ABSTRACT
This systematic review was aimed at examining the impact of extraction methods on the phytochemical profile of Moringa oleifera leaves to identify the most effective extraction technique for food application. Mainly, maceration, Soxhlet extraction, and ultrasound‐assisted extraction (UAE) are reviewed in this study for their efficiency in extracting key phytochemicals in M. oleifera leaves. Given the rich phytochemical profile of M. oleifera leaves, selecting an appropriate extraction method is important in preserving their functionality and ensuring the quality of fortified or enriched food products. Adhering to PRISMA guidelines, this review found that maceration is the most efficient method for the extraction of gallic acid, which can enhance certain food textures but may also increase hardness in products such as cream cheese; Soxhlet extraction is effective in the extraction of kaempferol but slightly diminishes the sensory attributes in beverages such as malt drinks; and the UAE method is efficient in achieving the highest yield of quercetin while maintaining desirable sensory and textural properties. Overall, these findings suggest that the interaction between phytochemicals from M. oleifera leaves and the food matrix can affect the sensory and functional properties of the final product. Further optimization of each extraction technique is required to maximize the potential of M. oleifera leaf extracts in food applications.
Keywords: bioactive, extraction yield, moringa leaf, phytochemicals
This PRISMA‐guided systematic review compared three extraction methods of maceration, Soxhlet, and ultrasound‐assisted extraction (UAE) to determine their impact on the phytochemical profile of Moringa oleifera leaves intended for food applications. The analysis suggested method‐specific differences in the yields of key bioactive compounds including gallic acid, kaempferol, and quercetin. The findings emphasize the importance of optimizing the extraction methods to increase both the sensory attributes and functional properties of food products fortified with M. oleifera phytochemicals.

1. Introduction
The World Food Programme identifies food fortification as an effective strategy for improving dietary nutrition. This has led to a growing demand for research on fortified foods (Ahmad and Ahmed 2019). Food fortification involves the incorporation of minerals, vitamins, or bioactive compounds in foods in order to improve the nutritional value of the final product (Raza et al. 2024). Phytochemicals have emerged as promising fortifying agents owing to their extensive health benefits in preventing disease and addressing malnutrition (Jobby et al. 2023). Phytochemicals are bioactive compounds naturally present in plant‐based foods such as fruits, vegetables, and grains, and their inclusion in food products is associated with improved nutritional value of the final product (Abera et al. 2022).
Moringa oleifera is a rich source of phytochemicals and has potential for food fortification (Gopalakrishnan et al. 2016), owing to its nutritional and antioxidant properties (Hassan et al. 2021). It has been incorporated into food products, including instant porridge, ice cream, bread, and yogurt (Katmawanti et al. 2021; Ademosun 2021; Adetola et al. 2022). The phytochemical constituents abundantly present in M. oleifera leaves are phenolic acids, including gallic acids, and flavonoids such as quercetin and kaempferol (Vergara‐Jimenez et al. 2017). However, their incorporation into food products can have substantial impacts on some aspects. For example, incorporating M. oleifera leaves into beef patties can increase the protein content and shelf life due to its antioxidant activity (Al‐Baidhani et al. 2024), while their incorporation into bread formulations resulted in a less fluffy texture and a decrease in height and volume. The phytochemicals in M. oleifera leaves may inhibit yeast growth during fermentation, leading to a denser dough and less fluffy texture in the bread (Sengev et al. 2021). In this regard, the extraction technique may play an important role, as variations in temperature, extraction time, and pressure during leaves preparation can influence the quantity and composition of phytochemicals extracted from M. oleifera leaves (Bitwell et al. 2023), some of which possess antimicrobial properties (Anzano et al. 2022).
Extraction is the initial step for any plant biomolecule studies. The extraction of bioactive compounds from M. oleifera leaves is typically performed through three main methods: maceration, Soxhlet extraction, and ultrasound‐assisted extraction (UAE). Some extraction methods may preserve or enhance the bioactive properties of the compounds in leaves, while others may result in degradation or loss of bioactivity, which in turn affects the sensory attributes and overall quality of the final food product (Sandeep et al. 2023a). In the maceration technique, coarse plant materials are immersed in organic solvents, typically performed at low temperatures. It is crucial for preserving heat‐sensitive phytochemicals such as polyphenols (Amirullah et al. 2023). However, maceration may result in incomplete solvent penetration, leading to a lack of sensory properties in food products (Putra et al. 2023). Another technique is Soxhlet, which is highly efficient for extraction of a wide range of bioactive compounds. The continuous cycling of the solvent through the sample ensures a thorough extraction process and repeated exposure of the solid material to fresh solvent (Quitério et al. 2022). But the high temperature used during the extraction process may degrade some heat‐sensitive compounds, impacting mouthfeel (Sandeep et al. 2023a). The UAE method usually requires less time to achieve the desired bioactive compound compared to maceration or Soxhlet extraction methods. However, the optimization of parameters is required to maximize the result (Louie et al. 2020).
Each extraction method has advantages and disadvantages that can affect the yield and quality of phytochemicals extracted (Sandeep et al. 2023a). It is thus imperative to identify the most effective technique for food applications to maintain their sensory attributes and overall acceptability. However, there is still a gap in the literature on this topic, and this systematic review was designed to fill this knowledge gap by focusing on the phytochemicals present in M. oleifera leaves obtained through three different extraction methods and correlates these findings with current knowledge on the application of M. oleifera leaves in food products. By comparing the effects of all described extraction methods, for example, maceration, Soxhlet extraction, and UAE, on phytochemicals such as gallic acid and flavonoids, specifically quercetin and kaempferol, this review aims to identify the most effective extraction method for enhancing the application of M. oleifera leaves in food products.
2. Methodology
2.1. Data Sources and Search Strategy
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Figure 1). The studies were collected from the following databases: ScienceDirect, Scopus, PubMed, Wiley Online, and SpringerLink. The search was conducted using the keywords “ Moringa oleifera leaves”, “yield”, “extraction,” “phytochemicals”, and “food application”. Duplicate entries were eliminated, and the original data was collected, compiled, and cited properly.
FIGURE 1.
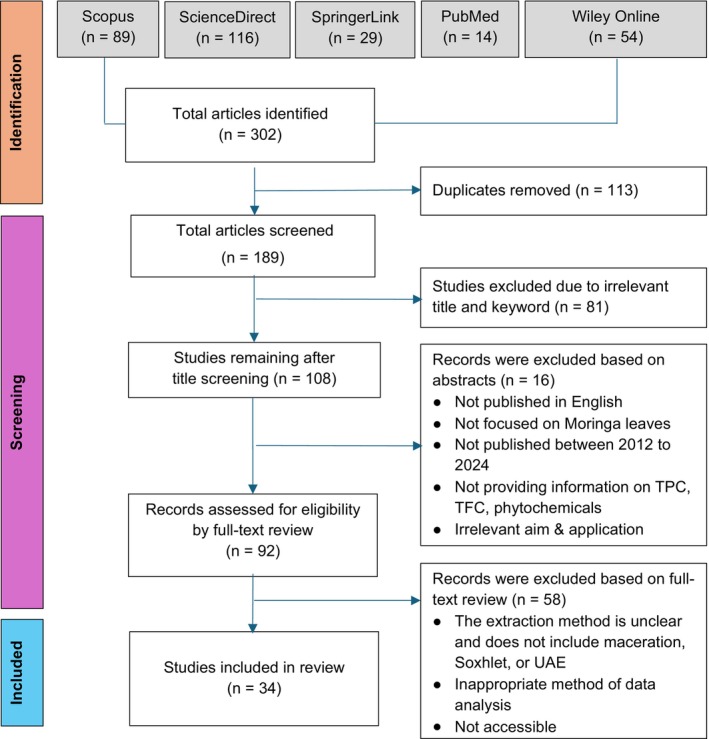
Overview of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart.
2.2. Study Selection, Inclusion, and Exclusion Criteria
The selection criteria used to assess full articles relied on the following required details: Initially, articles published in English from 2012 to 2024 were considered. Only research articles focused on the leaves part of M. oleifera were included, while articles examining any other parts were excluded. The articles needed to explain the specific extraction methods of M. oleifera leaves, including maceration, Soxhlet extraction, and UAE methods. Further, the articles should include information about total polyphenol content (TPC), total flavonoid content (TFC), and some key phytoconstituents, including gallic acid, quercetin, and kaempferol. Evidence regarding the application of M. oleifera in food products was also required. Articles addressing agricultural material, or veterinary applications, as well as other review articles, meta‐analyses, books, or articles unable to meet the inclusion criteria were excluded. All collected articles were screened based on the title, abstract, and subsequent full‐text analysis, adhering to the PRISMA flow diagram (Figure 1).
2.3. Data Extraction
To assess the three extraction methods for M. oleifera leaves and their relevance to food applications, two structured data collection tables were developed. The first table (Table 1) outlined the key parameters of the extraction method, including the solvent used, temperature, time, and how these parameters influenced the yield, TPC, TFC, and phytoconstituents in M. oleifera leaf extracts. The second table (Table 2) summarizes the existing knowledge about the incorporation of M. oleifera leaves in food products, highlighting both the benefits and potential limitations associated with their use in food formulations.
TABLE 1.
