Abstract
Aims
The primary objective of this study was to determine whether concomitant therapy with ticagrelor and rosuvastatin affects rosuvastatin plasma concentrations in patients receiving rosuvastatin 40 mg/day after myocardial infarction.
Methods
We included 93 patients who had experienced a myocardial infarction and were receiving high-dose rosuvastatin 40 mg/day and a P2Y12 receptor antagonist, either ticagrelor, prasugrel or clopidogrel. We used liquid chromatography with tandem mass spectrometry to measure rosuvastatin plasma concentrations after liquid–liquid extraction.
Results
Rosuvastatin plasma concentrations (9.7 ng/mL) were approximately twice as high in patients receiving ticagrelor therapy as in those receiving prasugrel (5.1 ng/mL, p < 0.001) or clopidogrel (5.0 ng/mL, p = 0.009), and ticagrelor was an independent factor influencing rosuvastatin concentrations. In addition, creatinine levels were associated with increased rosuvastatin concentrations (p = 0.039).
Conclusion
Our results suggest an important pharmacokinetic interaction between ticagrelor and rosuvastatin, leading to approximately two-fold higher rosuvastatin plasma concentrations in those receiving concomitant ticagrelor than in those receiving prasugrel or clopidogrel. The association is significant and independent of other potential factors influencing rosuvastatin levels, indicating its potential clinical relevance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-025-01489-1.
Key Points
| Ticagrelor independently increases rosuvastatin levels. |
| Patients taking ticagrelor and high doses of rosuvastatin had about twice the plasma concentrations of rosuvastatin than those taking prasugrel or clopidogrel. |
| Higher levels of creatinine were linked to higher rosuvastatin concentrations. |
Introduction
Secondary prevention, including high-intensity statin therapy (e.g. rosuvastatin 20–40 mg once daily) and P2Y12 receptor antagonists (e.g. ticagrelor, prasugrel or clopidogrel), is recommended for patients after myocardial infarction (MI). This guideline-directed therapy improves patient outcomes by effectively reducing the risk of further atherothrombotic events, including recurrent MI, stroke and cardiovascular death [1]. However, some reports have also indicated potential unfavorable interactions associated with the coadministration of these drugs [2–4].
Rosuvastatin is administered orally once daily to a maximum approved dose of 40 mg. Peak plasma concentration is reached after a median of 5 h (range 0.5–6 h), bioavailability is 20%, and the half-life is approximately 20 h, with considerable variation in absorption (48%) and low accumulation after continuous dosing [5]. Rosuvastatin undergoes limited metabolism as metabolizing enzymes have minimal impact on exposure, and several transporters such as organic anion transporters (1B1, 1B3, 2B1) and ABCG2 importantly influence its pharmacokinetics [2].
Because of its pronounced effect on low-density lipoprotein cholesterol and clinical risk reduction, rosuvastatin is considered a high-intensity statin therapy and so is commonly prescribed in patients at very high cardiovascular risk, such as patients after MI, wherein dual antiplatelet therapy (aspirin plus a P2Y12 antagonist) is also recommended [6, 7].
All three antiplatelet medications, ticagrelor, prasugrel and clopidogrel, are rapidly absorbed, and the time to peak plasma concentration is 1.3–2 h, 30–60 min, and 30 min, respectively. They each have relatively short elimination half-lives (7.7–13.1 h for ticagrelor, 7 h [range 2–15] for the active metabolite of prasugrel, and 30 min for the active metabolite of clopidogrel), and the half-life of clopidogrel after oral administration is 6 h [8, 9]. Ticagrelor does not require metabolic activation, whereas prasugrel and clopidogrel do, mainly via cytochrome P450 (CYP) enzymes [8]. Several reports of severe side effects after coadministration of ticagrelor and rosuvastatin have been published [2, 10–18], suggesting possible drug–drug interactions. Therefore, it is crucial to investigate possible pharmacokinetic explanations for these interactions to ensure patient safety and optimize therapeutic outcomes.
