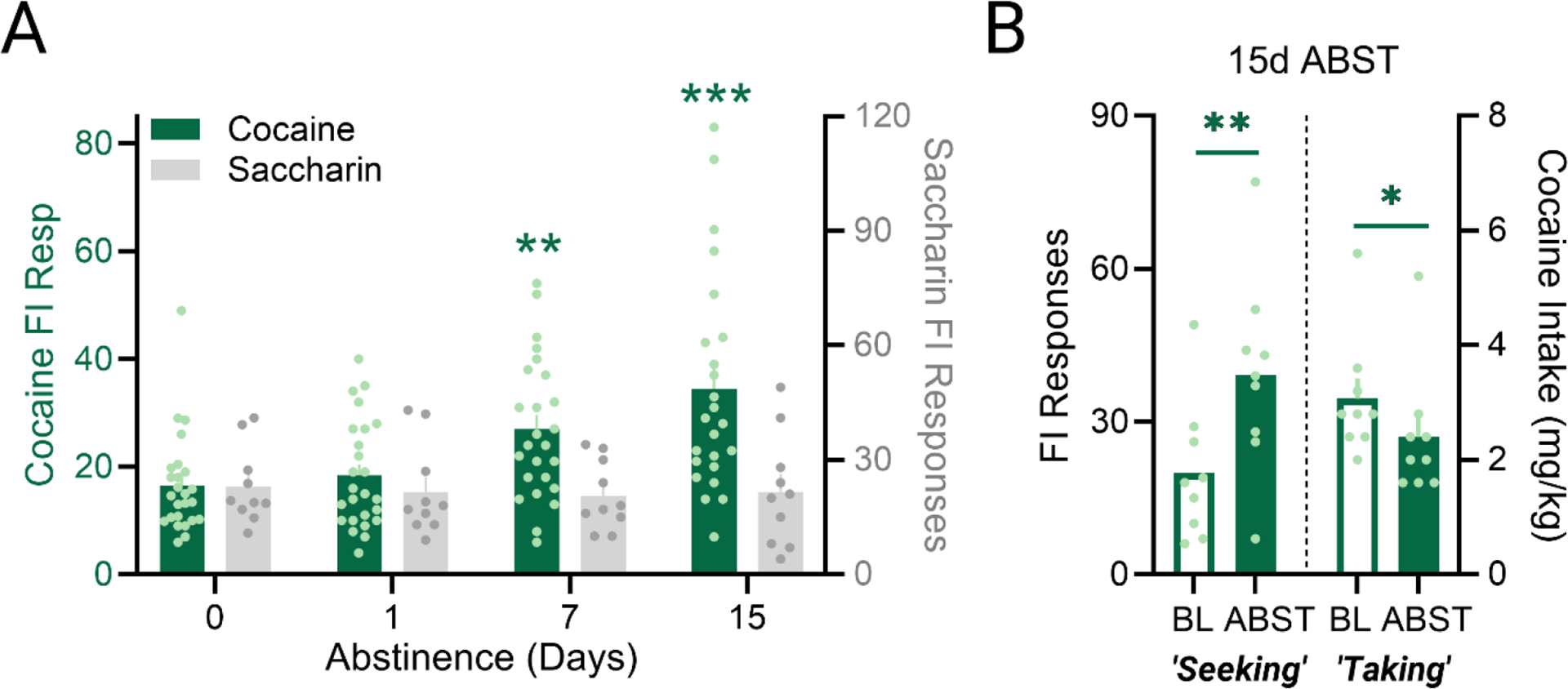
A) Incubation-like increases in fixed-interval responding for cocaine (solid bars; left y-axis), but not saccharin (striped bars; right-y axis) reinforcement. B) Renewed access to cocaine after a 15-day drug-free period resulted in increased FI responding (solid bar) and decreased cocaine intake (open bar). All values depicted as a mean (±SEM). *p<.05, ,**p < .01,***p < 0.001.
Seeking-lever responses and cocaine intake after 15-day ABST are shown in Figure 8B, for rats in which catheter patency was intact (n=24). A paired t-test shows a modest decrease in cocaine intake upon resumed drug-taking, when compared to the 0-day ABST baseline session (t=5.774, p<0.01). Saccharin intake was not affected by a 15-day withdrawal period (t=0.4332, n.s).
Factor Analysis
A factor analysis was conducted in order to clarify how each phase of the seeking-taking chain dimensionally relates to conventional measures of self-administration thought to capture features of addiction-like behavior. Three factors were extracted, which collectively account for 69.7% of the variance (Fig. 9 and Table 1). I line with our initial assumption, each link of the response chain loaded into orthogonal dimensions. The first extracted factor (Factor 1) accounts for 29.3% of the total variance and contains strong loadings (>0.6) from three of the behavioral variables: Basal Drug Anticipation (FI), ‘Incubation’ of Drug-Seeking (ABST) and Progressive Ratio performance (PR). A second component (Factor 2), which accounts for 21.6% of the variance, contains high factor loadings from Resistance to Extinction (EXT) and Yohimbine-Induced Reinstatement (Yoh). Interestingly, PR also loads onto Factor 2 with moderate strength (>0.3). The remaining behavioral variables could be further differentiated on the basis of high factor loading onto a third dimension (Factor 3), which explains 18.8% of the overall variance. Both Acute Cocaine Intake (FR) and Binge-like Consumption loaded onto this dimension with moderate and high loading values, respectively. These findings support the assumption that each phase of the FI-FR chain represents a distinct feature of self-administration behavior. And, furthermore, seeking-lever and taking-lever responding each appear to share dimensionality to with putative measures of appetitive and consummatory processes, respectively.
Fig. 9. Exploratory Factor Analysis of different cocaine self-administration modalities.