Bias analysis of in vitro studies on selected articles on M. oleifera leaves.
| Reference | Score | Total score | Risk | |||||
|---|---|---|---|---|---|---|---|---|
| Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | |||
| Abera et al. (2022) | 1 | 1 | 1 | 1 | 2 | 1 | 7 | M |
| Al‐Ghanayem et al. (2022) | 2 | 1 | 2 | 2 | 2 | 2 | 11 | L |
| Bennour et al. (2021) | 2 | 1 | 2 | 2 | 2 | 2 | 11 | L |
| da Silva et al. (2022) | 2 | 2 | 2 | 1 | 2 | 1 | 10 | L |
| García‐Beltrán et al. (2020) | 2 | 1 | 1 | 1 | 2 | 2 | 9 | M |
| Gomes, Leitão, et al. (2023) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Gomes, Albuquerque, et al. (2023) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Karim et al. (2018) | 2 | 2 | 2 | 2 | 1 | 2 | 11 | L |
| Kashaninejad et al. (2021) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Khalid et al. (2023a) | 2 | 2 | 2 | 2 | 1 | 2 | 11 | L |
| Khalid et al. (2023b) | 2 | 2 | 2 | 2 | 2 | 0 | 10 | L |
| Mabrok and Mohamed (2019) | 2 | 1 | 2 | 2 | 2 | 2 | 11 | L |
| Mabrouki et al. (2020) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Mahdi et al. (2016) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Mehganathan et al. (2024) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | L |
| Mohamed et al. (2018) | 2 | 2 | 2 | 1 | 1 | 1 | 9 | M |
| Pakade et al. (2013) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Pereira et al. (2021) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Pollini et al. (2020) | 2 | 2 | 2 | 1 | 1 | 1 | 9 | M |
| Rastogi et al. (2024) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Sandeep et al. (2023a) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Sandeep et al. (2023b) | 2 | 1 | 2 | 1 | 2 | 2 | 10 | L |
| Sandeep et al. (2022) | 2 | 1 | 1 | 1 | 2 | 2 | 9 | M |
| Setyani et al. (2023) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Shah et al. (2015) | 2 | 1 | 1 | 2 | 2 | 1 | 9 | M |
| Shervington et al. (2018) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Sulastri et al. (2018) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Thangaiah et al. (2024) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Thomas et al. (2020) | 2 | 1 | 1 | 2 | 2 | 1 | 9 | M |
| Virk et al. (2023) | 2 | 1 | 2 | 2 | 2 | 2 | 11 | L |
| Vongsak et al. (2013) | 2 | 1 | 2 | 2 | 2 | 2 | 11 | L |
| Wu et al. (2020) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Zhao and Zhang (2013) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
| Zhu et al. (2020) | 2 | 2 | 2 | 2 | 2 | 2 | 12 | L |
Note: Item 1 = clearly stated aim. Item 2 = accurate experimental design. Item 3 = identification and evaluation of sample. Item 4 = comparability and reproducibility. Item 5 = other bias. Item 6 = adequate statistical analysis. Item score 0 = not reported/described. Item score 1 = unclear/inadequately assessed. Item score 2 = briefly described/adequately assessed.
Abbreviations: L, low risk; M, moderate risk.
TABLE 2.
Effects of extraction methods on yield and phytochemicals in M. oleifera leaves.
| Items | Extraction methods | Solvent | Time | Temperature | Content | References |
|---|---|---|---|---|---|---|
| Yield | Maceration | 70% ethanol | 20 h | 40°C | 12.65% | Sandeep et al. (2023b) |
| Maceration | 70% ethanol | 24 h | RT | 15.55% | Sandeep et al. (2023a) | |
| Maceration | Water | 72 h | 40°C | 13.23% | Sandeep et al. (2023b) | |
| Soxhlet | 70% ethanol | 16 h | 40°C | 6.60% | Al‐Ghanayem et al. (2022) | |
| Soxhlet | Methanol | 16 h | NA | 18.56% | Zhao and Zhang (2013) | |
| Soxhlet | n‐Hexane | 8 h | 78°C | 9.30% | Pereira et al. (2021) | |
| UAE | 70% ethanol | 30 min | 30°C | 21.79% | Sandeep et al. (2023a) | |
| UAE | 14.6% ethanol | 5 min | 30°C | 31.0% | Thangaiah et al. (2024) | |
| UAE | 70% ethanol | 30 min | 35°C | 23.41% | Sandeep et al. (2022) | |
| TPC | Maceration | 70% ethanol | 24 h | RT | 130.16 mg QE/g | Bennour et al. (2021) |
| Maceration | Water | 24 h | NA | 101.81 mg GAE/g | Vongsak et al. (2013) | |
| Maceration | 70% ethanol | 72 h | RT | 132.30 mg CAE/g | Virk et al. (2023) | |
| Soxhlet | 70% ethanol | 16 h | 40°C | 123.17 mg QE/g | Bennour et al. (2021) | |
| Soxhlet | 70% ethanol | 20 h | NA | 45.5 mg CAE/g | Virk et al. (2023) | |
| Soxhlet | 50% ethanol | 20 h | NA | 44.6 mg CAE/g | Virk et al. (2023) | |
| UAE | 70% ethanol | 30 min | 30°C | 144.52 mg QE/g | Sandeep et al. (2023a) | |
| UAE | Methanol | 5 min | NA | 149.70 mg GAE/mL | García‐Beltrán et al. (2020) | |
| UAE | 70% ethanol | 30 min | 35°C | 144.90 mg GAE/mL | Sandeep et al. (2022) | |
| TFC | Maceration | 70% ethanol | 24 h | RT | 18.62 mg GAE/mL | Bennour et al. (2021) |
| Maceration | 80% ethanol | 20 h | RT | 26.33 mg QE/g | Mahdi et al. (2016) | |
| Maceration | 50% methanol | 48 h | 45°C | 12.06 mg QE/g | Wu et al. (2020) | |
| Soxhlet | 70% ethanol | 16 h | 40°C | 17.90 mg GAE/mL | Bennour et al. (2021) | |
| Soxhlet | 70% ethanol | 20 h | NA | 24.5 mg IQE/g | Virk et al. (2023) | |
| Soxhlet | 50% ethanol | 20 h | NA | 12.7 mg IQE/g | Virk et al. (2023) | |
| UAE | 70% ethanol | 30 min | 35°C | 24.23 mg GAE/mL | Sandeep et al. (2023a) | |
| UAE | Deep eutectic solvent | 30 min | 40°C | 48.90 mg RE/g | Setyani et al. (2023) | |
| UAE | 50% ethanol | 45 min | 35°C | 56.63 mg QE/g | Thomas et al. (2020) | |
| Gallic acid | Maceration | 70% ethanol | 72 h | RT | 150.0 μg/g | Mabrok and Mohamed (2019) |
| Maceration | 70% methanol | 72 h | NA | 51.20 μg/g | Vongsak et al. (2013) | |
| Soxhlet | Water | 20 min | NA | 46.00 μg/g | da Silva et al. (2022) | |
| Soxhlet | 99% ethanol | 6 h | 60°C | 23.00 μg/g | Zhu et al. (2020) | |
| UAE | 100% methanol | 40 min | 20°C | 4.24 μg/g | Rastogi et al. (2024) | |
| UAE | 50% methanol | 30 min | 25°C | 11.20 μg/g | Sulastri et al. (2018) | |
| Quercetin | Maceration | 96% ethanol | 72 h | RT | 64.80 μg/g | Khalid et al. (2023b) |
| Maceration | 70% ethanol | 72 h | RT | 45.01 μg/g | Pakade et al. (2013) | |
| Soxhlet | Acidified methanol | 14 h | 90°C | 21.50 μg/g | Mehganathan et al. (2024) | |
| Soxhlet | 99% ethanol | 6 h | 60°C | 13.00 μg/g | Zhu et al. (2020) | |
| UAE | 60% ethanol | 14 min | 48°C | 55.56 μg/g | Pollini et al. (2020) | |
| UAE | 50% methanol | 35 min | 45°C | 65.40 μg/g | Mabrouki et al. (2020) | |
| Kaempferol | Maceration | Water | 24 h | RT | 1.84 μg/g | Vongsak et al. (2013) |
| Maceration | Butanol | 24 h | RT | 1.02 μg/g | Shervington et al. (2018) | |
| Soxhlet | Acidified methanol | 14 h | 90°C | 79.40 μg/g | Mehganathan et al. (2024) | |
| Soxhlet | HCl 0.1 M | 3 h | 90°C | 84.93 μg/g | Kashaninejad et al. (2021) | |
| UAE | 50% methanol | 35 min | 45°C | 30.10 μg/g | Mabrouki et al. (2020) | |
| UAE | 20% ethanol | NA | 50°C | 16.90 μg/g | Pareek et al. (2023) |
Abbreviations: CAE, chlorogenic acid equivalent; GAE, gallic acid equivalent; IQE, isoquercetin equivalent; NA, information not given; QE, quercetin equivalent; RE, rutin equivalent; RT, room temperature.
2.4. Risk of Bias Assessment
A bias analysis for in vitro studies was conducted in accordance with the Methods Guide for Comparative Effectiveness Reviews as described by Viswanathan et al. (Viswanathan et al. 2008). The assessment included 6 items (Table 1): clearly stated aim considered as item 1, accurate experimental design as item 2, sample identification and evaluation as item 3, comparability and reproducibility as item 4, other bias as item 5, and adequate statistical analysis as item 6. Item 1 was scored 0 if the article's aim did not correspond to the research, 1 if the aim was unclear, and 2 if the aim was clearly reported. Item 2 (experimental design) was scored 0 if the experimental design was not described, 1 if there was unclear experimental design, and 2 if the experimental design was reported in detail and accurately. Item 3 (sample identification and evaluation) was scored 0 if any polyphenols were not reported, 1 if the value of some properties like TPC and TFC were reported, and 2 if the phytochemical constituents, including gallic acid, kaempferol, and quercetin, were reported in detail. Item 4 (comparability and reproducibility) was scored 0 if the extraction methods required non‐lab standards, 1 if the methods were less complex but had low reproducibility, and 2 if the methods could be easily practiced in standard labs with consistent results. Item 5 (other bias) was scored 0 if the abstract, methods, and conclusions were poorly or not described, 1 if they were too brief, and 2 if they were adequately detailed. Item 6 (statistical analysis) was scored 0 if not performed, 1 if partially done, and 2 if detailed (e.g., standard deviation). The total score determined the risk category: 0–6 for high risk, 7–9 for moderate risk, and 10–12 for low risk.
