Abstract
Background/Aim
Ischemic heart disease is a leading cause of death worldwide in comparison to malignant neoplasia. Myocardial infarction (MI) is the result of severe ischemia due to a low consumption of oxygen in the myocardium. The main pathophysiological reason is a progressive obstructive atherosclerotic endothelial lesion that causes reduction in coronary blood flow and increases the corresponding arterial stenosis. Our research aim was to investigate the role of altered expression connexin-43 (gene locus: 6q22.31) protein in MI tissue substrates with different clinico-pathological characteristics.
Materials and Methods
A set of fifty (n=50) MI archival tissue sections derived from a forensic pathology file were selected and micro-sectioned. Immunohistochemistry and digital image analysis assays were implemented for detecting and measuring the levels of connexin-43, respectively.
Results
Low expression of Connexin-43 protein was detected in 16/50 (32%) cases, biphasic expression pattern (low/medium) was identified in 10/50 (20%), whereas moderate and high levels of protein expression were observed in the rest of them (24/50-48%). Connexin-43 overall expression was significantly correlated with the timing of the MI onset (recent or past) (p=0.001).
Conclusion
Connexin-43 is a critical gap junction intermediate protein in MI pathology diagnosis and research. Different Connexin-43 expression levels, including single phase or biphasic patterns, should be a reliable biomarker for determining the timing of the MI lesions inside the corresponding tissue sections. Furthermore, implementation of sophisticated, accurate computerized techniques, such as digital image analysis provide very detailed, objective results regarding protein expression as modern precise (evidence-based) medicine requires.
Keywords: Myocardium, artery, connexin 43, infarction, immunohistochemistry
Introduction
Myocardial infarction (MI) is a severe clinical condition emerging as a result of a dramatic blood flow decrease in coronary arteries of the heart (1). In fact, the majority of MIs occur on the basis of persistent coronary artery disease (CAD), the main cause of severe heart failure (2). CAD is a chronic clinical syndrome based on a progressively increased atherosclerotic plaque development (3). Atherosclerosis affects large and medium-sized arteries due to the progressive accumulation of lipids and calcium in the intima of the arteries. Endothelial damage and dysfunction combined with irritation and inflammation of the arterial wall triggers the formation of atherosclerotic plaque (4). Chronic tobacco smoking combined or not with severe metabolic diseases including diabetes mellitus, obesity, dyslipidemia and arterial hypertension represent the prominent causes of atherosclerosis, whereas a genetic predisposition is detected in a limited subgroup of patients (5-7). Concerning the mechanism and progression of CAD, the atheromatous plaque does not modify negatively the blood flow in the early stages of this procedure. But, as the phenomenon evolves, the atheromatous plaque grows inside the arterial lumen restricting the blood flow (8). Destabilization and rupture of atherosclerotic plaque disturbs critically the coronary blood flow causing ischemia and acute thrombosis of the coronary arteries leads to the MI lesion (9,10).
Among the molecules that are crucially involved in myocardial homeostasis, connexin-43 (Cnx-43) is significantly involved in normal myocardial formation and function acting as a major mediator in the regulation of gap junctions that mediate inter-cardiomyocyte communication (11,12). Cnx-43 or Gap junction alpha-1 protein (GJA1) of 43.0 kDa and 382 amino acids is a component of gap junctions is encoded by the GJA1 gene (gene locus: 6q22.31) (13,14). In the current original research study, we analysed the Cnx-43 expression patterns on a series of MI forensic autopsy tissue sections exploring its potential impact on their specific clinicopathological characteristics.
Materials and Methods
Study group. A set of fifty (n=50) archival, formalin-fixed and paraffin-embedded MI tissue specimens obtained during a forensic autopsy procedure and filed was selected. The Department of Pathology and the corresponding Ethics Committee of National and Kapodistrian University of Athens consented to the use of these tissues for research purposes (Reference ID research protocol: 85678/06-03-21), according to the World Medical Association Declaration of Helsinki guidelines (2008, revised 2014). The extracted tissue sections were fixed in 10% neutral-buffered formalin. Hematoxylin and eosin (H&E)-stained slides were evaluated by two independent pathologists for the final categorization of the examined MI tissues according to the World Health Organization (WHO) pathology guidelines (15).