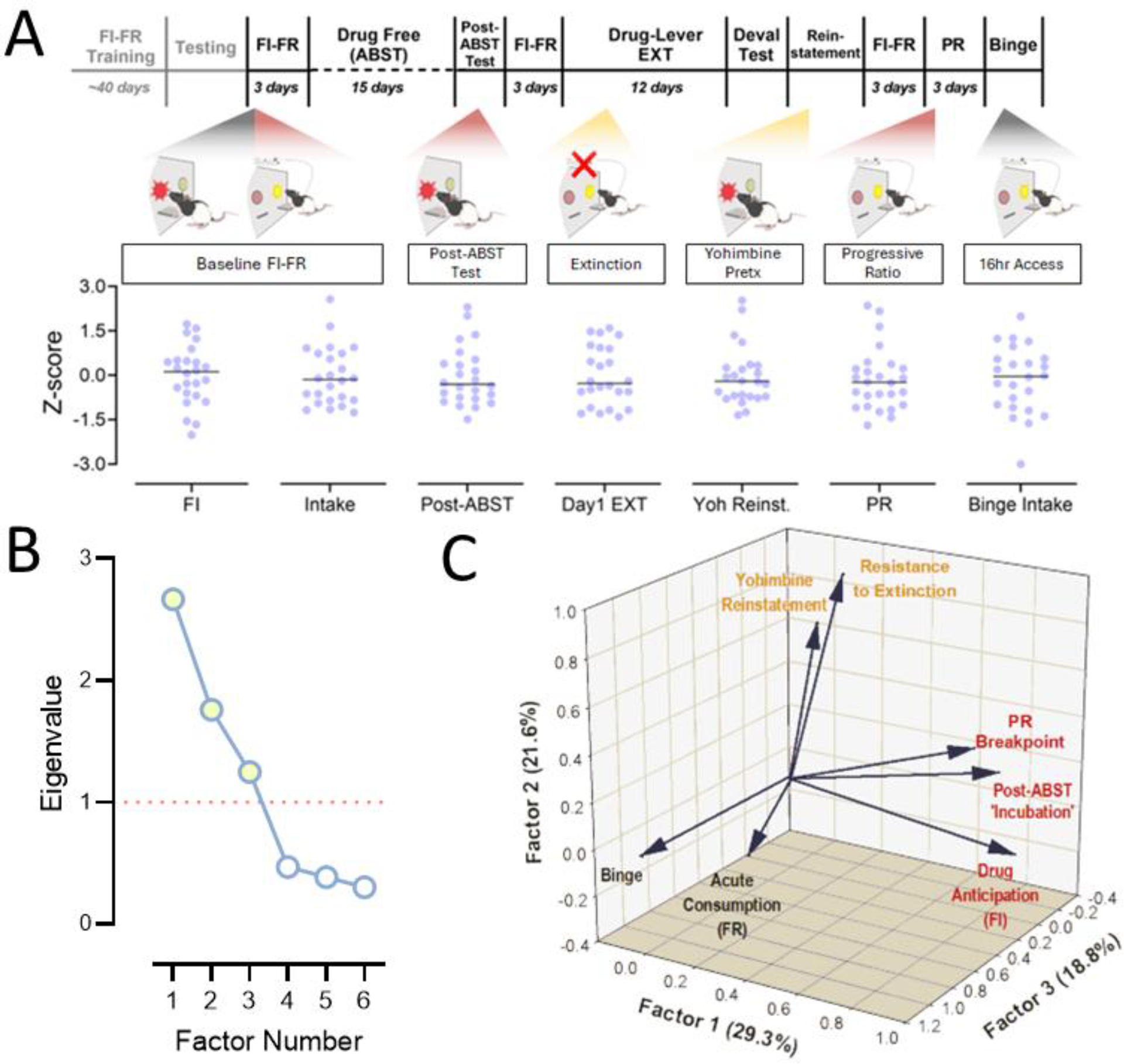
A) Schematic of behavioral measures used in factor analysis with individual z-scores. C) Visual representation of factor loadings onto the 3 extracted factors (of 6 identified), which collectively describe 70% of total variance. B) Scree plot depicts eigenvalues for all components extracted. Filled symbols denote significant values (eigenvalue > 1) selected for further analysis.
Table 1. Factor loadings for each variable used in EFA.
Bolded values denote loadings considered to be important for each factor.
| Factor | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Potential Behavioral Dimension | Appetitive/Motivation | Perseverative/Habitual | Consummatory/Hedonic |
| Variable | |||
| FI-FR Chain: Baseline FI Responses (per 5m) | 0.891 * | −0.112 | 0.0073 |
| FI-FR Chain: Baseline Cocaine Intake (mg/kg/5m) | 0.087 | −0.156 | 0.541 δ |
| Post-ABST Δ in FI Responses | 0.823 * | 0.236 | 0.0369 |
| Extinction (Day 1) Responses | 0.131 | 0.898 * | −0.191 |
| Reinstatement Responses (Yohimbine-Induced) | 0.127 | 0.733 * | 0.0358 |
| Binge Cocaine Intake (mg/kg/16hrs) | −0.119 | −0.0723 | 0.989 * |
| Progressive Ratio Responses | 0.723 * | 0.309 δ | 0.0121 |
| Eigenvalue | 2.666 | 1.759 | 1.247 |
| Proportion of Total Variance | 29.3 | 21.6 | 18.8 |
Strong loading (>0.6)
Moderate loading (>0.3)
DISCUSSION
Anticipated drug availability can engender heightened behavioral arousal and motivation to procure drugs, wherein the intensity of this response is thought to be a critical factor in relapse propensity in humans. In well-trained animals, the present procedure appears to provide sensitive and dissociable measures of anticipatory and consummatory drug use behavior both within a single experimental session, and over the course of prolonged self-administration. Importantly, we show that responding during the fixed-interval (i.e., seeking link) provides a useful operant index of anticipatory arousal prior to expected reward availability – whereby response rates tracked expected reward magnitude, degree of reward-specific satiety, and general motivational state. Moreover, extended periods of forced abstinence specifically enhanced responding to procure access to cocaine upon return to the drug-taking context, while markedly reducing ensuing cocaine consumption. These results, in part, highlight the divergent behavioral mechanisms that control the acquisition and consumption of both drug and non-drug rewards.
Fixed-Interval (Seeking) Performance Detects Incentive Value of Reward Access
The intensity of seeking-lever responses emitted during the FI systematically correlated with expected reward magnitude, which was manipulated by varying available drug dose (Figures 4 & 5). Since dose manipulations were conducted in isolation from the seeking link, no direct action-outcome (A-O) associations could be established between the seeking-lever response and primary drug reinforcement. Therefore, anticipatory behavior observed during revaluation testing can most easily be explained by the conditioned value of access to the drug-taking lever (Balleine and Dickinson 1991, 1998; Gollub 2022). Although the duration of access permitted during the drug-taking phase posed no practical constraints on the amount of drug available – and thus, drug access represent a fixed utility – both humans and animals show a clear preference for higher unit doses and a faster rate delivery (Balster and Schuster 1973; Panlilio et al. 1998; Abreu et al. 2001; Nelson et al. 2006; Schindler et al. 2009). It was presumed that higher doses would be more potently reinforcing, and thus carry higher incentive value. Accordingly, the observed increase in seeking-lever responding after training with the 1.0mg/kg dose of cocaine supports the premise that FIs may effectively describe an incentive process under the present conditions.
These findings are largely consistent with studies conducted under similar experimental parameters, wherein the effects of dose availability on putative drug-seeking behavior are only revealed when a sufficient timeout period is enforced between trials. When rats were allowed to freely initiate trials (i.e., free-operant conditions), cocaine dose had little impact on seeking-lever responding maintained by a random interval (RI-FR; Olmstead et al. 2000; Veeneman et al. 2012b), and was even found to be inversely proportional to response rates under a FI (Dougherty and Pickens 1973). Increasing cocaine doses produced concomitant shifts in the pattern of responding (i.e., index of curvature) and a potential loss of stimulus control, which we did not observe when behavior was measured in a drug-free state (Supplemental Figure S3). Conversely, it has otherwise been shown that drug-seeking responding under several schedules of reinforcement systematically increase with dose after extended inter-trial-intervals, which ensures that behavior is assayed without contamination by unconditioned effects of the self-administered cocaine (Balster and Schuster 1973; Corrigall and Coen 1989; Markou et al. 1999; Olmstead et al. 2000). In the midst of ongoing drug use, effort to procure additional drug is strongly influenced by on-board drug levels – and perhaps, a relative state of satiety – as well as the psychomotor stimulant properties of the drug itself (Wise and Bozarth 1987; Wise et al. 1995; Tsibulsky and Norman 1999). These factors are less relevant to the control of drug-seeking from an undrugged state.
