Abstract
Background:
The COVID-19 pandemic prompted researchers to develop new ways to design and launch studies and recruit and retain participants. Pregnant women and infants are considered vulnerable populations in research, and families affected by substance use are particularly difficult to recruit and retain. Recruitment for studies involving medical technologies such as MRI can also be difficult due to misconceptions and fear of the technologies.
Objectives:
To describe “lessons learned” during the launch of the Outcomes of Babies with Opioid Exposure (OBOE) study, including successes and challenges when working with high-risk infants and families and the importance of engaging participants through recruitment materials and retention efforts.
Methods:
The OBOE study is a multisite prospective longitudinal cohort study comparing infants with antenatal opioid exposure to unexposed controls from birth to 2 years of age. Chi-square tests were used to examine refusal reasons among caregivers of eligible infants by exposure group and differences in 6-month retention among subgroups based on social determinants of health.
Results:
Four factors were essential in establishing the Consortium, implementing the study, and retaining participants: (a) creating venues for collaboration, (b) pivoting from in-person to virtual training, (c) anticipating potential enrollment barriers and addressing them directly, and (d) engaging participants through recruitment materials and retention efforts. With these factors in place, only 5% of caregivers of eligible opioid-exposed infants and 8% of control infants declined to participate in the study because of MRIs. Of 310 enrolled infants, 234 infants had attended the 6-month visit. Subgroups of enrolled infants were similar in retention at 6 months.
Discussion:
Reporting our successes and challenges in setting up a nationwide consortium during the pandemic may help other consortia that need to be set up virtually. We anticipated that the serial MRIs would be a barrier to participation; however, few indicated they refused to participate because of MRIs, suggesting our efforts to address this potential barrier to enrollment were successful.
Keywords: COVID-19, longitudinal study, opioid crisis, recruiting
As the coronavirus disease 2019 (COVID-19) global pandemic became the primary focus of the world’s attention, the opioid crisis continued to adversely affect pregnant women and infants across the United States. Just prior to the COVID-19 pandemic, the maternal opioid-related diagnosis rate increased from 3.5 in 2010 to 8.2 per 1,000 delivery hospitalizations in 2017 (Haight et al., 2018; Hirai et al., 2021). Consequently, the risk for adverse maternal and infant outcomes such as preterm birth, neonatal opioid withdrawal syndrome (NOWS), low birth weight, and complications related to delivery also increased among this vulnerable population (Centers for Disease Control and Prevention, 2021; Patrick et al., 2017; Whiteman et al., 2014). One Level III neonatal intensive care unit (NICU) in Tennessee observed an increase in antenatal fentanyl exposure among substance-exposed infants during the pandemic (January 2020 to April 2021) compared to pre-pandemic (January 2018 to December 2019), including an increase in fentanyl use among mothers receiving medication-assisted treatment (Lien et al., 2023). There is currently a limited amount of information available on the intersection between maternal opioid use, the COVID-19 pandemic, and neonatal outcomes.
Beginning in March 2020, the pandemic forced the scientific community to change substantially in order to continue conducting important research. To advance science and support vulnerable populations, researchers and clinical care providers adapted to using alternative approaches for collaboration. This article focuses on establishing the Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome (ACT NOW) Longitudinal Study Consortium and launching the Outcomes of Babies with Opioid Exposure (OBOE) study through virtual meetings and training webinars, highlighting efforts to maximize participant recruitment and retention during the COVID-19 pandemic. We describe the “lessons learned” and successes with working with this population of high-risk infants and their families, the importance of engaging participants through recruitment materials and retention efforts, and 6-month retention rates for subgroups based on social determinants of health. We provide details on our experience, including successes and challenges, so other consortia that need to be set up virtually can build upon our strategies.
