Abstract
BACKGROUND:
The optimal anesthetic approach for patients with acute ischemic stroke with large vessel occlusion but low National Institutes of Health Stroke Scale receiving mechanical thrombectomy remains unclear. We aimed to evaluate the association of anesthetic strategies with procedural and clinical outcomes, hypothesizing that conscious sedation/local anesthesia (CS/LA) may offer a more favorable risk-benefit ratio than general anesthesia (GA).
METHODS:
Multicenter cohort study screening all thrombectomy patients prospectively enrolled in GSR-ET (German Stroke Registry-Endovascular Treatment) across 25 centers between 2015 and 2021. Patients with an admission National Institutes of Health Stroke Scale score of <6 and large vessel occlusion in the anterior circulation underwent 1:1 propensity score matching by their anesthetic strategy during mechanical thrombectomy (CS/LA versus GA). Outcome measures were an excellent functional outcome (modified Rankin Scale score of 0–1 at 90 days) and successful recanalization (modified Thrombolysis in Cerebral Infarction score of 2b-3).
RESULTS:
Of 13 082 thrombectomy cases, 814 had a National Institutes of Health Stroke Scale <6, of whom 36% received CS/LA and 64% received GA. Before matching, CS/LA patients were less often male (46% versus 54%; P=0.043), had lower National Institutes of Health Stroke Scale scores at admission (median, 3 versus 4; P=0.002), and the M1 segment of the middle cerebral artery was more often occluded (51% versus 39%; P<0.001). After matching, 582 patients were included, and baseline and imaging characteristics were balanced between CS/LA and GA. CS/LA and GA patients achieved similar rates of successful recanalization (85% versus 89%; P=0.14). However, complete recanalization (modified Thrombolysis in Cerebral Infarction score of 3) was less often observed in CS/LA patients (45% versus 61%; P<0.001; adjusted odds ratio, 0.44 [95% CI, 0.30–0.65]; P<0.001). CS/LA patients achieved more often excellent functional outcomes (59% versus 48%; P=0.005; adjusted odds ratio, 1.99 [95% CI, 1.34–2.95]; P=0.001).
CONCLUSIONS:
In thrombectomy patients with minor stroke, the rate of successful recanalization was comparable between CS/LA and GA. However, our results suggest a more favorable risk-benefit ratio of CS/LA, with an increased rate of excellent functional outcomes.
Keywords: angiography, cohort studies, registries, stroke, thrombectomy
Mechanical thrombectomy (MT) in patients with low National Institutes of Health Stroke Scale (NIHSS) scores (<6) on admission is being recognized as a potential next frontier of endovascular stroke treatment.1,2 Pending results from the ongoing randomized trials ENDOLOW (Endovascular Therapy for Low NIHSS Ischemic Strokes)3 and MOSTE (Minor Stroke Therapy Evaluation),4 various questions regarding optimal procedural treatment strategies persist, encompassing the optimal anesthetic approach.
Previous studies on patients with baseline NIHSS score ≥6 were inconclusive whether conscious sedation/local anesthesia (CS/LA) or general anesthesia (GA) serves best for patients in endovascular therapy.5–8 While a recently published randomized controlled trial found no difference in functional independence and major periprocedural complications,9 a meta-analysis of 7 randomized controlled trials indicated that applying GA resulted in higher recanalization rates and improved functional recovery 3 months after stroke.10 However, data on anesthetic strategies with respect to patients with an admission NIHSS score of <6 remain scarce.11 While minor stroke symptoms may have a favorable effect on the patients’ cooperativeness and, therefore, may facilitate CS/LA, the involvement of more peripheral vessel segments may mitigate this advantage and require GA due to more tortuous vessel segments and smaller diameters.12–15 However, in light of the unclear treatment effect of MT in patients with minor stroke, a careful risk-benefit assessment is essential when evaluating the potential benefits and risks of GA.
Hence, we aimed to evaluate the association of anesthetic strategies with procedure-related and clinical outcomes in thrombectomy patients with low admission NIHSS score and hypothesized that CS/LA may be associated with a more favorable benefit-risk ratio.
Methods
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request after approval of the GSR-ET (German Stroke Registry-Endovascular Treatment) centers.
Study Design and Participating Centers
This retrospective multicenter cohort study included patients who were prospectively enrolled in GSR-ET from June 2015 to December 2021. The GSR-ET is a continuing, prospective, open-label, multicenter registry that includes patients who underwent MT at 1 of 25 comprehensive stroke centers in Germany (URL: https://www.clinicaltrials.gov; Unique identifier: NCT03356392). All patients with acute ischemic stroke due to large vessel occlusion who subsequently received MT and were aged ≥18 years were included in the registry by the respective stroke center. The ethics committee at Ludwig Maximilian University, Munich, Germany (689-15), granted approval for the GSR-ET. Additionally, local ethics committees granted approval for all participating sites in accordance with their respective regulations. Informed consent for this study was waived after review of the ethics committee of each participating center. This research was conducted following the STROBE guidelines (Strengthening the Reporting of Observational Studies in Epidemiology) for observational studies.16 All procedures adhered to the guidelines set by the Health Insurance Portability and Accountability Act and the Declaration of Helsinki.
