Abstract
Background:
Quantitative computed tomography has emerged as a crucial tool for assessing the severity of emphysema in chronic obstructive pulmonary disease (COPD) patients. Vascular endothelial growth factor (VEGF) levels are significantly elevated in patients with chronic bronchitis but reduced in those with emphysema. Chronic inflammation is a key factor in the pathogenesis and progression of COPD, with cytokines such as Interleukin-1 beta playing a significant role.
Objective:
This study aimed to evaluate the characteristics of emphysema in patients with COPD using quantitative computed tomography (QCT) and to investigate the relationship between the extent of emphysema, clinical phenotypes, lung function, and plasma concentrations of VEGF and IL-1β in COPD patients.
Design:
A prospective cross-sectional study was conducted on 30 male patients with stable COPD at Military Hospital 175.
Methods:
The emphysema index (EI) was quantified using QCT of the chest and categorized into levels from 0 to 4. Data on acute exacerbation frequency, CAT scores, mMRC, pulmonary function indices, arterial blood gas measurements, and plasma concentrations of VEGF and IL-1β were collected and analyzed to determine their relationship with EI.
Results:
The study found an average EI of 12.8% ± 11.64%, with 96.7% of patients exhibiting a bronchitis-dominant phenotype. The severity of airflow obstruction, PaCO2 levels, mMRC scores, and the number of exacerbations per year increased with the degree of emphysema. Conversely, FEV1% and the FEV1/FVC ratio significantly decreased with increasing emphysema severity. Plasma VEGF concentration was inversely correlated with the EI. In GOLD 3 and 4 stages, plasma VEGF levels decreased in proportion to emphysema severity, indicating that more advanced emphysema was associated with a more rapid decline in VEGF concentrations. Notably, when emphysema exceeded 25%, a significant reduction in both VEGF and IL-1β concentrations was observed.
Conclusion:
The EI determined by QCT is a valuable tool for identifying COPD phenotypes and assessing disease severity. It can also provide insights into the prognosis regarding the risk of exacerbations, clinical symptom burden, and lung function decline. The significant inverse correlation between plasma VEGF concentration and EI indicates that decreased VEGF levels may be a crucial factor in the pathogenesis of emphysema, suggesting a potential target for research on “treatable” factors in COPD management.
Trial registration:
The study was approved by an independent ethics committee (Ethics Committee of Military Hospital 175, No. 003/QĐ-IRB-VN01.055) and conducted in accordance with the Declaration of Helsinki and Guidelines for Good Clinical Practice.
Keywords: COPD, emphysema, emphysema index, IL-1β, quantitative computed tomography, VEGF
Introduction
Chronic obstructive pulmonary disease (COPD) is a major global health challenge, characterized by significant morbidity, mortality, and socioeconomic impact. 1 COPD is a progressive lung disease marked by airflow limitation that is not fully reversible, primarily due to a combination of small airway disease and emphysema. This results in increased airflow resistance and decreased lung compliance. Emphysema, characterized by the destruction of alveolar structures, involves the permanent dilation of air spaces beyond the terminal bronchioles and is often accompanied by the destruction of alveolar walls without evident fibrosis. 2
Quantitative computed tomography (QCT) has emerged as a crucial tool for assessing the severity of emphysema in COPD patients. QCT not only detects the presence of emphysema but also quantifies the extent of lung tissue involvement, facilitating the classification of COPD phenotypes.3 –5
The role of vascular endothelial growth factor (VEGF) in the development of various COPD phenotypes has been widely studied. In vitro studies have shown that cigarette smoke reduces the expression and signaling of VEGF and its receptors, potentially leading to emphysema by causing pulmonary endothelial cell death and subsequent apoptosis of alveolar septa. Inhibition of VEGF receptors induces alveolar septal cell apoptosis and contributes to airspace enlargement, a hallmark of emphysema. This inhibition also increases markers of oxidative stress, which are central to COPD development. Histological examinations reveal that alveolar septa in centrilobular emphysema are very thin and nearly avascular, whereas bronchial vascularity is increased in chronic bronchitis. VEGF levels are significantly elevated in patients with chronic bronchitis but reduced in those with emphysema. An inverse correlation between sputum VEGF levels and airflow limitation (measured by FEV1%) was observed in chronic bronchitis patients, whereas a positive correlation between VEGF levels in sputum and FEV1 and gas exchange (DLCO) was seen in patients with emphysema. These studies suggest a complex role for VEGF in COPD, with potential protective functions in the alveoli and harmful effects in the bronchi and bronchioles. 6
Chronic inflammation is a key factor in the pathogenesis and progression of COPD, with cytokines such as Interleukin-1 beta (IL-1β) playing a significant role. IL-1β is associated with inflammation, disease severity, and exacerbation frequency. 7 Elevated serum IL-1β levels may serve as a biomarker for assessing the progression of persistent neutrophilic airway inflammation and the risk of severe disease. 8
This study aims to evaluate the role of the degree of emphysema, as assessed by quantitative lung computed tomography, in predicting the risk of exacerbations and to explore the relationship between emphysema severity, plasma concentrations of VEGF and IL-1β, lung function, and clinical and paraclinical characteristics in patients with stable COPD.