Methods
Study Overview
The ACT NOW Longitudinal Study Consortium consists of four clinical sites (Case Western Reserve University, Children’s Hospital of Pennsylvania, Cincinnati Children’s Hospital Medical Center [CCHMC], and the University of Alabama at Birmingham), a data coordinating center (DCC) at RTI International—a Neuroimaging Core at Children’s National Hospital—and a steering committee chair (Clinical Trials.gov NCT04149509). The Consortium was funded in September 2019 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) through the Helping to End Addiction Long-term (HEAL) initiative. The OBOE study protocol is described elsewhere in detail (Bann et al., 2023). Briefly, the OBOE study is a multisite prospective longitudinal cohort study of infants with antenatal opioid exposure and controls (unexposed infants) from birth to 2 years of age. The primary objectives are to examine the effect of antenatal opioid exposure on brain development and neurodevelopmental outcomes over the first 2 years of life and explore whether family, home, and community factors modify developmental trajectories. The study consists of three in-person MRI visits at 0–1 month, 6 months, and 24 months; one home visit at 12 months; one telephone interview at 18 months; and developmental testing at 12 and 24 months. This paper focuses on retention at the first follow-up time point in visiting series (6 months), given enrollment was completed in December 2023 and the full cohort had aged out of the 6- to 8-month follow-up window when this paper was written in August 2024. Not enough infants aged out of the subsequent visit time points (e.g., 12, 18, and 24 months) to include in this analysis due to the time it took (August 2020 to December 2023) to enroll in the complete cohort.
Participant Recruitment
Infants were prescreened at birth by the research coordinator in birth hospitals using the electronic medical record, and parents/guardians of eligible infants were approached for participation. To address slower-than-anticipated enrollment, two clinical sites approached pregnant women before birth to tell them about the study. Once eligibility was confirmed at birth, as Bann et al. (2023) detailed, research coordinators obtained written informed consent.
Consent and Enrollment
Through a single institutional review board at CCHMC, all four contributing clinical sites, the Neuroimaging Core, and the DCC at RTI received approval for human subjects research activities for this study, and written informed consent was obtained for all participants. For those who refused to participate, study coordinators asked the reason for refusal and documented the response on the study’s eligibility form.
Measures
Retention
Infants were considered retained at 6 months if they were seen for the 6-month follow-up visit.
Food Insecurity
Food insecurity was assessed as an indicator of socioeconomic status at 6-month intervals. Families screened positive for food insecurity if the mother/caregiver responded “often true” or “sometimes true” to either or both statements: “During the past 6 months, (a) we worried whether our food would run out before we got money to buy more,” and (b) “The food we bought just didn’t last, and we didn’t have money to get more” (American Academy of Pediatrics, 2021). Clinical site coordinators provided referrals to caregivers if they screened positive for food insecurity.
Depression
The Patient-Reported Outcomes Measurement Information System (PROMIS) measures were administered directly to the mother (or primary caregiver if the infant was not in the mother’s custody) to assess caregiver well-being and mental health (Cella et al., 2010). Participants’ responses resulting in a standard t-score > 70 on the depression scale indicated that the participant was above the clinically significant threshold for depression symptoms, and the clinical site took prompt action to refer/triage mothers for maternal mental health concerns.
Statistical Analysis
All analyses were performed using SAS (Version 9.4). Chi-square tests were used to examine (a) refusal reasons among caregivers of eligible infants by exposure group, and (b) differences in 6-month retention among subgroups based on social determinants of health.
Results
We established the Consortium remotely and changed during the virtual launch and conduct of the study to adapt to challenges of the COVID-19 pandemic. Our first Steering Committee meeting and first Scientific and Safety Monitoring Committee (SSMC) meeting to review the final protocol and informed consent form were held virtually in December 2019 within 3 months of the award to accommodate clinicians on service schedules. Rapid development and approval of a Consortium policy and procedures document and an SSMC charter provided a framework for these governing bodies to operate.
The first infant was enrolled into the OBOE study in August 2020 within the first year of funding with only a 6-month delay in study start despite clinic closures, social distancing measures, and local institutional restrictions in conducting research-only visits in 2020 as a result of the COVID-19 pandemic. Enrollment was completed in December 2023 with 310 infants enrolled (207 opioid-exposed infants; 103 unexposed infants).
Demographic characteristics of participants are shown in Table 1. Most participants had opioid exposure, were discharged to their mother, had public insurance, were aged 30 years or older (maternal age), were not married, spent more than 50% of their income on housing, were White and multiparous, and lived in households with four people or fewer.