3. Results and Discussion
3.1. PRISMA Flow Selection
As presented in the PRISMA flow chart (Figure 1), the initial search resulted in the identification of 301 related records through the database searches. After removing duplicates, 188 records remained. Of these, 92 articles were selected for full‐text assessment. Ninety‐six records were excluded for reasons including: they were not published in English, did not focus on Moringa leaves, did not provide information on TPC, TFC, phytoconstituents, food application, or were not published between 2012 and 2024. Following the application of pre‐determined criteria, 58 articles were removed, leaving 34 eligible articles that investigated the effect of extraction method on TPC, TFC, phytoconstituents, and food application of M. oleifera. The distribution of studies selected for this review shows distinctive trends across several dimensions (Figure 2). Most of the sources were published recently, especially in 2023, an indication of recent interest in the subject matter. Tunisia emerges as a major source of studies, with a substantial number of publications exploring the phytochemical composition and food application of Moringa leaves.
FIGURE 2.

Distribution of selected studies based on publication year (a) and country of origin (b).
3.2. Bias Analysis
The risk of bias of in vitro studies is tabulated in Table 1. The overall bias risk analysis of the studies indicates a moderate to low risk of bias across multiple parameters.
Almost all studies are relevant and closely related to the aim of the present topic. Over 80% of studies provided detailed information on the phytochemical constituents in M. oleifera leaves, including gallic acid, kaempferol, and quercetin, with adequate descriptions of abstract, methods, and conclusion. More than 70% use extraction methods that are reproducible in standard laboratory settings without requiring specialized equipment, and they report robust statistical analyses. Lastly, above 65% of studies provided a detailed description of the experimental methods.
3.3. Effects of Extraction Methods on Yield, TPC, and TFC
In general, M. oleifera crude extracts are obtained using maceration, Soxhlet, and UAE techniques. Maceration is one of the simplest methods involving immersing coarse plant materials in organic solvents such as methanol, acetone, ethanol, ethyl acetate, hexane, or water, typically at room temperature. During maceration, the plant cells are ruptured (Figure 3), allowing the bioactive components to come into contact with the solvent and be extracted (Farooq et al. 2022).
FIGURE 3.
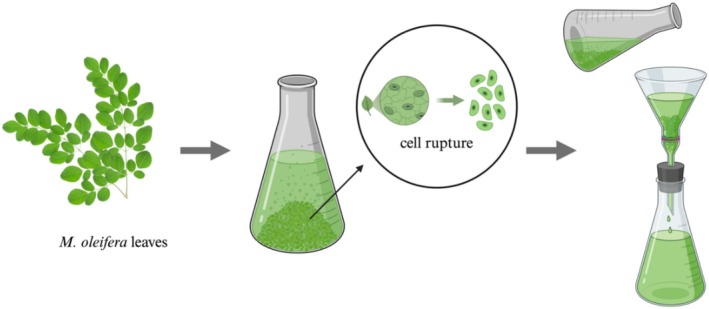
Technique of Moringa oleifera leaves extraction using the maceration method. The figure was created using BioRender (https://BioRender.com, accessed on 30 April 2024).
Soxhlet extraction is another widely used technique for the extraction of plant phytochemicals and is performed using a specialized apparatus known as the Soxhlet apparatus (Figure 4). In this extraction method, the solvent is heated in a round‐bottom flask, and the vapor rises to the condenser and passes through the extraction chamber. The condensed droplets then drip down into the porous thimble containing the sample. The solvent dissolves the target compounds from the plant material, gradually filling the extraction chamber. Once the level of solvent reaches above the siphon bend, the solvent returns to the round‐bottom flask, where it accumulates (Malik and Mandal 2022; Tian et al. 2016; Qin et al. 2023). The continuous cycling of the solvent through the sample ensures a thorough extraction process and repeated exposure of the solid material to fresh solvent repeatedly (Quitério et al. 2022). Compared to the maceration method, Soxhlet extraction uses less solvent and enhances solute diffusion, which accelerates the extraction process (Amirullah et al. 2023).
FIGURE 4.

Technique of Moringa oleifera leaves extraction using Soxhlet extraction. The figure was created using BioRender (https://BioRender.com, accessed on 30 April 2024).
A modern and widely used technique for the phytochemical extraction in M. oleifera leaves is UAE. In the UAE method, high‐frequency ultrasound pulses generate localized hotspots on a macroscopic scale (Figure 5), inducing high shear stress and temperature through the generation of cavitation bubbles. These bubbles expand and then collapse, releasing energy that produces localized high temperatures and pressures. The energy released by the collapsing bubbles creates shock waves and microjets, which break down the plant cell walls. When these bubbles collapse, they generate localized high pressure and temperature, which can disrupt cell walls and enhance mass transfer. This mechanism improves solvent penetration into plant material, allowing for a more thorough extraction of polyphenols (Vernès et al. 2020). The UAE method can be more energy‐efficient than other extraction methods, as it operates at lower temperatures and achieves faster extraction rates. Owing to the enhanced extraction efficiency, UAE typically requires less time to achieve the desired yield of bioactive compounds compared to other methods such as maceration or Soxhlet extraction. Also, its capability to operate under room temperature plays a crucial role in preventing oxidation and decomposition of target natural products (Louie et al. 2020). These mechanisms make UAE the most effective method, yielding the highest TPC, TFC, and overall extract yield from M. oleifera leaves. Maceration ranked second in efficiency, while Soxhlet extraction produced the lowest outcomes for these parameters. The comparative results are reported in Table 2.
FIGURE 5.

Technique of Moringa oleifera leaves extraction using ultrasound‐assisted extraction (UAE). The figure was created using BioRender (https://BioRender.com, accessed on 30 April 2024).
M. oleifera leaves contain various phytochemicals that are sensitive to high temperatures (Pareek et al. 2023), including phenolic acids and flavonoids. For example, gallic acid begins to degrade at temperatures above 60°C (Antony and Farid 2022), while flavonoids such as quercetin and kaempferol may experience thermal degradation at approximately 70°C (158°F) (Speisky et al. 2022). The UAE method typically operates at controlled lower temperatures (often below 50°C) (Kumar et al. 2021). This mild condition helps maintain the stability of sensitive compounds such as gallic acid, quercetin, and kaempferol. The high energy of ultrasound waves causes a faster extraction process. In this mechanism, there is a quick release of polyphenols from the plant material. The faster process may minimize the risk of degradation of sensitive compounds, thereby retaining a higher concentration of TPC.
Prior research has reported the efficacy of ultrasonication in advancing food processing and enhancing the physicochemical attributes and quality of foods. The high‐intensity ultrasound technology can potentially serve as a direct, efficient, swift, and eco‐friendly approach for the extraction of polyphenols. For example, employing a high ultrasonic intensity of 700 W was effective in the extraction of mangiferin from Phaleria macrocarpa fruits (Lim et al. 2019). Similarly, ultrasonication increased the TPC in sorghum flour. In these studies, a high‐energy ultrasonic device operating at 1000 W and an ultrasonic processor at 500 W were used, with the increase in free phenolic acid content attributed to the increased release of bound polyphenols (Lohani and Muthukumarappan 2021). Similarly, the combination of acid with ultrasonic extraction for Rubus idaeus L. enhanced the efficiency of extracting bound phenolic acids, even when using a relatively low ultrasonic power of 320 W (Wang et al. 2019).
In contrast, Soxhlet extraction requires continuous heating of the solvent, often at temperatures above 80°C (Tzanova et al. 2020). This high‐temperature exposure can rapidly degrade sensitive compounds such as quercetin and gallic acid. This leads to a lower yield of TPC and TFC. Though extraction using the maceration method preserves more sensitive compounds due to the lower temperature employed, the prolonged extraction time associated with this method may result in the gradual degradation or transformation of compounds over time, potentially reducing the yield (Shen et al. 2023).
As reported in Table 2, the extraction solvent significantly influences the extraction yield. Maceration and UAE methods resulted in higher yields as ethanol concentration increased. The polarity of ethanol allows it to effectively dissolve a wide range of compounds from the plant material (Lim et al. 2019). Polar solvents can dissolve polar compounds such as phenolic acids and flavonoids, resulting in increased efficiency of extraction (Lohani and Muthukumarappan 2021). However, in the Soxhlet extraction method, methanol produced a higher yield, likely because methanol is less prone to oxidation and degradation compared to ethanol (Wang et al. 2019). In the Soxhlet extraction method, where the solvent is continuously heated over a prolonged period, the stability of the solvent becomes crucial. Greater chemical stability of methanol makes it more suitable for extended extraction processes, resulting in minimal degradation of the solvent and higher yields of bioactive compounds.
The highest TPC value was observed with UAE when methanol was utilized as a solvent. However, direct comparison with other methods is difficult owing to the specific extraction parameters employed in the study. Stability of methanol facilitates superior extraction of polyphenols at high temperatures, resulting in a higher TPC (Wang et al. 2019). It is important to consider that the exceptionally short extraction period used in this study may have influenced the extraction efficiency. This highlights the significance of extraction conditions in interpreting TPC values.
M. oleifera leaves macerated with ethanol exhibited higher flavonoid content compared to methanol. It is likely because ethanol is capable of dissolving both polar and non‐polar compounds, thus effective for flavonoids extraction (Tzanova et al. 2020). Moreover, ethanol has the ability to solubilize quercetin and kaempferol (Shen et al. 2023). Consequently, ethanol yields higher TFC from M. oleifera leaves. However, the UAE method achieved the highest TFC using lower ethanol concentrations. This may be influenced by the cavitation phenomenon induced by ultrasound, which creates intense localized pressure and temperature gradients within the solvent. These fluctuations may enhance the interactions between the solvent molecules and the solutes in the plant material (Vinatoru 2001). In the case of lower ethanol concentrations, where solvent molecules may be less prone to clustering, the cavitation‐induced turbulence and microstreaming promote more uniform and intimate contact between the solvent and the plant surface (Vinatoru 2001). This facilitates the dissolution of target compounds, such as flavonoids.
