Abstract
INTRODUCTION:
Social determinants of health (SDOH) may impact chronic liver disease (CLD) outcomes but are not clearly understood. We conducted a systematic review to describe the associations of SDOH with mortality, hospitalizations, and readmissions among patients with CLD.
METHODS:
This review was registered (PROSPERO ID: CRD42022346654) and identified articles through MEDLINE, Embase, Cochrane Library, and Scopus databases. The review included studies that reported SDOH characteristics within the domains of economic stability, healthcare access, education, social and community context, and the neighborhood-built environment. Associated outcomes of interest were mortality, hospitalizations, or readmissions. The Cochrane Risk of Bias in Nonrandomized Studies for Exposure was used to assess study quality and risk of bias.
RESULTS:
A total of 5,205 abstracts were screened, 60 articles underwent full-text review, and 27 articles were included in the final review. Poor economic stability, healthcare access, social support, and household/environmental conditions were associated with higher mortality and hospital readmissions among patients with CLD. Increasing distance (≥25 miles away) from a liver transplantation center was associated with higher mortality, despite increasing access to the liver transplantation waitlist. When assessing the overall risk of bias among included studies, most had “some concern” (N = 13, 48.1%) or “high risk” (N = 11, 40.7%), whereas a minority had “very high risk” (N = 3, 11.1%). No studies were categorized as “low risk.”
DISCUSSION:
Unfavorable SDOH were associated with increased mortality and hospital readmissions among patients with CLD. Rigorous empirical research is needed to identify evidence-based strategies that aim to mitigate disparities among vulnerable populations.
KEYWORDS: social determinants of health, chronic liver disease, transplant, waitlist, community, socioeconomic status
INTRODUCTION
Social determinants of health (SDOH) such as economic stability, healthcare access, education, household characteristics, and the community environment significantly influence chronic disease (1,2). Poor socioeconomic status (SES) and exposure to resource-impoverished neighborhoods are consistently associated with higher rates of cardiovascular disease (3–5). In fact, SDOH may account for as much as 40% of the variance observed in health outcomes (1). Health disparities often reflect poor access to care (e.g., low density of healthcare providers and fewer healthy food options) and delineate needs for public health investment to provide crucial health services (6–8). Examining patient-specific SDOH may also help clinicians recognize risk factors and deploy targeted interventions earlier in the continuum of care (2,9). Ultimately, addressing SDOH is crucial for national public health goals, including the US Surgeon General's Healthy People initiative, to eliminate health disparities (Figure 1) (10,11).
Figure 1.
Healthy People 2030 framework for social determinants of health.
Despite advances in SDOH research, the relationship between SDOH and chronic liver disease (CLD) outcomes remains unknown. Understanding these associations is clinically important, given that over the past 2 decades, CLD prevalence has increased by 200%. The increased burden of CLD has disproportionately affected vulnerable populations, including SES-deprived areas and marginalized communities (12–15). These trends are further complicated given that liver transplantation (LT), the only curative treatment for CLD, is a highly coveted resource involving an extensive referral processes, with one's candidacy also heavily influenced by SDOH (16).
Evidence from the broader chronic disease literature suggests that patients with CLD living in disadvantaged environments may face complex barriers to care in the pre-LT and post-LT setting (2,17). This may further exacerbate the trajectory of poor clinical outcomes and extensive healthcare burden. To clarify these topics and to synthesize the quality of existing literature, we conducted a systematic review to evaluate the relationship between SDOH with mortality, hospital admissions, and readmissions among patients with CLD.
METHODS
Search strategy
The review protocol was registered (PROSPERO ID: CRD42022346654) and follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (18). An experienced librarian conducted the search strategy through the MEDLINE, Embase, Cochrane Library, and Scopus databases (see Supplementary Materials, Supplementary Digital Content 1, http://links.lww.com/AJG/D448). All searches spanned from database inception until January 8, 2023. Studies found in a manual search after this date and reference lists of included articles were included.
Study selection and eligibility criteria
Two authors (B.J.H. and A.H.) independently screened each study's title and abstract and full text using an online standardized tool. Discrepancies were reconciled through discussion or referral to a third reviewer (P.P.). Data were extracted using a predefined data extraction form.
The study population included all adult (≥ 18 years old) patients living in the United States with CLD. We included journal articles published in the English language only. We excluded studies with acute pathologies of liver disease (e.g., alcoholic hepatitis and hepatic failure) as they may have different clinical trajectories compared with CLD. We also excluded studies that were not published in a peer-reviewed journal (e.g., preprint letters, editorials, dissertations, and opinion articles) or those involving case reports, case series, or basic science research. Studies examining SDOH that used an area unit larger than the county level (e.g., state and national level) were excluded from the study. We restricted this study to those conducted in the United States to (i) minimize confounders from international healthcare system differences and (ii) to account for the specific US geopolitical, historical context of systemic and structural inequity against marginalized groups (19).
Study population and outcomes of interest
Outcomes of interest were mortality, hospital admissions, and readmissions. Outcomes that occurred in the waitlist or post-LT setting were noted for studies involving cohorts on the LT waitlist or receiving LT.
SDOH characteristics and composite scores
This systematic review followed the Healthy People 2023 framework, which includes 5 major domains of SDOH: neighborhood and built environment (e.g., availability of resources and housing quality), healthcare access and quality, education access and quality, economic stability, and social and community context (e.g., community and personal support) (11). SDOH characteristics belonging to each domain are not mutually exclusive and may overlap. Few studies used composite SDOH indices or scores that aggregate multiple SDOH domains to describe geographic areas and can often be extracted from publicly available data (e.g., American Community Survey and Census Bureau) (9,20–38).
