Abstract
Rationale
Cystic fibrosis (CF) is a genetic disease leading to progressive lung function loss and early mortality. Many clinical and demographic variables are associated with lung function decline, but little is known about the effects of prolonged periods of missed care.
Objectives
To determine if missed care in the Cystic Fibrosis Foundation Patient Registry (CFFPR) is associated with decreased lung function at follow-up visits.
Methods
Deidentified CFFPR data for 2004–2016 were analyzed, with the exposure of interest being ⩾12-month gap in CFFPR data. We modeled percentage predicted forced expiratory volume in 1 second using longitudinal semiparametric modeling with natural cubic splines for age (knots at quantiles) and with subject-specific random effects, adjusted for sex and CFTR (cystic fibrosis transmembrane conductance regulator) genotype, race, and ethnicity and included time-varying covariates for gaps in care, insurance type, underweight body mass index, CF-related diabetes status, and chronic infections.
Results
A total of 24,328 individuals with 1,082,899 encounters in the CFFPR met inclusion criteria. In the cohort, 8,413 (35%) individuals had at least a single ⩾12-month episode of discontinuity, whereas 15,915 (65%) had continuous care. Of the encounters preceded by a 12-month gap, 75.8% occurred in patients 18 years and older. Compared with those with continuous care, those with a discontinuous care episode had a lower follow-up percentage predicted forced expiratory volume in 1 second at the index visit (−0.81%; 95% confidence interval, −1.00, −0.61) after adjustment for other variables. The magnitude of this difference was much greater (−2.1%; 95% confidence interval, −1.5, −2.7) in young adult F508del homozygotes.
Conclusions
There was a high rate of ⩾12-month gap in care, especially in adults, documented in the CFFPR. Discontinuous care identified in the CFFPR was strongly associated with decreased lung function, especially in adolescents and young adults homozygous for the F508del CFTR mutation. This may have implications for identifying and treating people with lengthy gaps in care and may have implications for CFF care recommendations.
Keywords: cystic fibrosis, care fragmentation, lung function decline
Cystic fibrosis (CF) is a genetic disease that leads, in most cases, to progressive lung function decline and increased risk of premature death. Accelerated decline in lung function is associated with early mortality in this disease (1). A national registry of people with CF (PwCF) receiving care from Cystic Fibrosis Foundation (CFF) care centers in the United States, known as the Cystic Fibrosis Foundation Patient Registry (CFFPR), was established in the 1960 s (2) and has tracked encounter-based data since 2003. The CFFPR is a large, rich dataset that aims to capture information on all encounters within the healthcare system, including clinic visits and hospitalizations. It also holds longitudinal measures of pulmonary function testing (PFT) and a wide variety of clinical and demographic metrics.
The CFF currently recommends that at least four in-clinic assessments be performed on each patient yearly (3), as derived from expert consensus (4). Given this standard, identification and quantification of associations between key clinical outcomes and visit frequency would help establish a data-driven evidence base for this guideline. Previous analyses of the CFFPR and other large CF datasets have associated multiple patient characteristics with increased risk for accelerated decline in lung function, including female sex (5–10); older age (6, 8, 10–12); severe CFTR (cystic fibrosis transmembrane conductance regulator) genotype (5–7, 9, 13–15); pancreatic insufficiency (5, 9, 10, 13, 16); infection with methicillin-resistant Staphylococcus aureus (MRSA) (7, 8, 17), Burkholderia spp. (8, 10), and/or Pseudomonas aeruginosa (8–11, 18); higher baseline forced expiratory volume in 1 second (FEV1) (7, 16, 19); CF-related diabetes (CFRD) (5, 8, 9); and nutritional failure (6, 7, 20, 21). Insurance status has also been found to influence long-term lung function and other important clinical outcomes (22, 23). Studies have reported associations between socioeconomic status and loss of lung function, though no difference in access to care or frequency of visits was found to account for this relationship (24). Encounter-level data from the ESCF (Epidemiologic Study of CF) from the mid-1990 s showed a positive correlation between CF center visit frequency and center-based average lung function quartiles (25, 26); however, there is minimal published information from the CFFPR about the effects of care continuity or frequency on patient-level key clinical outcomes.
Thus, the purpose of the present study was to use encounter-based data from the CFFPR and rigorous statistical methodology to evaluate the association between care continuity and lung function over time (8). Our hypothesis was that there would be a significant association between care discontinuity and lower lung function and that this association would be reshaped by two important, nonmodifiable factors: age and genotype. Specifically, we hypothesized that discontinuous care would be associated with worse age-adjusted lung function during early adolescence and young adulthood and in the setting of more severe CF genotype.
A portion of the work contained in this manuscript has been previously published in abstract form (27).
Methods
Study Population
Our project was approved by the CFFPR committee and reviewed and found to be exempt by the Maine Medical Center Institutional Review Board. We analyzed data from the CFFPR between 2004 and 2016 for patients at least 6 years of age who had ⩾3 years of PFT data available and had not undergone transplant. Patients aged <6 years were excluded because of a lack of reliable PFT data. Patients older than 45 years of age were also excluded on the basis of concerns regarding the heterogeneity of this population (e.g., frequency of delayed diagnosis and unusual genotypes) (28). Patients with a history of lung transplant before the study period were excluded. Patients were censored at death and in the year before transplant because transplant care is often shared between the primary CF center and the transplant center after listing.
