Significance
Accumulation of essential metals in the brain induces incurable neuromotor disease. Mechanisms are unknown, which has limited therapeutic development. Here, we focus on manganese-induced motor disease, which is a major public health problem. Using a combination of unbiased transcriptomics and metabolomics followed by functional assays, we define upregulation of the kynurenine pathway of tryptophan degradation, dependent in part on hypoxia-inducible factor signaling, as a fundamental mechanism of manganese-induced motor disease in mice. Pharmacological inhibition of kynurenine metabolism attenuates manganese-induced neuromotor deficits. These findings identify an unexpected mechanism of manganese-induced motor disease that may be amenable to pharmacological intervention for therapy and that may widely influence investigations on mechanisms of neuromotor disease induced by other essential metals.
Keywords: manganese, motor disease, metal homeostasis, metabolism, multi-omics
Abstract
Elevated brain levels of the essential metals manganese (Mn), copper, or iron induce motor disease. However, mechanisms of metal-induced motor disease are unclear and treatments are lacking. Elucidating the mechanisms of Mn-induced motor disease is particularly important because occupational and environmental Mn overexposure is a global public health problem. To address this, here we combined unbiased transcriptomics and metabolomics with functional studies in a mouse model of human environmental Mn exposure. Transcriptomics unexpectedly revealed that Mn exposure up-regulated expression of metabolic pathways in the brain and liver. Notably, genes in the kynurenine pathway of tryptophan metabolism, which produces neuroactive metabolites that impact neurological function, were up-regulated by Mn. Subsequent unbiased metabolomics revealed that Mn treatment altered kynurenine pathway metabolites in the brain and liver. Functional experiments then demonstrated that pharmacological inhibition of the first and rate-limiting step of the kynurenine pathway fully rescued Mn-induced motor deficits. Finally, elevated Mn directly activates hypoxia-inducible factor (HIF) transcription factors, and additional mechanistic assays identified a role for HIF1, but not HIF2, in regulating expression of hepatic kynurenine pathway genes under physiological or Mn exposure conditions, suggesting that Mn-induced HIF1 activation may contribute to the dysregulation of the kynurenine pathway in Mn toxicity. These findings (1) identify the upregulation of the kynurenine pathway by elevated Mn as a fundamental mechanism of Mn-induced motor deficits; (2) provide a pharmacological approach to treat Mn-induced motor disease; and (3) should broadly advance understanding of the general principles underlying neuromotor deficits caused by metal toxicity.
The essential metals manganese (Mn), iron (Fe), and copper (Cu) are necessary for life but induce toxicity at elevated levels. In humans, at elevated levels, Mn, Fe, or Cu accumulate in the brain, particularly in the basal ganglia, and induce movement/motor disorders (1–4). Dystonia and parkinsonism with basal ganglia Cu accumulation are a characteristic feature of the neurological presentation of Wilson’s disease, which occurs due to a genetic defect in Cu excretion (1). Basal ganglia Fe accumulation and an increased risk of movement disorders associate with the most important genetic risk factor for hereditary hemochromatosis, an Fe overload disease (2). Diseases that constitute neurodegeneration with brain iron accumulation are also characterized by basal ganglia Fe accumulation, and parkinsonism/movement disorders are among the prominent clinical manifestations of these diseases (3). Finally, elevated brain Mn levels are a well-established cause of parkinsonism and motor deficits (4). Despite the causal relationship between brain metal accumulation and motor disorders, underlying mechanisms of metal-induced motor deficits are unclear. Consequently, definitive treatments are not available, and current therapeutic options (e.g., chelation) have limited efficacy.
Among the essential metals, Mn-induced motor disease has high public health relevance. Individuals may be overexposed to Mn, and therefore at risk of developing Mn-induced motor deficits, from occupational (e.g. welding, mining, etc.) or environmental (e.g., drinking water) sources (4). Occupationally exposed adults usually develop a parkinsonian-like disease while environmentally exposed children and adolescents develop substantial motor function impairments, such as deficits in motor coordination, skilled motor function, tremor intensity, postural stability, etc. (4–9) (for the remainder of this manuscript, we use the term “motor disease” to encompass all aspects of motor deficits induced by Mn at all ages). Mn is widely used in modern industry with estimates suggesting that >100,000 welders in America are overexposed to Mn (4, 10, 11). Moreover, environmental Mn overexposure, particularly from drinking water in developmentally sensitive early life periods, has emerged as a significant global public health problem (4). In the United States, it is estimated that ~36.4 million people obtain drinking water from domestic wells, and the Mn content in the water for ~7% of this population (i.e., ~2.6 million people) is elevated (i.e., exceeds the Environmental Protection Agency health reference level of 0.3 mg Mn/L) (12). In addition to elevated exposure, because hepatic excretion is a major route of clearing systemic Mn, individuals with liver disease (e.g., alcoholic cirrhosis) may retain excess Mn and develop Mn-induced motor disease in the absence of elevated Mn exposure (13, 14). Finally, homozygous mutations in the critical Mn transporters SLC30A10 or SLC39A14 that regulate systemic and brain Mn homeostasis lead to the retention of Mn in the body and brain and the onset of hereditary Mn-induced motor disease (4, 13). Defining a mechanism by which elevated brain Mn levels induce motor disease is essential for rational therapeutic development.
We recently discovered that the primary homeostatic response of mammalian systems to elevated Mn is transcriptional upregulation of the Mn efflux transporter SLC30A10 via hypoxia-inducible factor (HIF) 1 and HIF2 transcription factors, which provides a pathway to reduce cellular/organismal Mn levels and protect against Mn toxicity (15). The SLC30A10 promoter contains a hypoxia response element that is necessary and sufficient for the Mn-induced transcriptional response (15). HIFs are heterodimeric transcription factors formed by the association of an α subunit with a common β subunit, and HIF1α and HIF2α are the best characterized α isoforms (16) (heterodimeric HIFs are named after their α subunits). Under physiological conditions, HIFα subunits are targeted for degradation by prolyl hydroxylase domain (PHD) enzymes (16). Our recent studies also elucidated the mechanism of Mn-induced HIF1/HIF2 activation by characterizing PHD2, the major PHD isoform (17), as an intracellular Mn sensor (18). We showed that elevated Mn inhibits PHD2 by outcompeting a catalytic Fe atom of PHD2, and Mn-induced PHD2 inhibition subsequently leads to the induction of HIF1/HIF2-dependent transcription (18). However, while activation of HIF1/HIF2 by elevated Mn performs an essential homeostatic function in up-regulating SLC30A10 and controlling Mn levels (15, 18), HIFs regulate transcription of numerous other target genes in diverse biological pathways (19). In particular, HIFs are prominent regulators of metabolism (19), and metabolic alterations have been linked to neurodegenerative diseases (20). Thus, there is a possibility that HIF1/HIF2-mediated transcription may contribute not only to the homeostatic control of Mn but also to the disease phenotype. The established effects (1) of Mn on the HIF1/HIF2 pathway (15, 18); (2) of HIF signaling on metabolism (19); and (3) between metabolic alterations and neurological disease (20) led to the overarching hypothesis of this study – transcriptional and metabolic reprogramming, driven perhaps in part by HIF1/HIF2, may be an important component of Mn-induced motor disease.
The essential amino acid tryptophan is catabolized by three pathways: kynurenine, serotonin, and indole (Fig. 1) (21). The kynurenine pathway is the major degradative pathway, metabolizes >95% of tryptophan, and is responsible for the de novo synthesis of NAD+ in the body (21, 22). Importantly, the kynurenine pathway also generates several neuroactive metabolites, prominent among which are quinolinic acid, a well-established endogenous neurotoxin that functions as an N-methyl-D-aspartate (NMDA) receptor agonist and induces excitotoxicity, and kynurenic acid, which is neuroprotective with antioxidant and receptor antagonist, including NMDA antagonist, properties (Fig. 1) (21–23). Alterations in the kynurenine pathway that lead to an imbalance of neurotoxic and neuroprotective metabolites are linked to several neurological diseases, including Parkinson’s disease (21–24). More recently, a relationship between HIFs and the kynurenine pathway has also emerged. A study on kidney injury revealed that hypoxic or pharmacological inhibition of PHD, which should enhance HIF activity, increases the activity of the kynurenine pathway (25). Another study on natural killer cells showed that depletion of HIF1α decreases the activity of the kynurenine pathway (26). Whether regulation of the kynurenine pathway by HIFs impacts neurological function is unknown, but based on the available evidence (21–26), it is plausible that factors that activate HIFs, such as Mn, may induce neurological sequelae via effects on the kynurenine pathway.
Fig. 1.
Tryptophan metabolism pathways. Kynurenine pathway metabolites and enzymes are in blue and red, respectively.
