Abstract
Conventional treatments for glioblastoma (GBM) are hindered by systemic toxicity, limited blood–brain barrier penetration, and therapeutic resistance. To address these challenges, we developed dual-functionalized gold nanoparticles (AuNPs) conjugated with a biotinylated NFL-TBS.40-63 peptide and the chemotherapeutic agent doxorubicin. This platform integrates targeted delivery and therapeutic action to enhance efficacy while minimising off-target effects. Our findings reveal superior cellular uptake, dose- and time-dependent cytotoxicity, and apoptosis induction in GBM cells compared to mono-functionalized counterparts. Furthermore, pH-sensitive drug release profiles underscore the system’s potential to exploit the tumour microenvironment’s acidic conditions for precise drug delivery. Comprehensive characterisation confirmed the stability, biocompatibility, and functional efficacy of the dual-functionalized AuNPs. This study highlights the promise of these nanoconjugates as a multimodal approach to GBM therapy, paving the way for further translational research in nanomedicine.
Supplementary Information
The online version contains supplementary material available at 10.1186/s11671-025-04249-z.
Keywords: Gold Nanoparticles, Dual-functionalized nanoparticles, Drug uptake enhancement, Glioblastoma, Therapeutic nanomedicine
Introduction
Glioblastoma (GBM) remains the most aggressive primary brain tumour, characterised by rapid progression, high recurrence rates, and poor prognosis [1]. Despite advances in multimodal treatment strategies, including surgical resection, radiotherapy, and chemotherapy, the median survival for GBM patients remains less than 15 months [2]. Current standard-of-care therapies, including surgical resection, radiotherapy, and chemotherapy, are often limited by systemic toxicity, poor blood–brain barrier (BBB) penetration, and the heterogeneity of tumour cells [3]. These challenges necessitate innovative approaches to improve drug delivery and efficacy while minimising off-target effects. Nanotechnology has emerged as a transformative tool in oncology, providing platforms for precise drug delivery, tumour targeting, and enhanced therapeutic efficacy [4, 5]. Among these, gold nanoparticles (AuNPs) offer unique advantages, including high biocompatibility, stability, and the ability to be conjugated with various therapeutic agents [6, 7]. Functionalised AuNPs have been explored in preclinical studies to deliver chemotherapeutics such as paclitaxel [8], temozolomide [9], 5-fluorouracil [10] and doxorubicin (DOX) [11], with promising results, increasing tumour accumulation and reducing systemic side effects [12, 13]. However, clinical translation has been limited, underscoring the need for novel multifunctional formulations that offer enhanced biocompatibility and efficacy.
Ligands like peptides represent a particularly attractive strategy for cancer targeting due to their ability to selectively bind overexpressed receptors on tumour cells [14]. Among these, the NFL (Neuro Filament Low subunit Tubulin Binding Site 40–63) peptide stands out for its ability to disrupt glioblastoma microtubule networks without affecting healthy cells[15–18]. Previous studies have shown the efficacy of mono functionalised AuNPs, carrying either BIOT-NFL or DOX, showing a dose-dependent reduced mitochondrial activity of various glioblastoma cells [19–22] and significantly increased levels of reactive oxygen species (ROS) in pancreatic cancer cells [23].
Dual targeting delivery systems incorporating both targeting and therapeutic agents could provide synergistic benefits, enhancing specificity, efficacy, and tumour cell killing. Various studies have reported an improved delivery of therapeutic agents to glioma through mediated penetration of the BBB, retainment at the GBM parenchyma and enhanced anti-glioma activity [24–28]. Polymeric micelles, which contained the peptide sensitive to the matrix metalloproteinases 2 enzyme and the peptide GFLG sensitive to the cathepsin B enzyme, were reported to have a significant dual-responsive feature toward both extracellular and intracellular enzymes [29]. DOX was released from the peptide through activation of cathepsin B overexpression in lysosomes, cleaving the GFLG sequence. Co-delivery of liposomes containing DOX and erlotinib showed enhanced translocation across the BBB, resulting in regression of tumour in the in vitro brain tumour model [30]. Chen and co-workers reported AuNPs conjugated with DOX via a thiol–Au bond and using a peptide substrate, CPLGLAGG [31]. The thiol exchanging reaction between the thiol–Au bond and intracellular glutathione (GSH) increased the intracellular release of DOX and enhanced the efficacy of in vivo tumour imaging and tumour growth inhibition. Zhan used diselenide-containing co-polymers (mPEG-Se–Se-PEGm) anchored onto the surface of AuNPs via Au-Se interactions and loaded DOX for combined PTT and chemotherapic treatment [32].
The study herein aims to develop and evaluate dual functionalised BIOT-NFL-DOX NPs as a novel approach for glioblastoma therapy with a design that integrates the microtubule-disrupting properties of NFL with the cytotoxic efficacy of DOX to create a robust platform. A comparative study in terms of different dosages and time of exposure was carried out towards singularly functionalised AuNPs (mono with DOX and NFL) and dual functionalised AuNPs (dual BIOT-NFL-DOX) to advance the understanding of the development of drug-eluting nanocarriers. Additionally, its pH-sensitive drug release highlights its potential for selective delivery in acidic tumour microenvironments. Internalisation through uptake and cytotoxicity (MTT and SRB bioassays), were investigated at different time intervals and concentrations, followed by measurement of intracellular ROS (by DCFH-DA probe) and mitochondrial ROS (by MITOSOX probe) in live cells. Membrane integrity was evaluated through the Trypan Blue assay. Mode of cell death was determined by triple staining (Hoechst/Annexin V/PI triple staining). The relative efficiencies of the mono and dually functionalised AuNPs showed enhanced uptake and efficacy for the dual functionalised formulation over their mono counterparts. Gaining insight into how cells respond to different doses with AuNP exposure over time is essential to understanding the interactions between nanomaterials and cells fully. By bridging therapeutic and diagnostic capabilities, this platform offers a novel approach to overcoming the limitations of current GBM treatments and advancing the field of nanomedicine.
Results
Physical and chemical characterisation of the dual and mono formulations.
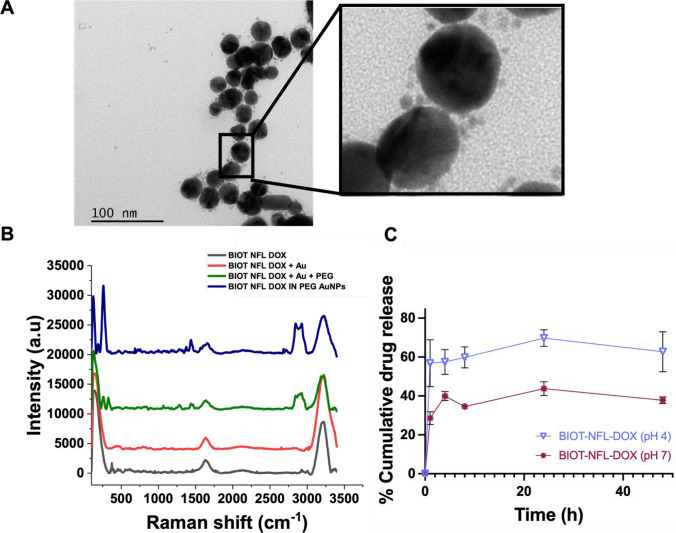
Mono functionalised NFL and DOX AuNP conjugates were prepared as previously reported [20, 21]. Dual functionalised NFL-DOX conjugates were prepared via a previously described methodology, referred to as Methodology IN [20–23, 33] and characterised in size through TEM and in zeta potential and polydispersity index (PDI) by dynamic light scattering (DLS) (Tables S1, Supporting Information). DLS results showed a hydrodynamic diameter of the BIOT -NFL-DOX around 62.74 nm and zeta potential was around − 22.3 mV (Table S1), suggesting that the dual functionalised AuNPs are capable of avoiding the interaction with most of the negatively charged proteins present within the biological circulation. TEM images confirmed a heterogeneous size and distribution of the dual functionalised AuNPs, and a further magnification highlighted the presence of smaller particles around the AuNPs (Fig. 1A) associated with DOX-NFL complexes. As expected, the NPs hydrodynamic diameter, measured by DLS, was higher than the TEM measurements. This was attributed to an electric dipole layer that forms around the particle’s surface, whereas sample preparation for TEM requires a drying process [34].