The aim of our study was to determine whether ticagrelor, compared with prasugrel and clopidogrel, affects plasma concentrations of rosuvastatin in patients who have experienced MI and are receiving high doses of rosuvastatin.
Methods
Subjects and Study Design
We conducted a prospective observational monocentric study including 93 patients who had experienced an MI and who had been receiving oral rosuvastatin 40 mg/day and an antiplatelet agent (ticagrelor 90 mg/12 h, prasugrel 10 mg/day or clopidogrel 75 mg/day) for at least 1 month before blood sampling. The choice of antiplatelet agent was at the discretion of the treating physician and was not changed in the 1 month before blood sampling. Data on associated medical conditions and lifestyle factors were collected for statistical analysis.
Sample Collections and Measurement of Plasma Rosuvastatin Concentrations
Blood samples were collected from all patients at trough (approximately 24 h after taking the last rosuvastatin dose) into vacuum tubes containing K3-EDTA to measure rosuvastatin concentrations and into serum-separating tubes for biochemical testing for creatine kinase, alanine aminotransferase (ALT), creatinine, and lactate dehydrogenase (LDH). Samples were centrifuged within 30 min of collection. Liver enzymes and creatinine were measured in serum according to standard procedures, and plasma was immediately stored at − 70 °C until analysis.
Rosuvastatin was measured using a method based on liquid chromatography-tandem mass spectrometry, validated in accordance with International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use M10 guidelines [19]. Briefly, 100 µL serum samples with added isotope-labelled rosuvastatin as internal standard were extracted using tert-butyl methyl ether with 10% isopropanol as an extraction solvent. The samples were then dried and reconstituted in 100 μL of 70% methanol with 0.1% formic acid. Instrumental analysis was performed on an Agilent 1290 Infinity II ultra-high-performance liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA), coupled to a Sciex QTRAP 5500+ triple quadrupole/linear ion trap mass spectrometer (Sciex, Framingham, MA, USA).
Statistical Analyses
We conducted statistical analyses using IBM SPSS version 29.0. We tested the normality of distribution with the Shapiro–Wilk test and calculated medians, with first and third quartiles (Q1–Q3). We analyzed the baseline characteristics of the patients, and comparisons among the groups based on prescribed medications, using the Kruskal–Wallis rank sum test and Pearson’s chi-squared test and used the Mann–Whitney U test to compare two groups in the analysis of the medication effects. P values < 0.05 were considered statistically significant. To identify predictors of rosuvastatin concentration, we performed both univariate and multivariate linear regression analyses after logarithmically transforming the rosuvastatin concentrations. In the multivariate analysis, we used ticagrelor as the reference drug.
Results
Study Subjects
All patients completed the study and were included in the analysis. Most of the patients were men (80%, n = 74). The median age of the patients was 58 years (Q1–Q3 52–64 years), and the median weight was 85 kg (79–94). In total, 48 (52%) patients were receiving ticagrelor, 30 (32%) were on prasugrel and 15 (16%) were on clopidogrel (Table 1). The median trough rosuvastatin concentration was 7 ng/mL (Q1–Q3 5–11 ng/mL).
Table 1.