Methods
Study design and subjects
This prospective, cross-sectional study included 30 male patients with stable COPD who were recruited from October 2021 to December 2023 at Military Hospital 175. We did not perform a sample size calculation due to limited financial resources for the testing techniques used in the study. Instead, we employed a convenience sampling method, selecting a sample size of 30 to achieve statistical significance in our research findings. COPD diagnosis was based on the criteria set by the GOLD 2020 and confirmed by spirometry, with a post-bronchodilator FEV1/FVC ratio of <70%. 9 Patients were required to be stable, with no exacerbations within the month preceding enrollment. Exclusion criteria were a history of asthma or allergies, cancer, systemic infections or inflammation, and the use of immunotherapy or stem cell therapies.
Data acquisition
Epidemiological, demographic, clinical, and therapeutic data were collected at the time of admission using a standardized medical record format. The primary outcomes focused on exploring associations between the emphysema index (EI) assessed by QCT and clinical characteristics, lung function, and VEGF and IL-1β levels. Secondary outcomes were analyzed using descriptive statistics to characterize patient demographics, plasma VEGF and IL-1β levels, and the severity of emphysema as measured by QCT.
Sampling and testing of plasma VEGF and IL-1β
Blood samples were collected in 5 mL EDTA tubes for plasma separation. The samples were centrifuged at 3500 rpm for 15 min at 4°C, and the plasma was stored at −80°C until analysis. Cytokine levels of VEGF and IL-1β were measured using the ELISA method with commercial kits (Human VEGF ELISA Kit, Human IL-1β ELISA Kit, Thermo Fisher, USA), following the manufacturer’s instructions.
Quantitative lung CT scan and analysis
Computed tomography (CT) of the entire lung was performed during deep inspiration using a standardized protocol, covering the area from the lung apex to the adrenal glands without contrast enhancement, with a slice thickness of 5 mm and reconstruction of 1 mm. Quantitative analysis of emphysema was conducted on segmented lung images using Thoracic VCAR software. The EI was calculated as the percentage of lung voxels with attenuation values below −950 HU. The degree of emphysema was classified based on EI levels. The classification of emphysema severity, proposed by Goddard et al. in 1982, has gained widespread consensus and is now extensively used in both research and clinical practice. 3
| EI levels | EI |
|---|---|
| Level 0 | EI ⩽ 5% |
| Level 1 | 5% < EI ⩽ 25% |
| Level 2 | 25% < EI ⩽ 50% |
| Level 3 | 50% < EI ⩽ 75% |
| Level 4 | EI > 75% |
EI, emphysema index.
Two COPD phenotypes were identified: predominantly emphysematous COPD (EI ⩾ 35%, <1.75 mm segmental bronchial wall thickness) and predominantly airway COPD (EI < 35%, ⩾1.75 mm segmental bronchial wall thickness). 10
Outcome measures
The primary outcome measure was the association between VEGF and IL-1β concentrations with the EI. Secondary outcome measures included the relationship between plasma VEGF and IL-1β concentrations, as well as EI, with various clinical characteristics such as exacerbation frequency and severity, lung function, and GOLD stages.
Statistical analysis
Data were analyzed using SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA). The normality of the data distribution was assessed using the Kolmogorov–Smirnov test (K–S test). Numerical data were expressed as mean ± standard deviation (SD) or as percentages. Correlations between the severity of emphysema and other variables were examined using Spearman correlation coefficients. The Mann–Whitney U-test was used to compare continuous data, such as VEGF, IL-1β, and EI levels across different subgroups. A p-value of less than 0.05 was considered statistically significant.
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Supplemental Material). 11
Results
Baseline characteristics and QCT findings in patients with COPD
The study included 30 male patients with a mean age of 67.5 ± 6.9 years, all with a history of smoking. The average duration since COPD diagnosis was 7.07 ± 5.6 years. Over the past 12 months, the patients had a mean of 2.2 ± 1.2 exacerbations, with 1.63 ± 0.8 of these requiring hospitalization. Based on GOLD classification, 46.7% of the patients were categorized as GOLD 3 and 16.7% as GOLD 4. The mean mMRC dyspnea score was 2.23 ± 0.57, and the average distance covered in the 6-minute walk test was 326.7 ± 88.2 m (Table 1).
Table 1.
Demographics and biomedical, QCT characteristics of the study population.
| Indices | Patients (n = 30) |
|---|---|
| Age (years), ± SD | 67.5 ± 6.9 |
| Smoking History (yes), n (%) | 30 (100) |
| Duration of disease (years), ± SD | 7.07 ± 5.6 |
| AECOPD in last 12 months, ± SD | 2.2 ± 1.2 |
| AECOPD relate hospitalization/12 months, ± SD | 1.63 ± 0.8 |
| BMI (kg/m2), ± SD | 20.7 ± 4.3 |
| mMRC (scores), ± SD | 2.23 ± 0.57 |
| 6MWT (m), ± SD | 326.7 ± 88.2 |
| CAT (scores), ± SD | 18.1 ± 4.9 |
| BODE Index (scores), ± SD | 4.33 ± 1.62 |
| FVC (L), ± SD | 2.12 ± 0.5 |
| Post-bronchodilator FEV1 (%), ± SD | 45.1 ± 14.9 |
| FEV1/FVC (%), ± SD | 45.3 ± 9.4 |
| GOLD stages, n (%) | |
| GOLD 2 | 11 (36.7) |
| GOLD 3 | 14 (46.7) |
| GOLD 4 | 05 (16.7) |
| White blood cells (G/L) | 8.7 ± 2.99 |
| Albumin (g/L), ± SD | 37.2 ± 3.2 |
| CRP (mg/dL), ± SD | 15.3 ± 27.4 |
| Arterial blood gas | |
| PaO2 (mmHg), ± SD | 81.8 ± 11.3 |
| PaCO2 (mmHg), ± SD | 44.6 ± 5.56 |
| SaO2 (%), ± SD | 95.5 ± 1.92 |
| pH, ± SD | 7.42 ± 0.03 |
| VEGF (pg/ml), ± SD | 70 ± 46.6 |
| IL-1 (pg/ml), ± SD | 11.2 ± 11 |
| Emphysema percentage (%), ± SD | |
| Right lung | 13.25 ± 13.97 |
| Left lung | 13.04 ± 11.05 |
| Total | 12.8 ± 11.64 |
| Emphysema—predominant, n (%) | 01 (3.3) |
Data are presented as ± SD and n (%).