Table 1. Demographic Characteristics of Infants Enrolled in the Study by Exposure Group (N=310).
| Characteristic | Opioid Exposed N (%) |
Control N (%) |
|---|---|---|
| Maternal age | ||
| <25 | 21 (10.2) | 25 (24.3) |
| 25–29 | 60 (29.1) | 33 (32.0) |
| 30–34 | 81 (39.3) | 27 (26.2) |
| ≥35 | 44 (21.4) | 18 (17.5) |
| Race | ||
| White | 168 (82.0) | 69 (68.3) |
| Non-White | 37 (18.0) | 32 (31.7) |
| Maternal marital status | ||
| Married | 22 (11.0) | 31 (31.3) |
| Not married | 178 (89.0) | 68 (68.7) |
| Maternal education | ||
| Less than high school | 44 (22.0) | 6 (6.0) |
| High school diploma | 77 (38.5) | 35 (35.0) |
| More than high school | 79 (39.5) | 59 (59.0) |
| Public insurance | ||
| Yes | 189 (92.6) | 78 (77.2) |
| No | 15 (7.4) | 23 (22.8) |
| Parity | ||
| 1 | 32 (15.5) | 35 (34.0) |
| 2+ | 174 (84.5) | 68 (66.0) |
| Discharged to mother | ||
| Yes | 159 (77.2) | 103 (100.0) |
| No | 47 (22.8) | 0 (0.0) |
| Food insecurity (during pregnancy) | ||
| Yes | 66 (34.0) | 14 (14.1) |
| No | 128 (66.0) | 85 (85.9) |
| Spending excessive (> 50%) income on housing costs (0–1 month) | ||
| Yes | 113 (64.2) | 51 (53.7) |
| No | 63 (35.8) | 44 (46.3) |
| Number of people in household (0–1 month) | ||
| 5 or more | 78 (40.6) | 40 (40.4) |
| 4 or fewer | 114 (59.4) | 59 (59.6) |
| Moved in last 6 months (0–1 month) | ||
| Yes | 38 (19.8) | 26 (26.3) |
| No | 154 (80.2) | 73 (73.7) |
| Child and Family Services involvement (0–1 month) | ||
| Yes | 87 (45.3) | 4 (4.0) |
| No | 105 (54.7) | 95 (96.0) |
NOTE. The following variables have missing values: maternal age (n=1), race (n=4), marital status (n=11), education (n=10), public insurance (n=5), and parity (n=1), discharge to mother (n=1), food insecurity(n=17), excessive spending (n=39), number in household (n=19), moved in last 6-months (n=19), child and family services involvement (n=19).
Lessons Learned
Throughout this process, we found four factors to be necessary to establishing the Consortium, developing the protocol, implementing the study, and retaining participants (Figure 1), described in detail below.
Figure 1.
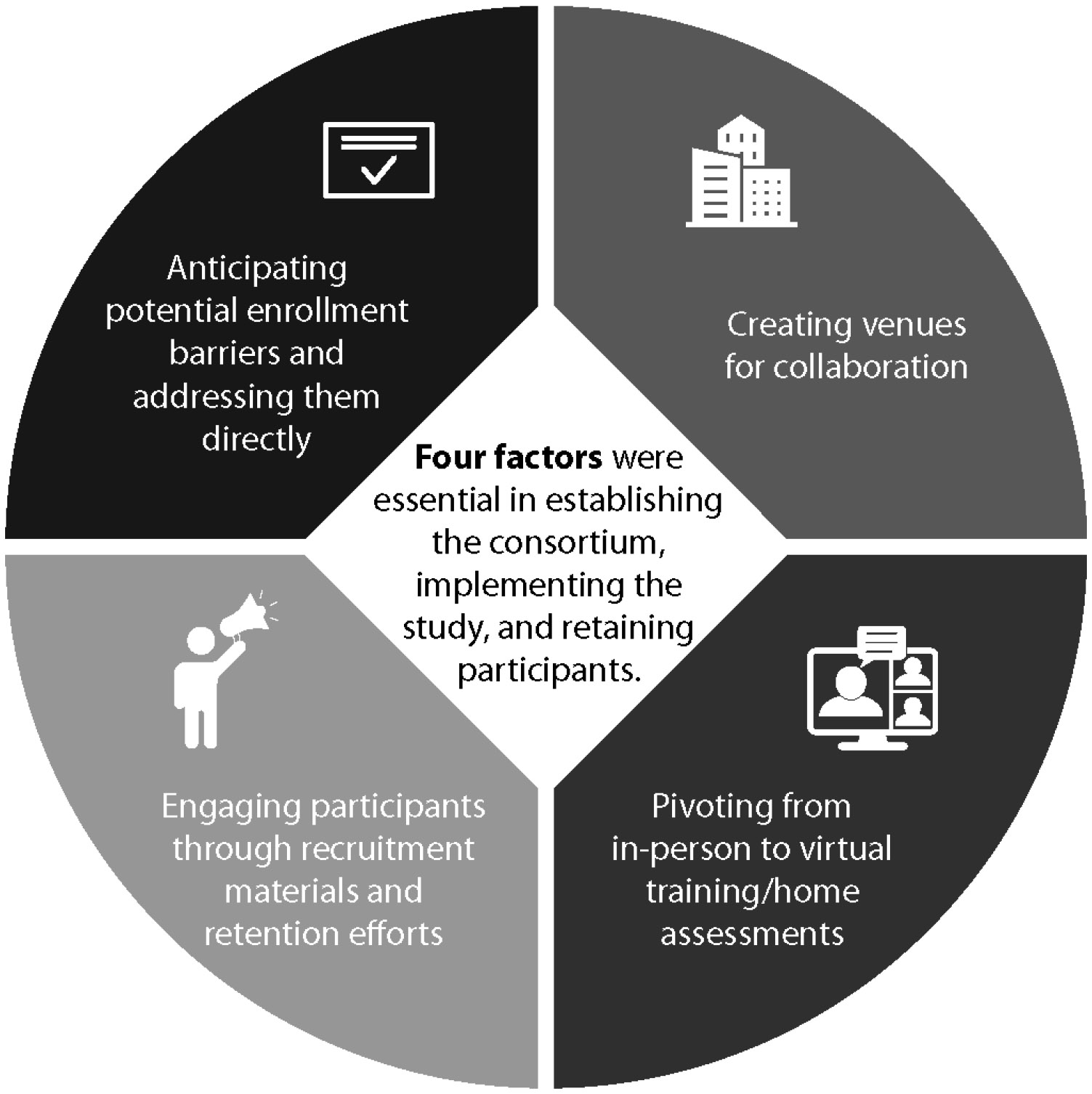
Four Factors Essential to the OBOE Study
Creating Venues for Collaboration
Creating venues for collaboration was vital to establishing the Consortium, developing the protocol, and conducting the study. Meetings were held via videoconference, and participants were encouraged to have their cameras on to enhance communication through nonverbal cues, promote active listening, and discourage multitasking. Whenever possible during virtual meetings, agenda topics were presented by different investigators and staff members, instilling