Abstract
Background/Aim
Hepatic steatosis is a significant independent risk factor for liver surgery because of its vulnerability to ischemia-reperfusion (IR) injury. Invariant natural killer T (iNKT) cells contribute to IR injury in healthy liver. We previously reported that activated iNKT cells mitigate liver IR injury and facilitate liver repair by interacting with macrophages. This study aimed to assess the role of activated iNKT cells in IR injury in steatotic livers.
Materials and Methods
Male C57/BL6 mice were fed a normal diet (ND) or high-fat diet (HFD) for 12 weeks before liver IR. iNKT cells were activated by intraperitoneal injection of α-galactosylceramide (α-GC) into HFD-fed mice. This study assessed liver injury, cytokine levels, and immune cell accumulation.
Results
HFD-fed mice exhibited increased levels of liver injury, pro-inflammatory mediators, and macrophages compared to those of ND-fed mice. Administration of α-GC to HFD-fed mice enhanced liver IR injury that was associated with increased numbers of iNKT cells and pro-inflammatory macrophages compared with those in the vehicle-treated group. Additionally, liver repair was delayed in α-GC-treated HFD-fed mice, as demonstrated by the increased necrotic area and decreased proliferating cell nuclear antigen expression. This was accompanied by reduced levels of anti-inflammatory mediators and reparative macrophages. Pro-inflammatory cytokine levels were increased in activated hepatic iNKT cells co-cultured with macrophages isolated from HFD-fed mice.
Conclusion
The activation of hepatic iNKT cells aggravates steatotic liver IR injury by upregulating pro-inflammatory mediators and macrophages, while suppressing anti-inflammatory mediators and reparative macrophages.
Keywords: Fatty liver, iNKT cells, inflammation, macrophages, ischemia, reperfusion
Introduction
Obesity has become a significant global health concern and is closely associated with various metabolic abnormalities, including metabolic syndrome, type 2 diabetes mellitus, and metabolic dysfunction-associated steatotic liver disease (MASLD). Recent advancements in the treatment of viral hepatitis have highlighted MASLD as the most prevalent chronic liver disorder, affecting an estimated 25% of the global population (1).
Liver ischemia-reperfusion (IR) injury during liver transplantation and resection is a significant concern. Additionally, the presence of hepatic steatosis increases the risk of liver failure. Steatotic livers are more vulnerable to IR injury and are an independent risk factor for liver surgery-associated complications, including early graft dysfunction, liver failure, and postoperative mortality (2). The increasing risk of liver failure and postoperative complications following hepatectomy limits the surgical options for patients with MASLD. Moreover, studies using rodent models have consistently demonstrated that hepatic steatosis exacerbates liver IR injury (3,4). Due to the growing need for liver transplants and the limited availability of suitable donor organs, suboptimal grafts, including those with hepatic steatosis, are being considered as viable options despite their increased susceptibility to IR injury (5). These circumstances have resulted in significant research into identifying modifiable immune mediators to minimize IR injury in these marginal organs. Therefore, hepatic steatosis presents a novel challenge for surgical patients who require liver resection and liver transplantation. However, the mechanisms by which the steatotic liver exacerbates liver IR injury remain unclear.
Invariant natural killer T (iNKT) cells recognize glycolipid antigens expressed on the surface molecule CD1d through the invariant T cell receptor (TCR). Upon activation, iNKT cells secrete significant amounts of cytokines, including interferon (IFN)-γ and interleukin (IL)-4, and chemokines that regulate subsequent immune responses, recruit innate effectors, and remove infected or damaged cells in physiological and pathological settings (6,7). A prototypic ligand for the iNKT cell TCR, α-galactosylceramide (α-GC) (8), has been widely employed to stimulate iNKT cells, specifically to rapidly induce significant amounts of various cytokines and chemokines (9).
Although activated iNKT cells can have protective or deleterious roles during liver IR injury, we previously demonstrated that iNKT cells mitigate liver IR injury and facilitate liver repair after IR injury by interacting with recruited macrophages in healthy mouse livers (10,11). This beneficial effect is mediated by accelerated macrophage polarization driven by IFN-γ and IL-4 production by iNKT cells. Similar effects of iNKT cells on tissue injury and repair were observed in mice with cardiac IR injury (12) and thermal liver injury (13). The findings indicate a synergistic relationship between iNKT cells and macrophages in alleviating liver inflammation and promoting recovery following acute liver injury. However, there remains a paucity of research examining the effects of activated iNKT cells on IR injury in steatotic livers. Therefore, this study aimed to assess whether activated iNKT cells also contribute to the attenuation of IR injury in steatotic livers and facilitate liver repair after steatotic liver injury.
Materials and Methods
Animals. Eight-week-old male C57BL/6 mice, weighing 20-23 g, were obtained from CLEA Japan (Tokyo, Japan). The mice were kept in a pathogen-free facility with controlled environmental conditions: humidity at 50±5%, temperature at 25±1˚C, and a 12-hour light-dark cycle. Animals had unlimited access to food and water. The Kitasato University School of Medicine’s Institutional Animal Care and Use Committee reviewed and approved the experimental protocols (2024-021). All experimental procedures followed the institution’s guidelines for animal research, which are based on the Science Council of Japan’s Guidelines for Proper Conduct of Animal Experiments.