Immunohistochemistry assay (IHC). Ready-to-use anti-Cnx-43 antibody (rabbit monoclonal, clone GJA1[EPR21153]-Abcam Limited, Cambridge, UK) at the dilution of 1:500 was applied in the corresponding tissue sections. The IHC assay was performed as described in our previous study (16). Membranous and sub-membranous staining was considered acceptable for Cnx-43 mono- or biphasic protein expression patterns (Figure 1, Figure 2).
Figure 1.
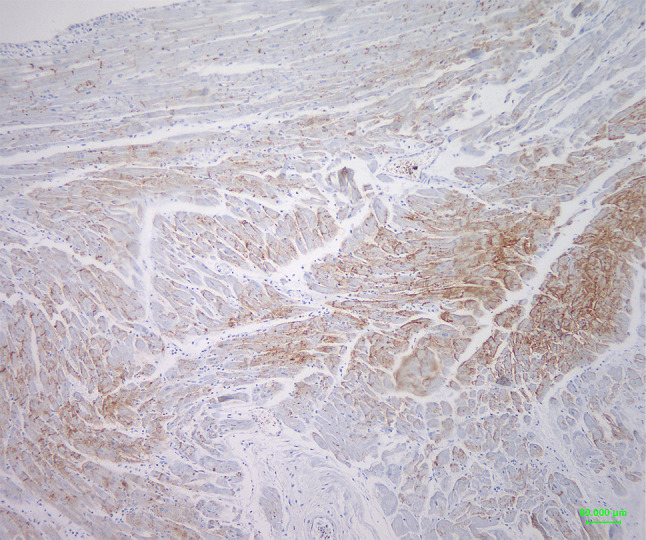
Connexin-43 biphasic (high/moderate) expression pattern in a case of MI. Note areas with dense brown membranous staining (right part of the image) and areas with a slighter staining (centre and left part of the image), respectively (original magnification 100×, Diaminobenzidine-DAB- chromogen).
Figure 2.
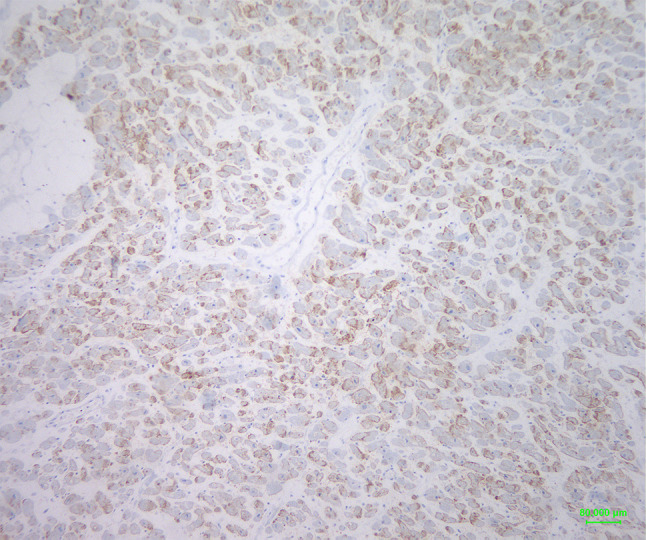
Connexin-43 biphasic (moderate/low) expression pattern in a case of MI. Note mixed areas with medium brown membranous and areas with a slighter staining, respectively (original magnification 100×, Diaminobenzidine-DAB-chromogen).
Digital Image Analysis assay (DIA). Cnx-43 protein expression levels were evaluated quantitatively by measuring the corresponding staining intensity levels (densitometry evaluation) in the immunostained tissue sections. We performed a DIA assay (ImageProPlus v.6, Media Cybernetics, Rockville, MD, USA) as we have previously described (17) (Figure 3). All results and DIA values are demonstrated in Table I.
Figure 3.

Digitized evaluation of connexin-43 protein expression. Red areas correspond to the different levels of protein expression that lead to specific staining intensity levels in a spectrum of 0-255 continuous grey scale RGB values.