Accordingly, our data reflect that both noncontingent and contingent administration of a cocaine dose that approximates the rat’s typical intake levels results in an apparent satiation or disruptive effect on anticipatory arousal, which is reflected in diminished responding during the FI component, as well as a reduction in cocaine intake once access is acquired (Figure 5; Supplemental Figure S6). Nevertheless, the rate-suppressing effects of pre-session cocaine are absent during later trials, once estimated drug levels subsided. These findings follow an established precedent for cocaine-seeking behavior under second-order schedules in rats and primates (Markou et al. 1999; Howell and Fantegrossi 2009). Although drug ‘satiety’ is not entirely understood, these findings resemble the satiety-like effects on reward-seeking and consumption that have been well established under instrumental chains for food reinforcement (Colwill and Rescorla 1985; Balleine and Dickinson 1998; Corbit et al. 2001), and were herein demonstrated by pre-feeding rats with saccharin prior to measuring for saccharin self-administration under the FI-FR procedure (Figure 6). The fact that rats adjust drug intake in order to achieve similar drug levels after a pretreatment dose further demonstrates a propensity to maintain a ‘set-point’ level of on-board cocaine, and provides an important validation for the continuous access (FR) period as a sensitive intake measure. Collectively, these findings highlight that both phases of the seeking-taking chain are sufficiently sensitive to effects of satiety, whereby the incentive to acquire drug is presumably reduced.
Fixed-Interval Performance Resembles Goal-Directed Action
Implicit knowledge of the contingencies between seeking-lever responding and access to a reinforced operandum (i.e., A-O association) appear critical to reward-seeking behavior under these conditions, whereby degradation of the A-O contingency by extinction of the terminal link was sufficient to suppress responding during the fixed-interval for nearly every animal – even after >60 days of training (Figure 4). Since extinction training was conducted in the absence of the drug-seeking component, the predictive reliability for a seeking-lever responses should have remained intact. As such, FI responses reliably produced access to a less valuable reinforcer (i.e., saline), which is reflected in reduced seeking-lever engagement. Fixed-interval performance under the present conditioned, therefore, appears to comprise a goal-directed process (Balleine and Dickinson 1998).
Prolonged cocaine self-administration under chained schedules has previously been shown to produce inflexible operant behavior that is insensitive to devaluation by punishment (Vanderschuren and Everitt 2004; Pelloux et al. 2007) or extinction of the drug-taking response (Zapata et al. 2010), which has largely been interpreted to reflect a pathological shift towards habit-like or compulsive behavior (Lüscher et al. 2020). The observation that A-O behavioral control was preserved throughout the self-administration is likely a consequence of the fixed-interval contingency, as opposed to random interval (RI). Indeed, the extent to which behavior remains goal-directed positively correlates with the predictability of reward availability in proximity to action (DeRusso 2010; Garr et al. 2020). Whereas temporal uncertainty, such as in a random or variable schedule, tends to promote instrumental behavior that is largely divorced from value of the goal. This is supported by the finding that mean IoC values strongly predicted whether an individual rat would be sensitive to outcome devaluation (Supplemental Figure S4), where animals that demonstrated effective interval timing also maintained the highest degree of goal-directedness.
Procedural Considerations: Preclinical Models of Drug-Seeking
The motivational properties of self-administered drugs have most commonly been studied using procedures that systematically alter the number of responses required to achieve a unit of reward. Protocols such as Progressive Ratio (Hodos 1961; Richardson and Roberts 1996), or those designed for behavioral economic analysis (i.e., Threshold Procedures; see Oleson and Roberts 2009), rely on peak behavioral output in the face of increasing effort requirements as a metric of reinforcing strength. However, in the context of intravenous self-administration, the procurement and consumption of a reward are achieved by the same action, which runs in contrast to the discrete processes of obtaining and consuming drugs in the human condition (Roberts et al. 2013). As these two forms of behavior are differentially controlled (see Supplemental Figure S2), occasionally in opposing directions (See Figure 8B), single-lever procedures can yield ambiguous interpretations about the processes underlying drug self-administration.
Indeed, several previous approaches have been explored as a means of dissociating the appetitive and consummatory behavior in IV self-administration models (See Roberts et al. 2013, for review). While the present FI-FR seeking-taking chain shares several important commonalities with previously described procedures, it is important to acknowledge a few key differences which meaningfully contribute to the stimulus control of behavior, and underlying interpretations; most notably, the on-board drug status and role of action-outcome contingencies in behavioral control.
Assessing Behavior Under Drugged vs. Drug-Free Conditions
The vast majority of procedures designed to assess drug-seeking behavior, including conventional seeking-taking chains (Olmstead et al. 2000, 2001; Vanderschuren and Everitt 2004; Veeneman et al. 2012a, b) and second order schedules of reinforcement (Goldberg et al. 1975; Arroyo et al. 1998; Markou et al. 1999), are conducted under free-operant conditions. That is, drug is continuously available for subjects to initiate a seeking/taking sequence at will. With the notable exception of the initial seeking event, all subsequent drug-seeking behavior is inseparable from the unconditioned activational effects of accumulating infusions achieved throughout the session. Indeed, it has been shown that progressive ratio breakpoint measures are dramatically influenced by cumulative reinforcement across testing. Whereas breakpoints for cocaine can be collected entirely in a drug-free state by presenting increasing ratio requirements as a contingency to initiate self-administration on successive days, rats were shown to achieve ~2.5-fold higher ratios when evaluated within-session (Gancarz et al. 2012). Because it is likely that drug-seeking behavior in a ‘drugged’ state involves distinct neural substrates, these conditions may not be appropriate for the characterization of the motivational mechanisms that drive the initiation of drug use from a drug-free state.