Animal procedures. For a period of 12 weeks, C57BL/6 mice were provided with either a normal diet (ND) or a high-fat diet (HFD). Obesity was induced in mice by ad libitum feeding of HFD (60% kcal from fat; HFD32, CLEA Japan) for 12 weeks (14). ND (344.9 kcal/100 g, with 4.6% of calories from fat; CLEA Japan) was administered to age-matched animals, serving as a control group. Body weight was monitored weekly.
Following a previously described method (15), a mouse model of partial warm liver IR injury was established. To induce anesthesia, the mice received an intraperitoneal (i.p.) injection consisting of three components: 0.3 mg/kg medetomidine hydrochloride (Nippon Zenyaku Kogyo, Fukushima, Japan), 4.0 mg/kg midazolam (Astellas Pharma, Tokyo, Japan), and 5.0 mg/kg butorphanol (Meiji Seika Pharma, Tokyo, Japan). Liver ischemia was achieved by applying vascular clamps (ROBOZ Surgical Instrument, Washington, DC, USA) to restrict blood flow to the left and median liver lobes. Following 60 minutes of ischemia, the clamps were removed to initiate reperfusion. To reverse the effects of medetomidine, an i.p. injection of atipamezole (0.75 mg/kg; Nippon Zenyaku Kogyo) was administered. Mice in the sham group underwent identical surgical procedures but without occluding the blood vessels.
During the initiation of reperfusion, mice were given an i.p. injection of α-GC (Funakoshi, Tokyo, Japan) at a dose of 0.1 mg/kg body weight. The α-GC was dissolved in 0.1% dimethyl sulfoxide (DMSO) and then diluted in phosphate-buffered saline (PBS). The vehicle consisted of PBS containing 0.1% DMSO.
Experimental protocols. The mice underwent anesthesia through i.p. injection of a combination of anesthetic agents containing midazolam, medetomidine hydrochloride, and butorphanol at specified reperfusion periods. Cardiac blood samples were obtained and analyzed for alanine aminotransferase (ALT) activity, total cholesterol, and triglyceride levels in the serum using a Dri-Chem 7000 Chemistry Analyzer System (Fujifilm, Tokyo, Japan). The measurement of blood glucose levels was conducted using a glucose meter (TERUMO Corp., Tokyo, Japan). Following the collection of blood samples, the livers were removed. A small portion of each liver was preserved in 10% formaldehyde, and the residual liver specimen was placed in RNAiso Plus (Takara Bio, Shiga, Japan) for RNA extraction and polymerase chain reaction (PCR) quantification. After this process, the animals were euthanized humanely using the cervical dislocation method.
Histology and immunohistochemistry. Sections (3.5 μm thick) were prepared from paraffin-embedded tissues and subjected to either hematoxylin and eosin staining or immunostaining. The procedures for conducting histological and immunohistochemical evaluations, as well as the subsequent image analysis, were performed in accordance with previously described protocols (11).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. For the detection of IR-induced necrosis in steatotic livers, we used an in situ apoptosis detection kit (in Situ Cell Death Detection Kit; Roche Diagnostics, Mannheim, Germany). The TUNEL staining procedure was conducted following the instructions provided by the manufacturer. The level of necrosis was quantified by measuring the TUNEL-positive area as a percentage of the total area in five 200× magnification fields per animal. Using ImageJ (version 1.50i; National Institutes of Health, Bethesda, MD, USA), the size of the necrotic region was calculated in relation to the total area of the histological section. Results were reported as percentages of the total area.
Proliferating cell nuclear antigen (PCNA) staining. Sections of liver tissue were immunostained for PCNA using a rabbit monoclonal antibody (1:200, cat. no 13-3900; Thermo Fisher Scientific, Waltham, MA, USA). Histofine Simple Stain MAX PO (MULTI) (Nichirei, Tokyo, Japan) was employed to visualize the immune complexes. A Biozero BZ-700 Series microscope was employed to obtain images of the immunostained sections. Quantification of PCNA+ hepatocytes was performed in five 200× magnification fields per animal using the ImageJ software. The findings were expressed as the percentage of PCNA+ hepatocytes.
Isolation of intrahepatic leukocytes. The procedures for isolating leukocytes from the livers of animals were conducted according to previously established protocols (10). In brief, perfused livers were incubated in Roswell Park Memorial Institute (RPMI) containing 0.05% collagenase (Type IV; Sigma Chemical Co., St. Louis, MO, USA) for 20 min at 37˚C. Non-parenchymal cells were purified using density-gradient centrifugation with 33% Percoll (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Flow cytometry. The extracted non-parenchymal cells underwent treatment with an anti-mouse CD16/32 antibody (TruStain FcX; cat. no 101320; BioLegend, San Diego, CA, USA). This step was performed to prevent non-specific binding of the primary monoclonal antibody to the cell samples. The cells were then subjected to a staining procedure using a combination of specific reagents. These included phycoerythrin (PE)-conjugated anti-CD45 (30-F11; BioLegend), allophycocyanin/cyanine 7 (CY7)- conjugated anti-Ly6G (1A8; BioLegend), PE/Cy7-conjugated anti-CD11b (M1/70; BioLegend), Brilliant Violet 510-conjugated anti-Ly6C (HK1.4; BioLegend), anti-F4/80 (BM8; BioLegend), fluorescein isothiocyanate- conjugated anti-TCRβ (H57-597; BioLegend), and α-GalCer (PBS-57)-loaded CD1d tetramer. The NIH Tetramer Core Facility at Emory University (Atlanta, GE, USA) supplied the control CD1d-tetramer. The analysis excluded cells exhibiting positivity for 7-aminoactinomycin D (BioLegend). Sample examination was conducted using a FACS Verse cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data analysis was performed with Kaluza software v2.1 (Beckman Coulter, Brea, CA, USA). Cell quantification was carried out and normalized to cells per gram of liver tissue weight (cells/g).