ownership in topics and enhancing engagement during meetings. The clinical site coordinators nominated a coordinator liaison to assist with setting meeting agendas and to provide coordinator updates to the steering committee. This was important to ensure that the coordinator’s perspective was included when Consortium decisions were being made. The steering committee—consisting of the principal investigators from the four clinical sites, DCC, Neuroimaging Core, an independent chair, and funding agency (NICHD)—met twice a month via videoconference, providing a venue for sites to discuss challenges/obstacles and share ideas/strategies so that solutions could be collectively developed. In addition, the site MRI teams met monthly via videoconference with the Neuroimaging Core and the DCC to share their experiences and provide ongoing communication with MRI staff. The OBOE site clinical coordinators met monthly via videoconference to address frequently asked questions and share their experiences recruiting and conducting the study visits with their counterparts at other sites. Coordinator meetings facilitated swift troubleshooting of common roadblocks in recruitment and implementing study procedures at the clinical sites.
OBOE Study Website
The study website consisted of two parts. A members-only portion of the OBOE study website served as a communication and collaboration hub for research staff, facilitating cross-site communication, document sharing, consensus for Consortium management, and multisite study implementation. The public website served as a gateway and provided details about the study, requirements for participating, and answers to frequently asked questions. Print materials designed for participants typically included a quick response (QR) code that directed them to the public website to obtain more information about the study.
Pivoting from In-Person to Virtual Training and Virtual Home Assessments
As COVID-19 travel restrictions and social distancing measures precluded in-person training, we rapidly shifted to holding virtual training webinars and certifying examiners via recorded certification videos. Curriculum modifications were required to support remote learning of the NICU Network Neurobehavioral Scale, Second Edition (NNNS–II; Lester et al., 2004), which had traditionally required in-person training and certification. We conducted a series of interactive training webinars with one-on-one support from expert trainers as examiners recorded themselves performing exams. Trainers reviewed videos and debriefed with examiners after each recorded practice exam by sharing detailed feedback and troubleshooting challenges.