While we did not initiate this study with a focus on the kynurenine pathway, through unbiased multiomics assays and targeted functional experiments, we unexpectedly identify upregulation of the kynurenine pathway as a central mechanism of Mn-induced motor disease that is amenable to pharmacological manipulation for treatment. Our results further suggest that HIF1 is a critical mediator of the dysregulation of the kynurenine pathway in Mn toxicity. These findings represent a fundamental advancement in the understanding of the pathobiology of Mn-induced motor disease and provide a rational therapeutic approach for the treatment of this condition.
Results
Elevated Mn Exposure Up-Regulates Expression of Metabolic Genes, Including Genes Regulating the Kynurenine Pathway, in the Brain.
As the first test of the hypothesis that transcriptional and metabolic changes underlie Mn-induced motor disease, we performed unbiased transcriptomics in the brain, using a part of the middle of the brain that contains the basal ganglia, of mice treated with or without an oral Mn regimen that models human environmental Mn exposure (4). In humans, environmental Mn exposure is most relevant in the developmentally sensitive early life stages of infancy, childhood, and adolescence (4), which correspond to preweaning/early postnatal (PND 1–21) and early/late adolescence (PND 21–56) periods in mice (27, 28). We exposed mice to Mn over PND 1-56 and harvested tissue for transcriptomics at the end of the Mn exposure so that we could assess the cumulative effects of Mn treatment over the entire early life period. Mn levels, analyzed in a subset of mice in the transcriptomics cohort, in the brain, liver, small intestine, and blood of the Mn-exposed mice were modestly elevated at PND 56 (SI Appendix, Fig. S1 A–D). Transcriptomic analyses revealed that Mn exposure up-regulated expression of ~450 genes in the brain with a trend toward an upregulation of SLC30A10 expression (Fig. 2 A–C and SI Appendix, Fig. S2). The lack of a statistically significant upregulation of SLC30A10 expression in the brain of the Mn-exposed mice is consistent with our previously reported qRT-PCR data from the same animals (15). We had initially expected that expression of genes regulating synaptic transmission or neuronal function will be altered by Mn. Surprisingly, however, analyses of differentially expressed genes and pathways revealed that primary upregulatory changes occurred in the expression of genes controlling diverse metabolic processes (Fig. 2D, Table 1 and Datasets S1 and S2). Notable upregulatory changes were in genes controlling glycolysis, gluconeogenesis, urea cycle, amino acid degradation (phenylalanine, tyrosine, histidine, and tryptophan via the kynurenine pathway), folate/one-carbon metabolism, transsulfuration/transmethylation, and lipid metabolism (Table 1 and Datasets S1 and S2). Importantly, critical genes of the kynurenine pathway involved in the production of the neurotoxic metabolite quinolinic acid (21–23) were up-regulated – Tdo2, which codes for tryptophan 2,3 dioxygenase (TDO2), the enzyme that catalyzes the first and rate-liming step of the pathway (21–23); Kynu, which codes for kynureninase, the enzyme that converts 3-hydroxykynurenine to 3-hydroxyanthranilic acid (21–23); and Haao, which codes for 3-hydroxyanthranilic acid 3,4 dioxygenase, the enzyme that converts 3-hydroxyanthranilic acid to 2-amino-3-carboxymuconic acid-6-semialdehyde for subsequent nonenzymatic cyclization to quinolinic acid (21–23) (Table 1 and Fig. 1). In contrast to the upregulatory changes, downregulatory changes were limited (Datasets S1 and S2), suggesting that transcriptional downregulation was unlikely to be a major driver of the disease phenotype. Overall, the unexpected upregulation of genes controlling metabolism suggests that changes in metabolism in general, and in the kynurenine pathway in particular, may modulate Mn-induced motor disease.
Fig. 2.
Mn exposure alters expression of metabolic genes in the mouse brain. N = 7 vehicle and 8 Mn (sex break-up in SI Appendix). (A) Volcano plot. Red dots—differentially expressed genes (DEGs) (FDR <0.05) in the brain of Mn-exposed mice. Labels—top DEGs. (B) Total number of up-regulated and down-regulated DEGs in the brain of Mn-exposed mice (FDR <0.05). (C) Slc30a10 expression. (D) Pathway enrichment analyses showing major categories of up-regulated DEGs in the brain of Mn-exposed mice. Enrichment is a function of -log10(P-value).
Table 1.
Mn-induced changes in expression of critical metabolic genes in the brain
|
Gene |
Full gene or protein name | Function/Pathway | Fold Change (log2) |
|---|---|---|---|
| Aldob | Aldolase | Glycolysis | 4.932 |
| Pklr | Pyruvate kinase | Glycolysis | 3.629 |
| Fbp1 | fructose-bisphosphatase 1 | Gluconeogenesis | 4.511 |
| G6pc | glucose-6-phosphatase | Gluconeogenesis | 4.502 |
| Pck1 | Phosphoenolpyruvate carboxykinase 1 | Gluconeogenesis | 5.394 |
| Arg1 | Arginase | Urea cycle | 5.608 |
| Otc | Ornithine transcarbamylase | Urea cycle | 5.215 |
| Ass1 | Argininosuccinate synthase 1 | Urea cycle | 0.833 |
| Nags | N-acetylglutamate synthase | Urea cycle | 3.577 |
| Cps1 | Carbamoyl phosphate synthetase I | Urea cycle | 5.644 |
| Pah | Phenylalanine hydroxylase | Phenylalanine degradation | 5.152 |
| Hgd | Homogentisate oxidase | Tyrosine/Phenylalanine degradation | 4.173 |
| Hpd | 4-Hydroxyphenylpyruvate dioxygenase | Tyrosine/Phenylalanine degradation | 5.175 |
| Tat | Tyrosine aminotransferase | Tyrosine/Phenylalanine degradation | 5.338 |
| Fah | Fumarylacetoacetate hydrolase | Tyrosine/Phenylalanine degradation | 0.510 |
| Hal | Histidine ammonia-lyase | Histidine degradation | 4.080 |
| Uroc1 | Urocanate hydratase 1 | Histidine degradation | 4.773 |
| Amdhd1 | Aminohydroxylase domain containing 1 | Histidine degradation | 3.064 |
| Tdo2 | Tryptophan 2,3-dioxygenase | Tryptophan degradation/Kynurenine pathway | 5.395 |
| Kynu | Kynureninase | Tryptophan degradation/Kynurenine pathway | 3.563 |
| Haao | 3-hydroxyanthranilate 3,4-dioxygenase | Tryptophan degradation/Kynurenine pathway | 2.672 |
| Aldh8a1 | Aldehyde dehydrogenase 8 family member A1 | Tryptophan degradation/Kynurenine pathway | 3.646 |
| Gpt | Alanine aminotransaminase 1 | Alanine degradation | 0.647 |
| Prodh2 | Proline Dehydrogenase 2 | Hydroxyproline degradation | 5.176 |
| Shmt1 | Serine hydroxymethyltransferase | Folate/1 carbon metabolism | 2.121 |
| Ftcd | Formimidoyltransferase cyclodeaminase | Folate/1 carbon metabolism | 4.449 |
| Cth | Cystathionine gamma-lyase | Methionine–Cysteine metabolism | 1.064 |
| Ahcy | Adenosylhomocysteinase | Transmethylation/homocysteine pathway | 0.838 |
| Bhmt2 | Betaine--homocysteine S-methyltransferase 2 | Methyl transferase | 4.348 |
| Bhmt | Betaine--homocysteine S-methyltransferase | Methyl transferase/Methionine metabolism | 6.495 |
| Mat1a | Methionine adenosyltransferase 1A | S-adenosylmethionine generation | 4.956 |
| Glyat | Glycine-N-acyltransferase | Glycine/xenobiotic metabolism | 3.531 |
| Cdo1 | Cysteine dioxygenase type 1 | Cysteine degradation | 0.770 |
| Acox2 | Acyl-CoA oxidase 2 | Degradation of long branched fatty acids | 3.911 |
| ACSM1 | Acyl-CoA synthetase medium chain family member 1 | Fatty acid biosynthesis | 5.649 |
| Acat3 | Acetyl-Coenzyme A acetyltransferase 3 | Fatty acid beta-oxidation | 0.865 |
| SLC27A2 | Long-chain fatty-acid-coenzyme A ligase | Fatty acid degradation | 1.245 |
| HMGCS2 | 3-hydroxy-3-methylglutaryl-CoA synthase 2 | Ketogenesis | 1.542 |
Log2 fold change for expression change in Mn-exposed mice compared with vehicle-treated controls is indicated for each gene.
Weighted Gene Coexpression Network Analysis (WGCNA) Identifies Coordinated Changes in Metabolic Networks in the Brain of Mn-Exposed Mice.