Preparation and co-culture. Bone marrow (BM) cells were harvested from the femurs and tibias of HFD-fed mice for the generation of BM-derived macrophages. The procedures for the culture of BM-derived macrophages were carried out as described previously (10). Following a 7-day culture period of BM-derived macrophages, NKT cells were extracted from the livers of HFD-fed mice using the mouse NKT cells isolation kit (Miltenyi Biotec, Auburn, CA, USA). These cells (1×105/well) were then co-cultured with BM-derived macrophages (3×105/well) in 12-well plates. The culture medium consisted of RPMI 1640 enriched with 5% fetal calf serum. The co-culture was incubated for 72 h in the presence of α-GC (100 ng/ml) (10). Following a 72-hour incubation period, the supernatants were harvested for cytokine level assessment using the Cytometric Bead Array (BD Bioscience). The resulting data were subsequently analyzed with the BD Biosciences FCAP software (V3.0).
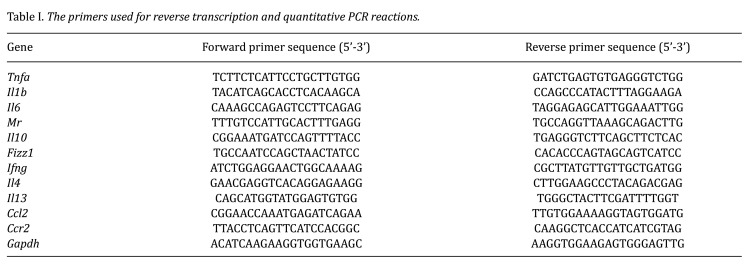
Real-time quantitative PCR analysis. RNA was isolated from liver samples using RNAiso Plus (Takara Bio, Inc., Shiga, Japan). The ReverTra Ace qPCR RT Kit (TOYOBO Co., Ltd., Osaka, Japan) was employed to synthesize cDNA from 1 μg of the extracted RNA. Quantitative PCR was performed using TB Green Premix Ex Taq II (Tli RNase H Plus; Takara Bio, Inc.) with the following thermal cycling conditions: 10 s at 95˚C, then 40 cycles of 3 s at 95˚C and 20 s at 60˚C. The comparative threshold cycle method was used to calculate mRNA expression levels, which were then normalized to glyceraldehyde-3-phosphate dehydrogenase expression in each sample. The primer sequences used are provided in Table I.
Table I. The primers used for reverse transcription and quantitative PCR reactions.
Statistical analysis. All results are expressed as the mean±standard deviation. All statistical analyses were performed using GraphPad Prism version 8 (GraphPad Software, La Jolla, CA, USA). Comparisons between two groups and multiple groups were performed using unpaired two-tailed Student’s t-test and one-way analysis of variance, respectively, followed by Tukey’s post hoc test. Statistical significance was set at p<0.05.
Results
HFD-fed mice exhibited MASLD. To assess the vulnerability of steatotic livers to IR injury, C57BL/6 mice were fed an HFD for 12 weeks. At the termination of the feeding period, the mice exhibited obesity, significant weight gain, and increased liver weight with fat droplets in the liver (Figure 1A-C). Additionally, they exhibited higher levels of ALT, total cholesterol, and triglycerides than those of ND-fed mice (Figure 1D). Blood glucose levels were higher in HFD-fed mice, and their glucose tolerance was impaired compared to that in ND-fed mice (Figure 1E). These data indicate that the livers of HFD-fed mice after 12 weeks exhibited MASLD.
Figure 1.
High-fat diet (HFD)-fed mice exhibited metabolic-dysfunction associated with steatotic liver disease. (A) Representative images of mice fed a normal diet (ND) or HFD for 12 weeks. Alterations in body weight gain during ND and HFD feeding. (B) Representative images of livers in the abdomen (left panel), extirpated livers (middle panel), and liver weights (right panel) of ND- and HFD-fed mice. (C) Representative photomicrographs of hematoxylin and eosin-stained liver sections from ND- and HFD-fed mice. Scale bar, 200 μm. (D) Alanine aminotransferase (ALT), total cholesterol, and triglyceride levels in ND- and HFD-fed mice. (E) Fasting blood glucose levels in ND- and HFD-fed mice. Alterations in blood glucose levels after intraperitoneal glucose tolerance test (2 g/kg BW glucose administration) in ND- and HFD-fed mice (n=7 mice/group). Data are expressed as the mean±standard deviation (SD). ****p<0.0001, using analysis of variance (ANOVA) followed by Tukey’s test (A) and Student’s t-test (B, D, E).