Quality assessment and data synthesis
Two reviewers (B.J.H. and A.H.) independently assessed the risk of bias for each study using the Cochrane Risk of Bias in Nonrandomized Studies for Exposure tool (version 6/2022) (39). A third reviewer (P.P.) was consulted if there were discrepant scores to reach a consensus. There were 4 stratifications for risk of bias: “low risk,” “some concerns,” “high risk,” and “very high risk.” Each study was evaluated on the 7 domains of bias that contribute to an overall risk score: confounding, exposure measurement, participant selection/analysis, postexposure interventions, missing data, outcome measurement, and reported results selection.
RESULTS
Overview of the study selection process
Of 5,205 articles identified through database retrieval, 27 manuscripts were included in the final review (Figure 2). There were 24 (88.9%) retrospective cohort studies and 3 (11.1%) cross-sectional studies. Geographic areas were defined using different administrative boundaries: counties (N = 4, 14.8%) (22,26,40,41), ZIP codes (N = 18, 66.7%) (20,21,27–30,32–38,42–46), and census tracts (N = 4, 14.8%) (9,23–25). These studies included patients with CLD in the general population (N = 12, 44.4%), those on the LT waitlist (N = 10, 37.0%), and LT recipients (N = 5, 18.5%) (Table 1). Table 2 provides a summary of each study included in the review.
Figure 2.
Search results and application of eligibility criteria. SDOH, social determinants of health.
Table 1.
Characteristics of studies reviewed

Table 2.
Characteristics of each study included in the review
Demographic differences between SDOH indices/scores
SES-deprived areas had higher proportions of non-Hispanic Black (NHB) or Hispanic patients (20,22–25,29,31–35,38,47) and had higher rates of public or no insurance (20,22–25,30,31,34,35,37). These patients were younger (30), traveled further distances to receive care (36,37,44), had lower family support (35,47), and received care at lower volume LT centers (20,37). There were differences in management strategies for patients from low SES areas: They received lower usage of living donor liver transplant (LDLT), split grafts, and nationally shared organs (20,21,31,46).
There were 4 studies that analyzed the interaction between SES-deprived areas and race (24,28,29,35). Associations of poor outcomes among NHB and Hispanic patients may be mitigated by higher household income or residing in high SES areas (28,35). Underrepresented populations from low SES areas were particularly vulnerable, having a 31% higher risk of being denied from the LT waitlist (24).
Patients from more socially deprived areas had higher rates of metabolic dysfunction-associated steatohepatitis (MASH) and hepatitis C virus (HCV) (20,21,23,30,31,34,35,37,46,47). Disease severity of CLD was also much higher as shown by their higher model for end-stage liver disease scores (20,33,35,37) and rates of decompensation events (35), especially ascites (31,35,36). Patients with cholestatic liver disease (9,42,44), HCV (9,42,44), autoimmune (32), alcohol-related (44,45) CLD, and hepatocellular carcinoma (HCC) (24,44) lived further away and traveled longer distances to LT centers to receive care. CLD etiologies with the lowest rates of LT waitlisting were HCV and alcoholic liver disease, whereas the highest rates of LT waitlisting were MASH, autoimmune, cholestatic CLD, and HCC (25,47). Bitterman et al (40) reported that autoimmune, viral hepatitis, and MASH CLD had 8%, 41%, and 56% higher odds of facing food insecurity respectively, compared with alcoholic liver disease. Other CLD-related predictors of high food insecurity included hypoalbuminemia and hepatic encephalopathy (40).
Economic stability
There were 19 (70.4%) studies (9,20–22,24–26,28–30,32–38,40,42,48) that investigated the association between income/poverty with mortality (Table 3). Of these, 10 (52.6%) studies (9,20–22,26,34–36,38,40,48) found an association between low economic stability with high risks of mortality. Economic stability had the highest impact on poor morbidity and high mortality compared with other domains of SDOH (34). Karpati et al (26) found that the most impactful elements of economic stability associated with all-cause mortality were employment and poverty status (48).
Table 3.
Covariates of social determinants of health investigated by studies included
Studies reporting the associations between SDOHs and admission or readmission rates are limited (N = 1, 5.9%). A single study using the Nationwide Readmission Database found that patients residing in the lowest SES quartile were more likely to be readmitted within 30, 90, and 180 days compared with those in the highest income quartile (30).
Healthcare access and quality
Insurance status
There were 14 (51.9%) (20,21,24,25,27,28,30–34,36,37) studies that investigated the associations of insurance status and outcomes (Table 3). There was a correlation between lower SES and public insurance (e.g., Medicare and Medicaid) status and, conversely, high SES with private insurance status (20,21,24,25,27,28,30–34,36,37). Public insurance was associated with lower odds of LT (25), higher risks of mortality (32,36), and odds of 30-, 90-, and 180-day readmissions (30).
Distance to LT center
There were 9 studies (33.3%) that investigated the association of LT center proximity with mortality (9,20,23,27,42–46). Multicenter collaborations found that increasing distance from LT centers had lower survival (20,27,42–46). There was a dose-response relationship between increasing distance to LT center and mortality at 1, 3, and 5 years of follow-up starting at a threshold of 25 miles (20,27,42–46). This risk increased with every 50-mile interval, culminating in the largest risk of mortality being observed at >150 miles away. Increasing distance was also associated with lower odds of LT, despite being listed at multiple LT centers (23,43,44).
Education access and quality
Education status and highest level of education attained were reported in 17 (63.0%) (9,20–22,24–26,28,29,32–38,40,48) studies, although they were rarely the primary SDOH covariate of interest (Table 3). Lower education attainment was associated with increased risk of death (32,35) and lower likelihood of waitlisting (35).
Social and community context
Social and community context SDOH covariates were the least of all SDOH domains that were studied (N = 11; 40.7%) (9,24,25,32–38,40) and were often lumped into composite SDOH indices (Table 3). Patients with risk factors within social and community context domains had lower likelihood of waitlisting (25,35), although there were no associated differences in likelihood of LT once on the waitlist (25). Specifically, patients residing in areas with higher proportions of single-parent households with children had higher risks of all-cause mortality (25,35).
