Abstract
Background
We hypothesized treatment with nivolumab and stereotactic radiosurgery (SRS) would be feasible, well tolerated, and may improve intracranial tumor control over SRS alone for breast cancer brain metastases (BCBM).
Methods
The study is a phase Ib trial of nivolumab and SRS for BCBM. Clinical trial information: NCT03807765. Key eligibility criteria include BCBM of all subtypes, age ≥18, Eastern Cooperative Oncology Group Performace Status (ECOG-PS)≤2 with ≤10 brain metastases. Treatment was initiated with a dose of nivolumab (480 mg intravenously) that was repeated every 4 weeks. The initial dose of nivolumab was followed 1 week later by SRS. Blood was collected at baseline and every 4 weeks for flow cytometry and cell-free DNA (cfDNA) assessment.
Results
A total of 12 patients received SRS to 17 brain metastases. Breast cancer subtypes included triple negative (50%), hormone receptor (HR)+/HER2− (33%), and HR−/HER2+ (17%). Median follow-up from start of protocol therapy is 56 months. No cases of radionecrosis were noted. Two lesions were noted to undergo local failure, both pathologically confirmed, for a 12-month local control of 94%. Median distant intracranial control was 7.4 months with a 12-month control rate of 33%. Median systemic progression-free survival was 7.7 months with a 12-month rate of 42%. Median overall survival (OS) was 24.7 months with a 12-month OS of 75%. Most patients were noted to have an increase in cfDNA throughout study treatment, at week 5 compared with baseline (83%), week 25 compared with baseline (89%), and 100% at first follow-up. Intracranial control was associated with lower levels of CD4 regulatory T cells (Treg) (p=0.03) and higher levels of CD4 T effector memory (p=0.04).
Conclusions
Nivolumab and SRS is a safe and feasible treatment option in BCBM. Long-term follow-up revealed no cases of radiation necrosis.
Trial registration number
Keywords: Nivolumab, Breast Cancer, Radiotherapy/radioimmunotherapy, Immunotherapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Anti-programmed cell death protein-1/programmed cell death ligand-1 (PD-1/PD-L1) therapy has demonstrated intracranial responses in non-small cell lung cancer and melanoma brain metastases.
Pembrolizumab is approved in the management of metastatic and high-risk, early-stage triple-negative breast cancer.
Retrospective studies have suggested a potentially increased risk of radiation necrosis with the combination of stereotactic radiosurgery (SRS) and anti-PD-1/PD-L1 therapy.
WHAT THIS STUDY ADDS
The study noted combination therapy with nivolumab and SRS to be safe and feasible in the management of breast cancer brain metastases.
No cases of radiation necrosis were noted with long-term follow-up.
Improved overall survival and systemic progression-free survival were noted with increased levels of effector memory cells and lower levels of lymphocyte activation gene 3 (LAG3).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Further study is required to assess the potential synergism between SRS and nivolumab to improve intracranial control for breast cancer brain metastases.
Circulating systemic biomarkers require further study in assessing treatment outcomes in breast cancer brain metastases.
Introduction
Breast cancer brain metastases (BCBM) are becoming an increasingly common diagnosis as patients live longer due to improved systemic control.1 Although numerous treatment options exist for the management of systemic disease, once metastases travel to the brain, patients with BCBM have limited options, particularly those without HER2+disease. Local approaches including surgical resection and radiation therapy have a critical role in the management of BCBM. Standard-of-care treatments for patients include local treatments, such as surgical resection, stereotactic radiosurgery (SRS), or whole brain radiotherapy (WBRT).2 Anti-programmed cell death protein-1 and programmed cell death ligand-1 (anti-PD-1/PD-L1) therapy has shown promise in the management of specific subtypes of metastatic breast cancer and in the neoadjuvant setting when combined with conventional chemotherapy.3,5
The use of pembrolizumab has demonstrated objective intracranial responses in one-third of patients with non-small cell lung cancer (NSCLC) BM and a quarter of patients with melanoma BM.6 Long-term results from CheckMate 204, with the combination of nivolumab and ipilimumab alone in patients with melanoma BM produced an overall response rate of 56%.7 However, rates of control were significantly lower in patients with symptomatic BM. Combining radiation therapy with immune checkpoint inhibitors may hold promise.8 Preclinical data suggests radiation therapy may upregulate PD-L1 expression leading to improved responses from immune checkpoint inhibitors.9 The local control provided by SRS also has clear benefits. Given the encouraging data in NSCLC and melanoma BM as well as data revealing systemic control in the management of breast cancer, particularly the triple-negative subtype,4 10 we conducted a study of SRS and nivolumab in the management of BCBM. Early clinical results were previously reported.11
We report long-term clinical results as well as a biomarker analysis of a phase Ib study to evaluate safety and preliminary activity of nivolumab and SRS in the management of BCBM.