Encounter Inclusion Criteria
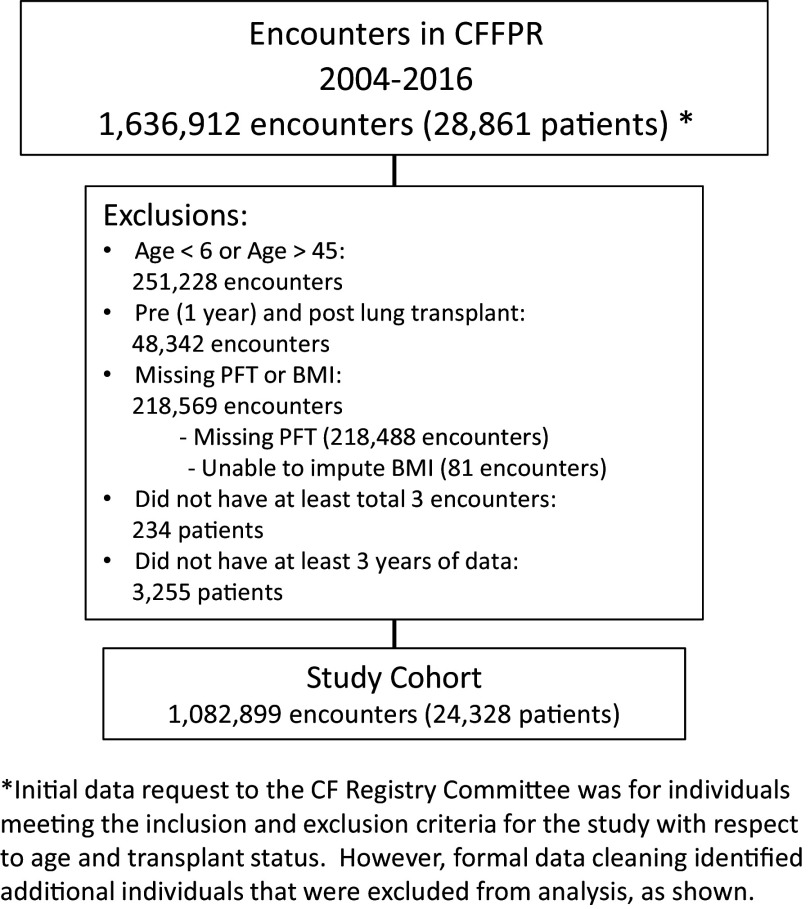
Subsequently, a second level of exclusions was applied at the encounter level, including visits lacking percentage predicted forced expiratory volume in 1 second (FEV1PP) data and body mass index (BMI) data that could not be imputed. Patients who had at least three encounter records across at least 3 years were included in our final cohort. A visual representation of our cohort selection process is provided in Figure 1, and a comparison of those included in our study and those excluded is provided in Table E2 in the data supplement.
Figure 1.
Description of the study cohort, derived from people with cystic fibrosis in the CFFPR between 2004 and 2016. The figure includes a description of patient-level and encounter-level exclusions. BMI = body mass index; CF = cystic fibrosis; CFFPR = Cystic Fibrosis Foundation Patient Registry; FEV1PP = percentage predicted forced expiratory volume in 1 second; PFT = pulmonary lung function using FEV1PP.
Exposure Definition
After patient exclusions (but before encounter exclusions), each encounter was categorized as following a period of continuous or discontinuous care. This was done before encounter-level exclusions to ensure that encounters where lung function or BMI was not recorded were not considered missed visits. Discontinuous care was defined as an absence of data in the CFFPR with respect to patient encounters for a span ⩾12 months in the primary analysis, although this gap length was varied in a sensitivity analysis. Continuous care was characterized by the absence of 12-month gaps in CFFPR data. Patients could have more than one discontinuous care episode during or outside of the study period. Encounter-level data from the CFFPR were used, which should capture clinic visits, hospitalizations, and home intravenous antibiotic treatment episodes.
A 12-month gap was chosen because it represents a common break point after which prescriptions and other therapies may not be refilled and would represent missing three consecutive appointments if following current CFF care center guidelines. It has also been used by other groups looking at gaps in CF care in other contexts (29). We performed sensitivity analyses with discontinuous care lengths of either 6 or 18 months.
Outcome Definition
Our outcome of interest, lung function, was calculated using the Global Lung Initiative FEV1PP at each clinical encounter (30).
Variables of Interest
Categorical variables included in our analysis were sex, genotype, race, ethnicity, underweight (based on BMI), insurance type, CFRD, and chronic infection status. Age at each encounter was included as a continuous variable. Chronic infections were defined as follows: P. aeruginosa: mucoid phenotype or three separate positive culture results (which did not have to be consecutive); MRSA: three separate positive culture results; Burkholderia spp.: two separate positive culture results. In all cases, patients were considered infected as of the date of the first positive culture result (see Table E1 for additional study definitions and details).
Statistical Analysis
Purely for the purposes of describing population demographics, we divided the cohort into those ever having a yearlong gap in care and those who did not. We summarized individual characteristics for fixed variables and “ever present” for time-varying covariates. For descriptive purposes, we also categorized the age ranges in which yearlong gaps were seen.