Study Inclusion and Exclusion Criteria
The inclusion criteria for this study were as follows: (1) patients with acute ischemic stroke with an occlusion in the anterior circulation due to an isolated occlusion of the intracranial internal carotid artery or the M1 or M2 segment of the middle cerebral artery; (2) baseline NIHSS score of <6; and (3) complete data on baseline NIHSS, anesthetic regimen, recanalization status, and modified Rankin Scale (mRS) at 90 days. Patients with conversion from CS/LA to GA were excluded from the main analyses. CS and LA were grouped together as CS/LA. A detailed illustration of the inclusion and exclusion criteria can be found in Figure S1.
Clinical and Radiological Data Acquisition
Patient characteristics, radiological parameters, and functional outcomes were obtained from the GSR-ET database. Local investigators at each participating center assessed baseline imaging, digital subtraction angiograms, and follow-up imaging. The recanalization status was determined by the treating neurointerventionalist using the modified Thrombolysis in Cerebral Infarction (mTICI) score (without mTICI score of 2c).17 Successful recanalization was defined as a final mTICI score of 2b-3. The patient’s clinical status was assessed on admission, after 24 hours, and after 90 days using the NIHSS and mRS.
Outcome Measures
The primary outcome was an excellent functional outcome, as defined by an mRS score of 0 to 1 at 90 days after treatment.18,19
Secondary outcomes were successful (mTICI score of 2b-3) and complete (mTICI score of 3) recanalization, functional independence (mRS score of 0–2), and an ordinal shift across the mRS at 90 days.
Safety outcomes were a symptomatic intracranial hemorrhage, defined according to ECASS II (European Cooperative Acute Stroke Study-II) as any intracranial hemorrhage within 24 hours accompanied by an increase of at least 4 points on the NIHSS20 and mortality within 90 days after stroke.
Statistical Analysis
Propensity score matching was conducted to minimize the influence of potential confounders and to create comparable groups of patients who received GA and patients who received CS/LA (Table 1). We used the psmatch2 command in Stata (Stata/MP18; StataCorp, TX) using 1:1 matching without replacement, using the nearest neighbor matching algorithm with a caliper of 0.25 and no replacement.21 The covariates used to generate the propensity scores were age, sex, prestroke mRS score, NIHSS at admission, and vessel occlusion site. Absolute standardized difference plots were assessed to compare the distribution of baseline characteristics pre- and post-matching (Figure S2). Adequate balancing was presumed as a standardized difference of <0.1. Standard descriptive statistics were performed to compare anesthetic regimens (CS/LA versus GA) before and after propensity score matching. Continuous variables were analyzed using the Mann-Whitney U test, while categorical variables were examined using the χ2 test. The normality of data distribution was assessed with Shapiro-Wilk tests. Continuous variables are reported as medians with interquartile ranges (IQRs), and categorical variables are presented as counts and percentages. Unadjusted (odds ratio) and adjusted odds ratios were reported, along with their P values and 95% CIs. Analyses on propensity score–matched data were performed with robust SE for clustered data to account for the matched design of the data. The adjusted common odds ratio was estimated using multivariable ordinal logistic regression. The proportional odds assumption was met (Brant test, P=0.48). After propensity score matching, multivariable logistic regression models with the dependent variables successful recanalization (mTICI score of 2b-3) and complete recanalization (mTICI score of 3) were additionally adjusted for Alberta Stroke Program Early CT Score, administration of tPA (tissue-type plasminogen activator), and time from symptom onset/last seen well to admission. Adjustments for all other outcome parameters additionally included successful recanalization (mTICI score of 2b-3; Table S1). We conducted a sensitivity analysis using the aforementioned statistical tests, which included patients who converted from CS/LA to GA. The results are provided in Tables S2 and S3. Statistical significance was defined as a P value of <0.05. The data analysis was conducted using Stata (Stata/MP18).
Table 1.
Patients’ Baseline, Procedural, and Outcome Characteristics
Results
Patients’ Baseline Characteristics
Of 13 082 patients included in GSR-ET, 814 met the inclusion criteria (Figure S1), of whom 36% received CS/LA and 64% received GA. Before matching, CS/LA patients were less often male (46% versus 54%; P=0.043), had lower NIHSS scores at admission (median, 3 versus 4; P=0.002), and the M1 segment of the middle cerebral artery was more often occluded (51% versus 39%; P<0.001), while the M2 segment was less often affected (39% versus 48%; P=0.021). After propensity score matching, 582 patients were included. Of those, 291 patients received GA and 291 patients received CS/LA during endovascular treatment. The distribution of the anesthetic regimen stratified by each respective stroke center can be found in the appendix (Table S4). Baseline characteristics were balanced with a median age of 73 (IQR, 63–81) years, 45% were male, median NIHSS at admission was 3 (IQR, 2–4), and median time from symptom onset/last seen well to admission was 184 (IQR, 77–401) minutes. The median baseline Alberta Stroke Program Early CT Score was 9 (IQR, 8–10), the M1 segment of the middle cerebral artery was the most frequent vessel occlusion site (53%), and intravenous tPA was administered in 41% of cases. Please refer to Table 1 for further statistics on baseline and procedural characteristics.