Factors that influence drug consumption, on the other hand, were clearly delineated via factor analysis (Figure 9). Behavior measured under the continuous reinforcement phase of the FI-FR chain was strongly associated with a latent variable (i.e., Factor 1) that similarly correlated with cocaine intake over a 16-hr, unlimited access ‘binge’. Although collected over dramatically different timescales, both intake variables occupied were closely related and occupied a distinct dimensional space from putative drug-seeking measures. This aligns with our finding that drug taking was tightly controlled by on-board drug level (Figure 5), and the propensity for rats to titrate cocaine intake about a ‘set-point’ level under unlimited access conditions (Pickens and Thompson 1968; Gerber and Wise 1989; Wise et al. 1995; Morgan et al. 2009; Zimmer et al. 2011) – though, this relationship begins to degrade beyond 12–16hrs of self-administration, with the onset of circadian phase transition (Bozarth and Wise 1985; Fitch and Roberts 1993; Tornatzky and Miczek 2000; Covington et al. 2005; Bass et al. 2010; Leonard et al. 2017; Valenza et al. 2020; Stowe et al. 2022)
Under the present conditions, independent behavioral dimensions of drug-seeking and -taking were carefully determined within a single session. This provides a significant advantage towards the measurement and manipulation of appetitive and consummatory drug-motivated processes under uniform experimental conditions. Nevertheless, although estimated cocaine levels in the brain were presumed to be negligeable prior to the onset of each trial (Figure 1D), it may be necessary to constrain analyses to the first trial. In some instances, fixed-interval response rates tended to increase across consecutive trials regardless of inter-trial interval durations (i.e., drug-washout). Accordingly, ongoing drug-seeking to maintain drug consumption within a bout of drug-taking (i.e., a binge) may be further dissociated from drug procurement, beyond the acute stimulant effects of cocaine. This may be related to transient dysregulation of dopamine pharmacodynamics, which have been shown to persist for hours beyond the offset of cocaine’s direct pharmacological actions (Mateo et al. 2005; Zimmer et al. 2012).
Assessing Behavior Under Extinction vs. Non-Extinction Conditions
Perhaps the most ubiquitous approach to evaluating drug-seeking in preclinical models are reinstatement procedures, which ostensibly aim to recapitulate relapse-like behavior in animal models (Venniro et al. 2016; Mantsch et al. 2016; Fredriksson et al. 2021). These procedures are particularly attractive due to the consistent finding that previously extinguished responding for various drugs of abuse can be reactivated by exposure to stimuli that are known to precipitate relapse in humans, such as acute exposure to the self-administered dug (De Wit and Stewart 1981; Leonard et al. 2020), drug-associated cues (Meil and See 1996; Bossert et al. 2007), contexts (Fuchs et al. 2006; Holly et al. 2016) or stressors (Erb et al. 1996; Mantsch et al. 2016). Despite the apparent face-validity, there are reasonable concerns regarding whether extinction training approximates the conditions in which human dug users abstain from use (Hughes 2002; Katz and Higgins 2003; Epstein et al. 2006). Extinction is an active learning process that has been shown to mediate unique neuroadaptations that are not produced by cocaine self-administration alone (Self 2004; Knackstedt et al. 2010). Additionally, reinstatement tests necessarily assay drug-seeking behavior under extinction conditions – wherein drug reinforcement cannot be obtained – under the questionable assumption that all non-reinforced responses recorded under unfamiliar contingencies are fundamentally equivalent. Indeed, cue-elicited responding engages distinct neural circuitry depending on testing followed extinction training or a period of abstinence (Fuchs et al. 2006).
Perseverative modes of behavior – which persist in spite of devalued outcome contingencies – represent a prominent latent factor undergirding our behavioral observations (Figure 9). Most notably, both the responding under extinction and yohimbine-reinstated responding after extinction/devaluation occupied this dimensional space. Since the yohimbine-evoked responses were collected during the FI component, it seems unlikely that this factor represents the capacity for the drug-CS to drive behavior (i.e., conditioned reinforcement), as the drug-paired CS was only present during the drug-taking link. While yohimbine has consistently been shown to reinstate extinguished cocaine-seeking behaviors (Lee et al. 2004; Feltenstein and See 2006; Kupferschmidt et al. 2009), its effects can be nonspecific to prior cue-reward history (Chen et al. 2015), and has been shown to generally exacerbate perseverative behaviors (Caetano et al. 2013; Schwager et al. 2014). That yohimbine also abolished the temporal control of FI responding further suggests that the reinstatement of extinguished responding may fundamentally deviate from what is typically captured by the FI (Figure 7C). Indeed, yohimbine failed to influence basal rates of behavior when administered acutely prior to devaluation training, wherein the structure of responding was intact (Supplemental Figure S7). Thus, yohimbine does not appear to produce a generalized disruption of behavioral control, but may drive previously extinguished actions in a unique manner.
One should not discount the observable associations between stimulant dependence with inflexible response perseveration in the clinical population – which are theorized to underlie compulsive drug use (Ersche et al. 2011, 2016; Everitt and Robbins 2016). Yet, there is considerable uncertainty as to which aspects of drug use are subserved by habit-like actions like those which violate established action-outcome contingencies, such as in extinction (Silverman et al. 1999; Hogarth et al. 2019; Epstein 2020).
Anticipatory Arousal Reflects Discriminative Stimulus Control of Drug-Seeking
‘Drug-seeking’ under the FI-FR chain schedule is better understood as a function of discriminative stimuli which, by definition, elicits behavior directed at procuring a reinforcer by indicating its availability (Weiss 2017) – in this case, the lever-protraction and cue light that is illuminated throughout the interval, as well as internal timing processes that provide a running estimate of impending reward availability. Indeed, while propensity to seek out drug may be influenced by exposure to discrete contexts or stimuli associated with acute drug experience (i.e., conditioned stimuli; CS), the mere perception of an imminent drug use opportunity is sufficient to evoke intense subjective cravings, attentional bias and physiological arousal in clinical observations (Wertz and Sayette 2001; Yamamoto et al. 2007; Jedras et al. 2013). Under naturalistic condition, drug-users report suppressed cravings in contexts which drugs are inaccessible, such as the workplace or during air travel, yet experience intensifying cravings in the hours and minutes leading to prospective drug-taking opportunities (Dar et al. 2010; Phillips et al. 2013). This is recapitulated in the sparse human self-administration literature, where cocaine available under a fixed-interval engenders an accelerated response scallop similar to what we demonstrate in rats (see Figure 2; Henningfield et al. 1987; Panlilio et al. 2005; Risinger et al. 2005), and which coincides with escalating self-reported craving scores across the interval (Risinger et al. 2005). Thus, we contend that responding during the fixed-interval captures an important dimension of behavior that is relevant to the processes by which subjective craving motivates action, in humans.
It is well-established that SD or CS exposure engages distinct drug-seeking modalities (Di Ciano and Everitt 2003; Namba et al. 2018; Batten and Beckmann 2018; Collins et al. 2023), which are illustrated by the capacity for a cocaine priming injection to reinstate behavior under the FI-FR chain.. As described above, extinction of the drug-taking response fundamentally redefines the ‘goal’ of reward procurement behavior (Thrailkill and Bouton 2016; 2017), and it is therefore unsurprising that cocaine failed to further elicit responding during the FI. Indeed, while SDs can be effective in reinstating responding when isolated from the extinction training in a single lever task (Weiss et al. 2000; Ciccocioppo et al. 2001) – presumably sparing its predictive value – SDs lose their response-eliciting efficacy over repeated extinction trials, which cannot be recovered by cocaine priming (Ndiaye et al. 2024). On the other hand, non-contingent cocaine potently re-invigorated drug-lever responses, likely reflecting the potentiation of conditioned reinforcement (Figure 7B). It is notable that the CS was presented throughout extinction training and, therefore, on-board cocaine may serve as an interoceptive stimulus that sets the occasion for reinforced behavior by restoring the internal conditions on which response-CS associations were initially established (Thompson and Pickens 1971; Huang and Riley 2024).