Methods and materials
Study design and participants
Study methodology has been previously described.11 Briefly, the study was designed as a prospective, single-arm, phase Ib trial of nivolumab and SRS among patients with metastatic BCBM, online supplemental file 2. All procedures were performed in compliance with relevant laws and institutional guidelines and were approved by the Moffitt Cancer Center Central Institutional Review Board (Advarra IRB #00000971). Clinical trial information: NCT03807765. Informed consent was obtained from all patients.
Key eligibility criteria included patients with BCBM of all subtypes, ≤10 BM with an Eastern Cooperative Oncology Group Performance Status (ECOG-PS) ≤2 and a maximum diameter of the largest intact BM≤4 cm. There was no limit on lines of previous systemic therapies. A 2-week wash-out period for investigational systemic treatments was required before starting study treatment.
Exclusion criteria included receipt of prior WBRT, presence of leptomeningeal disease (LMD), or prior treatment with immune checkpoint therapy.
Procedures
Nivolumab (480 mg intravenously) was given every 4 weeks, starting 1-week prior to SRS. For continued systemic disease control, patients were allowed to continue prior systemic therapies per treating physicians’ discretion if BM progression was noted on these agents for continued systemic disease control. SRS was given to sites of BM or postoperative cavities.
Imaging, including a brain MRI and CT thorax, abdomen, and pelvis, was obtained every 8 weeks for the first year followed by every 12 weeks thereafter to assess intracranial and systemic responses, respectively. BM response was assessed via Response Assessment in Neuro-Oncology (RANO) and extracranial disease was assessed via immune-related Response Evaluation Criteria in Solid Tumors (irRECIST) criteria.12 13
Flow cytometry, cytokine, and cell-free DNA analysis
For analysis of the immune phenotype of the peripheral blood mononuclear cells (PBMCs), cryopreserved human PBMC samples collected at week 1, week 5, week 9, and week 17 were assessed. Cells were thawed in media and subsequently stained in phosphate-buffered saline containing 5% fetal bovine serum (vol/vol, fluorescence-activated cell sorting (FACS) buffer) with CD3 (BUV496), CD4 (BUV737), CD8 (BUV395), CD14 (BV605), and CD19 (BV605) from BD Biosciences. Dead cells were excluded using the Zombie NIR Fixable Viability Kit from BioLegend, incubated at 4°C for 1 hour, washed twice with FACS buffer, and finally fixed in phosphate-buffered saline containing 1% paraformaldehyde before undergoing flow cytometry. Cells were acquired on a BD FACSymphony A5, and data were analyzed with FlowJo V.10.0 software. All cell gates were drawn uniformly for analysis across patients and time points. Collected plasma was analyzed for Th1, Th2 and Th17 cytokines and chemokines using the Luminex Multiplex System at week 1, week 5, week 9, and week 17.
Yields of circulating tumor DNA (ctDNA) extracted from plasma specimens were estimated using the Agilent TapeStation 4200 with the Agilent Cell-free DNA ScreenTape Assay (Agilent Technologies, California, USA). Cell-free DNA (cfDNA) samples were analyzed on this instrument and the yields of mononucleosome, dinucleosomes, and trinucleosomes appearing in the 50–700 bp region of the electropherogram have been estimated and expressed as %cfDNA. This approach subtracts the mass of any carryover high molecular weight genomic DNA, which appears as a peak larger than 700 bp.