HFD-fed mice are susceptible to liver IR injury. To assess whether steatotic livers are susceptible to IR, we compared IR injury between steatotic and healthy livers. ALT levels, a marker of liver injury, were significantly higher in HFD-fed mice than in ND-fed mice (Figure 2A). TUNEL staining confirmed necrotic cell death in mouse livers after IR, with a diffuse TUNEL staining pattern, as reported previously (16). Based on these findings, we quantified the necrotic areas as indicated by the TUNEL-positive area. The hepatic necrotic areas in the HFD-fed mice were larger than those in the ND-fed mice (Figure 2A). Additionally, the mRNA levels of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6, were higher in HFD-fed mice than in ND-fed mice (Figure 2B). Moreover, the numbers of pro-inflammatory (Ly6Chigh/F4/80high cells) and reparative macrophages (Ly6Clow/F4/80high cells) were higher in steatotic livers than in healthy livers (Figure 2C). These results indicated that HFD exacerbated IR-induced inflammation in steatotic livers.
Figure 2.
The steatotic liver is vulnerable to ischemia-reperfusion (IR) injury. (A) Alanine aminotransferase (ALT) levels and the percentage of hepatic necrotic area during IR injury in normal diet (ND)- and high-fat diet (HFD)-fed mice. Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of liver sections from the indicated groups. Scale bar, 200 μm. (B) Expression of mRNA encoding genes associated with a pro-inflammatory macrophage phenotype, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6 in the livers of ND- and HFD-fed mice after liver IR injury. (C) Representative dot plots for pro-inflammatory and reparative macrophages in the livers of ND- and HFD-fed mice at 48 h post-reperfusion. After gating out Ly6Ghigh and CD11bhigh cells, cells were separated into these subsets based on Ly6C and F4/80 expression. The percentage of hepatic pro-inflammatory (Ly6Chigh/F4/80high cells) and reparative macrophages (Ly6Clow/F4/80high cells) in ND- and HFD-fed mice 48 h post-reperfusion. Data are expressed as the mean±SD. *p<0.05, ***p<0.001, and ****p<0.0001, using analysis of variance (ANOVA) followed by Tukey’s test (A, B) and Student’s t-test (C).
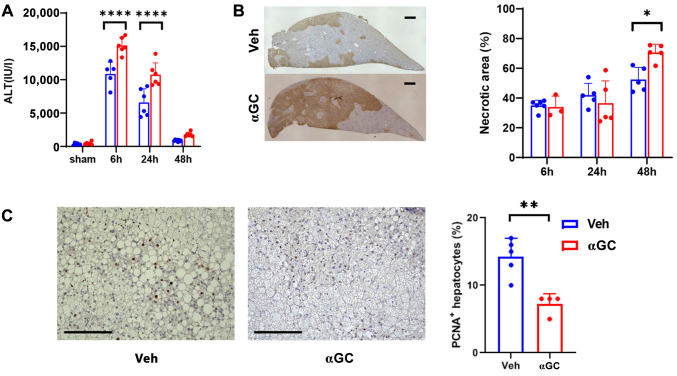
Activated iNKT cells stimulated with αGC increased IR injury in steatotic livers. We previously demonstrated that activated iNKT cells stimulated with αGC attenuated IR injury in normal livers and facilitated liver repair by interacting with accumulated macrophages in mice (10,11). Therefore, we assessed whether αGC-induced activation of iNKT cells mitigates IR injury in steatotic livers. αGC was administered to HFD-fed IR mice. We observed that the ALT levels at 6 and 24 h post-reperfusion and hepatic necrotic area at 48 h post-reperfusion in αGC-treated HFD-fed mice were higher than those in vehicle-treated HFD-fed mice (Figure 3A). The hepatic necrotic areas in αGC-treated HFD-fed mice were larger than those in vehicle-treated HFD-fed mice (Figure 3B). Immunocytochemistry for PCNA revealed hepatocyte proliferation during liver IR injury. The number of PCNA+ hepatocytes in αGC-treated HFD-fed mice was lower than that in vehicle-treated HFD-fed ones (Figure 3C). These results indicated that activated iNKT cells aggravate IR injury in steatotic livers and delay liver repair after IR injury.
Figure 3.
α-galactosylceramide (α-GC) enhances steatotic liver injury in ischemia-reperfusion (IR). (A, B) Alterations in alanine aminotransferase (ALT) levels (A) and the percentage of hepatic necrotic area (B) in high-fat diet (HFD)-fed mice treated with α-GC or vehicle after liver IR injury. Representative photomicrographs of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-stained liver sections from HFD-fed mice treated with α-GC or vehicle 48 h post-reperfusion. Scale bar, 200 μm. (C) Proliferating cell nuclear antigen (PCNA)+-hepatocytes in HFD-fed mice treated with α-GC or vehicle 48 h post-reperfusion. Representative PCNA staining of liver sections from HFD-fed mice treated with α-GC. Scale bars, 200 μm. (D) Expression of mRNA encoding genes associated with a pro-inflammatory macrophage phenotype, including TNF-α, IL-1β, and IL-6; a reparative macrophage phenotype including mannose receptor (MR), IL-10, and Fizz1; type 1 helper T cells (Th1), including interferon gamma (IFN-γ); and Th2, including IL-4 and IL-13 cytokines in the livers of HFD-fed mice treated with α-GC vehicle after liver IR injury. Data are expressed as the mean±standard deviation (SD). *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001, using analysis of variance (ANOVA) followed by Tukey’s test (A, B, D) and Student’s t-test (C).