Outcome Parameters
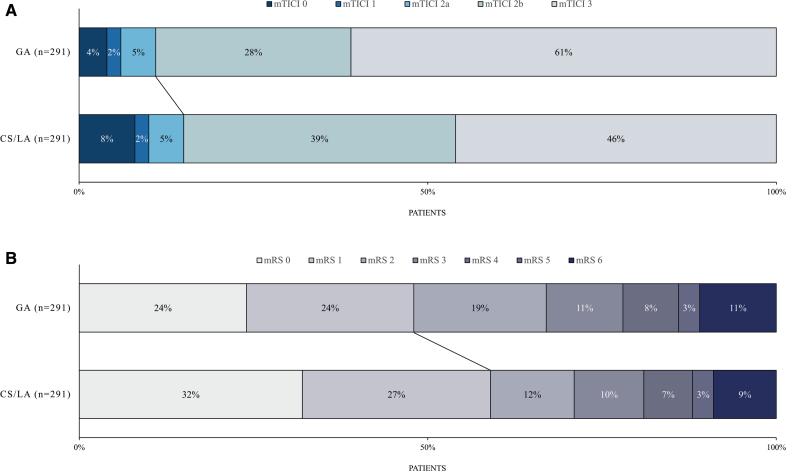
After propensity score matching, CS/LA patients had shorter time intervals from admission to groin puncture (median, 75 versus 87 minutes; P<0.001) and shorter time intervals from admission to flow restoration (median, 120 versus 134 minutes; P<0.001) compared with GA patients. There was no significant difference in the rate of successful recanalization (mTICI score of 2b-3) between patients who underwent CS/LA and those who underwent GA (85% versus 89%; P=0.14; adjusted odds ratio, 0.63 [95% CI, 0.36–1.11]; P=0.11; Table 2). However, complete recanalization (mTICI score of 3) was more often achieved in patients who underwent GA (61% versus 45%; P<0.001; adjusted odds ratio, 0.44 [95% CI, 0.30–0.65]; P<0.001). Patients who were treated under CS/LA exhibited more often excellent functional outcomes at 90 days (59% versus 48%; P=0.005; adjusted odds ratio, 1.99 [95% CI, 1.34–2.95]; P=0.001), as defined by a 90-day mRS score of 0 to 1. In addition, CS/LA patients had higher rates of functional independence (mRS score of 0–2) 90 days after treatment (71% versus 67%; P=0.28; adjusted odds ratio, 1.53 [95% CI, 1.01–2.33]; P=0.04). Patients with CS/LA had lower mRS scores 90 days after treatment (median, 1 versus 2; P=0.018) and exhibited lower odds of a shift in the distribution of mRS scores toward higher values (adjusted common odds ratio, 0.63 [95% CI, 0.45–0.87]; P=0.006). Please also refer to the Figure, which displays the distribution of final angiographic results and mRS scores 90 days after treatment stratified by the anesthetic regimen. A sensitivity analysis, including patients who converted from CS/LA to GA, revealed comparable results (Tables S1 and S2).
Table 2.
Functional and Safety Outcomes After Propensity Score Matching
Figure.
Final recanalization results and functional outcomes stratified by the anesthestic strategy. Procedural and functional outcomes of patients with minor stroke undergoing mechanical thrombectomy. Distribution of the final recanalization result (A) and functional outcome 90 days after treatment (B) stratified by anesthetic strategy during endovascular procedure. Please note that there was no significant difference in successful recanalization (modified Thrombolysis in Cerebral Infarction [mTICI] score of 2b-3) between patients who underwent general anesthesia (GA) and those with conscious sedation/local anesthesia (CS/LA; straight line in A, 89% vs 85%; P=0.14). However, patients with CS/LA had more often excellent functional outcomes 90 days after treatment (straight line in B, 59% vs 48%; P=0.005). mRS indicates modified Rankin Scale.
Safety Outcomes
A comparison of CS/LA patients with GA patients revealed no significant differences in the incidence of symptomatic intracranial hemorrhage within 24 hours (4% versus 4%; P=0.22) or in mortality within 90 days (9% versus 11%; P=0.41; adjusted odds ratio, 0.64 [95% CI, 0.34–1.19]; P=0.16).
Discussion
In this multicenter propensity score–matched study of low NIHSS patients undergoing MT, we observed no significant difference between the anesthetic procedure used and successful recanalization (mTICI score of 2b-3). CS/LA was associated with shorter time intervals from admission to groin puncture and higher odds of excellent functional outcomes 90 days after treatment. There were no significant differences with regard to safety outcomes between the 2 anesthetic regimens.