Optimal yields of polyphenols in UAE method depend on the selection of an appropriate solvent. Methanol is the preferred solvent for extracting polyphenols from Centaurea sp. leaves, with ethanol following closely. Conversely, ethanol is the most effective solvent for the extraction of phenolic acids from mango peels (Martínez‐Ramos et al. 2020). Similarly, the ethanolic extracts of Laurus nobilis exhibited the highest TPC compared to water and methanolic extracts (Rincón et al. 2019). However, it is important to note that geographical origin and plant species or cultivars may also influence the yield of extracted polyphenols (Zainol et al. 2020).
3.4. Effects of Extraction Methods on Phytoconstituents
The three most abundant phytoconstituents in M. oleifera leaves are gallic acid, quercetin, and kaempferol. Each extraction method influenced the major phytoconstituents in M. oleifera leaves. M. oleifera leaves extracted using the maceration technique yield the highest concentration of gallic acid (Table 2). Maceration involves soaking the plant material in a solvent at room temperature or slightly higher (Subramanian and Anandharamakrishnan 2023). The solvents, typically ethanol, methanol, or water, diffuse into the plant cells, breaking down the cell wall and releasing gallic acid into the solvent (Bitwell et al. 2023). The chemical structure of gallic acid further supports this mechanism. Gallic acid contains a benzene ring with three hydroxyl groups (–OH) (Figure 6) (Charlton et al. 2023), which can form hydrogen bonds with polar solvents such as water and alcohol (Spange et al. 2022). These interactions help dissolve gallic acid into the solvent by reducing the intermolecular forces between gallic acid molecules and allowing it to disperse more easily during maceration (Park and Lee 2024). The aromatic ring in gallic acid contributes significantly to its antioxidant properties; however, its hydroxyl groups make it highly susceptible to oxidation. Exposure to heat, light, or strong mechanical stress can trigger oxidative degradation, leading to the formation of reactive oxygen species (ROS). This process not only compromises the structural integrity of gallic acid but also reduces its antioxidant efficacy, ultimately impacting its stability and functional benefits in food and pharmaceutical applications (van Lith and Ameer 2016; Qu et al. 2024). In oxidative conditions, gallic acid may undergo auto‐oxidation, leading to the formation of quinones (Tan et al. 2023) or participate in oxidative coupling reactions with other polyphenols (Kieserling et al. 2024), potentially modifying the texture, digestibility, and bioavailability of both nutrient and gallic acid (Tan et al. 2023; Sun et al. 2023). Thus, the conditions of maceration help preserve gallic acid from harsh exposure, that commonly occurs in Soxhlet and UAE methods (Bitwell et al. 2023; Gil‐Martín et al. 2022). Soxhlet extraction involves continuous boiling and condensation (Nafiu et al. 2019), while UAE uses ultrasound waves (Weggler et al. 2020). Both methods carry a higher risk of thermal or mechanical degradation of gallic acid.
FIGURE 6.
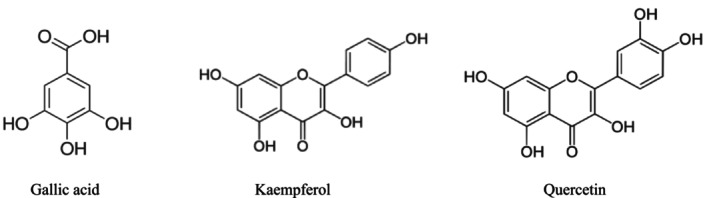
Chemical structure of phytoconstituents in M. oleifera leaves.
M. oleifera leaves extracted using the Soxhlet extraction technique yield the highest concentration of kaempferol (Table 2). Kaempferol's structure contains four hydroxyl groups (Dabeek and Marra 2019) contributing to its strong antioxidant activity, anti‐inflammatory, anticancer, and neuroprotective effects (Kaur et al. 2024). This compound is poorly soluble in water but slightly polar, allowing it to dissolve well in polar solvents like ethanol (Cid‐Ortega and Monroy‐Rivera 2018), which is commonly used in Soxhlet extraction. Moreover, although high temperatures and prolonged extraction times in Soxhlet extraction may potentially degrade some compounds, kaempferol has a relatively stable molecular structure, allowing it to withstand higher temperatures without significant degradation (de Oliveira et al. 2017). Kaempferol has a typical flavonol backbone, consisting of two benzene rings connected by a heterocyclic oxygen‐containing ring (Figure 6). The presence of a C–ring and hydroxyl groups at the 3 and 4 positions allows electron delocalization, stabilizing ROS more effectively than gallic acid with a simpler structure (Jomova et al. 2023). This stability allows kaempferol to be effectively extracted using the Soxhlet method.
M. oleifera leaves extracted using the UAE technique yield the highest concentration of quercetin. Quercetin is a flavonol with a diphenylpropane skeleton and five hydroxyl groups (Figure 6) (Michala and Pritsa 2022). Quercetin has a catechol (ortho‐dihydroxy) structure and a C–ring heterocycle, which stabilizes radicals via electron delocalization (Carrillo‐Martinez et al. 2024). This structural feature makes quercetin one of the most potent antioxidants, surpassing kaempferol and gallic acid in antioxidative efficacy (Madiha et al. 2021). However, its high reactivity also increases its susceptibility to oxidation, making it prone to degradation under excessive heat or prolonged exposure to oxidative environments (Cao et al. 2022). The UAE method offers an advantage by operating at lower temperatures (Kumar et al. 2021) compared to Soxhlet extraction, which helps maintain the stability of quercetin, as prolonged heat (≥ 60°C) can lead to dihydroxylation or oxidation of quercetin into quinone derivatives (Bhatia et al. 2022). The presence of cavitation in UAE does not generate excess oxygen radicals that could modify the structure of quercetin. Moreover, UAE is a faster extraction method (Shen et al. 2023), reducing the exposure of quercetin to potentially harmful conditions such as extended heat or mechanical stress.
3.5. Effects of Extraction Methods on Food Application of M. oleifera Leaves
As moringa‐fortified food products continue to grow in popularity, careful consideration must be given to both their health benefits and sensory properties. The extraction method used for M. oleifera leaves impacts their incorporation into food products, particularly affecting sensory attributes (Table 3). Differences in extraction conditions for example, solvent type, temperature, and mechanical forces influence the yield and composition of bioactive compounds, which in turn affect the color, flavor, texture, and functional properties of the final product (Trigo et al. 2023). For example, the maceration method employs soaking plant material in a solvent (such as ethanol or water) at room temperature for an extended duration (Subramanian and Anandharamakrishnan 2023). Due to its broad extraction range, this method potentially results in a high concentration of anti‐nutrient phytochemicals, including phytate (Guan et al. 2021). These compounds, while beneficial in some contexts, can increase hardness and decrease cohesiveness in products like cream cheese, and also promote undesirable flavor in atmosphere‐packaged raw beef (Table 3).
TABLE 3.
Benefits and drawbacks of different extraction methods for the application of M. oleifera leaves in food products.
| Extraction Methods | Food Product | Benefits | Drawbacks | References |
|---|---|---|---|---|
| Maceration | Cream cheese |
|
|
Karim et al. (2018) |
| Atmosphere packaged raw beef |
|
|
Gomes, Leitão, et al. (2023) | |
| Soxhlet | Giant Freshwater Prawn ( M. rosenbergii ) |
|
None | Gomes, Albuquerque, et al. (2023) |
| Malt drink |
|
|
Abera et al. (2022) | |
| UAE | Yogurt |
|
None | Wu et al. (2024) |
| Pasta |
|
None | Bell et al. (2018) |
Phytate chelates and affects the bioavailability of calcium ions in cream cheese incorporated with M. oleifera leaves extract (Guan et al. 2021). This ion binding results in protein–protein interaction (Guan et al. 2021), which in turn tightens the structure into a more complex matrix, increasing the hardness of the cream cheese (Guan et al. 2021) In addition, phytates present in M. oleifera leaves have metal‐chelating properties, binding prooxidant metal ions such as iron and copper. This reduces their availability, which could otherwise catalyze oxidative degradation, thereby lowering the oxidation level in atmosphere‐packaged raw beef modified with M. oleifera extract (Scarano et al. 2023). However, this strong metal‐binding property can also contribute to the development of metallic or astringent flavors, which are generally considered undesirable in food applications (Ömür‐Özbek et al. 2012).
Phenolic acids in M. oleifera leaves may also reduce essential amino acids, thus weakening the stabilization of protein structures in food (Karabulut et al. 2024). For example, lysine is involved in cross‐linking collagen fibers, while cysteine forms disulfide bonds stabilizing protein structure. Also, tryptophan contributes to protein stability and folding (Rawel et al. 2002). The lack of these essential amino acids results in a less cohesive and more crumbly texture in food products.
Soxhlet extraction uses organic solvents at elevated temperatures to continuously extract compounds over several cycles (Tzanova et al. 2020). However, the high temperatures and prolonged exposure associated with this method can enhance the extraction of volatile compounds, including isothiocyanates present in M. oleifera leaves (Wu et al. 2024; Bell et al. 2018). Isothiocyanates are known to activate bitter taste receptors (TAS2Rs), particularly TAS2R38 on the human tongue, contributing to the bitterness of malt drinks containing M. oleifera leaf extract (Tran et al. 2021). Moreover, polyphenols in M. oleifera leaves, such as kaempferol, have planar structures that may further contribute to bitterness by interacting with receptors involved in bitter taste perception (Nejabati and Roshangar 2022; Tarragon and Moreno 2020). The extended exposure to high temperature during Soxhlet extraction potentially promotes the release of bound tannins from the plant matrix (Das et al. 2020). Tannins can bind proteins in saliva and mucous membranes in the mouth, causing them to precipitate or aggregate. This leads to the dry and puckering sensation, which is associated with astringency (Soares et al. 2020).