Additionally, the mRNA levels of pro-inflammatory mediators, including TNF-α, IL-1β, and IL-6, were higher in αGC-treated HFD-fed mice than in vehicle-treated HFD-fed mice (Figure 3D). In contrast, the mRNA levels of anti-inflammatory mediators, including mannose receptor (MR), IL-10, and Fizz1 in αGC-treated HFD-fed mice, were lower than those in vehicle-treated HFD-fed mice (Figure 3D). Because activated iNKT cells produce a mixture of T helper type 1 (Th1) and Th2 cytokines, such as IFN-γ, IL-4, and IL-13, we determined the mRNA levels of these cytokines in the livers 48 h post-reperfusion (Figure 3D). The mRNA levels of IFN-γ in αGC-treated HFD-fed mice increased by 7.6-fold compared to those in vehicle-treated HFD-fed mice. In contrast, the mRNA levels of IL-4 in αGC-treated HFD-fed mice increased by 1.8-fold compared to those in vehicle-treated HFD-fed mice, and no difference in IL-13 mRNA levels was observed between HFD-fed mice treated with α-GC and vehicle.
In summary, these results indicate that αGC-induced aggravated IR injury in steatotic livers appears to be caused by enhanced pro-inflammatory and suppressed anti-inflammatory mediators. Additionally, these results indicate that αGC treatment increased the mRNA levels of pro-inflammatory macrophages and decreased those of reparative macrophages during IR injury in the steatotic livers of HFD-fed mice.
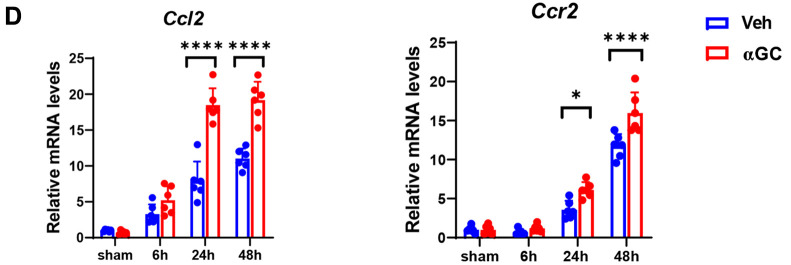
Changes in iNKT cells and macrophages during IR injury in steatotic livers. To understand the role of iNKT cells during IR injury in steatotic livers, we measured iNKT cells 48 h post-reperfusion. iNKT cells in steatotic livers were identified as TCR-β/CD1d tetramer cells through flow cytometry. The number of iNKT cells in αGC-treated HFD-fed mice was higher than that in vehicle-treated HFD-fed mice (Figure 4A). iNKT cells regulate liver IR injury by interacting with macrophages (10). Additionally, enhanced expression of genes associated with pro-inflammatory macrophages and reduced expression of genes associated with reparative macrophages were observed during IR injury in steatotic livers. At 48 h post-reperfusion, αGC-treated HFD-fed mice had a higher number of pro-inflammatory macrophages (Ly6Chigh/F4/80high cells) and fewer reparative macrophages (Ly6Clow/F4/80high cells) than those of vehicle-treated HFD-fed mice (Figure 4B, C).
Figure 4.
α-galactosylceramide (α-GC) increased the number of invariant natural killer T (iNKT) cells and pro-inflammatory macrophages and decreased the number of reparative macrophages after ischemia-reperfusion (IR) injury in steatotic livers. (A) Representative dot plots for iNKT cells [T cell receptor (TCR)-β+/CD1d tetramer+] in the livers of high-fat diet (HFD)-fed mice treated with α-GC or vehicle at 48 h post-reperfusion (left). The number of hepatic iNKT cells in HFD-fed mice treated with α-GC or vehicle (right). (B) Representative dot plots and gating strategies for pro-inflammatory and reparative macrophages. (C) Representative dot plots for pro-inflammatory (Ly6Chigh/F4/80high cells) and reparative macrophages (Ly6Clow/F4/80high cells) in the livers of HFD-fed mice treated with α-GC or vehicle at 48 h post-reperfusion (left). The number of hepatic pro-inflammatory and reparative macrophages in HFD-fed mice treated with α-GC or vehicle (right). (D) mRNA levels of chemokines and their receptors, C-C motif chemokine ligand 2 (CCL2) and C-C chemokine receptor type 2 (CCR2) in the livers of HFD-fed mice treated with α-GC or vehicle after liver IR injury. Data are expressed as the mean±standard deviation (SD). *p<0.05, **p<0.01, and ****p<0.0001, using analysis of variance (ANOVA) followed by Tukey’s test.