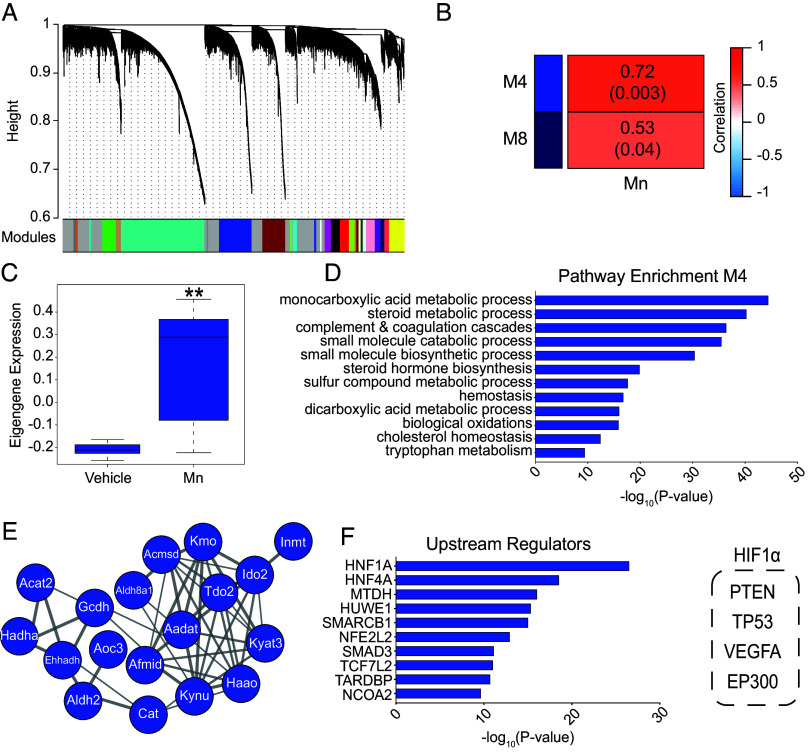
To further analyze Mn-induced transcriptomic changes at the systems level in the brain, we performed WGCNA, which identified several gene expression modules associated with Mn treatment (Fig. 3A and SI Appendix, Fig. S3). Two modules of interest significantly positively correlated with Mn exposure (Fig. 3B and SI Appendix, Fig. S3 and Dataset S3). Subsequent analyses focused on the positively correlated module M4, 2051 genes (R = 0.72, P = 0.003), which exhibited a strong Mn-induced upregulation of gene coexpression (Fig. 3C). This module was enriched in genes regulating numerous metabolic pathways, including tryptophan metabolism (Fig. 3D and Dataset S3). Specifically, there was a strong enrichment of critical genes related to the kynurenine pathway in this network, including Tdo2, Kynu, and Haao (Fig. 3E). Ingenuity pathway analysis for upstream regulators of this module included several transcription factors, and importantly, HIF1 was a critical upstream regulator (Fig. 3F). HIF1 also emerged as a critical upstream regulator in the entire transcriptomic dataset (SI Appendix, Fig. S4A and B). Overall, WGCNA outcomes (Fig. 3 and Dataset S3) are consistent with and expand the results of the differential gene expression analyses (Fig. 2, Table 1 and Datasets S1 and S2), and results of transcriptomic analyses, together, indicate that (1) Mn exposure induces profound changes in the expression of metabolic genes, pathways, and networks in the brain; (2) changes in expression of genes regulating tryptophan metabolism via the kynurenine pathway are an important component of the Mn-induced transcriptomic changes; and (3) HIF1 is a critical upstream regulator of the Mn-induced changes in gene expression.
Fig. 3.
WGCNA of transcriptomic data reveal that Mn exposure induces coordinated changes in the expression of genes, networks, and pathways regulating metabolism in the brain. (A) A hierarchical clustering tree of genes was constructed based on the coexpression network analysis from the transcriptome of brains from vehicle and Mn-exposed animals. Genes in each module are assigned the same color, shown in the color band below the dendrogram. Genes not assigned to any of the modules are colored gray. (B) A heatmap of selected correlations and significance between modules and vehicle and Mn-exposed animals. (C) The module eigengene (ME) expression of module 4 (M4, blue). Column: mean values. Bars: SEM. **P < 0.01. (D) Pathway enrichment of coexpressed genes in module 4 (M4). Enrichment is a function of -log10(P-value). (E) Module 4 enriched tryptophan and kynurenine pathway genes visualized as a protein–protein interaction cluster. (F) Ingenuity Pathway Analysis (IPA) upstream regulators enriched in module 4. HIF1α upstream regulators are highlighted in the dashed box on the Right.
Expression of Metabolic Genes Is Also Altered in the Liver and Small Intestines of Mn-Exposed Mice.
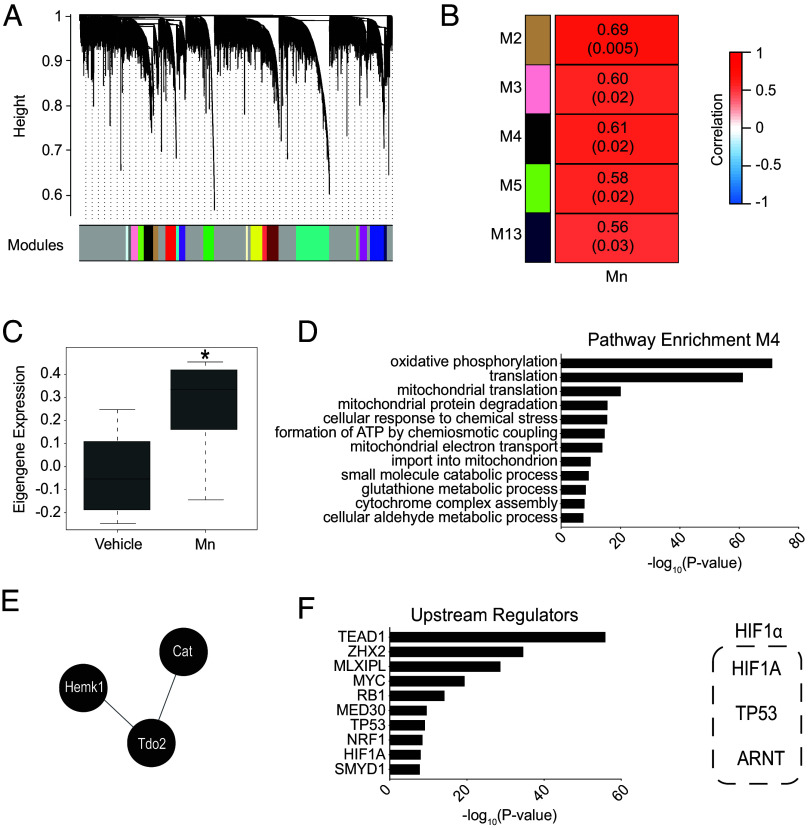
As the next step, we performed unbiased transcriptomics in the liver and intestine of mice that were used for brain transcriptomics because (1) hepatic and intestinal Mn excretion play an essential role in controlling brain Mn (13, 14, 29); and (2) the liver is the primary organ that mediates tryptophan degradation via the kynurenine pathway (22, 30), and peripherally produced kynurenine is permissible across the blood–brain barrier (23). In the liver and intestines, unlike the brain, Mn exposure produced a more even distribution of up and downregulated genes, but similar to the brain, changes in genes regulating metabolism were present (Fig. 4 A–D, Table 2, SI Appendix, Fig. S2, Fig. S5 A–D and Datasets S1 and S2). SLC30A10 expression was up-regulated in the small intestine (SI Appendix, Fig. S5C) but not the liver (Fig. 4C) after Mn exposure, which is consistent with the organ-specific effect of Mn on SLC30A10 expression in this cohort of C57BL/6 mice that we previously reported using qRT-PCR analyses (15). Mn treatment increased expression of the critical kynurenine pathway gene Tdo2 in the liver, but not the intestines (Table 2 and Datasets S1 and S2), providing evidence for induction of the kynurenine pathway in the liver and, together with the results of the SLC30A10 expression, indicating that organ specificity was an important feature of Mn-induced changes in expression of critical regulatory genes in the liver and intestines. Subsequent WGCNA in the liver identified several gene expression modules that significantly positively correlated with Mn treatment (Fig. 5 A and B, SI Appendix, Fig. S6 and Dataset S3), although fewer modules were identified in the liver compared to the brain. In particular, one module (M4, 524 genes, R = 0.61, P = 0.02) included networks enriched in genes responsible for regulating metabolism, similar to the brain networks, including key kynurenine gene Tdo2 (Fig. 5 C–E and Dataset S3). Additionally, liver-specific pathway enrichment in M4 revealed changes in mitochondrial function and cellular response to stress (Fig. 5D and Dataset S3) further reinforcing the presence of both core Mn-induced and organ-specific Mn-induced changes in gene expression. HIF1 was again identified as a critical upstream regulator, along with other transcription factors, of the Mn-induced changes in the liver networks (Fig. 5F and SI Appendix, Fig. S4A and B). Finally, comparison of gene and pathway expression changes across the three organs revealed limited overlap of differentially expressed genes (DEGs) between organs, but an overall conservation of changes in genes regulating metabolic pathways in all three organs (SI Appendix, Fig. S7 A–C). Specifically, genes key to tryptophan metabolism such as Tdo2 were conserved between brain and liver (SI Appendix, Fig. S7A). Thus, similar to the brain, Mn exposure alters expression of genes, pathways, and networks regulating metabolism in the liver and intestines, including the kynurenine pathway in the liver, and HIF1 is an important upstream regulator of the transcriptional changes in the liver.