Fig. 1.
Physical and chemical characterisation of synthesised BIOT-NFL-DOX AuNPs. A Representative TEM image of the dual functionalised BIOT-NFL-DOX AuNPs, the further magnification shows the presence of DOX-NFL complex. B Raman spectra of BIOT-NFL-DOX AuNPs (NFL-DOX diacide are also reported for comparison). The spectra were normalised on the intensity of the band at 990 cm−1. Experimental conditions: λexc = 785 nm; laser power 20 mW; 1200 Tof 180 s. C Cumulative drug release profiles of the BIOT-NFL, DOX PEG-Au in PBS (pH 4.0) and PBS (pH 7.4). Results are expressed as the mean ± S.E.M across three independent trials (n = 3)
Raman spectroscopy confirmed successful encapsulation of NFL-DOX into the AuNPs..As shown, the Raman spectrum of BIOT NFL-DOX (Fig. 1B; grey line) and BIOT NFL-DOX AuNPs (Fig. 1B; blue line) presented several bands in the spectral region 200–3300 cm−1, corresponding to the specific skeletal ring vibrations. In particular, we observed the characteristic peaks of DOX during the formation of AuNPs (403 cm−1 and several bands between 600 and 1000 cm−1), Fig. 1B (red line and green line).
After reduction with NaBH4, the SERS (Surface Enhanced Raman scattering due to the gold nanoparticle formation) spectra of BIOT NFL-DOX NPs exhibited improvement of the peak at 480 cm−1 (Fig. 1B, blue line). This peak is due to the gold chloride stretches, δ (O–Au–O) and δ (–C–O–C) in the DOX ring, confirming the presence of the complex DOX-AuCl2. The peak at 860 cm−1 was attributed to the C–O and C–N vibrations in peptide bonds. The spectral bands from 1200 to 1350 cm−1 correspond to the vibrations of N–H bending and C–N stretching, while the peak at 1550 cm−1 corresponds to the C=O stretching mode. Additionally, the amide III peak was also visible at 1230 cm−1, as well as amide II and I at 1560 and 1680 cm−1.
The Raman spectra of each step (Fig. 1B, red line and green line) displayed several peaks in the region 500–2000 cm−1 with a typical band at 1650 cm−1 due to the presence of water. In particular, the vibrations at 1250 cm−1 and 1400 cm−1 were related to the ν C–O–C stretching, and the vibration at 1655 cm−1 was due to protonated NH3.
The surface modification of the dual conjugated Au compared to the mono was monitored by UV–Vis absorption spectra. Based on the UV–vis spectrum (Figure S1A), the resultant mono and dual functionalised AuNPs composites all exhibited a plasmon peak at ≈ 550 nm due to the Surface Plasmon Resonance (SPR) of AuNPs, with no obvious peak shift. This suggested that the structure of the dual functionalised NPs remained relatively stable during the surface modification process.
As stability is an essential requisite for drug-delivery therapeutic and biomedical applications, the dual formulations were monitored for long-term stability by using UV–Vis absorption spectroscopy. As shown in Figure S1B, the intensity of Surface Plasmon resonance (SPR) peak increases slightly as the reaction time increases. Despite the slight increase in the SPR intensity, which suggested a tendency to agglomerate over time, we observed no shift in the SPR band which indicated dearth of degradation. In fact, the characteristic absorption peak of BIOT NFL-DOX NPs was detected at 550 nm and the intensity for over 30 days, confirming the stability of the synthesised conjugates.
In vitro drug release profiles of the dual functionalised AuNPS
Prior to the drug release test, the drug loading content (w/w%) and drug entrapment efficiency (%) were calculated and found to be 30% and 80%, respectively.
The in vitro release profiles of the mono and dually functionalised AuNPs were performed in both physiological (pH 7) PBS and acidic (pH 4) PBS at 37 °C. As shown in Fig. 1C, the rate and amount of drug released from the dual functionalised AuNPs was pH-dependent. All conjugations (data not shown) showed much faster drug release at pH 4.0 than at pH 7.4, while a significantly faster rate was observed for the dual functionalised AuNPs compared to mono-conjugations, indicating the excellent stability of the BIOT-NFL-DOX NPs under the physiological condition. In fact, after an initial burst release within the initial 2 h, the release rate for BIOT-NFL-DOX reached by 24 h, 69.7% ± 7.4.%, in acidic PBS, whereas 43.7% ± 6.19.%, was released in normal PBS. The increased release observed under these acidic conditions is similar to previous reports with DOX NPs in acidic tumour microenvironments and was hypothetically associated with the protonation of the carboxylate groups present in the chemical structure of COOH-terminated PEG molecules [11, 33]. Such protonated groups reduce the electrostatic interaction between PEG and DOX, stabilising the DOX IN-PEG-AuNPs structure [33]. Taken together, our data indicates that the rapid release under acidic conditions of the dual functionalised AuNPs supports their potential for intelligent drug delivery due to the formation of a slightly acidic environment in tumour tissues.
Time-dependent behaviour of the dual and single functionalised AuNPs
As the stability in physiological environments represents a limitation in the translation from bench to clinic [35], we investigated the interaction of the mono and dually functionalised AuNPs of different concentrations (100 and 200 μM) for different time points (0, 2, 6, 24, 48 and 72 h), with serum-free and complete medium, incubated at 37 °C. The selected concentrations were chosen to identify and optimise the dose–response relationship, which influences their cellular interactions and bioavailability. As shown in Figure S2, the absorbance at the SPR peak wavelength changed with AuNP concentration, in accordance with the Beer-Lambert Law, with no obvious peak shift. All formulations changed their absorbance within 2 h of incubation and with respect to the different media. Indeed, in the presence of complete medium, the SPR band was reduced, which indicated the formation of larger non-spherical aggregates [36, 37]. No further significant increase in the intensity of the SPR band was observed after additional incubation (6 h, 24 h, 48 h, 72 h). This might indicate that 2 h is the maximum time after which the AuNPs have reached the maximum amount of absorbed serum proteins on their surface.
Next, we investigated the osmolality of the different concentrations of AuNPs within the serum-free and complete medium. As shown in Fig. 2, all concentrations reported an initial osmolarity of approximately 280–300 mOsmol/kg, slightly lower than the osmolarity of serum-free and complete medium (without AuNPs). Serum-free and complete medium with AuNPs with a lower concentration exhibited a higher osmolarity, regardless of formulations. However, all formulations of AuNPs exhibited an increase within 72 h of incubation, which was associated with the change in the concentration by release of the molecules in the medium.
Fig. 2.
Dose- and time-dependent analysis of Osmolality of the two concentrations 100–200 μM of A BIOT-NFL NPs, B DOX Au-PEG NPs and C BIOT-NFL-DOX NPs after incubation for 0, 2, 6, 24, 48 and 72 h in serum-free (DMEM) and complete cell culture (CM) media at 37 °C. Results are expressed as the mean ± S.E.M across six independent trials (n = 6)
Overall, our data suggests that the dual functionalised BIOT NFL-DOX NPs exhibited the same resistance of the mono to nonspecific protein binding and maintained stability under biologically relevant conditions.
Dual conjugated AuNPs increased in vitro cellular uptake
The efficacy and efficiency of uptake of the different AuNP formulations were quantified to assess the cellular AuNP uptake in GBM cells for either concentration and various time points (2, 6, 24, 48 or 72 h). As expected, intracellular AuNP content, measured in U87-MG cells, was dose-dependent, showing a statistically higher cellular uptake after 24 h of exposure to all AuNPs for a concentration of 200 μM compared to a lower (100 μM) (Fig. 3A, p < 0.001). Uptake showed to be also time-dependent, showing a higher uptake for the dual formulation of BIOT NFL-DOX NPs compared to single formulations for both concentrations. However, prolonged time of exposure to formulations (48 h, 72 h) decreased the uptake for higher concentrations, whereas it remained stable for lower.
Fig. 3.