Statistical analysis
Local brain metastasis failure was defined by RANO-brain metastases (BM) criteria in which there was a ≥20% increase that remained consistent or demonstrated continued progression on subsequent imaging.12 Distant intracranial control (DIC) was defined as freedom from the development of new BM outside of the irradiated field and freedom from the development of LMD. Intracranial control was defined as freedom from local and distant failure. Systemic progression-free survival (PFS) was defined by extracranial progression by irRECIST criteria13 or death. The rate of local control (LC) and DIC of brain lesions, as well as biomarker assessments, was calculated using the Kaplan-Meier curve method with differences assessed via log-rank. The association between cfDNA and outcomes was explored by identifying a clinically relevant cfDNA cutpoint using the MaxStat method, which maximizes the difference in outcomes between the resulting two groups.14
Flow cytometry assessments were completed with a split at the median. Events were summarized descriptively using frequencies and percentages. Demographics and baseline laboratory results were summarized using descriptive statistics for all participants.
Results
Patient and treatment characteristics
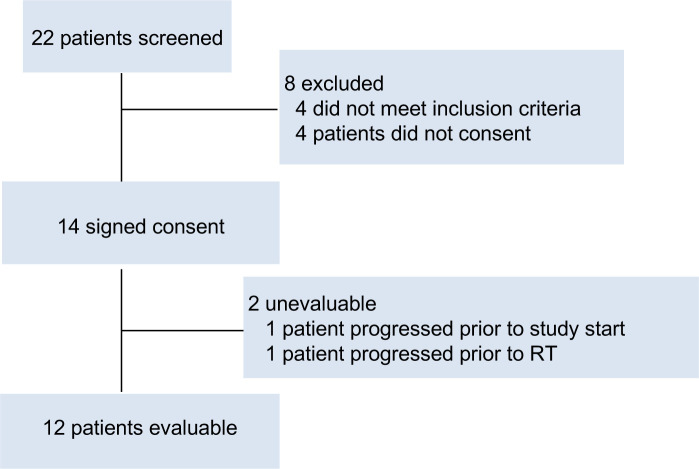
Between February 2019 and July 2020, a total of 12 patients treated to 17 BM were enrolled, figure 1. Data from the study was assessed in January 2024. Patient and treatment characteristics are detailed in table 1. The median age of patients was 58 (range: 26–67) and the majority of patients had triple-negative disease (n=6; 50%). Six patients (50%) underwent prior central nervous system-directed local therapy and the majority of patients had a singular lesion treated (n=8; 67%).
Figure 1. Consolidated Standards of Reporting Trials flow diagram. RT, Radiation therapy.
Table 1. Patient and treatment characteristics.
| Variable | n | % |
|---|---|---|
| No. of patients | 12 | |
| Number of lesions Irradiated | 17 | |
| Age, median (range) | 58 (26–67) | |
| ECOG performance status | ||
| 0 | 8 | 67 |
| 1 | 4 | 33 |
| Receptors | ||
| HR+/HER2− | 4 | 33 |
| HR−/HER2+ | 2 | 17 |
| TN | 6 | 50 |
| Number of previous systemic therapy regimens | ||
| 1 | 8 | 67 |
| 2 | 2 | 17 |
| ≥4 | 2 | 17 |
| Previous CNS therapy | ||
| None | 6 | 50 |
| Surgical resection+stereotactic radiation therapy | 1 | 8 |
| Stereotactic radiation therapy | 2 | 17 |
| Surgery | 3 | 25 |
| Number of lesions irradiated | ||
| 1 | 8 | 67 |
| 2 | 3 | 25 |
| 3 | 1 | 8 |
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group ; HR, hormone receptor; TN, triple negative.