The decision as to whether MT should be performed under GA or CS/LA has been investigated predominantly in large vessel occlusion patients with NIHSS scores ≥6. However, the results were ambiguous and inconclusive. While a post hoc analysis of the MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) and the HERMES (Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials) meta-analysis favored CS/LA over GA,22,23 it must be noted that these trials were not intended for this comparison, and there is a potential selection bias due to the requirement of GA for medical reasons (eg, more severe strokes or comorbidities). Consequently, a number of randomized controlled trials have been published in previous years, yet the results remain inconclusive.9,24–31 Most recently, a meta-analysis of 7 randomized controlled trials concluded that there is level A evidence supporting improved recanalization status and level B evidence supporting improved functional recovery when the procedure is conducted under GA. However, these results must be interpreted with caution, as 6 of the 7 included trials were single-center and the availability of specialized neuro-anesthesiologic or neurocritical care teams may not reflect real-world conditions and daily clinical practice. In addition, the most recently published randomized controlled trial, which showed no advantage of GA or CS/LA over the other procedure, was not included in the meta-analysis.9 The lack of clarity persists in the guidelines set forth by the leading stroke associations, as the European Stroke Organization suggests that local anesthesia or CS/LA may be favored over GA, if the patient is able to undergo MT without GA, while the American Heart Association/American Stroke Association states that either method of procedural sedation for acute stroke endovascular procedures is reasonable.32,33
Data comparing anesthetic strategies in thrombectomy patients with low admission NIHSS remain scarce. Given the lack of clarity regarding the efficacy of MT in this patient population, it is of paramount importance to minimize procedural risks and complications, to avoid a treatment-related worsening of the initial functional status before MT. A recently published study of 94 patients with minor stroke found no significant differences in clinical outcomes, rates of reperfusion, and safety outcomes between patients receiving GA or CS/LA. Of note, this study solely included patients with M2 occlusion, and there was no significant difference in door-to-groin times between GA and CS/LA. We observed a longer time interval from admission to groin puncture in patients who had undergone GA. The observed delay of a median of 12 minutes in the GA group is in accordance with the findings of previously published studies and is primarily attributable to the induction and intubation phase.26,28,34 It is conceivable that this time delay may offer a partial explanation for the reduced likelihood of excellent functional outcome observed in patients with GA.8,35 Another hypothesis that has been raised with regard to GA patients and worse clinical outcomes is the potentially transient reduction and variability of blood pressure during induction and extubation. In patients with acute ischemic stroke, particularly those with large vessel occlusion and minor stroke symptoms, cerebral perfusion is contingent upon the presence of favorable collaterals.36–40 Consequently, a reduction in blood pressure may result in the exhaustion of collateral blood supply, which may subsequently lead to infarct progression and worse functional outcomes.41,42
While we did not find a significant difference between the applied anesthetic regimen and successful recanalization (mTICI score of 2b-3), our results yield an independent association between GA and complete recanalization (mTICI score of 3). The immobilization of the patient during GA ensures optimal image quality and facilitates demanding catheter navigation, which may allow for a precise recanalization of even the smallest vascular branches.10,43 Of interest, the higher rate of complete recanalization observed in patients who underwent GA did not seem to translate into superior functional outcomes. Previous research, predominantly comprising patients with an initial NIHSS score of ≥6, has indicated that achieving mTICI 3 recanalization yields superior functional outcomes compared with mTICI 2b recanalization.44,45 However, this may not apply to patients with minor stroke who usually exhibit smaller infarct volumes. Recanalization of the most distal vessel branches might have a limited effect on the already mild symptoms, especially when compared with patients with more severe symptoms. Future research is warranted to determine whether the continuation of MT beyond mTICI 2b is reasonable in patients with minor stroke.
Our study analyzed data from 25 distinct stroke centers in Germany. Given the variability in the availability of specialized neuroanesthesia teams in real-world clinical settings, and the divergence in clinical standards regarding the preferred anesthetic strategy during MT, it is important to underscore that we observed no significant difference with regard to safety outcomes between patients who received CS/LA and those who received GA.
Limitations
This study has limitations. It is conceivable that the observational design may introduce unknown bias and potentially limit the generalizability of our findings. Despite the use of propensity score matching, unidentified confounding factors may have influenced the treatment decision of interventionalists, for instance, in favoring patients for GA who were perceived to be at a higher risk of complications. The anesthetic regimen was not uniformly distributed across centers, with certain hospitals predominantly using GA during MT. This clustering might indicate that center-specific factors, such as institutional protocols and preferences of the neurointerventional teams, may have influenced the choice of anesthesia and could partially explain differences in outcomes. In addition, the retrospective design influences data completeness and accuracy, which we aimed to address by carefully curating our data set. The GSR-ET database is primarily managed from a neurological and neuroradiological perspective and was not specifically designed to capture more procedural anesthetic details, including the agents used, sedation quality, and blood pressure control during GA induction. Moreover our database does not differentiate between patients who received only LA from those who received LA and CS. In our study, only patients with internal carotid artery, M1, and M2, occlusions were included; considering a possible new frontier of MT for even more distal vessel occlusions (eg, M3 or M4 occlusions), it is important to mention that our results have limited generalizability for these patients.
Conclusions
CS/LA and GA yielded similar rates of successful recanalization in patients with minor stroke undergoing MT. However, CS/LA may offer a more favorable benefit-risk ratio, as indicated by higher rates of excellent functional outcome 90 days after treatment.
Article Information
Acknowledgments
The authors acknowledge the GSR-ET (German Stroke Registry-Endovascular Treatment) investigators and the GSR-ET steering committee (Table S5).
Sources of Funding
None.