Cellular uptake of the different AuNPs and doses showed to be time and dose dependent. Quantification of cellular uptake of AuNPs where uptake is expressed as A the absolute number of internalised AuNPs in pmole/cell and B uptake efficiency of the different concentrations and formulations of NPs. Results are expressed as the mean ± S.E.M across five independent trials (n = 5), *p < 0.5, ***p < 0.001; obtained using two-way ANOVA with a Tukey post-test
An analysis of the efficacy of uptake, which is independent of the number of cells, highlighted the time-dependent concentration effect (Fig. 3B). Following 24, 48, and 72 h time of co-incubation, the spectrophotometric analysis revealed that uptake efficiency plateaued at higher concentrations after 24 h, while lower concentrations showed (although not statistically significant) a slight tendency to increase over time.
Taken together, these results confirmed that the dual conjugation of BIOT NFL-DOX NPs enhanced cellular uptake with respect to mono conjugations.
GBM cells exhibited concentration and time-dependent cytotoxicity
To test whether the increased cellular uptake led to an improved anticancer activity of dual functionalised AuNPs, cytotoxicity studies of the BIOT-NFL, BIOT-NFL-DOX and DOX nanocomposites on GBM cells were performed by using two different viability tests: the MTT assay, which is correlated to cell metabolism and vitality (Fig. 4A), and the SRB assay, which is based on the measurement of cellular protein content and correlated to cell proliferation (Fig. 4B). Exposure to increasing concentrations of the different AuNP formulations (100–200 μM) showed a concentration and time-dependent decrease of cell viability, roughly with the same sensitivity in both tests (Fig. 4A, B). Indeed, compared to the control (cells without AuNPs), a 200 μM solution caused a more significantly reduced absorbance, compared to controls, in both tests, especially after 72 h of treatment. This is consistent with previous studies showing that the cytotoxicity of different conjugated AuNPs on cells is dose-dependent and increases with the concentration of AuNPs over time [38].
Fig. 4.
Viability of U87-MG cells after treatment with BIOT-NFL, DOX PEG-Au and BIOT-NFL-DOX NPs at the concentrations of 100–200 µM for 24 h (black columns), 48 h (grey columns) and 72 h (light grey columns) analysed via MTT assay (A) and SRB assay (B). Experiments were run in triplicate, and fluorescence at 560 nm is expressed as % of control (without AuNPs). Results are expressed as the mean ± S.E.M, across three independent trials (n = 3). *p < 0.05 and **p < 0.01; obtained using two-way ANOVA with a Tukey post-test
Cell death due to the different formulations of AuNPs was apoptosis-dose dependent
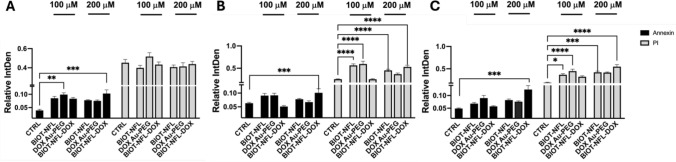
To identify the mechanism of death induced by the different concentrations and formulations of AuNPs in U87-MG cells, the Annexin V assay was performed with the co-localization of DAPI and the vital dye Propidium Iodide (PI), to exclude necrotic cells and cells of late apoptosis, Fig. 5, S3. FITC-conjugated Annexin V has a high affinity for phosphatidylserine, making it a sensitive probe for detecting apoptotic cells. This staining occurs before the loss of membrane integrity, which is associated with both apoptotic and necrotic processes [39, 40]. Viable, non-apoptotic cells did not exhibit a positive Annexin V signal or PI. Cells which exhibited early apoptotic cells appeared green due to FITC staining.
Fig. 5.
Bar graph analyses of apoptosis and necrosis after 24 h (A), 48 h (B) and 72 h (C). Results are expressed as the mean ± S.E.M across three independent trials (n = 3), compared to the control (cells without NPs), obtained using One-way ANOVA plus Tukey’s multiple comparison, *p < 0.05; **p < 0.01; ***p < 0.001
Our results confirmed qualitatively that the AuNP formulations, induced cell death (Fig. 5, S3). In particular, the fluorescence of early apoptotic cells was substantially lower than that of late apoptotic and dead cells (Fig. 5, p < 0.05). Noticeably, cells incubated with a 200 μM concentration of BIOT-NFL-DOX reported a considerably higher fluorescence of apoptotic compared with untreated cells (CTRL) and with respect to all other formulations regardless time of treatment. Furthermore, significantly higher PI-positive cells, regardless of concentration after 48, 72 h of treatment, were detected using BIOT-NFL NPs (p > 0.05; Fig. 5 B, C).
These results illustrate that the dual functionalised BIOT-NFL-DOX NPs have superior therapeutic efficacy than single formulations.
The integrity of the cell membrane was confirmed through Trypan blue exclusion assay (Figure S4). A minor reduction in viability was observed in the GBM cell line for lower concentrations, confirming that, after exposure to mono and dual formulations of AuNP, the survival rate of GBM cells underwent substantial decreases in a time- and dose-dependent manner, correlating with the MTT/SRB assays.
AuNP formulations on oxidative stress was time dependant
AuNP complexes are reported to induce cell death through the increase of cellular ROS levels. High ROS concentrations damage lipids, proteins, DNA, RNA, and carbohydrates, whereas low ROS concentrations play a role in cellular functions and signalling.
The overall intracellular ROS was first evaluated using 2′,7′-dichlorofluorescein diacetate (DCFH-DA), which ROS can rapidly oxidise to generate green fluorescent dichlorofluorescein (DCF). As shown in Fig. 6A, intracellular ROS showed a similar trend to increase after 72 h treatment with all AuNPs formulations compared to cells with no treatment, specifically for the DOX Au-PEG group at higher concentrations.
Fig. 6.
ROS production of U87 MG cells exposed to the different formulations and doses. AuNPs. Cells were incubated with the AuNPs s for 24, 48 and 72 h and treated with A 2’,7’-dichlorofluorescin diacetate (DCFDA) and B MitoSOX-red fluorescence based mitochondrial superoxide indicator. Data presented is the respective mean value of three independent experiments of six replicates normalised to total protein determined by the SRB assay. Results are expressed as the mean ± S.E.M across three or more independent trials (n ≥ 3), *p < 0.05; **p < 0.01; ***p < 0.001
Using MitoSOX Red, which specifically labels mitochondrial ROS production, we observed a significative time-dependent increase of ROS levels and the status of oxidative stress, specifically after 72 h treatment with AuNPs, regardless of AuNP formulation (Fig. 6B). A higher concentration of AuNPs slightly induced higher mitochondrial ROS expression.
Discussion
The development of dual functionalised BIOT-NFL-DOX AuNPs presents a significant advancement in GBM therapy. We have previously reported synthesised nanocomposites capped by the anticancer drug DOX showing increased antitumor efficacy with repression of tumour growth and higher stimulation of the immune system [23, 33]. Moreover, our previous studies using the NFL-peptide conjugated with the AuNPs, showed to decrease the mitochondrial activity of various glioblastoma cells in a dose-dependent manner [16, 21]. However, single ligands for dual targeting to overcome BBB and GBM targeting challenges offers a technological advantage, simplifying formulation and streamlining characterisation, making it an attractive strategy for future clinical applications. For instance, Ruan and his group developed a novel stapled peptide (ST-RAP12) derived from the RAP protein to target the LRP1 receptor, which is overexpressed in both BBB and GBM cells, showing improved brain and GBM accumulation compared to nontargeted formulations [41]. The therapeutic potential of this approach was supported by increased survival, apoptosis, and angiogenesis inhibition following paclitaxel-loaded ST-RAP12 micelle administration in glioma-bearing mice. Huo and his team exploited PEI-coated mesoporous silica nanospheres, conjugated with a peptide (T10), with high transferrin (Tf) affinity to explore the upregulation of Tf receptors in both brain endothelial and GBM cells [42]. The BBB- and GBM-targeting ability was demonstrated after administration, corroborated by a decrease in IC50 value and a prolonged mice survival rate compared to the free drug, the nonmodified formulation, and a commercial liposomal DOX. A sequential targeting strategy was proposed by Kuo’s group, who developed PEGylated poly(ethylene glycol)–poly(ε-caprolactone) polymeric NPs functionalised with wheat germ agglutinin and folic acid to target the BBB and GBM [43]. These NPs were loaded with anticancer drugs such as etoposide, carmustine, or DOX and evidenced that the dual-targeted NPs exhibited enhanced BBB penetration and GBM internalization in vitro, highlighting a synergistic effect between the two ligands that led to greater accumulation compared to single-ligand or nontargeted NPs. A novel dual targeted system involving the modification of cabazitaxel-loaded PEGylated nanocrystal liposomes composed of hydrogenated soy phosphatidylcholine and mPEG2000-DSPE showed superior BBB penetration and GBM spheroid accumulation compared to the free drug and monofunctionalized NPs [44]. In orthotopic GBM mouse models, these NPs successfully crossed the BBB and exhibited antiangiogenic and apoptotic effects following drug release.