Of the 17 lesions treated on the study, the majority were treated with single fraction radiosurgery (n=15; 88%) to a median dose of 21 Gy (range: 16–24 Gy) while 2 lesions were treated to a dose of 30 Gy in five fractions. Four postoperative cavities were treated.
Toxicity
Study therapy was well tolerated with no dose limiting toxicities (DLTs) noted in our patient population, table 2. No cases of radionecrosis have been noted with extended follow-up. Neurologic toxicities unrelated to study therapy included grade 4 encephalopathy and grade 3 seizures in the same patient noted in follow-up due to leptomeningeal disease progression noted on MRI. Another patient experienced an episode of grade 3 ataxia, paresthesia, and headache unrelated to study treatment in follow-up after intracranial leptomeningeal progression was noted and Ommaya reservoir placement had occurred. One patient experienced grade 3 cerebral edema leading to word-finding difficulties, lethargy, and decreased oral intake thought to be possibly related to study treatment during week 9 of treatment, which resolved 2 days later with oral steroids. Another patient experienced grade 3 cerebral edema due to disease progression in follow-up that was unrelated to study treatment. The most common neurologic toxicity was grade 1–2 headaches in eight patients (66%) and dizziness in 6 (50%). The most common non-neurologic adverse effects at least possibly attributable to study therapy included grade 1–2 fatigue (n=7; 58%), nausea (n=6; 50%), and hypothyroidism (n=6; 50%). No treatment-related deaths were noted.
Table 2. Adverse events in all treated patients.
| Grade 1–2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Neurological adverse events, regardless of attribution | |||
| Ataxia | 1 (8%) | ||
| Stroke | 1 (8%) | ||
| Muscle weakness right-sided | 1 (8%) | ||
| Memory impairment | 1 (8%) | ||
| Headache | 8 (66%) | 1 (8%) | |
| Dizziness | 6 (50%) | ||
| Seizure | 1 (8%) | ||
| Peripheral sensory neuropathy | 2 (17%) | ||
| Paresthesia | 1 (8%) | 1 (8%) | |
| Nystagmus | 1 (8%) | ||
| Dysgeusia | 1 (8%) | ||
| Syncope | 1 (8%) | ||
| Cerebral edema | 1 (8%) | 2 (17%) | |
| Encephalopathy | 1 (8%) | ||
| Non-neurological adverse events, treatment related | |||
| Hypotension | 1 (8%) | ||
| Decreased lymphocyte count | 4 (33%) | 3 (25%) | |
| Weight loss | 1 (8%) | ||
| Thromboembolic event | 1 (8%) | ||
| Pneumonitis | 1 (8%) | ||
| Adrenal insufficiency | 2 (17%) | ||
| Alanine aminotransferase increased | 2 (17%) | ||
| Anemia | 3 (25%) | 1 (8%) | |
| Aspartate aminotransferase increased | 3 (25%) | ||
| Blood lactate dehydrogenase increased | 2 (17%) | ||
| Diarrhea | 4 (33%) | ||
| Dyspnea | 2 (17%) | ||
| Fatigue | 7 (58%) | ||
| Hyperglycemia | 2 (17%) | ||
| Hypothyroidism | 6 (50%) | ||
| Nausea | 6 (50%) | ||
| Platelet count decreased | 2 (17%) | ||
| Alkaline phosphatase increased | 2 (17%) | ||
| Arthralgia | 2 (17%) | ||
| Hypercalcemia | 2 (17%) | ||
| Neutrophil count decreased | 2 (17%) | ||
Adverse events are included if they are grade 3–5 severity or occurred in at least 10% of patients, and were considered at least possibly related to study therapy. Neurological events are included regardless of attribution to study therapy.