Neighborhood and built environment
Living and environmental conditions
There were 13 (48.1%) studies that investigated household living and environmental conditions (9,20–22,24,29,33–38,44), although most were included in composite SDOH indices (Table 3). Poor household conditions (e.g., household crowding and lack of complete plumbing) were associated with lower likelihood of waitlisting (25,35) and LT (25). Hasjim et al (35) also found that patients residing in areas with lower home values, mortgages, or gross rent were associated with higher risks of all-cause mortality.
Food insecurity and food deserts
Studies examining access to fresh foods were minimal. Two (7.4%) (40,41) studies investigated the associations between food insecurity and food deserts using linked data from the national LT registry (Table 3). Food insecurity was estimated using data from the Census and US Department of Agriculture through Map the Meal Gap, a national effort by Feeding America. Food deserts were defined through the Food Environment Atlas and identified counties with limited access to fresh produce based on their distance to the nearest supermarket/grocery store. Patients with steatotic CLD were more likely to reside in areas high in food insecurity (40). Food deserts were associated with higher rates of CLD-related mortality (40,41) and lower access to LT in this cohort (41).
Composite SDOH indices/scores
Five studies (18.5%) (9,20,21,26,34,48) created their own study-specific index, whereas seven (25.9%) (23,24,32,33,35–37) used the Area Deprivation Index. The Community Health Score (46), County Health Ranking (31), Distressed Community Index (22), Social Deprivation Index (38), and Social Vulnerability Index (25) were each used once (Table 3) (9,20–38). Low composite SES scores were correlated with rural areas, areas with fewer densities of primary care physicians, and treatment at safety net hospitals (22–24,49).
SDOH indices/scores and mortality
Five studies (18.5%) did not find significant differences between area SDOH index and survival (22,25,29,38,49). However, most studies found that patients with CLD residing in low SES areas had higher rates of mortality. After adjusting for confounders, patients residing in the lowest SES quartile experienced higher mortality rates, with 1 study reporting as high as a 25% increased risk of death (20–24,32,34–38). The impact of area SES is more pronounced in LT candidates hoping to find a living donor. Kanneganti et al (38) found that patients residing in more deprived areas were less likely to receive an LDLT and had a 19% increased risk of waitlist mortality.
SDOH indices/scores and hospital admission/readmission
There were no studies examining hospital admission and a limited number of studies assessing readmission (20,21,30,36) (N = 4, 14.8%). The majority of studies (N = 3) involved post-LT cohorts and did not show significant differences in readmission rates between SDOH index strata (20,21,36).
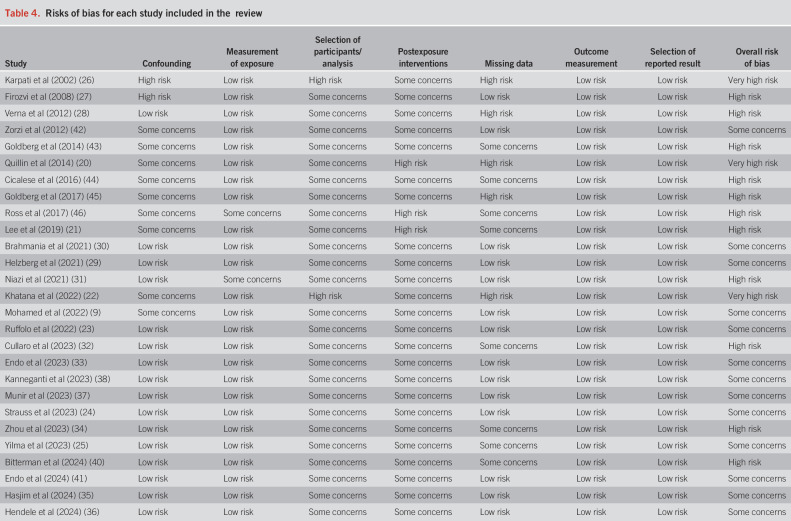
Study quality
The included studies for this systematic review varied in quality, and there were no studies evaluated to have an overall “low risk of bias.” The majority of studies had “some concerns” of bias (N = 13, 48.1%), followed by “high risk” (N = 11, 40.7%) and “very high risk” (N = 3, 11.1%). There were no studies that had “low risk” of bias with respect to participant selection and postexposure interventions—that is, the true length of time a patient was exposed to various SDOH factors were often not accounted for in the analysis nor captured during the observation period of the study. Bias in relation to confounding factors also had “some concerns” (N = 9, 33.3%) or “high risk” (N = 2, 7.4%) because many studies were limited in their ability to account for the same clinicodemographic covariates in multivariable statistical models (Table 4).
Table 4.
Risks of bias for each study included in the review
DISCUSSION
This is the first systematic review to synthesize the impact of SDOH on CLD outcomes. Collectively, these studies showed that patients with CLD who face unfavorable SDOH or reside in SES-deprived areas were associated with higher mortality and hospital readmissions in the United States (20–24,32,34–38,40,41). In addition, increasing distance from LT centers was associated with higher mortality and lower LT rates, despite having access to multiple LT waitlists (20,27,42–46). This systematic review underscores the influence of SDOH on CLD outcomes, which have not been previously synthesized in the literature.