Hu’s team reported nanocarriers formed through combination of a pH-sensitive DOX prodrug and an RGD peptide derivative [45]. By adjusting their ratios, the team selectively generated either vesicles or micelles, both of which demonstrated preferential biodistribution to GBM in vivo and improved anticancer efficacy through controlled DOX release. Building on this approach, Qi and colleagues developed a dual-targeted liposomal nanosystem by combining RGD and lactoferrin (Lf) ligands [46]. The expected synergistic effect improved BBB transport and deeper tumour spheroid penetration. Tf-conjugated magnetic silica poly(d,l-lactic-co-glycolic acid) (PLGA) NPs loaded with DOX and paclitaxel, a mitotic inhibitor which interferes with the normal breakdown of microtubules during cell division, were reported for brain glioma treatment with enhanced delivery and cellular uptake with the presence of a magnetic field [47]. The study reported herein highlights the potential of combining the selective targeting capabilities of the BIOT-NFL peptide with the cytotoxic effects of DOX to overcome the limitations of conventional treatments. The hydrodynamic size, PDI, and morphology, showed mono-dispersed, round, negatively charged AuNPs (Fig. 1, Table S1). Strategically these are key parameters when designing AuNPs as they may influence their delivery and migration properties. Indeed, non-spherical foreign particles would be eliminated by filtration and eradication through the lymphatic and vascular systems [48] and positively charged AuNPs are generally more toxic than negatively charged or neutral AuNPs due to the attraction between the negatively charged cell membrane and positively charged AuNPs.
Additionally, the observed stability of the dual functionalised AuNPs under physiological conditions (no shift in the SPR band) supports their potential for clinical applications. In fact, stability of the drug delivery carrier is an important factor for good efficacy of drug delivery systems. The stability of the BIOT-NFL-DOX AuNPs was also evaluated indirectly by examining the in vitro time-dependent drug release of DOX. One of the major limitations in DOX’s clinical use, despite its potency as an anticancer agent, is its poor penetration through the blood–brain barrier due to its low lipophilicity and high molecular weight [49]. Reduced side effects and increased treatment efficiency have been obtained through conjugation with NPs [50]. Moreover, tumour cells report a lower pH due to the accumulation of lactic acid [51]. Thus, subsequent internalisation of NP-drug conjugates, the drug release can be obtained under the acidic environment through breakage of the hydrazone bond. In our work, drug release rate was studied using two different pH environments, 4.0 and 7.4, to represent tumour and healthy cells, respectively. The dual functionalised BIOT-NFL-DOX AuNPs exhibited pH-dependent drug release, with significantly higher release rates under acidic conditions mimicking the tumour microenvironment. These results are particularly advantageous as they ensure selective drug release at the tumour site while minimising systemic toxicity [52, 53]. Previous studies have reported similar pH-responsive behaviour with DOX-conjugated nanoparticles, attributing the effect to the protonation of functional groups in acidic environments [11, 33]. This selective release mechanism facilitates more precise and controlled drug delivery, ultimately improving treatment outcomes, minimising side effects and reduces off-target effects, a critical requirement for GBM therapy. The findings of this study have significant implications for targeted cancer therapy as they demonstrate the potential of pH-responsive drug delivery systems for selective release within the acidic tumour microenvironment.
The osmolality of the different AuNPs formulations was assessed to rule out any impact on cell damage. Cell membranes are, in fact, sensitive to changes relative to parameters such as pH and osmolality. We observed that all AuNPs in complete medium (or serum-free) initially exhibited an osmolality slightly lower than that of the medium, which, however, increased over time, prompting a hyperosmolar media (in both complete medium and serum-free after 72 h of incubation). Both hyperosmolar and hypo-osmolar stress have been shown to influence cellular processes such as signal transduction, ion homeostasis, volume regulation, cytoskeletal organisation, cell cycle progression, and energy metabolism [54, 55]. Work in the literature showed that exposure of adherent GBM cell lines to a hyperosmolar media, reported an increase of matrix-degrading enzyme production and invasiveness with a phenotype reminiscent of EMT [56]. A better understanding of the molecular and cellular mechanisms which promote GBM invasiveness may help to identify innovative therapeutic approaches to decrease its aggressiveness.
One of the key findings of this study is the enhanced cellular uptake of dual functionalised AuNPs compared to mono functionalised formulations. UV/Vis spectroscopy as a macroscopic quantitative approach was applied to verify the real uptake extent of NP internalised in the cells as loosely adsorbed NP, on the outer surface of the cell membrane, were washed away. We quantitatively observed that the dual functionalised AuNPs showed a higher uptake compared to the mono. Since the BIOT-NFL peptide targets glioblastoma-specific microtubule networks, it may enhance cellular recognition and binding. Previous studies suggest that NFL-TBS.40-63 interacts with tubulin-associated proteins, triggering clathrin- and caveolae-mediated endocytosis for nanoparticle uptake [57, 58]. This targeted mechanism enables the nanoparticles to efficiently bypass non-specific cellular barriers, leading to enhanced internalization. Additionally, the biotinylation of the NFL peptide may further promote cellular recognition and receptor binding, potentially engaging biotin-avidin-like interactions, which are known to enhance endocytic uptake [59]. Moreover, the negatively charged dual-functionalized AuNPs (− 22.3 mV) may also allow internalization via macropinocytosis, an energy-dependent process where cells engulf extracellular fluid containing NPs [60, 61]. We thus hypothesise a synergistic effect of the BIOT-NFL peptide, which facilitates receptor-mediated endocytosis [22, 62], and the chemical properties of DOX, which improve intracellular delivery. This enhanced cellular internalization is crucial for improving therapeutic efficacy, ensuring higher intracellular drug concentrations, and optimizing glioblastoma-targeted drug delivery. Further analyses are required to test this hypothesis. However, our results align with previous reports demonstrating increased cellular uptake with functionalised AuNPs, particularly those designed with tumour-targeting ligands.
Cytotoxicity assays revealed that dual functionalised AuNPs induced dose- and time-dependent reductions in GBM cell viability. The superior efficacy of BIOT-NFL-DOX AuNPs compared to mono functionalised formulations is consistent with the hypothesis that combining targeting and therapeutic agents enhances treatment outcomes. We, thus, hypothesised that the different AuNPs induced cytotoxicity through oxidative stress. The generation of ROS and the resulting oxidative stress are key mechanisms contributing to nanotoxicity, leading to DNA damage, apoptosis, and carcinogenesis [63–65]. Mitochondria, as the primary site of cellular ROS production, are particularly susceptible to dysfunction, and any structural or functional impairments can result in excessive mitochondrial ROS accumulation, thereby acting as a critical trigger for apoptosis [66]. Upon mitochondrial infiltration, NPs stimulate ROS production through mechanisms such as disruption of the electron transport chain, structural damage to mitochondrial components, activation of NADPH-like enzyme systems, and depolarization of the mitochondrial membrane [63]. In general, the generation of ROS can be associated with apoptosis and the resulting oxidative damage of cell components, especially in the case of NPs [67]. Besides, ROS production has been also recognised as an early cellular response to NP internalisation, eventually leading to cell death [68]. Our findings indicated that ROS levels, particularly mitochondrial superoxide production, increased significantly after 72 h of treatment with all AuNP formulations, regardless of the concentration and conjugation. The increase in mitochondrial ROS levels aligns with the observed dose and time-dependent cytotoxic effects. and may be attributed to mitochondrial damage, which contributes to the late phase of O2·-production. Thus, the different formulations did not interfere with the ROS production, suggesting a different mechanism of action. ROS mediated via NP-cell interaction involves mechanisms including immune cell activation, mitochondrial respiration, and NADPH oxidase system. As ROS plays a dual role in cell fate, acting both as a trigger for cell death and as second messengers in adaptive responses, these findings highlight the complex relationship between oxidative stress, NP properties, and cell type–dependent toxicity, emphasizing the role of ROS in both cytotoxic effects and potential cellular defence mechanisms. Nano-metal particles can enhance ROS generation, inducing oxidative stress, DNA damage, and unregulated cell signalling, and eventually leading to changes in cell motility, apoptosis, and even carcinogenesis. We identified apoptosis as the primary mode of cell death, driven by the production of ROS. Since most tumour cells are believed to have disabled apoptosis to attain their malignant state, they are generally expected to exhibit greater resistance to DNA-damaging anticancer agents compared to the normal cells from which they originate [69].