Fig. 4.
Expression of metabolic genes is also altered in the liver of Mn-exposed mice. (A–D) Transcriptomic analyses were performed in liver tissue of mice used for brain transcriptomics in Fig. 2. Volcano plot (A); total DEGs in the liver (B); Slc30a10 expression (C); and pathway enrichment (D) as described for Fig. 2 A–D.
Table 2.
Mn-induced changes in expression of selected metabolic genes in the liver
| Gene | Full gene or protein name | Function/Pathway | Fold Change (log2) |
|---|---|---|---|
| Eno1 | Enolase | Glycolysis | 0.498 |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Glycolysis | 0.388 |
| Fbp1 | Fructose-bisphosphatase 1 | Gluconeogenesis | 0.349 |
| Mdh1 | Malate dehydrogenase 1 | TCA cycle | 0.328 |
| Slc25a10 | Mitochondrial metabolite transporter | TCA cycle/fatty acid synthesis | 0.371 |
| Sdhd | Succinate dehydrogenase complex subunit D | TCA cycle | 0.317 |
| Slc25a15 | Ornithine translocase | Urea cycle | 0.316 |
| Otc | Ornithine transcarbamylase | Urea cycle | 0.307 |
| Pah | Phenylalanine hydroxylase | Phenylalanine degradation | 0.493 |
| Hgd | Homogentisate oxidase | Tyrosine/Phenylalanine degradation | 0.364 |
| Hpd | 4-Hydroxyphenylpyruvate dioxygenase | Tyrosine/Phenylalanine degradation | 0.444 |
| Tdo2 | Tryptophan 2,3-dioxygenase | Tryptophan degradation/Kynurenine pathway | 0.619 |
| Gpt | Alanine aminotransaminase 1 | Alanine degradation | 0.467 |
| Aass | Aminoadipate-semialdehyde synthase | Lysine degradation | 0.391 |
| Dhfr | Dihydrofolate reductase | Folate/1 carbon metabolism | 0.377 |
| Cth | Cystathionine gamma-lyase | Transsulfuration | 0.660 |
| Ahcy | Adenosylhomocysteinase | Transmethylation/homocysteine pathway | 0.362 |
| Gls2 | Glutaminase | Glutamine degradation | 0.594 |
| Sptssa | Serine palmitoyltransferase small subunit A | Sphingolipid biosynthesis | 0.302 |
| Acat3 | Acetyl-Coenzyme A acetyltransferase 3 | Fatty acid beta-oxidation | 0.326 |
| Mcee | Methylmalonyl-CoA epimerase | Fatty acid catabolism | 0.276 |
Log2 fold change in Mn-exposed mice compared with vehicle-treated controls is indicated.
Fig. 5.
WGCNA of liver transcriptomic data. Hierarchical clustering tree of genes (A); heatmap (B); module eigengene (ME) expression of module 4 (M4: black, column: mean values, bars: SEM, *P < 0.05) (C); pathway enrichment of coexpressed genes in M4 (D); protein interaction cluster of M4 enriched tryptophan and kynurenine pathway genes (E); and IPA upstream regulators enriched in M4 (F) as described in Fig. 3 A–F.
Unbiased Metabolomics Identifies Upregulation of Kynurenine Pathway Metabolites in the Brain of Mn-Exposed Mice in Early Postnatal Life.
The transcriptomic analyses suggested that changes in metabolism in general, and in the kynurenine pathway in particular, may contribute to Mn-induced motor deficits. To directly determine whether Mn exposure altered metabolism, we performed unbiased metabolomics in the brain and liver of a new cohort of vehicle or Mn-treated C57BL/6 mice. We did not include the intestines because of logistical limitations and the lack of Mn-induced transcriptional changes in the kynurenine pathway in the intestines. We used an unbiased, rather than targeted, approach to ensure wide detection of metabolic changes. Finally, we performed the metabolomics assay at two time points, PND 21 or 56, because of the following reason. Tissue Mn levels of mice exposed to daily oral Mn are higher in early postnatal life than in late adolescence/early adulthood despite ongoing Mn treatment (14, 31) likely because of an increase in Mn excretion capacity that occurs around weaning (13) and/or a reduced efficiency of Mn absorption postweaning. We confirmed the age-dependent effect of Mn exposure on tissue Mn in the mice used for the metabolomics assay – i.e., tissue Mn levels of Mn-exposed mice were higher than vehicle-treated counterparts at PND 21, but not PND 56 (SI Appendix, Fig. S8A and B). Note that the absolute Mn levels in the brain and liver of the Mn-exposed mice at PND 56 in the metabolomics and transcriptomics cohorts were essentially similar (~3 and ~7 μg Mn/g tissue, respectively, for brain and liver) (SI Appendix, Figs. S1 and S8). The difference in statistical significance for comparisons of tissue Mn levels between vehicle or Mn-treated conditions of the two cohorts at PND 56 (i.e., P < 0.05 in the transcriptomics but not the metabolomics cohort; SI Appendix, Figs. S1 and S8) may be reflective of minimal cohort-to-cohort variation in absolute values of Mn in different subject animals or difference in statistical tests used (t test in SI Appendix, Fig. S1; two-way ANOVA in SI Appendix, Fig. S8) and unlikely to be of biological relevance. Because tissue Mn load may modulate Mn-induced metabolic changes, inclusion of both the PND 21 and PND 56 time points increased the likelihood of detecting pathophysiologically relevant metabolomic alterations in the Mn-exposed mice. We did not repeat the transcriptomic assay at PND 21 because data at the PND 56 time point was sufficient to progress to the metabolomics experiment as the next step of the study.
In the brain, Mn exposure induced changes in several metabolic pathways at PND 21 (e.g., amino acid degradation, gluconeogenesis, pentose phosphate pathway, glycolysis, and fatty acid metabolism) that mostly normalized at PND 56 (Dataset S4). There were significant Mn-induced changes in the levels of byproducts of tryptophan, histidine, phenylalanine, and tyrosine catabolism in the brain at PND 21 (Table 3 and Dataset S4), indicative of widespread changes in amino acid degradation. Specific to tryptophan metabolism and the kynurenine pathway, brain levels of the critical metabolite kynurenine were highly elevated by Mn exposure at PND 21 (Table 3 and Dataset S4), suggesting that Mn exposure increased the flux of tryptophan degradation via the kynurenine pathway. Brain tryptophan levels of PND 21 Mn-treated mice were also modestly elevated (Table 3 and Dataset S4), raising the possibility of an increase in brain tryptophan import to counter enhanced tryptophan degradation. There was a trend toward a reduction of brain NAD+ levels in the PND 21 Mn-exposed mice as well (Dataset S4), which may partly explain the increase in tryptophan degradation because the kynurenine pathway mediates de novo NAD+ synthesis (21, 22) (Fig. 1). We did not detect most other kynurenine pathway metabolites (e.g., kynurenic acid or quinolinic acid) in the brain, perhaps because of short half-lives and/or low concentrations [e.g., half-life of quinolinic acid under physiological conditions is ~20 min (23)]. But levels of xanthurenic acid, which is produced by a branch of the kynurenine pathway (Fig. 1), were reduced in the brain of the PND 21 Mn-exposed mice (Table 3 and Dataset S4). The simultaneous increase of kynurenine and decrease of xanthurenic acid suggests that the flux of the downstream metabolism of kynurenine in the brain of the PND 21 Mn-exposed mice may be directed toward kynurenic acid or quinolinic acid (Fig. 1), which are neuroactive (21–23). Finally, trending or significant changes in metabolites of the serotonin or indole pathways of tryptophan degradation were also evident in the brain of the Mn-treated mice at PND 21 (Table 3 and Dataset S4), indicating that Mn exposure profoundly altered tryptophan metabolism.