For our primary analysis, we used longitudinal mixed-effect semiparametric modeling of the relationship between care gap and FEV1PP with natural cubic splines for age with six knots at the quantiles and subject-specific random effects. We fit three such models including fixed covariates for race, ethnicity, sex, CFTR genotype, and time-varying covariates for insurance coverage; underweight status; CFRD status; and infection with P. aeruginosa, MRSA, and/or Burkholderia spp. To estimate the overall effect of 1 year or more of discontinuous care, we fit the first model as described with no additional interaction terms (Model 1). In our second model, we included an interaction term between age and care gap (Model 2), and in our third model, we included a three-way interaction between age, care gap, and genotype (Model 3).
We calculated the estimated FEV1PP for those with and without gaps for strata of covariates of interest by averaging and conditioning on covariates. Details of our modeling approach and R code can be found in the data supplement (see Model Specification and Code). We performed all analyses in R version 4.0.2 (R Core Team) (31), employing R packages gtsummary for table creation, nlme and splines for data analysis, and effects and ggplot2 for model predictions and visualizations.
Results
A total of 24,328 PwCF with 1,082,899 separate encounters in the CFFPR met criteria (Figure 1) for inclusion in our main longitudinal parametric model. Subjects entered the study at any point from January 1, 2004, to December 31, 2014. During the study window, 8,413 individuals experienced at least a single ⩾12-month gap in CF registry data, whereas 15,915 had continuous care (Table 1).
Table 1.
Descriptive characteristics of people with cystic fibrosis aged 6 to 45 years in the Cystic Fibrosis Foundation Patient Registry between 2004 and 2016, by continuity of care
| Characteristic | Continuity of Care |
|||
|---|---|---|---|---|
| Continuous (n = 15,915; 65%)* |
Discontinuous (n = 8,413; 35%)* |
Difference† | 95% CI† | |
| Demographics | ||||
| Female sex | 8,090 (51%) | 3,609 (43%) | 0.16 | 0.13, 0.19 |
| Race/ethnicity‡ | ||||
| Hispanic | 1,125 (7.3%) | 536 (6.6%) | 0.03 | 0.00, 0.05 |
| White | 15,111 (95%) | 7,842 (93%) | 0.07 | 0.05, 0.10 |
| Black or African American | 608 (3.8%) | 450 (5.3%) | −0.07 | −0.10, −0.05 |
| Asian | 81 (0.5%) | 52 (0.6%) | −0.01 | −0.04, 0.01 |
| Other race (AIAN, NHOPI, other) | 323 (2.0%) | 176 (2.1%) | 0.00 | −0.03, 0.02 |
| Age at CF diagnosis, yr | 0.4 (0.1, 2.6) | 0.6 (0.2, 3.7) | −0.17 | −0.20, −0.14 |
| Genotype | ||||
| F508del heterozygote | 5,909 (37%) | 3,220 (38%) | −0.02 | −0.05, 0.00 |
| F508del homozygote | 7,900 (50%) | 3,636 (43%) | 0.13 | 0.10, 0.16 |
| Other/unknown | 2,106 (13%) | 1,557 (19%) | −0.14 | −0.17, −0.12 |
| Died during study period | 1,239 (7.8%) | 438 (5.2%) | 0.10 | 0.08, 0.13 |
| Ever experienced | ||||
| Underweight | 5,498 (35%) | 2,730 (32%) | 0.04 | 0.02, 0.07 |
| Insurance coverage | ||||
| Private insurance | 13,109 (82%) | 6,977 (83%) | −0.01 | −0.04, 0.01 |
| Public insurance | 10,536 (66%) | 5,314 (63%) | 0.06 | 0.04, 0.09 |
| Other insurance | 1,909 (12%) | 1,303 (15%) | −0.10 | −0.13, −0.08 |
| Unknown insurance | 294 (1.8%) | 140 (1.7%) | 0.01 | −0.01, 0.04 |
| No insurance | 535 (3.4%) | 858 (10%) | −0.27 | −0.30, −0.25 |
| Chronic infection | ||||
| Pseudomonas aeruginosa | 10,527 (66%) | 5,519 (66%) | 0.01 | −0.01, 0.04 |
| MRSA | 5,956 (37%) | 2,467 (29%) | 0.17 | 0.15, 0.20 |
| Burkholderia spp. | 889 (5.6%) | 435 (5.2%) | 0.02 | −0.01, 0.04 |
| CF-related diabetes | 5,595 (35%) | 2,777 (33%) | 0.05 | 0.02, 0.07 |
Definition of abbreviations: AIAN = American Indian and Alaskan Native; CF = cystic fibrosis; CI = confidence interval; IHS = Indian Health Service; MRSA = methicillin-resistant Staphylococcus aureus; NHOPI = Native Hawaiian and other Pacific Islanders.
Descriptive characteristics of people with CF aged 6 to 45 years in the Cystic Fibrosis Foundation Patient Registry between 2004 and 2016 by continuity of care. The cohort is divided into those ever having a ⩾12-month yearlong gap in care and those who did not. Individual characteristics are summarized for fixed variables and “ever present” for time-varying covariates. Variable definitions are available in Table E1.