Intracranial and systemic response
Median follow-up from start of protocol therapy is 56 months. 2 (12%) of 17 treated lesions have been noted to undergo local failure both of which were confirmed by surgical resection for a 12-month local control of 94%. Median distant intracranial control was 7.4 months with a 12-month control rate of 33%, figure 2A. A total of 10 patients had distant intracranial failure, of which 6 patients received additional SRS, 3 received WBRT, and 1 patient received craniospinal irradiation for LMD progression. Median intracranial control was 5.8 months (95% CI 2.8 to 14 months) with 6 and 12-month control rates of 42% and 25%, respectively. Best RANO intracranial responses on study therapy were categorized as complete response (CR) in six patients (50%), partial response (PR) in five patients (42%), and progressive disease (PD) in one patient (8%); intracranial response (92%).
Figure 2. Kaplan-Meier curves of (A) distant intracranial control and (B) overall survival.
Median systemic PFS was 7.7 months with a 12-month rate of 42%. Median overall survival (OS) was 24.7 months with a 12-month OS of 75%, figure 2B.
Biomarker assessment
In the flow cytometry analysis, improved OS was associated with higher levels of CD4 T EM effector memory (EM) at week 17 (p<0.03) and higher levels of CD8 T EM week 1 (p=0.01), week 5 (p<0.01), week 9 (p=0.03). In addition, smaller changes in CD4 T lymphocyte activation gene 3 (LAG3) from week 9 to baseline during study therapy were associated with longer OS (p=0.04), figure 3A. Longer systemic PFS was associated with higher levels of CD4 T EM at week 9 (p=0.03) and week 17 (p=0.04), CD8 T EM week 1 (p=0.03) and lower levels of CD8 T LAG3 at week 9 (p=0.04), figure 3B. Intracranial control was associated with lower levels of CD4 regulatory T cells (Treg) at week 1 (p=0.03) and higher levels of CD4 T EM at week 17 (p=0.04). Assessment of cytokine levels revealed only mild effects on clinical endpoints.
Figure 3. Kaplan-Meier curves of (A) overall survival stratified by CD4 T LAG3 changes from week 9 to baseline and (B) systemic progression-free survival stratified by CD8 T LAG3 levels at week 9. High and low levels are stratified at the median. LAG3, lymphocyte activation gene 3.
Most patients were noted to have an increase in cfDNA throughout study treatment, at week 5 compared with baseline (83%), week 25 compared with baseline (89%), and 100% at first follow-up. There were trends towards higher cfDNA (MaxStat split 86%) at week 25 predicting shorter OS (p=0.09), online supplemental figure 1A, and higher levels of cfDNA (MaxStat split 83%) at screening predicting distant intracranial failure (p=0.08), online supplemental figure 1B.
Discussion
In this long-term analysis of a prospective phase Ib study of stereotactic radiation and nivolumab in the management of BCBM, we note several findings. First, study therapy was well tolerated with no cases of radionecrosis noted with extended follow-up. Second, several patients were noted to have sustained responses to study therapy. Third, we note the association of effector memory cells with OS and systemic PFS and the need for further investigation into the role of LAG3 inhibitors to improve responses to study treatment. Trends were also noted in worse OS and DIC with higher levels of cfDNA.
Immune checkpoint inhibitors have shown efficacy in the management of BM. Long-term follow-up from CheckMate 204 in the management of melanoma BM with nivolumab and ipilimumab revealed a 36-month intracranial PFS of 54.1% in asymptomatic patients and OS of 71.9%.15 In patients with symptomatic BM, outcomes were notably less pronounced with a 36-month intracranial PFS and OS of 18.9% and 36.6%, respectively. In patients with NSCLC BM, Goldberg et al reported an approximate 30% response rate with pembrolizumab in NSCLC BM with PD-L1 expression of ≥1%.6 However, no responses were noted in patients with a PD-L1 expression of <1%. Stereotactic radiation has a well-defined role in the local management of BM and studies have pointed to the potential synergy with immune checkpoint inhibition 16–20.9 16 Anti-PD-L1 inhibitors are used commonly in the upfront management of triple-negative breast cancer (TNBC) and metastatic breast cancer. The study was initiated prior to approvals for pembrolizumab in TNBC to assess the safety of SRS and nivolumab and to assess initial intracranial efficacy.