Table 3.
Mn-induced changes in metabolites of selected amino acids in the brain
| Fold Change (Mn/Vehicle) | ||
|---|---|---|
| Metabolite | PND 21 | PND 56 |
| Histidine Metabolism | ||
| 1-methylhistidine | 2.31 | 0.89 |
| 3-methylhistidine | 4.59 | 0.97 |
| N-acetyl-1-methylhistidine | 2.47 | 1 |
| imidazole propionate | 0.69 | 1.39 |
| formiminoglutamate | 3.15 | 0.77 |
| 1-methyl-5-imidazoleacetate | 3.88 | 1.04 |
| 1-methyl-5-imidazolelactate | 3.78 | 1.02 |
| 1-ribosyl-imidazoleacetate | 2.4 | 1.05 |
| 4-imidazoleacetate | 2.26 | 1 |
| Phenylalanine Metabolism | ||
| phenylalanine | 1.32 | 1.01 |
| N-acetylphenylalanine | 2.5 | 1.14 |
| phenyllactate (PLA) | 1.75 | 0.91 |
| Tyrosine Metabolism | ||
| tyrosine | 1.19 | 0.95 |
| N-acetyltyrosine | 1.38 | 1.09 |
| 4-hydroxyphenylpyruvate | 1.56 | 1.23 |
| phenol sulfate | 2.18 | 0.66 |
| o-tyrosine | 2.12 | 1.62 |
| N-formylphenylalanine | 2.16 | 1.64 |
| Tryptophan Metabolism | ||
| tryptophan | 1.29 | 1.01 |
| N-acetyltryptophan | 1.64 | 1.05 |
| oxindolylalanine | 2.77 | 2.31 |
| kynurenine | 3.32 | 0.78 |
| N-acetylkynurenine (2) | 9.81 | 1 |
| xanthurenate | 0.42 | 0.46 |
| 5-hydroxyindoleacetate | 0.7 | 0.87 |
| indolelactate | 2.36 | 0.69 |
| indoleacetate | 1.79 | 1.1 |
Fold change is the ratio of the mean scaled metabolite intensity of Mn-exposed mice to corresponding vehicle mice at PND 21 or PND 56.
WGCNA Metabolome Analysis Confirms Mn-Induced Changes in Tryptophan Metabolism in the Brain in Early Postnatal Life.
Due to the targeted but limited coverage of metabolomic analysis, it is typically difficult to analyze large datasets from heterogeneous sources to systematically decipher cellular response and identify critical pathways and metabolites relevant to conditions (32). However, applications of WGCNA for metabolome data have recently provided a fuller view of biological responses than individual metabolite-focused strategies (32, 33). Therefore, we took a network-based WGCNA approach to identify meaningful biological responses in metabolic pathways of the brain after Mn exposure (32). We performed the metabolite network analyses at the PND 21 time point because metabolic changes were largely limited to this time point (Dataset S4). We identified several metabolic modules significantly associated with Mn treatment (Fig. 6 A and B, SI Appendix, Fig. S9 and Dataset S3). Four metabolic modules significantly positively correlated with Mn treatment (modules M3, M6, M7, and M8) (Fig. 6B and Dataset S3). The up-regulated modules related to diverse metabolic superpathways, including amino acid, carbohydrate, and energy metabolism, and importantly, included tryptophan metabolism (Fig. 6 C–F and Dataset S3). Preliminary analyses also suggested that an overlap between transcriptomic and metabolomic changes across time points was likely present in the brain of the Mn-treated mice (e.g., in the kynurenine pathway; Tables 1 and 3). To validate this, we used a WGCNA module overlap approach to quantify the consistency and module overlap between Mn-induced metabolic changes at PND 21 and transcriptomic changes at PND 56. Although the time points for the changes in metabolites (PND 21) and gene expression (PND 56) for the overlap analyses were different, it was likely that gene expression changes persisted after normalization of metabolite levels, and changes in metabolites at PND 21 could lead to lasting neurological consequences despite normalization later in life. There was a strong overlap of transcriptional and metabolic modules that were up-regulated by Mn exposure in the brain based on pathway enrichment (Fig. 6G and Dataset S3). The strongest overlap was between RNA modules M4 and M8 with metabolomic module M7, which was highly enriched in tryptophan metabolism and the kynurenine pathway (Fig. 6 G and H and Dataset S3). In totality, (1) Mn exposure induces extensive metabolic changes in the brain in early postnatal life that normalize in later life; (2) changes in tryptophan metabolism and the kynurenine pathway are a prominent feature of the Mn-induced metabolomic alterations in the brain; and (3) Mn-induced transcriptomic and metabolomic changes in the brain are strongly conserved, albeit across life-stages.
Fig. 6.
WGCNA of Mn-induced metabolomic changes at PND 21 and overlap between metabolic changes at PND 21 and transcriptomic changes at PND 56 in the brain. For metabolomics, N = 6 per group (sex break-up in SI Appendix). (A) A hierarchical clustering tree of metabolites was constructed based on the coexpression network analysis from the metabolome of brains from vehicle and Mn-exposed animals. Metabolites in each module are assigned the same color, shown in the color band below the dendrogram. Metabolites not assigned to any of the modules are colored gray. (B) A heatmap of selected correlations and significance between modules and vehicle and Mn-exposed animals. (C) The module eigenmetabolite (ME) expression of module 7 (M7, tan) module. Column: mean values. Bars: SEM. **P < 0.01. (D) Left: barplot of metabolite superpathway categories for module 7. Right: circle chart of amino acid superpathway minor categories. (E) The module eigenmetabolite (ME) expression of module 8 (M8, dark turquoise) module. Column: mean values. Bars: SEM. **P < 0.01. (F) Left: Barplot of metabolite superpathway categories for module 8. Right: circle chart of amino acid superpathway minor categories. (G) Overlap between the brain transcriptome and metabolite modules based on pathway membership. The modules contained in the box show high overlap between the transcriptome and metabolome and likely represent shared processes. Significance of overlap (Fisher exact test, corrected for multiple comparisons using an FDR<0.05) is represented as a function of -log10 (P-value). (H) Highlighted key overlapping pathways between the brain transcriptome and metabolome.
Mn Exposure Also Alters Kynurenine Metabolites in the Liver in Early Postnatal Life.
Similar analyses in the liver revealed that Mn exposure altered several metabolic pathways and networks at PND 21, including amino acid, carbohydrate, and energy metabolism (SI Appendix, Figs. S10 A–H and S11 and Dataset S5). We detected more kynurenine pathway metabolites in the liver than in the brain (e.g., anthranilic acid, kynurenic acid, etc.; Table 3 and Datasets S4 and S5). Importantly, similar to the brain, Mn-induced alterations in kynurenine pathway metabolites were prominent in the liver at PND 21 with significant or trending increases in kynurenine and its downstream metabolites anthranilic acid and kynurenic acid, and a significant decrease in xanthurenic acid (Dataset S5). The directionality of the Mn-induced change in kynurenine and xanthurenic acid at PND 21 in the liver was similar to that in the brain (Table 3 and Datasets S4 and S5), suggesting a conserved effect of Mn on the kynurenine pathway across organs. Also similar to the brain, Mn-induced changes in critical kynurenine pathway metabolites at PND 21 in the liver normalized at PND 56 (Dataset S5), further supporting the idea that the increased tissue Mn load at PND 21 in the Mn-exposed mice led to the observed changes in kynurenine metabolism. Metabolites of the serotonin and indole pathways of tryptophan metabolism were also altered in the liver of the PND 21 Mn-exposed mice (Dataset S5), but these changes were not necessarily similar to the brain (Table 3 and Dataset S4), suggesting that, in addition to conserved effects across tissues, Mn also exerts organ-specific effects on tryptophan metabolism. Finally, the WGCNA module overlap approach confirmed strong overlap of Mn-induced changes in liver transcriptional networks at PND 56 and metabolomic networks at PND 21 (SI Appendix, Figs. S10G and H and S11 and Dataset S3), which was also consistent with changes we observed in the brain (Fig. 6 G and H and Dataset S3). Overall, Mn exposure alters diverse metabolic pathways in the liver, and a transient upregulation of kynurenine pathway metabolites in early postnatal life is a common feature of Mn exposure in the liver and brain.
Inhibition of the Kynurenine Pathway Rescues Mn-Induced Motor Deficits.