Associations of low SES with poor clinical outcomes were similar in CLD as those previously shown in cardiometabolic chronic diseases (50,51). Especially since recent advances in our understanding of CLD have notable overlap with cardiometabolic syndromes through steatotic CLD etiologies of cirrhosis, similar associations can be expected with CLD. Because of the maturity of SDOH research within the cardiovascular chronic disease literature, where significant progress has been made in implementing social needs screening, these findings may be translatable to the CLD population (3). For example, the American Heart Association has called for better SDOH cardiovascular health education across providers of all levels and promoted policies that will expand social care resources to address upstream determinants (3,52). Such policies include leveraging technology and the electronic health record (EHR) to automate screening protocols to identify patients with social needs more efficiently (53,54). In a single-center study that implemented autogenerated medical codes from physician notes of patients who may have social needs, 86% of screened patients were able to receive tailored resources that were embedded into the EHR (54). A meta-analysis on the effectiveness of SDOH-focused hepatitis B virus (HBV) interventions found that at least 66% of all interventions were effective in addressing disparate HBV-related care (55). These interventions included narrative videos to engage and educate at-risk populations (56), automated EHR alerts to increase HBV surface antigen testing in the outpatient and emergency department settings (57,58), and programming to increase HCC screening and education among subpopulations (55,59,60). Likewise, research on the implementation of interventions for CLD should focus on the following themes: knowledge and education, diagnosis and screening, care initiation, engagement with clinical care, and upstream prevention (55). Although inclusion of SDOH is suggested in many clinical guidelines, few provide clear guidance for how clinicians should identify and address actionable social risk factors in the context of care delivery (61). Further research on the implementation of these interventions among patients with CLD is essential next step toward health equity.
Along with worse survival, patients residing in more SES-deprived areas have lower rates of LT referral and care (62). Systemic or implicit biases may be linked to area-level social disadvantage that may ultimately influence management strategies for these patients (63,64). There were significant demographic differences between those residing in high and low SES areas. Patients with CLD in low area-level SES were more likely from underrepresented minority groups and had lower access to healthcare (22–24,49). These patients also have higher rates of alcoholic use disorder, viral hepatitis cirrhosis etiology, and treatment at safety net hospitals (22–24). These associations may lead to lower rates of LT referral, usage of LDLT, split grafts, and nationally shared organs (20,21,31,46). Compared with non-Hispanic White, NHB and Hispanic patients have been reported to have 64% and 41% lower rates of LT waitlisting, respectively (35). Meanwhile, patients from the highest SES neighborhoods exhibited higher utilization of resources, incurring 7.6% more costs, and were more often discharged to a facility (20). These findings highlight that significant disparities in LT evaluation and assessment of treatment options may exist. Addressing these disparities requires institutional investment and engagement. Initiatives such as Northwestern Medicine's Hispanic Liver Transplant Program (65–68), African American Transplant Access Program (69), and the University of California San Francisco's DeLIVER Care Van have provided aid to disenfranchised populations in navigating the complex CLD-care pathway (2,70,71). Inspiration can also be drawn from the kidney transplant community, which has been laying the groundwork for rigorous research (e.g., randomized control trials and prospective studies) and outreach initiatives to improve transplant access (72–74).
Although published composite SDOH indices are valuable for capturing multiple dimensions of SDOH and their associations with poor outcomes, significant heterogeneity exists in their definitions. For example, Strauss et al's Area Deprivation Index excludes age- and race-related covariates in its composite score unlike Yilma et al's Social Vulnerability Index (24,25). These indices may also combine individual- and area-level characteristics that are difficult to disentangle when considering targeted interventions. Identifying specific SDOH risk factors that drive these associations within composite SDOH indices is important to explain and may differ by geographic area. In a multicenter study in Chicago, a city with a history of redlining, housing conditions (e.g., crowding and availability of plumbing) were linked with higher risks for mortality, hepatic decompensation, and delays in LT waitlisting (35). Selection of SDOH covariates for research should align with study aims and community needs because different indices suit different research questions. A community-engaged approach is crucial for implementing interventions that address personalized structural barriers. Although telemedicine has gained in popularity to overcome geographical barriers, structural barriers such as lack of internet/cellular data coverage and access to privacy for telehealth visits may still persist (17). As digital tools are integrated into patient care, future investigations are needed to identify structural barriers that prevent equitable access to care. Disparities must be contextualized within specific geopolitical and healthcare systems to identify structural barriers that perpetuate disparities.
International cohorts can reveal similarities and differences in challenges to address SDOH. The Chronic Liver Disease Evolution and Registry for Events and Decompensation Consortium, an international multicenter effort from 90 tertiary care hospitals across 25 countries from 6 continents, found that patients who received care in lower-income countries were associated with twice the odds of death during or within 30 days of hospitalization compared with high-income countries (75). These associations were independent of known medical risk factors (75). Similarly, in cohorts of patients from Canada, patients residing in unstable neighborhoods and who had negative risk factors of SDOH were associated with more than 40% reduced rates of LT and lower likelihood of access to LDLT (76,77). However, these associations can be mitigated by neighborhoods with diverse immigrant or racial minority populations, age, and labor force (76,77). It is paramount for implementation strategies to consider the accessibility of specialized services (e.g., LT and intensive care) and to tailor interventions to each country's sociocultural context (75,78). For example, India has one of the largest gender disparities in receipt of LT, which may be due to sociocultural disadvantages and pressures of the female sex (79). Meanwhile, differences in sociocultural attitudes toward utilization of DDLT and LDLT between Western and Asian countries will also pose unique downstream challenges (78,80). Establishing a nationwide regulatory body with more uniform definitions for transplant candidacy among developing countries can help mitigate disparities from center to center (81–84).
There are several limitations to our systematic review. This comprehensive review may be susceptible to publication bias because it does not include unpublished reports, meeting abstracts, or preprint letters. However, all peer-reviewed manuscripts were considered in this review. Second, the definition of SDOH was heterogenous across studies and may lead to imprecision, indirectness, and hindered our ability to conduct pooled estimates for a meta-analysis. Third, some SDOH characteristics reported (such as education and income) may be more precisely evaluated at the patient level, rather than the area. However, SDOH that are measured at an area level serve as a critical proxy for environmental conditions, safety, quality of housing, and community capital that remains closely linked with a range of chronic disease outcomes (85). Fourth, although descriptive statistics were available on the prevalence of CLD etiologies among SES-deprived areas, interaction analyses between disease entities and their outcomes were limited. Previous work has shown that different CLD-related factors may face their own unique SDOH challenges (15,40,86). Future research is needed to investigate the differential impact of SDOH as they relate to distinct subgroups of CLD. Last, defining the appropriate area unit for measuring SDOH posed many challenges because current studies heavily rely on large geographic units that were usually developed for administrative purposes (87). Nevertheless, this review provides insightful opportunities and direction for future SDOH research.