Having a virtual option for the home visit was a necessary component for infants aging out of the 12- to 14-month window during the COVID-19 surge from December 2021 to February 2022. We incorporated the virtual version of Home Observation for Measurement of the Environment (HOME; Caldwell & Bradley, 2003) into the study protocol, enabling clinical sites to conduct the assessment virtually by video when an in-person visit was not feasible or if the parents refused the in-person home visit. Of 211 12-month home visits conducted as of August 2024, 18% had been completed virtually via their cell phones (internet access was not required).
In addition to COVID-19 transmission concerns, having the virtual option was helpful for families who preferred research staff not to go to their homes for other reasons. Despite coordinators having a close bond with families who had already been participating in the study for nearly a year, some families were reluctant to participate in the HOME component, either in person or virtual. Some families declined the in-person home visit because they lived with friends or family members or in a group home with unfavorable beliefs about research or were uncomfortable with strangers. Mothers in this situation typically did not disclose they were participating in a research study to those they were living with. Other families declined the in-person home visit because of the unpredictability of home life and inability to schedule with certainty. One clinical site with staff availability in the evening addressed this obstacle by texting the mother in the late afternoon or early evening, “Would this evening work for a virtual 12-month visit?” which resulted in several “yes” responses because the mother was available at that moment. Virtual visits enabled study teams to be flexible in offering when the visit could occur, maximizing the number of families completing this component of the study.
Engaging Participants Through Recruitment Materials and Retention Efforts
Our four clinical sites—all associated with Level III NICUs at academic medical centers—observed a reduction in the number of opioid-exposed infants born in their hospitals as opioid-exposed infants were increasingly being cared for in lower-level pediatric units during the COVID-19 pandemic as was observed elsewhere in the U.S. (MacMillan et al., 2022). To offset the reduction in the number of eligible participants at our clinical sites, the following efforts helped engage participants and maximize recruitment and retention.
Brochures and Poster, Including Those Tailored to Unexposed Infants
To assist with participant recruitment, we assembled a working group of coordinators and clinical site staff to develop public-facing materials to raise awareness of the study, including two brochures for parents/caregivers of exposed and unexposed infants and a poster tailored to parents of unexposed infants. The brochures were developed using plain language for lay audiences and were designed to facilitate communication between study staff and potential participants. The brochures covered topics such as the study objectives, participation benefits, what participants would need to do, payment, local site contact information, and a weblink and QR code directing the viewer to the study website to learn more. The brochure tailored to parents of unexposed infants also included details on why healthy babies are needed for research studies. The brochures and a poster contained images of infants and children of various races up to 2 years of age engaged in nurturing interactions with friendly health care providers.
Brochures and a poster helped the research teams engage families and initiate conversations about the study. The conversations these printed materials prompted helped staff to build trust and foster relationships with potential participants. The printed materials included easy-to-understand information about the study in a visually appealing format and used plain language best practices. The printed materials—which used NICHD and HEAL Initiative logos—offered credibility in legitimizing the study for potential participants and staff members in the locations where the brochures were distributed and the poster was displayed. When study coordinators approached other clinical staff members and potential participants about the study, many indicated they had seen the brochures or the poster.
Greeting Cards
The OBOE study developed four nonreligious greeting cards (i.e., Thank You, Happy Birthday, Happy New Year, Happy Thanksgiving) with the goals of maintaining contact with families, prompting families to update contact information if it had changed, expressing appreciation for their continued involvement, and enhancing participant engagement and retention. We purposely did not include the study name on the cards to avoid involuntary disclosure of antenatal opioid exposure. The cards were helpful for building and maintaining rapport with families between study visits. Several families whom coordinators were having difficulty contacting reconnected after receiving the cards.
Other Lessons Learned
Placing “opioid” front and center in the study name—Outcomes of Babies with Opioid Exposure Study—was confusing for women in recovery who were taking medications to treat opioid use disorder as well as those who were prescribed opioids for a medical condition. To address this issue, coordinators made a concerted effort to explain that infants of mothers who took any opioids, including medication used to treat opioid use disorder, were eligible for the study. Additionally, to avoid using stigmatizing language and be inclusive of women who were prescribed opioids for a medical condition during pregnancy, we consistently used phrasing such as “women who took opioids” rather than “women who used opioids.” Including opioid in the study name also made it challenging to recruit unexposed (control) infants. Many mothers of unexposed infants asked why they were being approached for an opioid-related study and did not want to participate for fear that they would be perceived as taking opioids. To address this concern, we added a description of why healthy infants are needed for research studies to recruitment materials to explain why control groups are vital to observational studies.