In a phase II trial of pembrolizumab in the management of BM of various histologies assessing 9 patients with untreated BMs (cohort A) and 48 patients with recurrent and progressive BMs (cohort B), median intracranial PFS was noted to be 1.6 months in cohort A and 2.2 months in cohort B.17 4 patients with TNBC were enrolled in cohort A and 7 patients with TNBC in cohort B of the 31 patients with breast cancer enrolled in B. Six patients (50%) in our study had TNBC. Although cross-trial comparisons are difficult, intracranial PFS in our study was higher at 5.8 months which points to the role of SRS in providing local control of lesions. Local control of lesions in our study was 94%. SRS was also well tolerated in our study.
One of the concerns for combining SRS with immunotherapy has been the risk of radiation necrosis. The current study was undertaken to assess this risk. There have been a number of mechanisms proposed for radiation necrosis, including vascular injury and hypoxia, injury to oligodendrocytes, and chronic inflammation in response to these injuries.18 Retrospective studies revealed a potential increased risk of radiation necrosis with the use of immune checkpoint inhibitors particularly ipilimumab and SRS.19 However, recent meta-analyses and retrospective series have failed to confirm this risk.20 21 Our study is among the first to assess this risk and now with long-term follow-up we can confirm no cases of radiation necrosis occurred in our cohort. Radiation necrosis can often require pathologic evaluation to differentiate it from local progression. Both cases of local progression of the 17 lesions treated in this series were confirmed by pathology without radiation necrosis noted. Our study supports the data of recent meta-analyses indicating the risk of radiation necrosis to be low with the use of anti-PD-1 inhibitors and SRS.
Although the sample size in this study was limited, we noted several findings in the flow cytometry analysis to be associated with clinical endpoints. Improved OS was associated with the presence of CD4 and CD8 effector memory cells, which correlates to responses seen with immune-rich breast cancers when treated with immunotherapy. This also extended to intracranial control with higher levels of CD4 T EM associated with improved intracranial control. In addition, lower levels of LAG3, the clinical target of the recent Food and Drug Administration approved drug in melanoma, relatlimab,22 were noted to be associated with OS and improved systemic PFS. LAG3 may be another target for the immunomodulation of breast cancer23,25 and is currently being assessed in prospective trials.
Numerous studies have revealed the correlation of ctDNA in early and metastatic breast cancer to systemic response rates.26,29 Data on the correlation of cfDNA and ctDNA to intracranial disease control remains limited, particularly in the setting of BCBM. A study of 72 patients with stage IV melanoma with brain metastasis treated with anti-PD-1 therapy found ctDNA to be a prognostic biomarker with higher levels associated with worse survival.30 Although our study is limited by patient numbers, we note trends in cfDNA predicting worse OS and intracranial control. Further investigation is needed to determine the optimal approach to use these systemic tests in tracking intracranial control for BM.
Conclusions
In summary, long-term data reveals the combination of stereotactic radiation with nivolumab to be safe and well tolerated in BCBM. No cases of radionecrosis were noted with long-term follow-up. Given the exploratory nature of the study, circulating systemic biomarkers require further study in assessing treatment outcomes in BCBM.
Supplementary material
Acknowledgements
We thank the patients who participated in this study and the clinical study teams who were involved in data collection and analyses.
Footnotes
Funding: This study was funded by Bristol-Myers Squibb (CA209-8NK) and Moffitt Cancer Center (P30-CA076292).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Moffitt Cancer Center, Advarra IRB
#00000971. Participants gave informed consent to participate in the study before taking part.
Data availability statement
Data are available upon reasonable request.