Disclosures
Dr Flottmann reported receiving personal fees from Eppdata GmbH outside the submitted work. Dr Kniep reported an ownership stake in Eppdata GmbH and compensation from Eppdata GmbH for consultant services outside the submitted work. Dr Faizy reported grants from the German Research Foundation/Deutsche Forschungsgesellschaft (DFG; project number: 411621970) and personal fees from Eppdata GmbH outside the submitted work. Dr Broocks reported grants from the American Society of Neuroradiology and receiving compensation as a speaker from Balt and personal fees from Eppdata GmbH outside the submitted work. Dr Meyer reported receiving compensation as a speaker from Balt and personal fees from Eppdata GmbH outside the submitted work. Dr Thaler reported receiving personal fees from Eppdata GmbH outside the submitted work. Dr Thomalla reported grants from FP7 Health and grants from AstraZeneca. In addition, he reported receiving personal fees from Acandis, Alexion, Amarin, Bayer, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Daiichi Sankyo, Portola, and Stryker outside the submitted work. Dr Fiehler reported stock holdings in Tegus Medical; compensation from Tonbridge for consultant services; stock holdings in Vastrax; compensation from Roche for consultant services; compensation from TG Medical for consultant services; and compensation from Penumbra Inc for consultant services. In addition, he reported an ownership stake in Eppdata GmbH and grants and personal fees from Acandis, Cerenovus, MicroVention, Medtronic, Stryker, and Phenox and grants from Route 92 outside the submitted work. The other authors report no conflicts.
Supplemental Material
Tables S1–S5
Figures S1–S2
STROBE Checklist
APPENDIX
GSR-ET (German Stroke Registry-Endovascular Treatment) investigators:
PD Dr med Anna Alegiani, Prof Dr med Jörg Berrouschot, PD Dr med Tobias Boeckh-Behrens, Dr med Georg Bohner, Prof Dr med Jan Borggrefe, Dr med Albrecht Bormann, Dr med Michael Braun, Prof Dr med Franziska Dorn, Prof Dr med Bernd Eckert, Prof Dr med Ulrike Ernemann, PD Dr med Marielle Ernst, Prof Dr med Jens Fiehler, Prof Dr med Christian Gerloff, Prof Dr med Klaus Gröschel, Prof Dr med Gerhard F. Hamann, Dr med Jörg Hattingen, Dr med Karl-Heinz Henn, Dr med Fee Keil, Prof Dr med Lars Kellert, Dr med Christoffer Kraemer, PD Dr med Ruben Mühl-Benninghaus, Prof Dr med Jan Liman, Dr med Alexander Ludolph, Prof Dr med Christian Nolte, Prof Dr med Omid Nikoubashman, Dr med Martina Petersen, Prof Dr med Gabor Petzold, Prof Dr med Sven Poli, PD Dr med Arno Reich, Prof Dr med Joachim Röther, Dr med Jan Hendrik Schäfer, Maximilian Schell, Prof Dr med Peter Schellinger, PD Dr med Eberhard Siebert, Prof Dr med Florian Stögbauer, Prof Dr med Götz Thomalla, Dr med Steffen Tiedt, Prof Dr med Christoph Trumm, Prof Dr med Timo Uphaus, Dr med Silke Wunderlich, Dr med Sarah Zweynert
Nonstandard Abbreviations and Acronyms
- CS/LA
- conscious sedation/local anesthesia
- ECASS II
- European Cooperative Acute Stroke Study-II
- ENDOLOW
- Endovascular Therapy for Low NIHSS Ischemic Strokes
- GA
- general anesthesia
- IQR
- interquartile range
- MOSTE
- Minor Stroke Therapy Evaluation
- mRS
- modified Rankin Scale
- MT
- mechanical thrombectomy
- mTICI
- modified Thrombolysis in Cerebral Infarction
- NIHSS
- National Institutes of Health Stroke Scale
- tPA
- tissue-type plasminogen activator
A list of all GSR-ET investigators is given in the Appendix.
For Sources of Funding and Disclosures, see page 1197.
Presented in part at the Annual Meeting of the European Society of Neuroradiology, Paris, France, September 21, 2024.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.124.049358.
Contributor Information
Alexander Heitkamp, Email: a.heitkamp@uke.de.
Christian Thaler, Email: c.thaler@uke.de.
Vincent Geest, Email: v.geest@uke.de.
Maximilian Schell, Email: M.Schell@uke.de.
Helge C. Kniep, Email: h.kniep@uke.de.
Gabriel Broocks, Email: gabriel.broocks@gmx.de.
Matthias Bechstein, Email: m.bechstein@uke.de.
Lukas Meyer, Email: lu.meyer@uke.de.
Uta Hanning, Email: u.hanning@uke.de.
Götz Thomalla, Email: thomalla@uke.de.
Jens Fiehler, Email: fiehler@uke.de.
Laurens Winkelmeier, Email: l.winkelmeier@uke.de.