BMI, body mass index; QCT, quantitative computed tomography.
The post-bronchodilator FEV1% was 45.1% ± 14.9%, indicating moderate to severe airflow obstruction. Arterial blood gas analysis revealed that most patients had chronic hypercapnia, with a mean partial pressure of carbon dioxide (PaCO2) of 44.6 ± 5.56 mmHg. The mean plasma concentration of VEGF was 70 ± 46.6 pg/mL, and IL-1β was 11.2 ± 11 pg/mL (Table 1).
QCT showed that the mean EI in the right lung was 13.25% ± 13.97%, in the left lung was 13.04% ± 11.05%, and for the total lung was 12.8% ± 11.64%. Only one patient exhibited an emphysema-predominant phenotype, with EI of 44.9% (Table 1). The classification of emphysema severity based on QCT revealed that 46.7% of patients had level 2, 16.6% had level 1, and 36.7% had level 0 (Figures 1 and 2).
Figure 1.
Emphysema regions visualized as a blue spectrum on QCT using Thoracic VCAR software. The blue spectrum indicates areas of reduced lung density, characteristic of emphysematous tissue, providing detailed insight into the extent and severity of lung damage.
QCT, quantitative computed tomography.
Figure 2.

Levels of emphysema on QCT.
QCT, quantitative computed tomography.
Associations of emphysema levels with clinical and subclinical characteristics, and serum VEGF and IL-1β concentrations in COPD patients
The analysis demonstrated that Body Mass Index (BMI), FEV1%, FEV1/FVC%, and serum albumin concentrations decreased progressively with increasing severity of emphysema (p < 0.05). In addition, the degree of airflow obstruction, as classified by GOLD stages, and the PaCO2 index showed a statistically significant increase corresponding to the severity of emphysema (p < 0.05). Other factors, including mean age, disease duration, mMRC score, C-reactive protein (CRP) concentration, IL-1β levels, the number of acute episodes per 12 months, and the number of acute episodes requiring hospitalization per 12 months, also tended to increase in line with the degree of emphysema. Notably, VEGF concentration decreased progressively with increasing emphysema severity (Table 2).
Table 2.
Comparison of clinical, subclinical characteristics, and cytokine concentrations across different emphysema levels.
| Indices | Level 0 | Level 1 | Level 2 | p-Values |
|---|---|---|---|---|
| Age (years), ± SD | 64.9 ± 6.5 | 69.4 ± 7.3 | 67.8 ± 6.2 | 0.3 |
| Duration of disease (year), ± SD | 5.8 ± 5.6 | 6.8 ± 4.6 | 10.6 ± 7.6 | 0.3 |
| AECOPD/12 months, ± SD | 1.9 ± 0.9 | 2.3 ± 1.4 | 2.6 ± 0.9 | 0.5 |
| AECOPD hospitalization/12 months, ± SD | 1.5 ± 0.7 | 1.6 ± 0.9 | 1.8 ± 0.8 | 0.8 |
| BMI (kg/m2), ± SD | 23.3 ± 4.5 | 19.7 ± 3.4 | 17.8 ± 3.1 | 0.02 |
| mMRC (scores), ± SD | 2.1 ± 0.5 | 2.1 ± 0.6 | 2.6 ± 0.5 | 0.2 |
| CAT (scores), ± SD | 18.1 ± 6.4 | 18.1 ± 4.6 | 18 ± 2.5 | 0.9 |
| 6MWT (m), ± SD | 319.1 ± 99.1 | 331.4 ± 96 | 330 ± 41.1 | 0.9 |
| FEV1 (%), ± SD | 55.3 ± 12.9 | 42.2 ± 13.6 | 31 ± 5.8 | 0.003 |
| FEV1/FVC (%), ± SD | 52.4 ± 10.3 | 42.6 ± 5.3 | 37 ± 5.3 | 0.001 |
| FVC (L), ± SD | 2.3 ± 0.4 | 1.9 ± 0.5 | 2.1 ± 0.4 | 0.1 |
| GOLD stages, n (%) | ||||
| GOLD 2 | 07 (63.6) | 04 (28.6) | 0 | |
| GOLD 3 | 04 (36.4) | 08 (57.1) | 02 (40) | 0.01 |
| GOLD 4 | 0 | 02 (14.3) | 03 (60) | |
| White blood cells (G/L), ± SD | 8.5 ± 2.2 | 7.9 ± 3 | 11.2 ± 3.4 | 0.09 |
| Albumin (g/L), ± SD | 39.2 ± 2.8 | 35.9 ± 2.8 | 36.6 ± 3 | 0.02 |
| CRP (mg/dL), ± SD | 15 ± 27.3 | 14.3 ± 24.3 | 18.8 ± 40.5 | 0.9 |
| Arterial blood gas | ||||
| PaO2 (mmHg), ± SD | 84.3 ± 9.8 | 80.3 ± 10.1 | 80.3 ± 18.2 | 0.7 |
| PaCO2 (mmHg), ± SD | 43.8 ± 3.7 | 43.2 ± 5.1 | 50.6 ± 6.9 | 0.02 |
| SaO2 (%), ± SD | 96 ± 1.2 | 95.4 ± 1.6 | 94.5 ± 3.5 | 0.3 |
| pH, ± SD | 7.42 ± 0.02 | 7.44 ± 0.04 | 7.41 ± 0.02 | 0.1 |
| VEGF (pg/mL), ± SD | 76.4 ± 37.4 | 78.8 ± 55.3 | 32.8 ± 20.6 | 0.1 |
| IL-1β (pg/mL), ± SD | 15.7 ± 14.5 | 7.1 ± 7.5 | 9.4 ± 0.7 | 0.3 |
Data are presented as median (IQR) and n (%).