Anticipating Potential Enrollment and Retention Barriers and Addressing Them Directly
Family Concerns About MRI Scans
When explaining the OBOE study, coordinators spent a considerable amount of time describing what would happen during each visit time point and how the MRI scans would be conducted. Coordinators also clarified that MRIs are safe, there is no radiation, there would be steps to protect infants’ hearing (e.g., earplugs, earmuffs), no sedation would be used, and the study team would wait for the infant to fall asleep before starting the MRI. As a result of these efforts, only 5% of caregivers of eligible opioid-exposed infants and 8% of caregivers of eligible control infants refused to participate due to the MRI requirements (p = 0.328; Table 2). Taking the time to explain what an MRI is and how it is performed (i.e., no sedation will be used), dispelling common misperceptions (such as MRI contains radiation), and explaining how infants’ hearing would be protected were successful strategies for overcoming this potential barrier to study enrollment.
Table 2.
Refusal Reasons among Parents/Caregivers of Eligible Infants, by Exposure Group
| Reason Why Parent/Caregiver Refused | Opioid-Exposed Infants (n = 173) |
Control Infants (n= 290) |
|---|---|---|
| Time commitment of follow-up visits/questionnaires | 35 (20%) | 104 (36%) |
| Does not trust information will be kept safe | 1 (1%) | 0 (0%) |
| Not interested in sharing information for research | 68 (39%) | 65 (22%) |
| Lives too far from follow-up clinic | 5 (3%) | 14 (5%) |
| MRI | 9 (5%) | 22 (8%) |
| Did not give reason | 36 (21%) | 60 (21%) |
| Other | 19 (11%) | 25 (9%) |
Note. MRI=magnetic resonance imaging
Screening Referrals
One quarter (26%) of mothers screened positive for food insecurity during pregnancy. Clinical site coordinators provided them with referrals for federal nutrition programs [i.e., Special Supplemental Nutrition Program for Women, Infants and Children (WIC), Supplemental Nutrition Assistance Program (SNAP)] and information on local food resources such as food banks/pantries. As of August 2024, three mothers had exceeded the cutoff (standard t-score >70) on the depression scale of the PROMIS instrument, indicating extreme symptoms of depression, prompting staff to take immediate action as per study procedures. In addition to speaking at length with the staff members responsible for referring mothers for mental health concerns, these mothers were provided phone numbers for local mental health resources, crisis hotlines, and social workers.
Troubleshooting Barriers to Attending Study Visits
In addition to providing an incentive for each study visit, giving families a meal voucher to use at the hospital cafeteria during the visit, a pack of diapers, and an age-appropriate book or toy allowed research staff to express their appreciation for the families’ time commitment in participating in this longitudinal study. Study staff problem-solved families’ obstacles to getting to study visits and provided support to families who needed assistance with transportation. This support resulted in a sense of trust and collaboration between families and study staff. Providing funds for babysitters for siblings or having study staff present to serve as babysitters while the mother put the infant to sleep for the MRI was helpful for single parents and parents with multiple children.
Retention at 6 Months
As of August 2024, the full cohort had aged out of their 6-month follow-up window, with 234 (75%) infants attending the 6-month visit. A larger proportion of control infants attended the 6-month visit than opioid-exposed infants (83% vs 72% respectively, p = 0.0421). Social determinants of health that could affect 6-month retention, such as food insecurity, housing instability (frequent moving, crowding, spending excessive amounts of household income on housing), maternal education, and Child and Family Services involvement, are provided in Table 3. Subgroups had similar 6-month retention rates when examined among the overall cohort and by exposure group.
Table 3.