Baseline characteristics of patients receiving ticagrelor, prasugrel, or clopidogrel
| Variables | All patients (n = 93) | Ticagrelor (n = 48) | Prasugrel (n = 30) | Clopidogrel (n = 15) | p value |
|---|---|---|---|---|---|
| Male | 74 (80) | 39 (81) | 25 (83) | 10 (67) | NS |
| Age, years | 58 (52–64) | 59 (53–64) | 57 (50–59) | 57 (53–68) | NS |
| Weight, kg | 85 (79–94) | 84 (80–94) | 86 (82–94) | 83 (79–93) | NS |
| BMI, kg/m2 | 28.0 (25.7–30.3) | 28.1 (26.0–30.5) | 27.1 (25.2–29.2) | 29.0 (26.7–30.8) | NS |
| ALT, µkat/L | 0.77 (0.54–1.21) | 0.85 (0.58–1.40) | 0.90 (0.58–1.19) | 0.44 (0.38–0.63) | < 0.001 |
| LDH, µkat/L | 3.09 (2.83–3.58) | 3.28 (2.95–3.65) | 2.96 (2.75–3.30) | 3.08 (2.72–3.72) | NS |
| CK, µkat/L | 2.64 (1.56–3.75) | 2.82 (1.55–3.8) | 2.3 (1.6–3.58) | 2.21 (1.30–2.96) | NS |
| Creatinine, µmol/L | 80 (71–90) | 79 (71–92) | 81 (76–85) | 89 (76–90) | NS |
| Alcohol consumptiona | 30 (32) | 14 (29) | 13 (42) | 3 (20) | NS |
| Physical activityb | 74 (80) | 37 (77) | 25 (83) | 12 (80) | NS |
| Therapy | |||||
| Acetylsalicylic acid | 93 (100) | 48 (100) | 30 (100) | 15 (100) | NS |
| Ezetimibe | 70 (75) | 29 (60) | 28 (93) | 13 (87) | 0.002 |
| Rosuvastatin | 93 (100) | 48 (100) | 30 (100) | 15 (100) | NS |
| ACE inhibitors | 58 (62) | 31 (65) | 19 (63) | 8 (53) | NS |
| ARBs | 12 (13) | 5 (10) | 4 (13) | 3 (20) | NS |
| Β-blockers | 85 (91) | 43 (90) | 29 (97) | 13(87) | NS |
| Proton pump inhibitors | 93 (100) | 48 (100) | 30 (100) | 15 (100) | NS |
| Calcium channel blockers | 10 (11) | 8 (17) | 1 (3.3) | 1 (6.7) | NS |
Data are presented as N (%) or median (first quartile–third quartile)
ACE angiotensin-converting enzyme, ALT alanine aminotransferase, ARB angiotensin receptor blocker, BMI body mass index, CK creatine kinase, LDH lactate dehydrogenase, NA not applicable, NS not significant
aAlcohol consumption of at least one dose per day
bPhysical activity for at least 2 h per week
Rosuvastatin Concentrations by P2Y12 Antagonists
Patients were categorized based on their prescribed antiplatelet agent. Trough rosuvastatin concentrations were approximately 1.9 times higher with ticagrelor than with either prasugrel (p < 0.001) or clopidogrel (p = 0.009), with median concentrations of 9.7 ng/mL (Q1–Q3 6.9–13.3), 5.1 ng/mL (3.1–7.5), and 5.0 ng/mL (4.1–9.2), respectively (Fig. 1). There were no statistical differences in rosuvastatin concentration between the prasugrel and the clopidogrel groups (p = 0.7). After combining clopidogrel and prasugrel groups, the difference in median concentrations remained unchanged (see Fig. 1 in the electronic supplementary material [ESM]).
Fig. 1.