According to Table 3, the UAE method did not show any effects or drawbacks on food products. The ultrasonic waves in the UAE method effectively break down plant cell walls, allowing for an efficient release of bioactive compounds. This process enhances the yield of desired phytochemicals without the need for prolonged exposure to solvents or high temperatures (Vernès et al. 2020). Furthermore, the UAE method can be optimized to selectively extract specific phytochemicals while minimizing the extraction of undesirable compounds that negatively affect sensory properties (Raghunath et al. 2023). The UAE often requires shorter extraction times compared to other methods (Shen et al. 2023) and can reduce the likelihood of extracting excessive amounts of polyphenols that can contribute to bitterness and astringency.
3.6. Work Limitation and Future Direction
This study highlights how UAE, maceration, and Soxhlet extraction methods impact the bioactive compounds in M. oleifera leaves. By understanding how these methods influence compound stability and yield, an optimal extraction approach can be identified to maximize bioactive retention while minimizing degradation. Further, this study explores the incorporation of M. oleifera extracts into various food products, such as dairy, meat, and beverages, providing practical information into how different extraction methods may affect sensory attributes, stability, and bioactivity. However, the impact of M. oleifera extracts on texture, flavor, and stability varies depending on food matrices.
This study also presents several limitations. The variability in solvent type, temperature, and extraction time can lead to complexity in standardizing optimal conditions, making it challenging to compare all phytoconstituents in M. oleifera leaves. Further, the influence of geographic origin and plant species or cultivar may cause variability, making it difficult to establish universally applicable conclusions regarding extraction protocols. This review is limited to the three most abundant phytoconstituents in M. oleifera leaves, without systematically evaluating the impact of various extraction methods on anti‐nutritional factors, which could influence the nutritional quality and functional properties of M. oleifera leaves in food application. Future research should expand on a broader range of these aspects to provide a more comprehensive understanding of the role of M. oleifera leaves in the food industry. Comparing studies with other extraction techniques, such as supercritical fluid extraction or microwave‐assisted extraction, along with other factors such as pH, sample‐to‐solvent ratio, and exploring various solvents with different polarities may offer opportunities to maximize the results of this review. In addition, interaction within food matrices may lead to the efficacy of phytochemicals and influence the sensory properties of food products. Therefore, the systematic evaluation of the impact of various extraction techniques on the interaction between phytochemicals and other food components could provide a better understanding of the optimization of M. oleifera incorporation in food products. Furthermore, investigating more examples of M. oleifera leaf applications in food products is essential to promote the wider adoption and greater impact of M. oleifera leaf extract in the food industry.
4. Conclusions
The extraction method used for M. oleifera leaves can have a substantial impact on the bioactive compounds and their benefits in food products. UAE outperforms traditional methods such as maceration and Soxhlet extraction with respect to extraction yield, TPC, and TFC. In particular, maceration is the most efficient for extracting gallic acid, UAE is best for quercetin, and Soxhlet extraction is particularly effective for obtaining kaempferol. Incorporating M. oleifera into food products enhances probiotic growth, flavor, shelf life, and antimicrobial and antioxidant properties. Importantly, maceration increases hardness in cream cheese, direct addition to raw beef causes undesirable flavors, and Soxhlet reduces sensory qualities in malt drinks. UAE, on the other hand, preserves sensory and textural attributes, making it a superior choice. Thus, selecting the appropriate extraction method is essential to maximize the benefits of M. oleifera leaves in food systems, ensuring both optimal sensory and functional qualities.
Author Contributions
Zulfa Ajrina Fitri: methodology (equal), visualization (equal), writing – original draft (equal). Farhad Ahmadi: conceptualization (equal), data curation (equal), supervision (equal), visualization (equal), writing – review and editing (equal). M. Ashraful Islam: validation (equal), writing – review and editing (equal). Eric N. Ponnampalam: validation (equal), writing – review and editing (equal). Frank R. Dunshea: resources (equal), validation (equal), writing – review and editing (equal). Hafiz A. R. Suleria: conceptualization (equal), funding acquisition (equal), project administration (equal), resources (equal), supervision (equal), validation (equal), writing – review and editing (equal).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The transformation of Australian grown Moringa into a high value feed ingredient for human and animal consumption project has been funded by AgriFutures Australia as part of the Emerging Industries Program, which focuses on new and emerging industries with high growth potential. This study was conducted at the School of Agriculture, Food, and Ecosystem Science, Faculty of Science, The University of Melbourne, Australia and was supported by the Indonesia Endowment Fund for Education (LPDP).
Funding: This work was supported by Agrifutures Australia.
Data Availability Statement
The authors have nothing to report.
References
- Abera, T. , Tamtam M. R., Koutavarapu R., and Shim J.. 2022. “Low‐Cost Production and Healthy Preservation of Malt Drink Using Melkassa‐7 and Moringa oleifera Leaf Extract.” International Journal of Gastronomy and Food Science 29: 100–568. 10.1016/j.ijgfs.2022.100568. [DOI] [Google Scholar]
- Ademosun, A. O. 2021. “Glycemic Properties of Soursop‐Based Ice Cream Enriched With Moringa Leaf Powder.” Foods and Raw Materials 9, no. 2: 207–214. 10.21603/2308-4057-2021-2-207-214. [DOI] [Google Scholar]
- Adetola, O. Y. , Kruger J., Ferruzzi M. G., Hamaker B. R., and Taylor J. R. N.. 2022. “Potential of Moringa Leaf and Baobab Fruit Food‐To‐Food Fortification of Wholegrain Maize Porridge to Improve Iron and Zinc Bioaccessibility.” International Journal of Food Sciences and Nutrition 73, no. 1: 15–27. 10.1080/09637486.2021.1911962. [DOI] [PubMed] [Google Scholar]
- Ahmad, A. , and Ahmed Z.. 2019. “Fortification in Beverages.” In Production and Management of Beverages, 85–122. Elsevier. 10.1016/B978-0-12-815260-7.00003-1. [DOI] [Google Scholar]
- Al‐Baidhani, A. M. S. , Hashim A. Z., Al‐Qutaifi H. K., et al. 2024. “Ultrasound‐Assisted Extraction of Bioactive Compounds From Moringa oleifera Leaves for Beef Patties Preservation: Antioxidant and Inhibitory Activities, Half‐Life, and Sensory Attributes.” Food Science & Nutrition 12, no. 10: 7737–7750. 10.1002/fsn3.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Ghanayem, A. A. , Alhussaini M. S., Asad M., and Joseph B.. 2022. “ Moringa oleifera Leaf Extract Promotes Healing of Infected Wounds in Diabetic Rats: Evidence of Antimicrobial, Antioxidant and Proliferative Properties.” Pharmaceuticals 15, no. 5: 528. 10.3390/ph15050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirullah, N. A. , Abdullah E., Zainal Abidin N., Abdullah N., and Manickam S.. 2023. “Influence of Extraction Technologies on the Therapeutic Properties of Pleurotus Spp. (Oyster Mushrooms)—A Critical Review.” Food Bioscience 56: 103352. 10.1016/j.fbio.2023.103352. [DOI] [Google Scholar]
- Antony, A. , and Farid M.. 2022. “Effect of Temperatures on Polyphenols During Extraction.” Applied Sciences 12, no. 4: 2107. 10.3390/app12042107. [DOI] [Google Scholar]
- Anzano, A. , de Falco B., Ammar M., et al. 2022. “Chemical Analysis and Antimicrobial Activity of Moringa oleifera Lam. Leaves and Seeds.” Molecules 27, no. 24: 8920. 10.3390/molecules27248920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, L. , Oloyede O. O., Lignou S., Wagstaff C., and Methven L.. 2018. “Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds.” Molecular Nutrition & Food Research 62, no. 18: e1700990. 10.1002/mnfr.201700990. [DOI] [PubMed] [Google Scholar]
- Bennour, N. , Mighri H., Bouhamda T., et al. 2021. “ Moringa oleifera Leaves: Could Solvent and Extraction Method Affect Phenolic Composition and Bioactivities?” Preparative Biochemistry and Biotechnology 51, no. 10: 1018–1025. 10.1080/10826068.2021.1891550. [DOI] [PubMed] [Google Scholar]