Table I. Clinicopathological parameters and total Cnx-43 expression results.
MI: Myocardial infarction; Cnx-43: Connexin 43; Cnx-43.DIA mean value was 142.7 (SD=13.7), with a range of 113.6 to 163.4; LE: Low expression staining intensity mean value at stained cells 151.2; MLE: moderate-low expression staining intensity mean value at stained cells 146.2; MHE: moderate-high expression staining intensity mean values at stained cells 129.7; MLE & MHE represent biphasic Cnx-43 expression patterns. Bold values indicate statistical significance.
Statistical analysis. Descriptive statistics were carried out using the statistical package SPSS vr 21.00 (IBM Corporation, Armonk, NY, USA). Data are expressed as mean±SD for quantitative variables and as percentages for qualitative variables. The Kolmogorov – Smirnov test was utilized for normality analysis of the quantitative variables. Unifactorial analyses were made using the Student t-test. All tests are two-sided, and statistical significance was set at p<0.05.
Results
The study included fifty (n=50) specimens, of which 10 (20%) were derived from female and 40 (80%) from male subjects, respectively. The mean age was 54.9 years (SD=14.0), with an age range of 36 to 92 years. Chronic hypertrophic cardiomyopathy was present in 24 participants (48%), while 26 participants (52%) did not present this abnormal condition. In terms of infarct timing, six (12%) had a past MI, whereas 44 (88%) had a recent MI. Concerning the MI tissue extent damage, 34 (68%) cases demonstrated limited damage, whereas the rest 16 (32%) had extensive damage. Regarding the severity of hypertrophic cardiomyopathy, early-stage hypertrophic cardiomyopathy was observed in one (2%) case, chronic hypertrophic cardiomyopathy in five (10%), secondary hypertrophic cardiomyopathy in one (2%), ischemic hypertrophic cardiomyopathy in 17 (34%), and lack of hypertrophic cardiomyopathy lesions in 26 (52%). In terms of localization, hypertrophic cardiomyopathy was observed in the interventricular septum and posterior wall of the left ventricle in two (4%) cases, the posterior wall of the left ventricle in nine (18%), the posterior wall of the left ventricle in seven (14%), the anterior wall of the left ventricle in 20 (40%), the anterolateral wall of the left abdomen in one (2%), the anterolateral wall of the left ventricle in seven (14%), the anterior wall of the left ventricle and apex in one (2%), and finally the anterior wall of the left ventricle in three (6%).
Based on the digitized IHC analysis, Cnx-43 protein low expression was detected in 16/50 (32%) cases, biphasic expression pattern (low/medium) in 10/50 (20%) a, whereas moderate and high levels of protein expression in the rest of them (24/50-48%). Cnx-43 overall expression was statistically significantly correlated with the timing of the MI, recent or past, (p=0.001). In fact, past MI tissues were characterized predominantly by low or biphasic moderate-low expression levels, whereas recent ones by moderate or high-moderate values. More specifically, the mean Cnx-43 DIA value was 142.7 (SD=13.7), with a range of 113.6 to 163.4. The Kolmogorov-Smirnov test indicated that Cnx-43 DIA did not follow a normal distribution (KS=0.015, p<0.005). Consequently, a logarithmic transformation was applied to Cnx-43 DIA for statistical analysis. The logarithmic scores of Cnx-43 DIA had a mean of 1.2 (SD=0.5), with a range of 0.0 to 1.7.