Many gaps in the literature remain regarding the impact of SDOH on CLD-related outcomes. In particular, there were no studies examining the impact of SDOH and hospital admission risk. Since the clinical progression of CLD is often insidious, secondary end points such as hospitalizations, LT referrals (62), or waitlisting (35,88) will be critical for identifying upstream SDOH risk factors compared with traditional end points (e.g., mortality and LT). Moreover, future research should broaden its scope to other aspects of SDOH and warrants more rigorous research methodologies. Other elements of a patient's lived environment, such as social cohesion, exposure to pollution, neighborhood walkability, immigration, and transportation, are all factors that had been previously identified in other literature to impact chronic disease (89–92). In addition, one of the biggest challenges in approaching SDOH research is characterizing the temporal nature between environmental exposure and clinical outcomes, which contributes to a considerable risk of bias. Over time, patients may move to different environments, and neighborhood characteristics may also change (e.g., gentrification). Differences or changes in the exposure (e.g., neighborhood features) may not be captured since they are measured at a single point in time through a cross-sectional approach. Thresholds for exposure time resulting in clinically significant outcomes may exist but are largely unknown (93,94). The environment may take decades to have an associated clinically significant outcome, and some exposures may be seen even before birth. A dose-dependent effect, like those observed in Goldberg et al's study on distance to LT center (43), may exist based on the length of time a patient has been exposed to an environment. This underscores the importance of longitudinal datasets for future SDOH and health outcomes research. Last, qualitative studies will be important to crystalize the challenges patients face on an individual level and should be integrated into the quantitative analysis in these studies (24,95,96). Leveraging data extraction tools such as Natural Language Processing to extract unstructured clinical data from the electronic health record can be a promising tool (97). Future work that engages the community and addresses the temporality of environmental exposure to precisely assess patients' risk is warranted.
Current literature on the association between SDOH and CLD-related outcomes remains limited. Patients with CLD exposed to unfavorable SDOH, particularly those who reside in SES-deprived areas or reside farther from LT centers, were associated with higher mortality and hospital readmission rates. Rigorous empirical research assessing additional dimensions of SDOH is needed to develop evidence-based strategies that mitigate health disparities among vulnerable populations.
CONFLICTS OF INTEREST
Guarantor of the article: Daniela P. Ladner, MD, MPH.
Specific author contributions: B.J.H., A.H., and D.P.L.: concept and design. B.J.H., A.H., K.N.K., M.W.L.T., D.P.L., S.B., and J.E.O.: drafting of the manuscript. B.J.H., A.H., P.P., and M.B.: statistical analysis. D.P.L. and S.M.: obtained funding and supervision. B.J.H., O.C.D., M.B., and M.P.: administrative, technical, or material support. All authors: acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content.
Financial support: This work was supported by the “LIVOPT” award (R01AG070194) (D.P.L., A.H., M.P., M.M., S.M., and S.B.), the Transplant Surgery Scientist Training Program (T32DK077662) (B.J.H. and J.E.O.) and the Stryker Endowment Grant (P.P.).
Potential competing interests: None to report.
Amendments: This protocol does not represent an amendment of a previously completed or published protocol. Any future amendments to the protocol will be submitted and registered through PROSPERO.
Study Highlights.
WHAT IS KNOWN
✓ Social determinants of health (SDOH) impacts the incidence of chronic disease and their outcomes.
✓ Chronic liver disease (CLD) is an increasing public health burden.
✓ The relationship between SDOH and CLD is unclear.
WHAT IS NEW HERE
✓ SDOH risk factors in CLD are multifactorial and impact CLD throughout the continuum of care.
✓ Poor SDOH risk factors are associated with early re-hospitalization and mortality rates.
✓ There are limited data on the associations of SDOH and hospitalizations as a clinical endpoint.
✓ There are opportunities for future research to investigate additional dimensions of SDOH and its temporal associations.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/D448
Contributor Information
Bima J. Hasjim, Email: bima.hasjim@northwestern.edu.
Alexandra Harris, Email: alexandra.harris1@northwestern.edu.
Salva N. Balbale, Email: salva.balbale@northwestern.edu.
Joy E. Obayemi, Email: joy.obayemi@northwestern.edu.
Molly Beestrum, Email: molly.beestrum@northwestern.edu.
Praneet Polineni, Email: praneet.polineni@northwestern.edu.
Mitchell Paukner, Email: mitchell.paukner@northwestern.edu.
Mohsen Mohammadi, Email: mohsen.mohammadi@northwestern.edu.
Oriana C. Dentici, Email: oriana.dentici@northwestern.edu.
Kiarri N. Kershaw, Email: k-kershaw@northwestern.edu.
Marquita W. Lewis-Thames, Email: marquita.lewis-thames@northwestern.edu.
Sanjay Mehrotra, Email: mehrotra@northwestern.edu.