Retention of Infants Expected for 6-Month Visit, Overall and by Exposure Group
| Characteristic | Overall Retained at 6 Months1 n/N (%) |
p | Opioid- Exposed Retained at 6 Months1 n/N (%) |
p | Control Retained at 6 Months1 n/N (%) |
p |
|---|---|---|---|---|---|---|
| Exposure status | ||||||
| Control (Unexposed) | 85/103 (83) | .0421 | ||||
| Opioid exposed | 149/207 (72) | |||||
| Maternal age | ||||||
| <25 | 36/46 (78) | .7692 | 16/21 (76) | .8584 | 20/25 (80) | .1587 |
| 25–29 | 65/93 (70) | 40/60 (67) | 25/33 (76) | |||
| 30–34 | 87/108 (81) | 64/81 (79) | 23/27 (85) | |||
| ≥35 | 46/62 (74) | 29/44 (66) | 17/18 (94) | |||
| Maternal marital status | ||||||
| Married | 42/53 (79.2) | .4941 | 14/22 (63.6) | .3544 | 28/31 (90.3) | .1819 |
| Not married | 184/246 (74.8) | 130/178 (73.0) | 54/68 (79.4) | |||
| Maternal education | ||||||
| Less than high school | 38/50 (76.0) | .1248 | 33/44 (75.0) | .5143 | 5/6 (83.3) | .1894 |
| High school diploma | 79/112 (70.5) | 53/77 (68.8) | 26/35 (74.3) | |||
| More than high school | 114/138 (82.6) | 62/79 (78.5) | 52/59 (88.1) | |||
| Public insurance | ||||||
| Yes | 197/267 (73.8) | .0803 | 136/189 (72.0) | .9090 | 61/78 (78.2) | .0547 |
| No | 33/38 (86.8) | 11/15 (73.3) | 22/23 (95.7) | |||
| Parity | ||||||
| 1 | 50/67 (74.6) | .8122 | 20/32 (62.5) | .1762 | 30/35 (85.7) | .5408 |
| 2+ | 184/242 (76.0) | 129/174 (74.1) | 55/68 (80.9) | |||
| Discharged to mother | ||||||
| Yes | 200/262 (76.3) | .5563 | 115/159 (72.3) | .9986 | 85/103 (82.5) | |
| No | 34/47 (72.3) | 34/47 (72.3) | 0/0 (0.0) | |||
| Food insecurity (during pregnancy) | ||||||
| Yes | 65/80 (81.3) | .6533 | 53/66 (80.3) | .4071 | 12/14 (85.7) | .9223 |
| No | 168/213 (78.9) | 96/128 (75.0) | 72/85 (84.7) | |||
| Spending excessive (> 50%) of income on housing costs (0–1 month) | ||||||
| Yes | 131/164 (79.9) | .9204 | 87/113 (77.0) | .9050 | 44/51 (86.3) | .7646 |
| No | 86/107 (80.4) | 49/63 (77.8) | 37/44 (84.1) | |||
| Number of people in household (0–1 month) | ||||||
| 5 or more | 89/118 (75.4) | .1317 | 58/78 (74.4) | .4575 | 31/40 (77.5) | .0931 |
| 4 or fewer | 143/173 (82.7) | 90/114 (78.9) | 53/59 (89.8) | |||
| Moved in last 6 months (0–1 month) | ||||||
| Yes | 52/64 (81.3) | .7312 | 32/38 (84.2) | .2431 | 20/26 (76.9) | .1893 |
| No | 180/227 (79.3) | 116/154 (75.3) | 64/73 (87.7) | |||
| Child and Family Services involvement (0–1 month) | ||||||
| Yes | 71/91 (78.0) | .6259 | 67/87 (77.0) | .9828 | 4/4 (100.0) | .3883 |
| No | 161/200 (80.5) | 81/105 (77.1) | 80/95 (84.2) |
Note. 1 Retained at 6 months if seen for 6-month visit.
Discussion
Research consortia are challenging to establish and maintain through virtual platforms because of difficulties in promoting collaboration and partnerships without face-to-face interaction. The Consortium largely consisted of investigators and coordinators who had previously collaborated on other research studies, providing a foundation of trust and collaboration. Prior to study launch, we anticipated that the serial MRIs would be a barrier to participation. However, only 5% of caregivers of opioid-exposed infants and 8% of caregivers of control infants indicated that they chose not to participate in the study because of MRIs, suggesting that our efforts to address this potential barrier to enrollment were successful.
The OBOE study successfully recruited and retained a complex population that frequently “falls through the cracks” of both the recovery health care system and research studies. The OBOE study families were frequently in difficult financial situations that made well-child medical visits secondary to getting to work, keeping a job, and having an unpredictable or demanding schedule. Most health care providers do not schedule families who miss appointments more than twice, and they will not attempt to reach alternative contacts when trying to schedule a patient. However, if enrolled in a research study, coordinators identify information for close contacts and go to great lengths to locate families and schedule them for visits—even if there are multiple cancellations. OBOE study coordinators often kept families of these high-risk infants engaged in the system by reaching them when they had been lost to follow-up and connecting them with services when gaps in care or concerns were identified. These families often needed extra reminders, assistance, and chances to complete study visits. We found that coordinator persistence was instrumental in helping families maintain their involvement in the study.