The conserved upregulation of the kynurenine pathway in the brain and liver of Mn-treated mice that we observed together with the established role of kynurenine pathway dysregulation in neurological disease (21–24) led us to test whether Mn-induced changes in the kynurenine pathway modulated the motor sequelae of Mn exposure. For this experiment, we used a new cohort of mice in which we pharmacologically inhibited TDO2 enzyme using two chemically distinct inhibitors, NTRC 3531-0 or LM10 (34, 35). We targeted TDO2 because TDO2 catalyzes the first and rate-limiting step of the kynurenine pathway (21–23) (Fig. 1), and Mn-treatment up-regulated expression of TDO2 in both the liver and the brain (Tables 1 and 2). Additionally, brain permeability of NTRC 3531-0 is substantially higher than LM10, but both inhibitors rescue motor deficits, neurodegeneration, and neuroinflammation in a rotenone-induced model of Parkinson’s disease in mice (34). Use of the two inhibitors was designed to assess whether effects of TDO2 inhibition on Mn-induced motor deficits were dependent on drug pharmacokinetic properties and increase the rigor of the study. Finally, Mn exposure preceded inhibitor treatment in the study (Fig. 7A). Thus, the experiment modeled a treatment, rather than a protection, modality in humans.
Fig. 7.
TDO2 inhibitors rescue Mn-induced motor deficits. (A) Experimental design. (B and C) qRT-PCR for indicated genes from brain tissue of a subset of mice in the experiment. Mean expression in the drug solvent group without Mn exposure was normalized to 1. N = 5 per group (sex break-up in SI Appendix). Mean ± SE. *, P <0.05 and n.s., not significant for indicated differences using two-way ANOVA and Tukey’s post hoc test. (D–F) Average (D) or total (E) missteps in all trials and average of the total time to complete all trials (F) from the beam balance test. N = 5 to 10 per group (sample size per group with sex break-up in SI Appendix). Mean ± SE. *, P <0.05 and n.s., not significant for indicated differences using two-way ANOVA and Tukey’s post hoc test. Kruskal–Wallis test followed by Dunn’s multiple comparison test confirmed a statistically significant effect of Mn exposure on missteps in the drug solvent-treated (P < 0.05) but not the LM10- or NTRC 3531-0-treated (P > 0.05) groups in D and E, and no significant difference between groups for total time in F. (G) Brain Mn levels of mice used for the beam balance test. Sample from one vehicle with drug solvent animal was not processed. Mean ± SE. *, P <0.05 and n.s., not significant for indicated differences using two-way ANOVA and Tukey’s post hoc test.
Gene expression analyses in the brain revealed that Mn exposure enhanced TDO2 expression (Fig. 7B), consistent with the unbiased transcriptomics. Importantly, both inhibitors fully blocked the Mn-induced upregulation of TDO2 (Fig. 7B). While the mechanism of reversal of the upregulation of TDO2 expression by the inhibitors is unclear, this result indicates that the inhibitors directly or indirectly impact the brain kynurenine pathway. Mn or inhibitor treatment did not affect brain expression of IDO1(Fig. 7C), an enzyme unrelated to TDO2 that also catalyzes the first step of the kynurenine pathway (21–23, 34, 35), indicating that Mn specifically affected some, but not all, kynurenine pathway enzymes and consistent with the previously reported strong selectivity of NTRC 3531-0 and LM10 in inhibiting TDO2 over IDO1 (34, 35). We then analyzed the effects of the inhibitors on Mn-induced motor deficits using the beam balance and open-field tests (36, 37). The beam balance test assays for the ability of a mouse to traverse a narrow beam and missteps are a highly sensitive indicator of motor dysfunction (36, 37). Two-way ANOVA revealed that, as expected, Mn-exposed mice that were not treated with either inhibitor exhibited increased missteps in the test (Fig. 7 D and E), indicating that Mn treatment-induced motor dysfunction (36, 37). Notably, both inhibitors fully rescued the Mn-induced increase in missteps (Fig. 7 D and E), and the inhibitors by themselves did not induce missteps (Fig. 7 D and E). Moreover, while the missteps data satisfied the normality of distribution assumptions of ANOVA (P > 0.05 for all groups in Fig. 7 D and E on Kolmogorov–Smirnov and Shapiro–Wilk tests for normality), we additionally confirmed the conclusions about the rescue effect of the inhibitors using the nonparametric Kruskal–Wallis test followed by Dunn’s multiple comparison test, which revealed that Mn exposure increased missteps only in mice that were not treated with either inhibitor (Fig. 7 D and E). Separately, we observed that the total time taken to traverse the beam in the beam balance test and movement in the open-field test, which are measures of gross motor/locomotor function, were comparable between groups (Fig. 7F and SI Appendix, Fig. S12A and B), implying that Mn exposure or drug treatment did not impact gross motor/locomotor activity. The fine sensorimotor phenotype of the Mn-exposed mice characterized by an increase in missteps in the beam balance test without changes in gross motor/locomotor function (Fig. 7 D–F and SI Appendix, Fig. S12A and B) is consistent with the motor deficits produced by environmental Mn exposure in human children/adolescents (4–9) and reflective of the Mn dosing regimen we used that modeled the lower end of human environmental Mn exposure (4). Moreover, the lack of an effect of the inhibitors on gross motor/locomotor activity implies that the rescue of the Mn-induced missteps in the beam balance test by the inhibitors was reflective of a true rescue of Mn-induced motor dysfunction and not a consequence of an unrelated effect of the inhibitors on locomotion. Finally, we also confirmed that inhibitor treatment did not impact brain Mn levels with or without Mn exposure (Fig. 7G), implying that the rescue effect of the inhibitors on motor function was not due to an unexpected reduction of brain Mn in the inhibitor-treated mice. In sum, inhibition of the first step of the kynurenine pathway rescues developmental Mn-induced motor deficits. Put together, results of the functional, metabolomics, and transcriptomics assays indicate that upregulation of the kynurenine pathway is necessary for the onset of Mn-induced motor disease.
HIF1α, but Not HIF2α, Is Required for the Expression of Critical Kynurenine Pathway Genes in the Liver Under Basal and Elevated Mn Exposure Conditions.
Finally, we sought to gain insights into the mechanism of the Mn-induced upregulation of the kynurenine pathway. We focused on HIF1 and HIF2 because (1) our prior work established that elevated Mn directly activates HIF1/HIF2 by inhibiting PHD2 (15, 18); (2) consistent with our prior work, transcriptomic analyses in this study identified HIF1 to be a major upstream regulator of Mn-induced gene expression changes; and (3) a role for the HIF pathway in kynurenine metabolism has been previously reported in other model systems (25, 26). To determine the relationship between Mn exposure, HIF signaling, and the kynurenine pathway, we performed gene expression analyses using liver tissue harvested from conditional HIF1α or HIF2α knockout mice lacking expression of the specific HIFα isoform in the liver with or without Mn treatment in early postnatal life. We could not analyze effects of depletion of HIFα isoforms in the brain because live pan-neuronal/glial HIF1α knockout mice were not born, likely due to the developmental requirement of HIF1α in the brain. We confirmed the isoform- and tissue-specific depletion of HIF1α or HIF2α in the liver of the knockout mice (Fig. 8A). Notably, there was a significant main effect of HIF1α knockout genotype reducing expression of the critical kynurenine pathway genes Tdo2 and Kynu (Fig. 8 B and C). Furthermore, post hoc analyses revealed that expression of Kynu in HIF1α knockout mice treated with Mn, but not vehicle, was significantly lower than corresponding littermate controls (Fig. 8C). The Mn-induced fold change, relative to vehicle-treated mice, in Kynu gene expression of HIF1α knockout mice was also trending lower than that of littermate controls (P = ~0.2 on t test). In contrast to the effect of HIF1α knockout, expression of Tdo2 and Kynu were independent of HIF2α expression in vehicle or Mn-treated conditions (Fig. 8 D and E), and instead, there was a significant or trending main effect of Mn treatment increasing expression of both genes in HIF2α knockouts and their littermate controls (Fig. 8 D and E). Lentivirus-induced stable knockdown of HIF1α, but not HIF2α, in hepatic HepG2 cells also reduced Kynu expression compared with control knockdown with or without Mn exposure (Fig. 8 F–H), although, for unclear reasons, Mn treatment lowered Kynu expression in HepG2 cells (Fig. 8F) unlike the trending increase in Mn-treated mice (Fig. 8E). Despite the difference in the effect of Mn treatment on Kynu gene expression in mice and cell culture, the totality of the results in Fig. 8 indicate that HIF1α, but not HIF2α, is required for the optimal expression of critical kynurenine pathway genes in the liver, including after Mn exposure. Combined with the identification of HIF1 as an important upstream regulator of Mn-induced transcriptomic changes (Figs. 3F and 5F and SI Appendix, Fig. S4A and B), results from Fig. 8 are consistent with the interpretation that the Mn-induced upregulation of the kynurenine pathway in the liver depends in part on HIF1α, but not HIF2α. These results further suggest that HIF1α may also influence the effects of Mn on the brain kynurenine pathway and neuromotor function (1) indirectly via effects on liver kynurenine metabolism [because hepatic and brain kynurenine pathways are closely integrated (22, 23)]; and/or (2) by directly affecting the brain kynurenine pathway [because HIF1 was identified as a major upstream regulator of Mn-induced gene expression changes in the brain; (Fig. 3F and SI Appendix, Fig. S4A and B)].