Of significance, in regard to the relative saliency of drug-predictive stimuli controlling drug-seeking across protracted abstinence, rats exhibited a progressive enhancement of responding during the fixed-interval with increasing drug-deprivation. There were no appreciable changes to behavior maintained by access to saccharin. This parallels some previous observations, in that stimuli that set the occasion for cocaine availability appear to take on increasing incentive properties, reminiscent of an ‘incubation’-like effect (Weiss et al. 2000; Ciccocioppo et al. 2001; Madangopal et al. 2019; Ndiaye et al. 2024). There is still considerable uncertainty about the mechanics of such time-dependent increases in drug-directed behavior – or the extent to which they represent clinically-relevant features of ‘craving’ (Bergeria et al. 2024). However, it is notable that this phenomenon extended to an interval-based response component in which contingencies remained intact, as opposed to an extinction test by which it has been conventionally demonstrated. Further, the scale at which rats individual increased FI responding over abstinence shared dimensional likeness to pre-ABST drug-seeking and progressive ratio performance, which support the idea that withdrawal from cocaine self-administration augments instrumental action towards drug-procurement.
Interestingly, time-dependent increases in seeking-lever engagement were followed by attenuated cocaine intake upon renewed drug access. Future studies may clarify whether reduced drug consumption is a result of diminished tolerance after protracted abstinence, or sensitization to the effects of cocaine (Mutschler et al. 2001; Morgan and Roberts 2004; Calipari et al. 2015).
Summary and Conclusion
In summary, rats performed under a fixed-interval schedule in order to procure access to a period of cocaine or saccharin consumption. Under these conditions the opportunity to engage in the drug-taking response acquires incentive value corresponding to the primary reinforcer – delivery of either cocaine or saccharin. We demonstrate the fixed-interval component of the sequence to be a sensitive and reliable index of motivational changes induced by either the extrinsic incentive value of reinforcement (i.e., anticipated dose) or intrinsic motive states (i.e., satiety or deprivation). Indeed, examining the anticipatory behavioral antecedents of drug-taking may be particularly important to understanding factors that contribute to relapse vulnerability, yet few preclinical models are adequately positioned to assess these features. Furthermore, the temporal structure of the described procedure is well-suited to support in vivo measurement and manipulation of neural circuitry – towards a more precise interrogation of the neurobiological substrates of substance use disorders.
Supplementary Material
Acknowledgments
This work was funded by NIDA grant DA031734 to KAM.
Footnotes
Conflict of Interest
Authors report no conflict of interest.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL (2001) Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology 154:76–84. 10.1007/s002130000624 [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF (1998) Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science 282:298–300. 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- Allain F, Bouayad-Gervais K, Samaha A-N (2018) High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology 235:317–328. 10.1007/s00213-017-4773-8 [DOI] [PubMed] [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ (1998) Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology 140:331–344. 10.1007/s002130050774 [DOI] [PubMed] [Google Scholar]
- Balleine B, Dickinson A (1991) Instrumental performance following reinforcer devaluation depends upon incentive learning. The Quarterly Journal of Experimental Psychology 43:279–296 [Google Scholar]
- Balleine BW, Dickinson A (1998) Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37:407–419. 10.1016/S0028-3908(98)00033-1 [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR (1973) FIXED-INTERVAL SCHEDULE OF COCAINE REINFORCEMENT: EFFECT OF DOSE AND INFUSION DURATION 1. Journal of the Experimental Analysis of Behavior 20:119–129. 10.1901/jeab.1973.20-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Jansen HT, Roberts DCS (2010) FREE-RUNNING RHYTHMS OF COCAINE SELF-ADMINISTRATION IN RATS HELD UNDER CONSTANT LIGHTING CONDITIONS. Chronobiology International 27:535–548. 10.3109/07420521003664221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten SR, Beckmann JS (2018) Differential stimulus control of drug‐seeking: multimodal reinstatement. Addiction Biology 23:989–999. 10.1111/adb.12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, et al. (2011) Incubation of Cue-Induced Cigarette Craving During Abstinence in Human Smokers. Biological Psychiatry 69:708–711. 10.1016/j.biopsych.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeria CL, Gipson CD, Smith KE, et al. (2024) Opioid craving does not incubate over time in inpatient or outpatient treatment studies: Is the preclinical incubation of craving model lost in translation? Neuroscience & Biobehavioral Reviews 160:105618. 10.1016/j.neubiorev.2024.105618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2004) Motivation concepts in behavioral neuroscience. Physiology & Behavior 81:179–209. 10.1016/j.physbeh.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, et al. (2007) Differential Effects of Blockade of Dopamine D1-Family Receptors in Nucleus Accumbens Core or Shell on Reinstatement of Heroin Seeking Induced by Contextual and Discrete Cues. Journal of Neuroscience 27:12655–12663. 10.1523/JNEUROSCI.3926-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA (1985) Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA 254:81–83 [PubMed] [Google Scholar]
- Caetano MS, Jin LE, Harenberg L, et al. (2013) Noradrenergic control of error perseveration in medial prefrontal cortex. Front Integr Neurosci 6:. 10.3389/fnint.2012.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR (2014) Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128:224–232. 10.1111/jnc.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Siciliano CA, Zimmer BA, Jones SR (2015) Brief Intermittent Cocaine Self-Administration and Abstinence Sensitizes Cocaine Effects on the Dopamine Transporter and Increases Drug Seeking. Neuropsychopharmacol 40:728–735. 10.1038/npp.2014.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-W, Fiscella KA, Bacharach SZ, et al. (2015) Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history: Yohimbine and reward history. Addiction Biology 20:690–700. 10.1111/adb.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevée M, Kim CJ, Crow N, et al. (2023) Food Restriction Level and Reinforcement Schedule Differentially Influence Behavior during Acquisition and Devaluation Procedures in Mice. eNeuro 10:ENEURO.0063–23.2023. 10.1523/ENEURO.0063-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F (2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: Reversal by D1 antagonists. Proceedings of the National Academy of Sciences 98:1976–1981. 10.1073/pnas.98.4.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins V, Bornhoft KN, Wolff A, et al. (2023) Hierarchical cue control of cocaine seeking in the face of cost. Psychopharmacology 240:461–476. 10.1007/s00213-022-06218-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Rescorla RA (1985) Postconditioning devaluation of a reinforcer affects instrumental responding. Journal of experimental psychology: animal behavior processes 11:120. [PubMed] [Google Scholar]