Collaborators: Dr med Anna Alegiani, Prof Dr med Jörg Berrouschot, PD Dr med Tobias Boeckh-Behrens, Dr med Georg Bohner, Prof Dr med Jan Borggrefe, Dr med Albrecht Bormann, Dr med Michael Braun, Prof Dr med Franziska Dorn, Prof Dr med Bernd Eckert, Prof Dr med Ulrike Ernemann, PD Dr med Marielle Ernst, Prof Dr med Jens Fiehler, Prof Dr med Christian Gerloff, Prof Dr med Klaus Gröschel, Prof Dr med Gerhard F. Hamann, Dr med Jörg Hattingen, Dr med Karl-Heinz Henn, Dr med Fee Keil, Prof Dr med Lars Kellert, Dr med Christoffer Kraemer, PD Dr med Ruben Mühl-Benninghaus, Prof Dr med Jan Liman, Dr med Alexander Ludolph, Prof Dr med Christian Nolte, Prof Dr med Omid Nikoubashman, Dr med Martina Petersen, Prof Dr med Gabor Petzold, PD Dr med Sven Poli, PD Dr med Arno Reich, Prof Dr med Joachim Röther, Dr med Jan Hendrik Schäfer, Prof Dr med Peter Schellinger, PD Dr med Eberhard Siebert, Prof Dr med Florian Stögbauer, Dr med Steffen Tiedt, Prof Dr med Christoph Trumm, Dr med Timo Uphaus, Dr med Silke Wunderlich, and Dr med Sarah Zweynert
References
- 1.Seners P, Cereda CW. Thrombectomy in stroke with a large vessel occlusion and mild symptoms: “striving to better, oft we mar what’s well?”. Stroke. 2023;54:2276–2278. doi: 10.1161/STROKEAHA.123.044205 [DOI] [PubMed] [Google Scholar]
- 2.Sarraj A, Hassan A, Savitz SI, Grotta JC, Cai C, Parsha KN, Farrell CM, Imam B, Sitton CW, Reddy ST, et al. Endovascular thrombectomy for mild strokes: how low should we go? Stroke. 2018;49:2398–2405. doi: 10.1161/STROKEAHA.118.022114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira RG. Endovascular therapy for low NIHSS ischemic strokes. clinicaltrials.gov; 2022. [cited June 8, 2023]. https://clinicaltrials.gov/ct2/show/NCT04167527 [Google Scholar]
- 4.University Hospital, Montpellier. Evaluation of acute mechanical revascularization in large vessel occlusion stroke with minor symptoms (NIHSS<6) in patients last seen well < 24 hours. clinicaltrials.gov; 2022. [cited June 8, 2023]. https://clinicaltrials.gov/ct2/show/NCT03796468 [Google Scholar]
- 5.Schönenberger S, Hendén PL, Simonsen CZ, Uhlmann L, Klose C, Pfaff JAR, Yoo AJ, Sørensen LH, Ringleb PA, Wick W, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. 2019;322:1283–1293. doi: 10.1001/jama.2019.11455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal N, Malhotra K, Ishfaq MF, Tsivgoulis G, Nickele C, Hoit D, Arthur AS, Alexandrov AV, Elijovich L. Current evidence for anesthesia management during endovascular stroke therapy: updated systematic review and meta-analysis. J Neurointerv Surg. 2019;11:107–113. doi: 10.1136/neurintsurg-2018-013916 [DOI] [PubMed] [Google Scholar]
- 7.Meyer L, Stracke CP, Broocks G, Wallocha M, Elsharkawy M, Sporns PB, Piechowiak EI, Kaesmacher J, Maegerlein C, Petzsche MRH, et al. Effect of anesthetic strategies on distal stroke thrombectomy in the anterior and posterior cerebral artery. J NeuroInterv Surg. 2023;16:230–236. doi: 10.1136/jnis-2023-020210 [DOI] [PubMed] [Google Scholar]
- 8.Feil K, Herzberg M, Dorn F, Tiedt S, Küpper C, Thunstedt DC, Hinske LC, Mühlbauer K, Goss S, Liebig T, et al. ; GSR Investigators. General anesthesia versus conscious sedation in mechanical thrombectomy. J Stroke. 2021;23:103–112. doi: 10.5853/jos.2020.02404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabanne R, Geeraerts T, Begard M, Balança B, Rapido F, Degos V, Tavernier B, Molliex S, Velly L, Verdonk F, et al. ; ANARLF NetworkAMETIS Study Group. Outcomes after endovascular therapy with procedural sedation vs general anesthesia in patients with acute ischemic stroke: the AMETIS randomized clinical trial. JAMA Neurol. 2023;80:474–483. doi: 10.1001/jamaneurol.2023.0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell D, Butler E, Campbell RB, Ho J, Barber PA. General anesthesia compared with Non-GA in endovascular thrombectomy for ischemic stroke. Neurology. 2023;100:e1655–e1663. doi: 10.1212/WNL.0000000000207066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valente I, Alexandre AM, Colò F, Brunetti V, Frisullo G, Camilli A, Falcou A, Scarcia L, Gigli R, Scala I, et al. Effect of general anesthesia versus conscious sedation/local anesthesia on the outcome of patients with minor stroke and isolated M2 occlusion undergoing immediate thrombectomy: a retrospective multicenter matched analysis. World Neurosurg. 2023;183:e432–e439. doi: 10.1016/j.wneu.2023.12.117. [DOI] [PubMed] [Google Scholar]
- 12.Saver JL, Chapot R, Agid R, Hassan AE, Jadhav AP, Liebeskind DS, Lobotesis K, Meila D, Meyer L, Raphaeli G, et al. ; Distal Thrombectomy Summit Group*†. Thrombectomy for distal, medium vessel occlusions. Stroke. 2020;51:2872–2884. doi: 10.1161/STROKEAHA.120.028956 [DOI] [PubMed] [Google Scholar]