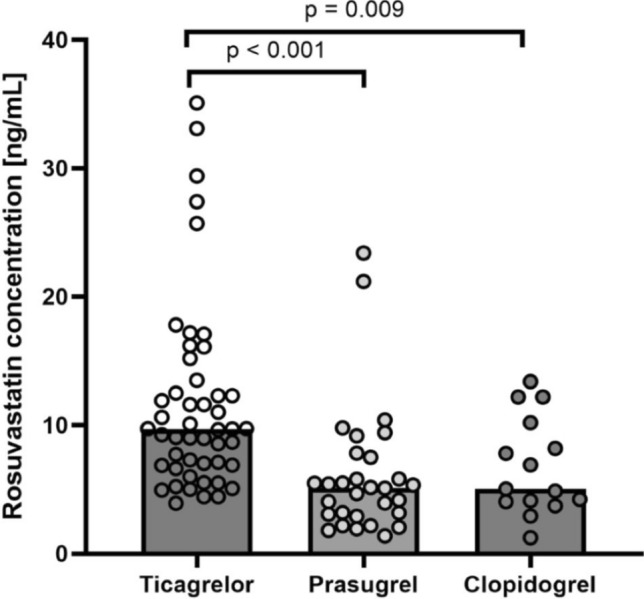
Rosuvastatin concentrations from each patient, represented as circles, grouped by P2Y12 antagonists: ticagrelor, prasugrel and clopidogrel. Column height represents the median value. Kruskal–Wallis rank sum test p value is indicated
Predictors of Rosuvastatin Concentrations
Significant univariate predictors of logarithmically transformed rosuvastatin concentrations were age, creatinine levels, ALT, LDH, concomitant therapy with ezetimibe, and type of P2Y12 antagonist (Table 2). We conducted an exploratory analysis focusing solely on patients taking ticagrelor to compare those aged < 65 years with older patients. This additional assessment aimed to evaluate the impact of older age on rosuvastatin concentrations, and we found no differences between the groups (Figure 2 in the ESM). As angiotensin II receptor blockers have distinct pharmacokinetic profiles, exploratory analysis was conducted solely analyzing valsartan. Results showed valsartan to be a significant predictor of logarithmically transformed rosuvastatin concentrations (Table 1 in the ESM).
Table 2.
Univariate and multivariate predictors of rosuvastatin concentrations
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p value | Beta | 95% CI | p value | |
| Sex | 0.317 | − 0.032 to 0.666 | NS | − 0.235 | − 0.129 to 0.598 | NS |
| Age | 0.020 | 0.007 to 0.037 | 0.004 | 0.006 | − 0.010 to 0.023 | NS |
| Creatinine | 0.009 | 0.001 to 0.017 | 0.033 | 0.009 | 0.001 to 0.016 | 0.024 |
| LDH | 0.337 | 0.147 to 0.526 | < 0.001 | 0.135 | − 0.060 to 0.329 | NS |
| ALT | 0.24 | 0.005 to 0.474 | 0.045 | 0.045 | − 0.195 to 0.286 | NS |
| Ezetimibe | − 0.371 | − 0.694 to − 0.048 | 0.025 | − 0.035 | − 0.335 to 0.266 | NS |
| Clopidogrel | − 0.562 | − 0.898 to − 0.225 | 0.001 | − 0.565 | − 0.936 to − 0.193 | 0.005 |
| Prasugrel | − 0.733 | − 1.011 to − 0.454 | < 0.001 | − 0.671 | − 0.966 to − 0.376 | < 0.001 |
ALT alanine aminotransferase, CI confidence interval, LDH lactate dehydrogenase, NS not significant
After multivariate adjustment, significant predictors of logarithmically transformed concentrations of rosuvastatin were creatinine levels (which slightly increased rosuvastatin concentrations), the type of P2Y12 antagonist (Table 2), and potentially concomitant therapy with valsartan (Table 1 in the ESM). Specifically, the analysis confirmed our hypothesis that ticagrelor independently affects rosuvastatin concentration. Sensitivity analysis combining non-ticagrelor medications into one group further validated these findings (Table 2 in the ESM).
Patient Characteristics Grouped by Medications
We compared the groups according to patients’ medical conditions and concomitant therapy. Patients receiving ticagrelor or prasugrel had approximately two-fold higher ALT activity than patients receiving clopidogrel (p < 0.001). The analysis also revealed that only 60% of patients receiving ticagrelor therapy also received ezetimibe, whereas co-therapy with ezetimibe was significantly (p = 0.002) more prevalent in patients receiving prasugrel (98%) or clopidogrel (87%). Detailed data on co-therapy are presented in Table 3 in the ESM.