- Corbit LH, Balleine BW (2003) Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. Journal of Experimental Psychology: Animal Behavior Processes 29:99–106. 10.1037/0097-7403.29.2.99 [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW (2001) The Role of the Nucleus Accumbens in Instrumental Conditioning: Evidence of a Functional Dissociation between Accumbens Core and Shell. J Neurosci 21:3251–3260. 10.1523/JNEUROSCI.21-09-03251.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM (1989) Fixed-interval schedules for drug self-administration in the rat. Psychopharmacology 99:136–139. 10.1007/BF00634468 [DOI] [PubMed] [Google Scholar]
- Covington HE, Kikusui T, Goodhue J, et al. (2005) Brief Social Defeat Stress: Long Lasting Effects on Cocaine Taking During a Binge and Zif268 mRNA Expression in the Amygdala and Prefrontal Cortex. Neuropsychopharmacol 30:310–321. 10.1038/sj.npp.1300587 [DOI] [PubMed] [Google Scholar]
- Covington III HE, Newman EL, Leonard MZ, Miczek KA (2019) Translational models of adaptive and excessive fighting: an emerging role for neural circuits in pathological aggression. F1000Res 8:963. 10.12688/f1000research.18883.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W (1917) Appetites and Aversions as Constituents of Instincts. Proceedings of the National Academy of Sciences 3:685–688. 10.1073/pnas.3.12.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CW, Sanabria F (2017) Interval timing under a behavioral microscope: Dissociating motivational and timing processes in fixed-interval performance. Learn Behav 45:29–48. 10.3758/s13420-016-0234-1 [DOI] [PubMed] [Google Scholar]
- Dar R, Rosen-Korakin N, Shapira O, et al. (2010) The craving to smoke in flight attendants: Relations with smoking deprivation, anticipation of smoking, and actual smoking. Journal of Abnormal Psychology 119:248–253. 10.1037/a0017778 [DOI] [PubMed] [Google Scholar]
- De Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143 [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for Addiction-like Behavior in the Rat. Science 305:1014–1017. 10.1126/science.1099020 [DOI] [PubMed] [Google Scholar]
- DeRusso AL (2010) Instrumental uncertainty as a determinant of behavior under interval schedules of reinforcement. Front Integr Neurosci 4:. 10.3389/fnint.2010.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PB (1978) Studies on responding under fixed-interval schedules of reinforcement: II. The scalloped pattern of the cumulative record. J Exp Anal Behav 29:67–75. 10.1901/jeab.1978.29-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ (2003) Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behavioral Neuroscience 117:952–960. 10.1037/0735-7044.117.5.952 [DOI] [PubMed] [Google Scholar]
- Dougherty J, Pickens R (1973) FIXED-INTERVAL SCHEDULES OF INTRAVENOUS COCAINE PRESENTATION IN RATS 1. Journal of the Experimental Analysis of Behavior 20:111–118. 10.1901/jeab.1973.20-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH (2020) Let’s agree to agree: a comment on Hogarth (2020), with a plea for not-so-competing theories of addiction. Neuropsychopharmacol 45:715–716. 10.1038/s41386-020-0618-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology 189:1–16. 10.1007/s00213-006-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J (1996) Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology 128:408–412. 10.1007/s002130050150 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Gillan CM, Jones PS, et al. (2016) Carrots and sticks fail to change behavior in cocaine addiction. Science 352:1468–1471. 10.1126/science.aaf3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, et al. (2011) Response Perseveration in Stimulant Dependence Is Associated with Striatal Dysfunction and Can Be Ameliorated by a D2/3 Receptor Agonist. Biological Psychiatry 70:754–762. 10.1016/j.biopsych.2011.06.033 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2016) Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol 67:23–50. 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- Feltenstein M, See R (2006) Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioural Brain Research 174:1–8. 10.1016/j.bbr.2006.06.039 [DOI] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF (1957) Schedules of reinforcement. Appleton-Century-Crofts, East Norwalk [Google Scholar]
- Fitch TE, Roberts DCS (1993) The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug and Alcohol Dependence 33:119–128. 10.1016/0376-8716(93)90053-S [DOI] [PubMed] [Google Scholar]
- Fredriksson I, Venniro M, Reiner DJ, et al. (2021) Animal Models of Drug Relapse and Craving after Voluntary Abstinence: A Review. Pharmacol Rev 73:1050–1083. 10.1124/pharmrev.120.000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry W, Kelleher RT, Cook L (1960) A MATHEMATICAL INDEX OF PERFORMANCE ON FIXED-INTERVAL SCHEDULES OF REINFORCEMENT. Journal of the Experimental Analysis of Behavior 3:193–199. 10.1901/jeab.1960.3-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE (2006) Different Neural Substrates Mediate Cocaine Seeking after Abstinence versus Extinction Training: A Critical Role for the Dorsolateral Caudate-Putamen. Journal of Neuroscience 26:3584–3588. 10.1523/JNEUROSCI.5146-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarz AM, Kausch MA, Lloyd DR, Richards JB (2012) Between-session progressive ratio performance in rats responding for cocaine and water reinforcers. Psychopharmacology 222:215–223. 10.1007/s00213-012-2637-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garr E, Bushra B, Tu N, Delamater AR (2020) Goal-directed control on interval schedules does not depend on the action–outcome correlation. Journal of Experimental Psychology: Animal Learning and Cognition 46:47–64. 10.1037/xan0000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH (1986) Abstinence Symptomatology and Psychiatric Diagnosis in Cocaine Abusers: Clinical Observations. Arch Gen Psychiatry 43:107. 10.1001/archpsyc.1986.01800020013003 [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Wise RA (1989) Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: A variable dose paradigm. Pharmacology Biochemistry and Behavior 32:527–531. 10.1016/0091-3057(89)90192-5 [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Kelleher RT, Morse WH (1975) Second-order schedules of drug injection. In: Weiss B, Laties VG (eds) Behavioral Pharmacology. Springer US, Boston, MA, pp 35–44 [Google Scholar]
- Gollub L (2022) Conditioned Reinforcement: Schedule Effects. In: Handbook of Operant Behavior. Routledge, pp 288–312 [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Incubation of cocaine craving after withdrawal. Nature 412:141–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhardi P, Church RM (2004) Measures of temporal discrimination in fixed-interval performance: A case study in archiving data. Behavior Research Methods, Instruments, & Computers 36:661–669. 10.3758/BF03206548 [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Nemeth-Coslett R, Katz JL, Goldberg SR (1987) Intravenous cocaine self-administration by human volunteers: second-order schedules of reinforcement. NIDA Res Monogr 76:266–273 [PubMed] [Google Scholar]
- Herrnstein RJ, Morse WH (1957) Some effects of response-independent positive reinforcement on maintained operant behavior. Journal of Comparative and Physiological Psychology 50:461–467. 10.1037/h0041506 [DOI] [PubMed] [Google Scholar]
- Hodos W (1961) Progressive Ratio as a Measure of Reward Strength. Science 134:943–944. 10.1126/science.134.3483.943 [DOI] [PubMed] [Google Scholar]