Median (interquartile range) or count (percent).
Standardized mean difference.
Individuals were able to endorse multiple race/ethnicity categories.
Basic Demographics
Descriptive demographics of the PwCF included in our model are shown in Table 1 (see Table E2 for characteristics of excluded patients). Patients with continuous care were more likely to be female, White, and underweight; to have CFRD; or to have chronic MRSA infection. They were also more likely to die during the study period. There were no significant between-group differences in occurrence of P. aeruginosa or Burkholderia spp. infection, and those with continuous care were more likely to be F508del homozygotes and less likely to have other/unknown genotype. PwCF with discontinuous care were also slightly older at the time of CF diagnosis.
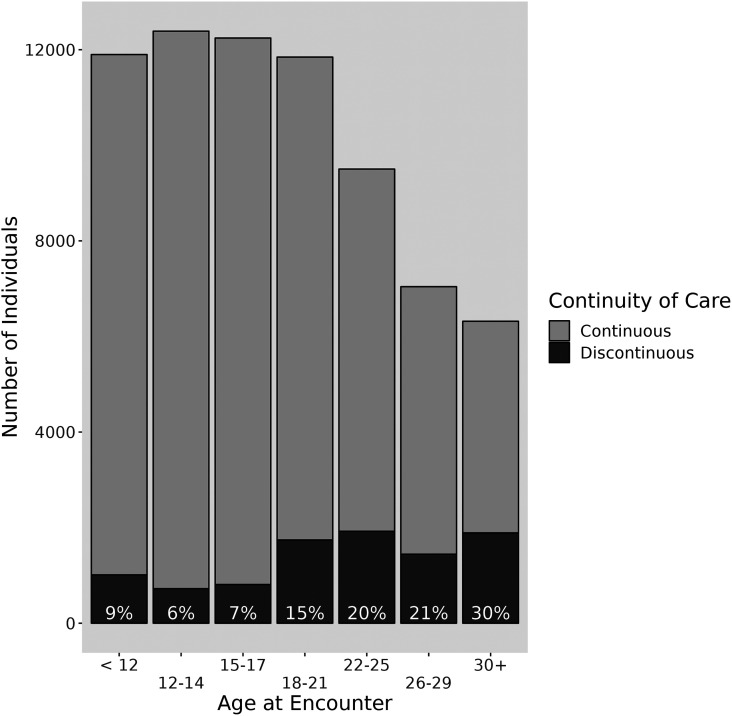
Age Distribution of Discontinuous Care
Periods of discontinuous care were more common in adults. Only 6–9% of those aged 6–17 years had documented care discontinuity; this rose to 15% in 18–21-year-olds and reached 30% in those over 30 years of age (Figure 2). Similarly, the proportion of encounters that occurred after ⩾12 months of discontinuous care tended to increase as PwCF aged (Table E3). Overall, 75.8% of all encounters preceded by a 12-month gap occurred in patients aged 18 years or older.
Figure 2.
Distribution of discontinuous care (⩾12-month gap) among people with cystic fibrosis by age group between 2004 and 2016. Reported proportions are restricted to each age stratum and do not reflect cumulative lifetime care continuity. Adapted from Reference (27).
Association of Discontinuous Care with Lung Function
PwCF with a preceding period of discontinuous care, on average, experienced a lower FEV1PP at their index visit than those who had continuous care (Model 1: absolute difference, −0.81%; 95% confidence interval [CI], −1.00, −0.61; P < 0.001). Model-derived FEV1PP values associated with continuous and discontinuous care for selected ages are presented in Tables E4A and E4B. Table 2 shows covariate estimates from Model 1, which demonstrates significant differences in lung function associated with other factors previously shown to associate with lung function in large cohorts. Consistent with prior literature, we found an association between MRSA and Burkholderia spp. infection and decreased lung function, though this effect was not seen with P. aeruginosa infection in our model (see Table E5 for details).
Table 2.
Multivariable analysis of the association with lung function (FEV1PP) in people with cystic fibrosis
| Variable | Estimate | Confidence Interval | P Value |
|---|---|---|---|
| Discontinuous care | −0.81 | −1.00, −0.61 | <0.001 |
| Race/ethnicity* | |||
| Hispanic | −5.93 | −6.91, −4.95 | <0.001 |
| White | 1.20 | −1.12, 3.53 | 0.3 |
| African American | −0.88 | −3.16, 1.40 | 0.4 |
| Other race | 4.83 | 2.54, 7.12 | <0.001 |
| Genotype | |||
| F508del heterozygotes | Reference | ||
| F508del homozygotes | −2.82 | −3.35, −2.28 | <0.001 |
| Other or unknown mutation | 0.26 | −0.50, 1.01 | 0.5 |
| Male sex | 0.72 | 0.23, 1.20 | 0.004 |
| Insurance coverage | −1.02 | −1.08, −0.95 | <0.001 |
| Private insurance/military/parent’s insurance | Reference | ||
| Medicare/Medicaid/state program/IHS | −0.84 | −0.92, −0.76 | <0.001 |
| Other insurance | −0.84 | −1.15, −0.53 | <0.001 |
| No insurance | −0.09 | −0.37, 0.19 | 0.5 |
| Unknown insurance status | −1.20 | −1.40, −1.01 | <0.001 |
| Underweight BMI | −8.20 | −8.29, −8.11 | <0.001 |
| CF-related diabetes | −3.12 | −3.21, −3.03 | <0.001 |
| Chronic infection | |||
| Pseudomonas aeruginosa | 0.14 | 0.04, 0.24 | 0.004 |
| MRSA | −1.19 | −1.28, −1.09 | <0.001 |
| Burkholderia spp. | −1.98 | −2.19, −1.76 | <0.001 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CFFPR = Cystic Fibrosis Foundation Patient Registry; FEV1PP = percentage predicted forced expiratory volume in 1 second; IHS = Indian Health Service; MRSA = methicillin-resistant Staphylococcus aureus.