Discussion
Our study is the first to show that concomitant therapy with ticagrelor is associated with increased blood concentrations of rosuvastatin compared with prasugrel or clopidogrel, whether analyzed separately or as a group. The association remained significant after adjusting for other potential predictors of rosuvastatin concentration such as age, sex, and renal or liver function. Our results provide important information on the concomitant guideline-directed administration of statins and P2Y12 antagonists. On one hand, our findings may explain recent reports of adverse rosuvastatin-related effects in patients on concomitant ticagrelor therapy [2, 10–18, 20, 21]. After a focused analysis of 2464 reports of rhabdomyolysis in patients treated with statins and antiplatelet drugs, an increase in rhabdomyolysis was observed when rosuvastatin and ticagrelor were co-administered. However, no difference was found when rosuvastatin was administered alone or co-administered with aspirin, clopidogrel, or prasugrel [22], which aligns with our results. On the other hand, research into the differences in cardiovascular outcomes between different P2Y12 regimens may benefit from further focusing on co-administration of specific statin therapies.
In our study, patients on ticagrelor had approximately two-fold higher trough plasma rosuvastatin concentrations than patients on either prasugrel or clopidogrel. Given the small group of patients on clopidogrel, we also performed a sensitivity analysis comparing patients on ticagrelor and those on non-ticagrelor therapy. The results remained consistent, further confirming the robustness of the results. Our results are in line with observations in healthy individuals, wherein peak rosuvastatin concentrations after a single dose of 10 mg was 2.6 times higher in participants taking ticagrelor than in those taking placebo [23]. Conversely, expected interactions with clopidogrel are smaller in magnitude (a 1.4-fold increase in the area under the plasma concentration-time curve of rosuvastatin was reported when administered concomitantly with clopidogrel 75 mg/day) [24], whereas no pharmacokinetic rosuvastatin–prasugrel interactions have been reported to date.
Possible mechanisms of rosuvastatin–ticagrelor pharmacokinetic interactions are more likely to involve transport mechanisms than metabolic enzymes [5, 25]. Rosuvastatin undergoes minimal metabolism by CYP2C9 and CYP2C19 and almost none by CYP3A4. It is primarily excreted through hepatic transporters, exhibiting high affinity to solute carrier organic anion transporter 1B1 and is also transported by adenosine triphosphate-binding cassette (ABC) transporters, such as ABCG2, ABCC2, and ABCB1, which supports its excretion via the bile. A static drug–drug interaction model predicted a 2.1-fold increase in rosuvastatin concentrations due to inhibition of ABCG2 by ticagrelor, whereas solute carrier organic anion transporter-mediated hepatic uptake of rosuvastatin should be unaffected because of the relatively low ticagrelor concentrations in the portal vein [2]. This inhibition was corroborated by similar effects observed with fostamatinib and febuxostat, where ABCG2 inhibition led to an approximately two-fold increase in rosuvastatin exposure [26, 27]. There are no pharmacokinetic studies describing how prasugrel affects rosuvastatin concentrations or ABCG2 function. Higher rosuvastatin concentrations observed in the ticagrelor group in our study could be explained by the extent of inhibition of ABCG2, as clopidogrel is expected to have a smaller effect on rosuvastatin concentrations. ABCG2 is a key transport protein involved in mediating rosuvastatin efflux in the small intestine [2]. Polymorphisms in the ABCG2 gene have been linked to increased rosuvastatin plasma concentrations [28, 29], supporting the hypothesis that ticagrelor’s inhibition of ABCG2 contributes to the elevated rosuvastatin levels observed in our study.