- 13.Feil K, Matusevicius M, Herzberg M, Tiedt S, Küpper C, Wischmann J, Schönecker S, Mengel A, Sartor-Pfeiffer J, Berger K, et al. Minor stroke in large vessel occlusion: a matched analysis of patients from the German Stroke Registry–Endovascular Treatment (GSR-ET) and patients from the Safe Implementation of Treatments in Stroke–International Stroke Thrombolysis Register (SITS-ISTR). Eur J Neurol. 2022;29:1619–1629. doi: 10.1111/ene.15272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radu RA, Costalat V, Romoli M, Musmar B, Siegler JE, Ghozy S, Khalife J, Salim H, Shaikh H, Adeeb N, et al. Outcomes with general anesthesia compared to conscious sedation for endovascular treatment of medium vessel occlusions: results of an international multicentric study. Clin Neuroradiol. 2024;34:761–769. doi: 10.1007/s00062-024-01415-1 [DOI] [PubMed] [Google Scholar]
- 15.Psychogios M-N, Tsogkas I, Blackham K, Schulze-Zachau V, Rusche T, Ntoulias N, Brehm A, Fischer U, Sporns PB. The quattro technique for medium distal vessel occlusion stroke. Clin Neuroradiol. 2024;34:257–262. doi: 10.1007/s00062-023-01317-8 [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 17.Heitkamp C, Heitkamp A, Winkelmeier L, Thaler C, Flottmann F, Schell M, Kniep H, Broocks G, Heit JJ, Albers GW, et al. Predictors of futile recanalization in ischemic stroke patients with low baseline NIHSS. Int J Stroke. 2024;19:1102–1112. doi: 10.1177/17474930241264737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safouris A, Palaiodimou L, Nardai S, Kargiotis O, Magoufis G, Psychogios K, Matusevicius M, Feil K, Ahmed N, Kellert L, et al. Medical management versus endovascular treatment for large-vessel occlusion anterior circulation stroke with low NIHSS. Stroke. 2023;54:2265–2275. doi: 10.1161/STROKEAHA.123.043937 [DOI] [PubMed] [Google Scholar]
- 19.Seners P, Arquizan C, Fontaine L, Ben Hassen W, Heldner MR, Strambo D, Nagel S, Carrera E, Mechtouff L, McCullough-Hicks M, et al. ; MINOR-STROKE-Perfusion Collaborators. Perfusion imaging and clinical outcome in acute minor stroke with large vessel occlusion. Stroke. 2022;53:3429–3438. doi: 10.1161/STROKEAHA.122.039182 [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 21.McDonald JS, Brinjikji W, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anaesthesia during mechanical thrombectomy for stroke: a propensity score analysis. J Neurointerv Surg. 2015;7:789–794. doi: 10.1136/neurintsurg-2014-011373 [DOI] [PubMed] [Google Scholar]
- 22.Berkhemer OA, van den Berg LA, Fransen PSS, Beumer D, Yoo AJ, Lingsma HF, Schonewille WJ, van den Berg R, Wermer MJH, Boiten J, et al. ; MR CLEAN investigators. The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87:656–664. doi: 10.1212/WNL.0000000000002976 [DOI] [PubMed] [Google Scholar]
- 23.Campbell BCV, van Zwam WH, Goyal M, Menon BK, Dippel DWJ, Demchuk AM, Bracard S, White P, Dávalos A, Majoie CBLM, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17:47–53. doi: 10.1016/S1474-4422(17)30407-6 [DOI] [PubMed] [Google Scholar]
- 24.Maurice A, Eugène F, Ronzière T, Devys J-M, Taylor G, Subileau A, Huet O, Gherbi H, Laffon M, Esvan M, et al. ; GASS (General Anesthesia Versus Sedation for Acute Stroke Treatment) Study Group and the French Society of Anesthesiologists (SFAR) Research Network. General anesthesia versus sedation, both with hemodynamic control, during intraarterial treatment for stroke: the GASS randomized trial. Anesthesiology. 2022;136:567–576. doi: 10.1097/ALN.0000000000004142 [DOI] [PubMed] [Google Scholar]
- 25.Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, Nagel S, Klose C, Pfaff J, Bendszus M, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316:1986–1996. doi: 10.1001/jama.2016.16623 [DOI] [PubMed] [Google Scholar]
- 26.Simonsen CZ, Yoo AJ, Sørensen LH, Juul N, Johnsen SP, Andersen G, Rasmussen M. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75:470–477. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Löwhagen Hendén P, Rentzos A, Karlsson J-E, Rosengren L, Leiram B, Sundeman H, Dunker D, Schnabel K, Wikholm G, Hellström M, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke. Stroke. 2017;48:1601–1607. doi: 10.1161/STROKEAHA.117.016554 [DOI] [PubMed] [Google Scholar]