Individuals were able to endorse multiple race/ethnicity categories. Estimates are from Model 1 (multivariable semiparametric regression analysis) which includes 24,328 individuals with cystic fibrosis in the CFFPR between 2004 and 2016.
Interactions between Genotype and Age and Care Continuity
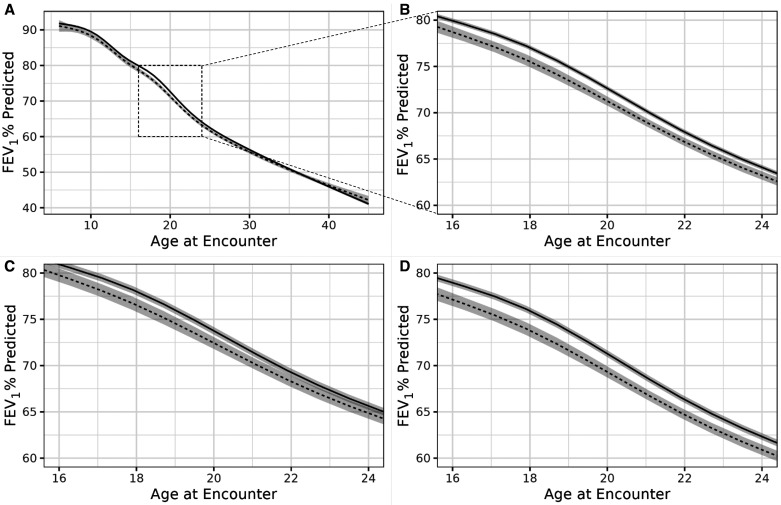
In an effort to explore potential interactions with selected nonmodifiable patient characteristics, we next examined modification of the relationship between discontinuous care and FEV1PP by age and genotype. Effect modification by age (Model 2) was minimal in preadolescents and adults over 30 years of age and more pronounced in adolescence and early adulthood (Figures 3A and 3B), where a clinically meaningful difference in FEV1PP became evident at the first appointment after a prolonged gap in care. For example, at age 16, there was a 1.2% (95% CI, −0.7, −1.8) decrease in FEV1PP associated with discontinuous care. There was a peak difference of 1.5% (95% CI, −1.0, −2.1) in FEV1PP between 18-year-olds with and without discontinuous care (Table E4A), the magnitude of which approximates the average annual rate of lung function decline in the United States in this age range (32). By age 28, the difference in overall lung function between groups declined to 0.7% (95% CI, −0.4, −1.1) FEV1PP.
Figure 3.
Estimated percentage predicted forced expiratory volume in 1 second (FEV1PP) values associated with continuous (solid line) and discontinuous (dashed line) care by age among 24,328 individuals with cystic fibrosis in the Cystic Fibrosis Foundation Patient Registry between 2004 and 2016. Shaded area indicates 95% confidence bounds. Estimated FEV1PP values are given for a typical person (i.e., at average values for covariates). (A) Estimated FEV1PP curves for ages 6 to 45 (Model 2). (B) Enlarged estimated FEV1PP curve showing ages 15–25 years (Model 2). (C) Estimated FEV1PP curves showing ages 15–25 years for F508del homozygotes (Model 3). (D) Estimated FEV1PP curves showing age 15–25 for F508del heterozygotes (Model 3). Adapted from Reference (27).
We next examined interactions of CFTR genotype and age with the relationship between lung function and care continuity (Model 3). The previously identified differences in lung function between continuous and discontinuous care in adolescence and early adulthood were magnified in F508del homozygotes compared with heterozygotes (Figures 3C and 3D). At age 10, there was a significant decrease in FEV1PP associated with discontinuous care of −1.7% (95% CI, −0.9, −2.4). The magnitude of this difference was much greater (−2.1%; 95% CI, −1.5, −2.7) in young adulthood, where, on average, an 18-year-old F508del homozygote with continuous care versus discontinuous care would have an FEV1PP of 75.9% (95% CI, 75.6%, 76.3%) and 73.8% (95% CI, 73.1%, 74.5%), respectively (Table E4B).
Sensitivity Analysis
We looked at the effect of varying the gap duration in our discontinuous care definition from 6 months to 18 months as a sensitivity analysis. Longer gaps in care were associated with larger absolute differences in lung function than those with continuous care. A 6-month gap duration was associated with −0.21 FEV1PP (95% CI, −0.31, −0.11; P < 0.001), a 12-month gap with −0.81 FEV1PP (95% CI, −0.61, −1.00; P < 0.001), and an 18-month gap with −1.17 FEV1PP (95% CI, −0.88, −1.47; P < 0.001).