Fig. 8.
Expression of critical kynurenine pathway genes in hepatic systems is reduced by knockout or knockdown of HIF1α. (A) RT-PCR from indicated tissue of mice of indicated genotypes. (B–E) HIF1α or HIF2α knockout mice and their littermate controls were exposed to daily vehicle or Mn from PND 1-14 and liver tissue processed for qRT-PCR. For each panel, mean expression in the control genotype without Mn exposure was normalized to 1. N = 5 to 6 per group (sample size per group with sex break-up in SI Appendix). Mean ± SE. *, P <0.05 for indicated differences using two-way ANOVA and Tukey’s post hoc test. P values for main effects of treatment and genotype are also provided. (F–H) HepG2 cells stably infected with lentivirus expressing shRNA targeting HIF1α or HIF2α or a scrambled sequence were treated with 0 or 500 µM Mn for 4 h and processed for qRT-PCR. Mean expression in cells infected with the scramble shRNA without Mn exposure was normalized to 1. N = 3 per group. Mean ± SE. *, P <0.05 and n.s., not significant for indicated differences using two-way ANOVA and Sidak’s post hoc test.
Discussion
Mn-induced motor disease is an important public health problem with millions of individuals at risk of elevated Mn exposure from environmental and occupational sources, heightened risk of excess Mn retention and subsequent motor disease in individuals with liver disease, and hereditary motor disease occurring due to Mn toxicity in patients harboring mutations in SLC30A10 or SLC39A14 (also see Introduction) (4–13). Mechanisms of Mn-induced motor disease have been unclear, and the disease lacks treatments. The major outcome of the current work is to establish the upregulation of the kynurenine pathway as a central mechanism of Mn-induced motor disease that can be pharmacologically targeted for therapy. This conclusion is based on the observations that (1) elevated Mn up-regulates kynurenine pathway genes and metabolites; and (2) inhibition of the kynurenine pathway rescues Mn-induced motor deficits. These findings substantially advance understanding of the biology and therapeutics of Mn-induced motor disease. Additionally, by providing a strong rationale for investigating the role of metabolic reprogramming in motor disease induced by other essential metals (e.g., Cu or Fe), these results are expected to widely inform investigations on metal-induced motor disease in general.
We previously established that Mn-induced HIF1/HIF2 activation serves a fundamental homeostatic role by increasing expression of the Mn efflux transporter SLC30A10 to reduce Mn levels (15, 18). Current results indicate that, by controlling kynurenine pathway gene expression, HIF1α may also play a role in Mn-induced motor disease. Thus, HIF1/HIF2 activation by elevated Mn may be leading to opposing outcomes – protecting against Mn toxicity by increasing SLC30A10 expression to reduce Mn levels (refs. 15 and 18), and inducing Mn toxicity by regulating kynurenine pathway genes (this study). A potential role for HIFs in Mn neurotoxicity is also supported by the recent characterization of HIF signaling as an important upstream regulator of transcriptomic changes in the basal ganglia of Slc30a10 knockout mice (38). Delineating the contrasting effects of HIF1/HIF2 activation on Mn homeostasis and neurotoxicity is an important direction for future work.
Alterations in the kynurenine pathway have been linked to several neurodegenerative diseases. As examples, in Huntington’s disease, brain levels of quinolinic acid and 3-hydroxykynurenine, which are neurotoxic, increase while those of the neuroprotective kynurenic acid decrease (23). In Parkinson’s disease, an increase in levels of 3-hydroxykynurenine and decrease of kynurenic acid and kynurenine in the basal ganglia are reported (23). Quinolinic acid-mediated toxicity is also implicated in the biology of Alzheimer’s disease (23). The established relationship between kynurenine metabolism and other neurologic diseases is consistent with our identification of kynurenine pathway dysregulation as critical to the onset of Mn-induced motor disease. Moreover, our characterization of HIF1 as a modulator of the kynurenine pathway in Mn toxicity is particularly significant because HIF control of kynurenine metabolism is not widely recognized as a mechanism of neurologic disease [increased production of neurotoxic kynurenine metabolites in neurodegenerative diseases is generally linked to immune responses (23)]. Determining the role of the HIF pathway in other neurologic diseases characterized by dysregulation of kynurenine metabolism is another important dimension of future work that emerges from the current study. We also note that our results do not imply that HIF signaling is the only mechanism of kynurenine pathway dysfunction in Mn toxicity, and HIF-independent processes that are yet to be discovered may also contribute to Mn-induced changes in the kynurenine pathway.
The kynurenine pathway in the brain exhibits complex intercellular and interorgan features. Within the brain, the pathway is primarily active in glial cells (23), and expression of kynurenine pathway enzymes is segregated in astrocytes and microglia leading to the production of neuroprotective kynurenic acid in astrocytes and neurotoxic 3-hydroxykynurenine and quinolinic acid in microglia (23). Peripheral organs also play a major role in modulating the brain kynurenine pathway because peripherally produced kynurenine reaches the brain by crossing the blood–brain barrier and subsequently participates in the brain kynurenine pathway (23). Estimates indicate that ~40% of brain kynurenine is locally produced and the remaining ~60% comes from other organs (23, 39). Among the peripheral organs, the liver is particularly important because it is the main site in the body for kynurenine pathway activity, tryptophan degradation, and kynurenine production (~90% of the total tryptophan degradation in the body occurs via the hepatic kynurenine pathway) (22, 30). While we did not attempt to investigate the effects of Mn on the kynurenine pathway at the organ or cellular level in this study, our results raise the possibility that Mn impacts both brain and peripheral kynurenine pathways. The Mn-induced increase in expression of brain kynurenine pathway enzymes that we observed likely reflects a direct effect of Mn on the brain kynurenine pathway. In contrast, the rescue of Mn-induced motor deficits with LM10, which has low blood–brain barrier permeability (34), suggests that part of the effect of Mn may be on peripheral, perhaps hepatic, kynurenine production. Clearly, an essential next step in defining the mechanisms by which Mn-induced alterations of kynurenine metabolism induce motor disease is to integrate organ- and cell-specific effects of Mn on kynurenine metabolism with neuromotor function. These future studies need to include targeted quantification approaches to analyze Mn-induced changes in brain levels of the neuroactive kynurenine metabolites (e.g., quinolinic acid, kynurenic acid, and 3-hydroxykynurenine) because the kynurenine pathway impacts neurological function through these metabolites (23) and we did not detect these metabolites in the brain in our metabolomics assay.
Pharmacological targeting of kynurenine pathway enzymes is being pursued as a strategy for the treatment of several diseases (22). Our finding that TDO2 inhibitors fully rescue Mn-induced motor deficits suggests that targeting TDO2 enzyme may be a promising strategy for the management of Mn-induced motor disease, which is currently untreatable. Notably, we initiated TDO2 inhibitor treatment after Mn exposure in our study. Thus, TDO2 inhibitors may be effective not only to prevent the onset of motor disease in individuals with ongoing/current Mn exposure but also to treat individuals with persistent Mn-induced motor deficits due to prior elevated Mn exposure. The reported efficacy of TDO2 inhibition in a model of Parkinson’s disease (34) further supports the idea of using TDO2 inhibitors for treatment of Mn-induced motor disease [although there are pathological differences between Parkinson’s disease and motor disease induced by Mn (40)]. Overall, in addition to providing mechanistic insights about disease biology, our results also identify a possible pharmacological approach to treat Mn-induced motor disease.
Methods
All experiments with mice were approved by the Institutional Animal Care and Use Committee of the University of Texas at Austin. Behavior assays, metal analyses, drug and Mn treatments in mice, cell culture assays, transcriptomics, and PCR-based assays were performed using standard procedures essentially similar to those described by us previously (14, 15, 18, 37, 38). Metabolomics was performed at Metabolon (North Carolina, USA). Primers are in SI Appendix, Table S1. Procedural details, including details of bioinformatics for transcriptomics and metabolomics, are in SI Appendix. GraphPad 8 was used for other statistical analyses.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Acknowledgments
This work was supported by NIH/NIEHS R01 ES024812 and R01 ES031574 (both to S.M.). We thank Dr. Stefano Tiziani and Dr. Dean Appling (University of Texas at Austin) for helpful discussions. TagSeq was performed by the Genomic Sequencing and Analysis Facility at University of Texas at Austin, Center for Biomedical Research Support (RRID#: SCR_021713). Metabolomics was performed at Metabolon (North Carolina).