Other determinants of rosuvastatin concentrations were increasing age, renal function, ALT and LDH activity, and concomitant therapy with ezetimibe and/or potentially also valsartan. As expected, renal function—an important determinant of rosuvastatin clearance [30]—was an independent predictor of rosuvastatin concentration regardless of ticagrelor therapy. Although age has been identified as an important factor in the occurrence of rhabdomyolysis, particularly in patients aged ≥ 75 years [31], our exploratory analysis did not find an association between age and rosuvastatin concentrations after multivariate analysis; however, it is noteworthy that our patient population primarily comprised patients who had experienced MI at < 75 years. When comparing rosuvastatin concentrations in patients on ticagrelor aged < 65 years with those > 65 years, no statistical difference was observed, which could be due to the low number of patients (N = 10) in the group aged > 65 years. During the analysis of group comparisons, we also observed that patients receiving ticagrelor and prasugrel had slightly elevated ALT levels, an incidental finding also reported by others [32, 33]. Given a distinct effect of P2Y12 inhibitors on ALT levels and the potential interaction between liver function and rosuvastatin concentration, we adjusted our results for ALT in the multivariate model to minimize a potential confounding effect. Nonetheless, this hypothesis should be further validated in a larger cohort. Conversely, ALT and LDH were only associated with rosuvastatin concentration in the univariate model. Statin therapy increases liver enzyme activity by 0.5–2% [6], so a correlation with rosuvastatin concentration is an expected finding. Conversely, rosuvastatin concentrations were lower in patients on concomitant therapy with ezetimibe and/or valsartan but not when angiotensin II receptor blockers were analyzed as a combined group. Although the rosuvastatin–ezetimibe interaction was not significant after multivariate adjustment, valsartan emerged as a potential independent predictor of lower rosuvastatin concentrations. Previous studies have already reported non-significantly lower rosuvastatin concentrations in healthy volunteers taking valsartan [34, 35]. However, our patients were receiving higher doses of rosuvastatin, which may influence the observed effect. More importantly, given the very small number of patients on valsartan in our study, this finding should be regarded as hypothesis generating but merits further exploration in future studies.
Our study has some limitations. First, this was an observational study to compare rosuvastatin concentrations between patients taking different P2Y12 receptor antagonists, which precludes adjustment for unmeasured confounders. Second, we only performed a single sample measurement of rosuvastatin concentration, which precludes time-dependent analysis and may lead to a bias due to sampling errors or biological variability. Additionally, the number of patients was too small to draw definitive conclusions regarding the comparison of statin intolerance between the groups. Moreover, the groups were not completely homogeneous in terms of medication use, although this is unlikely to have had a significant impact on the results. The strength of our study is that it was a homogenous real-life patient population treated in accordance with the international guidelines.
Nonetheless, our study has shown that rosuvastatin concentrations are higher in patients on concomitant therapy with ticagrelor than in those receiving prasugrel or clopidogrel. The association is significant, independent of other potential determinants of rosuvastatin concentrations, and may be clinically relevant. Possible interactions between ticagrelor and rosuvastatin should be taken into account in the clinical setting, as both medications are commonly recommended together as part of guideline-directed therapy in patients after MI.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the physicians for their key role in recruiting patients for this study, as well as the hospital staff for their assistance with blood sample collection and laboratory analysis. We also acknowledge the patients for their participation in the study.
Declarations
Funding
The study was supported by the Slovenian Research Agency (grant no. P3-0308).
Conflict of interest
The authors have no competing interests relevant to the content of this article.
Availability of data and material:
The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics approval
The study was approved by Commission for Medical Ethics of the Republic of Slovenia (grant nr.: 0120-124/2023/7) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
NA.
Code availability
NA.
Author contributions
Tjaša Dermota and Mojca Božič Mijovski designed the study. Tjaša Dermota and Jurij Trontelj prepared the material and collected the data. All authors contributed to the data analysis. Tjaša Dermota wrote the first draft of the manuscript, and all authors reviewed it. All authors read and approved the final manuscript.