Discussion
Our analysis of encounters recorded in the CFFPR shows that 35% of patients analyzed had at least one significant gap in care and that there was a pattern of gaps in care that increased with age, despite long-standing recommendations by the CFF for visits four times per year and intense nationwide quality improvement monitoring of contact frequency of PwCF at accredited centers. Of note, prior research has shown rates similar to ours of yearlong gaps in young adults (29), although there is not extensive reporting about this in other age groups.
Not surprisingly, gaps are far more common in adult PwCF, many of whom must balance competing responsibilities with the time and cost of healthcare appointments. We found that the frequency of study-defined care discontinuity rose to 30% in those over age 30. This may be partially explained by the loss of those with severe genotypes to death and lung transplant, resulting in enrichment of older cohorts with those having milder genotypes (28). A sizable fraction of these people may believe that they do not need frequent clinic visits.
Higher percentages of PwCF who are underweight, have CFRD, have chronic MRSA infection, and have died during the study period in the continuous care versus discontinuous care groups likely reflect the need for more frequent monitoring of complex medical problems. Older average age at diagnosis in those with discontinuous care may relate to the different genetic mix within the two care cohorts because milder genotypes are more frequently diagnosed at a later age.
Our analysis incorporates the aforementioned factors and shows a significant association between discontinuous care and reduced lung function at follow-up (index) visits, especially during late adolescence and young adulthood. This relationship was more pronounced in F508del homozygotes than in heterozygotes or those with other mutations. Although the absolute magnitude of lung function reduction attributable to these gaps in care was modest, in young adult F508del homozygotes, it is similar to the annual decrement in lung function historically observed in many CF populations (8, 32) (Table E4B).
Our analysis confirms associations between reduced lung function and factors such as older age, non-White race and ethnicity, female sex, malnutrition, severe genotype, CFRD, nonprivate insurance coverage, and certain chronic infections. Our evaluation detected negative correlations of lung function with both MRSA and Burkholderia spp. infections but did not find a negative association with P. aeruginosa infection. It is possible that aggressive treatment of P. aeruginosa with chronic suppressive medications after approval of inhaled tobramycin in 1998 has abrogated the injurious effects of P. aeruginosa respiratory infection.
Analyses of the ESCF registry examined factors associated with higher-performing CF centers, as judged by median FEV1, and found that higher-performing centers had more frequent office visits (25, 26). Although these important findings have served as a basis for the CFF recommendations, it is important to note their limitations. Both studies simplify center-based FEV1PP into quartiles, a study design choice that assumes homogeneity of risk within groups and can lead to inaccurate estimation (33). In addition, these studies used Mantel-Haenszel and Wilcoxon rank-sum tests to evaluate the association between FEV1PP and visit frequency, methods that do not control for important confounding causes of decreased lung function. Our study seeks to answer a similar question but uses the encounter-level data in the CFFPR, spans a much longer time frame, uses a richer dataset, and adjusts for confounding causes of lung function decline.
There are multiple reasons for gaps in care, and those factors are likely different in pediatric patients, adult patients, and those transitioning from pediatric to adult programs. Pediatric–adult transition is a critical period and occurs in our model during the interval of greatest decrement in lung function associated with discontinuous care. This raises the possibility that transition itself may have an independent effect on lung function. A study by Sawicki and colleagues, using data from the CFFPR over a time period similar to our study, investigated risk factors for prolonged gaps in care among PwCF transitioning from pediatric to adult care and found that those within a cohort with prolonged gaps (⩾1 yr), without adjustment for genotype, had a similar median FEV1PP before transition to those with shorter gaps (29). In contrast to this study which evaluated if lower FEV1PP predicts a longer gap in care, our study assessed whether gaps in care predict lower FEV1PP after the gap. That our model shows a significant difference in lung function associated with prolonged care gaps in 10-year-old F508del homozygotes, long before the usual transition period, supports the concept that discontinuous care is an independent contributor to reduced lung function. Detailed assessment of the impact of pediatric-to-adult transition warrants further study.
Strengths and Limitations
A strength of our analysis is the large size of the dataset, including 13 years of encounter-based information from the CFFPR, comprising more than 24,000 PwCF and more than 1 million clinical encounters. We identified the covariates included in our model a priori on the basis of previous research showing an association with both our exposure (gaps in care) and our outcome (FEV1PP) (34). Finally, our use of a longitudinal random effects model with time-varying covariates helps accommodate repeated measures, incomplete data, and the combination of discrete and continuous factors that vary over time, which increases our confidence in the validity of our models.
An important limitation of our approach is its retrospective nature and its reliance on data entered in the CFFPR. Lack of data in the registry is not equivalent to absence of care. While the CFF and care centers make every effort to collect information, data may not be correctly entered, and some people may seek and not report care outside the care center system. Care that was not recorded in the registry, however, would tend to bias our analysis toward finding no difference. We also did not include patterns of medication use in our models, because the CFFPR only records medication prescription data, not pharmacy refill or use data. We therefore cannot exclude the contribution of reduced CF medical therapy to the association between fragmented care and lower lung function.