REFERENCES
- 1.Kolak M, Bhatt J, Park YH, et al. Quantification of neighborhood-level social determinants of health in the continental United States. JAMA Netw Open 2020;3(1):e1919928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kardashian A, Wilder J, Terrault NA, et al. Addressing social determinants of liver disease during the COVID-19 pandemic and beyond: A call to action. Hepatology 2021;73(2):811–20. [DOI] [PubMed] [Google Scholar]
- 3.Powell-Wiley TM, Baumer Y, Baah FO, et al. Social determinants of cardiovascular disease. Circ Res 2022;130(5):782–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teshale AB, Htun HL, Owen A, et al. The role of social determinants of health in cardiovascular diseases: An umbrella review. J Am Heart Assoc 2023;12(13):e029765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2015;132(9):873–98. [DOI] [PubMed] [Google Scholar]
- 6.Kershaw KN, Albrecht SS, Carnethon MR. Racial and ethnic residential segregation, the neighborhood socioeconomic environment, and obesity among blacks and Mexican Americans. Am J Epidemiol 2013;177(4):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kershaw KN, Osypuk TL, Do DP, et al. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease the multi-ethnic study of atherosclerosis. Circulation 2015;131(2):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurani SS, Lampman MA, Funni SA, et al. Association between area-level socioeconomic deprivation and Diabetes care quality in US primary care practices. JAMA Netw Open 2021;4(12):e2138438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamed KA, Ghabril M, Desai A, et al. Neighborhood poverty is associated with failure to be waitlisted and death during liver transplantation evaluation. Liver Transplant 2022;28(9):1441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson S, Gold MR, Baciu A. Rethinking the leading health indicators for healthy people 2030. JAMA Health Forum 2020;1(5):e200426. [DOI] [PubMed] [Google Scholar]
- 11.Hasbrouck LM. Healthy people 2030: An improved framework. Health Education Behav 2021;48(2):113–4. [DOI] [PubMed] [Google Scholar]
- 12.Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149(6):1471–e18. [DOI] [PubMed] [Google Scholar]
- 13.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. BMJ 2018;362:2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladner DP, Gmeiner M, Hasjim BJ, et al. Increasing prevalence of cirrhosis among insured adults in the United States, 2012–2018. PLoS One 2024;19(2):e0298887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kardashian A, Serper M, Terrault N, et al. Health disparities in chronic liver disease. Hepatology 2023;77(4):1382–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder JJ, Schaffhausen CR, Hart A, et al. Stakeholders' perspectives on transplant metrics: The 2022 Scientific Registry of Transplant Recipients' consensus conference. Am J Transplant 2023;23(7):875–90. [DOI] [PubMed] [Google Scholar]
- 17.Rosenblatt R, Lee H, Liapakis AM, et al. Equitable access to liver transplant: Bridging the gaps in the social determinants of health. Hepatology 2021;74(5):2808–12. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017;389(10077):1453–63. [DOI] [PubMed] [Google Scholar]
- 20.Quillin RC, Wilson GC, Wima K, et al. Neighborhood level effects of socioeconomic status on liver transplant selection and recipient survival. Clin Gastroenterol Hepatol 2014;12(11):1934–41. [DOI] [PubMed] [Google Scholar]
- 21.Lee TC, Dhar VK, Hoehn RS, et al. Liver transplantation at safety net hospitals: Potentially vulnerable patients with noninferior outcomes. Surgery 2019;166(6):1135–41. [DOI] [PubMed] [Google Scholar]
- 22.Khatana SAM, Goldberg DS. Changes in county-level economic prosperity are associated with liver disease–related mortality among working-age adults. Clin Gastroenterol Hepatol 2022;20(5):1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruffolo LI, Zambrano D, Dale BS, et al. Inferior survival is associated with socioeconomic deprivation in hepatocellular carcinoma. J Surg Res 2022;279:228–39. [DOI] [PubMed] [Google Scholar]
- 24.Strauss AT, Moughames E, Jackson JW, et al. Critical interactions between race and the highly granular area deprivation index in liver transplant evaluation. Clin Transplant 2023;37(5):e14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilma M, Cogan R, Shui AM, et al. Community-level social vulnerability and individual socioeconomic status on liver transplant referral outcome. Hepatol Commun 2023;7:e00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karpati A, Galea S, Awerbuch T, et al. Variability and vulnerability at the ecological level: Implications for understanding the social determinants of health. Am J Public Health 2002;92(11):1768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firozvi AA, Lee CH, Hayashi PH. Greater travel time to a liver transplant center does not adversely affect clinical outcomes. Liver Transpl 2008;14(1):18–24. [DOI] [PubMed] [Google Scholar]
- 28.Verna EC, Valadao R, Farrand E, et al. Effects of ethnicity and socioeconomic status on survival and severity of fibrosis in liver transplant recipients with hepatitis C virus. Liver Transplant 2012;18(4):461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helzberg JH, Dai R, Muir AJ, et al. Socioeconomic status is associated with the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol 2021;32(7):950–60.e1. [DOI] [PubMed] [Google Scholar]
- 30.Brahmania M, Wiskar K, Walley KR, et al. Lower household income is associated with an increased risk of hospital readmission in patients with decompensated cirrhosis. J Gastroenterol Hepatol 2021;36(4):1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niazi SK, Vargas E, Spaulding A, et al. Impact of county health rankings on nationwide liver transplant outcomes. Transplantation 2021;105(11):2411–9. [DOI] [PubMed] [Google Scholar]
- 32.Cullaro G, Ge J, Lee BP, et al. Association between neighborhood-based material deprivation and liver transplant waitlist registrants demographics and mortality. Clin Transplant 2024;38(1):e15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endo Y, Sasaki K, Moazzam Z, et al. Liver transplantation access and outcomes: Impact of variations in liver-specific specialty care. Surgery 2024;175(3):868–76. [DOI] [PubMed] [Google Scholar]