- Bhatia, N. K. , Tomar V. R., Kishor S., and Deep S.. 2022. “Effect of pH and Temperature on Physicochemical Properties, Aggregation Behaviour and Degradation Kinetics of Quercetin and Baicalein in Nearly Aqueous Media.” Journal of Molecular Liquids 366: 120236. 10.1016/j.molliq.2022.120236. [DOI] [Google Scholar]
- Bitwell, C. , Indra S. S., Luke C., and Kakoma M. K.. 2023. “A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals From Plants.” Scientific African 19: e01585. 10.1016/j.sciaf.2023.e01585. [DOI] [Google Scholar]
- Cao, H. , Högger P., Prieto M. A., Simal‐Gandara J., and Xiao J.. 2022. “Stability of Quercetin in DMEM and Cell Culture With A549 Cells.” eFood 3: 3. 10.1002/efd2.13. [DOI] [Google Scholar]
- Carrillo‐Martinez, E. J. , Flores‐Hernández F. Y., Salazar‐Montes A. M., Nario‐Chaidez H. F., and Hernández‐Ortega L. D.. 2024. “Quercetin, a Flavonoid With Great Pharmacological Capacity.” Molecules 29, no. 5: 1000. 10.3390/molecules29051000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton, N. C. , Mastyugin M., Török B., and Török M.. 2023. “Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity.” Molecules 28, no. 3: 1057. 10.3390/molecules28031057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid‐Ortega, S. , and Monroy‐Rivera J. A.. 2018. “Extraction of Kaempferol and Its Glycosides Using Supercritical Fluids From Plant Sources: A Review.” Food Technology and Biotechnology 56, no. 4: 480–493. 10.17113/ftb.56.04.18.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva, M. , Trancoso J., Tormen L., Bombardelli M. M., Corazza M. L., and Bainy E. M.. 2022. “Extraction of Compounds From Moringa oleifera Leaves Using Supercritical CO2 Plus Ethanol as a Cosolvent.” Journal of Food Process Engineering 45, no. 3: e13979. 10.1111/jfpe.13979. [DOI] [Google Scholar]
- Dabeek, W. M. , and Marra M. V.. 2019. “Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular‐Related Bioactivity in Humans.” Nutrients 11, no. 10: 2288. 10.3390/nu11102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, A. K. , Islam M. N., Faruk M. O., Ashaduzzaman M., and Dungani R.. 2020. “Review on Tannins: Extraction Processes, Applications and Possibilities.” South African Journal of Botany 135: 58–70. 10.1016/j.sajb.2020.08.008. [DOI] [Google Scholar]
- de Oliveira, A. H. , Leite R. D. S., Dantas F. H., et al. 2017. “Thermal Degradation Kinetics of Kaempferol and Quercetin in the Pre‐Formulated of the Standardized Extracts of Poincianella Pyramidalis (Tul.) L. P. Queiroz Obtained by Spray Dryer.” International Journal of Pharmacy and Pharmaceutical Sciences 9, no. 6: 123. 10.22159/ijpps.2017v9i6.16935. [DOI] [Google Scholar]
- Farooq, S. , Mir S. A., Shah M. A., and Manickavasagan A.. 2022. “Extraction Techniques.” In Plant Extracts: Applications in the Food Industry, edited by Manickavasagan A., 23–37. Academic Press. 10.1016/B978-0-12-822475-5.00005-3. [DOI] [Google Scholar]
- García‐Beltrán, J. M. , Mansour A. T., Alsaqufi A. S., Ali H. M., and Esteban M. Á.. 2020. “Effects of Aqueous and Ethanolic Leaf Extracts From Drumstick Tree (Moringa oleifera) on Gilthead Seabream (Sparus Aurata L.) Leucocytes, and Their Cytotoxic, Antitumor, Bactericidal and Antioxidant Activities.” Fish & Shellfish Immunology 106: 44–55. 10.1016/j.fsi.2020.06.054. [DOI] [PubMed] [Google Scholar]
- Gil‐Martín, E. , Forbes‐Hernández T., Romero A., Cianciosi D., Giampieri F., and Battino M.. 2022. “Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds From Food Industry By‐Products.” Food Chemistry 378: 131–918. 10.1016/j.foodchem.2021.131918. [DOI] [PubMed] [Google Scholar]
- Gomes, S. M. , Albuquerque D., and Santos L.. 2023. “Innovative Approaches for Food: Using Natural Phenolic‐Rich Extracts to Produce Value‐Added Fresh Pasta.” International Journal of Molecular Sciences 24, no. 15: 12451. 10.3390/ijms241512451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, S. M. , Leitão A., Alves A., and Santos L.. 2023. “Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children's Malnutrition in Developing Countries.” Molecules 28, no. 6: 2526. 10.3390/molecules28062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan, L. , Doriya K., and Kumar D. S.. 2016. “ Moringa oleifera: A Review on Nutritive Importance and Its Medicinal Application.” Food Science and Human Wellness 5, no. 2: 49–56. 10.1016/j.fshw.2016.04.001. [DOI] [Google Scholar]
- Guan, H. , Zhang W., Sun‐Waterhouse D., et al. 2021. “Phenolic‐Protein Interactions in Foods and Post Ingestion: Switches Empowering Health Outcomes.” Trends in Food Science & Technology 118: 71–86. 10.1016/j.tifs.2021.08.033. [DOI] [Google Scholar]
- Hassan, M. A. , Xu T., Tian Y., et al. 2021. “Health Benefits and Phenolic Compounds of Moringa oleifera Leaves: A Comprehensive Review.” Phytomedicine 93: 153771. 10.1016/j.phymed.2021.153771. [DOI] [PubMed] [Google Scholar]
- Jobby, R. , Nair S. P., Murugan V., Khera S., and Tungare K.. 2023. “Phytochemicals—A Safe Fortification Agent in the Fermented Food Industry.” In Recent Frontiers of Phytochemicals, 535–544. Elsevier. 10.1016/B978-0-443-19143-5.00016-5. [DOI] [Google Scholar]
- Jomova, K. , Raptova R., Alomar S. Y., et al. 2023. “Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging.” Archives of Toxicology 97, no. 10: 2499–2574. 10.1007/s00204-023-03562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabulut, G. , Goksen G., and Mousavi Khaneghah A.. 2024. “Plant‐Based Protein Modification Strategies Towards Challenges.” Journal of Agriculture and Food Research 15: 101017. 10.1016/j.jafr.2024.101017. [DOI] [Google Scholar]
- Karim, N. U. , Siddiq U. S. A. A., Razak M. R. M., Zainol M. K. M., and Abdullah M. I.. 2018. “Effects of Moringa Leaves (Moringa oleifera) Extraction on Quality Changes and Melanosis of Giant Freshwater Prawn (Macrobrachium Rosenbergii) During Chilled Storage.” Italian Journal of Food Safety 7, no. 3: 6846. 10.4081/ijfs.2018.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashaninejad, M. , Blanco B., Benito‐Román O., Beltrán S., Niknam S. M., and Sanz M. T.. 2021. “Maximizing the Freeze‐Dried Extract Yield by Considering the Solvent Retention Index: Extraction Kinetics and Characterization of Moringa oleifera Leaves Extracts.” Food and Bioproducts Processing 130: 132–142. 10.1016/j.fbp.2021.09.008. [DOI] [Google Scholar]
- Katmawanti, S. , Supriyadi S., and Mariroh F.. 2021. “Is Instant Porridge With a High Calcium Content Based on Moringa oleifera as an Alternative Baby Food to Prevent Stunting in Indonesia?” Journal of Public Health Research 10, no. 2: jphr.2021.2233. 10.4081/jphr.2021.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, S. , Mendonca P., and Soliman K. F. A.. 2024. “The Anticancer Effects and Therapeutic Potential of Kaempferol in Triple‐Negative Breast Cancer.” Nutrients 16, no. 15: 2392. 10.3390/nu16152392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid, S. , Arshad M., Mahmood S., et al. 2023a. “Nutritional and Phytochemical Screening of Moringa oleifera Leaf Powder in Aqueous and Ethanol Extract.” International Journal of Food Properties 26, no. 1: 2338–2348. 10.1080/10942912.2023.2246685. [DOI] [Google Scholar]
- Khalid, S. , Arshad M., Mahmood S., et al. 2023b. “Extraction and Quantification of Moringa oleifera Leaf Powder Extracts by HPLC and FTIR.” Food Analytical Methods 16, no. 4: 787–797. 10.1007/s12161-023-02470-z. [DOI] [Google Scholar]
- Kieserling, H. , de Bruijn W. J. C., Keppler J., et al. 2024. “Protein–Phenolic Interactions and Reactions: Discrepancies, Challenges, and Opportunities.” Comprehensive Reviews in Food Science and Food Safety 23, no. 5: e70015. 10.1111/1541-4337.70015. [DOI] [PubMed] [Google Scholar]
- Kumar, K. , Srivastav S., and Sharanagat V. S.. 2021. “Ultrasound Assisted Extraction (UAE) of Bioactive Compounds From Fruit and Vegetable Processing By‐Products: A Review.” Ultrasonics Sonochemistry 70: 105–325. 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Y. P. , Pang S. F., Yusoff M. M., Abdul Mudalip S. K., and Gimbun J.. 2019. “Correlation Between the Extraction Yield of Mangiferin to the Antioxidant Activity, Total Phenolic and Total Flavonoid Content of Phaleria Macrocarpa Fruits.” Journal of Applied Research on Medicinal and Aromatic Plants 14: 100–224. 10.1016/j.jarmap.2019.100224. [DOI] [Google Scholar]
- Lohani, U. C. , and Muthukumarappan K.. 2021. “Study of Continuous Flow Ultrasonication to Improve Total Phenolic Content and Antioxidant Activity in Sorghum Flour and Its Comparison With Batch Ultrasonication.” Ultrasonics Sonochemistry 71: 105. 10.1016/j.ultsonch.2020.105402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie, K. B. , Kosina S. M., Hu Y., et al. 2020. “6.12—Mass Spectrometry for Natural Product Discovery.” In Comprehensive Natural Products III, edited by Liu H.‐W. and Begley T. P., 263–306. Elsevier. 10.1016/B978-0-12-409547-2.14834-6. [DOI] [Google Scholar]