Author contributions
S.M. designed research; A.S.W., N.S., S.H., C.L., N.R.H., K.C.G., and T.J. performed research; A.S.W., N.S., N.R.H., T.J., D.R.S., R.D.M., and S.M. analyzed data; and A.S.W., D.R.S., R.D.M., and S.M. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information. Sequencing data are available on the Gene Expression Omnibus (GEO; accession ID GSE292533) (41). Metabolomics data have been deposited on MetaboLights (study number MTBLS975) (42).
Supporting Information
References
- 1.Bandmann O., Weiss K. H., Kaler S. G., Wilson’s disease and other neurological copper disorders. Lancet Neurol. 14, 103–113 (2015), 10.1016/S1474-4422(14)70190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loughnan R., et al. , Association of genetic variant linked to hemochromatosis with brain magnetic resonance imaging measures of iron and movement disorders. JAMA Neurol. 79, 919–928 (2022), 10.1001/jamaneurol.2022.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregory A., Hayflick S., “Neurodegeneration with brain iron accumulation disorders overview” in GeneReviews®, Adam M. P., et al. Eds., (University of Washington, Seattle, WA, 1993). [PubMed] [Google Scholar]
- 4.Taylor C. A., et al. , Maintaining translational relevance in animal models of manganese neurotoxicity. J. Nutr. 150, 1360–1369 (2020), 10.1093/jn/nxaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucchini R. G., et al. , Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology 33, 687–696 (2012), 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugless F., et al. , Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in Southeastern Ohio. Neurotoxicol. Teratol. 41, 71–79 (2014), 10.1016/j.ntt.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takser L., Mergler D., Hellier G., Sahuquillo J., Huel G., Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology 24, 667–674 (2003), 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu W., et al. , Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: A systematic review and meta-analysis. Environ Health 19, 104 (2020), 10.1186/s12940-020-00659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oulhote Y., et al. , Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ. Health Perspect. 122, 1343–1350 (2014), 10.1289/ehp.1307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racette B. A., et al. , Increased risk of parkinsonism associated with welding exposure. Neurotoxicology 33, 1356–1361 (2012), 10.1016/j.neuro.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P., Culbreth M., Aschner M., Exposure, epidemiology, and mechanism of the environmental toxicant manganese. Environ. Sci. Pollut. Res. Int. 23, 13802–13810 (2016), 10.1007/s11356-016-6687-0. [DOI] [PubMed] [Google Scholar]
- 12.McMahon P. B., Belitz K., Reddy J. E., Johnson T. D., Elevated manganese concentrations in United States groundwater, role of land surface-soil-aquifer connections. Environ. Sci. Technol. 53, 29–38 (2019), 10.1021/acs.est.8b04055. [DOI] [PubMed] [Google Scholar]
- 13.Gurol K. C., Aschner M., Smith D. R., Mukhopadhyay S., Role of excretion in manganese homeostasis and neurotoxicity: A historical perspective. Am. J. Physiol. Gastrointest. Liver Physiol. 322, G79–G92 (2022), 10.1152/ajpgi.00299.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchens S., et al. , Hepatic and intestinal manganese excretion are both required to regulate brain manganese during elevated manganese exposure. Am. J. Physiol. Gastrointest. Liver Physiol. 325, G251–G264 (2023), 10.1152/ajpgi.00047.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C., Jursa T., Aschner M., Smith D. R., Mukhopadhyay S., Up-regulation of the manganese transporter SLC30A10 by hypoxia-inducible factors defines a homeostatic response to manganese toxicity. Proc. Natl. Acad. Sci. U.S.A. 118, e2107673118 (2021), 10.1073/pnas.2107673118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majmundar A. J., Wong W. J., Simon M. C., Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010), 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong G. H., Takeda K., Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 15, 635–641 (2008), 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- 18.Gurol K. C., et al. , PHD2 enzyme is an intracellular manganese sensor that initiates the homeostatic response against elevated manganese. Proc. Natl. Acad. Sci. U.S.A. 121, e2402538121 (2024), 10.1073/pnas.2402538121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dengler V. L., Galbraith M., Espinosa J. M., Transcriptional regulation by hypoxia inducible factors. Crit Rev. Biochem. Mol. Biol. 49, 1–15 (2014), 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camandola S., Mattson M. P., Brain metabolism in health, aging, and neurodegeneration. EMBO J. 36, 1474–1492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue C., et al. , Tryptophan metabolism in health and disease. Cell Metab. 35, 1304–1326 (2023), 10.1016/j.cmet.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Badawy A. A. K., Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 10, 1178646917691938 (2017), 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarcz R., Bruno J. P., Muchowski P. J., Wu H. Q., Kynurenines in the mammalian brain:When physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012), 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa T., et al. , Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 42, 1702–1706 (1992), 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- 25.Torosyan R., et al. , Hypoxic preconditioning protects against ischemic kidney injury through the IDO1/kynurenine pathway. Cell Rep. 36, 109547 (2021), 10.1016/j.celrep.2021.109547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelletier A., et al. , Resting natural killer cell homeostasis relies on tryptophan/NAD(+) metabolism and HIF-1alpha. EMBO Rep. 24, e56156 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semple B. D., Blomgren K., Gimlin K., Ferriero D. M., Noble-Haeusslein L. J., Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106–107, 1–16 (2013), 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brust V., Schindler P. M., Lewejohann L., Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool. 12, 17 (2015), 10.1186/1742-9994-12-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor C. A., et al. , SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity. J. Biol. Chem. 294, 1860–1876 (2019), 10.1074/jbc.RA118.005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marszalek-Grabska M., et al. , Kynurenine emerges from the shadows - current knowledge on its fate and function. Pharmacol. Ther 225, 107845 (2021), 10.1016/j.pharmthera.2021.107845. [DOI] [PubMed] [Google Scholar]
- 31.Hutchens S., et al. , Elevated thyroid manganese reduces thyroid iodine to induce hypothyroidism in mice, but not rats, lacking SLC30A10 transporter. Metallomics 16, mfae029 (2024), 10.1093/mtomcs/mfae029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei G., Chen L., Zhang W. W., WGCNA application to proteomic and metabolomic data analysis. Methods Enzymol 585, 135–158 (2017), 10.1016/bs.mie.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H., et al. , Metabolomics analysis coupled with weighted gene co-expression network analysis unravels the associations of tricarboxylic acid cycle-intermediates with edible pigments produced by Monascus purpureus (Hong Qu). Foods 11, 2168 (2022), 10.3390/foods11142168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Pardo P., et al. , Pharmacological validation of TDO as a target for Parkinson’s disease. FEBS J. 288, 4311–4331 (2021), 10.1111/febs.15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilotte L., et al. , Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 109, 2497–2502 (2012), 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luong T. N., Carlisle H. J., Southwell A., Patterson P. H., Assessment of motor balance and coordination in mice using the balance beam. J. Vis. Exp. 10, 2376 (2011), 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor C. A., et al. , SLC30A10 manganese transporter in the brain protects against deficits in motor function and dopaminergic neurotransmission under physiological conditions. Metallomics 15, mfad021 (2023), 10.1093/mtomcs/mfad021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warden A., et al. , Loss of SLC30A10 manganese transporter alters expression of neurotransmission genes and activates hypoxia-inducible factor signaling in mice. Metallomics 16, mfae007 (2024), 10.1093/mtomcs/mfae007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gal E. M., Sherman A. D., L-kynurenine: Its synthesis and possible regulatory function in brain. Neurochem. Res. 5, 223–239 (1980), 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- 40.Guilarte T. R., Gonzales K. K., Manganese-induced parkinsonism is not idiopathic Parkinson’s disease: Environmental and genetic evidence. Toxicol. Sci. 146, 204–212 (2015), 10.1093/toxsci/kfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warden A. S., et al. , Elevated brain manganese induces motor disease by up-regulating the kynurenine pathway of tryptophan metabolism. Gene Expression Omnibus (GEO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE292533. Deposited 20 March 2025. [DOI] [PMC free article] [PubMed]
- 42.Mukhopadhyay S., Elevated brain manganese induces motor disease by up-regulating the kynurenine pathway of tryptophan metabolism. MetaboLights. https://www.ebi.ac.uk/metabolights/MTBLS975. Deposited 26 March 2025. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Data Availability Statement
All study data are included in the article and/or supporting information. Sequencing data are available on the Gene Expression Omnibus (GEO; accession ID GSE292533) (41). Metabolomics data have been deposited on MetaboLights (study number MTBLS975) (42).