CRP, C-reactive protein.
A significant inverse relationship was observed between EI and lung function. As EI increased, both FEV1% and the FEV1/FVC ratio declined significantly (p < 0.01), suggesting that emphysema progression is a direct cause of lung function deterioration (Table 3). Furthermore, lung function decline was more pronounced in patients with severe emphysema as EI increased (Figure 3).
Table 3.
Correlation between emphysema severity and lung function parameters.
| Indices | FEV1 (%) | FVC/FEV1 (%) | FVC (L) | |||
|---|---|---|---|---|---|---|
| r | p-Values | r | p-Values | r | p-Values | |
| EI (%) | −0.7 | <0.01 | −0.6 | <0.01 | −0.4 | 0.04 |
| Total lung volume (L) | −0.5 | 0.004 | −0.6 | <0.01 | 0.2 | 0.3 |
Figure 3.
Correlation between emphysema severity and FEV1%, FEV1/FVC%.
There was an inverse correlation between EI and plasma VEGF levels, with VEGF concentrations decreasing as EI increased (r = −0.5, p = 0.02). This finding suggests that reduced plasma VEGF may contribute to the progression of emphysema in COPD patients. However, no significant correlation was found between plasma VEGF levels and the risk of exacerbations. Similarly, IL-1β levels did not show a clear significant correlation with EI or the risk of exacerbations (Table 4).
Table 4.
Correlation between serum VEGF, IL-1β concentrations and emphysema index, and acute exacerbations of COPD.
| Indices | EI (%) | AECOPD/12 months | AECOPD hospitalization/12 months | |||
|---|---|---|---|---|---|---|
| r | p-Values | r | p-Values | r | p-Values | |
| VEGF (pg/mL) | −0.5 | 0.02 | −0.14 | 0.5 | 0.14 | 0.5 |
| IL-1β (pg/mL) | −0.12 | 0.6 | 0.16 | 0.5 | 0.15 | 0.6 |
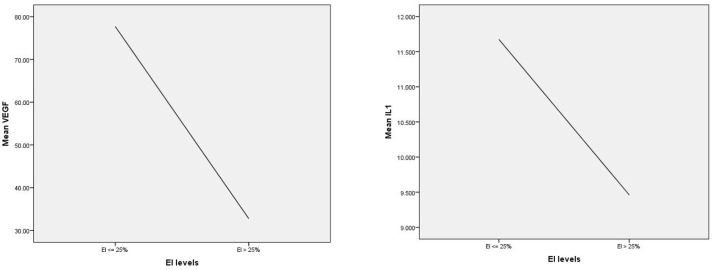
Further analysis revealed that plasma VEGF concentrations in GOLD 3 and 4 patients decreased inversely with the severity of emphysema; the more severe the emphysema, the more rapid the decline in VEGF levels (Figure 4). At emphysema levels of 25% or higher, a significant decrease in both VEGF and IL-1β concentrations was observed (Figure 5). In addition, the frequency of exacerbations increases proportionally with the degree of emphysema across all GOLD stages. In the GOLD 4 group, a significant correlation is observed between the degree of emphysema, the frequency of exacerbations, and the number of hospitalizations (Figure 6).
Figure 4.
Correlation between emphysema severity and plasma levels of VEGF and IL-1β according to GOLD stages.
Figure 5.
Changes in serum levels of VEGF and IL-1β in patients with an emphysema level above 25%
Figure 6.
Correlation between emphysema severity and frequency of exacerbations and hospitalizations over 12 months according to GOLD stages.