An independent samples t-test revealed no significant difference between males (M=1.1, SD=0.5) and females (M=1.3, SD=0.3) in Cnx-43 DIA, t(20.99)=1.0, p=0.344, d=0.30. The correlation between age and Cnx-43 DIA was not statistically significant, r(50)=–0.03, p=0.834. There were no significant differences in Cnx-43 DIA between participants with hypertrophic cardiomyopathy (M=1.1, SD=0.5) and those without hypertrophic cardiomyopathy (M=1.2, SD=0.4), t(42.46)=1.1, p=0.266, d=0.22. Significant differences were observed in Cnx-43 DIA based on infarct timing. Cases characterized by past MI (M=1.4, SD=0.1) had significantly higher Cnx-43 DIA values compared to the cases with a recent MI (M=1.1, SD=0.5), t(37.14)=3.83, p<0.001, d=1.00. Regarding the extent of infarct damage, no significant differences were observed between participants with limited damage (M=1.2, SD=0.4) and those with extended damage or the anatomic localization of the lesions (M=1.1, SD=0.5), t(48)=1.1, p=0.273, p=0.436, d=0.22), respectively. Table I presents clinic-pathological features of the examined tissues, Cnx-43 results and also statistical associations.
Discussion
Cnx-43 enhances the optimal embryonic, histological development and functionality of the myocardium by regulating the intra-cardio myocyte gap junction adhesion and signal transduction (18). In contrast, Cnx-43 inactivation is detected by a progressive loss of its expression in the majority of MI affected tissues. Concerning the genetic mechanisms that trigger Cnx-43 down regulation, a variety of mutated genes are implicated. Casein kinase-1 mutations reduce the normal Cnx-43 expression and activity. In fact, a molecular study reported phospha-tase-mediated mutations in three phosphorylated sites of the casein kinase-1 molecule that negatively affect Cnx-43 functionality in cardiomyocyte-to-cardiomyocyte adhesion junctions (18). Additionally, another study group explored the role of a B-Raf proto-oncogene (BRAF) specific mutation (BRAF-V600E) in the expression of Cnx-43. They observed that activating BRAF kinase mutations reduces also the Cnx-43 normal expression in cardiac tissues (19).
In the current experimental study, we focused on the importance of Cnx-43 in CAD/MI onset and progression by investigating its expression patterns on a series of MI tissue sections and the potential correlation with the corresponding clinicopathological features implementing a combination of IHC and DIA analytical methods. We report Cnx-43 low expression in a significant sub-group of the examined specimens, whereas a diphasic expression pattern (low/moderate – moderate/high levels) was also detected. Interestingly, overall Cnx-43 expression was found to be strongly associated with the timing of the MI (recent or past). No other statistically significant differences were assessed regarding the sex, age, hypertrophic cardio-myopathy history, or MI-depended damage tissue area. In conjunction with our IHC-DIA based Cnx-43 protein expression analysis, some other studies performed modern, micro-nano methods such as electron and super-resolution light microscopy, in order to detect and analyze nano-structures, and also, nanocellulose based electroactive tissue engineering scaffolds (20,21). Besides them, novel multi-tissue microdissection techniques using a grooved polydimethylsiloxane (PDMS) membrane as a substrate for immunohistochemical and/or immunofluorescence analysis of the corresponding Cnx-43 protein expression have also been used (22-24). Independently of the implemented method, an interesting point of view is the detection of different Cnx-43 expression patterns depended on the timing of the MI onset. Although the normal Cnx-43 expression is detected in the cell membrane and the gap junction between cardio myocytes, its translocation inside the cytoplasmic environment is a remarkable event. Interestingly, elevated nuclear concentration of the protein in cardiomyocytes and cardiomyoblasts has been already detected and this translocation to the nucleus, via intra-nuclear envelope channels, boosts the continuous biogenesis of myoblasts and cardiomyocyte differentiation (25). Besides the differences of Cnx-43 expression that are observed on the basis of the timing of MI establishment in the myocardial epithelium, its correlation with the CAD stability or instability is under investigation. In a clinicomolecular study based on the joined Cnx-43 and zonula occludens-1 (ZO1) molecule mRNA analysis in a series of forensic autopsy tissue sections, the researchers reported a combined decrease of the two proteins, especially in the cases characterized by acute ischemia in the corresponding epithelia due to chronic coronary atherosclerosis, but without features of myocardial necrosis (26). Additionally, another study group explored the role of Cnx-43 and ZO1 loss of expression, detected by immunohistochemistry, in myocardial dysfunction (27). They reported a strong correlation with the extent of the examined lesions considering also them as potential reliable markers for determining early ischemia condition in the MI abnormal myocardial epithelia. In fact, both mRNA and protein Cnx-43 expression levels seem to be implicated in the MI onset and progression that leads critically to the sudden cardiac death event. Similarly, Cnx-43 interacts with other molecules inside the cell microenvironment affecting the homeostasis of the myocardium. Cnx-43 and NADPH oxidase (NOX)/ROS co-expression analysis showed that their desynchronization may explain the high risk for a sudden MI-depended arrhythmic death that characterizes a sub-group of patients suffered by atherosclerosis (28).