Additionally, we measured the mRNA levels of chemokine (C-C motif) ligand 2 and C-C chemokine receptor type 2 in steatotic livers. Their hepatic expression was higher in the αGC-treated mice than in the vehicle-treated mice (Figure 4D). In summary, these results indicate that αGC-induced activation of iNKT cells exacerbates IR injury in steatotic livers by increasing the number of pro-inflammatory macrophages and reducing those of reparative macrophages.
Interaction of cultured iNKT cells with macrophages. We assessed whether the interaction between iNKT cells and macrophages increases pro-inflammatory cytokine levels in vitro. Co-culture of iNKT cells with BM-derived macrophages from HFD-fed mice increased the levels of TNF-α and IL-6, but not IL-1β, in the presence of αGC (Figure 5).
Figure 5.

The interaction of cultured hepatic invariant natural killer T (iNKT) cells with macrophages increases the levels of pro-inflammatory cytokines. Cytokine (tumor necrosis factor-alpha (TNF-α,), interleukin (IL)-1β, and IL-6 levels in supernatants from the co-culture of hepatic iNKT cells and bone marrow-derived macrophages stimulated with α-galactosylceramide (GC) or vehicle isolated from high-fat diet (HFD)-fed mice. Representative data from two independent experiments are shown. Data are expressed as the mean±standard deviation (SD). ****p<0.0001, using Student’s t-test.
Discussion
Current studies demonstrate that iNKT cell activation exacerbates IR injury in steatotic livers and impairs liver repair after IR injury. Significantly enhanced pro-inflammatory mediators and attenuated anti-inflammatory mediators were associated with aggravated IR injury in steatotic livers of mice treated with αGC. Increased activation of iNKT cells was associated with an increased number of pro-inflammatory macrophages and decreased numbers of reparative macrophages in the steatotic livers of αGC-treated IR mice. These results indicated that iNKT cell activation exacerbates steatotic liver inflammation by increasing the number of pro-inflammatory macrophages and decreasing those of reparative macrophages.
Obesity is a global health issue, affecting 25% of the population, and is associated with MASLD (1). Obesity-induced MASLD contributes to the development of hepatocellular carcinoma (HCC) (17). Furthermore, excessive accumulation of cholesterol resulting from obesity impairs the anti-tumor cytotoxic function of NKT cells within MASLD-affected livers, potentially facilitating the progression of HCC (18). In this study, the mice fed HFD for 12 weeks exhibited increased liver weight, ALT, total cholesterol, triglycerides, and blood sugar levels, as well as impaired glucose intolerance, which are characteristics of MASLD. Additionally, patients with obesity undergoing liver surgery demonstrated high morbidity and mortality rates. This is attributed to the susceptibility to IR injury in steatotic livers, accompanied by the impairment of liver repair and regeneration. Recent single-cell RNA sequencing analysis has revealed that transplanted fatty donor livers exhibit pro-inflammatory macrophage accumulation compared to that of non-fatty donor livers (19). Additionally, our data confirmed that IR stress exacerbated fatty liver damage, delayed liver repair, and enhanced pro-inflammatory cytokine expression in the livers of mice, consistent with previous findings (20). Moreover, this study demonstrated that a 12-week HFD feeding increased the number of pro-inflammatory and reparative macrophages in steatotic livers before inducing liver IR compared with that of ND feeding. Recent evidence indicates that monocyte-derived macrophages accumulate in steatotic livers, exhibiting a distribution distinct from that observed in healthy livers (21). Increased basal levels of monocyte-derived macrophages during HFD feeding may exacerbate IR injury in steatotic livers in response to IR.
Various immune cells contribute to liver IR injury followed by liver repair (22). iNKT cells mitigate liver IR injury and facilitate liver repair after IR injury by interacting with macrophages in healthy murine livers (10,11). In this study, we assessed whether iNKT cells have beneficial effects on inflammation in steatotic liver. The number of iNKT cells in HFD-fed mice were reduced compared to that in ND-fed mice (23). This is consistent with other reports, although certain studies have indicated iNKT cell recruitment (24). However, studies of NKT cells and their association with MASLD in humans are limited. Despite a reduction in the number of NKT cells in steatotic livers, activated NKT cells stimulated with αGC exacerbated IR injury in steatotic livers and delayed liver repair after IR injury. In αGC-treated HFD-fed mice, the mRNA levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, were increased, and the mRNA levels of anti-inflammatory mediators, including MR, IL10 and Fizz1, were attenuated compared to those in vehicle-treated HFD-fed mice. Concomitantly, the number of pro-inflammatory macrophages increased, whereas the number of reparative macrophages decreased in αGC-treated HFD-fed mice. Our previous data indicate that liver IR injury followed by liver repair is regulated by activated iNKT cell-macrophage interactions (10,11). This study indicated that activated iNKT cells interact with macrophages to enhance the inflammatory response to IR in steatotic livers. To support this possibility, co-culturing activated iNKT cells with macrophages isolated from steatotic livers of mice resulted in enhanced production of inflammatory cytokines, including TNF-α and IL-6.