- Mabrok, H. B. , and Mohamed M. S.. 2019. “Induction of COX‐1, Suppression of COX‐2 and Pro‐Inflammatory Cytokines Gene Expression by Moringa Leaves and Its Aqueous Extract in Aspirin‐Induced Gastric Ulcer Rats.” Molecular Biology Reports 46, no. 4: 4213–4224. 10.1007/s11033-019-04874-9. [DOI] [PubMed] [Google Scholar]
- Mabrouki, L. , Rjeibi I., Taleb J., and Zourgui L.. 2020. “Cardiac Ameliorative Effect of Moringa oleifera Leaf Extract in High‐Fat Diet‐Induced Obesity in Rat Model.” BioMed Research International 2020: 6583603. 10.1155/2020/6583603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiha, S. , Batool Z., Tabassum S., et al. 2021. “Quercetin Exhibits Potent Antioxidant Activity, Restores Motor and Non‐Motor Deficits Induced by Rotenone Toxicity.” PLoS One 16, no. 11: e0258928. 10.1371/journal.pone.0258928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi, H. , Yousif E., Khan N., Mahmud R., Murugaiyah V., and Asmawi M.. 2016. “Optimizing Extraction Conditions of Moringa oleifera Lam Leaf for Percent Yield, Total Phenolics Content, Total Flavonoids Content and Total Radical Scavenging Activity.” International Journal of Advanced Research 4, no. 11: 682–695. 10.21474/IJAR01/2133. [DOI] [Google Scholar]
- Malik, J. , and Mandal S. C.. 2022. “Chapter 2—Extraction of Herbal Biomolecules.” In Herbal Biomolecules in Healthcare Applications, edited by Mandal S. C., Nayak A. K., and Dhara A. K., 21–46. Academic Press. 10.1016/B978-0-323-85852-6.00015-9. [DOI] [Google Scholar]
- Martínez‐Ramos, T. , Benedito‐Fort J., Watson N. J., Ruiz‐López I. I., Che‐Galicia G., and Corona‐Jiménez E.. 2020. “Effect of Solvent Composition and Its Interaction With Ultrasonic Energy on the Ultrasound‐Assisted Extraction of Phenolic Compounds From Mango Peels (Mangifera indica L.).” Food and Bioproducts Processing 122: 41–54. 10.1016/j.fbp.2020.03.011. [DOI] [Google Scholar]
- Mehganathan, P. , Rosli N. A., Abd Karim K., and Soon Huat T.. 2024. “Optimizing Quercetin and Kaempferol Extraction From Moringa oleifera Leaves via Ultrasonic Assisted Extraction: Kinetic Modeling and Process Optimization.” Journal of Engineering Research (Kuwait). 10.1016/j.jer.2024.06.022. [DOI] [Google Scholar]
- Michala, A.‐S. , and Pritsa A.. 2022. “Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer.” Diseases 10, no. 3: 37. 10.3390/diseases10030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, F. A. E.‐F. , Salama H. H., El‐Sayed S. M., El‐Sayed H. S., and Abdel‐Hady Zahran H.. 2018. “Utilization of Natural Antimicrobial and Antioxidant of Moringa oleifera Leaves Extract in Manufacture of Cream Cheese.” Journal of Biological Sciences 18, no. 2: 92–106. [Google Scholar]
- Nafiu, A. O. , Akomolafe R. O., Alabi Q. K., Idowu C. O., and Odujoko O. O.. 2019. “Effect of Fatty Acids From Ethanol Extract of Moringa oleifera Seeds on Kidney Function Impairment and Oxidative Stress Induced by Gentamicin in Rats.” Biomedicine & Pharmacotherapy 117: 109–154. 10.1016/j.biopha.2019.109154. [DOI] [PubMed] [Google Scholar]
- Nejabati, H. R. , and Roshangar L.. 2022. “Kaempferol: A Potential Agent in the Prevention of Colorectal Cancer.” Physiological Reports 10, no. 20: e15488. 10.14814/phy2.15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ömür‐Özbek, P. , Dietrich A. M., Duncan S. E., and Lee Y.. 2012. “Role of Lipid Oxidation, Chelating Agents, and Antioxidants in Metallic Flavor Development in the Oral Cavity.” Journal of Agricultural and Food Chemistry 60, no. 9: 2274–2280. 10.1021/jf204277v. [DOI] [PubMed] [Google Scholar]
- Pakade, V. , Cukrowska E., Lindahl S., Turner C., and Chimuka L.. 2013. “Molecular Imprinted Polymer for Solid‐Phase Extraction of Flavonol Aglycones From Moringa oleifera Extracts.” Journal of Separation Science 36, no. 3: 548–555. 10.1002/jssc.201200576. [DOI] [PubMed] [Google Scholar]
- Pareek, A. , Pant M., Gupta M. M., et al. 2023. “ Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects.” International Journal of Molecular Sciences 24, no. 3: 2098. 10.3390/ijms24032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y.‐R. , and Lee B.‐S.. 2024. “Solubility of Gallic Acid in Single and Mixed Solvents.” Separations 11, no. 1: 36. 10.3390/separations11010036. [DOI] [Google Scholar]
- Pereira, J. M. G. , Viell F. L. G., de Lima P. C., et al. 2021. “Optimization of the Extraction of Antioxidants From Moringa Leaves: A Comparative Study Between Ultrasound‐ and Ultra‐Homogenizer‐Assisted Extractions.” Journal of Food Processing and Preservation 45: 6. 10.1111/jfpp.15512. [DOI] [Google Scholar]
- Pollini, L. , Tringaniello C., Ianni F., Blasi F., Manes J., and Cossignani L.. 2020. “Impact of Ultrasound Extraction Parameters on the Antioxidant Properties of Moringa oleifera Leaves.” Antioxidants 9, no. 4: 277. 10.3390/antiox9040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putra, N. R. , Yustisia Y., Heryanto R. B., et al. 2023. “Advancements and Challenges in Green Extraction Techniques for Indonesian Natural Products: A Review.” South African Journal of Chemical Engineering 46: 88–98. 10.1016/j.sajce.2023.08.002. [DOI] [Google Scholar]
- Qin, P. , Li T., Liu C., et al. 2023. “Extraction and Utilization of Active Substances From Edible Fungi Substrate and Residue: A Review.” Food Chemistry 398: 133–872. 10.1016/j.foodchem.2022.133872. [DOI] [PubMed] [Google Scholar]
- Qu, G. , Ma Z., and Jia T.. 2024. “Influence of Hydroxyl Groups on the Oxidative Reaction Characteristics of Active Groups in Lignite at Room Temperature.” ACS Omega 9, no. 14: 16,237–16,248. 10.1021/acsomega.3c10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitério, E. , Grosso C., Ferraz R., Delerue‐Matos C., and Soares C.. 2022. “A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health‐Promoting Compounds From Seaweeds.” Marine Drugs 20, no. 11: 677. 10.3390/md20110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath, S. , Budaraju S., Gharibzahedi S. M. T., Koubaa M., Roohinejad S., and Mallikarjunan K.. 2023. “Processing Technologies for the Extraction of Value‐Added Bioactive Compounds From Tea.” Food Engineering Reviews 15, no. 2: 276–308. 10.1007/s12393-023-09338-2. [DOI] [Google Scholar]
- Rastogi, S. , Farswan T. S., and Pandey M. M.. 2024. “Seasonal Variation in the Phytoconstituents and Antioxidant Activity in Moringa oleifera Lam. Leaves of North India.” South African Journal of Botany 166: 492–502. 10.1016/j.sajb.2024.01.054. [DOI] [Google Scholar]
- Rawel, H. M. , Czajka D., Rohn S., and Kroll J.. 2002. “Interactions of Different Phenolic Acids and Flavonoids With Soy Proteins.” International Journal of Biological Macromolecules 30, no. 3–4: 137–150. 10.1016/S0141-8130(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Raza, Q. , Azhar M. T., Rana I. A., Ahmad M. Q., and Atif R. M.. 2024. “Biofortification of Crops to Achieve Food and Nutritional Security.” In Biofortification of Grain and Vegetable Crops, 1–17. Elsevier. 10.1016/B978-0-323-91735-3.00001-7. [DOI] [Google Scholar]
- Rincón, E. , Balu A. M., Luque R., and Serrano L.. 2019. “Mechanochemical Extraction of Antioxidant Phenolic Compounds From Mediterranean and Medicinal Laurus Nobilis: A Comparative Study With Other Traditional and Green Novel Techniques.” Industrial Crops and Products 141: 111–805. 10.1016/j.indcrop.2019.111805. [DOI] [Google Scholar]
- Sandeep, G. , Arumugam T., Janavi G. J., Anitha T., Senthil K., and Lakshmanan A.. 2022. “A Comparative Study on Conventional and Non‐Conventional Extraction Methodologies for Yield, Quality and Antibacterial Investigation of Moringa oleifera Lam.” Medicinal Plants 14, no. 4: 614–625. 10.5958/0975-6892.2022.00068.5. [DOI] [Google Scholar]
- Sandeep, G. , Arumugam T., Janavi G. J., Anitha T., Senthil K., and Lakshmanan A.. 2023a. “Optimization of Microwave‐Assisted Extraction Method for the Yield of Extraction and Total Phenol Content From Moringa Leaves (Moringa oleifera Lam.) var. PKM 1.” Annals of Biology 39, no. 1: 73–81. [Google Scholar]
- Sandeep, G. , Arumugam T., Janavi G. J., Anitha T., Senthil K., and Lakshmanan A.. 2023b. “A Comparative Study on Conventional and Non‐Conventional Extraction Methodologies for Extraction Yield, Quality and Antibacterial Properties of Moringa (Moringa oleifera Lam.).” Journal of Applied Horticulture 25, no. 1: 17–24. 10.37855/jah.2023.v25i01.03. [DOI] [Google Scholar]
- Scarano, A. , Laddomada B., Blando F., et al. 2023. “The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota.” Antioxidants 12, no. 3: 630. 10.3390/antiox12030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengev, I. , MM A., and Sule S.. 2021. “Effect of Dry Shredded Moringa oleifera Leaves and Vitamin C on the Physicochemical Properties of the Dough and Bread.” Journal of Current Research in Food Science 2, no. 1: 35–39. [Google Scholar]