- 28.Liang F, Wu Y, Wang X, Yan L, Zhang S, Jian M, Liu H, Wang A, Wang F, Han R; CANVAS II Group. General anesthesia vs conscious sedation for endovascular treatment in patients with posterior circulation acute ischemic stroke: an exploratory randomized clinical trial. JAMA Neurol. 2023;80:64–72. doi: 10.1001/jamaneurol.2022.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren C, Xu G, Liu Y, Liu G, Wang J, Gao J. Effect of conscious sedation vs. general anesthesia on outcomes in patients undergoing mechanical thrombectomy for acute ischemic stroke: a prospective randomized clinical trial. Front Neurol. 2020;11. doi: 10.3389/fneur.2020.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu G, Shi Z, Li B, Shao W, Xu B. General anesthesia versus monitored anesthesia care during endovascular therapy for vertebrobasilar stroke. Am J Transl Res. 2021;13:1558–1567. [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen LH, Speiser L, Karabegovic S, Yoo AJ, Rasmussen M, Sørensen KE, Simonsen CZ. Safety and quality of endovascular therapy under general anesthesia and conscious sedation are comparable: results from the GOLIATH trial. J Neurointerv Surg. 2019;11:1070–1072. doi: 10.1136/neurintsurg-2019-014712 [DOI] [PubMed] [Google Scholar]
- 32.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, Vries J de, White P, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J NeuroInterv Surg. 2023;15:e8–e8. doi: 10.1136/neurintsurg-2018-014569 [DOI] [PubMed] [Google Scholar]
- 33.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Liang F, Wu Y, Zhao Y, Miao Z, Zhang L, Gelb AW, Chan MTV, Peng Y, Han R; CANVAS Pilot Trial Investigators. Choice of Anesthesia for Endovascular Treatment of Acute Ischemic Stroke (CANVAS): results of the CANVAS pilot randomized controlled trial. J Neurosurg Anesthesiol. 2020;32:41–47. doi: 10.1097/ANA.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 35.Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36:525–529. doi: 10.3174/ajnr.A4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seners P, Turc G, Oppenheim C, Baron J-C. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015;86:87–94. doi: 10.1136/jnnp-2014-308327 [DOI] [PubMed] [Google Scholar]
- 37.Yedavalli V, Koneru M, Hamam O, Hoseinyazdi M, Marsh EB, Llinas R, Urrutia V, Leigh R, Gonzalez F, Xu R, et al. Pretreatment CTP collateral parameters predict good outcomes in successfully recanalized middle cerebral artery distal medium vessel occlusions. Clin Neuroradiol. 2024;34:341–349. doi: 10.1007/s00062-023-01371-2 [DOI] [PubMed] [Google Scholar]
- 38.Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Broocks G, Flottmann F, Meyer L, Leischner H, Lansberg MG, et al. Distinct intra-arterial clot localization affects tissue-level collaterals and venous outflow profiles. Eur J Neurol. 2021;28:4109–4116. doi: 10.1111/ene.15079 [DOI] [PubMed] [Google Scholar]
- 39.Heitkamp C, Winkelmeier L, Heit JJ, Flottmann F, Thaler C, Kniep H, Broocks G, Meyer L, Geest V, Albers GW, et al. The negative effect of aging on cerebral venous outflow in acute ischemic stroke. J Cereb Blood Flow Metab. 2023;43:1648–1655. doi: 10.1177/0271678x231179558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heitkamp C, Winkelmeier L, Heit JJ, Albers GW, Lansberg MG, Wintermark M, Broocks G, van Horn N, Kniep HC, Sporns PB, et al. Unfavorable cerebral venous outflow is associated with futile recanalization in acute ischemic stroke patients. Eur J Neurol. 2023;30:2684–2692. doi: 10.1111/ene.15898 [DOI] [PubMed] [Google Scholar]
- 41.Petersen NH, Ortega-Gutierrez S, Wang A, Lopez GV, Strander S, Kodali S, Silverman A, Zheng-Lin B, Dandapat S, Sansing LH, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke. 2019;50:1797–1804. doi: 10.1161/STROKEAHA.118.024286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heitkamp C, Winkelmeier L, Flottmann F, Schell M, Kniep H, Broocks G, Thaler C, Steffen P, Thomalla G, Fiehler J, et al. Thrombectomy patients with minor stroke: factors of early neurological deterioration. J Neurointerv Surg. 2024;jnis-2024-021930. doi: 10.1136/jnis-2024-021930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kniep H, Meyer L, Broocks G, Bechstein M, Heitkamp C, Winkelmeier L, Faizy T, Brekenfeld C, Flottmann F, Deb-Chatterji M, et al. ; German Stroke Registry-Endovascular Treatment (GSR-ET). Thrombectomy for M2 occlusions: predictors of successful and futile recanalization. Stroke. 2023;54:2002–2012. doi: 10.1161/STROKEAHA.123.043285 [DOI] [PubMed] [Google Scholar]
- 44.Kaesmacher J, Dobrocky T, Heldner MR, Bellwald S, Mosimann PJ, Mordasini P, Bigi S, Arnold M, Gralla J, Fischer U. Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: success revisited. J Neurol Neurosurg Psychiatry. 2018;89:910–917. doi: 10.1136/jnnp-2017-317602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleine JF, Wunderlich S, Zimmer C, Kaesmacher J. Time to redefine success? TICI 3 versus TICI 2b recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg. 2017;9:117–121. doi: 10.1136/neurintsurg-2015-012218 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request after approval of the GSR-ET (German Stroke Registry-Endovascular Treatment) centers.