The results of this study revealed that αGC-treated mice exhibited increased mRNA levels of IFN-γ and IL-4 in steatotic livers compared to their vehicle-treated counterparts. This increase in mRNA levels coincided with the enhanced accumulation of iNKT cells in the αGC-treated HFD-fed mice. Activated iNKT cells are believed to produce both IFN-γ and IL-4 during liver IR injury (22). Notably, the increase in IFN-γ levels was more significant than that of IL-4 levels in αGC-treated mice. These observations indicate that IFN-γ, along with pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, plays a role in exacerbating IR injury in steatotic livers. This is consistent with recent research demonstrating that IFN-γ produced by iNKT cells in steatotic livers aggravates IR injury (25).
In this study, we observed that iNKT cells and pro-inflammatory macrophages accumulate in inflamed steatotic livers. These findings indicate that activated iNKT cells stimulated with αGC induce macrophage polarization toward a pro-inflammatory phenotype, thereby advancing inflammation during IR injury in steatotic livers. Additionally, IFN-γ produced by iNKT cells facilitates macrophage polarization toward a pro-inflammatory phenotype during liver IR injury (10). Therefore, IFN-γ is implicated in pro-inflammatory macrophage polarization to induce IR injury in steatotic livers. Although activated iNKT cells interact with macrophages that express CD1d during IR injury in steatotic livers, CD1d is also expressed by other immune cells, including NK cells, dendritic cells, B and T lymphocytes, hepatocytes, hepatic stellate cells, and liver sinusoidal endothelial cells (7,26). Additionally, CD1d expression in steatotic livers was reduced in both patients and mice with metabolic dysfunction-associated steatohepatitis (26). However, the relevance and potential effects of CD1d-expressing cells other than macrophages on IR injury in steatotic livers remain unclear.
These experimental data indicated that the interaction between activated hepatic iNKT cells and macrophages in steatotic livers plays a role in exacerbating liver IR injury in murine models. Conversely, these results suggest that disrupting the communication between iNKT cells and macrophages can potentially mitigate IR injury in steatotic livers. Additional research is required to further elucidate this phenomenon. The current data were derived from mouse studies and not human subjects. Consequently, future assessments are essential to confirm these observations in patients with obesity who have undergone liver IR.
Conclusion
Our studies demonstrated that activated iNKT cells stimulated with αGC exacerbated IR injury in steatotic livers by increasing pro-inflammatory mediators and macrophages, and delayed liver repair by reducing anti-inflammatory mediators and macrophages. Therefore, our results indicate that inhibition of the interaction between iNKT cells and macrophages may mitigate IR injury in the steatotic liver.
Funding
This research was supported by a research grant (23K08178) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Conflicts of Interest
The Authors declare that there are no conflicts of interest in relation to this study.
Authors’ Contributions
YK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft; YI: Formal analysis, Investigation, Writing - review & editing. NN, MT, TG, AY, and KH: Data curation, Investigation, Methodology; MS: Methodology, Resources; YK, NH, and HA: Supervision, Validation, Writing - review & editing.
Acknowledgements
The Authors thank Michiko Ogino for the technical assistance.
References
- 1.Henry L, Paik J, Younossi ZM. Review article: the epidemiologic burden of non‐alcoholic fatty liver disease across the world. Aliment Pharmacol Ther. 2022;56(6):942–956. doi: 10.1111/apt.17158. [DOI] [PubMed] [Google Scholar]
- 2.Gehrau RC, Mas VR, Dumur CI, Suh JL, Sharma AK, Cathro HP, Maluf DG. Donor hepatic steatosis induce exacerbated ischemia-reperfusion injury through activation of innate immune response molecular pathways. Transplantation. 2015;99(12):2523–2533. doi: 10.1097/TP.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa T, Ito Y, Wijeweera J, Liu J, Malle E, Farhood A, McCuskey RS, Jaeschke H. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1385–G1395. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liss KHH, McCommis KS, Chambers KT, Pietka TA, Schweitzer GG, Park SL, Nalbantoglu I, Weinheimer CJ, Hall AM, Finck BN. The impact of diet-induced hepatic steatosis in a murine model of hepatic ischemia/reperfusion injury. Liver Transpl. 2018;24(7):908–921. doi: 10.1002/lt.25189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinaixa C, Selzner N, Berenguer M. Fat and liver transplantation: clinical implications. Transpl Int. 2018;31(8):828–837. doi: 10.1111/tri.13288. [DOI] [PubMed] [Google Scholar]