- Hogarth L, Lam‐Cassettari C, Pacitti H, et al. (2019) Intact goal‐directed control in treatment‐seeking drug users indexed by outcome‐devaluation and Pavlovian to instrumental transfer: critique of habit theory. Eur J Neurosci 50:2513–2525. 10.1111/ejn.13961 [DOI] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, et al. (2016) Episodic Social Stress-Escalated Cocaine Self-Administration: Role of Phasic and Tonic Corticotropin Releasing Factor in the Anterior and Posterior Ventral Tegmental Area. J Neurosci 36:4093–4105. 10.1523/JNEUROSCI.2232-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Fantegrossi WE (2009) Intravenous drug self-administration in nonhuman primates. Methods of behavior analysis in neuroscience 2: [PubMed] [Google Scholar]
- Huang S, Riley AL (2024) Drug discrimination learning: Interoceptive stimulus control of behavior and its implications for regulated and dysregulated drug intake. Pharmacology Biochemistry and Behavior 244:173848. 10.1016/j.pbb.2024.173848 [DOI] [PubMed] [Google Scholar]
- Hughes JR (2002) Is Extinction in Animals The Same as Abstinence in Humans?: Letters to the Editor. Addiction 97:1219–1219. 10.1046/j.1360-0443.2002.00206.x [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J (1996) Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behavioral Neuroscience 110:331–345. 10.1037/0735-7044.110.2.331 [DOI] [PubMed] [Google Scholar]
- Jedras P, Jones A, Field M (2013) The role of anticipation in drug addiction and reward. NAN 1. 10.2147/NAN.S35917 [DOI] [Google Scholar]
- Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168:21–30. 10.1007/s00213-003-1441-y [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233:3587–3602. 10.1007/s00213-016-4393-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, et al. (2010) Extinction Training after Cocaine Self-Administration Induces Glutamatergic Plasticity to Inhibit Cocaine Seeking. Journal of Neuroscience 30:7984–7992. 10.1523/JNEUROSCI.1244-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S (2009) Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacology Biochemistry and Behavior 91:473–480. 10.1016/j.pbb.2008.08.026 [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD (2004) Pharmacological Blockade of α2-Adrenoceptors Induces Reinstatement of Cocaine-Seeking Behavior in Squirrel Monkeys. Neuropsychopharmacol 29:686–693. 10.1038/sj.npp.1300391 [DOI] [PubMed] [Google Scholar]
- Leonard MZ, DeBold JF, Miczek KA (2017) Escalated cocaine “binges” in rats: enduring effects of social defeat stress or intra-VTA CRF. Psychopharmacology 234:2823–2836. 10.1007/s00213-017-4677-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MZ, Rostin P, Hill KP, et al. (2020) The Molecular-Container Calabadion-2 Prevents Methamphetamine-Induced Reinstatement in Rats: A Potential Approach to Relapse Prevention? International Journal of Neuropsychopharmacology 23:401–405. 10.1093/ijnp/pyz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Caprioli D, Marchant NJ (2015) Recent updates on incubation of drug craving: a mini‐review. Addiction Biology 20:872–876. 10.1111/adb.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Robbins TW, Everitt BJ (2020) The transition to compulsion in addiction. Nat Rev Neurosci 21:247–263. 10.1038/s41583-020-0289-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madangopal R, Tunstall BJ, Komer LE, et al. (2019) Discriminative stimuli are sufficient for incubation of cocaine craving. eLife 8:e44427. 10.7554/eLife.44427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, et al. (2016) Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacol 41:335–356. 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Arroyo M, Everitt BJ (1999) Effects of Contingent and Non-Contingent Cocaine on Drug-Seeking Behavior Measured Using a Second-Order Schedule of Cocaine Reinforcement in Rats. Neuropsychopharmacology 20:542–555. 10.1016/S0893-133X(98)00080-3 [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, et al. (2005) Reduced Dopamine Terminal Function and Insensitivity to Cocaine Following Cocaine Binge Self-Administration and Deprivation. Neuropsychopharmacol 30:1455–1463. 10.1038/sj.npp.1300687 [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE (1996) Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behavioural pharmacology [PubMed] [Google Scholar]
- Morgan D, Liu Y, Oleson EB, Roberts DCS (2009) Cocaine self-administration on a hold-down schedule of reinforcement in rats. Psychopharmacology 201:601–609. 10.1007/s00213-008-1328-z [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DCS (2004) Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neuroscience & Biobehavioral Reviews 27:803–812. 10.1016/j.neubiorev.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Covington Iii HE, Miczek KA (2001) Repeated self-administered cocaine “binges” in rats: effects on cocaine intake and withdrawal. Psychopharmacology 154:292–300. 10.1007/s002130000646 [DOI] [PubMed] [Google Scholar]
- Namba MD, Tomek SE, Olive MF, et al. (2018) The Winding Road to Relapse: Forging a New Understanding of Cue-Induced Reinstatement Models and Their Associated Neural Mechanisms. Front Behav Neurosci 12:17. 10.3389/fnbeh.2018.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, et al. (2012) Prefrontal D1 dopamine signaling is required for temporal control. Proceedings of the National Academy of Sciences 109:20726–20731. 10.1073/pnas.1211258109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye NA, Shamleh SA, Casale D, et al. (2024) Relapse after intermittent access to cocaine: Discriminative cues more effectively trigger drug seeking than do conditioned cues. Psychopharmacology. 10.1007/s00213-024-06614-9 [DOI] [PubMed] [Google Scholar]
- Nelson A, Killcross AS (2006) Amphetamine Exposure Enhances Habit Formation. Journal of Neuroscience 26:3805–3812. 10.1523/JNEUROSCI.4305-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, et al. (2006) Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug and Alcohol Dependence 82:19–24. 10.1016/j.drugalcdep.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA (2000) Firing Rate of Nucleus Accumbens Neurons Is Dopamine-Dependent and Reflects the Timing of Cocaine-Seeking Behavior in Rats on a Progressive Ratio Schedule of Reinforcement. J Neurosci 20:5526–5537. 10.1523/JNEUROSCI.20-14-05526.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15–22. 10.1177/026988119801200103 [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC (2009) Behavioral Economic Assessment of Price and Cocaine Consumption Following Self-Administration Histories that Produce Escalation of Either Final Ratios or Intake. Neuropsychopharmacol 34:796–804. 10.1038/npp.2008.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Lafond MV, Everitt BJ, Dickinson A (2001) Cocaine seeking by rats is a goal-directed action. Behavioral Neuroscience 115:394–402. 10.1037/0735-7044.115.2.394 [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, et al. (2000) Cocaine-seeking by rats: regulation, reinforcement and activation. Psychopharmacology 152:123–131. 10.1007/s002130000498 [DOI] [PubMed] [Google Scholar]
- Pan H-T, Menacherry S, Justice JB (1991) Differences in the Pharmacokinetics of Cocaine in Naive and Cocaine-Experienced Rats. J Neurochem 56:1299–1306. 10.1111/j.1471-4159.1991.tb11425.x [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, et al. (1998) Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology 137:253–258. 10.1007/s002130050618 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Nemeth-Coslett R, et al. (2005) Human Cocaine-Seeking Behavior and its Control by Drug-Associated Stimuli in the Laboratory. Neuropsychopharmacol 30:433–443. 10.1038/sj.npp.1300599 [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ (2016) Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry 73:1127. 10.1001/jamapsychiatry.2016.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A (2007) Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology 194:127–137. 10.1007/s00213-007-0805-0 [DOI] [PubMed] [Google Scholar]