References
- 1.Mills MN, Figura NB, Arrington JA, et al. Management of brain metastases in breast cancer: a review of current practices and emerging treatments. Breast Cancer Res Treat. 2020;180:279–300. doi: 10.1007/s10549-020-05552-2. [DOI] [PubMed] [Google Scholar]
- 2.Witzel I, Oliveira-Ferrer L, Pantel K, et al. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 2016;18:8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanda R, Liu MC, Yau C, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020;6:676–84. doi: 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460–7. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379:2108–21. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:655–63. doi: 10.1016/S1470-2045(20)30111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379:722–30. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27:434–41. doi: 10.1093/annonc/mdv622. [DOI] [PubMed] [Google Scholar]
- 9.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. JCO. 2017;35:506. doi: 10.1200/JCO.2017.35.15_suppl.506. [DOI] [Google Scholar]
- 11.Ahmed KA, Kim Y, Arrington JA, et al. Nivolumab and Stereotactic Radiosurgery for Patients With Breast Cancer Brain Metastases: A Nonrandomized, Open-Label Phase 1b Study. Adv Radiat Oncol. 2021;6:100798. doi: 10.1016/j.adro.2021.100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16:e270–8. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 14.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43:121–37. doi: 10.1016/S0167-9473(02)00225-6. [DOI] [Google Scholar]
- 15.Tawbi HA, Forsyth PA, Hodi FS, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021;22:1692–704. doi: 10.1016/S1470-2045(21)00545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brastianos PK, Kim AE, Giobbie-Hurder A, et al. Pembrolizumab in brain metastases of diverse histologies: phase 2 trial results. Nat Med. 2023;29:1728–37. doi: 10.1038/s41591-023-02392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Rhun E, Dhermain F, Vogin G, et al. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. 2016;16:903–14. doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 19.Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and Symptomatic Radiation Necrosis in Patients With Brain Metastases Treated With Stereotactic Radiation. JAMA Oncol. 2018;4:1123–4. doi: 10.1001/jamaoncol.2017.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehrer EJ, Kowalchuk RO, Gurewitz J, et al. Concurrent Administration of Immune Checkpoint Inhibitors and Single Fraction Stereotactic Radiosurgery in Patients With Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma Brain Metastases. Int J Radiat Oncol Biol Phys. 2023;116:858–68. doi: 10.1016/j.ijrobp.2023.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Dohm AE, Nakashima JY, Kalagotla H, et al. Stereotactic radiosurgery and anti-PD-1 + CTLA-4 therapy, anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitors, or conventional chemotherapy for the management of melanoma brain metastases. Eur J Cancer. 2023;192:113287. doi: 10.1016/j.ejca.2023.113287. [DOI] [PubMed] [Google Scholar]
- 22.Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burugu S, Gao D, Leung S, et al. LAG-3+ tumor infiltrating lymphocytes in breast cancer: clinical correlates and association with PD-1/PD-L1+ tumors. Ann Oncol. 2017;28:2977–84. doi: 10.1093/annonc/mdx557. [DOI] [PubMed] [Google Scholar]
- 24.Rivoltini L, Camisaschi C, Fucà G, et al. Immunological characterization of a long-lasting response in a patient with metastatic triple-negative breast cancer treated with PD-1 and LAG-3 blockade. Sci Rep. 2024;14:3379. doi: 10.1038/s41598-024-54041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Qi Y, Zhai J, et al. Molecular and Clinical Characterization of LAG3 in Breast Cancer Through 2994 Samples. Front Immunol. 2021;12:599207. doi: 10.3389/fimmu.2021.599207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cullinane C, Fleming C, O’Leary DP, et al. Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open . 2020;3:e2026921. doi: 10.1001/jamanetworkopen.2020.26921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Murillas I, Chopra N, Comino-Méndez I, et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol. 2019;5:1473–8. doi: 10.1001/jamaoncol.2019.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 29.Stover DG, Parsons HA, Ha G, et al. Association of Cell-Free DNA Tumor Fraction and Somatic Copy Number Alterations With Survival in Metastatic Triple-Negative Breast Cancer. J Clin Oncol. 2018;36:543–53. doi: 10.1200/JCO.2017.76.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Menzies AM, Carlino MS, et al. Longitudinal Monitoring of ctDNA in Patients with Melanoma and Brain Metastases Treated with Immune Checkpoint Inhibitors. Clin Cancer Res. 2020;26:4064–71. doi: 10.1158/1078-0432.CCR-19-3926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.