Discussion
Baseline characteristics and QCT in patients with COPD
All patients in this study were male, with a mean age of 67.5 ± 6.9 years and a history of smoking, reflecting the typical epidemiological profile observed in Southeast Asia, particularly in Vietnam. 12 The mean number of exacerbations in the last 12 months was 2.2 ± 1.2, with 1.63 ± 0.8 requiring hospitalization. These figures are higher than those reported in other regional studies.13,14 This discrepancy could be attributed to the fact that most participants were classified as GOLD stage 2 or higher and were of advanced age, both of which are associated with more frequent exacerbations. In addition, suboptimal management of treatment could have contributed to the higher exacerbation rates. 15
The mean mMRC dyspnea score was 2.23 ± 0.57, and the mean distance in the 6-minute walk test (6MWT) was 326.7 ± 88.2 m. These results align with the observed degree of airflow obstruction in the study population, which had a mean post-bronchodilator FEV1% of 45.1 ± 14.9%. The 6MWT is a straightforward and effective tool for assessing overall exercise capacity without requiring specialized equipment or extensive technician training. It evaluates the combined function of several body systems involved in exercise, including respiratory, cardiovascular, circulatory, peripheral blood circulation, neuromuscular, and muscle metabolism. This test is particularly useful in evaluating exercise capacity in patients with moderate to severe COPD. 16
In this study, the average rate of emphysema was 13.25% ± 13.97% in the right lung, 13.04% ± 11.05% in the left lung, and 12.8% ± 11.64% for the total lung. One patient presented with an emphysema-predominant phenotype, with an EI of 44.9%. Classification of emphysema severity using QCT revealed that 46.7% of patients had level 2 emphysema, 36.7% had level 0, and 16.6% had level 1. Emphysema acts both as a cause and a consequence of recurrent infections and systemic inflammation in the pathogenesis of COPD. Therefore, whether a patient presents with emphysema or chronic bronchitis, the processes of alveolar destruction and the development of emphysematous areas in the lungs will continue. The severity of emphysema typically progresses over time and, along with airway remodeling, is a significant contributor to declining lung function.
Emphysema is a key phenotype of COPD, characterized by the abnormal and irreversible destruction of alveoli and terminal bronchioles, accompanied by the destruction of bronchial walls without evident fibrosis. QCT is essential for assessing lung structure and function in patients with emphysema. QCT can distinguish between subgroups with predominant emphysema phenotypes and those with predominant airway inflammation phenotypes in COPD. It also provides detailed imaging and analysis of lung parenchyma, which aids in predicting the prognosis of COPD patients. The EI derived from CT has recently been reported as a valuable predictor of FEV1/FVC ratios.3,10,17 According to Qi Ding et al., an EI threshold of 16.2% can be considered a diagnostic marker for COPD in patients with chronic bronchitis. 4
While spirometry remains the gold standard for diagnosing, classifying severity, and monitoring treatment response in COPD, low-dose CT with quantitative analysis is increasingly recognized for its role in the early detection of lung parenchymal and airway abnormalities, especially in high-risk populations. 1 QCT is effective in evaluating the extent and severity of damage caused by emphysema, which can be categorized into centrilobular emphysema, panlobular emphysema (PLE), and paraseptal emphysema (PSE). Combining qualitative and quantitative assessments enhances the ability to provide personalized care for COPD patients. In addition, QCT can assess other lung and airway changes, such as bronchial wall thickening, small airway inflammation, tracheal abnormalities, and bronchiectasis, in detail.18,19
Associations of emphysema levels with clinical and subclinical characteristics, and serum VEGF and IL-1β concentrations in COPD patients
The analysis revealed that the frequency of exacerbations increased proportionally with the severity of emphysema across all GOLD stages. Notably, in the GOLD 4 group, there was a significant correlation between the frequency of exacerbations, the number of hospitalizations, and the severity of emphysema. In addition, mMRC score significantly increased with the severity of emphysema. These findings suggest that the degree of emphysema visualized on QCT images could serve as a reliable predictor for the management and treatment of COPD, especially in patients with severe airflow obstruction.
The study also showed that BMI, FEV1%, FEV1/FVC ratio, and serum albumin concentrations progressively decreased as the severity of emphysema increased. This observation aligns with the classic classification of COPD phenotypes based on clinical characteristics proposed in 1955, which divided patients into two groups: “blue bloaters” and “pink puffers.” The “pink puffer” phenotype is characterized by predominant emphysema, a lean body type, and pink lips due to chronic hypercapnia. 20 This longstanding recognition of the inverse relationship between BMI, blood albumin concentration, and the degree of emphysema further supports these findings. Although some studies have reported no significant differences in age or BMI between emphysema and non-emphysema groups, 21 these discrepancies could be due to differences in study design, subject selection, and population characteristics.
Our study found a strong inverse correlation between EI and both FEV1% and FEV1/FVC ratios. Specifically, when the EI reaches level 2 or higher, lung function tends to decline more rapidly. This suggests that emphysema progression is a direct cause of lung function deterioration.