Conclusions
In summary, the present study highlighted that the dual conjugation of BIOT-NFL-DOX AuNP compared to mono functionalised NPs, demonstrated to be a superior drug delivery system. The synthesised dual conjugation of BIOT-NFL-DOX AuNP was characterised with UV–vis spectroscopy, zeta potential, dynamic light scattering, and fluorescence spectroscopy. The BIOT-NFL-DOX AuNPs exhibited improved pH responsiveness of drug release and cytotoxic effect against the U87 MG cell line. Moreover, analysis of induced apoptosis implied it to be the main mechanism of cell destruction, which could be a major contributor to anticancer therapy-induced killing of GBM cells. This innovative formulation can introduce both tumour-specific targeting and enhanced therapeutic efficacy by leveraging the NFL peptide’s selective interaction with glioblastoma microtubule networks while also delivering DOX, a widely used chemotherapeutic drug. By combining the coupling of enhanced oxidative stress induced by DOX and the mitochondrial damage by the NFL peptide in glioma cells, an innovative strategy to support the development of drug delivery system targeting intrinsic apoptosis pathway is warranted.
Experimental section
Materials
Tetrachloroauric acid (HAuCl4*H2O), sodium borohydride (NaBH4), dicarboxylic polyethylene glycol-600 (PEG-diacid), phosphate-buffered saline (PBS, 0.1 M, pH 4–13), Doxorubicin hydrochloride (DOX), (98%; 50 mg/mL), were purchased from Sigma-Aldrich. All reagents were of maximum purity grade. All solvents were used without any further purification. The biotinylated NFL-TBS.40-63 peptide (NH2-YSSYSAPVSSSLSVRRSYSSSSGS-CONH2), also called BIOT-NFL-peptide, was synthesised by PolyPeptide Group (Strasbourg, France). Experiments were performed at room temperature unless otherwise specified.
Synthesis of AuNPs nanocomposites
AuNP formulations were synthesised using protocols described previously [20, 21, 70].
Briefly, DOX Au-PEG NPs were synthesised mixing 20 mL of HAuCl4 solution (0.08 M) with DOX (5 mL, 0.1 M). Subsequent stirring, 250 μl of dicarboxylic PEG and 800 μl of aqueous 0.03 M NaBH4 was added immediately until the formation of a red colour.
BIOT-NFL NPS were obtained by diluting BIOT-NFL powder in water and ethanol (100/900 μM) at 1.8 nM of concentration. A DOX solution was prepared at 17 μM of concentration, subsequently 20 μl of BIOT NFL was then diluted to 980 μl of DOX.
BIOT-NFL-DOX NPs were obtained by adding 20 ml HAuCl4 aqueous solution (0.08 M) to 200 μl of NFL-DOX solution with subsequent stirring for 20 min. After this time, 250 μl of dicarboxylic PEG was added and mixed by magnetic stirring for 20 min at room temperature. Finally, 1.7 ml of aqueous 0.01 M NaBH4 was added at once. The formation of the BIOT NFL DOX IN PEG AuNPs was confirmed by a colour change of the solution after reduction (NaBH4) and proved by UV–VIS and Raman Spectroscopy (Fig. 1). The “as-prepared” BIOT-NFL-DOX NP solution was centrifuged at 9000 rpm for 15 min with subsequent discard of the supernatant. This was repeated twice to remove excess of non-conjugated dicarboxylic PEG.
BIOT-NFL-DOX AuNPs were observed by transmission electron microscopy (TEM) at the Service Commund’Imageries et d’Analyses Microscopiques (SCIAM; University of Angers, France) using a 120 kV Jeol JEM-1400 electron microscope (Jeol, Japan) equipped with a Gatan SC1000 ORIUS® CCD camera (11 Megapixel) from the USA. All DLS measurements were performed with a Malvern Instrument Zetasizer Nano ZS (Malvern Instruments, Westborough, MA, USA) equipped with a He–Ne laser (633 nm, a fixed scattering angle of 173) at room temperature. Size, zeta potential and TEM measurements were performed as previously described [20]. Long term stability was carried out storing the NPs at 5 °C for 6 months and were characterized through UV–Vis spectrophotometry.
Osmolarity measurements
Osmolarity (mOsm/Kg) of the different AuNPs with the different concentrations in both DMEM- and FBS rich medium was measured using an Osmometer (Osmomat 3000, Gonotec, Germany) at different time points. Samples incubated for 2 h, 6 h, 24 h, 48 h, 72 h were kept at 37 ℃ and 5% CO2 and were measured in triplicates. The osmolarity measured at the end of each time point were expressed as the mean ± standard error and plotted using GraphPad Prism 9 (GraphPad Software).
Drug loading and release from AuNPs
The drug loading content (w/w%) and drug entrapment efficiency (%) were calculated according to following equations:
The released amount of drug from the AuNPs was investigated at two pH values in PBS (pH 7.4 to mimic physiological pH, and pH 4.0 to mimic the acidic intracellular compartments such as endosomes) at 37°. First, the different formulations were dispersed in conic tubes (1.5 mL) and kept in a horizontal laboratory shaker, maintaining a constant temperature and stirring (100 rpm). Aliquots (500 μl) of the supernatant were taken at different time intervals (pH 4: 1, 2, 4, 8, 24, 48 and 72 h; pH 7.4: 1, 2, 4, 8, 24, 48, 72 h) followed by re-suspension with fresh 1X PBS solution of the same volume subtracted to maintain the total volume. The absorbance of released drugs was measured using a microplate reader (GloMax Discover, Promega). All the release experiments were performed in triplicates.
Determination of cell viability
The human glioblastoma cell line U87-MG (passage 15–36) was grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco), 100 unit/ml penicillin G sodium and 100 mg/ml streptomycin sulfate at 37 °C in humidified air with 5% CO2.
Cell viability was determined by SRB or MTT bioassay. In brief, cells were seeded in 96-well cell culture plates (Corning Inc., Corning, NY, USA) at a density of 3000–5000 cells per well and were cultured 24 h prior AuNP treatment for 24 h, 48 h, 72 h.
In the MTT assay, after the indicated period, the medium was discarded and replaced with an equal volume (200 μl) of fresh medium containing MTT and incubated at 37 °C for 2 h in the dark. Next, the MTT medium was discarded, and 100 μl of DMSO was added to dissolve the produced formazan. Cell viability was determined by a colorimetric method using a microplate reader (GloMax Discover, Promega) at the absorption wavelength of 560 nm. Cell metabolic activity was reported as the percentage of the Abs of AuNPs treated cells compared to untreated controls.
Cytotoxicity determination based on the measurement of live cell protein content, was assessed with the SRB assay. In brief, 100 μl of 10% TCA was added to each well and refrigerated at 4 °C for 12 h, then the supernatant was discarded, and the plate was washed 3 to 5 times with water and air dried. 100 μl of SRB solution 0.4% (w/v) in 1% acetic acid was added to each well and incubated for 30 min at room temperature. Unbounded SRB was removed by washing with 1% acetic acid and air dried. Protein-bound dye was solubilised in basic 10 mM Tris-solution, and the optical density (OD) at 560 nm wavelength was measured on a microplate reader. The ratio of cell viability to the control group was calculated from the SRB data. Assays were performed in triplicate on five independent experiments.