- 6.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19(3):354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18(9):559–574. doi: 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of V α 14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20(3):358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto T, Ito Y, Satoh M, Nakamoto S, Nishizawa N, Hosono K, Naitoh T, Eshima K, Iwabuchi K, Hiki N, Amano H. Activation of iNKT cells facilitates liver repair after hepatic ischemia reperfusion injury through acceleration of macrophage polarization. Front Immunol. 2021;12:754106. doi: 10.3389/fimmu.2021.754106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto T, Ito Y, Nishizawa N, Kuroda YU, Nakamoto S, Hosono K, Naitoh T, Hiki N, Amano H. Expansion of iNKT cells promotes liver repair following hepatic ischemia reperfusion injury. In Vivo. 2022;36(6):2604–2614. doi: 10.21873/invivo.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, Taniguchi M, Nakayama T, Ishimori N, Iwabuchi K, Tsutsui H. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res. 2012;111(8):1037–1047. doi: 10.1161/CIRCRESAHA.112.270132. [DOI] [PubMed] [Google Scholar]
- 13.Liew PX, Lee WY, Kubes P. iNKT cells orchestrate a switch from inflammation to resolution of sterile liver injury. Immunity. 2017;47(4):752–765.e5. doi: 10.1016/j.immuni.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Hosono K, Yamashita A, Tanabe M, Ito Y, Majima M, Tsujikawa K, Amano H. Deletion of RAMP1 signaling enhances diet-induced obesity and fat absorption via intestinal lacteals in mice. In Vivo. 2024;38(1):160–173. doi: 10.21873/invivo.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishizawa N, Ito Y, Eshima K, Ohkubo H, Kojo K, Inoue T, Raouf J, Jakobsson PJ, Uematsu S, Akira S, Narumiya S, Watanabe M, Majima M. Inhibition of microsomal prostaglandin E synthase-1 facilitates liver repair after hepatic injury in mice. J Hepatol. 2018;69(1):110–120. doi: 10.1016/j.jhep.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Ni HM, Chao X, Kaseff J, Deng F, Wang S, Shi YH, Li T, Ding WX, Jaeschke H. Receptor-interacting serine/threonine-protein kinase 3 (RIPK3)-mixed lineage kinase domain-like protein (MLKL)-mediated necroptosis contributes to ischemia-reperfusion injury of steatotic livers. Am J Pathol. 2019;189(7):1363–1374. doi: 10.1016/j.ajpath.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyashita S, Shimizu T, Niki M, Sato S, Tanaka G, Yamaguchi T, Park KH, Matsumoto T, Shiraki T, Mori S, Aoki T. Clinical impact of cellular senescence and RNA dysregulation in HCC is associated with MASLD and HCV-SVR. Anticancer Res. 2025;45(2):651–659. doi: 10.21873/anticanres.17452. [DOI] [PubMed] [Google Scholar]
- 18.Tang W, Zhou J, Yang W, Feng Y, Wu H, Mok MTS, Zhang L, Liang Z, Liu X, Xiong Z, Zeng X, Wang J, Lu J, Li J, Sun H, Tian X, Yeung PC, Hou Y, Lee HM, Lam CCH, Leung HHW, Chan AWH, To KF, Wong J, Lai PBS, Ng KKC, Wong SKH, Wong VWS, Kong APS, Sung JJY, Cheng ASL. Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver. Cell Mol Immunol. 2022;19(7):834–847. doi: 10.1038/s41423-022-00872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Lu D, Wang R, Lian Z, Lin Z, Zhuo J, Chen H, Yang M, Tan W, Yang M, Wei X, Wei Q, Zheng S, Xu X. Single-cell profiling reveals distinct immune phenotypes that contribute to ischaemia-reperfusion injury after steatotic liver transplantation. Cell Prolif. 2021;54(10):e13116. doi: 10.1111/cpr.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng M, Weng Y, Cao Y, Zhang C, Lin Y, Yu W. Caspase 6/NR4A1/SOX9 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed fatty liver. Cell Death Discov. 2023;9(1):106. doi: 10.1038/s41420-023-01396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease – novel insights into cellular communication circuits. J Hepatol. 2022;77(4):1136–1160. doi: 10.1016/j.jhep.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y, Hosono K, Amano H. Responses of hepatic sinusoidal cells to liver ischemia-reperfusion injury. Front Cell Dev Biol. 2023;11:1171317. doi: 10.3389/fcell.2023.1171317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, Tsutsui H, Onoé K, Iwabuchi K. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One. 2012;7(2):e30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Herck MA, Weyler J, Kwanten WJ, Dirinck EL, De Winter BY, Francque SM, Vonghia L. The differential roles of T cells in non-alcoholic fatty liver disease and obesity. Front Immunol. 2019;10:82. doi: 10.3389/fimmu.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liggett JR, Kang J, Ranjit S, Rodriguez O, Loh K, Patil D, Cui Y, Duttargi A, Nguyen S, He B, Lee Y, Oza K, Frank BS, Kwon D, Li HH, Kallakury B, Libby A, Levi M, Robson SC, Fishbein TM, Cui W, Albanese C, Khan K, Kroemer A. Oral N-acetylcysteine decreases IFN-γ production and ameliorates ischemia-reperfusion injury in steatotic livers. Front Immunol. 2022;13:898799. doi: 10.3389/fimmu.2022.898799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei Z, Yu J, Wu Y, Shen J, Lin S, Xue W, Mao C, Tang R, Sun H, Qi X, Wang X, Xu L, Wei C, Wang X, Chen H, Hao P, Yin W, Zhu J, Li Y, Wu Y, Liu S, Liang H, Chen X, Su C, Zhou S. CD1d protects against hepatocyte apoptosis in non-alcoholic steatohepatitis. J Hepatol. 2024;80(2):194–208. doi: 10.1016/j.jhep.2023.10.025. [DOI] [PubMed] [Google Scholar]