References
- 1.Rossello X, Dan G-A, Dweck MR, et al. ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;2023:3720–826. [DOI] [PubMed] [Google Scholar]
- 2.Lehtisalo M, Kiander W, Filppula AM, et al. Rhabdomyolysis during concomitant ticagrelor and rosuvastatin: a breast cancer resistance protein-mediated drug interaction? Br J Clin Pharmacol. 2023;89:2309–15. 10.1111/bcp.15684. [DOI] [PubMed] [Google Scholar]
- 3.Danielak D, Karaźniewicz-Łada M, Główka F. Assessment of the risk of rhabdomyolysis and myopathy during concomitant treatment with ticagrelor and statins. Drugs. 2018;78:1105–12. 10.1007/s40265-018-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ning C, Su S, Li J, et al. Evaluation of a clinically relevant drug-drug interaction between rosuvastatin and clopidogrel and the risk of hepatotoxicity. Front Pharmacol. 2021;12: 715577. 10.3389/fphar.2021.715577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanukula R, Salam A, Rodgers A, et al. Pharmacokinetics of rosuvastatin: a systematic review of randomised controlled trials in healthy adults. Clin Pharmacokinet. 2021;60:165–75. 10.1007/s40262-020-00978-9. [DOI] [PubMed] [Google Scholar]
- 6.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;73. 10.1161/CIR.0000000000000625
- 8.Teng R. Ticagrelor: pharmacokinetic, pharmacodynamic and pharmacogenetic profile: an update. Clin Pharmacokinet. 2015;54:1125–38. 10.1007/s40262-015-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achar S. Pharmacokinetics, drug metabolism, and safety of prasugrel and clopidogrel. Postgrad Med. 2011;123:73–9. 10.3810/pgm.2011.01.2247. [DOI] [PubMed] [Google Scholar]
- 10.New J, Le K, Wong KA, et al. A case of acute renal failure and rhabdomyolysis associated with the concomitant use of ticagrelor, rosuvastatin, and losartan. JSM Intern Med. 2017;2:1004. [Google Scholar]
- 11.Osborn H, Grossman D, Kochhar S, et al. A rare case of delayed onset multi-drug interaction resulting in rhabdomyolysis in a 66-year-old male. Cureus. 2021;13: e20035. 10.7759/cureus.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel G, Atanda AC, Onyemeh A, et al. A unique case of drug interaction between ticagrelor and statin leading to acute renal failure. Cureus. 2017;9: e1633. 10.7759/cureus.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrkić Kirhmajer M, Macolić Šarinić V, Šimičević L, et al. Rosuvastatin-induced rhabdomyolysis—possible role of ticagrelor and patients’ pharmacogenetic profile. Basic Clin Pharmacol Toxicol. 2018;123:509–18. 10.1111/bcpt.13035. [DOI] [PubMed] [Google Scholar]
- 14.Sibley RA, Katz A, Papadopoulos J. The interaction between rosuvastatin and ticagrelor leading to rhabdomyolysis: a case report and narrative review. Hosp Pharm. 2021;56:537–42. 10.1177/0018578720928262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park IS. Ticagrelor-induced acute kidney injury can increase serum concentration of statin and lead to concurrence of rhabdomyolysis. Anatol J Cardiol. 2018;19:225–7. 10.14744/AnatolJCardiol.2017.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vuren AJ, de Jong B, Bootsma HPR. Ticagrelor-induced renal failure leading to statin-induced rhabdomyolysis. Neth J Med. 2015;73. [PubMed]
- 17.Elsby R, Martin P, Surry D, et al. Solitary inhibition of the breast cancer resistance protein efflux transporter results in a clinically significant drug-drug interaction with rosuvastatin by causing up to a 2-fold increase in statin exposure. Drug Metab Dispos. 2016;44:398–408. 10.1124/dmd.115.066795. [DOI] [PubMed] [Google Scholar]
- 18.Patel R, Sharma JB, Rajput S. Statins ticagrelor and rhabdomyolysis: a coincidence or a drug interaction? J Lipid Atheroscler. 2024;13:61. 10.12997/jla.2024.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICH guideline M10 on bioanalytical method validation and study sample analysis. https://www.ema.europa.eu/en/ich-m10-bioanalytical-method-validation-scientific-guideline. Accessed Jan 2025