Trypan blue exclusion assay was used to evaluate membrane integrity by differentiating viable cells and dead cells. In brief, subsequent AuNP incubation, cells were trypsinised with 0.25% trypsin–EDTA solution (Sigma), centrifuged, resuspended in PBS and mixed within an equal volume (5 μl) of 0.4% Trypan blue, incubated for 1–2 min. The total number of cells, including both unstained (viable) and blue-stained (non-viable) cells, was counted in a Brand™ Bürker Counting Chamber. The percentage of cell viability was calculated using the following formula: (number of viable cells / total cells) × 100.
Evaluation of intracellular ROS production and mitochondrial superoxide level
To determine the level of intracellular ROS production, cells were incubated with 2′,7′-dichlorofluorescein diacetate (DCF-DA) (Cat. no. 6883; Sigma Aldrich GmbH, Schnelldorf, Germany). Briefly, cells were seeded 4 × 104 cells/well in a 96 well plate and treated after 24 h with the AuNPs for different concentrations and time points. Subsequently, samples were incubated in DMEM supplemented with DCF-DA (10 μM) in the dark at 37 °C for 30 min. Fluorescence was detected using a microplate reader (GloMax Discover, Promega) with 485 nm excitation and 538 nm emission filters and values were subtracted of blank (cells with medium only) and normalised by protein content (SRB assay) for each well. Each experiment was performed in triplicate and at least three biological replicates were carried out.
The level of mitochondrial superoxide was measured using the ROS-sensitive fluorescent probe MitoSox (Invitrogen, Molecular Probes, Eugene, Oregon, USA). Cells were seeded at a density of 3 × 104 cells/well in a 96 well plate, overnight and treated with the indicated concentrations (100–200 μM) for mono and dual formulations of AuNPs for 24/48/72 h. After treatment, cells were washed twice to remove the medium and incubated for 10 min. at 37 °C in the presence of 5 μM MitoSox in PBS containing. Next, cells were washed twice with PBS, and the fluorescence was recorded using a multiwell plate reader (GloMax Discover, Promega). Subsequently, cells were fixed for the evaluation of cell number in individual wells using SRB procedure in order to normalize the results by the total cell number. The relative change in the mean fluorescence intensity was calculated as the ratio between the mean fluorescence intensity in the channel of the treated cells and that in the channel of the control cells (cells without AuNP).
Nanoparticle uptake
Experiments for exploring the efficacy and efficiency of cellular uptake of AuNPs were carried out by UV/Vis spectroscopy, as previously described, with minor modifications [71, 72]. In brief, U87-MG cells were seeded in 35 mm Petri dishes at a concentration of 104 cells/cm2 and incubated at 37 °C in a humidified incubator containing 5% CO2. Subsequent incubation (2 h, 6 h, 24 h, 48 h, 72 h) with the different AuNP formulations and concentrations (100 μM and 200 μM), cells were harvested, washed with PBS to remove excess particles, centrifuged and resuspended. Cell count was carried out by Brand™ Bürker Counting Chambers using the Trypan Blue assay, as reported above. The absorbance (Abs) of the resultant cellular suspension was measured at λ = 560 nm using a GloMax Discover Microplate Reader (Promega, Madrid, Spain) and corrected to the negative control represented by the cell lysate of non-treated cells and blank. Calibration curves for AuNPs and of cell lysate of non-treated cells were determined in different concentrations and relevant solutions. Finally, the intracellular concentration of AuNPs was normalised to the volume of the sample and number of cells and expressed as pMol per cell, according to the following equations [71]:
where a and b are the constants from the calibration curve: y = ax + b
Moreover, the percentage uptake of AuNPs was calculated by applying the following formula [72]:
where AM+N is the absorbance of endocytosed NPs at λ = 560 nm, and AN is the absorbance of the reference NP solution at the same wavelength.
Apoptosis evaluation
U87-MG cells were seeded at a density of 1 × 104 cells per cm2 on a chamber slide (µ-Slide 8 Well––ibidi) and cultured at 37 °C in a humidified incubator containing 5% CO2 in complete culture medium. After treatment with 100uM and 200uM of various AuNPs for 24 h, 48 h, and 72 h, cells were harvested and washed with PBS. Subsequently, staining with Annexin V-FITC and PI was performed according to the manufacturer’s instructions with 1 μg/μl Hoechst (Sigma). Cells were then incubated for 15–20 min in the dark, and the images were acquired using a confocal laser scanning microscope (ZEISS 900 LM). The fluorescence intensities were identified by ImageJ software using the IntDen value. The green and red fluorescence intensity changes were analysed, normalising the IntDen with the blue IntDen nuclear staining.
Statistical analysis
All data are represented as mean ± SEM otherwise stated. All experiments are repeated at least three times. Statistical analyses were performed using GraphPad Prism 9.1 (GraphPad Software, San Diego, USA). The asterisks indicate significant level *p < 0.05; **p < 0.01 and ***p < 0.001.
Supplementary Information
Acknowledgements
The research leading to these results has received funding from AIRC under IG 2021—ID. 26328 project – P.I. Cortese Barbara. The authors are also grateful to the "Tecnopolo per la medicina di precisione" (TecnoMed Puglia)—Regione Puglia: DGR n.2117 del 21/11/2018, CUP: B84I18000540002 and "Tecnopolo di Nanotecnologia e Fotonica per la medicina di precisione" (TECNOMED)—FISR/MIUR-CNR: delibera CIPE n.3449 del 7-08-2017, CUP: B83B17000010001. We also thank Irene Iacuitto, Giovanna Loffredo and Manuela Marchetti for practical administrative support.
Author contributions
All authors contributed to the study. In particular: M. E. M. and M. K. carried out AuNP formulations and synthesis as well as their investigation and characterization. M. G. L. performed the initial cellular investigation, MTT, Trypan Blue, Annexin PI and UV–vis uptake. B.C., R.G. and S.D. performed the Osmolarity, UV characterizations, SRB and ROS measurements. J.E. carried out synthesis of the peptide. O.U. and R.G. contributed to the investigation, methodology and formal analysis. M.G. carried out the confocal imaging. B.C. and J.S. wrote the main manuscript text and B.C., R.G. and S.D. prepared all figures and carried out the revision of the contributions. All authors reviewed the manuscript.
Funding
The research leading to these results has received funding from AIRC under IG 2021—ID. 26328 project––P.I. Cortese Barbara. The authors are also grateful to the "Tecnopolo per la medicina di precisione" (TecnoMed Puglia)—Regione Puglia: DGR n.2117 del 21/11/2018, CUP: B84I18000540002 and "Tecnopolo di Nanotecnologia e Fotonica per la medicina di precisione" (TECNOMED)—FISR/MIUR-CNR: delibera CIPE n.3449 del 7–08-2017, CUP: B83B17000010001.
Data availability
Data is provided within the manuscript or supplementary information files.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Myriam El Moutaoukil, Maria Grazia Lolli and Stefania D’Amone have contributed equally to this work.