While there is heterogeneity in lung damage among different COPD patients, studies have consistently identified two primary types of damage that play critical roles in the development and progression of the disease: airway remodeling, especially of the small airways, and the irreversible destruction of alveoli and terminal bronchioles. Based on these understandings, studies using QCT to evaluate COPD phenotypes have consistently categorized patients into two groups: those with predominant chronic bronchitis and those with predominant emphysema. Furthermore, the poor response to anti-inflammatory therapies in patients with a predominant emphysema phenotype presents a significant challenge in their management.4,10,21
Patients with emphysema had significantly lower FEV1 and FEV1/FVC ratios than those without emphysema, indicating a higher degree of airflow obstruction in this group. In addition, the diffusion capacity for carbon monoxide was significantly reduced in patients with emphysema, reflecting impaired gas exchange—a key physiological characteristic of pulmonary emphysema. 21
The mean plasma VEGF concentration in our study was 70 ± 46.6 pg/mL. An inverse correlation was observed between VEGF concentration and emphysema severity, with VEGF levels gradually decreasing as EI increased. In the GOLD 3 and 4 groups, VEGF levels declined more rapidly with increasing emphysema severity. This suggests that a reduction in plasma VEGF may contribute to the progression of emphysema in COPD. VEGF, a cytokine involved in vascular permeability, remodeling, and angiogenesis, is produced by immune cells such as macrophages and neutrophils and is thought to play a crucial role in maintaining structural balance in the adult lung. Serum VEGF levels are higher in COPD patients compared to controls and are positively correlated with CRP levels and peripheral blood neutrophil counts. In addition, VEGF levels have been linked to inflammation, lung function, and exercise capacity in COPD patients.22,23
Experimental studies have demonstrated that inhibiting VEGF receptors in animal models leads to significant apoptosis of alveolar cells, resulting in the development of emphysema. This finding underscores the importance of VEGF in maintaining alveolar structure, suggesting that decreased expression or activity of VEGF could lead to emphysematous changes in the lung. 24
Further studies have investigated changes in serum VEGF levels over time in COPD patients, both in stable conditions and during exacerbations. These studies have shown that lower serum VEGF levels are associated with more severe forms of emphysema and greater declines in lung function. This suggests that serum VEGF could serve as a biomarker for identifying patients at high risk of developing emphysema. Although more research is needed to fully understand this relationship, current evidence supports the potential of serum VEGF as a biomarker and therapeutic target in managing emphysema in COPD patients. Monitoring serum VEGF levels may facilitate early detection and individualized treatment strategies for COPD. Interestingly, while decreased VEGF levels are associated with emphysema, increased VEGF expression has been observed in patients with chronic bronchitis. This highlights the complex role of VEGF in different forms of COPD—where it may contribute to airway remodeling in chronic bronchitis, while its deficiency exacerbates alveolar destruction in emphysema. 6
VEGF is crucial for regulating vascular permeability, remodeling, and angiogenesis. It is produced by immune cells such as macrophages and neutrophils and is essential for maintaining lung structural integrity in adults. Studies have shown that serum VEGF levels are higher in COPD patients than in healthy controls and are positively associated with inflammatory markers like CRP and peripheral blood neutrophil counts. VEGF levels also correlate with inflammation, lung function, and exercise capacity in COPD patients, underscoring its multifaceted role in the disease’s pathophysiology.23,25
The study found that the mean plasma IL-1β concentration in COPD patients was 11.2 ± 11 pg/mL, with no clear correlation with EI or the risk of acute exacerbations. However, a significant decrease in IL-1β concentration was observed in patients with emphysema levels of 25% or higher. Chronic airway inflammation is a central mechanism in the pathogenesis and progression of COPD, with neutrophil-mediated inflammation playing a key role.
This inflammatory process is thought to be driven by pro-inflammatory cytokines such as IL-1β and IL-17, which are critical in initiating and sustaining chronic airway inflammation. Research highlights the pivotal role of IL-1β in the progression of chronic inflammation and emphysema, suggesting that an imbalance in the cytokine network is a crucial pathogenic mechanism in the development and persistence of chronic inflammation in COPD patients.8,26
IL-1β promotes airway inflammation by recruiting inflammatory cells, such as neutrophils and macrophages, to lung tissue. This inflammatory response triggers the release of proteases and reactive oxygen species, which damage the alveolar structure and contribute to emphysema. 27 Moreover, the cytokine has been shown to induce apoptosis in alveolar epithelial cells (a key feature of emphysema), disrupting lung tissue integrity and impairing the lung’s ability to repair itself.28,29
Cigarette smoke, a major risk factor for COPD, directly activates the IL-1β pathway. Studies have demonstrated that cigarette smoke exposure increases IL-1β production through NLRP3 inflammasome activation and caspase-1 signaling. 30
IL-1β levels are believed to be associated with inflammation, disease severity, and the frequency of exacerbations. 7 Elevated serum IL-1β levels during exacerbations are significantly higher in COPD patients compared to stable COPD patients or healthy controls. These elevated levels are positively correlated with serum CRP levels, neutrophil percentages, and smoking status, while negatively correlated with FEV1% in COPD patients. Therefore, elevated serum IL-1β levels could potentially serve as a biomarker for assessing the progression of persistent neutrophilic airway inflammation and the risk of severe disease. 8
Given the central role of IL-1β in COPD pathogenesis, targeting this cytokine or its signaling pathway presents a promising therapeutic strategy. Studies have shown that blocking IL-1β or its receptor (IL-1R1) significantly reduces inflammation and emphysema in animal models.31,32 Furthermore, inhibiting NLRP3 inflammasome activation or caspase-1 activity has been found to decrease IL-1β production and mitigate COPD-like symptoms. 30 In addition to pharmacological approaches, reducing exposure to environmental triggers such as cigarette smoke and pollutants remains a cornerstone of COPD management.