In conclusion, based on this systematic and precise analysis, we showed that Cnx-43 abnormal protein expression is an important marker for the relative determination of the timing of MI development in the corresponding affected myocardial epithelia. Interestingly, a variety of expression patterns including mono-biphasic (low/moderate, moderate/high) are present in the examined forensic autopsy tissues revealing different aspects of its deregulation regarding the impact of its progressive deregulation in gap junctions between the cardiomyocytes. For these reasons, Cnx-43 is a significant target for specific therapeutic approaches including agents such as muscone, saracatinib or specific conditions (hygrothermal stress, post-translational modifications) that positively or negatively modify its protein expression (29-32).
Funding
The Authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Authors’ Contributions
All Authors contributed to the study conception, design and review. AT, DR, SM, PF, SP: materials selection, preparation, data collection; ET: digital image analysis procedure; GT: statistical analysis; AT, ET: Draft writing; ACL, NK, GA, NK: draft reviewing, academic advisors. All Authors read and approved the final manuscript.
Conflicts of Interest
The Authors confirm that there are no conflicts of interest in regard to this study.
References
- 1.Jenča D, Melenovský V, Stehlik J, Staněk V, Kettner J, Kautzner J, Adámková V, Wohlfahrt P. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021;8(1):222–237. doi: 10.1002/ehf2.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satya Sai Venkata Jagadeesh K, Shaik TA, Mayow AH, Sompalli S, Arsalan M, Chaudhari SS, Habib I, Ali N. Factors associated with the development of heart failure following acute coronary syndrome: a systematic review and meta-analysis. Cureus. 2024;16(12):e75999. doi: 10.7759/cureus.75999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attar A, Sayadi M, Hosseinpour A, Assadian K, Beykihosseinabadi M, Abtahian J, Aldavood D, Nasri M, Khosravi A, Sarrafzadegan N, Noohi F, Assareh A, Kazemi T, Farshidi H, Khaledifar A, Abbaszadeh M, Boshtam M, Jannati M. Severe left main coronary artery stenosis as the first finding in newly diagnosed chronic coronary syndrome: incidence and clinical predictors. Angiology. 2025:33197241312940. doi: 10.1177/00033197241312940. [DOI] [PubMed] [Google Scholar]
- 4.Kattamuri L, Duggal S, Aparece JP, Sairam S. Cardiovascular risk factor and atherosclerosis in rheumatoid arthritis (RA) Curr Cardiol Rep. 2025;27(1):31. doi: 10.1007/s11886-025-02198-8. [DOI] [PubMed] [Google Scholar]
- 5.Giao DM, Col H, Larbi Kwapong F, Turkson-Ocran RA, Ngo LH, Cluett JL, Wagenknecht L, Windham BG, Selvin E, Lutsey PL, Juraschek SP. Supine blood pressure and risk of cardiovascular disease and mortality. JAMA Cardiol. 2025 doi: 10.1001/jamacardio.2024.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supriami K, Urbut SM, Tello-Ayala JR, Unlu O, Friedman SF, Abou-Karam R, Koyama S, Uddin MM, Pomerantsev E, Lu MT, Honigberg MC, Aragam KG, Doshi-Velez F, Patel AP, Natarajan P, Ellinor PT, Fahed AC. Genomic drivers of coronary artery disease and risk of future outcomes after coronary angiography. JAMA Netw Open. 2025;8(1):e2455368. doi: 10.1001/jamanetworkopen.2024.55368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao Y, Wang Y, Wan Q, Tong N. The fibrosis-4 index and its association with carotid atherosclerosis in type 2 diabetes: a cross-sectional study in China. BMC Cardiovasc Disord. 