- 34.Zhou K, Lit A, Kuo LS, et al. Neighborhood-level social determinants of health and waitlist mortality for liver transplantation: The liver outcomes and equity index. Transplantation 2024;108(7):1558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasjim BJ, Huang AA, Paukner M, et al. Where you live matters: Area deprivation predicts poor survival and liver transplant waitlisting. Am J Transplant 2024;24(5):803–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendele JB, Nichols JT, Vutien P, et al. A retrospective cohort study of socioeconomic deprivation and post-liver transplant survival in adults. Liver Transplant 2024;30(8):816–25. [DOI] [PubMed] [Google Scholar]
- 37.Munir MM, Endo Y, Mehdi Khan MM, et al. Association of neighborhood deprivation and transplant center quality with liver transplantation outcomes. J Am Coll Surg 2024;238(3):291–302. [DOI] [PubMed] [Google Scholar]
- 38.Kanneganti M, Byhoff E, Serper M, et al. Neighborhood-level social determinants of health measures independently predict receipt of living donor liver transplantation in the United States. Liver Transplant 2023;30(6):618–27. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bittermann T, Goldberg DS, Rudel RK, et al. Liver disease etiology and race/ethnicity are associated with neighborhood food insecurity risk in US candidates for liver transplant. Liver Transplant 2024;30(10):1086–90. [DOI] [PubMed] [Google Scholar]
- 41.Endo Y, Tsilimigras DI, Khalil M, et al. The impact of county-level food access on the mortality and post-transplant survival among patients with steatotic liver disease. Surgery 2024;176(1):196–204. [DOI] [PubMed] [Google Scholar]
- 42.Zorzi D, Rastellini C, Freeman DH, et al. Increase in mortality rate of liver transplant candidates residing in specific geographic areas: Analysis of UNOS data. Am J Transplant 2012;12(8):2188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA 2014;311(12):1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cicalese L, Shirafkan A, Jennings K, et al. Increased risk of death for patients on the waitlist for liver transplant residing at greater distance from specialized liver transplant centers in the United States. Transplantation 2016;100(10):2146–52. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg DS, Newcomb C, Gilroy R, et al. Increased distance to a liver transplant center is associated with higher mortality for patients with chronic liver failure. Clin Gastroenterol Hepatol 2017;15(6):958–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross K, Patzer RE, Goldberg DS, et al. Sociodemographic determinants of waitlist and posttransplant survival among end-stage liver disease patients. Am J Transplant 2017;17(11):2879–89. [DOI] [PubMed] [Google Scholar]
- 47.Mohammed SH, Habtewold TD, Birhanu MM, et al. Neighbourhood socioeconomic status and overweight/obesity: A systematic review and meta-analysis of epidemiological studies. BMJ Open 2019;9(11):e028238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karpati A, Galea S, Awerbuch T, et al. Variability and vulnerability at the ecological level: Implications for understanding the social determinants of health. Am J Public Health 2002;92:1768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Endo Y, Alaimo L, Sasaki K, et al. Liver transplantation for colorectal liver metastases: Hazard function analysis of data from the organ procurement and transplantation network. J Gastrointest Surg 2023;27(8):1720–2. [DOI] [PubMed] [Google Scholar]
- 50.Abreu TC, Mackenbach JD, Heuvelman F, et al. Associations between dimensions of the social environment and cardiometabolic risk factors: Systematic review and meta-analysis. SSM Popul Health 2024;25:101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toms R, Bonney A, Mayne DJ, et al. Geographic and area-level socioeconomic variation in cardiometabolic risk factor distribution: A systematic review of the literature. Int J Health Geogr 2019;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White-Williams C, Rossi LP, Bittner VA, et al. Addressing social determinants of health in the care of patients with Heart failure: A scientific statement from the American Heart Association. Circulation 2020;141(22):e841–63. [DOI] [PubMed] [Google Scholar]
- 53.Davidson KW, McGinn T. Screening for social determinants of health: The known and unknown. JAMA 2019;322(11):1037–8. [DOI] [PubMed] [Google Scholar]
- 54.Buitron de la Vega P, Losi S, Sprague Martinez L, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care 2019;57(Suppl 6 Suppl 2):S133–9. [DOI] [PubMed] [Google Scholar]
- 55.Anyiwe K, Erman A, Hassan M, et al. Characterising the effectiveness of social determinants of health-focused hepatitis B interventions: A systematic review. Lancet Infect Dis 2024;24(6):e366–85. [DOI] [PubMed] [Google Scholar]
- 56.Alber JM, Cohen C, Bleakley A, et al. Comparing the effects of different story types and speakers in hepatitis B storytelling videos. Health Promot Pract 2020;21(5):811–21. [DOI] [PubMed] [Google Scholar]
- 57.Chak E, Taefi A, Li C-S, et al. Electronic medical alerts increase screening for chronic hepatitis B: A randomized, double-blind, controlled trial. Cancer Epidemiol Biomarkers Prev 2018;27(11):1352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brock C, Yi Y, Papaluca T, et al. Exploring the feasibility of targeted chronic hepatitis B screening in the emergency department: A pilot study. Emerg Med Australas 2018;30(6):864–6. [DOI] [PubMed] [Google Scholar]
- 59.Ching LK, Gounder PP, Bulkow L, et al. Incidence of hepatocellular carcinoma according to hepatitis B virus genotype in Alaska Native people. Liver Int 2016;36(10):1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weir RC, Toyoji M, McKee M, et al. Assessing the impact of electronic health record interventions on hepatitis B screening and vaccination. J Health Care Poor Underserved 2018;29(4):1587–605. [DOI] [PubMed] [Google Scholar]
- 61.Razon N, Hessler-Jones D, Bibbins-Domingo K, et al. How hypertension guidelines address social determinants of health: A systematic scoping review. Med Care 2021;59(12):1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henson JB, Chan NW, Wilder JM, et al. Characterization of social determinants of health of a liver transplant referral population. Liver Transplant 2023;29(11):1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. Am J Public Health 2015;105(12):e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maina IW, Belton TD, Ginzberg S, et al. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc Sci Med 2018;199:219–29. [DOI] [PubMed] [Google Scholar]
- 65.Gordon EJ, Lee J, Kang R, et al. Hispanic/latino disparities in living donor kidney transplantation: Role of a culturally competent transplant Program. Transpl Direct 2015;1(8):e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon EJ, Feinglass J, Carney P, et al. A culturally targeted website for Hispanics/Latinos about living kidney donation and transplantation: A randomized controlled trial of increased knowledge. Transplantation 2016;100(5):1149–60. [DOI] [PubMed] [Google Scholar]