The CFFPR requires consent (obtained initially from parents for minors) and reconsent at age 18; PwCF who decline participation in the registry at the time of reconsent would show a loss of data after the age of 18 years. However, the CFFPR reportedly contains 90–95% of clinic visits and hospitalizations in the medical record, and the demographic, anthropometric, and microbiologic data contained in the CFFPR (2) have accuracies of 95.2–99.9% (2). The accuracy of insurance and other socioeconomic status data has not been examined, however, and is likely much lower.
Another important limitation of our approach is that we estimate the immediate impact of gaps in care on lung function. Therefore, further analysis would be needed to draw conclusions about the effect of repeated gaps or to provide information on the long-term effects of discontinuous care. As with any retrospective statistical analysis, we cannot prove a causal link between exposure (care discontinuity) and outcome (lung function), although the “dose–response” relationship between the length of discontinuous care and the magnitude of the corresponding difference in lung function is consistent with such a link.
Our results are based on data through 2016 and therefore will not reflect recent trends, such as Food and Drug Administration approval of elexacaftor-tezacaftor-ivacaftor therapy in late 2019 or the coronavirus disease (COVID-19) pandemic starting in early 2020. In particular, CFTR modulator therapy may reduce the interaction of genotype on the effect of care gaps. This period also did not permit us to evaluate the effects of the widespread transition to telehealth care, which came about during the COVID-19 pandemic, on our primary outcome. However, dramatic shifts in patient outcomes due to these recent changes will complicate any assessment of the effects of gaps in care, and our results will serve as a baseline for future studies of the effect of these events on care continuity and its impact on lung function.
Our findings are provocative in the context of rising healthcare costs and increasing use of remote approaches to health maintenance. Because of the degree of education and training necessary to gain mastery of self-care of this complex disease, emphasis on continued access of PwCF to specialists with CF expertise is critically important, particularly as PwCF enter late adolescence and early adulthood. This concept is supported by the degree of reduction in lung function we observed with discontinuous care when young PwCF move into young adulthood. The lesser effect of prolonged gaps in care in those with milder genotypes may also have implications moving forward. The vast majority of PwCF who are receiving modulator therapy should begin to exhibit milder phenotypes that mimic those of people born with milder genotypes. Our data suggest that there may be opportunities to further tailor the frequency of CF center encounters in those who respond well to modulator therapy. We expect that PwCF will continue to need more frequent follow-up than the general population, but there should be room for a more personalized approach to scheduling, calibrated by weighted individual risk factors and trajectory of disease progression.
Conclusions
In summary, our analysis demonstrates that gaps in care ⩾12 months are associated with lower lung function in the general CF population, even after controlling for other known factors associated with pulmonary compromise. The effect is most pronounced in late adolescence and early adulthood in those who are homozygous for the F508del CFTR mutation. Our findings also warrant reassessment as we enter the era of advanced therapies for the disease.
Acknowledgments
Acknowledgment
The authors thank the CFF for the use of CFFPR data to conduct this study. In addition, the authors thank the people with CF, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CFFPR. The authors thank Rhonda Szczesniak, Ph.D., of Cincinnati Children’s Hospital, for reviewing a copy of the manuscript and providing input. The authors also thank Bernard F. Cole, Ph.D., of the University of Vermont for assistance with preliminary analysis of the data, supported by grant U54 GM115516 from the National Institutes of Health for the Northern New England Clinical and Translational Research Network, as well as John Dziodzio for his preliminary data analyses.
Footnotes
Early work on this paper was supported by a Cystic Fibrosis Foundation PACE Award (SEARS14AC0).
Data sharing statement: Data are available upon request through the Cystic Fibrosis Foundation Patient Registry Comparative Effectiveness Research Committee. The committee can be contacted at datarequests@cff.org.