References
- 1.Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi M, Mason W, van den Bent M, Taphoorn M, Janzer R, Ludwin S, Allgeier A, Fisher B, Belanger K, Hau P, Brandes A, Gijtenbeek J, Marosi C, Vecht C, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross J, Mirimanoff R, E.O.R. Treatment, C.B.T. Grp, R.O. Grp, N.C.I.C.C. Trials, E.O.R. Treatment, C.B.T. Grp, R.O. Grp, N.C.I.C.C. Trials, Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial, Lancet Oncol. 2009;10(5):459–66. [DOI] [PubMed]
- 3.Cagel M, Grotz E, Bernabeu E, Moretton MA, Chiappetta DA. Doxorubicin: nanotechnological overviews from bench to bedside. Drug Discov Today. 2017;22(2):270–81. [DOI] [PubMed] [Google Scholar]
- 4.Fan D, Cao Y, Cao M, Wang Y, Gong T. Nanomedicine in cancer therapy. Signal Transduct Target Ther. 2023;8(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shergalis A, Bankhead A, Luesakul U, Muangsin N, Neamati N. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70(3):412–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen NC, Chauhan R, Bates PJ, O’Toole MG. Optimization of tumor targeting gold nanoparticles for glioblastoma applications. Nanomaterials (Basel). 2022;12(21):3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norouzi M. Gold nanoparticles in glioma theranostics. Pharmacol Res. 2020;156: 104753. [DOI] [PubMed] [Google Scholar]
- 8.Heo DN, Yang DH, Moon HJ, Lee JB, Bae MS, Lee SC, Lee WJ, Sun IC, Kwon IK. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials. 2012;33(3):856–66. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Wang A, Wang S, Sun Y, Chu L, Zhou L, Yang X, Liu X, Sha C, Sun K, Xu L. Efficacy of temozolomide-conjugated gold nanoparticle photothermal therapy of drug-resistant glioblastoma and its mechanism study. Mol Pharm. 2022;19(4):1219–29. [DOI] [PubMed] [Google Scholar]
- 10.Chinnaiyan SK, Soloman AM, Perumal RK, Gopinath A, Balaraman M. 5 Fluorouracil-loaded biosynthesised gold nanoparticles for the in vitro treatment of human pancreatic cancer cell. IET Nanobiotechnol. 2019;13(8):824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X, Zhang Q, Yang Y, He Q, Gao H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–35. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Zheng X, Chen L, Gong X, Yang H, Duan X, Zhu Y. Multifunctional gold nanoparticles in cancer diagnosis and treatment. Int J Nanomed. 2022;17:2041–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi S, Anjum S, Hussain M, Sheikh A, Gupta G, Almoyad MAA, Wahab S, Kesharwani P. A recent insight of applications of gold nanoparticles in glioblastoma multiforme therapy. Int J Pharm. 2024;660: 124301. [DOI] [PubMed] [Google Scholar]
- 14.Raucher D. Tumor targeting peptides: novel therapeutic strategies in glioblastoma. Curr Opin Pharmacol. 2019;47:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lépinoux-Chambaud C, Eyer J. The NFL-TBS.40-63 peptide targets and kills glioblastoma stem cells derived from human patients and also targets nanocapsules into these cells. Int J Pharm. 2019;566:218–28. [DOI] [PubMed] [Google Scholar]
- 16.Balzeau J, Pinier M, Berges R, Saulnier P, Benoit JP, Eyer J. The effect of functionalizing lipid nanocapsules with NFL-TBS.40-63 peptide on their uptake by glioblastoma cells. Biomaterials. 2013;34(13):3381–9. [DOI] [PubMed] [Google Scholar]
- 17.Bocquet A, Berges R, Frank R, Robert P, Peterson AC, Eyer J. Neurofilaments bind tubulin and modulate its polymerization. J Neurosci. 2009;29(35):11043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivalin R, Lepinoux-Chambaud C, Eyer J, Savagner F. The NFL-TBS.40-63 anti-glioblastoma peptide disrupts microtubule and mitochondrial networks in the T98G glioma cell line. PLoS ONE. 2014;9(6):e98473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alnemeh-Al Ali H, Griveau A, Artzner F, Dupont A, Lautram N, Jourdain MA, Eyer J. Investigation on the self-assembly of the NFL-TBS.40-63 peptide and its interaction with gold nanoparticles as a delivery agent for glioblastoma. Int J Pharm X. 2022;4:100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arib C, Griveau A, Eyer J, Spadavecchia J. Cell penetrating peptide (CPP) gold(iii)–complex–bioconjugates: from chemical design to interaction with cancer cells for nanomedicine applications. Nanoscale Adv. 2022;4(14):3010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Liu H, Griveau A, Li X, Eyer J, Arib C, Spadavecchia J. NFL-TBS.40-63 peptide gold complex nanovector: a novel therapeutic approach to increase anticancer activity by breakdown of microtubules in pancreatic adenocarcinoma (PDAC). ACS Pharmacol Transl Sci. 2022;5(12):1267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griveau A, Arib C, Spadavecchia J, Eyer J. Biological activity of gold nanoparticles combined with the NFL-TBS.40-63 peptide, or with other cell penetrating peptides, on rat glioblastoma cells. Int J Pharm X. 2022;4:100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Liu H, Sacco P, Djaker N, Lamy de la Chapelle M, Marsich E, Li X, Spadavecchia J. CTL-doxorubicin (DOX)-gold complex nanoparticles (DOX-AuGCs): from synthesis to enhancement of therapeutic effect on liver cancer model. Nanoscale Adv. 2020;2(11):5231–41. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Markoutsa E, Papadia K, Giannou AD, Spella M, Cagnotto A, Salmona M, Stathopoulos GT, Antimisiaris SG. Mono and dually decorated nanoliposomes for brain targeting, in vitro and in vivo studies. Pharm Res. 2014;31(5):1275–89. [DOI] [PubMed] [Google Scholar]
- 25.Xin H, Jiang X, Gu J, Sha X, Chen L, Law K, Chen Y, Wang X, Jiang Y, Fang X. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32(18):4293–305. [DOI] [PubMed] [Google Scholar]
- 26.Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33(32):8167–76. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X, Xin H, Ren Q, Gu J, Zhu L, Du F, Feng C, Xie Y, Sha X, Fang X. Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials. 2014;35(1):518–29. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Wen G, Wang D, Zhang X, Lu Y, Wang J, Zhong L, Cai H, Wang Y. A complementary strategy for enhancement of nanoparticle intracellular uptake. Pharm Res. 2014;31(8):2054–64. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Ren Z, Zhang X, Zhao Z, Ma G, Pei Y, Zhao W, Wan D, Pan J. Dual-triggered peptide-based polymeric micelles enhance doxorubicin delivery for targeted cancer therapy. Acs Appl Nano Mater. 2024;7(12):14380–91. [Google Scholar]
- 30.Lakkadwala S, Dos Santos Rodrigues B, Sun C, Singh J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J Control Release. 2019;307:247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WH, Xu XD, Jia HZ, Lei Q, Luo GF, Cheng SX, Zhuo RX, Zhang XZ. Therapeutic nanomedicine based on dual-intelligent functionalized gold nanoparticles for cancer imaging and therapy in vivo. Biomaterials. 2013;34(34):8798–807. [DOI] [PubMed] [Google Scholar]
- 32.Zhan R, Xu X, Cui Y, Ma J, Liu H, Wang Y, Zhang G, Tian G. Dual-functional nano-carrier system based on NIR-laser-triggered dynamic Au-Se interaction for chemo-photothermal synergistic tumor therapy. Colloid Interface Sci Commun. 2024;59:100774. [Google Scholar]
- 33.Moustaoui H, Movia D, Dupont N, Bouchemal N, Casale S, Djaker N, Savarin P, Prina-Mello A, de la Chapelle ML, Spadavecchia J. Tunable design of gold(III)-doxorubicin complex-PEGylated nanocarrier. The golden doxorubicin for oncological applications. ACS Appl Mater Interfaces. 2016;8(31):19946–57. [DOI] [PubMed] [Google Scholar]
- 34.von Maltzahn G, Ren Y, Park JH, Min DH, Kotamraju VR, Jayakumar J, Fogal V, Sailor MJ, Ruoslahti E, Bhatia SN. In vivo tumor cell targeting with “click” nanoparticles. Bioconjug Chem. 2008;19(8):1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross AM, Kennedy T, McNulty D, Leahy CI, Walsh DR, Murray P, Grabrucker AM, Mulvihill JJE. Comparing nanoparticles for drug delivery: the effect of physiological dispersion media on nanoparticle properties. Mater Sci Eng C Mater Biol Appl. 2020;113: 110985. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch V, Kinnear C, Moniatte M, Rothen-Rutishauser B, Clift MJ, Fink A. Surface charge of polymer coated SPIONs influences the serum protein adsorption, colloidal stability and subsequent cell interaction in vitro. Nanoscale. 2013;5(9):3723–32. [DOI] [PubMed] [Google Scholar]