- 20.Calderon-Ospina CA, Hernández-Sómerson M, Garcia AM, et al. A pharmacogenomic dissection of a rosuvastatin-induced rhabdomyolysis case evokes the polygenic nature of adverse drug reactions. Pharmacogenom Pers Med. 2020;13:59–70. 10.2147/PGPM.S228709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik SM, Shukla S, Sethi J, et al. Acute kidney injury post coronary angioplasty. BMJ Case Rep. 2022;15: e249294. 10.1136/bcr-2022-249294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roule V, Alexandre J, Lemaitre A, et al. Rhabdomyolysis with co-administration of statins and antiplatelet therapies—analysis of the WHO Pharmacovigilance Database. Cardiovasc Drugs Ther. 2024;38:1191–9. 10.1007/s10557-023-07459-8. [DOI] [PubMed] [Google Scholar]
- 23.Lehtisalo M, Tarkiainen EK, Neuvonen M, et al. Ticagrelor increases exposure to the breast cancer resistance protein substrate rosuvastatin. Clin Pharmacol Ther. 2024;115:71–9. 10.1002/cpt.3067. [DOI] [PubMed] [Google Scholar]
- 24.Pinheiro LF, França CN, Izar MC, et al. Pharmacokinetic interactions between clopidogrel and rosuvastatin: effects on vascular protection in subjects with coronary heart disease. Int J Cardiol. 2012;158:125–9. 10.1016/j.ijcard.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 25.Hirota T, Fujita Y, Ieiri I. An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin Drug Metab Toxicol. 2020;16:809–22. 10.1080/17425255.2020.1801634. [DOI] [PubMed] [Google Scholar]
- 26.Martin P, Gillen M, Ritter J, et al. Effects of fostamatinib on the pharmacokinetics of oral contraceptive, warfarin, and the statins rosuvastatin and simvastatin: results from phase I clinical studies. Drugs RD. 2016;16:93–107. 10.1007/s40268-015-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehtisalo M, Keskitalo JE, Tornio A, et al. Febuxostat, but not allopurinol, markedly raises the plasma concentrations of the breast cancer resistance protein substrate rosuvastatin. Clin Transl Sci. 2020;13:1236–43. 10.1111/cts.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Lim H-H, Yee J, et al. The association between ABCG2 421C>A (rs2231142) polymorphism and rosuvastatin pharmacokinetics: a systematic review and meta-analysis. Pharmaceutics. 2022;14:501. 10.3390/pharmaceutics14030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehtisalo M, Taskinen S, Tarkiainen EK, et al. A comprehensive pharmacogenomic study indicates roles for SLCO1B1, ABCG2 and SLCO2B1 in rosuvastatin pharmacokinetics. Br J Clin Pharmacol. 2023;89:242–52. 10.1111/bcp.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crouse JR. An evaluation of rosuvastatin: pharmacokinetics, clinical efficacy and tolerability. Expert Opin Drug Metab Toxicol. 2008;4:287–304. 10.1517/17425255.4.3.287. [DOI] [PubMed] [Google Scholar]
- 31.Rocca B, Bigagli E, Cerbai E. Ticagrelor and statins: dangerous liaisons? Cardiovasc Drugs Ther. 2024;38:1103–9. 10.1007/s10557-024-07624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanau RM, Delzor F, Neuman MG. Efficacy and safety of prasugrel in acute coronary syndrome patients. Clin Biochem. 2014;47:516–28. 10.1016/j.clinbiochem.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Sharma N, Bose A, Kumar D. Ticagrelor induced hepatitis:a rare entity in patients with coronary artery disease on dual antiplatelet therapy. J Am Coll Cardiol. 2021;77:2541. 10.1016/S0735-1097(21)03896-1. [Google Scholar]
- 34.Jung JA, Lee S-Y, Kim J-R, et al. A pharmacokinetic and pharmacodynamic drug interaction between rosuvastatin and valsartan in healthy subjects. Drug Des Dev Ther. 2015;9:745–52. 10.2147/DDDT.S76942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seong SJ, Ohk B, Kang WY, et al. Pharmacokinetic drug interactions between amlodipine, valsartan, and rosuvastatin in healthy volunteers. Adv Ther. 2019;36:1642–56. 10.1007/s12325-019-00976-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