Author Contributions: E.H.S.: conceptualization, writing – original draft preparation, supervision, writing – reviewing and editing. A.C.H.: methodology, data analysis, writing – reviewing and editing. S.L.-P.: methodology, supervision. C.W.L.: methodology, supervision, writing – reviewing and editing. J.B.Z.: conceptualization, writing – reviewing and editing, supervision.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med . 1992;326:1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 2. Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc . 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation. 2021. https://www.cff.org/managing-cf/cf-care-center-visits#:~:text=CF%20Foundation%20clinical%20care%20guidelines,tests%20(PFTs)%20per%20year
- 4. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest . 2004;125:1S–39S. doi: 10.1378/chest.125.1_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 5. Schaedel C, de Monestrol I, Hjelte L, Johannesson M, Kornfält R, Lindblad A, et al. Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol . 2002;33:483–491. doi: 10.1002/ppul.10100. [DOI] [PubMed] [Google Scholar]
- 6. Sanders DB, Li Z, Laxova A, Rock MJ, Levy H, Collins J, et al. Risk factors for the progression of cystic fibrosis lung disease throughout childhood. Ann Am Thorac Soc . 2014;11:63–72. doi: 10.1513/AnnalsATS.201309-303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cogen J, Emerson J, Sanders DB, Ren C, Schechter MS, Gibson RL, et al. EPIC Study Group Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr Pulmonol . 2015;50:763–770. doi: 10.1002/ppul.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Szczesniak RD, McPhail GL, Duan LL, Macaluso M, Amin RS, Clancy JP. A semiparametric approach to estimate rapid lung function decline in cystic fibrosis. Ann Epidemiol . 2013;23:771–777. doi: 10.1016/j.annepidem.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 9. Taylor-Robinson D, Whitehead M, Diderichsen F, Olesen HV, Pressler T, Smyth RL, et al. Understanding the natural progression in %FEV1 decline in patients with cystic fibrosis: a longitudinal study. Thorax . 2012;67:860–866. doi: 10.1136/thoraxjnl-2011-200953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Konstan MW, Wagener JS, Vandevanter DR, Pasta DJ, Yegin A, Rasouliyan L, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros . 2012;11:405–411. doi: 10.1016/j.jcf.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vandenbranden SL, McMullen A, Schechter MS, Pasta DJ, Michaelis RL, Konstan MW, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Lung function decline from adolescence to young adulthood in cystic fibrosis. Pediatr Pulmonol . 2012;47:135–143. doi: 10.1002/ppul.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liou TG, Elkin EP, Pasta DJ, Jacobs JR, Konstan MW, Morgan WJ, et al. Year-to-year changes in lung function in individuals with cystic fibrosis. J Cyst Fibros . 2010;9:250–256. doi: 10.1016/j.jcf.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr . 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 14. Kerem E, Corey M, Kerem BS, Rommens J, Markiewicz D, Levison H, et al. The relation between genotype and phenotype in cystic fibrosis—analysis of the most common mutation (ΔF508) N Engl J Med . 1990;323:1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 15. Schibler A, Bolt I, Gallati S, Schöni MH, Kraemer R. High morbidity and mortality in cystic fibrosis patients compound heterozygous for 3905insT and deltaF508. Eur Respir J . 2001;17:1181–1186. doi: 10.1183/09031936.01.00034601. [DOI] [PubMed] [Google Scholar]
- 16. Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr . 2007;151:134–139.e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17. Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med . 2008;178:814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 18. Kerem E, Corey M, Gold R, Levison H. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr . 1990;116:714–719. doi: 10.1016/s0022-3476(05)82653-8. [DOI] [PubMed] [Google Scholar]
- 19. McPhail GL, Acton JD, Fenchel MC, Amin RS, Seid M. Improvements in lung function outcomes in children with cystic fibrosis are associated with better nutrition, fewer chronic Pseudomonas aeruginosa infections, and dornase alfa use. J Pediatr . 2008;153:752–757. doi: 10.1016/j.jpeds.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 20. Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF Quality Assurance (CFQA) project. Thorax . 2002;57:596–601. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr . 2013;162:530–535.e1. doi: 10.1016/j.jpeds.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 22. Tumin D, Crowley EM, Li SS, Wooten W, Ren CL, Hayes D., Jr Patterns of health insurance coverage and lung disease progression in adolescents and young adults with cystic fibrosis. Ann Am Thorac Soc . 2021;18:290–299. doi: 10.1513/AnnalsATS.201911-839OC. [DOI] [PubMed] [Google Scholar]
- 23. Stephenson AL, Sykes J, Stanojevic S, Quon BS, Marshall BC, Petren K, et al. Survival comparison of patients with cystic fibrosis in Canada and the United States: a population-based cohort study. Ann Intern Med . 2017;166:537–546. doi: 10.7326/M16-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med . 2001;163:1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 25. Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest . 2003;123:20–27. doi: 10.1378/chest.123.1.20. [DOI] [PubMed] [Google Scholar]
- 26. Padman R, McColley SA, Miller DP, Konstan MW, Morgan WJ, Schechter MS, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Infant care patterns at epidemiologic study of cystic fibrosis sites that achieve superior childhood lung function. Pediatrics . 2007;119:e531–e537. doi: 10.1542/peds.2006-1414. [DOI] [PubMed] [Google Scholar]
- 27. Sears E, Hinton AC, Lary C, Zuckerman JB. Association between gaps in care and lung function decline in the U.S. CF foundation patient registry [abstract] J Cyst Fibros . 2021;20:S15–S16. [Google Scholar]
- 28. Rodman DM, Polis JM, Heltshe SL, Sontag MK, Chacon C, Rodman RV, et al. Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med . 2005;171:621–626. doi: 10.1164/rccm.200403-404OC. [DOI] [PubMed] [Google Scholar]
- 29. Sawicki GS, Ostrenga J, Petren K, Fink AK, D’Agostino E, Strassle C, et al. Risk factors for gaps in care during transfer from pediatric to adult cystic fibrosis programs in the United States. Ann Am Thorac Soc . 2018;15:234–240. doi: 10.1513/AnnalsATS.201705-357OC. [DOI] [PubMed] [Google Scholar]
- 30. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3–95-yr age range: the Global Lung Function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 32. Schlüter DK, Ostrenga JS, Carr SB, Fink AK, Faro A, Szczesniak RD, et al. Lung function in children with cystic fibrosis in the USA and UK: a comparative longitudinal analysis of national registry data. Thorax . 2022;77:136–142. doi: 10.1136/thoraxjnl-2021-216849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol . 2012;12:21. doi: 10.1186/1471-2288-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc . 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]