Conclusion
Four factors were essential in establishing the Consortium, implementing the study, and retaining participants. With these factors in place, we successfully completed enrollment and achieved a 6-month follow-up visit rate of 75%.
Acknowledgments
The authors acknowledge the infants, mothers, and their families who agreed to take part in this study: the medical, neuroimaging, and nursing colleagues at Case Western Reserve University, Cincinnati Children’s Hospital Medical Center, University of Alabama at Birmingham, and Children’s Hospital of Philadelphia; the Neuroimaging Core colleagues at Children’s National Hospital; the data coordinating center staff at RTI International; and National Institute of Child Health and Human Development colleagues Dr. Andrew Bremer, Dr. Nahida Chakhtoura, Dr. Michele Walsh, and Ms. Stephanie Archer.
This research was supported by the National Institutes of Health (NIH) through the NIH Helping to End Addiction Long-term® (HEAL) Initiative [award number 1PL1HD101059-01]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Footnotes
The Outcomes of Babies with Opioid Exposure study received institutional review board (IRB) approval under the single IRB at Cincinnati Children’s Hospital Medical Center, and written informed consent was obtained for all participants.
The research reports findings from an observational study registered on Clinical Trials.gov, Identifier: NCT04149509, https://clinicaltrials.gov/study/NCT04149509. This observational study was registered on November 1, 2019, and the first participant was enrolled on August 19, 2020.
The authors have no conflicts of interest to report.
References
- American Academy of Pediatrics. (2021, January). Screen and intervene: A toolkit for pediatricians to address food insecurity. Food Research & Action Center. Retrieved from https://frac.org/wp-content/uploads/FRAC_AAP_Toolkit_2021.pdf [Google Scholar]
- Bann CM, Newman JE, Poindexter B, Okoniewski K, DeMauro S, Lorch SA, Wilson-Costello D, Ambalavanan N, Peralta-Carcelen M, Limperopoulos C, Kapse K, Davis JM, Walsh M, & Merhar S (2023). Outcomes of babies with opioid exposure (OBOE): Protocol of a prospective longitudinal cohort study. Pediatric Research, 93, 1772–1779. 10.1038/s41390-022-02279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, & Bradley RH (2003). Home observation for measurement of the environment: Administration manual. Family & Human Dynamics Research Institute, Arizona State University, 23–33. [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, … Hays R (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63, 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2021). Addressing opioid use disorder to improve maternal and infant health.
- Haight SC, Ko JY, Tong VT, Bohm MK, & Callaghan WM (2018). Opioid use disorder documented at delivery hospitalization—United States, 1999–2014. Morbidity and Mortality Weekly Report, 67, 845–849. 10.15585/mmwr.mm6731a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai AH, Ko JY, Owens PL, Stocks C, & Patrick SW (2021). Neonatal abstinence syndrome and maternal opioid-related diagnoses in the US, 2010–2017. JAMA, 325, 146–155. 10.1001/jama.2020.24991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, & Berry Brazelton T (2004). The neonatal intensive care unit network neurobehavioral scale procedure. Pediatrics, 113, 641–667. 10.1542/peds.113.S2.641 [DOI] [PubMed] [Google Scholar]
- Lien J, Hayes T, Liu-Smith F, & Rana D (2023). Comparing maternal substance use and perinatal outcomes before and during the COVID-19 pandemic. Journal of Perinatology, 43, 664–669. 10.1038/s41372-023-01613-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan KDL, Morrison TM, Melvin P, Diop H, Gupta M, & Wachman EM (2022). Impact of coronavirus disease-2019 on hospital care for neonatal opioid withdrawal syndrome. Journal of Pediatrics, 245, 47–55. 10.1016/j.jpeds.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Schiff DM, Ryan SA, Quigley J, Gonzalez PK, & Walker LR (2017). A public health response to opioid use in pregnancy. Pediatrics, 139, e20164070. 10.1542/peds.2016-4070 [DOI] [PubMed] [Google Scholar]
- Whiteman VE, Salemi JL, Mogos MF, Cain MA, Aliyu MH, & Salihu HM (2014). Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. Journal of Pregnancy, 2014, 906723. 10.1155/2014/906723 [DOI] [PMC free article] [PubMed] [Google Scholar]