2025;25(1):35. doi: 10.1186/s12872-025-04491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sogbadji J, Kadry K, Poletti G, Berti F, Edelman ER, Nezami FR. Impact of lesion preparation-induced calcified plaque defects in vascular intervention for atherosclerotic disease: in silico assessment. Biomech Model Mechanobiol. 2025 doi: 10.1007/s10237-024-01923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yang J, Kashima Y, Hachinohe D, Sugie T, Xu S, Guo X, Li X, Hu X, Sun B, Nagraj S, Lymperopoulos A, Kim YH, Tu S, Dong H. The influence between plaque rupture and non-plaque rupture on clinical outcomes in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention: a prospective cohort study. J Thorac Dis. 2024;16(11):7771–7786. doi: 10.21037/jtd-24-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds HR, Page CB, Shaw LJ, Berman DS, Chaitman BR, Picard MH, Kwong RY, Min JK, Leipsic J, Mancini GBJ, Budoff MJ, Hague CJ, Senior R, Szwed H, Bhargava B, Celutkiene J, Gadkari M, Bainey KR, Doerr R, Ramos RB, Ong P, Naik SR, Steg PG, Goetschalckx K, Chow BJW, Scherrer-Crosbie M, Phillips L, Mark DB, Spertus JA, Alexander KP, O’Brien SM, Boden WE, Bangalore S, Stone GW, Maron DJ, Hochman JS, ISCHEMIA Research Group Relationship between severity of ischemia and coronary artery disease for different stress test modalities in the ISCHEMIA trial. Circ Cardiovasc Interv. 2024;17(12):e013743. doi: 10.1161/CIRCINTERVENTIONS.123.013743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62(2):228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84(3):381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 13.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394(Pt 3):527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans WH, Martin PEM. Gap junctions: structure and function (Review) Mol Membr Biol. 2002;19(2):121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 15.Pasotti M, Prati F, Arbustini E. The pathology of myocardial infarction in the pre- and post-interventional era. Heart. 2006;92(11):1552–1556. doi: 10.1136/hrt.2005.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrysovergis A, Papanikolaou V, Mastronikolis N, Tsiambas E, Katsinis S, Manoli A, Papouliakos S, Ragos V, Pantos P, Peschos D, Mastronikolis S, Fotiades P, Mamoulidis P, Spyropoulou D, Kyrodimos E. ALK protein expression patterns in squamous cell carcinoma of the oral cavity. In Vivo. 2022;36(3):1144–1149. doi: 10.21873/invivo.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roukas D, Kouzoupis A, Spyropoulou D, Papanastasiou G, Tsiambas E, Tsouvelas G, Falidas E, Ragos V, Peschos D, Manaios L, Katsinis S, Manoli A, Papouliakos S, Lazaris AC, Kavantzas N. P53 suppressor gene tissue microarray-based protein expression analysis in meningiomas. In Vivo. 2022;36(5):2205–2210. doi: 10.21873/invivo.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Attar R, Jargstorf J, Romagnuolo R, Jouni M, Alibhai FJ, Lampe PD, Solan JL, Laflamme MA. Casein kinase 1 phosphomimetic mutations negatively impact connexin-43 gap junctions in human pluripotent stem cell-derived cardiomyocytes. Biomolecules. 2024;14(1):61. doi: 10.3390/biom14010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strash N, DeLuca S, Janer Carattini GL, Chen Y, Wu T, Helfer A, Scherba J, Wang I, Jain M, Naseri R, Bursac N. Time-dependent effects of BRAF-V600E on cell cycling, metabolism, and function in engineered myocardium. Sci Adv. 2024;10(4):eadh2598. doi: 10.1126/sciadv.adh2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mezache L, Soltisz AM, Johnstone SR, Isakson BE, Veeraraghavan R. Vascular endothelial barrier protection prevents atrial fibrillation by preserving cardiac nanostructure. JACC Clin Electrophysiol. 