It is important to acknowledge the limitations of our study. First, it was a single-center study with a small sample size, limiting its representativeness. Second, we were unable to quantitatively measure the concentrations of VEGF and IL-1β in sputum or bronchoalveolar lavage fluid, which would provide a more comprehensive assessment of their relationship with serum concentrations. Finally, long-term follow-up is required to evaluate the progression of emphysema over time to accurately determine the relationship between plasma cytokine concentrations and emphysema development as seen on QCT. Future studies will expand on this research with a multicenter model and a larger sample size.
Conclusion
In a study involving 30 stable male COPD patients, we found that EI analyzed by QCT is a valuable tool for identifying COPD phenotypes and assessing disease severity. Additionally, EI can help predict the prognosis regarding the risk of acute exacerbations, clinical symptom burden, and lung function decline. There was a significant inverse correlation between plasma VEGF concentration and EI. The observed decrease in VEGF with increasing emphysema severity suggests that reduced VEGF levels may be an important factor in the pathogenesis of emphysema. This finding highlights a potential direction for future research into a “treatable” factor in COPD management. Future large-scale, multicenter, longitudinal studies are essential to further elucidate the precise role of plasma VEGF and IL-1β in the pathogenesis and progression of COPD. In addition, interventional trials based on current evidence linking VEGF levels to emphysema could be considered potential adjunctive therapies for patients with a predominant emphysema phenotype.
Supplemental Material
Supplemental material, sj-doc-1-tar-10.1177_17534666251332469 for Quantitative chest computed tomography in chronic obstructive pulmonary disease: assessing the role of emphysema severity and its correlation with clinical characteristics, lung function, and plasma levels of VEGF and IL-1β by Cong Nguyen Hai, Thanh Bui Duc, The Nguyen Minh, Loi Trinh Duc and Thang Tran Quyet in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors extend our sincere appreciation to the patients, staff at Military Hospital 175, and Dr. Nu Nguyet Anh Nguyen from the University of Social Sciences and Humanities, Viet Nam National University Ho Chi Minh City, for their invaluable support in bringing this article to publication.
Footnotes
ORCID iD: Cong Nguyen Hai  https://orcid.org/0000-0002-3828-506X
https://orcid.org/0000-0002-3828-506X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Cong Nguyen Hai, Department of Tuberculosis and Respiratory Diseases, Military Hospital 175, Ho Chi Minh City, Vietnam.
Thanh Bui Duc, Military Hospital 175, Ho Chi Minh City, Vietnam.
The Nguyen Minh, Department of Tuberculosis and Respiratory Diseases, Military Hospital 175, Ho Chi Minh City, Vietnam.
Loi Trinh Duc, Department of Tuberculosis and Respiratory Diseases, Military Hospital 175, Ho Chi Minh City, Vietnam.
Thang Tran Quyet, Department of Diagnostic Imaging, Military Hospital 175, Ho Chi Minh City, Vietnam.
Declarations
Ethics approval and consent to participate: The study protocol underwent review and received approval from the Ethics Committee of Military Hospital 175 (No. 003/QĐ-IRB-VN01.055), and all participants provided written informed consent. The methodology outlined in this manuscript strictly adheres to these approvals.
Consent for publication: Written informed consent was obtained from the patient for the publication of this study and any accompanying images. A preprint version of this manuscript has been published at https://doi.org/10.21203/rs.3.rs-5051887/v1.
Author contributions: Cong Nguyen Hai: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Software; Validation; Writing – original draft; Writing – review & editing.
Thanh Bui Duc: Project administration; Supervision.
The Nguyen Minh: Data curation; Formal analysis; Investigation; Supervision.
Loi Trinh Duc: Data curation; Investigation.
Thang Tran Quyet: Data curation.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is part of a Ministry-level project conducted at Military Hospital 175 and was funded by the Ministry of Defense of Vietnam, research code: IRB-VN01.055-003.
The authors declare that there is no conflict of interest.
Availability of data and materials: The data supporting the findings of this study are available from the corresponding author upon reasonable request. The data were not publicly available because of privacy and ethical restrictions.
References
- 1. 2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease – GOLD, https://goldcopd.org/2024-gold-report/ (accessed 22 August 2024).
- 2. Fernandes L, Fernandes Y, Mesquita AM. Quantitative computed tomography imaging in chronic obstructive pulmonary disease. Lung India 2016; 33(6): 646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goddard PR, Nicholson EM, Laszlo G, et al. Computed tomography in pulmonary emphysema. Clin Radiol 1982; 33(4): 379–387. [DOI] [PubMed] [Google Scholar]
- 4. Ding Q, Wei X, Li J, et al. Role of the emphysema index combined with the chronic obstructive pulmonary disease assessment test score in the evaluation of chronic obstructive pulmonary disease. Can Respir J 2021; 2021: 9996305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuoka S, Yamashiro T, Washko GR, et al. Quantitative CT assessment of chronic obstructive pulmonary disease. Radiogr Rev Publ Radiol Soc N Am Inc 2010; 30(1): 55–66. [DOI] [PubMed] [Google Scholar]
- 6. Papaioannou AI, Kostikas K, Kollia P, et al. Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir Res 2006; 7(1): 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2009; 41(6): 631–638. [DOI] [PubMed] [Google Scholar]
- 8. Zou Y, Chen X, Liu J, et al. Serum IL-1β and IL-17 levels in patients with COPD: associations with clinical parameters. Int J Chron Obstruct Pulmon Dis 2017; 12: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 2020 Gold Reports. Global initiative for chronic obstructive lung disease – GOLD, https://goldcopd.org/gold-reports/ (accessed 27 August 2024).