- 37.Doyen M, Goole J, Bartik K, Bruylants G. Amino acid induced fractal aggregation of gold nanoparticles: why and how. J Colloid Interface Sci. 2016;464:160–6. [DOI] [PubMed] [Google Scholar]
- 38.Salesa B, Ferrús-Manzano P, Tuñón-Molina A, Cano-Vicent A, Assis M, Andrés J, Serrano-Aroca A. Study of biological properties of gold nanoparticles: Low toxicity, no proliferative activity, no ability to induce cell gene expression and no antiviral activity. Chemico-Biol Interact. 2023;382:110646. [DOI] [PubMed] [Google Scholar]
- 39.Madeo F, Fröhlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH, Fröhlich KU. Oxygen stress: a regulator of apoptosis in yeast. J Cell Biol. 1999;145(4):757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludovico P, Sousa MJ, Silva MT, Leão CL, Côrte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology (Reading). 2001;147(Pt 9):2409–15. [DOI] [PubMed] [Google Scholar]
- 41.Ruan H, Yao S, Wang S, Wang R, Xie C, Guo H, Lu W. Stapled RAP12 peptide ligand of LRP1 for micelles-based multifunctional glioma-targeted drug delivery. Chem Eng J. 2021;403:126296. [Google Scholar]
- 42.Huo T, Yang Y, Qian M, Jiang H, Du Y, Zhang X, Xie Y, Huang R. Versatile hollow COF nanospheres via manipulating transferrin corona for precise glioma-targeted drug delivery. Biomaterials. 2020;260: 120305. [DOI] [PubMed] [Google Scholar]
- 43.Kuo YC, Chang YH, Rajesh R. Targeted delivery of etoposide, carmustine and doxorubicin to human glioblastoma cells using methoxy poly(ethylene glycol)-poly(ε-caprolactone) nanoparticles conjugated with wheat germ agglutinin and folic acid. Mater Sci Eng C Mater Biol Appl. 2019;96:114–28. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Lu L, Zhou J, Ran D, Wang S, Xu Q, Xu W, Wang J, Liu Y, Xie C, Luo Z, Lu W. All-stage targeted therapy for glioblastoma based on lipid membrane coated cabazitaxel nanocrystals. J Control Release. 2022;345:685–95. [DOI] [PubMed] [Google Scholar]
- 45.Hu XY, Gao L, Mosel S, Ehlers M, Zellermann E, Jiang H, Knauer SK, Wang L, Schmuck C. From supramolecular vesicles to micelles: controllable construction of tumor-targeting nanocarriers based on host-guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small. 2018;14(52): e1803952. [DOI] [PubMed] [Google Scholar]
- 46.Qi N, Zhang S, Zhou X, Duan W, Gao D, Feng J, Li A. Combined integrin α. J Nanobiotechnol. 2021;19(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34(33):8511–20. [DOI] [PubMed] [Google Scholar]
- 48.Ali O, Bekhit A, Khattab S, Helmy M, Abdel-Ghany Y, Teleb M, Elzoghby A. Synthesis of lactoferrin mesoporous silica nanoparticles for pemetrexed/ellagic acid synergistic breast cancer therapy. Colloids Surfaces B-Biointerfaces. 2020;188:110824. [DOI] [PubMed] [Google Scholar]
- 49.Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25(6B):3825–31. [PMC free article] [PubMed] [Google Scholar]
- 50.Imantay A, Mashurov N, Zhaisanbayeva BA, Mun EA. Doxorubicin-conjugated nanoparticles for potential use as drug delivery systems. Nanomaterials (Basel). 2025;15(2):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, Wang Y. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. 2020;21(21):8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asokan A, Cho MJ. Exploitation of intracellular pH gradients in the cellular delivery of macromolecules. J Pharm Sci. 2002;91(4):903–13. [DOI] [PubMed] [Google Scholar]
- 53.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5(5):1275–9. [DOI] [PubMed] [Google Scholar]
- 54.Evans TG, Somero GN. A microarray-based transcriptomic time-course of hyper- and hypo-osmotic stress signaling events in the euryhaline fish Gillichthys mirabilis: osmosensors to effectors. J Exp Biol. 2008;211(Pt 22):3636–49. [DOI] [PubMed] [Google Scholar]
- 55.Pagani F, Paolicelli RC, Murana E, Cortese B, Di Angelantonio S, Zurolo E, Guiducci E, Ferreira TA, Garofalo S, Catalano M, D’Alessandro G, Porzia A, Peruzzi G, Mainiero F, Limatola C, Gross CT, Ragozzino D. Defective microglial development in the hippocampus of Cx3cr1 deficient mice. Front Cell Neurosci. 2015;9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pu W, Qiu J, Riggins GJ, Parat MO. Matrix protease production, epithelial-to-mesenchymal transition marker expression and invasion of glioblastoma cells in response to osmotic or hydrostatic pressure. Sci Rep. 2020;10(1):2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mellinger A, Lubitz LJ, Gazaille C, Leneweit G, Bastiat G, Lépinoux-Chambaud C, Eyer J. The use of liposomes functionalized with the NFL-TBS.40-63 peptide as a targeting agent to cross the in vitro blood-brain barrier and target glioblastoma cells. Int J Pharm. 2023;646:123421. [DOI] [PubMed] [Google Scholar]
- 58.Audrey G, Claire LC, Joel E. Effect of the NFL-TBS.40-63 peptide on canine glioblastoma cells. Int J Pharm. 2021;605:120811. [DOI] [PubMed] [Google Scholar]
- 59.Fathi-karkan S, Sargazi S, Shojaei S, Far B, Mirinejad S, Cordani M, Khosravi A, Zarrabi A, Ghavami S. Biotin-functionalized nanoparticles: an overview of recent trends in cancer detection. Nanoscale. 2024;16(27):12750–92. [DOI] [PubMed] [Google Scholar]
- 60.Freese C, Gibson MI, Klok HA, Unger RE, Kirkpatrick CJ. Size- and coating-dependent uptake of polymer-coated gold nanoparticles in primary human dermal microvascular endothelial cells. Biomacromol. 2012;13(5):1533–43. [DOI] [PubMed] [Google Scholar]
- 61.Panzarini E, Mariano S, Carata E, Mura F, Rossi M, Dini L. Intracellular transport of silver and gold nanoparticles and biological responses: an update. Int J Mol Sci. 2018;19(5):1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lépinoux-Chambaud C, Eyer J. The NFL-TBS.40-63 anti-glioblastoma peptide enters selectively in glioma cells by endocytosis. Int J Pharm. 2013;454(2):738–47. [DOI] [PubMed] [Google Scholar]
- 63.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013;2013: 942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xuan L, Ju Z, Skonieczna M, Zhou PK, Huang R. Nanoparticles-induced potential toxicity on human health: applications, toxicity mechanisms, and evaluation models. MedComm. 2023;4(4):e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jawaid P, Rehman MU, Zhao QL, Misawa M, Ishikawa K, Hori M, Shimizu T, Saitoh JI, Noguchi K, Kondo T. Small size gold nanoparticles enhance apoptosis-induced by cold atmospheric plasma via depletion of intracellular GSH and modification of oxidative stress. Cell Death Discov. 2020;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner C, Kroemer G. Apoptosis. Mitochondria–the death signal integrators. Science. 2000;289(5482):1150–1. [DOI] [PubMed] [Google Scholar]
- 67.Li LS, Ren B, Yang X, Cai ZC, Zhao XJ, Zhao MX. Hyaluronic acid-modified and doxorubicin-loaded gold nanoparticles and evaluation of their bioactivity. Pharmaceuticals (Basel). 2021;14(2):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, Hester S, Lowry GV, Veronesi B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115(11):1631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valdés-Rives SA, Casique-Aguirre D, Germán-Castelán L, Velasco-Velázquez MA, González-Arenas A. Apoptotic signaling pathways in glioblastoma and therapeutic implications. Biomed Res Int. 2017;2017:7403747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan M, Cherni K, Dekhili R, Spadavecchia J. Spectroscopic assessment of doxorubicin (DOX)-gemcitabine (GEM) gold complex nanovector as diagnostic tool of galectin-1 biomarker. Nanotechnol Sci Appl. 2024;17:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Svitkova B, Selc M, Nemethova V, Razga F, Gabelova A, Ursinyova M, Babelova A. Plate reader spectroscopy as an alternative to atomic absorption spectroscopy for the assessment of nanoparticle cellular uptake. Heliyon. 2022;8(11): e11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christie C, Madsen SJ, Peng Q, Hirschberg H. Photothermal therapy employing gold nanoparticle- loaded macrophages as delivery vehicles: comparing the efficiency of nanoshells versus nanorods. J Environ Pathol Toxicol Oncol. 2017;36(3):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.
Not applicable.