2023;9(12):2444–2458. doi: 10.1016/j.jacep.2023.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C, Xie Y, Zhu H, Zheng X, Hou R, Shi Z, Li J, Yang Q. Highly electroactive tissue engineering scaffolds based on nanocellulose/sulfonated carbon nanotube composite hydrogels for myocardial tissue repair. Biomacromolecules. 2023;24(12):5989–5997. doi: 10.1021/acs.biomac.3c01034. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs S, Racz B, Sotonyi P, Bakos Z. Morphological and histological investigation of the conduction system in the equine atrial muscle sleeve of pulmonary veins. Equine Vet J. 2024;56(5):1059–1067. doi: 10.1111/evj.13996. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Shanmugasundaram A, Lee CB, Kim JR, Park JJ, Kim E, Lee B, Lee D. Enhanced cardiomyocyte structural and functional anisotropy through synergetic combination of topographical, conductive, and mechanical stimulation. Lab Chip. 2023;23(20):4540–4551. doi: 10.1039/d3lc00451a. [DOI] [PubMed] [Google Scholar]
- 24.Villgrater HE, Xia R, Sharma Chivukula A, Tomsits P, Clauss S. Microdissection and immunofluorescence staining of myocardial sleeves in murine pulmonary veins. J Vis Exp. 2023;(201) doi: 10.3791/65836. [DOI] [PubMed] [Google Scholar]
- 25.Martins-Marques T, Witschas K, Ribeiro I, Zuzarte M, Catarino S, Ribeiro-Rodrigues T, Caramelo F, Aasen T, Carreira IM, Goncalves L, Leybaert L, Girao H. Cx43 can form functional channels at the nuclear envelope and modulate gene expression in cardiac cells. Open Biol. 2023;13(11):230258. doi: 10.1098/rsob.230258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue Y, Zhao R, Du SH, Zhao D, Li DR, Xu JT, Xie XL, Wang Q. Decreased mRNA levels of cardiac Cx43 and ZO1 in sudden cardiac death related to coronary atherosclerosis: a pilot study. Int J Legal Med. 2016;130(4):915–922. doi: 10.1007/s00414-016-1353-0. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto O, Michiue T, Ishikawa T, Maeda H. Immuno-histochemistry of connexin43 and zonula occludens-1 in the myocardium as markers of early ischemia in autopsy material. Histol Histopathol. 2014;29(6):767–775. doi: 10.14670/HH-29.767. [DOI] [PubMed] [Google Scholar]
- 28.Kelm NQ, Solinger JC, Piell KM, Cole MP. Conjugated linoleic acid-mediated connexin-43 remodeling and sudden arrhythmic death in myocardial infarction. Int J Mol Sci. 2023;24(13):11208. doi: 10.3390/ijms241311208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng L, Shi W, Liu B, Duan B, Sorgen PL. Evaluation of tyrosine kinase inhibitors loaded injectable hydrogels for improving connexin43 gap junction intercellular communication. ACS Appl Mater Interfaces. 2024;16(2):1985–1998. doi: 10.1021/acsami.3c10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang F, Zhang XL, Liu HH, Qian LL, Wang RX. Post translational modifications of connexin 43 in ventricular arrhythmias after myocardial infarction. Mol Biol Rep. 2024;51(1):329. doi: 10.1007/s11033-024-09290-2. [DOI] [PubMed] [Google Scholar]
- 31.Chi J, Wu N, Li P, Hu J, Cai H, Lin C, Lai Y, Yang H, Huang J, Li M, Xu L. Hygrothermal stress increases malignant arrhythmias susceptibility by inhibiting the LKB1-AMPK-Cx43 pathway. Sci Rep. 2024;14(1):5010. doi: 10.1038/s41598-024-55804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei T, Cao H, Zhang L, Cao Y, Ma T, Sun Z, Liu Z, Hu Y, Le W. 3D printed conductive hydrogel patch incorporated with MSC@GO for efficient myocardial infarction repair. ACS Biomater Sci Eng. 2024;10(4):2451–2462. doi: 10.1021/acsbiomaterials.3c01837. [DOI] [PubMed] [Google Scholar]