- Setyani, W. , Murwanti R., Sulaiman T. N. S., and Hertiani T.. 2023. “Application of Response Surface Methodology (RSM) for the Optimization of Ultrasound‐Assisted Extraction (UAE) of Moringa oleifera: Extraction Yield, Content of Bioactive Compounds, and Biological Effects In Vitro.” Plants 12, no. 13: 2455. 10.3390/plants12132455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, M. A. , Bosco S. J. D., and Mir S. A.. 2015. “Effect of Moringa oleifera Leaf Extract on the Physicochemical Properties of Modified Atmosphere Packaged Raw Beef.” Food Packaging and Shelf Life 3: 31–38. 10.1016/j.fpsl.2014.10.001. [DOI] [Google Scholar]
- Shen, L. , Pang S., Zhong M., et al. 2023. “A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies.” Ultrasonics Sonochemistry 101: 106–646. 10.1016/j.ultsonch.2023.106646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shervington, L. A. , Li B. S., Shervington A. A., et al. 2018. “A Comparative HPLC Analysis of Myricetin, Quercetin and Kaempferol Flavonoids Isolated From Gambian and Indian Moringa oleifera Leaves.” International Journal of Chemistry 10, no. 4: 28. 10.5539/ijc.v10n4p28. [DOI] [Google Scholar]
- Soares, S. , Brandão E., Guerreiro C., Soares S., Mateus N., and de Freitas V.. 2020. “Tannins in Food: Insights Into the Molecular Perception of Astringency and Bitter Taste.” Molecules 25, no. 11: 2590. 10.3390/molecules25112590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spange, S. , Weiß N., and Mayerhöfer T. G.. 2022. “The Global Polarity of Alcoholic Solvents and Water—Importance of the Collectively Acting Factors Density, Refractive Index and Hydrogen Bonding Forces.” ChemistryOpen 11, no. 10: e202200140. 10.1002/open.202200140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speisky, H. , Shahidi F., Costa de Camargo A., and Fuentes J.. 2022. “Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties.” Antioxidants 11, no. 1: 133. 10.3390/antiox11010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, P. , and Anandharamakrishnan C.. 2023. “Chapter Two—Extraction of Bioactive Compounds.” In Industrial Application of Functional Foods, Ingredients and Nutraceuticals, edited by Anandharamakrishnan C. and Subramanian P., 45–87. Academic Press. 10.1016/B978-0-12-824312-1.00002-9. [DOI] [Google Scholar]
- Sulastri, E. , Zubair M. S., Anas N. I., et al. 2018. “Total Phenolic, Total Flavonoid, Quercetin Content and Antioxidant Activity of Standardized Extract of Moringa oleifera Leaf From Regions With Different Elevation.” Pharmacognosy Journal 10: S104–S108. 10.5530/pj.2018.6s.20. [DOI] [Google Scholar]
- Sun, Q. , Kong B., Zheng O., Liu S., and Dong X.. 2023. “Effect of Protein Structure Changes During Different Power Ultrasound Thawing on Emulsification Properties of Common Carp (Cyprinus Carpio) Myofibrillar Protein.” Ultrasonics Sonochemistry 101: 106719. 10.1016/j.ultsonch.2023.106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, J. , Vincken J.‐P., van Zadelhoff A., Hilgers R., Lin Z., and de Bruijn W. J. C.. 2023. “Presence of Free Gallic Acid and Gallate Moieties Reduces Auto‐Oxidative Browning of Epicatechin (EC) and Epicatechin Gallate (ECg).” Food Chemistry 425: 136–446. 10.1016/j.foodchem.2023.136446. [DOI] [PubMed] [Google Scholar]
- Tarragon, E. , and Moreno J. J.. 2020. “Polyphenols and Taste 2 Receptors. Physiological, Pathophysiological and Pharmacological Implications.” Biochemical Pharmacology 178: 114086. 10.1016/j.bcp.2020.114086. [DOI] [PubMed] [Google Scholar]
- Thangaiah, A. , Gunalan S., Kulandaivelu Rathnasamy V., et al. 2024. “Optimization of Ultrasound‐Assisted Phytomolecules Extraction From Moringa Leaves (Moringa oleifera Lam) Using Response Surface Methodology.” Cogent Food & Agriculture 10, no. 1: 2309834. 10.1080/23311932.2024.2309834. [DOI] [Google Scholar]
- Thomas, A. , Kanakdhar A., Shirsat A., Deshkar S., and Kothapalli L.. 2020. “A High Performance Thin Layer Chromatographic Method Using a Design of Experiment Approach for Estimation of Phytochemicals in Extracts of Moringa oleifera Leaves.” Turkish Journal of Pharmaceutical Sciences 17, no. 2: 148–158. 10.4274/tjps.galenos.2018.80958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, B. , Qiao Y., Tian Y., Xie K., and Li D.. 2016. “Effect of Heat Reflux Extraction on the Structure and Composition of a High‐Volatile Bituminous Coal.” Applied Thermal Engineering 109: 560–568. 10.1016/j.applthermaleng.2016.08.104. [DOI] [Google Scholar]
- Tran, H. T. T. , Stetter R., Herz C., et al. 2021. “Allyl Isothiocyanate: A TAS2R38 Receptor‐Dependent Immune Modulator at the Interface Between Personalized Medicine and Nutrition.” Frontiers in Immunology 12: 669005. 10.3389/fimmu.2021.669005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo, C. , Castelló M. L., and Ortolá M. D.. 2023. “Potentiality of Moringa oleifera as a Nutritive Ingredient in Different Food Matrices.” Plant Foods for Human Nutrition 78, no. 1: 25–37. 10.1007/s11130-022-01023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanova, M. , Atanasov V., Yaneva Z., Ivanova D., and Dinev T.. 2020. “Selectivity of Current Extraction Techniques for Flavonoids From Plant Materials.” PRO 8, no. 10: 1–30. 10.3390/pr8101222. [DOI] [Google Scholar]
- van Lith, R. , and Ameer G. A.. 2016. “Antioxidant Polymers as Biomaterial.” In Oxidative Stress and Biomaterials, 251–296. Elsevier. 10.1016/B978-0-12-803269-5.00010-3. [DOI] [Google Scholar]
- Vergara‐Jimenez, M. , Almatrafi M., and Fernandez M.. 2017. “Bioactive Components in Moringa oleifera Leaves Protect Against Chronic Disease.” Antioxidants 6, no. 4: 91. 10.3390/antiox6040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernès, L. , Vian M., and Chemat F.. 2020. “Ultrasound and Microwave as Green Tools for Solid–Liquid Extraction.” In Liquid‐Phase Extraction, 355–374. Elsevier. 10.1016/B978-0-12-816911-7.00012-8. [DOI] [Google Scholar]
- Vinatoru, M. 2001. “An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles From Herbs.” Ultrasonics Sonochemistry 8, no. 3: 303–313. 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Virk, P. , Awad M. A., Saleh Abdu‐llah Alsaif S., et al. 2023. “Green Synthesis of Moringa oleifera Leaf Nanoparticles and an Assessment of Their Therapeutic Potential.” Journal of King Saud University, Science 35, no. 3: 102576. 10.1016/j.jksus.2023.102576. [DOI] [Google Scholar]
- Viswanathan, M. , Patnode C. D., Berkman N. D., et al. 2008. “Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions.” In Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- Vongsak, B. , Sithisarn P., Mangmool S., Thongpraditchote S., Wongkrajang Y., and Gritsanapan W.. 2013. “Maximizing Total Phenolics, Total Flavonoids Contents and Antioxidant Activity of Moringa oleifera Leaf Extract by the Appropriate Extraction Method.” Industrial Crops and Products 44: 566–571. 10.1016/j.indcrop.2012.09.021. [DOI] [Google Scholar]
- Wang, L. , Lin X., Zhang J., et al. 2019. “Extraction Methods for the Releasing of Bound Phenolics From Rubus Idaeus L. Leaves and Seeds.” Industrial Crops and Products 135: 1–9. 10.1016/j.indcrop.2019.04.003. [DOI] [Google Scholar]
- Weggler, B. A. , Gruber B., Teehan P., Jaramillo R., and Dorman F. L.. 2020. “Chapter 5—Inlets and Sampling.” In Basic Multidimensional Gas Chromatography, edited by Snow N. H., vol. 12, 141–203. Academic Press. 10.1016/B978-0-12-813745-1.00005-2. [DOI] [Google Scholar]
- Wu, L. , Li L., Chen S., Wang L., and Lin X.. 2020. “Deep Eutectic Solvent‐Based Ultrasonic‐Assisted Extraction of Phenolic Compounds From Moringa oleifera L. Leaves: Optimization, Comparison and Antioxidant Activity.” Separation and Purification Technology 247: 117014. 10.1016/j.seppur.2020.117014. [DOI] [Google Scholar]
- Wu, Q. , Zhou H.‐J., Sheng J., Su L.‐Y., and Tian Y.. 2024. “Extraction, Structural Properties, and Bioactivities of Moringa (Moringa oleifera Lam.) Isothiocyanates: A Review.” Food Bioscience 57: 103–447. 10.1016/j.fbio.2023.103447. [DOI] [Google Scholar]
- Zainol, M. K. , Mohd Subri I., Zamri A. I., Mohd Zin Z., Fisal A., and Mamat H.. 2020. “Antioxidative Properties and Proximate Analysis of Spent Coffee Ground (SCG) Extracted Using Ultrasonic‐Methanol Assisted Technique as a Potential Functional Food Ingredient.” Food Research 4, no. 3: 636–644. 10.26656/fr.2017.4(3)0.358. [DOI] [Google Scholar]
- Zhao, S. , and Zhang D.. 2013. “Supercritical Fluid Extraction and Characterisation of Moringa oleifera Leaves Oil.” Separation and Purification Technology 118: 497–502. 10.1016/j.seppur.2013.07.046. [DOI] [Google Scholar]
- Zhu, Y. , Yin Q., and Yang Y.. 2020. “Comprehensive Investigation of Moringa oleifera From Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC‐ESI‐MS/MS.” Molecules 25, no. 3: 676. 10.3390/molecules25030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors have nothing to report.