- 67.Gordon EJ, Reddy E, Gil S, et al. Culturally competent transplant program improves Hispanics' knowledge and attitudes about live kidney donation and transplant. Prog Transpl 2014;24(1):56–68. [DOI] [PubMed] [Google Scholar]
- 68.Gordon EJ, Mullee JO, Ramirez DI, et al. Hispanic/Latino concerns about living kidney donation: A focus group study. Prog Transpl 2014;24(2):152–62. [DOI] [PubMed] [Google Scholar]
- 69.Simpson DC, Obayemi JE, Kershaw KN, et al. The African American Transplant Access Program: Mitigating disparities in solid organ transplantation. NEJM Catalyst 2024;5(9):CAT.24.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price JC, Kanner R, Valadao E, et al. Mobile HCV screening in an at-risk urban population identifies significant fibrosis. In: Conference on Retroviruses and Opportunistic Infections (CROI); 2020. [Google Scholar]
- 71.Mera J, Williams MB, Essex W, et al. Evaluation of the Cherokee Nation Hepatitis C Virus Elimination Program in the first 22 months of implementation. JAMA Netw Open 2020;3(12):e2030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delman AM, Turner KM, Silski LS, et al. The kidney transplant equity index: Improving racial and ethnic minority access to transplantation. Ann Surg 2022;276(3):420–9. [DOI] [PubMed] [Google Scholar]
- 73.Patzer RE, Gander J, Sauls L, et al. The RaDIANT community study protocol: Community-based participatory research for reducing disparities in access to kidney transplantation. BMC Nephrol 2014;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patzer RE, Paul S, Plantinga L, et al. A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol 2017;28(3):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bajaj JS, Choudhury AK, Xie Q, et al. Global disparities in mortality and liver transplantation in hospitalised patients with cirrhosis: A prospective cohort study for the CLEARED Consortium. Lancet Gastroenterol Hepatol 2023;8(7):611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flemming JA, Muaddi H, Djerboua M, et al. Association between social determinants of health and rates of liver transplantation in individuals with cirrhosis. Hepatology 2022;76(4):1079–89. [DOI] [PubMed] [Google Scholar]
- 77.Leung KK, Kim A, Hansen BE, et al. The impact of primary liver disease and social determinants in a mixed donor liver transplant Program: A single-center analysis. Liver Transplant 2021;27(12):1733–46. [DOI] [PubMed] [Google Scholar]
- 78.Bajaj JS, Choudhury A, Kumaran V, et al. Geographic disparities in access to liver transplant for advanced cirrhosis: Time to ring the alarm! Am J Transplant 2024;24(5):733–42. [DOI] [PubMed] [Google Scholar]
- 79.Sahay M. Men are from mars, women are from venus: Gender disparity in transplantation. Indian J Transpl 2019;13(4):237. [Google Scholar]
- 80.Lo C-M. Deceased donation in Asia: Challenges and opportunities. Liver Transpl 2012;18(Suppl 2):S5–7. [DOI] [PubMed] [Google Scholar]
- 81.Narasimhan G, Kota V, Rela M. Liver transplantation in India. Liver Transpl 2016;22(7):1019–24. [DOI] [PubMed] [Google Scholar]
- 82.Contreras AG, McCormack L, Andraus W, et al. Current status of liver transplantation in Latin America. Int J Surg 2020;82S:14–21. [DOI] [PubMed] [Google Scholar]
- 83.Song E, Fabian J, Boshoff PE, et al. Adult liver transplantation in Johannesburg, South Africa (2004–2016): Balancing good outcomes, constrained resources and limited donors. S Afr Med J 2018;108(11):929–36. [DOI] [PubMed] [Google Scholar]
- 84.Peters DH, Garg A, Bloom G, et al. Poverty and access to health care in developing countries. Ann N Y Acad Sci 2008;1136:161–71. [DOI] [PubMed] [Google Scholar]
- 85.Social Determinants of Health Literature Summaries: Healthy People 2030. (https://health.gov/healthypeople/priority-areas/social-determinants-health/literature-summaries#block-sdohinfographics). Accessed April 18, 2024. [Google Scholar]
- 86.Spearman CW, Afihene M, Betiku O, et al. Epidemiology, risk factors, social determinants of health, and current management for non-alcoholic fatty liver disease in sub-Saharan Africa. Lancet Gastroenterol Hepatol 2021;6(12):1036–46. [DOI] [PubMed] [Google Scholar]
- 87.Kurani SS, McCoy RG, Lampman MA, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open 2020;3:e200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson WR, Rega SA, Feurer ID, et al. Associations between social determinants of health and abdominal solid organ transplant wait-lists in the United States. Clin Transplant 2022;36(11):e14784. [DOI] [PubMed] [Google Scholar]
- 89.Kaufman JD, Adar SD, Barr RG, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 2016;388(10045):696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eguchi N, Tantisattamo E, Chung D, et al. Outcomes among undocumented immigrant kidney transplant recipients in California. JAMA Netw Open 2023;6(2):e2254660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watson KB, Whitfield GP, Thomas JV, et al. Associations between the national walkability index and walking among US adults: National Health Interview Survey, 2015. Prev Med 2020;137:106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Syed ST, Gerber BS, Sharp LK. Traveling towards disease: Transportation barriers to health care access. J Commun Health 2013;38(5):976–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and Diabetes: A randomized social experiment. N Engl J Med 2011;365(16):1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ludwig J, Duncan GJ, Gennetian LA, et al. Neighborhood effects on the long-term well-being of low-income adults. Science 2012;337(6101):1505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Browne T, Amamoo A, Patzer RE, et al. Everybody needs a cheerleader to get a kidney transplant: A qualitative study of the patient barriers and facilitators to kidney transplantation in the Southeastern United States. BMC Nephrol 2016;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harding JL, Perez A, Snow K, et al. Non-medical barriers in access to early steps of kidney transplantation in the United States: A scoping review. Transplant Rev 2021;35(4):100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ge J, Lai JC, Wadhwani SI. Novel approaches are needed to study social determinants of health in liver transplantation. Liver Transplant 2023;29(3):241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]