- 10. Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology 2011; 261(1): 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. STROBE. STROBE, https://www.strobe-statement.org/ (accessed 11 February 2025). [Google Scholar]
- 12. Cong NH, Thang TB, Luc NH, et al. Study on prognostic values for mortality of clinical and subclinical factors in acute exacerbation of chronic obstructive pulmonary disease, http://220.231.117.26/TapChi_YDHQS/Data/TapTinBaiVietPDF/TCYDHQS%20s%E1%BB%91%202-2021_16.pdf (accessed 8 November 2024).
- 13. Lim S, Chi-Leung Lam D, Muttalif AR, et al. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: the EPIC Asia population-based survey. Asia Pac Fam Med 2015; 14(1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang WC, Wu MF, Chen HC, et al. Features of COPD patients by comparing CAT with mMRC: a retrospective, cross-sectional study. NPJ Prim Care Respir Med 2015; 25: 15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hai CN, Ba TT, Duc TB, et al. Serum immunoglobulin levels in group E of chronic obstructive pulmonary disease: insights for clinical management and immunoglobulin therapy strategies. BMC Pulm Med 2024; 24: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferreira JP, Metra M, Anker SD, et al. Clinical correlates and outcome associated with changes in 6-minute walking distance in patients with heart failure: findings from the BIOSTAT-CHF study. Eur J Heart Fail 2019; 21(2): 218–226. [DOI] [PubMed] [Google Scholar]
- 17. Farkas L, Farkas D, Warburton D, et al. Cigarette smoke exposure aggravates air space enlargement and alveolar cell apoptosis in Smad3 knockout mice. Am J Physiol – Lung Cell Mol Physiol 2011; 301(4): L391–L401. [DOI] [PubMed] [Google Scholar]
- 18. Lynch DA, Austin JHM, Hogg JC, et al. CT-Definable subtypes of chronic obstructive pulmonary disease: a statement of the fleischner society. Radiology 2015; 277(1): 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konietzke P, Brunner C, Konietzke M, et al. GOLD stage-specific phenotyping of emphysema and airway disease using quantitative computed tomography. Front Med 2023; 10: 1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dobnhoest AC. Respiratory insufficiency. Lancet 1955; 265(6876): 1185–1187. [PubMed] [Google Scholar]
- 21. Boschetto P, Miniati M, Miotto D, et al. Predominant emphysema phenotype in chronic obstructive pulmonary disease patients. Eur Respir J 2003; 21(3): 450–454. [DOI] [PubMed] [Google Scholar]
- 22. Chiş AF, Soritau O, Catana A, et al. VEGF serum levels in COPD patients without pulmonary hypertension – a case control study. Eur Respir J 52(Suppl. 62). [Google Scholar]
- 23. Farid Hosseini R, Jabbari Azad F, Yousefzadeh H, et al. Serum levels of vascular endothelial growth factor in chronic obstructive pulmonary disease. Med J Islam Repub Iran 2014; 28: 85. [PMC free article] [PubMed] [Google Scholar]
- 24. Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000; 106(11): 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chis AF, Râjnoveanu RM, Man MA, et al. Increased vascular endothelial growth factor serum level and the role of +936C/T gene polymorphism in chronic obstructive pulmonary disease. Medicina (Mex) 2021; 57(12): 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delieva A, Dolinina L, Trofimov V. The level of anti-inflammatory cytokine IL-10 in patients with chronic obstructive pulmonary disease of varying severity. Eur Respir J 2013; 42(Suppl. 57). [Google Scholar]
- 27. Churg A, Zhou S, Wang X, et al. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol 2009; 40(4): 482–490. [DOI] [PubMed] [Google Scholar]
- 28. Ruwanpura SM, McLeod L, Dousha LF, et al. Cross-talk between IL-6 trans-signaling and AIM2 inflammasome/IL-1β axes bridge innate immunity and epithelial apoptosis to promote emphysema. Proc Natl Acad Sci USA 2022; 119(36): e2201494119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ciminieri C, Woest ME, Reynaert NL, et al. IL-1β induces a proinflammatory fibroblast microenvironment that impairs lung progenitors’ function. Am J Respir Cell Mol Biol 2023; 68(4): 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eltom S, Belvisi MG, Stevenson CS, et al. Role of the inflammasome-caspase1/11-IL-1/18 axis in cigarette smoke driven airway inflammation: an insight into the pathogenesis of COPD. PLoS One 2014; 9(11): e112829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Couillin I, Vasseur V, Charron S, et al. IL-1R1/MyD88 signaling is critical for elastase-induced lung inflammation and emphysema. J Immunol Baltim Md 1950 2009; 183(12): 8195–8202. [DOI] [PubMed] [Google Scholar]
- 32. Pauwels NS, Bracke KR, Dupont LL, et al. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J 2011; 38(5): 1019–1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tar-10.1177_17534666251332469 for Quantitative chest computed tomography in chronic obstructive pulmonary disease: assessing the role of emphysema severity and its correlation with clinical characteristics, lung function, and plasma levels of VEGF and IL-1β by Cong Nguyen Hai, Thanh Bui Duc, The Nguyen Minh, Loi Trinh Duc and Thang Tran Quyet in Therapeutic Advances in Respiratory Disease