- Phillips KA, Epstein DH, Preston KL (2013) Daily temporal patterns of heroin and cocaine use and craving: Relationship with business hours regardless of actual employment status. Addictive Behaviors 38:2485–2491. 10.1016/j.addbeh.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson T (1968) Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther 161:122–129 [PubMed] [Google Scholar]
- Preston KL, Epstein DH (2011) Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl) 218:29–37. 10.1007/s00213-011-2183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods 66:1–11. 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, et al. (2005) Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage 26:1097–1108. 10.1016/j.neuroimage.2005.03.030 [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Gabriele A, Zimmer BA (2013) Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: Methodological and interpretational considerations. Neuroscience & Biobehavioral Reviews 37:2026–2036. 10.1016/j.neubiorev.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, Berridge KC (1993) The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews 18:247–291. 10.1016/0165-0173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Thorndike EB (2009) Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacology Biochemistry and Behavior 93:375–381. 10.1016/j.pbb.2009.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B (2005) Cocaine Makes Actions Insensitive to Outcomes but not Extinction: Implications for Altered Orbitofrontal–Amygdalar Function. Cerebral Cortex 15:1162–1169. 10.1093/cercor/bhh216 [DOI] [PubMed] [Google Scholar]
- Schwager AL, Haack AK, Taha SA (2014) Impaired flexibility in decision making in rats after administration of the pharmacological stressor yohimbine. Psychopharmacology 231:3941–3952. 10.1007/s00213-014-3529-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW (2004) Extinction Training Regulates Neuroadaptive Responses to Withdrawal from Chronic Cocaine Self-Administration. Learning & Memory 11:648–657. 10.1101/lm.81404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML (1999) Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology 146:128–138 [DOI] [PubMed] [Google Scholar]
- Stowe TA, Pitts EG, Leach AC, et al. (2022) Diurnal rhythms in cholinergic modulation of rapid dopamine signals and associative learning in the striatum. Cell Reports 39:110633. 10.1016/j.celrep.2022.110633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM (2005) Extinction of Cocaine Self-Administration Reveals Functionally and Temporally Distinct Dopaminergic Signals in the Nucleus Accumbens. Neuron 46:661–669. 10.1016/j.neuron.2005.04.036 [DOI] [PubMed] [Google Scholar]
- Thompson T, Pickens R (1971) Stimulus Properties of Drugs. Springer New York, Boston, MA [Google Scholar]
- Thrailkill EA, Bouton ME (2016) Extinction of chained instrumental behaviors: Effects of consumption extinction on procurement responding. Learn Behav 44:85–96. 10.3758/s13420-015-0193-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrailkill EA, Bouton ME (2017) Effects of outcome devaluation on instrumental behaviors in a discriminated heterogeneous chain. Journal of Experimental Psychology: Animal Learning and Cognition 43:88–95. 10.1037/xan0000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA (2000) Cocaine self-administration ”binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology 148:289–298. 10.1007/s002130050053 [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB (2021) Methodological and analytical issues of progressive ratio schedules: Definition and scaling of breakpoint. Journal of Neuroscience Methods 356:109146. 10.1016/j.jneumeth.2021.109146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB (1999) Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Research 839:85–93. 10.1016/S0006-8993(99)01717-5 [DOI] [PubMed] [Google Scholar]
- Valenza M, Windisch KA, Butelman ER, et al. (2020) Effects of Kappa opioid receptor blockade by LY2444296 HCl, a selective short-acting antagonist, during chronic extended access cocaine self-administration and re-exposure in rat. Psychopharmacology 237:1147–1160. 10.1007/s00213-019-05444-4 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ (2004) Drug Seeking Becomes Compulsive After Prolonged Cocaine Self-Administration. Science 305:1017–1019. 10.1126/science.1098975 [DOI] [PubMed] [Google Scholar]
- Veeneman MMJ, Broekhoven MH, Damsteegt R, Vanderschuren LJMJ (2012a) Distinct Contributions of Dopamine in the Dorsolateral Striatum and Nucleus Accumbens Shell to the Reinforcing Properties of Cocaine. Neuropsychopharmacol 37:487–498. 10.1038/npp.2011.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MMJ, van Ast M, Broekhoven MH, et al. (2012b) Seeking–taking chain schedules of cocaine and sucrose self-administration: effects of reward size, reward omission, and α-flupenthixol. Psychopharmacology 220:771–785. 10.1007/s00213-011-2525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, et al. (2020) Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci 21:625–643. 10.1038/s41583-020-0378-z [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving. In: Progress in Brain Research. Elsevier, pp 25–52 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M, Baler R (2019) The Neuroscience of Drug Reward and Addiction. Physiological Reviews 99:2115–2140. 10.1152/physrev.00014.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, et al. (2013) Effects of Length of Abstinence on Decision-Making and Craving in Methamphetamine Abusers. PLoS ONE 8:e68791. 10.1371/journal.pone.0068791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, et al. (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proceedings of the National Academy of Sciences 97:4321–4326. 10.1073/pnas.97.8.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Kearns DN, Cohn SI, et al. (2003) STIMULUS CONTROL OF COCAINE SELF-ADMINISTRATION. Journal of the Experimental Analysis of Behavior 79:111–135. 10.1901/jeab.2003.79-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA (2001) A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology 9:3–13. 10.1037/1064-1297.9.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1987) Intravenous Drug Self-Administration: A Special Case of Positive Reinforcement. In: Bozarth MA (ed) Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer New York, New York, NY, pp 117–141 [Google Scholar]
- Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492 [PubMed] [Google Scholar]
- Wise RA, Leeb K, Pocock D, et al. (1995) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 120:10–20 [DOI] [PubMed] [Google Scholar]
- Yamamoto RT, Karlsgodt KH, Rott D, et al. (2007) Effects of perceived cocaine availability on subjective and objective responses to the drug. Subst Abuse Treat Prev Policy 2:30. 10.1186/1747-597X-2-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS (2010) Shift from Goal-Directed to Habitual Cocaine Seeking after Prolonged Experience in Rats. Journal of Neuroscience 30:15457–15463. 10.1523/JNEUROSCI.4072-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Dobrin CV, Roberts DCS (2011) Brain-Cocaine Concentrations Determine the Dose Self-Administered by Rats on a Novel Behaviorally Dependent Dosing Schedule. Neuropsychopharmacol 36:2741–2749. 10.1038/npp.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC (2012) The Motivation to Self-Administer is Increased After a History of Spiking Brain Levels of Cocaine. Neuropsychopharmacol 37:1901–1910. 10.1038/npp.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


